Filing under Rule 425 under
the U.S. Securities Act of 1933
Filing by: Sankyo Company, Limited
Subject Company: Daiichi Pharmaceutical Co., Ltd.
and Sankyo Company, Limited
SEC File No. 132-02292

To Our Shareholders
We are providing this pamphlet to explain the key points of the business integration of Sankyo Company, Limited, and Daiichi Pharmaceutical Co., Ltd.
We would sincerely appreciate your understanding of the objectives of the business integration and ask that you give us your support by voting in favor of the integration proposal at the 151st General Meeting of Shareholders, to be held on June 29, 2005.
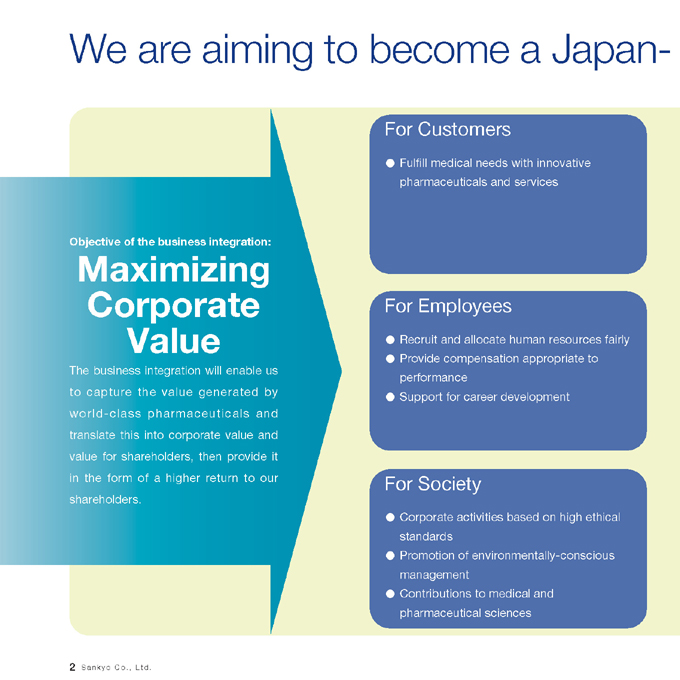
We are aiming to become a Japan-
Objective of the business integration:
Maximizing Corporate Value
The business integration will enable us to capture the value generated by world-class pharmaceuticals and translate this into corporate value and value for shareholders, then provide it in the form of a higher return to our shareholders.
For Customers
Fulfill medical needs with innovative pharmaceuticals and services
For Employees
Recruit and allocate human resources fairly
Provide compensation appropriate to performance
Support for career development
For Society
Corporate activities based on high ethical standards
Promotion of environmentally-conscious management
Contributions to medical and pharmaceutical sciences
2 Sankyo Co., Ltd.
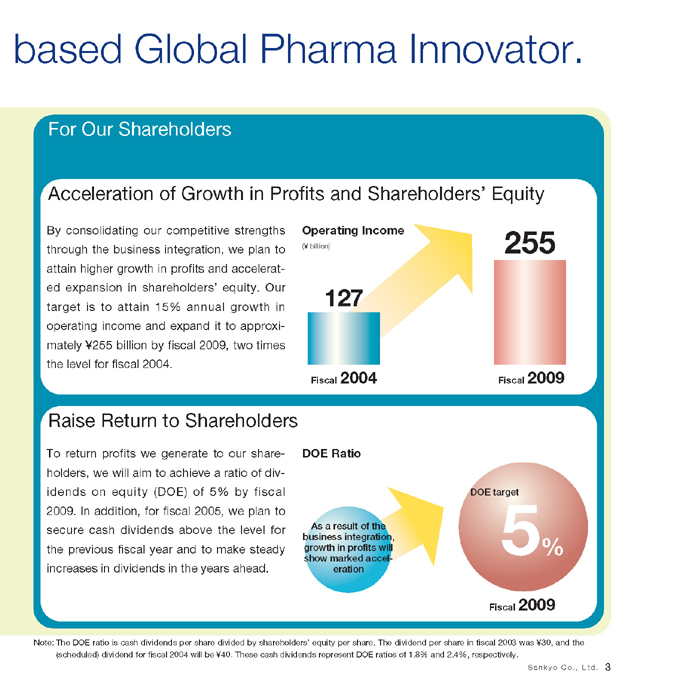
based Global Pharma Innovator.
