QuickLinks -- Click here to rapidly navigate through this document
Exhibit 1

ANNUAL INFORMATION FORM
FOR THE FINANCIAL YEAR ENDED DECEMBER 31, 2005
March 24, 2006
ITEM 1. |
|
CORPORATE STRUCTURE |
|
4 |
| | | 1.1 | | NAME AND INCORPORATION | | 4 |
| | | 1.2 | | INTERCORPORATE RELATIONSHIPS | | 4 |
ITEM 2. |
|
GENERAL DEVELOPMENT OF THE BUSINESS |
|
5 |
| | | 2.1 | | THREE YEAR HISTORY | | 5 |
| | | 2.2 | | SIGNIFICANT ACQUISITIONS AND SIGNIFICANT DISPOSITIONS | | 6 |
ITEM 3. |
|
DESCRIPTION OF THE BUSINESS |
|
6 |
| | | 3.1 | | BIOPHARMACEUTICAL ACTIVITIES | | 7 |
| | | | | 3.1.1 | | Pipeline Table | | 8 |
| | | | | 3.1.2 | | LHRH Antagonists | | 9 |
| | | | | 3.1.3 | | Signal Transduction Inhibitors | | 15 |
| | | | | 3.1.4 | | Cytotoxic Conjugates and Cytotoxics | | 19 |
| | | | | 3.1.5 | | Tubulin Inhibitors / Vascular Targeting Agents | | 21 |
| | | | | 3.1.6 | | GH-RH Modulators | | 22 |
| | | | | 3.1.7 | | Immunotherapy / Vaccines | | 23 |
| | | | | 3.1.8 | | Drug Discovery | | 24 |
| | | | | 3.1.9 | | Strategic Alliances | | 24 |
| | | | | 3.1.10 | | Growth Strategy | | 27 |
| | | 3.2 | | ATRIUM BIOTECHNOLOGIES INC. | | 27 |
| | | | | 3.2.1 | | Company Overview | | 27 |
| | | | | 3.2.2 | | Products | | 28 |
| | | | | 3.2.3 | | Competition | | 30 |
| | | | | 3.2.4 | | Manufacturing and Supply | | 31 |
| | | 3.3 | | RISK FACTORS | | 32 |
ITEM 4. |
|
DIVIDENDS |
|
32 |
| | | 4.1 | | DIVIDENDS | | 32 |
ITEM 5. |
|
GENERAL DESCRIPTION OF CAPITAL STRUCTURE |
|
32 |
| | | 5.1 | | GENERAL DESCRIPTION OF CAPITAL STRUCTURE | | 32 |
ITEM 6. |
|
MARKET FOR SECURITIES |
|
32 |
| | | 6.1 | | TRADING PRICE AND VOLUME | | 32 |
ITEM 7. |
|
DIRECTORS AND OFFICERS |
|
33 |
| | | 7.1 | | DIRECTORS | | 33 |
| | | 7.2 | | EXECUTIVE OFFICERS | | 35 |
ITEM 8. |
|
LEGAL PROCEEDINGS |
|
36 |
| | | 8.1 | | LEGAL PROCEEDINGS | | 36 |
ITEM 9. |
|
INTEREST OF MANAGEMENT AND OTHERS IN MATERIAL TRANSACTIONS |
|
36 |
ITEM 10. |
|
TRANSFER AGENT AND REGISTRAR |
|
36 |
| | | 10.1 | | TRANSFER AGENT AND REGISTRAR | | 36 |
ITEM 11. |
|
MATERIAL CONTRACTS |
|
37 |
| | | 11.1 | | MATERIAL CONTRACTS | �� | 37
|
| | | | | | | | | |
2
ITEM 12. |
|
INTERESTS OF EXPERTS AND AUDIT COMMITTEE DISCLOSURE |
|
37 |
| | | 12.1 | | NAMES AND INTEREST OF EXPERTS AND AUDIT COMMITTEE DISCLOSURE | | 37 |
ITEM 13. |
|
ADDITIONAL INFORMATION |
|
37 |
| | | 13.1 | | ADDITIONAL INFORMATION | | 37 |
ITEM 14. |
|
FORWARD-LOOKING STATEMENTS |
|
37 |
| | | 14.1 | | FORWARD-LOOKING STATEMENTS | | 37 |
As used in this Annual Information Form, all references to "Æterna Zentaris", the "Company", "we", "us", "our" or similar terms are to Æterna Zentaris Inc., and unless otherwise indicated, all dollar amounts are in U.S. currency and all information is presented as of March 24, 2006.
