Table of Contents
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
SCHEDULE 14A
(Rule 14a-101)
Information Required in Proxy Statement
Schedule 14A Information
Schedule 14A Information
Proxy Statement Pursuant to Section 14(a) of the Securities
Exchange Act of 1934 (Amendment No. )
Exchange Act of 1934 (Amendment No. )
Filed by the Registrant þ
Filed by a Party other than the Registrant o
Check the appropriate box:
| o | Preliminary Proxy Statement | |
| o | Confidential, for Use of the Commission Only (as permitted by Rule 14a-6(e)(2)) | |
| þ | Definitive Proxy Statement | |
| o | Definitive Additional Materials | |
| o | Soliciting Material Pursuant to §240.14a-12 |
IDENIX PHARMACEUTICALS, INC.
(Name of Registrant as Specified In Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
Payment of Filing Fee (Check the appropriate box):
| þ | No fee required. | |
| o | Fee computed on table below per Exchange Act Rules 14a-6(i)(1) and 0-11. |
| (1 | ) | Title of each class of securities to which transaction applies: | ||||
| (2 | ) | Aggregate number of securities to which transaction applies: | ||||
| (3 | ) | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (set forth the amount on which the filing fee is calculated and state how it was determined): | ||||
| (4 | ) | Proposed maximum aggregate value of transaction: | ||||
| (5 | ) | Total fee paid: | ||||
| o | Fee paid previously with preliminary materials. | |
| o | Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the Form or Schedule and the date of its filing. |
| (1 | ) | Amount Previously Paid: | ||||
| (2 | ) | Form, Schedule or Registration Statement No.: | ||||
| (3 | ) | Filing Party: | ||||
| (4 | ) | Date Filed: | ||||
Table of Contents

Dear Idenix Stockholder:
Please join us for the 2006 Annual Meeting of Stockholders of Idenix Pharmaceuticals, Inc. The meeting will be held on Wednesday, June 14, 2006, at 9:00 a.m., at the offices of WilmerHale LLP, located at 60 State Street, Boston, Massachusetts 02109.
At this year’s annual meeting, we will ask our stockholders to elect eight directors, ratify the appointment of our independent registered public accounting firm and transact any other business that may properly come before the meeting. Additional information about the items of business to be discussed at our annual meeting is given in the attached Notice of Annual Meeting and Proxy Statement.
I urge you to carefully review the proxy materials and to voteFORthe election of the director nominees, andFORratification of the appointment of our independent registered public accounting firm.
On behalf of the Idenix board of directors, employees and management, I thank you for your support and confidence. We look forward to seeing you at the annual meeting.
| Very truly yours, | |
 | |
| Jean-Pierre Sommadossi | |
| Chairman and Chief Executive Officer |
April 28, 2006
TABLE OF CONTENTS
Table of Contents
IDENIX PHARMACEUTICALS, INC.
60 Hampshire Street
Cambridge, Massachusetts 02139
NOTICE OF 2006 ANNUAL MEETING OF STOCKHOLDERS
| Date | June 14, 2006 | |
| Time | 9:00 a.m. (eastern daylight time) | |
| Place | WilmerHale LLP 60 State Street Boston, Massachusetts 02109 | |
| Items of Business | 1. To elect eight directors to serve until the next annual meeting of stockholders and until their successors are elected and qualified; | |
| 2. To ratify the selection of PricewaterhouseCoopers LLP as our independent registered public accounting firm for the current fiscal year ending December 31, 2006; and | ||
| 3. To transact such other business as may properly come before the meeting or any adjournment thereof. | ||
| Record Date | You are entitled to notice of, and to vote at the annual meeting and any adjournments of that meeting, if you were a stockholder of record at the close of business on April 18, 2006. | |
| Voting by Proxy | Please submit the enclosed proxy as soon as possible so that your shares can be voted at the annual meeting in accordance with your instructions. For specific instructions regarding voting, please refer to the Questions and Answers beginning on page 1 of the Proxy Statement and the instructions on your proxy card. | |
| Submitting your proxy will not affect your right to attend the meeting and vote. A stockholder who gives a proxy may revoke it at any time before it is exercised by voting in person at the annual meeting, by delivering a subsequent proxy or notifying the inspector of elections in writing of such revocation. |
| By Order of the Board of Directors, | |
| Andrea J. Corcoran | |
| Secretary |
Cambridge, Massachusetts
April 28, 2006
Table of Contents
IDENIX PHARMACEUTICALS, INC.
Proxy Statement for the 2006 Annual Meeting of Stockholders
To Be Held on June 14, 2006
PROXIES AND VOTING
This proxy statement contains information about the 2006 annual meeting of stockholders of Idenix Pharmaceuticals, Inc. We are holding the meeting on Wednesday, June 14, 2006 at 9:00 a.m. (eastern daylight time) at the offices of WilmerHale LLP, 60 State Street, Boston, Massachusetts 02109.
In this proxy statement, references to “Idenix,” “we,” “us” and “our” refer to Idenix Pharmaceuticals, Inc.
We are sending you this proxy statement in connection with the solicitation by our board of directors of proxies to be voted at the annual meeting or at any adjournment or postponement thereof.
We are mailing this proxy statement and proxy card together with our Annual Report for the year ended December 31, 2005 on or about May 5, 2006.
You may obtain additional printed copies of our Annual Report on Form 10-K, free of charge, by sending a written request to: Idenix Pharmaceuticals, Inc., attention: Investor Relations, 60 Hampshire Street, Cambridge, MA 02139. References to our website are inactive textual references only and the contents of our website should not be deemed to be incorporated by reference into this proxy statement.
| Q. | Who can vote at the annual meeting? | |
| A. | To be able to vote, you must have been a stockholder of record at the close of business on April 18, 2006, the record date for our annual meeting. On that date, 55,970,349 shares of common stock were issued and outstanding and entitled to vote at the annual meeting. | |
| If you were a stockholder of record on that date, you are entitled to vote all of the shares that you held on that date at the annual meeting, or any postponements or adjournments of the annual meeting. | ||
| Q. | What are the voting rights of the holders of common stock? | |
| A. | Each outstanding share of our common stock entitles the holder to one vote on each proposal considered at the annual meeting. We have no other securities entitled to vote at the meeting. | |
| Q. | What is a proxy card? | |
| A. | The proxy card enables you to appoint Jean-Pierre Sommadossi, David Arkowitz and Andrea Corcoran as your representatives at the annual meeting. By completing and returning the proxy card, you are authorizing Dr. Sommadossi, Mr. Arkowitz and Ms. Corcoran to vote your shares at the meeting as you have instructed on the proxy card. If you do not specify on the proxy card how your shares should be voted, they will be voted as recommended by our board of directors. This way, you can vote your shares whether or not you attend the meeting. | |
| Q. | What am I voting on? | |
| A. | We are asking you to vote on: |
| • | the election of directors for a one year term; and | |
| • | the ratification of PricewaterhouseCoopers LLP as our independent registered public accounting firm for the current fiscal year ending December 31, 2006. |
Table of Contents
| Q. | How do I vote? | |
| A. | If you are a record holder, meaning your shares are registered in your name, you may vote: | |
| (1) By Mail: Complete, date and sign the enclosed proxy card and mail it in the enclosed postage paid envelope. Your shares will be voted according to your instructions. If you do not specify how your shares should be voted, they will be voted as recommended by our board of directors. | ||
| (2) In Person at the Meeting: If you attend the meeting, you may deliver your completed proxy card in person or you may vote by completing a ballot, which we will provide to you at the meeting. | ||
| If your shares are held in “street name,” meaning they are held for you by a broker, bank or other nominee, you may vote: | ||
| (1) By Mail: You will receive instructions from your broker, bank or other nominee explaining how you can vote your shares by mail. You should follow those instructions. | ||
| (2) In Person at the Meeting: Contact the broker, bank or other nominee who holds your shares to obtain a proxy card and bring it with you to the meeting.You will not be able to vote in person at the meeting unless you have obtained from the broker, bank or other nominee a proxy issued in your name giving you the right to vote your shares. | ||
| Q. | How may I change or revoke my proxy? | |
| A. | You may change or revoke your proxy at any time before the meeting. To do so, you must do one of the following: | |
| (1) Provide written notice to us prior to the meeting that you wish to revoke your proxy. Such notice should be sent to us c/o Secretary, Idenix Pharmaceuticals, Inc., 60 Hampshire Street, Cambridge, Massachusetts 02139. | ||
| (2) Sign a new proxy and submit it to us c/o Secretary, Idenix Pharmaceuticals, Inc., 60 Hampshire Street, Cambridge, Massachusetts 02139 in time for receipt prior to the meeting. Only the most recently dated proxy will be counted. | ||
| (3) Attend the meeting, request that your proxy be revoked and vote in person as instructed above. Attending the meeting will not revoke your proxy unless you specifically request such revocation. | ||
| Q. | Will my shares be voted if I do not return my proxy? | |
| A. | If your shares are registered directly in your name, your shares will not be voted if you do not vote either by returning your proxy or voting in person by ballot at the meeting. | |
| If your shares are held in “street name,” we encourage you to provide voting instructions to your broker, bank or other nominee by giving your proxy to them. This ensures that your shares will be voted at the meeting according to your instructions. If you do not return a proxy to your broker, bank or other nominee to vote your shares, your broker, bank or other nominee may, with respect to the proposals to elect our directors and ratify the selection of our independent registered public accounting firm, either vote your shares or leave your shares unvoted, under their discretionary authority. | ||
| Q. | How many shares must be present to hold the meeting? | |
| A. | To establish a quorum, a majority of our outstanding shares of common stock must be present at the meeting. The presence of a quorum is a prerequisite to holding and conducting business at the meeting. We believe that Novartis Pharma AG, or Novartis, the holder of a majority of our issued and outstanding common stock, will be present at the meeting and that a quorum will be established as a result. |
2
Table of Contents
| Q. | What vote is required to approve each matter and how are votes counted? | |
| A. | Proposal 1 — Election of Directors. Since eight directors are to be elected at the annual meeting, the eight nominees for director who receive the highest number of votes FOR election will be elected as directors. Abstentions are not counted for purposes of electing directors. You may: |
| • | vote FOR all nominees; | |
| • | WITHHOLD your vote from all nominees; or | |
| • | vote FOR one or more nominees and WITHHOLD your vote from one or more of the others. |
| Votes that are withheld will not be included in the vote tally for the election of directors and will not affect the results of the vote. |
| Proposal 2 — Ratification of Selection of Independent Registered Public Accounting Firm. The affirmative vote of stockholders holding a majority of the votes cast on this proposal is required to ratify PricewaterhouseCoopers LLP as our independent registered public accounting firm for 2006. | ||
| If you vote to abstain on this proposal, your shares will not be voted in favor or against the proposal and will also not be counted as votes cast or shares voting on the proposal. As a result, voting to abstain will have no effect on the voting on the proposal. Although stockholder approval of our Audit Committee’s selection of PricewaterhouseCoopers LLP as our independent registered public accounting firm is not required, we believe that our stockholders should have an opportunity to ratify this selection. If this proposal is not approved at the annual meeting, our Audit Committee will reconsider its selection of PricewaterhouseCoopers LLP. | ||
| Proposal 3 — Other Matters. If any other matters are properly presented to the meeting, the persons named in the accompanying proxy will have the discretion to vote, or otherwise act for you, in accordance with their judgment on the matter. As of the date of this proxy statement, we do know of any other matters to be presented at the annual meeting. | ||
| We believe that Novartis intends to vote all of its shares FOR each proposal detailed above. On the record date, Novartis was the holder of approximately 56% of our outstanding common stock. | ||
| Q. | Where may I find the voting results? | |
| A. | We will announce preliminary voting results at the meeting. We will report the final voting results in our Quarterly Report on Form 10-Q for the second quarter ending June 30, 2006, which we expect to file with the Securities and Exchange Commission, or SEC, in August 2006. | |
| Q. | Who is soliciting the proxy and what are the costs of soliciting these proxies? | |
| A. | Our board of directors is soliciting the proxy accompanying this proxy statement. We will bear the cost of soliciting proxies. Our directors, officers and employees may solicit proxies by telephone,e-mail, facsimile and in person, without additional compensation. Upon request, we will also reimburse brokerage houses and other custodians, nominees and fiduciaries for their reasonableout-of-pocket expenses for distributing proxy materials. |
RELATIONSHIP WITH NOVARTIS PHARMA AG
In May 2003, we entered into a collaboration with Novartis relating to the worldwide development and commercialization of our product candidates. Simultaneously, Novartis purchased approximately 54% of our outstanding capital stock from our stockholders for $255 million in cash, with an aggregate amount of up to $357 million contingently payable to these stockholders if we achieve predetermined development milestones relating to a product candidate for the treatment of infections caused by hepatitis C virus, or HCV. Including shares acquired in 2005 from its affiliate, Novartis BioVentures Ltd., and shares acquired as a result of the exercise of its stock subscription rights, Novartis currently owns and as of the record date owned approximately 56% of our outstanding common stock. Novartis BioVentures Ltd. was an existing stockholder in May 2003 at the time of the Novartis stock purchase.
