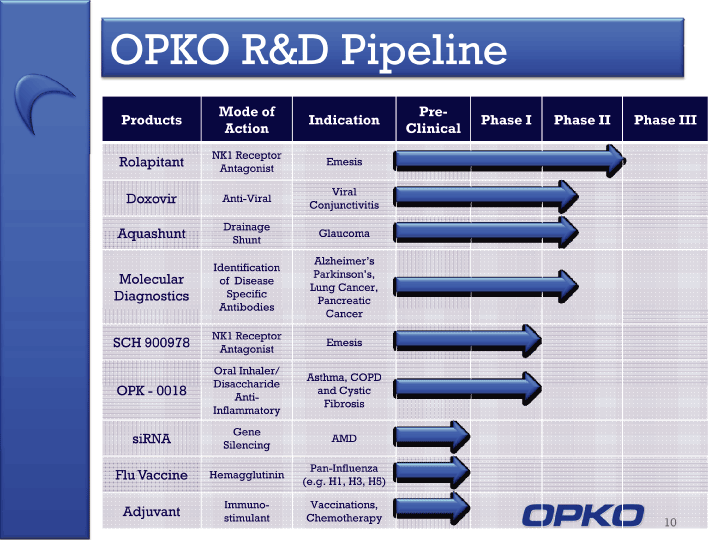
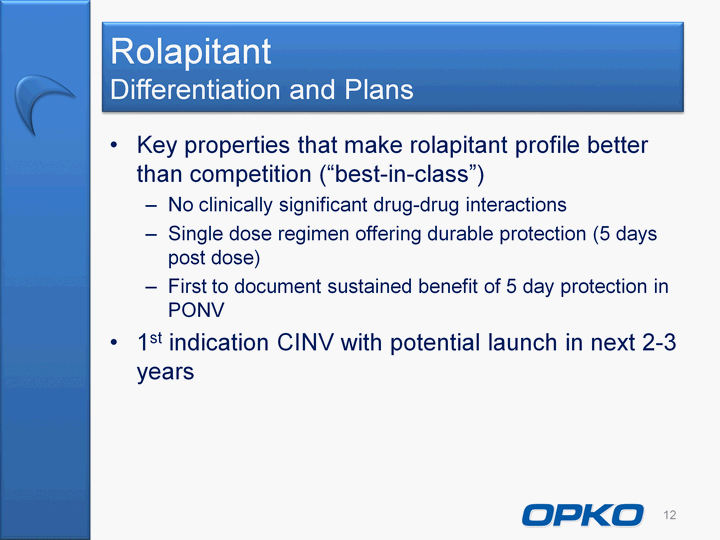
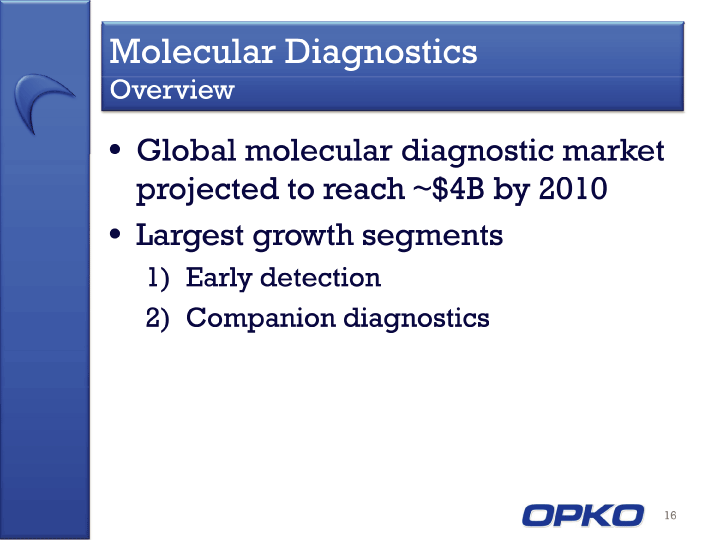
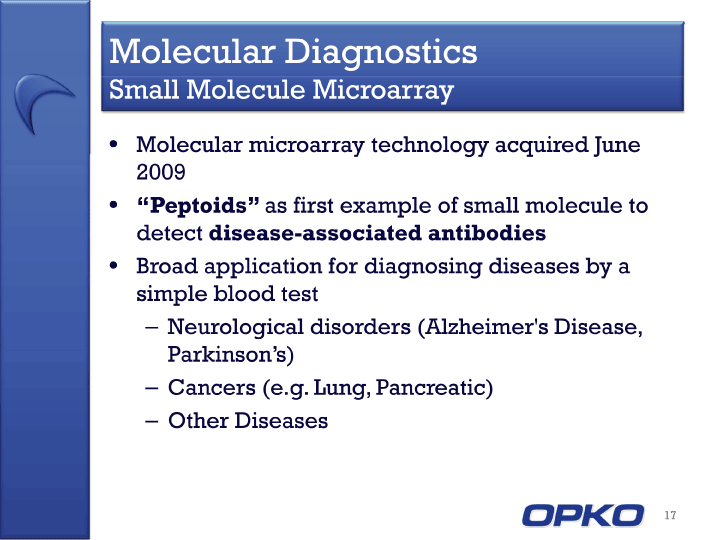
| Cautionary Statement This presentation contains "forward-looking statements," as that term is defined under the Private Securities Litigation Reform Act of 1995 (PSLRA), which statements may be identified by words such as "expects," "plans," "projects," "will," "may," "anticipates," "believes," "should," "intends," "estimates," and other words of similar meaning, including statements regarding our ability to develop oral and IV formulations of rolapitant and simple blood tests for neurological disorders, cancers and other disease, our product development efforts and expected timing thereof, the products' potential benefits, statements regarding rolapitant being a best-in-class product, the timing of clinical trials for our product candidates and the commercial launch of rolapitant and our other product candidates, and estimates regarding market potential and timing of regulatory approval for our product candidates, as well as other non-historical statements. These forward-looking statements are only predictions and reflect our views as of the date they were made, and we undertake no obligation to update such statements. Such statements are subject to many risks and uncertainties that could cause our actual activities or results to differ materially from the activities and results anticipated in forward-looking statements, including risks inherent in funding, developing and obtaining regulatory approvals of new, commercially-viable and competitive products and treatments, general market factors, competitive product development, product availability, federal and state regulations and legislation, the regulatory process for new products and indications, manufacturing issues that may arise, the possibility of infringing a third party's patents or other intellectual property rights, the uncertainty of obtaining patents covering our products and processes and in successfully enforcing them against third parties, and the possibility of litigation, among other factors. 2 |