Filing under Rule 425 under
the U.S. Securities Act of 1933
Filing by: Daiichi Pharmaceutical Co., Ltd.
Subject Company: Daiichi Pharmaceutical Co., Ltd.
and Sankyo Company, Limited
SEC File No. 132-02290

A Global Pharma Innovator
May 13, 2005 (Friday)

Focus of this Presentation
This presentation contains statements concerning forecasts for Sankyo Co., Ltd. (“Sankyo”), Daiichi Pharmaceutical Co., Ltd. (“Daiichi”) and their group companies. These statements are based on assumptions and judgments made with information available at the time of preparation, and as such are subject to existing and unforeseen risks, as well as uncertainties and other factors.
Risks, uncertainties and other factors may cause business performance, management actions and financial results to differ significantly from those presented in this material.
This presentation is not disclosure material in accordance with the Securities Exchange Law, and as such there is no guarantee as to the accuracy of the information.
Readers are therefore cautioned not to rely solely on this presentation when making an evaluation of the proposed business integration.
1

Contents
1 Transaction Overview
2 Benefits of Transaction
3 Integration Strategy and Global Growth
4 Management Structure and Integration Schedule
2

1-1 Profile of the New Company
Name DAIICHI SANKYO COMPANY, LIMITED
Head office location 3-5-1 Nihonbashi Honcho, Chuo-ku, Tokyo
Date of both companies’ delisting September 21, 2005
Stock transfer date September 28, 2005
Listing of the holding company September 28, 2005
Stock transfer ratio New company 1 : Sankyo 1 New company 1.159 : Daiichi 1
Stock exchange listings Tokyo, Osaka, Nagoya
3

1-2 Vision for Business Integration
Sankyo and Daiichi: Creating a global pharma innovator
Capability to create innovative products
Operating efficiency at highest industry standard Established global presence and growth potential Strengthen formidable position in Japan Accelerated earnings growth
Maximize Corporate Value
Customers
Fulfill medical needs with innovative pharmaceuticals and services
Employees
Equitable placement and promotion Appropriate compensation based on work and performance Support for career development
Society
Corporate activity based on high ethical standards Promotion of environmental management Contribute to medical and pharmaceutical science
Shareholders
Enhanced shareholder value
Return value to shareholders through dividend—highest levels in Japan
4
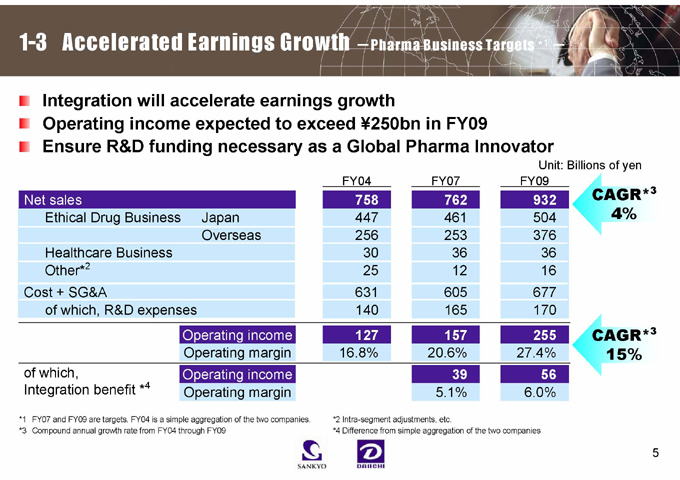
1-3 Accelerated Earnings Growth -Pharma Business Targets *1 -
Integration will accelerate earnings growth
Operating income expected to exceed ¥250bn in FY09 Ensure R&D funding necessary as a Global Pharma Innovator
Net sales Operating income 127 157 255 758 762 932
Unit: Billions of yen
FY04 FY07 FY09
Ethical Drug Business Japan 447 461 504
Overseas 256 253 376
Healthcare Business 30 36 36
Other*2 25 12 16
Cost + SG&A 631 605 677
of which, R&D expenses 140 165 170
Operating margin 16.8% 20.6% 27.4%
of which, Operating income 39 56
Integration benefit *4 Operating margin 5.1% 6.0%
CAGR*3 4%
CAGR*3 15%
*1 FY07 and FY09 are targets. FY04 is a simple aggregation of the two companies.
*3 Compound annual growth rate from FY04 through FY09
*2 Intra-segment adjustments, etc.
*4 Difference from simple aggregation of the two companies
5
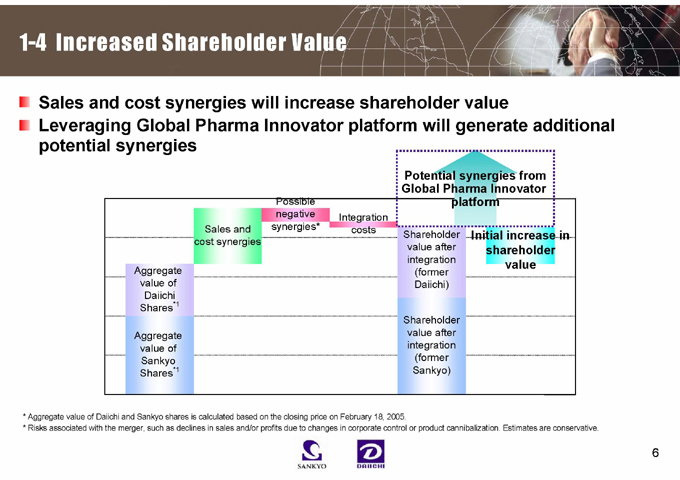
1-4 Increased Shareholder Value
Sales and cost synergies will increase shareholder value
Leveraging Global Pharma Innovator platform will generate additional potential synergies
Potential synergies from Global Pharma Innovator platform
Sales and cost synergies
Possible negative synergies*
Integration costs
Aggregate value of Daiichi Shares*1
Aggregate value of Sankyo Shares*1
Shareholder value after integration (former Daiichi)
Shareholder value after integration (former Sankyo)
Initial increase in shareholder value
* Aggregate value of Daiichi and Sankyo shares is calculated based on the closing price on February 18, 2005.
* Risks associated with the merger, such as declines in sales and/or profits due to changes in corporate control or product cannibalization. Estimates are conservative.
6