For Our Shareholders
Acceleration of Growth in Profits and Shareholders’ Equity
By consolidating our competitive strengths through the business integration, we plan to attain higher growth in profits and accelerated expansion in shareholders’ equity. Our target is to attain 15% annual growth in operating income and expand it to approximately ¥255 billion by fiscal 2009, two times the level for fiscal 2004.
Operating Income
(¥ billion)
127
Fiscal 2004
255
Fiscal 2009
Raise Return to Shareholders
To return profits we generate to our shareholders, we will aim to achieve a ratio of dividends on equity (DOE) of 5% by fiscal 2009. In addition, for fiscal 2005, we plan to secure cash dividends above the level for the previous fiscal year and to make steady increases in dividends in the years ahead.
DOE Ratio
As a result of the business integration, growth in profits will show marked acceleration
DOE target
5%
Fiscal 2009
Note: The DOE ratio is cash dividends per share divided by shareholders’ equity per share. The dividend per share in fiscal 2003 was ¥30, and the (scheduled) dividend for fiscal 2004 will be ¥40. These cash dividends represent DOE ratios of 1.8% and 2.4%, respectively.
Sankyo Co., Ltd. 3

Increasing Shareholder Value
The business integration will have the objective of creating a Japan-based Global Pharma Innovator and which will have the mission of providing a steady stream of innovative products and services that satisfy unmet medical needs and occupy a unique competitive position in the world’s major markets.
We believe positive sales and cost synergies resulting from the business integration will substantially outweigh the possible negative synergies and integration costs, and will increase shareholder value of Sankyo and Daiichi.
The most important objective of the business integration will be to create the potential synergies from Global Pharma Innovator platform. Specifically, the integration will combine the R&D capabilities of the two companies that created the antihyperlipidemic agent Mevalotin® (pravastatin) and the broad-spectrum oral antibacterial agent Cravit® (levofloxacin). We believe this combination will yield true synergies and enable the combined companies to bring promising drugs in their pipelines to market as quickly as possible.
As a result of the business integration, we are confident that the corporate value of Sankyo will expand on a sustained and stable basis and that this will lead to the maximization of shareholder value.
Aggregate value of Daiichi shares
Aggregate value of Sankyo shares
Sales and cost synergies
Possible negative synergies*
Integration costs
Estimated shareholder value after integration (Former Daiichi)
Estimated shareholder value after integration (Former Sankyo)
Targeted increase in shareholder value
Potential synergies from Global Pharma Innovator platform
Note: The aggregate values of Sankyo and Daiichi shares are calculated based on the closing prices on February 18, 2005.
* Risks associated with the merger, such as a decline in sales and/or profits due to changes in corporate control or product cannibalization
4 Sankyo Co., Ltd.

Capabilities for Creating Innovative New Drugs
Sankyo has managed its activities based on the policy of concentrating its management resources in prioritized domains, focusing especially on drugs in the cardiovascular field. Following the integration, the same policy will be continued.
Daiichi is a leading pharmaceutical company in Japan conducting an active R&D program not only in the infectious diseases field but also in the cardiovascular and other fields that Sankyo has prioritized. Combining the management of the product portfolios and drug development pipelines of the two companies will lead to major synergies, such as improvements in pharmaceutical development capabilities thereby strengthening R&D capacities.
Strong R&D Franchise Categories
Daiichi
Infectious diseases
Cancer
Immunological/allergic diseases
Cardiovascular diseases/Thrombosis and arteriosclerosis
Sankyo
Bone/joint diseases
Diabetes and metabolic disorders
Total R&D expenditure target for fiscal 2009 ¥170 billion
Global Drug Development Pipeline: Example of Activities in the United States
Development candidates Patient category Phase 1 Phase 2 Phase 3
CS-3030 SUN N8075 DU-176b CS-505 CS-747
DZ-697b Cardiovascular diseases SUN N4057 HGF
DX-9065a
CS-011 SNK-860 WelChol diabetes
Diabetes
CS-917
D100 DX-619 DU-6859a
Infectious diseases
CS-8958 CS-758
CS-1008 DJ-927
Cancer
CS-7017
CS-0777 Immunological/allergic diseases DW-908e CS-003
Bone/joint diseases CS-706
Note: Development candidates are clearly marked for U.S. development, and include those for which U.S. clinical trials have been scheduled.
