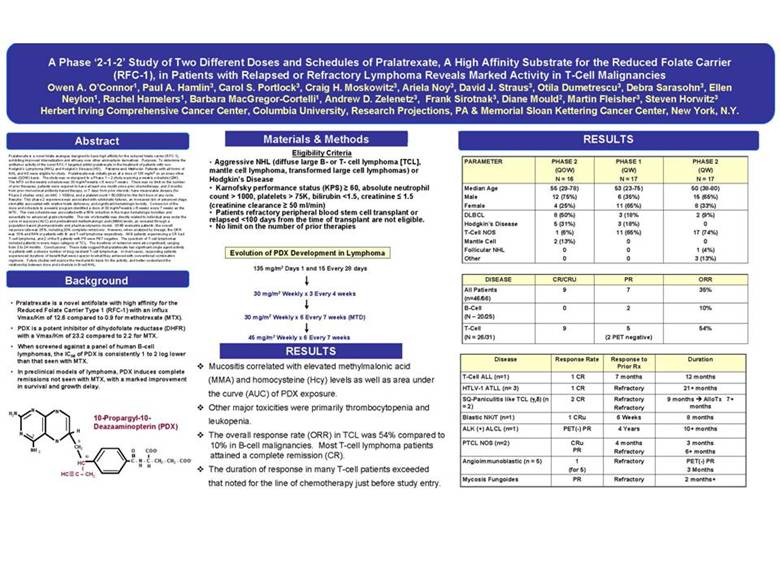
| A Phase ‘2-1-2’ Study of Two Different Doses and Schedules of Pralatrexate, A High Affinity Substrate for the Reduced Folate Carrier (RFC-1), in Patients with Relapsed or Refractory Lymphoma Reveals Marked Activity in T-Cell Malignancies Owen A. O’Connor1, Paul A. Hamlin3, Carol S. Portlock3, Craig H. Moskowitz3, Ariela Noy3, David J. Straus3, Otila Dumetrescu3, Debra Sarasohn3, Ellen Neylon1, Rachel Hamelers1, Barbara MacGregor-Cortelli1, Andrew D. Zelenetz3, Frank Sirotnak3, Diane Mould2, Martin Fleisher3, Steven Horwitz3 Herbert Irving Comprehensive Cancer Center, Columbia University,Research Projections, PA & Memorial Sloan Kettering Cancer Center, New York, N.Y. Abstract Pralatrexate is a novel folate analogue designed to have high affinity for the reduced folate carrier (RFC-1), exhibiting improved internalization and efficacy over other aminopterin derivatives. Purpose: To determine the antitumor activity of the novel RFC-1 targeted antifol pralatrexate in the treatment of patients with non-Hodgkin’s Lymphoma (NHL) and Hodgkin’s Disease (HD). Patients and Methods: Patients with all forms of NHL and HD were eligible for study. Pralatrexate was initially given at a dose of 135 mg/m2 on an every other week (QOW) basis. The study was re-designed to a Phase 1 – 2 study exploring a weekly schedule (QW). The MTD on the weekly schedule was 30 mg/m2 weekly x 6 every 7 weeks. There was no limit on the number of prior therapies, patients were required to have at least one month since prior chemotherapy; and 3 months from prior monoclonal antibody based therapy, or 7 days from prior steroids; have measurable disease (for Phase 2 studies only); an ANC > 1000/ul, and a platelet count > 50,000/ul for the first dose of any cycle. Results: This phase 2 experience was associated with cytokinetic failures, an increased risk of advanced stage stomatitis associated with relative folate deficiency, and significant hematologic toxicity. Conversion of the dose and schedule to a weekly program identified a dose of 30 mg/m2 weekly x 6 weeks every 7 weeks as the MTD. This new schedule was associated with a 50% reduction in the major hematologic toxicities and essentially no advanced grade stomatitis. The risk of stomatitis was directly related to individual area under the curve of exposure (AUC) and pretreatment methylmalongic acid (MMA) levels, as revealed through a population based pharmacokinetic and pharmacodynamic model. Of 46 evaluable patients, the overall response rate was 35%, including 20% complete remissions. However, when analyzed by lineage, the ORR was 10% and 54% in patients with B-and T-cell lymphoma respectively. All 9 patients experiencing a CR had T-cell lymphoma, and 2 of the 5 patients with PR were PET negative. The spectrum of T-cell lymphomas included patients in every major category of TCL. The durations of remission were also significant, ranging from 3 to 24 months. Conclusions: These data suggest that pralatrexate has significant single agent activity in patients with a diverse number of drug resistant T-cell lymphomas. In most