1-5 Shareholder Returns at Highest Levels in Japan
Committed to return value to shareholders at highest levels in Japan
Committed to growing DPS (dividend per share) in FY05 and increase further
Targeted to deliver DOE (Dividend on Equity) of 5% for FY09
7
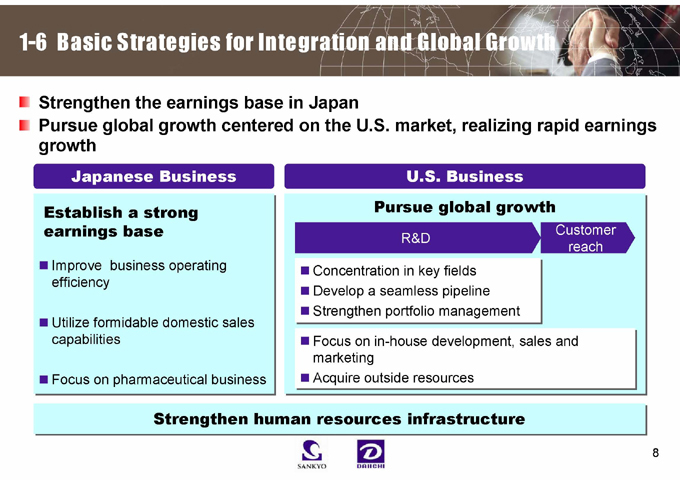
1-6 Basic Strategies for Integration and Global Growth
Strengthen the earnings base in Japan
Pursue global growth centered on the U.S. market, realizing rapid earnings growth
Japanese Business
Establish a strong earnings base
Improve business operating efficiency
Utilize formidable domestic sales capabilities
Focus on pharmaceutical business
U.S. Business
Pursue global growth
R&D
Customer reach
Concentration in key fields Develop a seamless pipeline Strengthen portfolio management
Focus on in-house development, sales and marketing Acquire outside resources
Strengthen human resources infrastructure
8