Sankyo Co., Ltd. 5
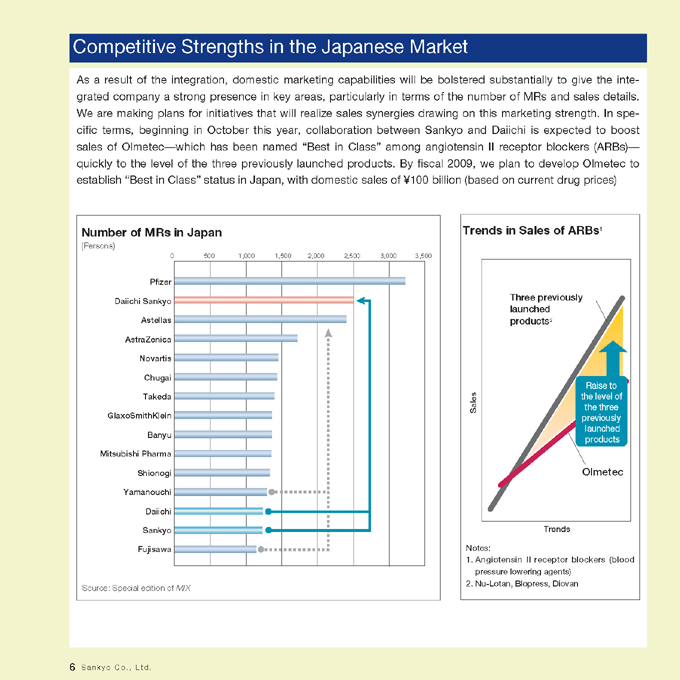
Competitive Strengths in the Japanese Market
As a result of the integration, domestic marketing capabilities will be bolstered substantially to give the integrated company a strong presence in key areas, particularly in terms of the number of MRs and sales details. We are making plans for initiatives that will realize sales synergies drawing on this marketing strength. In specific terms, beginning in October this year, collaboration between Sankyo and Daiichi is expected to boost sales of Olmetec—which has been named “Best in Class” among angiotensin II receptor blockers (ARBs)—quickly to the level of the three previously launched products. By fiscal 2009, we plan to develop Olmetec to establish “Best in Class” status in Japan, with domestic sales of ¥100 billion (based on current drug prices)
Number of MRs in Japan
(Persons)
0 500 1,000 1,500 2,000 2,500 3,000 3,500
Pfizer
Daiichi Sankyo
Astellas
AstraZenica
Novartis
Chugai
Takeda
GlaxoSmithKlein
Banyu
Mitsubishi Pharma
Shionogi
Yamanouchi
Daiichi
Sankyo
Fujisawa
Source: Special edition of MIX
Trends in Sales of ARBs1
Sales
Three previously launched products2
Raise to the level of the three previously launched products
Olmetec
Trends
Notes:
1. Angiotensin II receptor blockers (blood pressure lowering agents)
2. Nu-Lotan, Blopress, Diovan
6 Sankyo Co., Ltd.
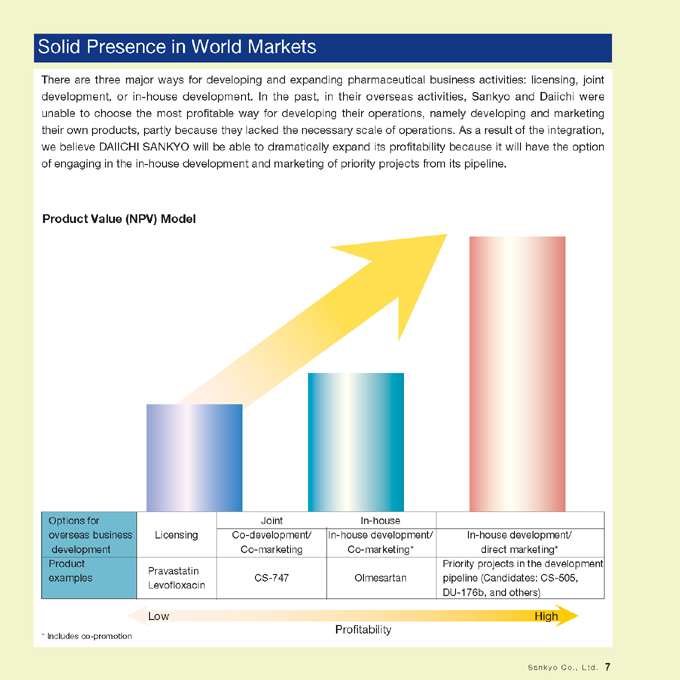
Solid Presence in World Markets
There are three major ways for developing and expanding pharmaceutical business activities: licensing, joint development, or in-house development. In the past, in their overseas activities, Sankyo and Daiichi were unable to choose the most profitable way for developing their operations, namely developing and marketing their own products, partly because they lacked the necessary scale of operations. As a result of the integration, we believe DAIICHI SANKYO will be able to dramatically expand its profitability because it will have the option of engaging in the in-house development and marketing of priority projects from its pipeline.
