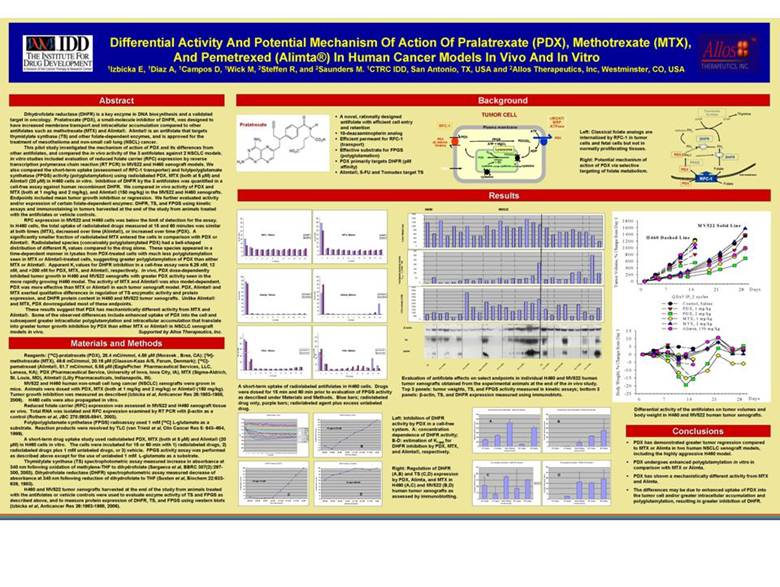
| Differential Activity And Potential Mechanism Of Action Of Pralatrexate (PDX), Methotrexate(MTX), And Pemetrexed (Alimta®) In Human Cancer Models In Vivo And In Vitro 1Izbicka E, 1Diaz A, 1Campos D, 1Wick M, 2Steffen R, and 2Saunders M. 1CTRC IDD, San Antonio, TX, USA and 2Allos Therapeutics, Inc, Westminster, CO, USA Abstract Dihydrofolate reductase (DHFR) is a key enzyme in DNA biosynthesis and a validated target in oncology. Pralatrexate (PDX), a small-molecule inhibitor of DHFR, was designed to have increased membrane transport and intracellular accumulation compared to other antifolates such as methotrexate (MTX) and Alimta®. Alimta®is an antifolate that targets thymidylate synthase (TS) and other folate-dependent enzymes, and is approved for the treatment of mesotheliomaand non-small cell lung (NSCL) cancer. This pilot study investigated the mechanism of action of PDX and its differences from other antifolates, and compared the in vivo activity of the 3 antifolates against 2 NSCLC models. In vitro studies included evaluation of reduced folate carrier (RFC) expression by reverse transcription polymerase chain reaction (RT PCR) in MV522 and H460 xenograft models. We also compared the short-term uptake (assessment of RFC-1 transporter) and folylpolyglutamate synthetase (FPGS) activity (polyglutamylation) using radiolabeled PDX, MTX (both at 5 µM) and Alimta®(20 µM) in H460 cells in vitro. Inhibition of DHFR by the 3 antifolates was quantified in a cell-free assay against human recombinant DHFR. We compared in vivo activity of PDX and MTX (both at 1 mg/kg and 2 mg/kg), and Alimta®(150 mg/kg) in the MV522 and H460 xenografts. Endpoints included mean tumor growth inhibition or regression. We further evaluated activity and/or expression of certain folate-dependent enzymes: DHFR, TS, and FPGS using kinetic assays and immunostainingin tumors harvested at the end of the study from animals treated with the antifolatesor vehicle controls. RFC expression in MV522 and H460 cells was below the limit of detection for the assay. In H460 cells, the total uptake of radiolabeled drugs measured at 15 and 60 minutes was similar at both times (MTX), decreased over time (Alimta®), or increased over time (PDX). A significantly smaller fraction of radiolabeled MTX entered the cells in comparison with PDX or Alimta®. Radiolabeled species (conceivably polyglutamylated PDX) had a bell-shaped distribution of different Rf values compared to the drug alone. These species appeared in a time-dependent manner in lysates from PDX-treated cells with much less polyglutamylation seen in MTX or Alimta®-treated cells, suggesting greater polyglutamylation of PDX than either MTX or Alimta®. Apparent Ki values for DHFR inhibition in a cell-free assay were 6.25 nM, 12 nM, and >200 nM for PDX, MTX, and Alimta®, respectively. In vivo, PDX dose-dependently inhibited tumor growth in H460 and MV522 xenografts with greater PDX activity seen in the more rapidly growing H460 model. The activity of MTX and Alimta® was also model-dependent. PDX was more effective than MTX or Alimta® in each tumor xenograft model. PDX, Alimta® and MTX exerted qualitative differences in regulation of TS enzymatic activity and protein expression, and DHFR protein content in H460 and MV522 tumor xenografts. Unlike Alimta® and MTX, PDX downregulatedmost of these endpoints. These results suggest that PDX has mechanistically