1 Transaction Overview
2 Benefits of Transaction
3 Integration Strategy and Global Growth
4 Management Structure and Integration Schedule
9
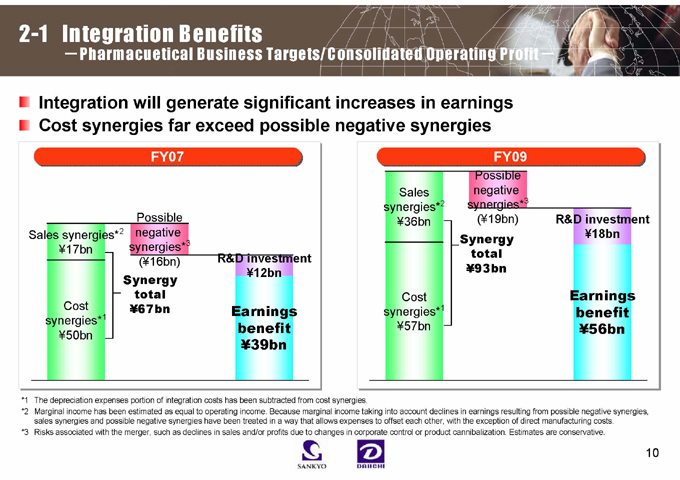
2-1 Integration Benefits
- Pharmacuetical Business Targets/Consolidated Operating Profit-
Integration will generate significant increases in earnings Cost synergies far exceed possible negative synergies
FY07
Sales synergies*2 ¥17bn
Possible negative synergies*3 (¥16bn)
R&D investment ¥12bn
Cost synergies*1 ¥50bn
Synergy total ¥67bn
Earnings benefit ¥39bn
FY09
Sales synergies*2 ¥36bn
Possible negative synergies*3 (¥19bn)
R&D investment ¥18bn
Synergy total ¥93bn
Cost synergies*1 ¥57bn
Earnings benefit ¥56bn
*1 The depreciation expenses portion of integration costs has been subtracted from cost synergies.
*2 Marginal income has been estimated as equal to operating income. Because marginal income taking into account declines in earnings resulting from possible negative synergies, sales synergies and possible negative synergies have been treated in a way that allows expenses to offset each other, with the exception of direct manufacturing costs.
*3 Risks associated with the merger, such as declines in sales and/or profits due to changes in corporate control or product cannibalization. Estimates are conservative.
10
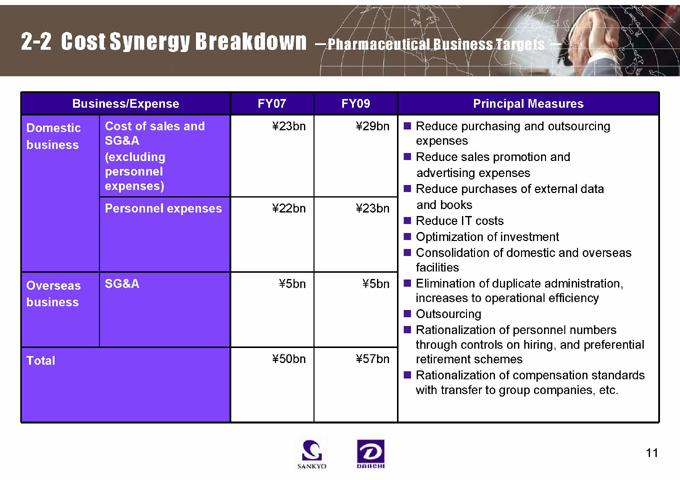
2-2 Cost Synergy Breakdown -Pharmaceutical Business Targets -
Business/Expense FY07 FY09
Domestic Cost of sales and ¥23bn ¥29bn
business SG&A
(excluding personnel expenses)
Personnel expenses ¥22bn ¥23bn
Overseas business SG&A ¥5bn ¥5bn
Total ¥50bn ¥57bn
Principal Measures
Reduce purchasing and outsourcing expenses Reduce sales promotion and advertising expenses Reduce purchases of external data and books Reduce IT costs Optimization of investment Consolidation of domestic and overseas facilities Elimination of duplicate administration, increases to operational efficiency Outsourcing Rationalization of personnel numbers through controls on hiring, and preferential retirement schemes Rationalization of compensation standards with transfer to group companies, etc.
11
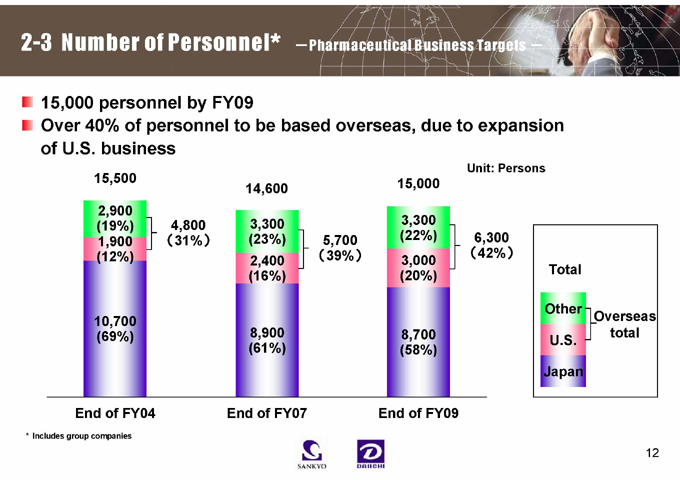
2-3 Number of Personnel* -Pharmaceutical Business Targets -
15,000 personnel by FY09
Over 40% of personnel to be based overseas, due to expansion of U.S. business
15,500
2,900 (19%) 1,900 (12%)
10,700 (69%)
4,800 (31%)
14,600
3,300 (23%) 2,400 (16%)
8,900 (61%)
5,700 (39%)
15,000
3,300 (22%) 3,000 (20%)
8,700 (58%)
Unit: Persons
6,300 (42%)
Total
Other U.S. Japan
Overseas total
End of FY04 End of FY07 End of FY09
* Includes group companies
12