Product Value (NPV) Model
Options for overseas business development
Joint
In-house
Licensing
Co-development/ Co-marketing
In-house development/Co-marketing*
In-house development/ direct marketing*
Product examples
Priority projects in the development pipeline (Candidates: CS-505, DU-176b, and others)
Pravastatin Levofloxacin
CS-747 Olmesartan
Low High Profitability
* Includes co-promotion
Sankyo Co., Ltd. 7
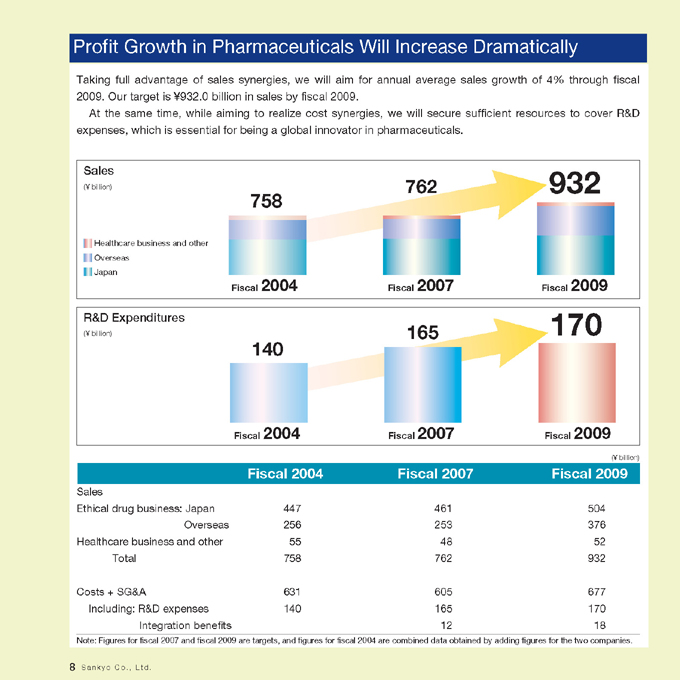
Profit Growth in Pharmaceuticals Will Increase Dramatically
Taking full advantage of sales synergies, we will aim for annual average sales growth of 4% through fiscal 2009. Our target is ¥932.0 billion in sales by fiscal 2009.
At the same time, while aiming to realize cost synergies, we will secure sufficient resources to cover R&D expenses, which is essential for being a global innovator in pharmaceuticals.
Sales
(¥ billion)
Healthcare business and other Overseas Japan
758
762
932
Fiscal 2004 Fiscal 2007 Fiscal 2009
R&D Expenditures
(¥ billion)
165
170
140
Fiscal 2004 Fiscal 2007 Fiscal 2009
(¥ billion)
Fiscal 2004 Fiscal 2007 Fiscal 2009
Sales
Ethical drug business: Japan 447 461 504
Overseas 256 253 376
Healthcare business and other 55 48 52
Total 758 762 932
Costs + SG&A 631 605 677
Including: R&D expenses 140 165 170
Integration benefits 12 18
Note: Figures for fiscal 2007 and fiscal 2009 are targets, and figures for fiscal 2004 are combined data obtained by adding figures for the two companies.