3
Table of Contents
Our relationship with Novartis includes a number of arrangements that affect our corporate governance and the research, development, manufacture and commercialization of our product candidates. The terms of these arrangements are set forth in the agreements described below to which we and Novartis are parties:
| • | a stockholders’ agreement, as amended and restated in July 2004 in connection with our initial public offering, provides for, among other things; the ability of Novartis to maintain its percentage ownership in our stock; rights of Novartis with respect to designation of nominees for election as director; rights of Novartis to approve specified material corporate activities of Idenix; and registration rights in favor of Novartis and certain of our other stockholders who held shares of our preferred stock prior to the conversion of such preferred stock into common stock in May 2003; | |
| • | a development agreement, as amended, under which we are collaborating with Novartis to develop, manufacture and commercialize our hepatitis B virus, or HBV, product candidates, valopicitabine, our HCV product candidate, and potentially other product candidates; and | |
| • | a supply agreement, under which Novartis will manufacture for us the active pharmaceutical ingredient, or API, for the clinical development supply of product candidates and potentially the API for the commercial supply of product candidates it has licensed from us and will perform the finishing and packaging of licensed products for commercial sale. |
| Stockholders’ Agreement |
Under the stockholders’ agreement, we have:
| • | agreed to use our reasonable best efforts to nominate for election as a director at least two designees of Novartis for so long as Novartis and its affiliates own at least 35% of our voting stock and at least one designee of Novartis for so long as Novartis and its affiliates own at least 19.4% of our voting stock; | |
| • | agreed that for so long as any designee of Novartis serves on our board of directors, a Novartis director designee is entitled to be a member of each committee of our board of directors or a non-voting observer to any such committee, if such committee membership is barred by applicable law, rule or regulation; | |
| • | required that, with certain limited exceptions, until May 8, 2008 or sooner if terminated pursuant to the terms of the stockholders’ agreement, Novartis and its affiliates not acquire additional shares of our voting stock unless a majority of our independent directors approves or requests the acquisition; | |
| • | granted to Novartis for so long as it and its affiliates continue to own at least 19.4% of our voting stock, approval rights over a number of corporate actions that we or our subsidiaries may take, including: |
| – | the authorization or issuance of additional shares of our capital stock or the capital stock of our subsidiaries, except for a limited number of specified issuances; | |
| – | any change or modification to the structure of our board of directors or a similar governing body of any of our subsidiaries; | |
| – | any amendment or modification to any of our organizational documents or those of our subsidiaries; | |
| – | the adoption of a three-year strategic plan or the adoption of an annual operating plan and budget, if there is no approved strategic plan; | |
| – | any decision that would result in a variance of total annual expenditures, capital or expense, in excess of 20% from the approved three-year strategic plan; | |
| – | any decision that would result in a variance in excess of the greater of $10 million or 20% of our profit or loss target in the strategic plan or operating plan; |
4
Table of Contents
| – | the acquisition of stock or assets of another entity that exceeds 10% of our consolidated net revenue, net income or net assets; | |
| – | the sale, lease, license or other disposition of any assets or business which exceeds 10% of our net revenue, net income or net assets; | |
| – | the incurrence of any indebtedness by us or our subsidiaries for borrowed money in excess of $2 million, other than in limited circumstances; | |
| – | any material change in the nature of our business or that of any of our subsidiaries; | |
| – | any change in control of Idenix or any subsidiary; and | |
| – | any dissolution or liquidation of Idenix or any subsidiary, or the commencement by us or any subsidiary of any action under applicable bankruptcy, insolvency, reorganization or liquidation laws; and |
| • | granted Novartis, together with certain other holders of our common stock, rights to cause us to register, under the Securities Act of 1933, or Securities Act, shares of our common stock held by such stockholders. |
| Novartis’ Ability to Maintain its Percentage Ownership Interest in our Capital Stock |
If we issue any shares of our capital stock, other than in certain situations, Novartis has the right to purchase such number of shares required to maintain its percentage ownership of our voting stock for the same consideration per share paid by others acquiring our stock. This right is applicable to equity awards under our stock incentive plans, including our 2005 Stock Incentive Plan.
Additionally, Novartis has the right to purchase, at par value of $0.001 per share, such number of shares as is required to maintain its percentage ownership of our voting stock if we issue shares of capital stock in connection with the acquisition or in-licensing of technology through the issuance of up to 5% of our stock in any24-month period. These additional purchase rights remain in effect until the earlier of the date that Novartis and its affiliates own less than 19.4% of our voting stock or the date that Novartis becomes obligated to make contingent payments of $357 million to those holders of our stock who sold shares to Novartis on May 8, 2003.
| Development, License and Commercialization Agreement |
Under the development, license and commercialization agreement, dated as of May 8, 2003 among us, our subsidiary Idenix (Cayman) Limited, or Idenix Cayman, and Novartis, as amended, which we refer to as the development agreement, we are collaborating with Novartis to develop, manufacture and commercialize our product candidates for the treatment of infections caused by the hepatitis B virus, or HBV, and valopicitabine, our HCV product candidate. In addition to providing development funding for these product candidates, Novartis will make payments to us of up to $45 million in connection with the advancement of valopicitabine into phase III clinical trials, up to $35 million and $455 million, respectively, upon the achievement of regulatory approval milestones for our HBV product candidates and regulatory filing and approval milestones for valopicitabine, as well as additional milestone payments based upon achievement of predetermined sales levels.
In addition, Novartis has the exclusive option to obtain rights to other product candidates we develop, or in some cases license, for so long as Novartis maintains ownership of 51% of our voting stock and for a specified period of time thereafter. The terms of these options, including license fees, milestone payments and payments in reimbursement of development expenses, vary according to the disease which the product candidate treats, the stage of development of the product candidate and Novartis’ ownership interest in Idenix.
| Development of Product Candidates and Regulatory Activities |
For most of our product candidates, Novartis has the right to approve, in its reasonable discretion, the development budget. We and Novartis will develop each product in accordance with a development plan approved by a joint operating committee that is comprised of an equal number of representatives of Idenix
5
Table of Contents
and Novartis. Novartis is solely responsible for the development expenses incurred in accordance with approved development budgets for our HBV product candidates and valopicitabine, or a replacement HCV product candidate. If valopicitabine fails to obtain regulatory approval in the U.S., Novartis will pay the development expenses for a replacement HCV product candidate if it has approved the corresponding development budget, up to a specified maximum. The development expense payments for any replacement HCV product candidate will be credited against the first sales milestone payment payable by Novartis to us for our initial HCV product. Novartis will also be primarily responsible for the development expenses for any other product candidate for which it exercises its option to obtain commercialization rights.
We have primary responsibility for preparing and filing regulatory submissions with respect to any licensed product in the U.S., and Novartis has primary responsibility for preparing and filing regulatory submissions with respect to any licensed product in all other countries in the world. Under certain circumstances, primary responsibilities for all or certain regulatory tasks in a particular country may be switched from one party to the other.
| Product Commercialization |
We have granted Novartis an exclusive, worldwide license to market and sell our HBV product candidates and valopicitabine. Additionally, we will grant Novartis such a license with respect to any other product candidates for which Novartis exercises its option. In each case we have retained the right to co-promote or co-market all licensed products in the U.S., the U.K., France, Germany, Italy and Spain. We will share equally the resulting net benefit with Novartis from the co-promotion in the U.S. from the date of product launch. In the U.K., France, Germany, Italy and Spain, the net benefit we expect to realize will increase incrementally during the first three years from the date of product launch, such that we will share equally with Novartis the net benefit from co-promotion beginning in the third year from the date of product launch.
In other countries, we will effectively sell products to Novartis for their further sale to third parties. Novartis will pay us to acquire such products at a price that is determined in part by the volume of product net sales under the terms of the supply agreement described below.
Novartis has agreed that it will not market, sell or promote, or grant a license to any third party to market, sell or promote, certain competing products. However, if Novartis seeks to engage in such activities, it must first inform us of the competitive product opportunity and, at our election, enter into good faith negotiations with us concerning such opportunity. If we either do not elect to enter into negotiations with respect to such opportunity or are unable to reach agreement within a specified period, Novartis would be free to proceed with its plans with respect to such competing product. The competitive restrictions on Novartis terminate on a country-by-country basis on the earlier of May 8, 2008 or the termination of the development agreement with respect to each particular country as described below.
| Termination |
Novartis and in certain circumstances, we, have the right to terminate the development agreement. Novartis may in its sole discretion terminate the development agreement with respect to a particular product, product candidate or country on not less than six months notice.
If Novartis terminates the development agreement for material breach by us, or for bankruptcy, insolvency or reorganization on our part, then Novartis may elect to retain licenses to our product candidates or products, in which case it will remain obligated to make payments to us in amounts to be negotiated in good faith at the time of termination. If we terminate part or all of the development agreement for material breach by Novartis, or for bankruptcy, insolvency or reorganization on the part of Novartis, or if Novartis terminates the development agreement unilaterally in the absence of a breach by us, we may be obligated to make payments to Novartis in amounts to be negotiated in good faith at the time of termination.
6
Table of Contents
| Master Manufacturing and Supply Agreement |
Under the master manufacturing and supply agreement, dated as of May 8, 2003, between Idenix Cayman and Novartis, which we refer to as the supply agreement, Idenix Cayman appointed Novartis to manufacture or have manufactured the clinical supply of the API for each product candidate licensed under the development agreement and certain other product candidates. Idenix Cayman will appoint Novartis or a third party to manufacture the commercial supply of the API based on a competitive bid process under which Novartis has the right to match the best third-party bid. Novartis will perform the finishing and packaging of the APIs into the final form for sale.
Idenix Cayman will pay Novartis for manufacturing the commercial supply of API, if Novartis manufactures the API, and finishing and packaging the products. Novartis will pay to Idenix Cayman a transfer price based on net sales of the products sold outside the co-commercialization countries. The parties will negotiate the transfer prices for the products to be sold in the co-commercialization countries.
| Indemnification |
We have agreed to indemnify Novartis and its affiliates against losses suffered as a result of our breach of representations and warranties in the development agreement and stock purchase agreement dated March 21, 2003 which we, Novartis and substantially all of our stockholders as of March 21, 2003 are a party. In these agreements, we made numerous representations and warranties to Novartis regarding our hepatitis C and hepatitis B product candidates, including representations regarding our ownership of the inventions and discoveries relating to such product candidates. If one or more of our representations or warranties were not true at the time we made them to Novartis, we would be in breach of these agreements. Novartis has the right to seek from us, and under certain circumstances, from us and our stockholders who sold shares to Novartis, who include many of our officers and directors, indemnification for damages suffered by Novartis as a result of a breach by us. For a further discussion of indemnification rights and obligations, please refer to our Annual Report on Form 10-K where a more in depth discussion is presented under the caption “Business — Collaborations — Relationship with Novartis — Development, License and Commercialization Agreement — Indemnification” “— Stock Purchase Agreement” “Risk Factors — Factors Related to our Relationship with Novartis” and “— Factors Related to Patents and Licenses”.
| Other Agreement |
We have also agreed that until such time as Novartis and its affiliates own less than 50% of our voting stock, Novartis’ consent is required for the selection and appointment of our chief financial officer. If in Novartis’ reasonable judgment our chief financial officer is not satisfactorily performing his duties, we are required to terminate his employment.
PROPOSAL 1 — ELECTION OF DIRECTORS
Our board of directors is elected each year at the annual meeting of stockholders. There are eight nominees for the nine presently authorized seats on our board of directors. Each director elected to hold office will do so until the 2007 annual meeting of stockholders and until her or his successor is elected and qualified, or until such director’s earlier death, resignation or removal.
If all the nominees for director are elected at the annual meeting, our board of directors will have one vacancy to be filled by a director who is considered independent under the NASDAQ regulations and the SEC rules. Under our bylaws, as well as Delaware law, any vacancy on our board of directors may be filled by a majority of the directors then in office, even if such number of directors is less than a quorum. We are actively searching for a new independent director who has the appropriate qualifications to make valuable contributions to our board of directors.
Each person nominated for election is currently serving as a director of Idenix. The board of directors, upon the recommendation of the Nominating and Corporate Governance Committee, has nominated each of the listed nominees for election as a director. Thomas Ebeling and Robert E. Pelzer have been nominated as the two designees of Novartis, pursuant to the stockholder’s agreement described under the
7
Table of Contents
caption “Corporate Governance — Director Independence — Relationship with Novartis.” There are no family relationships among any of our directors and our executive officers.
Each nominee has agreed to serve if elected and we do not know any reason why any nominee would be unable to serve. In the event that any nominee should be unavailable for election, proxies will be voted for the election of a substitute nominee designated by the board of directors or for election of only the remaining nominees.
Our board of directors met four times in 2005. No director attended fewer than 75% of the total number of meetings of the board of directors or the committees on which he or she served during 2005.
Unless authority to do so is withheld, shares represented by executed proxies will be voted for the election of the eight nominees named below. Proxies cannot be voted for a greater number of persons than the number of nominees standing for election. Since eight directors are to be elected at the annual meeting, the eight nominees for director who receive the highest number of votes for election will be elected as directors. Abstentions are not counted for purposes of electing directors.
| Our board of directors recommends a vote FOR each of the nominees named below. |
The name, age, years of service on our board of directors, and principal occupation and business experience of each director nominee is set forth below.