2-4.1 Sales Synergies -Pharmaceutical Business Targets-
Grow sales of the Company’s high-margin products, both in Japan and overseas
Income increase benefits (net sales)
¥20bn ¥4bn
¥16bn
¥40bn ¥5bn
¥35bn
Total Overseas Japan
FY07 FY09
Earnings increase benefits (operating income*)
¥17bn ¥3bn
¥14bn
¥36bn ¥4bn
¥32bn
FY07 FY09
* Marginal income has been estimated as equal to operating income. Because of marginal income taking into account declines in earnings resulting from possible negative synergies, sales synergies and possible negative synergies have been treated in a way that allows expenses to offset each other, with the exception of direct manufacturing costs.
13

2-4.2 Measures to Generate Sales Synergies
Domestic Ethical Drugs -
Collaboration*1 will begin in October 2005
Focus on leading products from each company, in major drug markets
Olmetec, Mevalotin, Cravit, Calblock, etc.
Strategic utilization of the formidable marketing abilities of both companies
Over 2,500 MRs provide more than ten million pieces of information annually*2
Emphasis on cardiovascular diseases
*1 Cooperative sales efforts, including co-promotion, and scientific lectures
*2 IMS JDI fiscal 2004
14

2-4.3 Emphasis on the Cardiovascular Disease Field
Numerous products and development projects
Extensive skills and knowledge in cardiovascular-related fields
Maximization of Olmetec at the earliest opportunity through prioritization in the cardiovascular field
Begin collaboration from October 2005
Provide the greatest number of details for ARB*1 in Japan Integrate and utilize expertise and know-how
*1 Angiotensin II receptor blocker (blood pressure lowering agent)
15
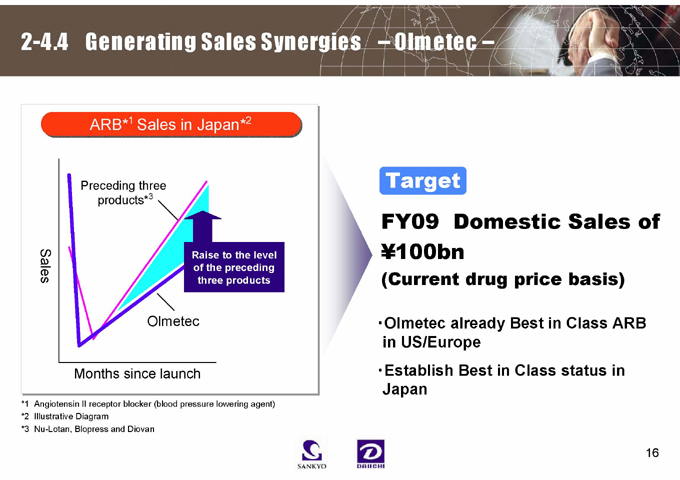
2-4.4 Generating Sales Synergies – Olmetec –
ARB*1Sales in Japan* 2
Preceding three products*3
Sales
Raise to the level of the preceding three products
Olmetec
Months since launch
*1 Angiotensin II receptor blocker (blood pressure lowering agent)
*2 Illustrative Diagram
*3 Nu-Lotan, Blopress and Diovan
Target
FY09 Domestic Sales of ¥100bn
(Current drug price basis)
·Olmetec already Best in Class ARB in US/Europe·Establish Best in Class status in Japan
16

2-5 Focus on the Pharmaceutical Business
The Daiichi Sankyo Group will focus on the pharmaceutical business All 15 non-pharma affiliates will be made wholly independent Target for completion March 2007
Affiliates currently represent
¥170bn in sales
¥9bn in operating income
17
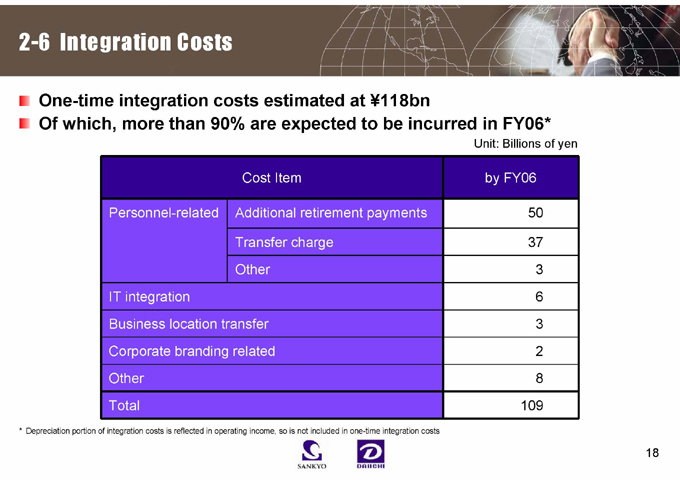
2-6 Integration Costs
One-time integration costs estimated at ¥118bn
Of which, more than 90% are expected to be incurred in FY06*
Unit: Billions of yen
Cost Item by FY06
Personnel-related Additional retirement payments 50
Transfer charge 37
Other 3
IT integration 6
Business location transfer 3
Corporate branding related 2
Other 8
Total 109
* Depreciation portion of integration costs is reflected in operating income, so is not included in one-time integration costs
18