8 Sankyo Co., Ltd.
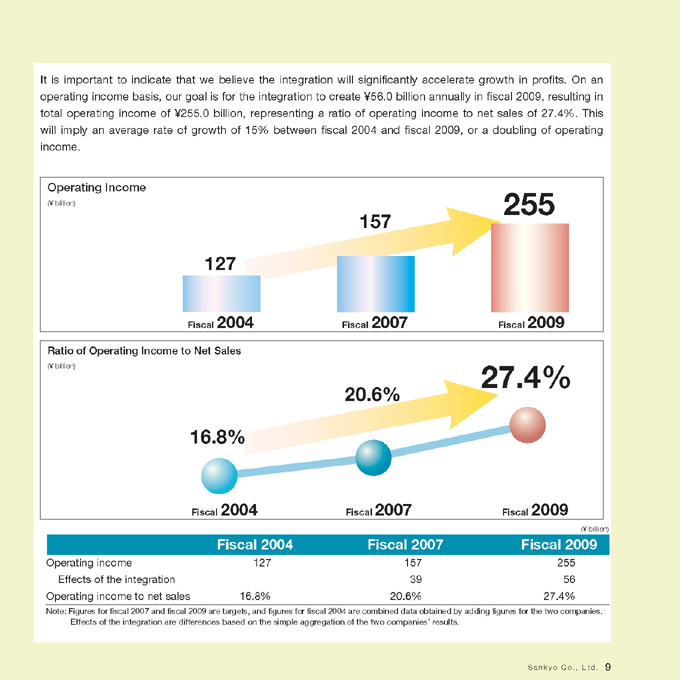
It is important to indicate that we believe the integration will significantly accelerate growth in profits. On an operating income basis, our goal is for the integration to create ¥56.0 billion annually in fiscal 2009, resulting in total operating income of ¥255.0 billion, representing a ratio of operating income to net sales of 27.4%. This will imply an average rate of growth of 15% between fiscal 2004 and fiscal 2009, or a doubling of operating income.
Operating Income
(¥ billion)
127
157
255
Fiscal 2004 Fiscal 2007 Fiscal 2009
Ratio of Operating Income to Net Sales
(¥ billion)
16.8%
20.6%
27.4%
Fiscal 2004 Fiscal 2007 Fiscal 2009
(¥ billion)
Fiscal 2004 Fiscal 2007 Fiscal 2009
Operating income 127 157 255
Effects of the integration 39 56
Operating income to net sales 16.8% 20.6% 27.4%
Note: Figures for fiscal 2007 and fiscal 2009 are targets, and figures for fiscal 2004 are combined data obtained by adding figures for the two companies.
Effects of the integration are differences based on the simple aggregation of the two companies’ results.
Sankyo Co., Ltd. 9
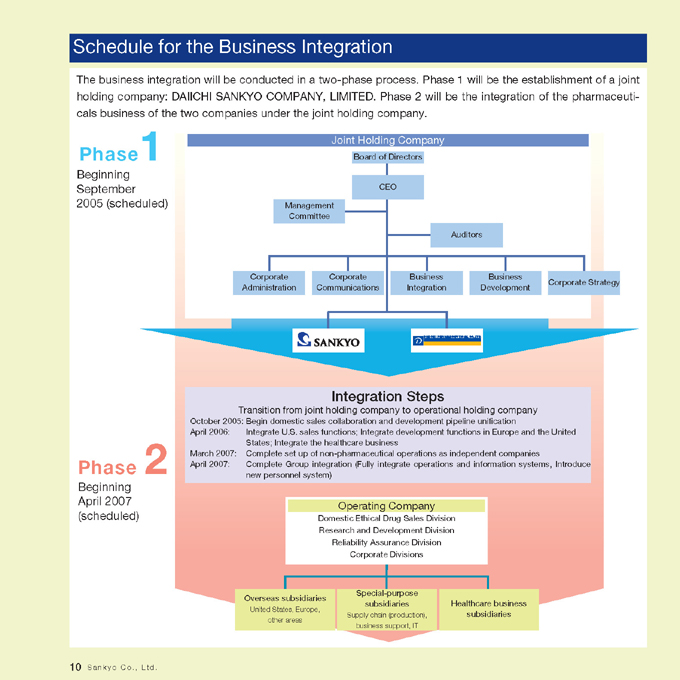
Schedule for the Business Integration
The business integration will be conducted in a two-phase process. Phase 1 will be the establishment of a joint holding company: DAIICHI SANKYO COMPANY, LIMITED. Phase 2 will be the integration of the pharmaceuticals business of the two companies under the joint holding company.