| Name and Age | Principal Occupation and Business Experience | Director Since | ||
| Jean-Pierre Sommadossi, Ph.D. (age 50) | Dr. Sommadossi is the principal founder of Idenix and has served as the chairman of our board of directors since our inception in 1998 and as our president and chief executive officer since November 2000. During the period from November 1999 to November 2000, Dr. Sommadossi served as our executive president and chief scientific officer. Prior to taking a sabbatical and then unpaid leave from November 1999 to November 2002, Dr. Sommadossi served as a professor of pharmacology, toxicology and clinical pharmacology and associate director of both the Center for AIDS Research and the Liver Center, University of Alabama at Birmingham School of Medicine from June 1992 to November 2000. From 1996 to 1999, Dr. Sommadossi served on the Research Agenda Committee of the AIDS Clinical Trial Group. Dr. Sommadossi holds a Pharm.D. and Ph.D. in Pharmacology from the University of Marseilles in France. | 1998 | ||
| Charles W. Cramb (age 59) | Mr. Cramb has served as the chief financial officer of The Avon Company, a global beauty company, since November 2005. Prior to joining The Avon Company, Mr. Cramb served as the chief financial officer at Gillette Company, a worldwide consumer products company, from July 1997 to November 2005. From July 1995 to July 1997, Mr. Cramb served as a corporate vice president and corporate controller of The Gillette Company. Mr. Cramb is a director and vice chairman of the Private Sector Council. He is also a member of the board of directors of Tenneco Automotive Inc. Mr. Cramb holds a B.A. from Dartmouth College and a M.B.A. from the University of Chicago. | 2003 | ||
| Thomas Ebeling (age 47) | Mr. Ebeling joined the Novartis Group, a multinational group of companies specializing in the research, development, manufacture, sale and distribution of innovative healthcare products, in January 1998 as chief executive officer of Novartis Nutrition World Wide. After serving as chief executive officer of Novartis’ global nutrition operations, Mr. Ebeling became chief executive officer of Novartis Consumer Health World Wide, and then chief operating officer of the Novartis Pharmaceuticals Division. Mr. Ebeling was appointed chief executive officer of Novartis Pharmaceuticals Division in July 2000. Prior to joining Novartis, Mr. Ebeling served as general manager of Pepsi-Cola Germany, where he began his career in 1991 as marketing manager. Prior to working for Pepsi-Cola, Mr. Ebeling held several positions at Reemstma Germany from 1987 to 1991. Mr. Ebeling holds a degree in psychology from the University of Hamburg, Hamburg, Germany. | 2003 | ||
8
Table of Contents
| Name and Age | Principal Occupation and Business Experience | Director Since | ||
| Wayne T. Hockmeyer, Ph.D. (age 61) | Dr. Hockmeyer founded MedImmune, Inc., a biotechnology company, in April 1988 and served until October 2000 as the chief executive officer of MedImmune. In October 2000, Dr. Hockmeyer relinquished his position as chief executive officer and now serves as chairman of the board of directors of MedImmune, Inc. and as president of MedImmune Ventures, Inc., a wholly-owned subsidiary of MedImmune, Inc. Dr. Hockmeyer also serves as a director of Advancis Pharmaceutical Corporation, GenVec, Inc., TolerRx, Inc. and Vanda Pharmaceuticals Inc. Dr. Hockmeyer was recognized, in 1998, by the University of Florida as a Distinguished Alumnus and in 2002 was awarded a Doctor of Science honoris causa from Purdue University. Dr. Hockmeyer holds a B.S. from Purdue University and a Ph.D. from the University of Florida. | 2002 | ||
| Thomas R. Hodgson (age 64) | Since January 1999, Mr. Hodgson has engaged in private investing activities. From September 1990 to January 1999, Mr. Hodgson served as the president and chief operating officer of Abbott Laboratories, a pharmaceutical company. From 1983 to 1990, Mr. Hodgson served as the president of Abbott International and from 1978 to 1983, Mr. Hodgson served as the president of the Hospital Products Division of Abbott Laboratories. Mr. Hodgson is a director of The St. Paul Travelers Inc. and Intermune, Inc. Mr. Hodgson holds a B.S. from Purdue University, an M.S. from the University of Michigan, an M.B.A. from Harvard Business School and an honorary doctorate degree in engineering awarded by Purdue University. | 2002 | ||
| Robert E. Pelzer (age 52) | Mr. Pelzer is general counsel of Novartis Pharmaceuticals Division, a part of the Novartis Group, a multinational group of companies specializing in the research, development, manufacture, sale and distribution of innovative healthcare products. Prior to this appointment at Novartis in March 2002, Mr. Pelzer was general counsel at DuPont Pharmaceuticals Company from 1998 to December 2001. Prior to that time, Mr. Pelzer held various positions with The DuPont Company. Mr. Pelzer started his legal career at the law firm of MacKimmie Matthews in Calgary, Alberta. Mr. Pelzer holds degrees in Commerce and in Law from the University of Alberta. He is admitted as barrister and solicitor in the Province of Alberta, Canada, and as Solicitor in England and Wales. | 2003 | ||
| Denise Pollard-Knight, Ph.D. (age 47) | Dr. Pollard-Knight has served since April 2004 as head of Nomura Phase4 Ventures, an affiliate of Nomura International plc. From January 1999 to March 2004, Dr. Pollard-Knight served as head of Healthcare Private Equity at Nomura International plc, a leading Japanese financial institution. From January 1997 to January 1999, Dr. Pollard-Knight was a member of Rothschild Asset Management Ltd., an investment management firm. Prior to that, Dr. Pollard-Knight held several research and development management positions at Amersham-Pharmacia and Fisons plc. Dr. Pollard-Knight is a director of ViaCell, Inc. Dr. Pollard-Knight holds a Ph.D. and BSc (Hons) from the University of Birmingham in England. Dr. Pollard-Knight completed postdoctorate work as a Fulbright Scholar at the University of California, Berkeley. | 2003 | ||
9
Table of Contents
| Name and Age | Principal Occupation and Business Experience | Director Since | ||
| Pamela Thomas-Graham (age 42) | Ms. Thomas-Graham has served as group president overseeing apparel for better and moderate department stores for Liz Claiborne, Inc. since October 2005. Prior to joining Liz Claiborne, Inc., an apparel and retail company, Ms. Thomas-Graham served most recently as chairman of CNBC from February 2005 to October 2005 and served as president and chief executive officer of CNBC from July 2001 to February 2005. From February 2001 to July 2001, Ms. Thomas-Graham served as president and chief operating officer of CNBC. From September 1999 to February 2001, Ms. Thomas-Graham served as an executive vice president of NBC, and president and chief executive officer of CNBC.com. Prior to joining NBC, Ms. Thomas-Graham was a partner at McKinsey & Company from December 1995 to September 1999. Ms. Thomas-Graham also serves as a director of the Clorox Company. Ms. Thomas-Graham holds a J.D., M.B.A. and B.A. from Harvard University. | 2005 | ||
CORPORATE GOVERNANCE
| Commitment |
Our board of directors strongly believes that good corporate governance policies and practices lead to management of Idenix in a manner that will result in successful business performance and benefit to our stockholders. We routinely review and update our corporate governance policies and practices. We expect to continue to seek and implement those corporate governance practices that will promote a high level of performance from our board of directors, officers and employees.
| Corporate Governance Principles and Policy on Business Conduct and Ethics |
Our board of directors has adopted corporate governance principles which include guidelines for, among other things, determining director independence, establishing criteria and qualifications of directors, conduct of meetings of the board and meetings of independent directors, access by the directors to management, independent consultants and professional advisors, and management evaluation and succession.
Our board of directors is committed to legal and ethical conduct in fulfilling its responsibilities. We expect all of our directors, officers and employees to act ethically, legally and with integrity and in compliance with our Policy on Business Conduct and Ethics as well as our other policies and standards of conduct. Our Policy on Business Conduct and Ethics includes the code of ethics that applies to our principal executive officer, principal financial officer, principal accounting officer and persons performing similar functions. Our Policy on Business Conduct and Ethics is posted on our website atwww.idenix.com under the caption “Investor Center — Our Leadership & Governance — Idenix Policy on Business Conduct and Ethics” and we intend to post on our website all disclosures that are required by law or NASDAQ® listing standards concerning any amendments to, or waivers from, any provision of our policy. No waivers from any provision of our policy have been granted.
| Information about our Nominating Process |
The Nominating and Corporate Governance Committee is responsible for identifying and evaluating individuals to become members of our board of directors, including the review of candidates recommended by our stockholders.
The process followed by the Nominating and Corporate Governance Committee to identify, evaluate and review candidates includes requests to members of our board of directors and others for recommendations, meeting from time to time to evaluate biographical information and background material relating to potential candidates, an assessment of such candidates qualifications vis-a-vis our director qualification standards described below, and interviews of selected candidates by members of the Nominating and Corporate Governance Committee and the board of directors. To assist in the
10
Table of Contents
identification and evaluation of potential director candidates, an executive search firm is retained by the Nominating and Corporate Governance Committee.
Stockholders may recommend to the Nominating and Corporate Governance Committee individuals for consideration as potential director nominees by submitting on a timely basis the name and background of the candidate to the Nominating and Corporate Governance Committee, c/o Secretary, Idenix Pharmaceuticals, Inc., 60 Hampshire Street, Cambridge, Massachusetts 02139. The Nominating and Corporate Governance Committee will consider a recommendation if appropriate biographical information and background material is provided. In addition to the biographical and background information, the stockholder making such recommendation must include a statement as to whether the stockholder or the group of stockholders making the recommendation has beneficially owned more than 5% of our common stock for at least a year as of the date such recommendation is made. Assuming that appropriate biographical and background material is timely provided for candidates recommended by stockholders, the Nominating and Corporate Governance Committee will evaluate those candidates by following substantially the same process, and applying substantially the same criteria, as for candidates submitted by members of our board of directors or by other persons. If our board of directors determines to nominate a stockholder recommended candidate, such nominee’s name will be included in our proxy statement and our proxy card for the stockholder meeting at which such nominee’s election is recommended.
Our stockholders also have the right to nominate director candidates themselves, without any prior review or recommendation by the Nominating and Corporate Governance Committee or the board of directors, by following the procedures set forth under “Stockholder Proposals for the 2007 Annual Meeting.” Director candidates nominated in accordance with such procedures will be included in our proxy materials but may not be included in our proxy card for the next annual meeting.
| Director Qualification Standards |
Directors should possess the highest personal and professional ethics and integrity, understand and be aligned with our core values, and be committed to representing the long-term interests of our stockholders. Directors must also be inquisitive, objective and have practical wisdom and mature judgment. We endeavor to have a diverse board of directors possessing strategic and policy-making experience and skills in business, healthcare, science and technology and in the international arena. In considering whether to recommend any candidate for inclusion in our board of director’s slate of recommended director nominees, including candidates recommended by stockholders, the Nominating and Corporate Governance Committee will apply the criteria set forth in our corporate governance guidelines. These criteria include the candidate’s integrity, business acumen, age, experience, diligence, conflicts of interest and the ability to act in the interests of all of our stockholders. The Nominating and Corporate Governance Committee does not assign specific weights to particular criteria and no particular criterion is necessarily applicable to all prospective nominees. Our board of directors believes that the backgrounds and qualifications of our directors, considered as a group, should provide diversity and a significant composite mix of experience, knowledge and abilities that will allow our board of directors to fulfill its responsibilities.
Directors must be willing to devote sufficient time to carrying out their duties and responsibilities effectively, and should be committed to serve on our board of directors for an extended period of time. Our board of directors does not believe that arbitrary term limits on directors’ service are appropriate since such term limits could result in the loss of directors who have developed insights into Idenix and our business and operations. Our board of directors annually engages in a self-evaluation process and a review of the requisite skills and criteria comprised by our board of directors and those to be sought in nominees for directors.
| Communicating with the Board of Directors |
We have established an Integrity Hotline for the confidential, anonymous submission by our directors, officers and employees of concerns regarding violations or suspected violations of our Policy on Business Conduct and Ethics, including matters relating to accounting and auditing matters. In addition, the Audit
11
Table of Contents
Committee has established procedures for the receipt, retention and treatment of communications received by us, our board of directors and the Audit Committee regarding accounting, internal controls or auditing matters. Written communications from our stockholders and employees may be sent to: Idenix Pharmaceuticals, Inc., Audit Committee Chair, 60 Hampshire Street, Cambridge, Massachusetts 02139.
Stockholders who wish to send other communications to our board of directors should address such communications to Board of Directors, c/o Secretary, Idenix Pharmaceuticals, Inc., 60 Hampshire Street, Cambridge, Massachusetts 02139.
Our board of directors will give attention to written communications that are submitted by our stockholders and other interested parties, and will respond to appropriate communications it receives. Absent unusual circumstances or as contemplated by committee charters and subject to any required assistance or advice from our counsel, the chair of the Nominating and Corporate Governance Committee is primarily responsible for monitoring communications from our stockholders and other interested parties and for providing copies or summaries to the other directors as he or she considers appropriate.
| Director Independence |
Relationship with Novartis. Under the terms of the stockholder’s agreement, we have agreed to use our reasonable best efforts to nominate for election as a director at least two designees of Novartis for so long as Novartis and its affiliates own at least 35% of our voting stock and at least one designee of Novartis for so long as Novartis and its affiliates own at least 19.4% of our voting stock. We have also agreed that for so long as one or more Novartis designees serve on our board of directors, to permit Novartis designated directors to serve on our board committees unless such committee service is prohibited by applicable law, rule or regulation, in which case, the Novartis designee is entitled to serve on our board committees as a non-voting observer. To enable us to fulfill these contractual obligations, we are relying upon an exemption provided to “controlled companies” by NASDAQ with respect to its requirements that a majority of the directors on the compensation and nominating and corporate governance committee be independent. Applicable NASDAQ rules define “controlled companies” as those which have more than 50% of their voting power held by an individual, group or entity. Since Novartis and its affiliates currently own more than 50% of our voting stock, Idenix is a controlled company.
Board of Directors. Our Board of Directors has determined that the following five directors satisfy NASDAQ’s independence requirements and the additional independence guidelines set forth in our corporate governance principles: Charles W. Cramb, Wayne T. Hockmeyer, Thomas R. Hodgson, Denise Pollard-Knight and Pamela Thomas-Graham.
Committees. Our Audit Committee, as required by NASDAQ and SEC rules, is composed entirely of independent directors. Our Compensation Committee and our Nominating and Corporate Governance Committee, with the exception of one Novartis designated director serving on each committee, is each composed of independent directors.
| Meetings of Independent Directors |
Our corporate governance principles require that our independent directors regularly meet without management being present. At each meeting of our board of directors, the independent directors meet in executive session without management.
| Attendance at Annual Meetings |
We expect all of our directors to attend our annual meetings of stockholders. All directors serving on our board as of the 2005 annual meeting of stockholders attended that meeting.
12
Table of Contents
Board of Directors and its Committees
| Board of Directors |
Our business, property and affairs are managed by or under the direction of our board of directors. Our board of directors has responsibility for establishing broad corporate policies and reviewing our overall performance. Among the primary responsibilities of our board of directors is the oversight of the management of our company. Our directors remain informed of our business and management’s activities by reviewing documents provided to them before each meeting and by attending presentations made by our chief executive officer and other members of management. At each meeting of the board of directors, our directors are advised of actions taken by each board committee. Directors have access to our books, records and reports and independent advisors. Members of our management frequently interact with and are at all times available to our directors.