3
ITEM 1. CORPORATE STRUCTURE
1.1 Name and Incorporation
The Company was incorporated on September 12, 1990, pursuant to theCanada Business Corporations Act under the corporate name of 171162 Canada Inc., which name was changed under Articles of Amendment dated September 26, 1991 to Les Laboratoires Æterna inc. On May 26, 2004, the Company modified its Articles of Amendment to change its name to Æterna Zentaris Inc. ("Æterna Zentaris") as well as to:
- i)
- create a new class of shares, being an unlimited number of Common Shares;
- ii)
- change each issued and outstanding Subordinate Voting Share into one Common Share; and
- iii)
- cancel the Subordinate Voting Shares and the Multiple Voting Shares as a class.
The authorized share capital of the Company consists of an unlimited number of Common Shares, an unlimited number of First Preferred Shares, issuable in series, and an unlimited number of Second Preferred Shares, issuable in series.
Our head office is located at 1405 Parc-Technologique Blvd., Quebec City, Quebec, Canada G1P 4P5. Our telephone number is (418) 652-8525 and our facsimile number is (418) 652-0881. Our Web site iswww.aeternazentaris.com. Any information or documents on our Web site are not, however, included in, nor shall any of such information or documents be deemed to be incorporated by reference into, this Annual Information Form.
1.2 Intercorporate Relationships
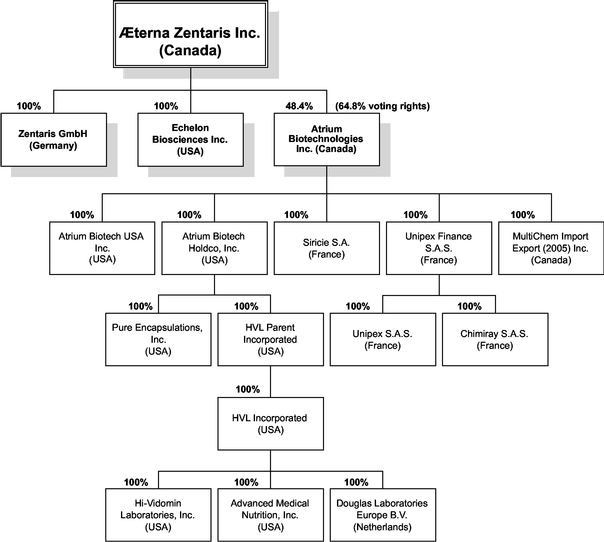
As indicated below, Æterna Zentaris, based in Canada, has three directly owned subsidiaries, being Zentaris GmbH ("Zentaris") based in Germany, Echelon Biosciences, Inc. ("Echelon") based in the United States and Atrium Biotechnologies Inc. ("Atrium") based in Canada. We are organized into three operating segments: biopharmaceutical, operated by Æterna Zentaris, Zentaris and Echelon; Active Ingredients & Specialty Chemicals and Health & Nutrition which are operated by our subsidiary Atrium.
Æterna Zentaris is the sole holder of Multiple Voting Shares issued by Atrium and these shares will be automatically converted into Subordinate Voting Shares on a one-for-one basis: i) upon any transfer thereof, subject to limited exceptions; ii) within five years from the closing date of Atrium's initial public offering, namely April 6, 2010; and iii) in certain other circumstances, including a change of control of the Company. Æterna Zentaris holds 64.8% of the voting rights and 48.4% of the equity interests in Atrium.
4
The following chart depicts Æterna Zentaris and its subsidiaries as of March 24, 2006:
ITEM 2. GENERAL DEVELOPMENT OF THE BUSINESS
2.1 Three Year History
On December 30, 2002, we acquired 100% of Zentaris AG, a biopharmaceutical company based in Germany. Zentaris is a spin-off of the former Asta-Medica and is involved in oncology, endocrine and anti-infective therapy. This acquisition brought us a broad product pipeline, which leverages six different therapeutic approaches, including the use of luteinizing hormone releasing hormone (LHRH) antagonists and signal transduction inhibitors. We have two lead LHRH antagonist compounds. The first one, cetrorelix, currently marketed forin vitro fertilization under the brand name Cetrotide®, is also in late stage clinical development for endometriosis and benign prostatic hyperplasia (BPH). The second compound, ozarelix, is currently in Phase 2 clinical trials for BPH and prostate cancer. The lead signal transduction inhibitor compound, perifosine, is an alkylphosphocholine which interacts in cancer cells with vital apoptotic and signal transduction mechanisms. Perifosine is in several Phase 2 trials for multiple cancers. Furthermore, the acquisition included several other preclinical and clinical programs that are under way with various potential development candidates, supported by a worldwide network of scientific and marketing partners, as well as by an integral drug discovery unit which includes a library of nearly 120,000 molecules.
5
In May 2004, we changed our name to Æterna Zentaris Inc. in order to recognize the contribution of the Zentaris pipeline and to reflect the new international scope of the Company.
In early January 2005, we acquired all of the issued and outstanding shares of Echelon, a privately-held biotech company based in Salt Lake City, Utah, USA. Echelon's product pipeline is focused on the rapidly emerging field of transduction signalling technology thus mainly providing us a complementary strategic fit for perifosine, our lead compound in our signal transduction inhibitor approach in oncology. Furthermore, Echelon markets chemical reagents and its sales reached nearly US$2.4 million during the twelve months ended December 31, 2005.