1 Transaction Overview
2 Benefits of Transaction
3 Integration Strategy and Global Growth
4 Management Structure and Integration Schedule
19

3-1 Enhanced R&D Capability
Exploratory Research
Developmental Research
Clinical Development
Search for development candidates that fulfill medical needs
Create highly competitive products
Reinforce research skills, resources and technologies
Proper selection of targets through mutual utilization of information and technologies* Approach focusing on new target proteins Increase hit rate through expansion of compound library Search for lead compounds using a varied approach
Enhancement of development resources and mutual utilization of development expertise
Concentration on high-priority products Verify the added value exceeds that of existing products Quickly assess product potential Train and retain in-house and outside experts
*Databases, genetically-modified animals, etc
20
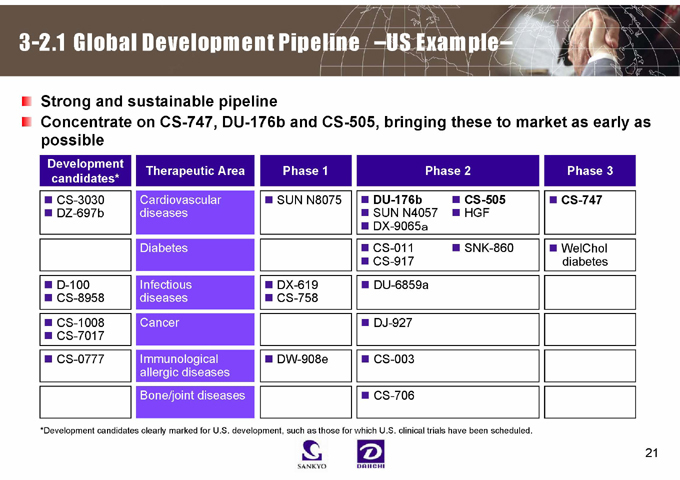
3-2.1 Global Development Pipeline –US Example–
Strong and sustainable pipeline
Concentrate on CS-747, DU-176b and CS-505, bringing these to market as early as possible
Development candidates* Therapeutic Area Phase 1 Phase 2 Phase 3
CS-3030 Cardiovascular SUN N8075 DU-176b CS-505 CS-747
DZ-697b diseases SUN N4057 HGF
DX-9065a
Diabetes CS-011 SNK-860 WelChol
CS-917 diabetes
D-100 Infectious DX-619 DU-6859a
CS-8958 diseases CS-758
CS-1008 Cancer DJ-927
CS-7017
CS-0777 Immunological DW-908e CS-003
allergic diseases
Bone/joint diseases CS-706
*Development candidates clearly marked for U.S. development, such as those for which U.S. clinical trials have been scheduled.
21
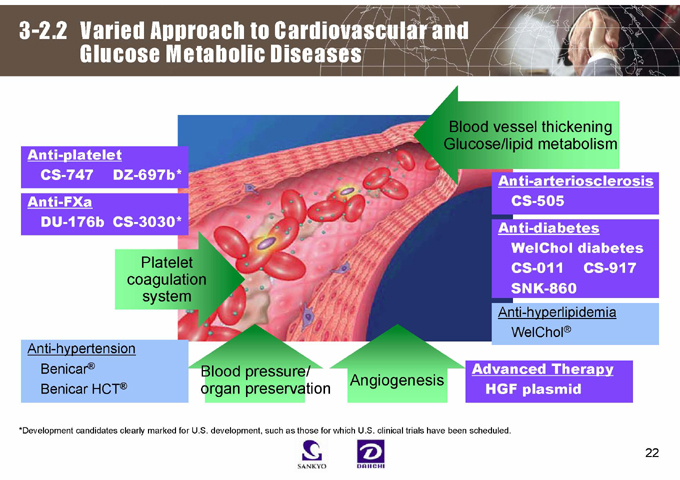
3-2.2 Varied Approach to Cardiovascular and Glucose Metabolic Diseases
Anti-platelet CS-747 DZ-697b*
Anti-FXa
DU-176b CS-3030*
Blood vessel thickening Glucose/lipid metabolism
Platelet coagulation system