Our corporate governance principles provide for the appointment of a lead director. Mr. Hodgson serves as our lead director. In such capacity, Mr. Hodgson, in consultation with the other independent directors, establishes agendas for the independent directors’ meetings, chairs such meetings and meets with directors to discuss their performance.
| Committees of our Board of Directors |
Our board of directors has three standing committees — Audit, Compensation and Nominating and Corporate Governance. Copies of each committee’s charter, as currently in effect, are posted on our website atwww.idenix.com under the caption “Investor Center — Our Leadership & Governance — Board of Directors, Committee Composition and Charters.” Additionally, a copy of the Audit Committee charter, as in effect on the date of this proxy statement, is attached to this proxy statement asAppendix A.
| • | The Audit Committee assists the board of directors in its oversight of the integrity of our financial statements, compliance with legal and regulatory requirements and understanding of our accounting and financial reporting processes. Our Audit Committee has the sole authority and responsibility to select, evaluate, compensate and replace our independent registered public accounting firm. Our board of directors has determined that Charles W. Cramb, the chair of the Audit Committee, is a financial expert under applicable SEC and NASDAQ rules. The Audit Committee held 8 meetings in 2005. The report of the Audit Committee appears on page 18. | |
| • | The Compensation Committee assists the board of directors with its overall responsibility relating to compensation and management development, recommends for approval by the board of directors the compensation of our chairman and chief executive officer and our non-employee directors, establishes annually the compensation of our other officers, effects the engagement of, and terms of employment agreements and arrangement with, and the termination of all our officers and administers our equity incentive plans. The Compensation Committee held 5 meetings in 2005. The report of the Compensation Committee appears on page 27. | |
| • | The Nominating and Corporate Governance Committee assists in developing and recommending to our board of directors sound corporate governance principles and practices, identifying qualified individuals to become members of our board of directors, recommending nominees to our board of directors and reviewing and making recommendations to our board of directors with respect to management succession planning. The Nominating and Corporate Governance Committee held 2 meetings in 2005. |
While each committee has its own charter and designated responsibilities, the committees act on behalf of the entire board of directors. The committees regularly report on their activities to the entire board of directors, and all members of our board of directors may receive copies of each committee’s agendas and minutes.
13
Table of Contents
None of the members of any committee of our board is or has been an officer of Idenix. Messrs. Pelzer and Ebeling are officers of Novartis. The members of the committees of our board of directors are set forth in the following table:
| Nominating and Corporate Governance | ||||
| Audit Committee | Compensation Committee | Committee | ||
| Charles W. Cramb (Chair) | Wayne T. Hockmeyer (Chair) | Pamela Thomas-Graham (Chair) | ||
| Wayne T. Hockmeyer | Charles W. Cramb | Wayne T. Hockmeyer | ||
| Thomas Hodgson | Thomas Ebeling | Robert Pelzer | ||
| Thomas Hodgson |
Security Ownership of Certain Beneficial Owners and Management
| Security Ownership of Directors and Management |
The following table sets forth information regarding the ownership of our common stock as of April 1, 2006 by:
| • | each of our directors, including our chief executive officer, and the other four most highly compensated executive officers of Idenix; and | |
| • | all of our executive officers and directors as a group. |
Unless otherwise indicated, to our knowledge, each of the persons named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned, subject to community property laws where applicable.
Except for Dr. Sommadossi, who beneficially owns 4.2% of the outstanding shares of Idenix common stock, no director or named executive officer beneficially owned more than 1% of the outstanding shares of Idenix common stock.
| Shares of | Option | ||||||||||||
| Name | Common Stock(1) | Shares(1) | Total | ||||||||||
Directors | |||||||||||||
| Jean-Pierre Sommadossi | 2,158,854 | (2) | 193,750 | 2,352,604 | |||||||||
| Charles W. Cramb | 7,200 | 60,000 | 67,200 | ||||||||||
| Thomas Ebeling | — | — | |||||||||||
| Wayne T. Hockmeyer | 41,708 | 20,000 | 61,708 | ||||||||||
| Thomas R. Hodgson | 42,324 | 20,000 | 62,324 | ||||||||||
| Robert Pelzer | — | — | — | ||||||||||
| Denise Pollard-Knight | — | — | — | ||||||||||
| Pamela Thomas-Graham | — | 35,000 | 35,000 | ||||||||||
Other Named Executive Officers | |||||||||||||
| David A. Arkowitz | 8,000 | 216,250 | 224,250 | ||||||||||
| Nathaniel A. Brown | — | 208,125 | 208,125 | ||||||||||
| Guy Macdonald | 10,000 | 216,250 | 226,250 | ||||||||||
| Andrea J. Corcoran | 63,324 | 144,563 | 207,887 | ||||||||||
| All directors and executive officers as a group (15 persons) | 2,685,261 | 1,248,126 | 3,933,387 | ||||||||||
| (1) | Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to shares of our common stock. Shares of our common stock issuable under stock options that are exercisable within 60 days after April 1, 2006 are deemed outstanding and are included for computing the percentage ownership of the person holding the options but are not deemed outstanding for computing the percentage ownership of any other person. |
| (2) | Includes 37,810 shares held by the Jean-Pierre Sommadossi 2002 Qualified Annuity Trust. |
14
Table of Contents
The address of each stockholder listed is in c/o Idenix Pharmaceuticals, Inc., 60 Hampshire Street, Cambridge, Massachusetts 02139.
| Security Ownership of Certain Beneficial Owners |
The following table sets forth information regarding the ownership of our common stock as of April 1, 2006 by persons known to us to be the beneficial owners of more than five percent of the outstanding shares of our common stock.
| Name and Address | Shares of Common Stock | Percentage | |||||||
| Novartis AG | 31,295,870 | (1) | 55.9 | % | |||||
| Lichtstrasse 35 | |||||||||
| CH-4002 Basel | |||||||||
| Switzerland | |||||||||
| MPM Capital | 3,339,645 | (2) | 6.0 | % | |||||
| 200 Clarendon Street | |||||||||
| Boston, Massachusetts 02116 | |||||||||
| (1) | Consists of 31,295,870 shares held by Novartis Pharma AG, a direct, wholly-owned subsidiary of Novartis AG. This information is based solely on information set forth in a Schedule 13D filed on November 2, 2005 jointly by Novartis AG and Novartis Pharma AG. |
| (2) | Consists of 2,965,957 shares held by BB BioVentures L.P., or BB BioVentures, 257,952 shares held by MPM Bioventures Parallel Fund L.P., or Parallel Fund, 37,508 shares held by MPM Asset Management Investors 1998 LLC, or Investor’s Fund, and 78,228 shares held by MPM Asset Management. This information is based solely on information set forth in a Schedule 13G/A filed by such entities on February 13, 2006. |
Equity Compensation Plan Information
The following table provides information about the securities authorized for issuance under our equity compensation plans as of December 31, 2005. In connection with a public offering of our common stock in October 2005, our board of directors reduced the number of shares of common stock authorized for issuance under the 2005 stock incentive plan to 1,400,000 shares. In March 2006, the board of directors authorized the reserve of 1,600,000 shares for issuance under the 2005 stock incentive plan with the effect that all shares approved by the Idenix stockholders at the 2005 annual meeting of stockholders for issuance pursuant to the 2005 stock incentive plan have been reserved for such purpose.
Equity Compensation Table
| Number of securities | ||||||||||||
| remaining available for | ||||||||||||
| future issuance under | ||||||||||||
| Number of securities | equity compensation | |||||||||||
| to be issued upon | Weighted average | plans (excluding | ||||||||||
| exercise of | exercise price of | securities reflected in | ||||||||||
| Plan Category | outstanding options | outstanding options | column (a)) | |||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders | 3,584,322 | (1) | $ | 12.20 | 338,608 | (2) | ||||||
| Equity compensation plans not approved by security holders | — | — | — | |||||||||
Total | 3,584,322 | 338,608 | ||||||||||
| (1) | Includes shares of our common stock issuable upon exercise of options to purchase common stock awarded under our 1998 equity incentive plan, as amended, and our 2005 stock incentive plan. |
| (2) | Includes shares of our common stock issuable under our 1998 equity incentive plan, as amended, and our 2005 stock incentive plan. |
15
Table of Contents
Comparative Stock Performance Graph
The comparative stock performance graph below compares the cumulative stockholder return on our common stock for the period from July 22, 2004 (the date our common stock began publicly trading) through December 31, 2005 with the cumulative total return on (i) the American Stock Exchange, or AMEX Biotech Index, (ii) the NASDAQ Stock Market (U.S. Companies), or the “NASDAQ Composite Index,” and (iii) the NASDAQ Biotech Index. The graph assumes $100 had been invested in our common stock, the AMEX Biotech Index, the NASDAQ Composite Index and the NASDAQ Biotech Index on July 22, 2004).
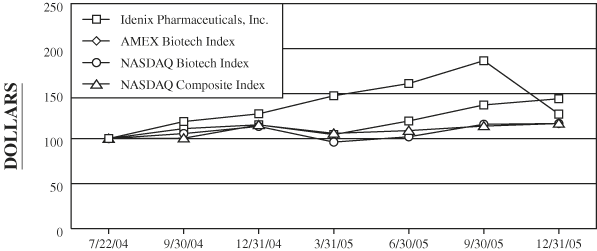
| 7/22/04 | 9/30/04 | 12/31/04 | 3/31/05 | 6/30/05 | 9/30/05 | 12/31/05 | ||||||||||||||||||||||||||||
| Idenix Pharmaceuticals, Inc. | $ | 100.00 | $ | 118.96 | $ | 127.51 | 147.58 | 161.19 | 186.62 | 127.21 | ||||||||||||||||||||||||
| AMEX Biotech Index | 100.00 | 111.09 | 115.32 | 104.43 | 119.60 | 137.40 | 144.27 | |||||||||||||||||||||||||||
| NASDAQ Composite Index | 100.00 | 100.41 | 115.16 | 105.83 | 108.89 | 113.90 | 116.74 | |||||||||||||||||||||||||||
| NASDAQ Biotech Index | 100.00 | 105.57 | 113.54 | 96.11 | 102.00 | 115.95 | 116.76 | |||||||||||||||||||||||||||
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
| Agreements with Novartis |
We have entered into agreements with Novartis, including a stockholders’ agreement, development agreement, supply agreement and stock purchase agreement. For a discussion of these agreements and related obligations, please refer to “Relationship with Novartis.”
Employment Agreements
All of our named executive officers are party to employment agreements with the Company. For a description of such employment agreements, please refer to “Employment Agreements.”
| Registration Rights |
As of April 1, 2006, the holders of 37,151,869 shares of our common stock are entitled to cause us to register their shares or participate in a registration by us under the Securities Act. These rights are
16
Table of Contents
provided under the terms of the stockholders’ agreement. These holders include the following directors, officer and holders of more than five percent of our voting securities and their affiliates:
| Number of | ||||
| Name of Holder | Registrable Shares | |||
| Novartis AG(1) | 31,295,870 | |||
| MPM Capital L.P. affiliated funds(2) | 3,261,417 | |||
| Jean-Pierre Sommadossi | 100,000 | |||
| Total | 34,657,287 | |||
| (1) | Represents 31,295,870 shares held by Novartis, a direct, wholly-owned subsidiary of Novartis AG. Mr. Ebeling, one of our directors, serves as chief executive officer of the Novartis Pharmaceuticals Division, an affiliate of Novartis, and Mr. Pelzer, also one of our directors, serves as general counsel to Novartis Pharmaceuticals Division. |
| (2) | Represents 2,965,957 shares held by BB BioVentures, 257,952 shares held by Parallel Fund, and 37,508 shares held by Investor’s Fund. Each of these funds is affiliated with MPM Capital L.P. affiliated funds. |
Compensation of Directors
For their service to Idenix, we compensate our non-employee directors, other than directors who are employees of Novartis, with a combination of cash and equity. We do not provide additional remuneration to Dr. Sommadossi, an officer of Idenix, for his service as a director. Additionally, prior to 2006, Dr. Pollard-Knight was not compensated for her service as a director.
The following table describes Idenix’s compensation practices for non-employee directors, other than the non-employee directors noted above, during 2005 and 2006:
| Options to purchase | |||||||||||||||||||
| Meeting Fees | common stock(1) | ||||||||||||||||||
| (per meeting | |||||||||||||||||||
| Year | Cash Retainer | attended) | Initial | Annual | |||||||||||||||
| Board Member | 2005 | $ | 30,000 | $ | — | 15,000 | (2) | 20,000 | (3) | ||||||||||
| 2006 | 30,000 | 2,000 | 15,000 | (2) | 20,000 | (3) | |||||||||||||
| Committee Chair (other than Audit) | 2005 | 5,000 | — | — | — | ||||||||||||||
| 2006 | 5,000 | 1,000 | — | — | |||||||||||||||
| Audit Committee Chair | 2005 | 5,000 | — | — | — | ||||||||||||||
| 2006 | 8,000 | 1,000 | — | — | |||||||||||||||
| Committee Members (other than chair) | 2005 | — | — | ||||||||||||||||
| 2006 | — | 1,000 | — | — | |||||||||||||||
| (1) | The exercise price of these options is equal to the fair market value of our common stock on the date of grant as reported by NASDAQ. Each option terminates on the earlier of ten years from the date of grant or 180 days after the optionee ceases to serve as a director, except in the case of death or disability, in which event the option terminates one year from the date of the director’s death or disability. |
| (2) | Each non-employee director is entitled to receive an award of stock options upon his or her election or appointment to our board of directors. The vesting period for options awarded in 2005 was 12 monthly equal installments from the date of grant. In 2006, the vesting period for any options awarded upon initial appointment will vest in 24 equal monthly installments from the date of grant. Ms. Thomas-Graham was the only director whose service to Idenix commenced in 2005. As a result, she was the only director in 2005 to receive an initial option award. |
| (3) | Each non-employee director is entitled to receive at each year’s annual meeting after which he or she continues to serve as a director, an additional option grant of 20,000 shares. The number of options to |
17
Table of Contents
| be awarded to new non-employee directors who are appointed to our board of directors at times other than immediately after the annual meeting of stockholders will be prorated for the period of service between date of appointment and the next annual meeting. The annual option grant vests in 12 equal monthly installments from the date of grant. |
In addition, members of our board of directors, other than directors affiliated with Novartis, are reimbursed for expenses incurred in connection with attendance at meetings of our board of directors and its committees and related activities in accordance with Idenix policy.
| Compensation Committee Interlocks and Insider Participation |
Mr. Ebeling serves on the Compensation Committee as a designee of Novartis pursuant to the terms of our stockholders’ agreement. No other member of the Compensation Committee had any relationship with us requiring disclosure under Item 404 of Regulation S-K under the Exchange Act.