We own 48.4% of the equity of our subsidiary Atrium Biotechnologies Inc. and 64.8% of its voting rights. Atrium was created at the end of 1999 to develop, manufacture and market active ingredients, specialty chemicals and finished products in the health and personal care industry. As part of its growth strategy, Atrium initiated an aggressive acquisition program and completed several acquisitions, including Unipex (France) in July 2001, Interchemical S.A. and Chimiray S.A. (France) in August 2003, Siricie S.A. (France) in November 2003, Pure Encapsulations, Inc. (United States) in March 2004, MultiChem (Canada) in January 2005, and, more recently, HVL Inc. (United States) in December 2005.
On April 6, 2005, Atrium completed an initial public offering and secondary offering of 6,250,000 subordinate voting shares in Canada at a price of CAN$12.00 per share, for total gross proceeds of $61.4 million (CAN$75 million). Atrium's subordinate voting shares now trade on the Toronto Stock Exchange under the symbol "ATB.sv". The gross proceeds from the treasury offering totalled approximately $41 million (CAN$50 million) and will be used by Atrium primarily to pursue its acquisition strategy and for general corporate purposes.
2.2 Significant Acquisitions and Significant Dispositions
On December 8, 2005, Atrium, through one of its U.S. subsidiaries, acquired all of the outstanding shares of HVL Parent Incorporated whose main brand is Douglas Laboratories. This company develops, manufactures and markets health and nutritional products through healthcare practitioners mainly in the United States.
This acquisition was made for a total consideration of $86,852,000, of which an amount of $73,906,000, including all acquisition-related costs, was or will be paid cash, net of cash and cash equivalents acquired of $3,182,000, and $8,632,000 was paid by Atrium in 917,532 subordinate voting shares issued to certain Douglas Laboratories management shareholders at a price of CAN$10.95 per share. The cash portion came from cash on hand and from Atrium's revolving credit facility renegotiated in November 2005.
ITEM 3. DESCRIPTION OF THE BUSINESS
The Company operates in three segments of operations which are: (i) Biopharmaceutical; (ii) Active Ingredients & Specialty Chemicals; and (iii) Health and Nutrition.
Æterna Zentaris, along with its wholly-owned subsidiaries, Zentaris and Echelon, constitute the Biopharmaceutical segment or activities. This segment focuses on the development and marketing of therapies for cancer, endocrine disorders and infectious diseases. We offer a broad product pipeline through five therapeutic approaches.
Our Atrium subsidiary, together with its subsidiaries, including Unipex, Multichem, Pure Encapsulations and Douglas Laboratories, operates the two other business segments. The Active Ingredients & Specialty Chemicals Division offers value-added products that include high-value proprietary active ingredients developed, acquired or in-licensed by Atrium. Through the Health & Nutrition Division, Atrium develops, manufactures and markets Health & Nutrition finished products.
6
3.1 Biopharmaceutical Activities
We are focused on advancing our product pipeline in oncology and endocrine therapy. We believe that we have a proven expertise in drug discovery, pharmaceutical development and commercialization.
We believe that the LHRH antagonists and the signal transduction inhibitors therapeutic approaches are the value drivers of our biopharmaceutical activities and they have the potential to target large market opportunities. Our LHRH antagonists include cetrorelix involved inin vitro fertilization (IVF), endometriosis and benign prostatic hyperplasia (BPH) and ozarelix (D-63153) which targets benign prostatic hyperplasia (BPH) and prostate cancer. In addition, our signal transduction inhibitors include our lead compound perifosine targeting multiple types of cancer.
Cetrorelix is our lead compound of the LHRH antagonist therapeutic area and is currently marketed by our partner Serono forin vitro fertilization under the brand name Cetrotide®.
Cetrorelix is also in late-stage development in endometriosis with our worldwide (ex-Japan) partner Solvay Pharmaceuticals (Solvay). We are planning to further advance the development of cetrorelix in late-stage clinical development in BPH in North America while our Japanese parter Shionogi/Nippon Kayaku is continuing a Phase 2 program in Japan. These trials were initiated following the results disclosed during 2004, of the extensive 7 Phase 2 - 735 patient program — in endometriosis (3 trials), BPH (3 trials) and uterine myoma (1 trial). All trials yielded significant to highly significant positive results with an excellent safety profile.
Ozarelix is our 4th generation LHRH antagonist aiming at extended suppression of testosterone levels that does not require a sophisticated depot formulation for long lasting activity. Phase 2 trials have now been initiated in 2005 in hormone-dependent prostate cancer as well as BPH and are fully funded by our partner Spectrum Pharmaceuticals (North America and India exclusive rights).