Anti-arteriosclerosis CS-505 Anti-diabetes WelChol diabetes CS-011 CS-917 SNK-860
Anti-hypertension Benicar® Benicar HCT®
Blood pressure/ organ preservation
Angiogenesis
Anti-hyperlipidemia WelChol®
Advanced Therapy HGF plasmid
*Development candidates clearly marked for U.S. development, such as those for which U.S. clinical trials have been scheduled.
22
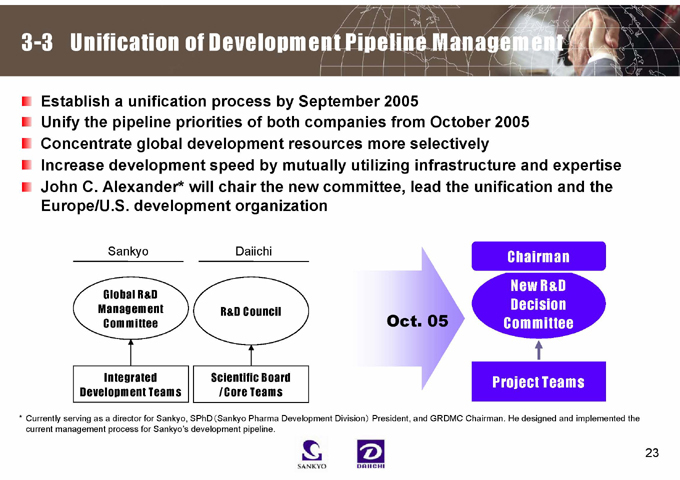
3-3 Unification of Development Pipeline Management
Establish a unification process by September 2005
Unify the pipeline priorities of both companies from October 2005 Concentrate global development resources more selectively
Increase development speed by mutually utilizing infrastructure and expertise John C. Alexander* will chair the new committee, lead the unification and the Europe/U.S. development organization
Sankyo
Global R&D Management Committee
Integrated Development Teams
Daiichi
R&D Council
Scientific Board /Core Teams
Oct. 05
Chairman
New R&D Decision Committee
Project Teams
* Currently serving as a director for Sankyo, SPhD (Sankyo Pharma Development Division) President, and GRDMC Chairman. He designed and implemented the current management process for Sankyo’s development pipeline.
23
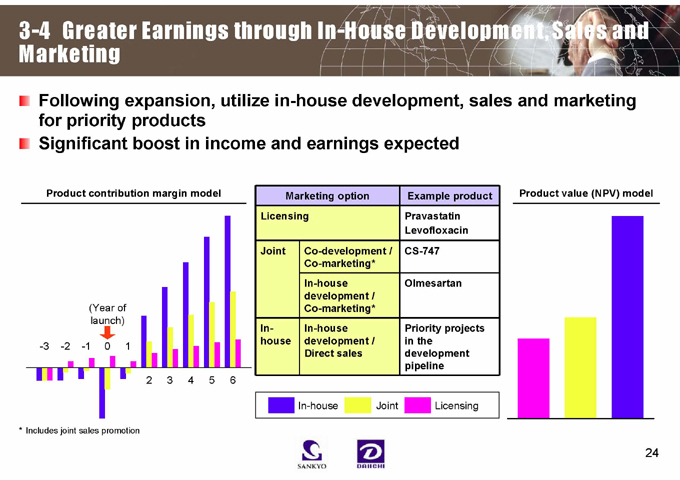
Following expansion, utilize in-house development, sales and marketing for priority products Significant boost in income and earnings expected
Product contribution margin model
(Year of launch)
-3 -2 -1 0 1
2 3 4 5 6
* Includes joint sales promotion
Marketing option Example product
Licensing Pravastatin
Levofloxacin
Joint Co-development / CS-747
Co-marketing*
In-house Olmesartan
development /
Co-marketing*
In- In-house Priority projects
house development / in the
Direct sales development
pipeline
Product value (NPV) model
In-house
Joint
Licensing
24
3-4 Greater Earnings through In-House Development, Sales and Marketing