None of our executive officers has served as a director or member of the compensation committee (or other committee serving an equivalent function) of any other entity, one of whose executive officers served as a director of Idenix or member of our Compensation Committee.
PROPOSAL 2 — RATIFICATION OF SELECTION OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Our Audit Committee has selected the firm of PricewaterhouseCoopers LLP as our independent registered public accounting firm for the current fiscal year ending December 31, 2006. PricewaterhouseCoopers LLP has served as our independent registered public accounting firm since 1998. Although stockholder approval of the selection of PricewaterhouseCoopers LLP is not required by law, our board of directors believes that it is advisable to give stockholders an opportunity to ratify this selection. If this proposal is not approved at the annual meeting, our Audit Committee will reconsider its selection of PricewaterhouseCoopers LLP. Representatives of PricewaterhouseCoopers LLP are expected to be present at the annual meeting and will have the opportunity to make a statement if they desire to do so and will also be available to respond to questions from stockholders.
Our board of directors believes that the selection of PricewaterhouseCoopers LLP as our independent registered public accounting firm is in our best interests and the best interests of our stockholders. Therefore, a vote FOR this proposal is recommended.
| Audit Committee Report |
The Audit Committee assists the board of directors in its oversight of the integrity of Idenix’s financial statements and Idenix’s financial reporting process, compliance with legal and regulatory requirements and monitoring the qualifications and independence of the Idenix independent registered public accounting firm. Management has the primary responsibility for the financial statements and the financial reporting process including the systems of internal controls. The independent registered public accounting firm, PricewaterhouseCoopers LLP, is responsible for performing an independent audit of Idenix’s financial statements in accordance with the standards of the Public Company Accounting Oversight Board (United States) and issuing a report on those financial statements. The Audit Committee is responsible for monitoring and overseeing these activities. The Board has adopted a charter for the Audit Committee. The Audit Committee reviews and assesses, not less frequently than annually, the adequacy of its committee charter and recommends any proposed changes to the Board for approval. A copy of the Audit Committee charter, as in effect, as of the date of this proxy statement, is attached asExhibit A to this proxy statement.
18
Table of Contents
As appropriate, the Audit Committee reviews and evaluates, and discusses with Idenix’s management and the independent registered public accounting firm, the following:
| • | the plan for, and the independent registered public accounting firm’s report on, each audit of Idenix’s financial statements, effectiveness of internal control over financial reporting and management’s assessment of the effectiveness of internal control over financial reporting; | |
| • | Idenix’s financial disclosure documents, including financial statements and reports filed with the SEC or sent to stockholders; | |
| • | analyses prepared by management and/or the independent registered public accounting firm setting forth significant financial reporting issues and judgments made in connection with the preparation of the financial statements, including analyses of the effects of alternative generally accepted accounting principles methods on Idenix’s financial statements; | |
| • | changes in Idenix’s accounting practices, principles or methodologies; | |
| • | significant developments or changes in regulatory and accounting rules applicable to Idenix and the effects of such developments and changes on Idenix’s financial statements; | |
| • | the adequacy of Idenix’s financial reporting processes, including systems of internal control over financial reporting; and | |
| • | the type and presentation of information to be included in earnings press releases, as well as any financial information and earnings guidance provided to analysts and rating agencies. |
Additionally, the Audit Committee meets regularly with the independent registered public accounting firm, outside the presence of management, to discuss Idenix’s accounting practices, principles and methodologies, and the adequacy of Idenix’s financial reporting processes, including systems of internal control over financial reporting.
The Audit Committee has discussed Idenix’s major risk exposures and the steps that management has taken to monitor and control such exposures. Management is required to advise the Audit Committee of any instances of fraud relating to employees who have a significant role in Idenix’s internal controls. The Audit Committee was advised that management was not aware of any such instances of fraud.
Management has represented to the Audit Committee that the financial statements have been prepared in accordance with accounting standards generally accepted in the United States of America. The Audit Committee reviewed the audited consolidated financial statements with both management and the independent registered public accounting firm and discussed with each of them the quality, not just the acceptability, of the accounting principles that were followed and the clarity of disclosures in, and the presentation of, the financial statements. The Audit Committee also discussed with the independent registered public accounting firm the matters required to be discussed by Statement on Auditing Standards No. 61,Communication with Audit Committee. These matters include:
| • | methods to account for significant unusual transactions; | |
| • | the effect of significant accounting policies in controversial or emerging areas for which there is a lack of authoritative guidance or consensus; | |
| • | the process used by management in formulating particularly sensitive accounting estimates and the basis for the independent registered public accounting firm’s conclusions regarding the reasonableness of those estimates; and | |
| • | disagreements with management, if any, over the application of accounting principles, the basis, if any, for management’s accounting estimates and the disclosures in the financial statements. |
The Audit Committee have received the written disclosures and the letter from the independent registered public accounting firm required by Independence Standards Board Standard No. 1,Independence Discussions with Audit Committees. Independence Standards Board Standard No. 1 requires the independent registered public accounting firm annually to disclose in writing all relationships that, in the independent registered public accounting firm’s professional opinion, may reasonably be thought to bear on independence, confirm their perceived independence and engage in a discussion of independence.
19
Table of Contents
The Audit Committee has discussed with the independent registered public accounting firm its objectivity and independence from Idenix.
During the fiscal year ended December 31, 2005, management completed the documentation, testing and evaluation of Idenix’s system of internal control over financial reporting in response to the requirements set forth in Section 404 of the Sarbanes-Oxley Act of 2002 and related regulations. The Audit Committee was kept apprised of the progress of the evaluation and provided oversight and advice to management during the process. In connection with this oversight, the Audit Committee received periodic updates from management and the independent registered public accounting firm. At the conclusion of the process, the Audit Committee reviewed a report by management on the effectiveness of Idenix’s internal control over financial reporting. The Audit Committee also reviewed the independent registered public accounting firm’s Report of Independent Registered Public Accounting Firm included in Idenix’s Annual Report on Form 10-K for the fiscal year ended December 31, 2005 related to its audit of the effectiveness of internal control over financial reporting and management’s assessment of the effectiveness of internal control over financial reporting.
Based on the discussions among the Audit Committee, management and the independent registered public accounting firm, and the Audit Committee’s review of the representations and information provided by management and the independent registered public accounting firm, the Audit Committee recommended to the board of directors that the audited financial statements be included in Idenix’s Annual Report on Form 10-K for the fiscal year ended December 31, 2005. The Audit Committee also recommended the retention of PricewaterhouseCoopers LLP as Idenix’s independent registered public accounting firm for the current fiscal year ending December 31, 2006.
| By the Audit Committee | |
| Charles W. Cramb, Chair | |
| Wayne T. Hockmeyer, Ph.D. | |
| Thomas R. Hodgson |
| Principal Accountant Fees and Services |
The following table summarizes the fees PricewaterhouseCoopers, LLP, our independent registered public accounting firm, billed to us for each of the last two fiscal years for audit and other services:
| Fee Category | 2005 | 2004 | |||||||
| Audit Fees(1) | $ | 645,788 | $ | 442,739 | |||||
| Audit-Related Fees(2) | 3,900 | 16,450 | |||||||
| Tax Fees(3) | 48,565 | 190,035 | |||||||
| All Other Fees | — | — | |||||||
| Total Fees | $ | 698,253 | $ | 649,224 | |||||
| (1) | Audit fees consist of fees for the audit of our financial statements, the review of the interim financial statements included in our quarterly reports on Form 10-Q, and other professional services provided in connection with statutory and regulatory filings or engagements. Audit fees included $75,000 in 2005 and $230,043 in 2004 for services rendered by our independent registered public accounting firm in connection with our public offering completed in November 2005 and our initial public offering completed in July 2004, respectively. |
| (2) | Audit-related fees consist of fees for assurance and related services that are reasonably related to the performance of the audit and the review of our financial statements and which are not reported under “Audit Fees.” These services relate to consultations concerning financial accounting and reporting standards. |
| (3) | Tax fees consist of fees for tax compliance, tax advice and tax planning services. Tax compliance services, which relate to services provided for preparation of original and amended tax returns, accounted for $28,505 of the total tax fees billed in 2004. Tax advice and tax planning services relate to United States federal and state and international tax planning and advice. |
20
Table of Contents
No audit-related or tax fees billed in 2005 or 2004 were provided under the de minimis exception to the Audit Committee pre-approval requirements.
| Pre-Approval Policies and Procedures |
The Audit Committee has adopted policies and procedures relating to the approval of all audit and non-audit services that are to be performed by our independent registered public accounting firm. This policy generally provides we will not engage our independent registered public accounting firm to render audit or non-audit services unless the service is specifically approved in advance by the Audit Committee or the engagement is entered into pursuant to one of the pre-approval procedures described below.
From time to time, the Audit Committee may pre-approve specified types of services that are expected to be provided to us by our independent registered public accounting firm during the next 12 months. Any such pre-approval is detailed as to the particular service or type of services to be provided and is also generally subject to a maximum dollar amount.
The Audit Committee has also delegated to the chair of the Audit Committee the authority to approve any audit or non-audit services to be provided to us by our independent registered public accounting firm. Any approval of services by the Audit Committee chair pursuant to this delegated authority is reported on at the next meeting of the Audit Committee.
Executive Officers
The following table sets forth information relating to the individuals who serve as executive officers as of April 15, 2006:
| Name | Age | Position | ||||
| Jean-Pierre Sommadossi, Ph.D. | 50 | President and Chief Executive Officer and Chairman of the Board of Directors | ||||
| David A. Arkowitz | 44 | Chief Financial Officer and Treasurer | ||||
| Guy Macdonald | 47 | Executive Vice President, Operations | ||||
| Nathaniel A. Brown, M.D. | 58 | Executive Vice President, Clinical Development, and Chief Medical Officer | ||||
| Andrea J. Corcoran | 43 | Executive Vice President, Legal and Administration and Secretary | ||||
| Paul J. Fanning | 48 | Vice President, Human Resources | ||||
| Susan Koppy | 44 | Senior Vice President, Business and Corporate Development | ||||
| David N. Standring, Ph.D. | 55 | Senior Vice President, Biology | ||||
| Richard Storer, Ph.D.* | 58 | Senior Vice President, Chemistry | ||||
| * | Dr. Storer has announced his intention to resign from Idenix effective May 1, 2006 |
Jean-Pierre Sommadossi, Ph.D. is the principal founder of Idenix and has served as the chairman of our board of directors since our inception in 1998 and as our president and chief executive officer since November 2000. During the period from November 1999 to November 2000, Dr. Sommadossi served as our executive president and chief scientific officer. Prior to taking a sabbatical and then unpaid leave from November 1999 to November 2002, Dr. Sommadossi served as a professor of pharmacology, toxicology and clinical pharmacology and associate director of both the Center for AIDS Research and the Liver Center, University of Alabama at Birmingham School of Medicine from June 1992 to November 2000. From 1996 to 1999, Dr. Sommadossi served on the Research Agenda Committee of the AIDS Clinical Trial Group. Dr. Sommadossi holds a Pharm.D. and Ph.D. in Pharmacology from the University of Marseilles in France.
21
Table of Contents
David A. Arkowitzhas served as our chief financial officer since December 2003 and as treasurer since January 2004. Prior to joining Idenix, Mr. Arkowitz was with Merck & Co., Inc., a pharmaceutical company, where he served as vice president and controller of the U.S. sales and marketing division from September 2002 to December 2003, controller of the global research and development division from April 2000 to September 2002, and as vice president finance and business development of Merck’s Canadian subsidiary from July 1997 to April 2000. Mr. Arkowitz holds an M.B.A. from Columbia University and a B.A. from Brandeis University.
Guy Macdonaldhas served as our executive vice president, operations since September 2003. Prior to joining Idenix, Mr. Macdonald was with Merck & Co., Inc., a pharmaceutical company, from November 1981 to August 2003, most recently as vice president, anti-infectives. During his tenure at Merck, Mr. Macdonald directed the launch of five anti-infective products and led global business strategy for Merck’s HIV franchise. Mr. Macdonald holds a B.S. from the University of Dundee in Scotland.
Nathaniel Brown, M.D. has served as our chief medical officer since September 2003 and as our executive vice president, clinical development since September 2004, previously serving as our senior vice president, hepatitis clinical research from January 2001 to September 2004. From September 1994 to January 2001, Dr. Brown served as principal clinical program head, hepatitis clinical research and as director, clinical and scientific affairs for GlaxoSmithKline plc, a pharmaceutical company. Dr. Brown served as section head, opportunistic infections for Burroughs Wellcome Co., a pharmaceutical company, from May 1989 to September 1994. Dr. Brown was the first recipient of the Hepatitis B Foundation’s Distinguished Leadership Award in 1997. He has held appointments as assistant professor at UCLA School of Medicine and associate professor at Cornell University Medical College. Dr. Brown holds an M.D. from Georgetown University School of Medicine and completed post-graduate clinical and research training at The New York Hospital-Cornell Medical Center and Yale University School of Medicine.
Andrea J. Corcoranhas served as our executive vice president, legal and administration, since February 2004 and as senior vice president, legal and administration from June 2001 until February 2004. From September 2000 until May 2003, Ms. Corcoran served as a member of our board of directors. Ms. Corcoran has served as our secretary since May 2000. From December 1999 to June 2001, Ms. Corcoran served as our vice president, legal and administration, and from December 1998 to December 1999 as our general counsel and administration. Prior to joining Idenix, Ms. Corcoran was associated with the law firm Kelley Drye & Warren LLP from June 1996 to September 1998 and with the law firm Edwards & Angell LLP from July 1990 to June 1996. Ms. Corcoran holds a J.D. from Boston College Law School and a B.S. from Providence College.