Another therapeutic approach of high interest is represented by our signal transduction inhibitors. Perifosine, an orally-active Akt inhibitor that is in several Phase 2 trials for multiple cancers, is a first-in-class alkylphosphocholine oral compound that demonstrated interactions with vital signal transduction mechanisms in tumor cells and showed induction of apoptosis. Perifosine has also shown anti-tumor activity in several monotherapy trials. Perifosine is being investigated in over ten Phase 1 and 2 clinical trials in monotherapy as well as in combination with chemotherapy, biologics or radiotherapy. Impavido® (miltefosine) originates from the same research approach and is marketed for visceral and cutaneous leishmaniasis, a parasitic infection.
We have several other therapeutic approaches and several other preclinical and clinical programs are under way with various potential development candidates, supported by a worldwide network of scientific and development partners. We also benefit from an important drug discovery unit which includes high throughput screening systems and a library of nearly 120,000 compounds.
We possess an extensive portfolio of over 20 active substances and product candidates at different phases of development from drug discovery to market. As part of our focused strategy, we intend to further expand and develop this portfolio with internal resources or through various forms of partnerships.
7
3.1.1 Pipeline Table
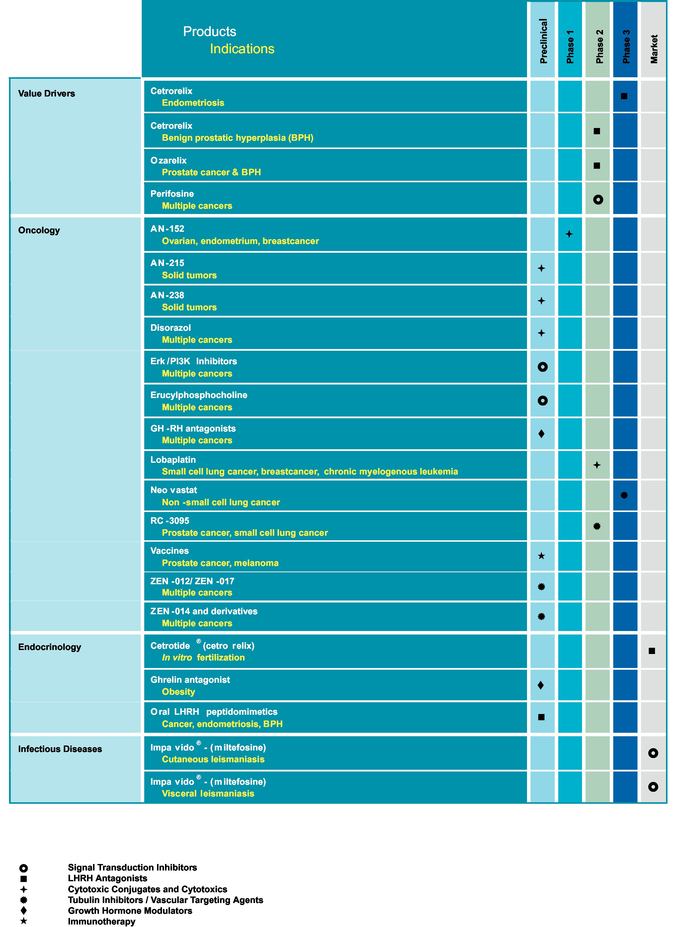
8
3.1.2 LHRH Antagonists
Cetrorelix
Cetrorelix is a peptide-based active substance which was developed in cooperation with Nobel Laureate Professor Andrew Schally of Tulane University in New Orleans. This compound is an LHRH antagonist (LHRH also known as GnRH) that blocks the pituitary LHRH receptors resulting in a rapid decrease of sexual hormone levels. Moreover, cetrorelix allows the LHRH receptors on the pituitary gland to be blocked gradually. Conversely, the side effects usually associated with the use of agonists and resulting from total hormone withdrawal can be avoided in conditions that do not require a castrating degree of hormone withdrawal. Therefore, in contrast to treatment with agonists, LHRH antagonists permit dose-dependent hormone suppression which is of critical importance for the tolerability of hormonal therapy.
The mode of action of cetrorelix and the distinction between LHRH antagonists and LHRH agonists
LHRH is released by the hypothalamus in the brain and controls the production of sex hormones (i.e. testosterone in the testes and estrogen and progesterone in ovaries) via the activation of LHRH receptors located on the pituitary gland (hypophysis).
When using LHRH agonists, the LHRH receptors on the pituitary gland are stimulated leading to an initial increased secretion of the hormones LH and FSH, which in turn regulate formation of testosterone and estrogens. The increase or surge of hormonal levels induces a "flare-up" effect that can last up to three weeks until the pituitary markedly decreases the release of LH and FSH by desensitization and depletion of LHRH receptors (i.e. down-regulation) resulting in a considerable drop in testosterone and estrogen levels. Though the initial flare-up effect is limited in time, it can sometimes cause, depending on the nature and stage of the particular disorder, considerable additional symptoms or even life-threatening complications, which in turn require additional therapeutic intervention. By simultaneous administration of further drugs, the flare-up effect can be attenuated. However, this additional treatment also bears the risk of certain side effects, e.g. disturbances of the function of the stomach, intestines and liver.
