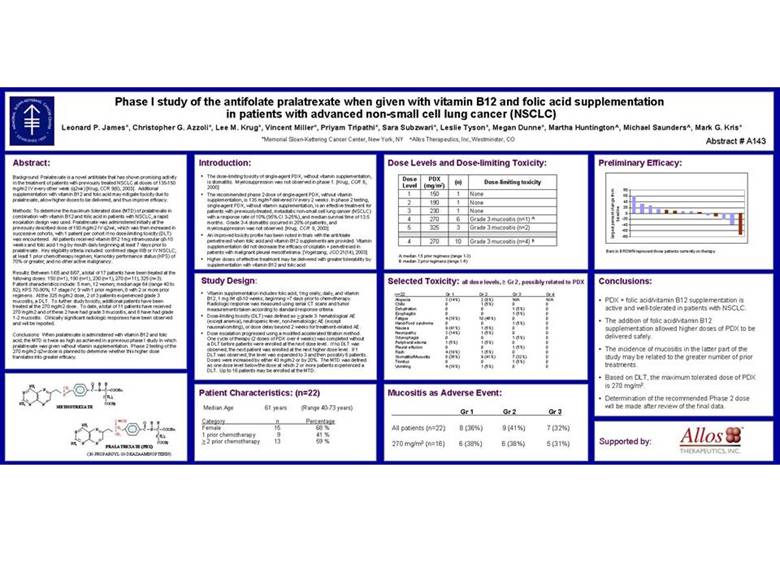
| Phase I study of the antifolate pralatrexate when given with vitamin B12 and folic acid supplementation in patients with advanced non-small cell lung cancer (NSCLC) Leonard P. James*, Christopher G. Azzoli*, Lee M. Krug*, Vincent Miller*, Priyam Tripathi*, Sara Subzwari*, Leslie Tyson*, Megan Dunne*, Martha Huntington^, Michael Saunders^, Mark G. Kris* *Memorial Sloan-Kettering Cancer Center, New York, NY ^Allos Therapeutics, Inc, Westminster, CO Abstract # A143 Abstract: Background: Pralatrexate is a novel antifolate that has shown promising activity in the treatment of patients with previously treated NSCLC at doses of 135-150 mg/m2 IV every other week (q2wk) [Krug, CCR 9(6), 2003]. Additional supplementation with vitamin B12 and folic acid may mitigate toxicity due to pralatrexate, allow higher doses to be delivered, and thus improve efficacy. Methods: To determine the maximum tolerated dose (MTD) of pralatrexate in combination with vitamin B12 and folic acid in patients with NSCLC, a rapid escalation design was used. Pralatrexate was administered initially at the previously described dose of 150 mg/m2 IV q2wk, which was then increased in successive cohorts, with 1 patient per cohort if no dose-limiting toxicity (DLT) was encountered. All patients received vitamin B12 1mg intramuscular q8-10 weeks and folic acid 1 mg by mouth daily beginning at least 7 days prior to pralatrexate. Key eligibility criteria included: confirmed stage IIIB or IV NSCLC; at least 1 prior chemotherapy regimen; Karnofsky performance status (KPS) of 70% or greater; and no other active malignancy. Results: Between 1/05 and 8/07, a total of 17 patients have been treated at the following doses: 150 (n=1), 190 (n=1), 230 (n=1), 270 (n=11), 325 (n=3). Patient characteristics include: 5 men, 12 women; median age 64 (range 40 to 82); KPS 70-90%; 17 stage IV; 9 with 1 prior regimen, 8 with 2 or more prior regimens. At the 325 mg/m2 dose, 2 of 3 patients experienced grade 3 mucositis, a DLT. To further study toxicity, additional patients have been treated at the 270 mg/m2 dose. To date, a total of 11 patients have received 270 mg/m2 and of these 2 have had grade 3 mucositis, and 8 have had grade 1-2 mucositis. Clinically significant radiologic responses have been observed and will be reported. Conclusions: When pralatrexate is administered with vitamin B12 and folic acid, the MTD is twice as high as achieved in a previous phase I study in which pralatrexate was given without vitamin supplementation. Phase 2 testing of the 270 mg/m2 q2w dose is planned to determine whether this higher dose translates into greater efficacy. O H H NH2 N CH3 C N C COOH a N N (CH2)2 NH2 N N COOHg METHOTREXATE C CH O H H NH2 N CH2 C N C COOHa N CH1 (CH2)2 N N COOHg NH2 PRALATREXATE (PDX) (10-PROPARGYL-10-DEAZAAMINOPTERIN) Introduction: • The dose-limiting toxicity of single-agent PDX, without vitamin supplementation, is stomatitis. Myelosuppression was not observed in phase 1. [Krug, CCR 6, 2000] • The recommended phase 2 dose of single-agent PDX, without vitamin supplementation, is 135 mg/m2 delivered IV every 2 weeks. In phase 2 testing, single-agent PDX, without vitamin supplementation, is an effective treatment for patients with previously-treated, metastatic non-small cell lung cancer (NSCLC) with a response rate of 10% (95% CI 3-25%), and median survival time