different activity from MTX and Alimta®. Some of the observed differences include enhanced uptake of PDX into the cell and subsequent greater intracellular polyglutamylation and intracellular accumulation that translate into greater tumor growth inhibition by PDX than either MTX or Alimta® in NSCLC xenograft models in vivo. Supported by Allos Therapeutics, Inc. Materials and Methods Reagents: [14C]-pralatrexate (PDX), 28.4 mCi/mmol, 4.58 µM (Moravek , Brea, CA); [3H]- %Total CPM's methotrexate (MTX), 49.6 mCi/mmol, 20.16 µM (Clauson-Kaas A/S, Farum, Denmark); [14C]-pemetrexed (Alimta®, 51.7 mCi/mmol, 5.55 µM (EaglePicher Pharmaceutical Services, LLC, Lenexa, KA); PDX (Pharmaceutical Service, University of Iowa, Iowa City, IA), MTX (Sigma-Aldrich, St. Louis, MO), Alimta®(Lilly Pharmaceuticals, Indianapolis, IN). MV522 and H460 human non-small cell lung cancer (NSCLC) xenografts were grown in mice. Animals were dosed with PDX, MTX (both at 1 mg/kg and 2 mg/kg) or Alimta®(150 mg/kg). Tumor growth inhibition was measured as described (Izbicka et al, Anticancer Res 26:1983-1988, 2006). H460 cells were also propagated in vitro. Reduced folate carrier (RFC) expression was assessed in MV522 and H460 xenografttissue ex vivo. Total RNA was isolated and RFC expression examined by RT PCR with β-actin as a control (Rothem et al, JBC 278:8935-8941, 2003). Folylpolyglutamate synthetase (FPGS) radioassayused 1 mM [14C] L-glutamate as a substrate. Reaction products were resolved by TLC (van Triest et al, ClinCancer Res 5: 643–654, 1999). A short-term drug uptake study used radiolabeled PDX, MTX (both at 5 µM) and Alimta®(20 µM) in H460 cells in vitro. The cells were incubated for 15 or 60 min with 1) radiolabeled drugs, 2) radiolabeleddrugs plus 1 mM unlabeled drugs, or 3) vehicle. FPGS activity assay was performed as described above except for the use of unlabeled 1 mM L-glutamate as a substrate. Thymidylate synthase (TS) spectrophotometric assay measured increase in absorbance at 340 nm following oxidation of methylene-THF to dihydrofolate (Sergeeva et al, BBRC 307(2):297-300, 2003). Dihydrofolate reductase (DHFR) spectrophotometric assay measured decrease of absorbance at 340 nm following reduction of dihydrofolate to THF (Susten et al, Biochem 22:633-639, 1983). H460 and MV522 tumor xenografts harvested at the end of the study from animals treated with the antifolates or vehicle controls were used to evaluate enzyme activity of TS and FPGS as described above, and to measure protein expression of DHFR, TS, and FPGS using western blots (Izbicka et al, Anticancer Res 26:1983-1988, 2006). Background Thymidylate TUMOR CELL Synthase Thymine A novel, rationally designed cMOAT/ dUMP dTMP antifolatewith efficient cell entry MRP and retention RFC-1 Plasma membrane ATPase ATP Left: Classical folate analogs are Methylene FH4 10-deazaaminopterin analog PDX PDX internalized by RFC-1 in tumor Efficient permeant for RFC-1 PDX FPGS DHFR (& natural PDX(G)n cells and fetal cells but not in (transport) folates) ATP + MgCl2 Effective substrate for FPGS Lysosome normally proliferating tissues. FH4 FH2 ADP Gn PDX (polyglutamation) PDX(G)n (Glun) DHFR PDX Right: Potential mechanism of PDX primarily targets DHFR (pM FPGH + SH FPGS affinity) cysteine cysteine ? cysteine action of PDX via selective TMTX targeting of folate metabolism. PDX Folate Alimta®, 5-FU and Tomudex target TS Cell membrane cysteine RFC-1 PDX Folate 45 45 40 40 35 