During full hormone suppression, LHRH agonists reduce the male sex hormones to ranges below castration level. In women, the hormone levels are far below the ranges observed after the end of the climacteric. Treatment with an LHRH agonist, therefore, is regularly associated with side effects such as hot flashes, depression, muscle weakness, loss of libido and, particularly in women, osteoporosis and ovarian cysts. At the end of treatment, it takes several weeks for the hormone function to return to normal ranges. At the same time, an excessive rebound effect can lead to renewed deterioration of the symptoms.
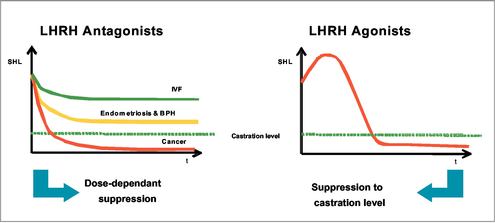
9
We believe that cetrorelix, an LHRH antagonist, because of its different mode of action, can avoid the side effects associated with the administration of agonists. Since LHRH antagonists have a rapid onset of action, the treatment time with cetrorelix can be much shorter than with agonists. Moreover, in various clinical studies, the effect of cetrorelix therapy lasted much longer than the hormone suppression, which consequently confirms the new therapeutic principle of intermittent treatment. Periods with moderate and well-tolerated hormonal suppression can be followed by intervals without treatment during which side effects are avoided. Since there is no necessity for long-term therapy and the overall treatment time is much shorter, the risks of side effects are also reduced. In particular, we also believe that the risk of developing osteoporosis in women taking the cetrorelix therapy regimen is diminished.
Cetrorelix might therefore be useful in a variety of malignant and non-malignant indications in which a suppression of the pituitary-gonadal axis is desired. The degree of suppression of gonadotrophins and sex steroids required is dependent on the clinical circumstances and disease treated. For example, in patients undergoing controlled ovarian stimulation (COS) for assisted reproductive techniques (ART), endogenous gonadotrophin secretion has to be controlled, whereas development of the follicle must not be adversely affected.
Cetrorelix inIn Vitro Fertilization (COS/ART)
Cetrorelix is the first LHRH antagonist which was approved for therapeutic use as part ofin vitro fertilization programs in Europe and was launched on the market under the trade name Cetrotide® (cetrorelix acetate) in 1999 by our partner Serono. In women who undergo controlled ovarian stimulation (COS) for recovery of ovocytes for subsequent fertilization, Cetrotide® prevents premature ovulation. LHRH is a naturally occurring hormone produced by the brain to control the secretion of LH and, therefore, final egg maturation and ovulation. Cetrotide® is designed to prevent LH production by the pituitary gland and to delay the hormonal event, known as the "LH Surge" which could cause eggs to be released too early in the cycle, reducing the opportunity to retrieve the eggs for the assisted reproductive techniques (ART) procedure.
In comparison with LHRH agonists that require a much longer pre-treatment, the use of our LHRH antagonist, Cetrotide®, permits the physician to interfere in the hormone regulation of the women undergoing treatment much more selectively and within a shorter time.
The effectiveness of Cetrotide® has been examined in five clinical trials (two Phase 2 and three Phase 3 trials). Two dose regimens were investigated in these trials: either a single dose per treatment cycle or multiple dosing. In the Phase 2 studies, a single dose of 3 mg was established as the minimal effective dose for the inhibition of premature LH surges with a protection period of at least four days. When Cetrotide® is administered in a multi-dose regimen, 0.25 mg was established as the minimal effective dose. The extent and duration of LH suppression was found to be dose dependent. In the Phase 3 program, efficacy of the single 3 mg dose regimen and the multiple 0.25 mg dose regimen was established separately in two controlled studies utilizing active comparators. A third non-comparative study evaluated only the multiple 0.25 mg dose regimen of Cetrotide®. In the five Phase 2 and Phase 3 trials, 184 pregnancies were reported out of a total of 732 patients (including 21 pregnancies following the replacement of frozen-thawed embryos). In these studies, drug related side effects were limited to a low incidence of injected site reactions; however, none of them was serious — like an allergic type of reaction — or required withdrawal from treatment. No drug-related allergic reactions were reported from these clinical studies.
Cetrotide® is the only LHRH antagonist that is available in two dosing regimens. With an immediate onset of action, Cetrotide® permits precise control — a single dose (3 mg), which controls the LH surge for up to four days, or a daily dose (0.25 mg) given over a short period of time (usually five to seven days). The treatment with Cetrotide® can be accomplished during a one-month cycle with a simplified, more convenient and shorter treatment requiring fewer injections than LHRH agonists.