of 13.5 months. Grade 3-4 stomatitis occurred in 20% of patients, and myelosuppression was not observed. [Krug, CCR 9, 2003] • An improved toxicity profile has been noted in trials with the antifolate pemetrexed when folic acid and vitamin B12 supplements are provided. Vitamin supplementation did not decrease the efficacy of cisplatin + pemetrexed in patients with malignant pleural mesothelioma. [Vogelzang, JCO 21(14), 2003] • Higher doses of effective treatment may be delivered with greater tolerability by supplementation with vitamin B12 and folic acid Study Design: • Vitamin supplementation includes folic acid, 1mg orally, daily, and vitamin B12, 1 mg IM q8-10 weeks, beginning >7 days prior to chemotherapy. Radiologic response was measured using serial CT scans and tumor measurements taken according to standard response criteria. • Dose-limiting toxicity (DLT) was defined as > grade 3: hematological AE (except anemia), neutropenic fever, non-hematologic AE (except nausea/vomiting), or dose delay beyond 2 weeks for treatment-related AE. • Dose escalation progressed using a modified accelerated titration method. One cycle of therapy (2 doses of PDX over 4 weeks) was completed without a DLT before patients were enrolled at the next dose level. If no DLT was observed, the next patient was enrolled at the next higher dose level. If 1 DLT was observed, the level was expanded to 3 and then possibly 6 patients. Doses were increased by either 40 mg/m2 or by 20%. The MTD was defined as one dose level below the dose at which 2 or more patients experienced a DLT. Up to 16 patients may be enrolled at the MTD. Patient Characteristics: (n=22) Median Age 61 years (Range 40-73 years) Category n Percentage Female 15 68 % 1 prior chemotherapy 9 41 % > 2 prior chemotherapy 13 59 % Dose Levels and Dose-limiting Toxicity: Dose PDX (n) Dose-limiting toxicity Level (mg/m2) 1 150 1 None 2 190 1 None 3 230 1 None 4 270 6 Grade 3 mucositis (n=1) A 5 325 3 Grade 3 mucositis (n=2) 4 270 10 Grade 3 mucositis (n=4) B A: median 1.5 prior regimens (range 1-3) B: median 3 prior regimens (range 1-6) Selected Toxicity: all dose levels, Gr 2, possibly related to PDX n=22 Gr 1 Gr 2 Gr 3 Gr 4 Alopecia 3 (14%) 2 (9%) N/A N/A Chills 0 1 (5%) 0 0 Dehydration 0 0 1 (5%) 0 Esophagitis 0 0 1 (5%) 0 Fatigue 4 (18%) 10 (46%) 0 0 Hand/Food syndrome 0 0 1 (5%) 0 Nausea 9 (41%) 1 (5%) 0 0 Neuropathy 3 (14%) 1 (5%) 0 0 Odynophagia 0 0 1 (5%) 0 Peripheral edema 1 (5%) 1 (5%) 0 0 Pleural effusion 0 0 1 (5%) 0 Rash 4 (19%) 1 (5%) 0 0 Stomatitis/Mucositis 8 (36%) 9 (41%) 7 (32%) 0 Tinnitus 0 0 1 (5%) 0 Vomiting 4 (18%) 1 (5%) 0 0 Mucositis as Adverse Event: Gr 1 Gr 2 Gr 3 All patients (n=22) 8 (36%) 9 (41%) 7 (32%) 270 mg/m2 (n=16) 6 (38%) 6 (38%) 5 (31%) Preliminary Efficacy: 80 60 40 20 0 baseline -20 -40 -60 largest percent change from -80 Bars in BROWN represent those patients currently on therapy Conclusions: • PDX + folic acid/vitamin B12 supplementation is active and well-tolerated in patients with NSCLC. • The addition of folic acid/vitamin B12 supplementation allowed higher doses of PDX to be delivered safely. • The incidence of mucositis in the latter part of the study may be related to the greater number of prior treatments. • Based on DLT, the maximum tolerated dose of PDX is 270 mg/m2. • Determination of the recommended Phase 2 dose will be made after review of the final data. Supported by: |