3-5 Strengthen Human Resources Infrastructure
Effectively utilize the exceptional employees of both companies through the introduction and strict adherence to a new HR system
Introduction of a new HR system
Build new classification, compensation and evaluation systems
Ensure equal employment opportunities and compensation reflecting work and results
Equitable placement and promotion
Based on ability, irrespective of former company affiliation or age Employ highly objective and transparent evaluation criteria
Self-responsibility and self-actualization
Full support of career development
25

1 Transaction Overview
2 Benefits of Transaction
3 Integration Strategy and Global Growth
4 Management Structure and Integration Schedule
26

27
4-1 Governance and Responsibilities
Adoption of the Corporate Officer System and Board of Auditors will provide for responsive management Oversight and conduct of operations will be kept separate, handled by the Board of the Directors and the CEO, respectively
Management bodies/positions
Shareholders’ Meeting
Board of Auditors
Board of Directors
CEO
(Chief Executive Officer)
Management Committee
Operating Officers
Responsibilities
Makes decisions regarding substantive legal matters regulated by the Commercial Code, and oversees conduct of operations Rules on matters deliberated by the Board of Directors in accordance with its regulations, in order to clarify the responsibilities of directors Presided over by the Chairman
Under supervision of the Board of Directors, holds all rights and responsibility regarding conduct of operations
Deliberates matters concerning conduct of operations Chaired by the CEO
Hold rights and responsibilities for conduct of operations granted by the CEO

28
4-2 Directors and Auditors (planned)
Directors
Chairman Kiyoshi Morita
President and CEO Takashi Shoda
Directors Hiroyuki Nagasako
Hideho Kawamura
Yasuhiro Ikegami
Tsutomu Une
Outside directors Kunio Nihira
Yoshifumi Nishikawa
Jotaro Yabe
Katsuyuki Sugita
Auditors
Standing auditors Kozo Wada
Atsuo Inoue
Outside auditors Kaoru Shimada
Koukei Higuchi
29
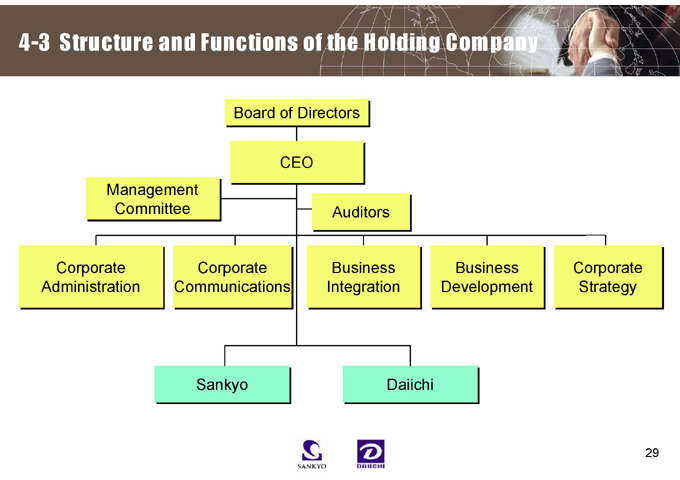
4-3 Structure and Functions of the Holding Company
Board of Directors
CEO
Management Committee
Auditors
Corporate Administration
Corporate Communications
Business Integration
Business Development
Corporate Strategy
Sankyo
Daiichi
30
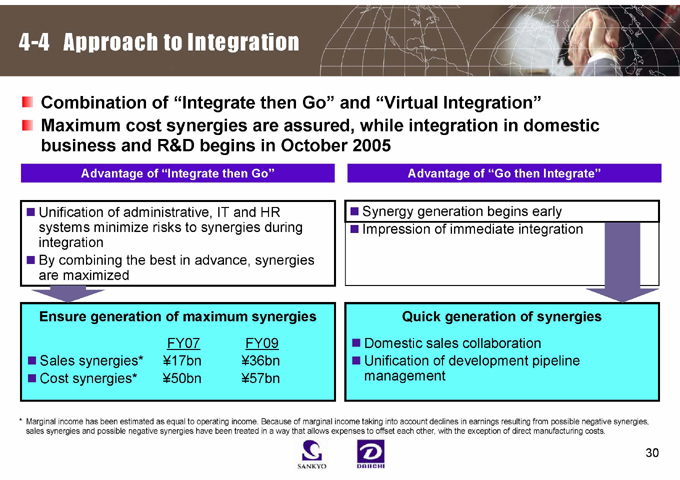
4-4 Approach to Integration
Combination of “Integrate then Go” and “Virtual Integration” Maximum cost synergies are assured, while integration in domestic business and R&D begins in October 2005
Advantage of “Integrate then Go”
Advantage of “Go then Integrate”
Unification of administrative, IT and HR systems minimize risks to synergies during integration By combining the best in advance, synergies are maximized
Synergy generation begins early
Impression of immediate integration
Ensure generation of maximum synergies
FY07 FY09
Sales synergies* ¥17bn ¥36bn Cost synergies* ¥50bn ¥57bn
Quick generation of synergies
Domestic sales collaboration Unification of development pipeline management
* Marginal income has been estimated as equal to operating income. Because of marginal income taking into account declines in earnings resulting from possible negative synergies, sales synergies and possible negative synergies have been treated in a way that allows expenses to offset each other, with the exception of direct manufacturing costs.
31
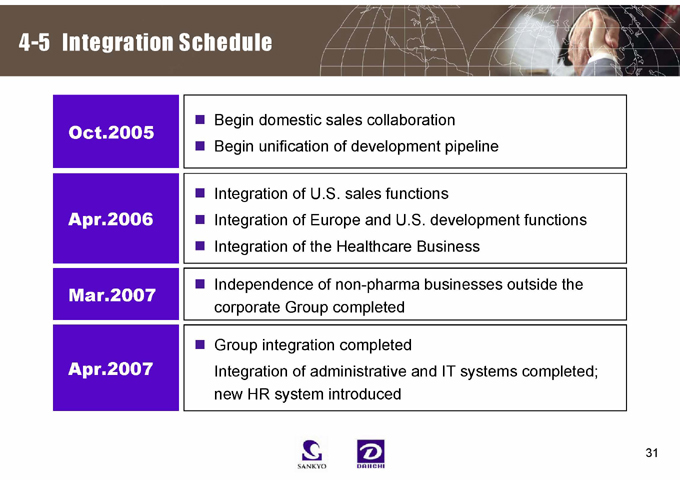
4-5 Integration Schedule
Oct.2005
Begin domestic sales collaboration Begin unification of development pipeline
Apr.2006
Integration of U.S. sales functions
Integration of Europe and U.S. development functions Integration of the Healthcare Business
Mar.2007
Independence of non-pharma businesses outside the corporate Group completed
Apr.2007
Group integration completed
Integration of administrative and IT systems completed; new HR system introduced