35 30 30 25 MTX, 15min. [3H]-MTX 25 MTX, 60min. [3H]-MTX [3H]-MTX+ [3H]-MTX+ %Total CPM's 20 %Total CPM's 20 15 15 10 10 5 5 0 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3 4 5 6 7 8 9 10 11 12 TLC Section TLC Section 100 100 90 90 80 80 70 70 60 60 Alimta, 15min. [14C]-Alimta Alimta, 60min. [14C]-Alimta 50 50 %Total CPM's [14C]-Alimta+ %Total CPM's [14C]-Alimta+ 40 40 30 30 20 20 10 10 0 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3 4 5 6 7 8 9 10 11 12 TLC Section TLC Section 70 70 60 60 50 50 PDX, 15min. [14C]-PDX PDX, 60min. [14C]-PDX [14C]-PDX+ [14C]-PDX+ %Total CPM's 35 20 %Total CPM's 20 10 10 0 0 1 2 3 4 5 6 7 8 9 10 11 12 1 2 3 4 5 6 7 8 9 10 11 12 TLC Section TLC Section A short-term uptake of radiolabeled antifolatesin H460 cells. Drugs were dosed for 15 min and 60 min prior to evaluation of FPGS activity as described under Materials and Methods. Blue bars; radiolabeled drug only, purple bars; radiolabeledagent plus excess unlabeled drug. DHFR Kinetics -PDX DHFR Kinetics (PDX) 0.18 100 0.16 90 80 0.14 70 PDX [200nM] Kiapp=6.25 nM 0.12 60 PDX [100nM] PDX [50nM] 50 0.1 O.D. PDX [25nM] 40 PDX [12.5nM] 0.08 30 PDX [6.25nM] PDX [3.13nM] 20 0.06 % Maximum Velocity PDX [0nM] 10 0.04 0 B 0.02 -10 -20 0 [200nM] [100nM] [50nM] [25nM] [12.5nM] [6.25nM] [3.13nM] [0nM] 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 Time Point PDX Concentration DHFR Kinetics (MTX) DHFR Kinetics (Alimta) 100 100 90 90 80 80 70 70 60 60 Kiapp=12 nM Kiapp>200 nM 50 50 40 40 % Maximum Velocity 30 % Maximum Velocity 30 20 20 10 C D 10 0 0 [200nM] [100nM] [50nM] [25nM] [12.5nM] [6.25nM] [3.13nM] [0nM] [200nM] [100nM] [50nM] [25nM] [12.5nM] [6.25nM] [3.13nM] [0nM] MTX Concentration Alimta Concentration H460 MV522 1600 1400 1200 1000 800 600 Tumor Weight (mg) 400 200 0 350 300 250 200 /Time * 1e6 150 0 –T 100 max 50 T ThymidylateSynthase Activity 0 5000 4500 4000 3500 3000 2500 2000 FPGS activity (CPM) 1500 1000 500 0 β -Actin TS DHFR g g g g g g g g g l r l g/k t r g/k t g/k g/k g/k g/k g/k m g/k g/k C m m m m C m m m m 2 0 1 2 2 0 1 2 1 5 5 X X 1 X X X 1 X X D D t a T T D t a T T P P m M M P i m M M l i l A A Evaluation of antifolate effects on select endpoints in individual H460 and MV522 human tumor xenografts obtained from the experimental animals at the end of the in vivo study. Top 3 panels: tumor weights, TS, and FPGS activity measured in kinetic assays; bottom 3 panels: β-actin, TS, and DHFR expression measured using immunoblots. Left: Inhibition of DHFR activity by PDX in a cell-free system. A: concentration dependence of DHFR activity; B-D: estimation of Ki app for DHFR inhibition by PDX, MTX, and Alimta®, respectively. Right: Regulation of DHFR (A,B) and TS (C,D) expression by PDX, Alimta, and MTX in H460 (A,C) and MV522 (B,D) human tumor xenografts as assessed by immunoblotting. Differential activity of the antifolates on tumor volumes and body weight in H460 and MV522 human tumor xenografts. Conclusions PDX has demonstrated greater tumor regression compared to MTX or Alimta in two human NSCLC xenograftmodels, including the highly aggressive H460 model. PDX undergoes enhanced polyglutamylation in vitro in comparison with MTX or Alimta. PDX has shown a mechanistically different activity from MTX and Alimta. The differences may be due to enhanced uptake of PDX into the tumor cell and/or greater intracellular accumulation and polyglutamylation, resulting in greater inhibition of DHFR. |