Paul J. Fanninghas served as our vice president, human resources since March 2004. Prior to joining Idenix, Mr. Fanning was employed by The Foxboro Company and its affiliates from 1984 to 2004, most recently as vice president, human resources at Invensys Process Systems from 2000 to 2004 and from 1998 to 2000 as vice president, human resources, Invensys Process Automation. Mr. Fanning holds an M.B.A. from Babson College and a B.S. from the University of Massachusetts.
Susan L. Koppyhas served as our Senior Vice President, Business and Corporate Development since January 2006. Prior to joining Idenix, from May 2004 to July 2005, Ms. Koppy served as vice president of strategy and business development at Applied Biosystems, Inc., a life sciences instrument discovery and commercialization company. Ms. Koppy served at Novartis Pharma AG as director of business development from July 2001 to May 2004 and as head of commercial and business intelligence from February 2000 to June 2001. From January 1996 to November 1999, Ms. Koppy served as marketing manager at Millipore Corp. Ms. Koppy holds a B.S. from the University of Minnesota.
David N. Standring, Ph.D. has served as our senior vice president, biology since March 2006 and previously served as vice president, biology from March 2002 to March 2006 and as our executive director of biology from September 2000 to March 2002. Prior to joining Idenix, Dr. Standring served from July 1999 to June 2000 as associate director, and from February 1998 to July 2000, Dr. Standring served as research fellow and then as associate director, virology department at Schering-Plough Research Institute, a division of Schering Plough Corporation, a pharmaceutical company. From November 1994 to January
22
Table of Contents
1998, Dr. Standring served as group leader, hepatitis, virology department at Bristol-Myers Squibb Research Institute. From 1984 to 1994, Dr. Standring was on the faculty of the University of California at San Francisco. Dr. Standring holds a B.S. from St. John’s College, Oxford University and a Ph.D. in Bioorganic Chemistry from Harvard University.
Richard Storer, Ph.D. has served as our vice president, chemistry since March 2002. From November 2001 to March 2002, Dr. Storer served as our executive director, chemistry. Prior to joining Idenix, Dr. Storer served as director of chemistry at BioChem Pharma Inc., a biopharmaceutical company, from August 1997 to August 2001. From 1996 to 1997, Dr. Storer served as head of the combinatorial medicinal chemistry research unit and as research manager from 1988 to 1996 at GlaxoWellcome, a pharmaceutical company. Dr. Storer is a co-recipient of the 1996 Canadian Prix Galien for the discovery of 3TC. Dr. Storer is a visiting professor at the University of Sussex and a fellow of the Royal Society of Chemistry (U.K.). Dr. Storer holds a Ph.D. in Synthetic Organic Chemistry from the University of Sussex and a B.S. from the University of Sussex.
Each of our executive officers is elected or appointed by, and serves at the discretion of, the board of directors. In addition, until such time as Novartis and its affiliates own less than 50% of our voting stock, Novartis’ consent is required for the selection and appointment of our chief financial officer. If in Novartis’ reasonable judgment our chief financial officer is not satisfactorily performing his duties, we are required to terminate the employment of our chief financial officer. Each of our executive officers devotes his or her full time to our affairs.
Compensation of Executive Officers
The following table shows, for the years ended December 31, 2005, 2004 and 2003, the compensation awarded or paid to, or earned by, our chief executive officer and our four other most highly compensated executive officers. We refer to this group as our named executive officers.
Summary Compensation Table
| Long Term | |||||||||||||||||||||||||
| Compensation | |||||||||||||||||||||||||
| Annual Compensation | |||||||||||||||||||||||||
| Shares | |||||||||||||||||||||||||
| Name and | Year Ended | Other Annual | Underlying | All Other | |||||||||||||||||||||
| Principal Position in 2005 | December 31, | Salary | Bonus(1) | Compensation | Options(#)(2) | Compensation | |||||||||||||||||||
| Jean-Pierre Sommadossi | 2005 | $ | 475,000 | $ | 350,000 | $ | — | 150,000 | $ | — | |||||||||||||||
| Chairman of the Board | 2004 | 421,383 | 225,000 | 64,694 | (3) | 100,000 | — | ||||||||||||||||||
| of Directors, President and | 2003 | 381,600 | 600,000 | (4) | — | 100,000 | — | ||||||||||||||||||
| Chief Executive Officer | |||||||||||||||||||||||||
| David A. Arkowitz(5) | 2005 | 298,700 | 134,415 | 24,861 | (3) | 30,000 | — | ||||||||||||||||||
| Chief Financial Officer and | 2004 | 290,000 | 120,000 | 62,937 | (3) | 30,000 | — | ||||||||||||||||||
| Treasurer | 2003 | 18,528 | 200,000 | (6) | — | 175,000 | — | ||||||||||||||||||
| Nathaniel A. Brown | 2005 | 290,000 | 159,500 | — | 30,000 | — | |||||||||||||||||||
| Executive Vice President, | 2004 | 260,836 | 100,000 | 21,439 | (7) | 30,000 | — | ||||||||||||||||||
| Clinical Research, and | 2003 | 251,114 | 80,000 | 21,346 | (7) | — | — | ||||||||||||||||||
| Chief Medical Officer | |||||||||||||||||||||||||
| Guy Macdonald(8) | 2005 | 298,700 | 134,415 | 129,907 | (3) | 30,000 | 3,170 | (9) | |||||||||||||||||
| Executive Vice President, | 2004 | 290,000 | 110,000 | 27,685 | (3) | 30,000 | 3,442 | (9) | |||||||||||||||||
| Operations | 2003 | 95,861 | 300,000 | (10) | 31,853 | (3) | 175,000 | — | |||||||||||||||||
| Andrea J. Corcoran | 2005 | 267,800 | 93,730 | — | 30,000 | — | |||||||||||||||||||
| Executive Vice President, | 2004 | 257,400 | 85,000 | — | 30,000 | — | |||||||||||||||||||
| Legal and Administration, | 2003 | 228,795 | 100,000 | — | — | — | |||||||||||||||||||
| and Secretary | |||||||||||||||||||||||||
| (1) | Bonus amounts for the year indicated are paid in the immediately following year. Amounts paid are determined based on the Compensation Committee’s review of corporate performance and individual achievements for the relevant year. |
23
Table of Contents
| (2) | We have not granted any stock appreciation rights, made any long-term incentive plan awards or made any restricted stock grants to any executive officer, including the named executive officers, or any other employee during the periods covered. |
| (3) | Represents amounts paid for reimbursement of relocation expenses, including amounts required to be paid to gross up such expenses for tax purposes. |
| (4) | Includes $400,000 bonus received upon consummation of our collaboration with Novartis. |
| (5) | Mr. Arkowitz became an executive officer in December 2003. |
| (6) | Consists of $200,000 sign-on bonus. |
| (7) | Represents forgiveness of principal and interest on $60,000 relocation loan made in 2001. Loan was extinguished in full in 2004. |
| (8) | Mr. Macdonald became an executive officer in September 2003. |
| (9) | Represents supplemental life insurance premium paid by Idenix. |
| (10) | Includes $200,000 sign-on bonus. |
| Stock Option Grants and Exercises |
In 2005, we granted both incentive stock options and nonstatutory stock options to our officers. The following tables show, for the year ended December 31, 2005, certain information regarding options granted to, exercised by and held at year-end by our named executive officers:
Option Grants in 2005
| Individual Grants | ||||||||||||||||||||||||
| Potential Realizable Value | ||||||||||||||||||||||||
| % of Total | at Assumed Annual Rates | |||||||||||||||||||||||
| Shares | Options | of Stock Price Appreciation | ||||||||||||||||||||||
| Underlying | Granted to | Exercise | for Option Term(4) | |||||||||||||||||||||
| Options | Employees in | Price Per | Expiration | |||||||||||||||||||||
| Name | Granted(1) | 2005(2) | Share(3) | Date | 5% | 10% | ||||||||||||||||||
| Jean-Pierre Sommadossi | 150,000 | 12.85 | % | $ | 19.14 | 2/27/15 | $ | 1,805,556 | $ | 4,575,635 | ||||||||||||||
| David A. Arkowitz | 30,000 | 2.57 | % | 19.14 | 2/27/15 | 361,111 | 915,127 | |||||||||||||||||
| Nathaniel A. Brown | 30,000 | 2.57 | % | 19.14 | 2/27/15 | 361,111 | 915,127 | |||||||||||||||||
| Guy Macdonald | 30,000 | 2.57 | % | 19.14 | 2/27/15 | 361,111 | 915,127 | |||||||||||||||||
| Andrea J. Corcoran | 30,000 | 2.57 | % | 19.14 | 2/27/15 | 361,111 | 915,127 | |||||||||||||||||
| (1) | The terms of such options, which were granted in February 2005, are substantially consistent with those of options granted to other employees under the Idenix Pharmaceuticals, Inc. 2005 Stock Incentive Plan, which we refer to as our 2005 Plan. For all persons other than Dr. Sommadossi, such options vest ratably over a period of 48 successive months beginning in the month of option grant. For Dr. Sommadossi, 25% of the total award vest on the first anniversary of option grant and the remaining 75% vest ratably over a period of 48 successive months. |
| (2) | Based on options to purchase 1,167,750 shares of common stock granted to employees, including executive officers, for the year ended December 31, 2005. |
| (3) | The exercise price per share represents the fair market value of our common stock on the grant date. |
| (4) | The potential realizable value is based on the term of the option at the date of the grant, which is ten years. It is calculated by assuming that the stock price on the date of grant appreciates at the indicated annual rate, compounded annually for the entire term, and that the option is exercised and sold on the last day of the option term for the appreciated stock price. Actual gains, if any, are dependent on the actual future performance of our common stock and the timing of exercise and sale transactions by the holder. These numbers are based on the SEC requirements and do not reflect our projection or estimate of future stock price growth. |
24
Table of Contents
Aggregated Option Exercises in 2005 and 2005 Year-End Option Values
| Exercised in 2005 | Unexercised at December 31, 2005 | |||||||||||||||||||||||
| Shares of Common Stock | ||||||||||||||||||||||||
| Underlying Unexercised | Value of Unexercised | |||||||||||||||||||||||
| Shares | Options(1) | In-the-Money Options(2) | ||||||||||||||||||||||
| Acquired | Value | |||||||||||||||||||||||
| on Exercise | Realized(1) | Exercisable | Unexercisable | Exercisable | Unexercisable | |||||||||||||||||||
| Jean-Pierre Sommadossi | — | $ | — | 77,605 | 228,645 | $ | 522,292 | $ | 475,833 | |||||||||||||||
| David A. Arkowitz | — | — | 111,771 | 123,229 | 589,343 | 554,457 | ||||||||||||||||||
| Nathaniel A. Brown | — | — | 186,458 | 40,417 | 2,561,758 | 88,867 | ||||||||||||||||||
| Guy Macdonald | — | — | 122,708 | 112,292 | 651,246 | 492,554 | ||||||||||||||||||
| Andrea J. Corcoran | — | — | 117,167 | 46,146 | 1,468,514 | 138,481 | ||||||||||||||||||
| (1) | The term “exercisable” in this column reflects such portion of the option that, if exercised, would be exercisable for fully vested shares. |
| (2) | The value of the unexercisedin-the-money options is based on the fair value of Idenix’s common stock at December 31, 2005 ($17.16 based on the average open and close price as reported by NASDAQ), less the exercise price of the options |
| Employment Agreements |
We have entered into employment agreements with each of Drs. Sommadossi and Brown, Messrs. Arkowitz and Macdonald and Ms. Corcoran.
The terms of employment under these agreements, subject to earlier termination, runs to May 2008 for Drs. Sommadossi and Brown and Ms. Corcoran and to September and December 2006, respectively, for Messrs. Macdonald and Arkowitz. The agreements are automatically renewable after the initial term for successive one-year periods unless either party gives written notice to the other 90 days, or in the case of Mr. Arkowitz 120 days, prior to the expiration of the term.
The employment agreements for each officer provide base salary in an amount annually reviewable for increase, but not decrease, at the discretion of our board of directors or a committee of the board of directors. The employment agreements also entitle each officer to receive an annual cash performance bonus in an amount that is expressed as percentage of base salary if the board of directors in its discretion determines that such officer has achieved or surpassed performance goals established by the board of directors in consultation with our management. The minimum target bonus percentages for each officer are set forth in each officer’s employment agreement. For Dr. Sommadossi, the minimum target percentage is 40% of base salary and for each other officer the minimum target percentage is 30% of base salary. Each officer is also eligible to participate in any of our equity incentive programs and has the opportunity, subject to approval of the board of directors, to be awarded annually an option to purchase shares of our common stock which vest over a four year period (except for awards granted to Dr. Sommadossi which vest over a five year period). The base salaries, the target bonus amount and target option award for 2006 are set forth below for each named executive officer. Pursuant to the terms of the employment agreements, the base salary and target bonus and equity award set forth below become the minimum amounts for future years for each executive officer:
| 2006 | ||||||||||||
| Target Bonus as a % | ||||||||||||
| Officer | Base Salary | of Base Salary | Target Option Award | |||||||||
| Jean-Pierre Sommadossi | $ | 525,000 | 60 | % | 150,000 | |||||||
| David A. Arkowitz | 313,635 | 50 | 40,000 | |||||||||
| Nathaniel A. Brown | 307,400 | 50 | 40,000 | |||||||||
| Guy Macdonald | 313,635 | 50 | 40,000 | |||||||||
| Andrea J. Corcoran | 275,834 | 35 | 30,000 | |||||||||
25
Table of Contents
The employment agreements include provisions that are effective upon termination of the employment of the officer in certain circumstances. In the event that we terminate an officer’s employment without cause, or if the officer terminates his employment for good reason:
| • | the officer is entitled to receive: |
| - | a lump-sum severance payment equal to one times his base salary (two times his base salary in the case of Dr. Sommadossi); and | |
| - | the greater of such officer’s current year target bonus amount or the bonus such officer earned for the year preceding the year in which the termination occurs; and |
| • | all outstanding equity awards held by the officer as of the time of termination will become immediately vested and exercisable. |
Additionally, in the case of Messrs. Macdonald and Arkowitz, if we elect not to extend such officer’s employment beyond its initial term, expiring in each case in 2006, the option to purchase 175,000 shares of our common stock awarded to each of Messrs. Macdonald and Arkowitz at the commencement of his employment will become immediately vested and exercisable for a period of 15 months following the date of employment termination.