Cetrotide® is marketed in a 3 mg and a 0.25 mg subcutaneous injection as cetrorelix acetate by Serono in the US and Europe. Approval for Cetrotide® in Japan is pending. The market competitor is ganirelix (Antagon™/Orgalutran®) from Akzo (Organon) indicated for the inhibition of premature LH surges in women undergoing controlled ovarian hyperstimulation.
10
Clinical Development Overview of Cetrorelix
In October 2004, cetrorelix completed an extensive seven Phase 2 trial program in urology and gynaecology, a significant part of which was sponsored by our partner Solvay Pharmaceuticals.
Cetrorelix in Benign Prostatic Hyperplasia (BPH)
BPH is a hormone-driven enlargement of the male prostate gland. The prostate is located directly at the vesicle outlet in the male surrounding the first part of the urethra. The enlargement puts pressure on the urethra, causing difficulty in urinating. BPH is classified into three stages according to symptoms: 1) the irritant phase, where the patient suffers dysuria (pain when urinating) and nocturia (the urge to urinate during the night); 2) residual urine occurring in the bladder thus increasing problems during urinating; and 3) overflow of the bladder. These can result in formation of bladder stones, congestion of urine, and engorged kidneys; which can in turn lead to life-threatening kidney damage. Enlargement of the male prostate is controlled by testosterone. Testosterone is generally responsible for the proper functioning of the prostate. With increasing age, testosterone can cause benign cell growth. The development of BPH is caused by an imbalance of testosterone and aging.
Because LHRH agonists decrease testosterone to castration levels, treatment of BPH with agonists is not convenient and therefore not the best approach. Drug therapy with plant-based drugs, alpha-receptor or alpha-reductase blocker is possible but the plant-based and alpha-receptor blockers cannot delay further prostate growth. They merely improve the symptoms in 50% of patients. Treatment with alpha-reductase blockers decreases the size of the prostate; however, this form of therapy is successful only in patients with a greatly increased prostate volume and only after a treatment period of at least 6 months. In contrast, cetrorelix improves the symptoms of BPH and reduces the size of the prostate after a short treatment period without chemical castration. The effects are independent of the prostate volume and are maintained for a long period following treatment withdrawal.
BPH Clinical Trials
All Phase 2 studies performed so far in patients with symptomatic BPH revealed that cetrorelix is therapeutically active in this indication as demonstrated by an improvement in symptoms as assessed primarily by the IPSS (International Prostate Symptom Score) as well as an increase in urinary peak flow rate and a reduction in prostate volume. Cetrorelix has been shown to suppress the formation of the male sex hormone testosterone, which plays a principal role in cell growth of the prostate.
On April 29 and May 25, 2004, we announced the results of two placebo-controlled Phase 2 trials that were conducted in BPH. As early as one month following initiation of therapy, both trials demonstrated improvement of clinical symptoms, classified and graded according to the IPSS which was paralleled by an increase in maximum uroflow in patients receiving cetrorelix treatment group, compared with patients on placebo group. The positive effect lasted three months without additional administration of cetrorelix. Furthermore, the use of cetrorelix was associated with a slight reduction of prostate size and moreover did not have an adverse influence on sexual activity or libido.
On October 7, 2004, we announced additional results for cetrorelix in BPH, which was a randomized, double-blind, placebo-controlled Phase 2 trial that enrolled patients with symptomatic and objectively defined BPH (decreased urine flow). This trial was conducted in Europe, under the coordination of Professor Frans MJ Debruyne from the Department of Urology, University Medical Center in Nijmegen. During a run-in period, all patients received two intramuscular injections of placebo, two weeks apart. Thereafter, 250 patients with persisting symptomatic BPH were randomized into five equal groups receiving either placebo injections or four different dosage regimens from 60 to 120 mg in two or three injections of a depot formulation of cetrorelix over the course of four weeks.
Patients were followed up for about six months after the last injection for efficacy and safety assessments, as well as for levels of testosterone and quality of life and sexual function. As early as one month following the initiation of therapy, the use of cetrorelix was associated with a dose-dependent, statistically significant improvement of clinical signs and symptoms, including IPSS and maximum uroflow, compared to placebo. Importantly, for all dosage regimens the therapeutic response lasted until the last observation point, i.e. 24 to 26 weeks following cessation of cetrorelix administration.
11
On March 16, 2005, we announced that our partners, Shionogi & Co., Ltd. and Nippon Kayaku Co., Ltd., are pursuing the development of cetrorelix by initiating the first Phase 2a trial in the Japanese market with cetrorelix in BPH. This trial will evaluate the safety (systemic and local tolerability) and explore efficacy (effects on BPH-related parameters such as the IPSS) of cetrorelix.
On January 30, 2006, we announced that we regained world wide rights (ex-Japan) from our partner Solvay to develop and potentially market Cetrorelix in BPH. This agreement was reached based upon Solvay's focus expertise and large presence in women's health. Therefore, Solvay will pursue the development of cetrorelix in endometriosis while we expect to initiate a late stage study in BPH in the United States either by ourselves or with a new pharmaceutical partner.