Phase1
Beginning September 2005 (scheduled)
Joint Holding Company
Board of Directors
CEO
Management Committee
Auditors
Corporate Administration
Corporate Communications
Business Integration
Business Development
Corporate Strategy
Phase 2
Beginning April 2007 (scheduled)
Integration Steps
Transition from joint holding company to operational holding company
October 2005: Begin domestic sales collaboration and development pipeline unification
April 2006: Integrate U.S. sales functions; Integrate development functions in Europe and the United States; Integrate the healthcare business
March 2007: Complete set up of non-pharmaceutical operations as independent companies
April 2007: Complete Group integration (Fully integrate operations and information systems, Introduce new personnel system)
Operating Company
Domestic Ethical Drug Sales Division Research and Development Division Reliability Assurance Division Corporate Divisions
Overseas subsidiaries
United States, Europe, other areas
Special-purpose subsidiaries
Supply chain (production), business support, IT
Healthcare business subsidiaries
10 Sankyo Co., Ltd.
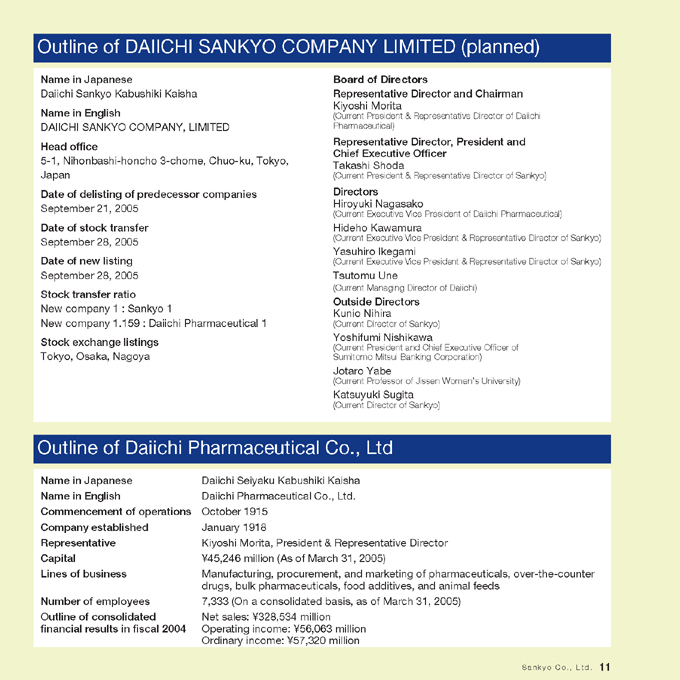
Outline of DAIICHI SANKYO COMPANY LIMITED (planned)
Name in Japanese
Daiichi Sankyo Kabushiki Kaisha
Name in English
DAIICHI SANKYO COMPANY, LIMITED
Head office
5-1, Nihonbashi-honcho 3-chome, Chuo-ku, Tokyo, Japan
Date of delisting of predecessor companies
September 21, 2005
Date of stock transfer
September 28, 2005
Date of new listing
September 28, 2005
Stock transfer ratio
New company 1 : Sankyo 1
New company 1.159 : Daiichi Pharmaceutical 1
Stock exchange listings
Tokyo, Osaka, Nagoya
Board of Directors
Representative Director and Chairman
Kiyoshi Morita
(Current President & Representative Director of Daiichi Pharmaceutical)
Representative Director, President and Chief Executive Officer
Takashi Shoda
(Current President & Representative Director of Sankyo)
Directors
Hiroyuki Nagasako
(Current Executive Vice President of Daiichi Pharmaceutical)
Hideho Kawamura
(Current Executive Vice President & Representative Director of Sankyo)
Yasuhiro Ikegami
(Current Executive Vice President & Representative Director of Sankyo)
Tsutomu Une
(Current Managing Director of Daiichi)
Outside Directors
Kunio Nihira
(Current Director of Sankyo)
Yoshifumi Nishikawa
(Current President and Chief Executive Officer of Sumitomo Mitsui Banking Corporation)
Jotaro Yabe
(Current Professor of Jissen Women’s University)
Katsuyuki Sugita
(Current Director of Sankyo)
Outline of Daiichi Pharmaceutical Co., Ltd
Name in Japanese Daiichi Seiyaku Kabushiki Kaisha Name in English Daiichi Pharmaceutical Co., Ltd.