cases, responding patients experienced durations of benefit that were superior to what they achieved with conventional combination regimens. Future studies will explore the mechanistic basis for the activity, and better understand the relationship between dose and schedule in B-cell NHL. Background • Pralatrexate is a novel antifolate with high affinity for the Reduced Folate Carrier Type 1 (RFC-1) with an influx Vmax/Km of 12.6 compared to 0.9 for methotrexate(MTX). • PDX is a potent inhibitor of dihydofolate reductase (DHFR) with a Vmax/Km of 23.2 compared to 2.2 for MTX. • When screened against a panel of human B-cell lymphomas, the IC50 of PDX is consistently 1 to 2 log lower than that seen with MTX. • In preclinical models of lymphoma, PDX induces complete remissions not seen with MTX, with a marked improvement in survival and growth delay. N N H2N 10-Propargyl-10-Deazaaminopterin (PDX) H N 3 N 9 CH2 O COO-NH 2 10 = HC C - N - C - CH2 - CH2 - COO-H H HC C – CH2 Materials & Methods Eligibility Criteria • Aggressive NHL (diffuse large B-or T- cell lymphoma [TCL], mantle cell lymphoma, transformed large cell lymphomas) or Hodgkin’s Disease • Karnofsky performance status (KPS) 60, absolute neutrophil count > 1000, platelets > 75K, bilirubin <1.5, creatinine 1.5 (creatinine clearance 50 ml/min) • Patients refractory peripheral blood stem cell transplant or relapsed <100 days from the time of transplant are not eligible. • No limit on the number of prior therapies Evolution of PDX Development in Lymphoma 135 mg/m2 Days 1 and 15 Every 28 days 30 mg/m2 Weekly x 3 Every 4 weeks 30 mg/m2 Weekly x 6 Every 7 weeks (MTD) 45 mg/m2 Weekly x 6 Every 7 weeks RESULTS Mucositis correlated with elevated methylmalonic acid (MMA) and homocysteine (Hcy) levels as well as area under the curve (AUC) of PDX exposure. Other major toxicities were primarily thrombocytopenia and leukopenia. The overall response rate (ORR) in TCL was 54% compared to 10% in B-cell malignancies. Most T-cell lymphoma patients attained a complete remission (CR). The duration of response in many T-cell patients exceeded that noted for the line of chemotherapy just before study entry. RESULTS PARAMETER PHASE 2 PHASE 1 PHASE 2 (QOW) (QW) (QW) N = 16 N = 17 N = 17 Median Age 55 (29-78) 53 (23-75) 50 (38-80) Male 12 (75%) 6 (35%) 15 (65%) Female 4 (25%) 11 (65%) 8 (33%) DLBCL 8 (50%) 3 (18% 2 (9%) Hodgkin’s Disease 5 (31%) 3 (18%) 0 T-Cell NOS 1 (6%) 11 (65%) 17 (74%) Mantle Cell 2 (13%) 0 0 Follicular NHL 0 0 1 (4%) Other 0 0 3 (13%) DISEASE CR/CRU PR ORR All Patients 9 7 35% (n=46/56) B-Cell 0 2 10% (N –20/25) T-Cell 9 5 54% (N = 26/31) (2 PET negative) Disease Response Rate Response to Duration Prior Rx T-Cell ALL (n=1) 1 CR 7 months 12 months HTLV-1 ATLL (n= 3) 1 CR Refractory 21+ months SQ-Paniculitislike TCL (γ,δ ) (n 2 CR Refractory 9 months AlloTx 7+ = 2) Refractory months BlasticNK/T (n=1) 1 CRu 6 Weeks 8 months ALK (+) ALCL (n=1) PET(-) PR 4 Years 10+ months PTCL NOS (n=2) CRu 4 months 3 months PR Refractory 6+ months Angioimmunoblastic(n = 5) 1 Refractory PET(-) PR (for 5) 3 Months Mycosis Fungoides PR Refractory 2 months+ |