Cetrorelix in Endometriosis
Endometriosis is the estrogen-driven displacement of endometrium-like tissue (tissue from the mucous membranes of the uterus) to other organs outside the womb. In the abdomen, the tissue can spread to the fallopian tubes, the ovaries, the bladder, the small and large intestines, the stomach, the lungs or the legs. Estrogen-dependent diseases often regress when estrogen production is reduced (endometriosis, and the pelvic pain associated with it, improves when estrogen production is reduced). Excessive and prolonged, reduction of estrogen production, however, is typically associated with adverse side effects, such as vasomotor symptoms and bone loss.
A similar, very low estrogen-level can be induced by oophorectomy (surgical removal of the ovaries) and by chronic LHRH agonist treatment. In both cases, estrogen replacement treatment is necessary to reduce the hypo-estrogenic effects (e.g. bone loss, climacteric symptoms) associated with these therapeutic approaches. Administration of LHRH agonists can initially lead to a deterioration of symptoms due to the flare-up effect, then, due to the complete suppression of estrogen to below castration levels values for many months. These symptoms can further deteriorate upon withdrawal of hormonal replacement. The longer the treatment period with traditional LHRH agonists, the higher the risk of developing osteoporosis. Its use is therefore restricted to six months and can be extended only if estrogens and progesterones are administered concomitantly.
We believe that these side effects can be avoided with cetrorelix, as LHRH antagonist, therapy due to the absence of flare-up effects and to the possibility of controlling estrogen levels at values comparable to the ones observed at the beginning of the regular monthly cycle. Since the controlled hormone withdrawal is achieved in a very short period of time, complaints from monthly bleeding are reduced while inflammatoryfoci of endometriosis are depleted of their basis. Therefore, we believe that treatment time can be reduced. Initial experiences show that the effect of therapy persists for many months. Since the effect of cetrorelix starts within a short period of time and the risk of developing osteoporosis is low, we believe that cetrorelix therapy can be repeated in several cycles.
Endometriosis Clinical Trials
In earlier Phase 2 clinical trials, cetrorelix was given at a rate of 3 mg per week over a period of eight weeks. All patients were free of pain during the course of treatment. A second laparoscopy was performed after eight weeks and an improvement of the disease was shown in 60% of the cases. The efficacy was comparable to agonists but with the benefit of an almost complete absence of side-effects. Cetrorelix allowed targeted control of the hormone level to show rapid effects, while avoiding the problems of menopause and risks (e.g. osteoporosis) associated with an otherwise complete and long-term withdrawal of hormones. We believe that the rapid onset of action would be ideal for intermittent therapies, allowing for treatment-free intervals with re-dosing at the time when the therapeutic effect starts to fade.
12
On April 29, 2004, we announced the results of Phase 2 placebo-controlled studies demonstrating that cetrorelix use was associated with a rapid and durable therapeutic response, namely improvement of endometriosis-related symptoms, such as pelvic pain, extending up to several months following only two intramuscular injections of cetrorelix with a one month interval.
On March 16, 2005, we announced that our worldwide (ex-Japan) exclusive development and marketing partner, Solvay Pharmaceuticals, is conducting a full development program for the potential treatment of endometriosis with cetrorelix.
Cetrorelix in Uterine Myoma
As part of the seven Phase 2 programs, cetrorelix was also evaluated for the indication of uterine myoma. A uterus myoma is a benign tumor of the uterine muscles. If the entire uterine wall is penetrated by myoma, one refers to uterus myomatosus. Depending upon the length and the direction, it is either referred to as a subserous myoma, which is located below the peritoneal covering of the uterus and grows towards the intestinal cavity, or a submucous myoma, which is located below the mucous membrane and grows into the uterine cavity. The most frequent form however, is the intramural myoma bound in the muscular layer of the uterus. Intramural myoma leads to pain in the lower abdomen and in some cases to prolonged or severe monthly bleeding outside the normal cycle. This can cause severe blood loss leading to anemia. Infertility and pregnancy problems such as miscarriage or premature delivery are also frequent consequences. When the myoma puts pressure on the intestine or the bladder, the result can be constipation, bladder pain, or a desire to urinate. If the myoma exerts pressure on nerves leaving the spinal cord, the result can be back and neuralgic pain in the legs.
Uterus Myoma Clinical Trials
On April 29, 2004, we disclosed positive Phase 2 results from a double-blind, placebo-controlled, multi-center trial evaluating the subcutaneous formulation of cetrorelix, administered weekly for four weeks, as a pre-surgical treatment in 109 women with uterine myomas. In addition to evaluating the safety and tolerability of different doses of the new formulation, the trial also evaluated whether cetrorelix use could lead to the reduction of myoma and uterine volumes within a shorter treatment period than that normally required for LHRH agonists. Data from this trial demonstrated that cetrorelix use led to a reduction of myoma and uterine volumes after a one-month treatment period, which is significantly shorter than the two- to six-month treatment period typically required for LHRH agonists. The best response rate was obtained at a dose of 10 mg cetrorelix per week. Cetrorelix use did not lead to castration-like symptoms.