Commencement of operations October 1915 Company established January 1918
Representative Kiyoshi Morita, President & Representative Director Capital ¥45,246 million (As of March 31, 2005)
Lines of business Manufacturing, procurement, and marketing of pharmaceuticals, over-the-counter drugs, bulk pharmaceuticals, food additives, and animal feeds Number of employees 7,333 (On a consolidated basis, as of March 31, 2005) Outline of consolidated Net sales: ¥328,534 million financial results in fiscal 2004 Operating income: ¥56,063 million Ordinary income: ¥57,320 million
Sankyo Co., Ltd. 11

Filings with the U.S. SEC Daiichi Pharmaceutical Co., Ltd. and Sankyo Company, Limited have filed a registration statement on Form F-4 with the U.S. SEC in connection with the proposed business integration of Daiichi and Sankyo under a new holding company by way of a joint stock transfer. The Form F-4 contains a prospectus and other documents. After the Form F-4 has been declared effective, Daiichi and Sankyo plan to mail the prospectus contained in the Form F-4 to their U.S. shareholders prior to the shareholders’ meetings at which the share exchange will be voted upon. The Form F-4 and prospectus contain important information about Daiichi and Sankyo, the joint stock transfer and related matters. U.S. shareholders of Daiichi and Sankyo are urged to read the Form F-4, the prospectus and the other documents filed with the U.S. SEC in connection with the joint stock transfer carefully before they make any decision at the shareholders’ meeting with respect to the joint stock transfer. The Form F-4, the prospectus and all other documents filed with the U.S. SEC in connection with the joint stock transfer are available, free of charge, on the U.S. SEC’s web site at www.sec.gov. In addition, the prospectus and all other documents filed with the U.S. SEC in connection with the business integration are available to shareholders, free of charge, by calling, writing or e-mailing:
Sankyo Company, Limited
Mr. Shigemichi Kondo
Corporate Communications Department
3-5-1, Nihonbashi Honcho
Chuo-ku, Tokyo 103-8426, Japan
Telephone: 81-3-5255-7034
E-mail: shige-k@sankyo.co.jp
Daiichi Pharmaceutical Co., Ltd.
Mr. Toshio Takahashi
Corporate Communications Department
14-10 Nihonbashi, 3-chome
Chuo-ku, Tokyo 103-8234,
Japan Telephone: 81-3-3273-7107
E-mail: andokb5o@daiichipharm.co.jp
You may read and copy any reports and other information filed with, or submitted to, the U.S. SEC at the U.S. SEC’s public reference rooms at 100 F Street, N.E., Washington, D.C. 20549 or at the other public reference rooms in New York, New York and Chicago, Illinois. Please call the U.S. SEC at 1-800-SEC-0330 for further information on public reference rooms. Filings with the U.S. SEC also are available to the public from commercial document-retrieval services and at the web site maintained by the U.S. SEC at http//www.sec.gov.
Forward-Looking Statements This communication contains forward-looking information and statements about Daiichi and Sankyo and their combined businesses after completion of the joint stock transfer. Forward-looking statements are statements that are not historical facts. These statements include financial projections and estimates and their underlying assumptions, statements regarding plans, objectives and expectations with respect to future operations, products and services, and statements regarding future performance. Forward-looking statements are generally identified by the words “expects,” “anticipates,” “believes,” “intends,” “estimates” and similar expressions. Although the management of Daiichi and Sankyo believe that the expectations reflected in such forward-looking statements are reasonable, investors and holders of Daiichi and Sankyo securities are cautioned that forward-looking information and statements are subject to various risks and uncertainties, many of which are difficult to predict and generally beyond the control of Daiichi and Sankyo, that could cause actual results and developments to differ materially from those expressed in, or implied or projected by, the forward-looking information and statements. These risks and uncertainties include those discussed or identified in the public filings with the SEC and the local filings made by Daiichi and Sankyo, including those listed under “Cautionary Statement Concerning Forward-Looking Statements” and “Risk Factors” in the prospectus included in the registration statement on Form F-4 that Daiichi and Sankyo have filed with the U.S. SEC. Other than as required by applicable law, neither Daiichi nor Sankyo undertakes any obligation to update or revise any forward-looking information or statements.