In the event an officer’s employment terminates as described above or due to disability or death, the officer or such officer’s estate, is entitled to receive a pro rata share of the officer’s annual performance bonus and equity option award, continued medical, dental and life insurance coverage for himself and eligible dependents for up to 12 months after termination (24 months in the case of Dr. Sommadossi) and all outstanding equity awards held by the officer as of the time of such death or disability will become immediately vested and exercisable. In the event of disability, each officer is entitled to an additional payment of 60% of his base salary per year until he reaches age 65. Additionally, Dr. Sommadossi is entitled to life and disability insurance benefits payable in the amount of $2 million in event of his death or disability, and Mr. Macdonald is entitled to life insurance benefits payable in the amount of $1 million in event of his death. In the event that Mr. Arkowitz’ employment terminates due to death or disability, all of his outstanding equity awards will become immediately vested and exercisable for a period of two years following the date of employment termination. Further, in the event of employment termination as a result of either death or the occurrence of death within six months after employment termination as a result of the condition which necessitated such termination, Mr. Arkowitz’s estate is entitled to a payment equal to one-half of his termination payment.
If, within one year following a change in control of Idenix, we terminate an officer’s employment without cause or if such officer terminates his or her employment for good reason, the officer is entitled to an additional lump-sum payment in an amount equal to:
| • | such officer’s base salary; and | |
| • | the greater of such officer’s target bonus amount or the bonus earned in the year preceding the year in which the termination occurs. |
We have also agreed to compensate each officer for excise taxes and associated penalties imposed by Section 4999 of the Internal Revenue Code by payinggross-up amounts on any applicable benefits such officer receives under his or her respective employment agreement.
The employment agreement Drs. Sommadossi and Brown and Ms. Corcoran have entered into include restrictive covenants prohibiting the sale, transfer or disposition of more than 50% of the Idenix capital stock owned by such officer on May 8, 2003, plus shares of common stock such officer acquires upon exercise of stock options outstanding as of that date, until May 8, 2008, unless:
| • | the officer’s employment is terminated without cause; | |
| • | the officer resigns for good reason; or | |
| • | the employment termination is a result of the officer’s death or disability. |
26
Table of Contents
In connection with the commencement of their employment with Idenix in 2003, each of Messrs. Macdonald and Arkowitz received a $200,000 sign-on bonus. Additionally, to facilitate their relocation to the Cambridge, Massachusetts area, we agreed to reimburse both officers for their respective expenses incurred in connection with their relocation to Massachusetts, including up to $75,000 for Mr. Macdonald and $85,000 for Mr. Arkowitz in relocation expenses, up to 6% of real estate commissions incurred, 90 days temporary housing, and such amounts required to gross up these expenses for tax purposes.
| Compensation Committee Report |
The Compensation Committee assists the board of directors in establishing its executive compensation philosophy and objectives and with other matters relating to compensation and management development. The responsibilities of the committee, which are set forth in detail in its charter, include:
| • | recommending to the board of directors the compensation payable to non-employee directors; | |
| • | determining the type and level of compensation for executive officers; | |
| • | recommending to the board of directors the type and level of compensation for the chief executive officer; | |
| • | overseeing the administration of Idenix’s equity incentive plans; and | |
| • | monitoring all general compensation programs. |
The Compensation Committee periodically review and assess, not less frequently than annually, the adequacy of the Compensation Committee’s charter and recommend any proposed changes to the Board for approval. The Compensation Committee’s charter is available for your review on Idenix’s website atwww.idenix.com.
| Overview of Executive Compensation Philosophy and Program |
The goals of Idenix’s executive compensation program are to attract, retain, and develop executive officers who contribute to the realization of Idenix’s business goals and objectives, reward performance, and link the interests of management and stockholders.
To achieve these objectives, the Compensation Committee believes that Idenix’s executive compensation program should:
| • | be closely linked to and deliver differentiated pay opportunities based upon Idenix’s and individual performance; | |
| • | have incentives based upon a broad range of challenging operational, strategic and other goals that are objective and measurable; | |
| • | be competitive with compensation of other employers with whom Idenix competes for talent; and | |
| • | be clearly communicated to participants and stockholders. |
The Compensation Committee administers the Idenix compensation program with the goal of providing total cash and equity-based compensation that is competitive in the marketplace, recognizing meaningful differences in individual performance and offering the opportunity to be rewarded when merited by corporate and individual performance. Cash bonus awards are directly linked to corporate and individual performance with actual awards varying according to Idenix’s overall performance and the individual officer’s contribution to the realization of corporate and individual goals. Stock option awards are intended to meaningfully align the interests of Idenix’s officers with the interests of Idenix’s stockholders.
27
Table of Contents
| Annual Compensation Review |
The Compensation Committee routinely evaluates and analyzes the type, level and mix of the components of the compensation offered to Idenix’s executive officers. At least once each year, the Compensation Committee reviews each executive officer’s total compensation package, consisting of base salary, cash incentive compensation and equity compensation. The Compensation Committee conducts this review to ensure that Idenix’s executive compensation policies and programs remain appropriate with respect to evolving business needs, best compensation practices and leading corporate governance principles.
The Compensation Committee engages an independent compensation consultant. This consultant advises the Compensation Committee with respect to developments in the design of compensation packages and provides benchmarks to compare the total compensation packages of Idenix officers to those of the companies with which Idenix competes. At the Compensation Committee’s request, the consulting firm prepared and reviewed with the committee a detailed report which compared each component within the Idenix executive compensation program, namely base salary, cash incentive bonus, equity programs and benefits, to a group of biotechnology and biopharmaceutical companies that are engaged in the discovery, development and commercialization of drug products. This group of companies is referred to as our peer group. The report also included a review of compensation methodologies, competitive practices, best practices and trends. In addition to this comparative report, the consultant, at the committee’s request, prepared for each executive officer a tally sheet setting forth the value of all components of 2005 compensation and the total compensation potentially payable to the executive.
In the first quarter of 2006, the Compensation Committee reviewed the 2005 performance of Idenix and the individual officers against criteria established by the Compensation Committee at the beginning of 2005 and approved the achievement percentage for each corporate goal, along with the overall percent of corporate and individual goal achievement for purposes of awarding actual bonuses. The goals and performance criteria that the Compensation Committee established at the beginning of 2005 included effective management of the operations of Idenix and continued development of Idenix’s product candidates in a timely and successful manner.
| Elements of Compensation |
Idenix’s compensation program for all its employees, including its executive officers, is comprised of three principal elements:
| • | base salary; | |
| • | cash incentive compensation; and | |
| • | equity compensation. |
| Base Salary |
Base salaries for Idenix’s executives are generally established by evaluating the responsibilities of the position, the experience of the individual, review of comparable positions in the market and the historical compensation levels of Idenix’s executives. The Compensation Committee strives to provide Idenix’s executive officers with compensation that is competitive, generally between the 50th and 75th percentile for total annual cash compensation paid by our peer group. The minimum base salaries of Drs. Sommadossi and Brown, Messrs. Arkowitz and Macdonald and Ms. Corcoran are established in their respective employment agreements. Base salary adjustments are determined annually by evaluating the factors above and the performance of the executive officer.
| Cash Incentive Compensation |
Idenix uses the annual bonus program to reflect the direct contribution made by the executive to Idenix’s achievement of its goals. The Compensation Committee annually establishes a target bonus
28
Table of Contents
opportunity for each officer. This target bonus is based upon our evaluation of the individual’s role in the organization and peer group analysis. For 2005, the target bonuses, expressed as a percentage of annual base salary ranged, from 35 to 40% of base salary. Actual bonus awards, which are dependent on corporate and individual performance, can range from zero to 200% above the target. The actual individual bonuses awarded for 2005 ranged from 100 to 133% of the respective officer’s target annual bonus award. This resulted in total cash compensation, on average, above the 50th and below the 75th percentile of the peer group for Idenix’s executive officers.
| Equity Compensation |
Equity compensation is a key component within Idenix’s compensation program. The Compensation Committee believes that equity awards significantly enhance Idenix’s ability to attract, retain and motivate executives and key employees, link pay with performance and align the interests of executive officers with those of stockholders. Idenix provides executive officers with a substantial economic interest in the long-term appreciation of Idenix’s common stock through the grant of stock options. Options provide value only if Idenix’s stock price increases (which benefits all stockholders) and only if the executive or employee remains with Idenix until his or her options vest. The minimum annual target equity awards are set forth in the employment agreements between Idenix and each executive officer. For services in 2005, the Compensation Committee awarded each of the Idenix executive officers, other than the chief executive officer, options to purchase 30,000 shares of Idenix common stock. These actual awards take into account the target amount, the executive’s position with Idenix and his or her contributions to the company. For persons other than the company’s chief executive officer, the Compensation Committee grants options that vest monthly over a four year period. The stock options awarded to the chief executive officer vest over a five year period with 25% of the award vesting on the first anniversary of the date of grant and the remaining 75% vesting on a monthly basis over the next four years.
| 2005 Compensation for our Chief Executive Officer |
The compensation paid to Dr. Sommadossi for services he rendered to Idenix in 2005 was determined in accordance with Idenix’s compensation philosophy and practices described above. Dr. Sommadossi is eligible to participate in the same compensation plans available to other officers and employees of Idenix.
At the beginning of 2005, the Compensation Committee recommended and the board of directors established Dr. Sommadossi’s 2005 base salary at $475,000, the target bonus amount at 60% of base salary and the equity target of options to purchase 150,000 shares of Idenix common stock.
For services rendered by Dr. Sommadossi in 2005, the Compensation Committee recommended and the board of directors awarded Dr. Sommadossi a bonus in the amount of $350,000, or 123% of his target bonus amount, and options to purchase 150,000 shares of Idenix common stock. The Compensation Committee made such recommendations based upon our evaluation of the leadership demonstrated by Dr. Sommadossi throughout 2005, Idenix’s corporate performance and achievement of 2005 corporate goals.
| Policy on Tax Deductibility of Compensation |
Section 162(m) of the Internal Revenue Code of 1986, as amended, limits a company’s tax deductibility of compensation in excess of $1 million paid to the company’s chief executive officer and the other four most highly compensated executive officers unless such compensation is, among other things, performance-based and has been approved by stockholders.
In its establishment of the Idenix executive compensation programs, the committee reviews the potential effects of Section 162(m) and generally seeks to structure such compensation in a manner intended to avoid the disallowance of tax deductions. However, to maintain the necessary flexibility to compensate Idenix’s executive officers in a manner that is consistent with our philosophy and in the best interests of Idenix and its stockholders, from time to time, the Compensation Committee may award compensation that is not fully deductible.
29
Table of Contents
| Conclusion |
The Compensation Committee believes that the continued commitment and leadership of Idenix’ executive officers in 2005 was and continues to be important factors in realizing Idenix’ achievements. Additionally, the Compensation Committee believes that its philosophy of linking a significant portion of executive compensation to corporate performance aligns executives’ interests with the interests of Idenix stockholders. The Compensation Committee intends to continue utilizing compensation as a strategic tool to attract, retain and motivate officers who contribute to the achievement of Idenix’s goals and prospects.
| By the Compensation Committee | |
| Wayne T. Hockmeyer, Chair | |
| Charles W. Cramb | |
| Thomas Ebeling | |
| Thomas R. Hodgson |
OTHER INFORMATION
Other Matters
Our board of directors does not know of any other matters which may come before the meeting. However, if any other matters are properly presented to the meeting, it is the intention of the persons named in the proxy card to vote, or otherwise act, in accordance with their judgment on such matters.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended, or Exchange Act, requires our directors, executive officers and holders of more than 10% of our common stock to file with the SEC initial reports of ownership and reports of changes in ownership of common stock and other of our equity securities. Based solely on our review of copies of Section 16(a) reports provided to us by the persons required to file such reports and written representations made to us by such persons, we believe that during 2005 all filings required to be made by our directors, executive officers and holders of more than 10% of our common stock were timely made in accordance with the Section 16(a) filing requirements.
Delivery of Security Holder Documents
Some brokers, banks and other nominee record holders may be participating in the practice of “householding” proxy statements and annual reports. This means that only one copy of our proxy statement or annual report may have been sent to multiple stockholders in your household. We will promptly deliver a separate copy of either document to you if you call or write us at the following address or telephone number: 60 Hampshire Street, Cambridge, Massachusetts, 02139, Attention: Investor Relations; 617-995-9800. If you want to receive separate copies of the annual report and proxy statement in the future, or if you are receiving multiple copies and would like to receive only one copy for your household, you should contact your broker, bank or other nominee record holder, or you may contact us at the above address and telephone number.
Stockholder Proposals for the 2007 Annual Meeting
Stockholder proposals submitted pursuant to Rule 14a-8 under the SEC rules for inclusion in our proxy materials for our 2007 annual meeting of stockholders must be received by us at our principal office in Cambridge, Massachusetts not later than January 5, 2007. We suggest that stockholder proponents submit their proposals by certified mail, return receipt requested, addressed to us c/o Secretary, 60 Hampshire Street, Cambridge, Massachusetts 02139.
30
Table of Contents
The persons designated in the proxy card will be granted discretionary authority with respect to any stockholder proposal for the 2007 annual meeting of stockholders not submitted pursuant to Rule 14a-8 if such proposal is not received by us by March 21, 2007.
| By Order of the Board of Directors, | |
| ANDREA J. CORCORAN | |
| Secretary |
April 28, 2006
OUR BOARD OF DIRECTORS ENCOURAGES STOCKHOLDERS TO ATTEND THE MEETING. WHETHER OR NOT YOU PLAN TO ATTEND, YOU ARE URGED TO COMPLETE, DATE, SIGN AND RETURN THE ENCLOSED PROXY IN THE ACCOMPANYING ENVELOPE.
31
Table of Contents
APPENDIX A
IDENIX PHARMACEUTICALS, INC.