32
Global Pharma Innovator
Daiichi Sankyo building a strong global competitor

33
Filings with the U.S. SEC
Daiichi Pharmaceutical Co., Ltd. and Sankyo Company, Limited may file a registration statement on Form F-4 with the U.S. SEC in connection with the proposed business combination of Daiichi and Sankyo under a new holding company by way of a joint stock transfer. The Form F-4 (if filed) will contain a prospectus and other documents. If a Form F-4 is filed and declared effective, Daiichi and Sankyo plan to mail the prospectus contained in the Form F-4 to their U.S. shareholders prior to the shareholders meetings at which the stock exchange will be voted upon. The Form F-4 (if filed) and prospectus will contain important information about Daiichi and Sankyo, the joint stock transfer and related matters. U.S. shareholders of Daiichi and Sankyo are urged to read the Form F-4, the prospectus and the other documents that may be filed with the U.S. SEC in connection with the joint stock transfer carefully before they make any decision at the shareholders meeting with respect to the joint stock transfer. The Form F-4 (if filed), the prospectus and all other documents filed with the U.S. SEC in connection with the joint stock transfer will be available when filed, free of charge, on the U.S. SEC’s web site at www.sec.gov. In addition, the prospectus and all other documents filed with the U.S. SEC in connection with the business combination will be made available to shareholders, free of charge, by calling, writing or e-mailing:
Sankyo Company, Limited
Mr. Shigemichi Kondo
Corporate Communications Department 3-5-1, Nihonbashi Honcho Chuo-ku, Tokyo 103-8426, Japan Telephone: 81-3-5255-7034 E-mail:shige-k@sankyo.co.jp
Daiichi Pharmaceutical Co., Ltd.
Mr. Toshio Takahashi
Corporate Communications Department
14-10 Nihonbashi, 3-chome
Chuo-ku, Tokyo 103-8234, Japan
Telephone: 81-3-3273-7107
E-mail: andokb5o@daiichipharm.co.jp
You may read and copy any reports and other information filed with, or submitted to, the U.S. SEC at the U.S. SEC’s public reference rooms at 100 F Street, N.E., Washington, D.C. 20549 or at the other public reference rooms in New York, New York and Chicago, Illinois. Please call the U.S. SEC at 1-800-SEC-0330 for further information on public reference rooms. Filings with the U.S. SEC also are available to the public from commercial document-retrieval services and at the web site maintained by the U.S. SEC at http//www.sec.gov.

34
Forward-Looking Statements
This communication contains forward-looking information and statements about Daiichi and Sankyo and their combined businesses after completion of the joint stock transfer. Forward-looking statements are statements that are not historical facts. These statements include financial projections and estimates and their underlying assumptions, statements regarding plans, objectives and expectations with respect to future operations, products and services, and statements regarding future performance. Forward-looking statements are generally identified by the words “expects,” “anticipates,” “believes,” “intends,” “estimates” and similar expressions. Although the management of Daiichi and Sankyo believe that the expectations reflected in such forward-looking statements are reasonable, investors and holders of Daiichi and Sankyo securities are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Daiichi and Sankyo, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include those discussed or identified in the public filings with the SEC and the local filings made by Daiichi and Sankyo, including those listed under “Cautionary Statement Concerning Forward-Looking Statements” and “Risk Factors” in the prospectus included in the registration statement on Form F-4 that Daiichi and Sankyo may file with the U.S. SEC. Other than as required by applicable law, neither Daiichi nor Sankyo undertakes any obligation to update or revise any forward-looking information or statements.


