AUDIT COMMITTEE CHARTER
| I. | PURPOSE |
The Audit Committee (the “Committee”) is appointed by the Board of Directors (the “Board”) of Idenix Pharmaceuticals, Inc. (the “Company”) to:
A. Assist the Board in its oversight of:
| 1. | the integrity of the Company’s financial statements |
| 2. | the Company’s compliance with legal and regulatory requirements |
| 3. | the independent auditor’s qualifications, independence and performance |
| 4. | the Company’s accounting and financial reporting processes |
| B. | Prepare the report required by the rules of the Securities and Exchange Commission to be included in the Company’s annual proxy statement |
| II. | STRUCTURE AND MEMBERSHIP REQUIREMENTS |
The Committee must be comprised of at least three directors. The members of the Committee shall meet the applicable requirements of the NASDAQ, the Sarbanes-Oxley Act of 2002 and the rules and regulations of the Securities and Exchange Commission, including the rules and regulations relating to independence and financial literacy. In addition, at least one member of the Committee shall be “an audit committee financial expert” (as defined in Securities and Exchange Commission rules and regulations).
No Committee member may accept any consulting, advisory or other compensatory fee from the Company, or its subsidiaries, other than the compensation relating to the member’s service on the Committee or the Board or any other committee thereof.
The members of the Committee shall be appointed annually, upon recommendation of the Nominating and Corporate Governance Committee, by resolution passed by the majority of the Board at its first meeting following the Annual Meeting of the Stockholders and shall serve until the next Annual Meeting of Stockholders and until their successors are elected and qualified or until their earlier death, resignation, removal, with or without cause, at the discretion of the Board. Unless a Chair is elected by the Board, the members of the Committee shall elect a Chair by majority vote of the full Committee membership.
The Committee may delegate its authority to a subcommittee or subcommittees.
The Committee shall provide an oral report to the Board of the actions taken or issues discussed at its meetings. This will generally take place at the Board meeting following the Committee meeting.
In order to fulfill the Company’s contractual obligations, Novartis Pharma AG shall be entitled to designate one non-voting observer to the Committee.
| III. | MEETINGS |
The Committee shall meet at least quarterly, or more frequently as circumstances dictate. Committee meetings shall be called and conducted in the manner provided for in the Amended and Restated By-Laws of the Company. The Committee shall meet in executive session periodically with the Chief Financial Officer (and other members of management as it deems appropriate) and the independent auditor separately.
A-1
Table of Contents
The Committee may request any officer or employee of the Company or the Company’s outside counsel or independent auditor to attend a meeting of the Committee or to meet with any members of, or consultants to, the Committee.
For the transaction of business at any Committee meeting, two members shall constitute a quorum. If the Chair is not present, the senior independent director who is present shall assume the Chair.
Minutes of each meeting of the Committee will be prepared by the individual who is appointed secretary of the meeting and submitted to Committee members for approval at the next meeting.
| IV. | RESPONSIBILITIES AND DUTIES |
| A. | General |
| 1. | Review and assess the adequacy of this Charter on an annual basis and submit any proposed amendments to the Board for approval. | |
| 2. | Review and discuss with management and the independent auditor: |
| a. | the selection, application and disclosure of critical accounting policies and practices. |
| b. | all alternative treatments for policies and practices related to material items within generally accepted accounting principles that have been discussed with management, the ramifications of using such alternative disclosures and treatments, and the treatment preferred by the independent auditor. |
| c. | the effects on the Company’s financial statements of regulatory and accounting initiatives. |
| d. | any material off-balance-sheet transactions, arrangements, obligations including contingent obligations, and any other relationships of the Company with unconsolidated entities that may have a current or future material effect on the Company’s financial statements. |
| e. | any pro-forma or non-GAAP information proposed to be included in the Company’s financial statements or any other public disclosure, and the reasons for such pro forma or non-GAAP information. | |
| f. | the annual audited financial statements and quarterly financial statements, including the Company’s disclosures under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in its SEC filings. | |
| g. | the presentation of the financial statements and significant judgments made in connection with the preparation of such financial statements. |
| 3. | Review and discuss with management the Company’s system of internal controls and policies relating to the assessment of risk. Discuss with the independent auditors any significant matters regarding internal controls over financial reporting that have come to their attention during the conduct of their audit. | |
| 4. | Recommend to the Board whether or not the audited, consolidated financial statements should be included in the Company’s Annual Report filed with the SEC on Form 10-K. | |
| 5. | Review and discuss the earnings press release, SEC Forms 10-K and 10-Q, as well as financial projections and earnings guidance (if any) given to analysts and rating agencies. | |
| 6. | Discuss policies with respect to risk assessment, risk management, the Company’s major financial and operational risk exposures and the steps that management has taken to monitor and control such exposures, including a review of the Company’s insurance program. | |
| 7. | Review and approve the Company’s investment policy and review the deployment and security of the Company’s liquid assets. |
A-2
Table of Contents
| 8. | Obtain reports from management that the Company and its subsidiaries are in conformity with applicable legal and regulatory requirements, the Foreign Corrupt Practices Act and the Company’s Business Conduct Policy. Review reports and disclosures of insider transactions and any conflicts of interest. Review and approve all “related party transactions” (defined as transactions required to be disclosed pursuant to Item 404 of Regulation S-K). Advise the Board with respect to the Company’s policies and procedures regarding compliance with applicable laws and regulations and with the Company’s Business Conduct Policy. |
| 9. | Review with the Company’s Executive Vice President, Legal and Administration: (a) any significant issues concerning litigation and contingencies and regulatory actions that could have a significant impact on the Company’s financial statements; and (b) the effectiveness of the Company’s compliance program in detecting and preventing violations of the Company’s Business Conduct Policy. |
| 10. | Review and approve the procedures established for the receipt, retention, and treatment on a confidential basis of complaints received by the Company, including the Committee and the Board, regarding accounting, internal controls, or auditing matters and the confidential, anonymous submissions by employees of concerns regarding questionable accounting or auditing matters. |
| 11. | Perform any other activities consistent with this Charter, the Company’s Amended and Restated By-Laws and Restated Certificate of Incorporation, as amended, as the Committee or the Board deems necessary or appropriate. |
| B. | Independent Auditor |
| 1. | The Committee shall have the sole authority to appoint or replace the independent auditor. The Committee shall be directly responsible for the compensation and oversight of the work of the independent auditor for the purpose of preparing or issuing an audit report or related work. The independent auditor shall report directly to the Committee. |
| 2. | The Committee shall annually evaluate the qualifications, performance and independence of the independent auditor and the lead partner of the independent auditor, taking into consideration: (a) the independent auditor’s work throughout the year; (b) the disclosures of the independent auditor required by the Independence Standards Board Standard No. 1 and all relationships between the independent auditor and the Company; and (c) the views of management and report its conclusions to the Board. |
| 3. | Review and approve the audit fees and any other compensation proposed to be paid to the independent auditor in accordance with the Committee’s Pre-Approval Policy and Procedures. |
| 4. | Pre-approve the retention of the independent auditor for any auditing service or any non-audit service that is not prohibited under Section 10A(g) of the Securities Exchange Act and the terms of engagement and fee for such service, it being understood that the Committee may delegate pre-approval authority to one or more of its members so long as the decisions made by such member(s) are presented to the Committee at its next meeting. |
| 5. | Discuss with the independent auditor any relationships or services that may affect the objectivity and independence of the independent auditor as stipulated in Independence Standards Board Standard No. 1, and matters relating to the conduct of audits required to be disclosed by Statement of Auditing Standards No. 61. |
| 6. | Discuss with the independent auditor: (a) significant consultations between the audit team and the firm’s national office relating to auditing or accounting issues on matters that otherwise are required to be disclosed to the Committee; (b) the “management letter” issued or proposed to be issued by the independent auditor to the Company and any other material written communications between the independent auditor and management; and (c) any issues identified or problems encountered by the independent auditor with management’s response to such communications or letter. |
A-3
Table of Contents
| 7. | Resolve any disagreements between management and the independent auditor. |
| 8. | Review the annual audit plan of the independent auditor. |
| 9. | Ensure the rotation of the audit partners every five years, as required by applicable regulatory requirements. |
| 10. | Review and approve the proposed hiring of former employees of the independent auditor. |
| C. | Financial Reporting Process |
| 1. | Review with management and the independent auditor any correspondence with regulators or government agencies and any employee complaints or published reports that raise material issues regarding the Company’s financial statements or accounting policies. | |
| 2. | Review any significant difficulties reported by the independent auditor in conducting the audit, including any restrictions on the scope of work or access to required information. | |
| 3. | Review any significant changes to the Company’s internal controls or in other factors that could significantly affect these controls. | |
| 4. | Review the reports of the CEO and CFO (in connection with their required certifications) regarding the internal controls and the independent auditor’s attestation of the reports prior to the filing of the Company’s Form 10-K, any significant deficiencies or material weaknesses in the design or operation of internal controls, and any fraud that involves the management or other employees who have a significant role in the Company’s internal controls. | |
| 5. | Review any significant issues identified regarding the adequacy of the Company’s internal controls and any special audit steps adopted in light of control deficiencies. |
| V. | ADVISORS AND AUTHORITY |
| A. | The Committee shall have the authority, at the expense of the Company, to retain such accounting, legal and other advisors as it deems appropriate without Board or management approval. | |
| B. | The Committee will receive from the Company appropriate funding, as determined by the Committee, for the payment of: (a) any advisors employed by the Committee, as described above; or (b) ordinary administrative expenses of the Committee that are necessary and appropriate in carrying out its duties. |
| VI. | PERFORMANCE EVALUATION |
| A. | Not less frequently than once a year, the Committee shall review and evaluate its performance. |
While the Committee has the responsibility and powers set forth in the Charter, it is not the duty of the Committee to plan or conduct audits or to determine that the Company’s financial statements are complete and accurate and are in accordance with generally accepted accounting principles. This is the responsibility of management.
A-4
Table of Contents
IDE-PS-1-06
Table of Contents
 | + | ||
Idenix Pharmaceuticals, Inc. | ||||||
 | MR A SAMPLE DESIGNATION (IF ANY) ADD 1 ADD 2 ADD 3 ADD 4 ADD 5 ADD 6  | 000004 Least Address Line | 000000000.000 ext 000000000.000 ext 000000000.000 ext 000000000.000 ext 000000000.000 ext 000000000.000 ext 000000000.000 ext C 1234567890 J N T | |||
 | ||||||
| o | Mark this box with an X if you have made changes to your name or address details above. | |||
Annual Meeting Proxy Card | ||||||||
A VOTE FOR THE DIRECTOR NOMINEES AND FOR PROPOSAL NUMBER 2 IS RECOMMENDED BY THE BOARD OF DIRECTORS.
| 1. | To elect eight directors to serve until the next annual meeting of stockholders and until their successors are elected and qualified. | ||||||||
Nominees: | |||||||||
| For | Withhold | For | Withhold | |||||||||||||
| 01 - Jean-Pierre Sommadossi, Ph.D. | o | o | 05 - Thomas R. Hodgson | o | o | |||||||||||
| 02 - Charles W. Cramb | o | o | 06 - Robert E. Pelzer | o | o | |||||||||||
| 03 - Thomas Ebeling | o | o | 07 - Denise Pollard-Knight, Ph.D. | o | o | |||||||||||
| 04 - Wayne T. Hockmeyer, Ph.D. | o | o | 08 - Pamela Thomas-Graham | o | o | |||||||||||
| The Board of Directors recommends a vote FOR the following proposals. | Mark this box with an X if you have made comments below. | o | ||||||||
| For | Against | Abstain | ||||||||
| 2. | To ratify the selection of PricewaterhouseCoopers LLP as the Company’s independent registered public accounting firm for the current fiscal year ending December 31, 2006. | o | o | o | ||||||
Authorized Signatures - Sign Here - This section must be completed for your instructions to be executed.
Please sign this proxy exactly as your name appears hereon. Joint owners should each sign personally. Trustees and other fiduciaries should indicate the capacity in which they sign. If a corporation or partnership, this signature should be that of an authorized officer who should state his or her title.
| Signature 1 - Please keep signature within the box | Signature 2 - Please keep signature within the box | Date (mm/dd/yyyy) | ||
| / / |
| + | ||||||||
| 0 0 9 3 7 3 1 | 1 U P X | C O Y |
001CD40001 00KIVB
Table of Contents
Proxy - Idenix Pharmaceuticals, Inc. | ||||||||
THIS PROXY IS SOLICITED ON BEHALF OF THE BOARD OF DIRECTORS
ANNUAL MEETING OF STOCKHOLDERS
JUNE 14, 2006
Those signing on the reverse side, revoking any prior proxies, hereby appoint(s) Jean-Pierre Sommadossi, David A. Arkowitz and Andrea J. Corcoran, and each of them with full power of substitution, as proxies for those signing on the reverse side to act and vote all shares of common stock, $0.001 par value per share, of Idenix Pharmaceuticals, Inc., a Delaware corporation (the “Company”), held by the undersigned as of the close of business on April 18, 2006 at the 2006 Annual Meeting of Stockholders and at any adjournments or postponements thereof as indicated herein upon all matters referred to on the reverse side and described in the Proxy Statement for the Annual Meeting and, in their discretion, upon any other matters which may properly come before the Annual Meeting. Each proposal included in this proxy has been proposed by the Company, and none of the proposals are conditioned upon approval of any other proposal.
THIS PROXY, WHEN PROPERLY EXECUTED, WILL BE VOTED AS DIRECTED BY THE UNDERSIGNED STOCKHOLDER. IF NO SUCH DIRECTION IS GIVEN, THIS PROXY WILL BE VOTED FOR THE ELECTION OF THE NOMINEES LISTED ON THE REVERSE SIDE FOR THE BOARD OF DIRECTORS AND FOR PROPOSAL NUMBER 2. Attendance of the undersigned at the meeting or any adjournment or postponement thereof will not be deemed to revoke this proxy unless the undersigned revokes this proxy in writing before it is exercised.
PLEASE MARK, SIGN, DATE, AND RETURN THIS PROXY CARD PROMPTLY IN ENCLOSED REPLY ENVELOPE
THIS PROXY CARD IS VALID ONLY WHEN SIGNED AND DATED
CONTINUED AND TO BE SIGNED ON REVERSE SIDE