Partners for Cetrorelix
Cetrorelix has been licensed exclusively to Solvay Pharmaceuticals worldwide (except Japan) for all indications with the exception of IVF/COS/ART, which rights belong to Serono, and BPH for which we regained exclusive worldwide (except Japan) rights. Japanese market rights are held by Shionogi and Nippon Kayaku for all potential indications.
Competition for Cetrorelix
The market leaders in the indication of BPH are Pfizer, Boehringer Ingelheim and Abbott with alpha-receptor blockers and Merck Inc. with an alpha-reductase blocker. Worldwide, there are four LHRH agonists for the treatment of endometriosis, including TAP Pharmaceutical Products (Abbott and Takeda), Astra Zeneca, Sanofi-Aventis and Pfizer.
Ozarelix (D-63153)
Ozarelix is a modified LHRH antagonist which is a linear decapeptide sequence. Ozarelix is a 4th generation LHRH antagonist aiming at extended suppression of testosterone levels that does not require a sophisticated depot formulation for long lasting activity. The aim of this project is to identify an active substance with superior properties for the development of longer-acting formulations that we believe are particularly suitable for tumor therapy.
13
Single doses of ozarelix depot were tested in healthy male volunteers. Ozarelix was well tolerated and produced a dose-dependent suppression of testosterone. An immediate decrease in testosterone plasma levels were observed in all dose groups reaching levels below 1 ng/ml within the first 12 hours after application. Duration of suppression was dose-dependent and at the highest dose of 60 mg caused testosterone suppression for one month.
On August 12, 2004, we entered into a licensing and collaboration agreement with Spectrum Pharmaceuticals for ozarelix and its potential to treat hormone-dependent cancers as well as benign proliferative disorders, like BPH and endometriosis. The agreement comes after regaining worldwide rights for ozarelix from Baxter Healthcare. Under the terms of the agreement, we granted to Spectrum an exclusive license to develop and commercialize ozarelix for all potential indications in North America (including Canada and Mexico) and India while keeping the rights for the rest of the world.
Phase 2 trials have now been initiated in hormone-dependent prostate cancer and BPH and are fully funded by Spectrum Pharmaceuticals. The Phase 2 trial for prostate cancer will further assess the ability of ozarelix to suppress testosterone levels in a dose-dependent manner and related antitumor activity, based on testosterone decrease level (castration level<0.5 ng/ml). The double-blind placebo-controlled BPH Phase 2 trial will evaluate the efficacy of ozarelix as measured by its effects on clinical signs and symptoms characteristic of BPH, including the International Prostate Symptom Score (IPSS) and maximum uroflow, as well as the durability of therapeutic response over several months. Additionally, Spectrum has initiated in the US a Phase1/2 trial in prostate cancer. Æterna Zentaris is conducting the European trials with full access to the data and owns the rights for Europe and Asia.
Teverelix
Teverelix has been developed as a short-acting lyophilisate and a long-acting depot formulation. The product has completed Phase 1 clinical trials as a sustained-release form and the development costs are assumed by Ardana Bioscience Ltd., which has worldwide rights for the development and marketing of this compound. As part of the agreement, we will provide certain development services and supply clinical samples to Ardana. On April 2, 2004, Ardana extended its agreement with us and acquired full global rights and has been assigned the intellectual property relating to teverelix and the underlying microcrystalline suspension technology for use with LHRH antagonists. In return, we received an up-front payment at signature and will receive fixed annual guaranteed payments until end of 2006, as well as potential royalties on future sales of teverelix.
Development of a Non-Peptide LHRH Antagonist
As outlined above, the LHRH receptor plays an important role in a number of benign and malignant tumors. The Company's drug discovery unit searches for small, non-peptide molecules which have the same effect on the receptor. Their advantage lies in the potential for oral administration and the ability to be produced in a cost-efficient manner. They represent the next generation of LHRH antagonists. A drug based on these substances could be especially useful for the treatment of BPH, breast cancer and prostate carcinoma.
The development of new orally bioavailable LHRH antagonists for hormonal therapy has yielded some promising compounds. The project has advanced to a pre-clinical stage where thein vivo activity has been confirmed for two compounds.
We have exclusive worldwide rights for all therapeutic areas for this compound since the recovery from Solvay in December 2005 of the worldwide rights that were licensed out to them in January 2004 for endocrinological indications.
14
3.1.3 Signal Transduction Inhibitors
Perifosine
Perifosine is an alkylphosphocholine compound with structural similarity to phospholipids that are main constituents of cellular membranes and is an active ingredient with anti-tumor capacities. In tumor cells, perifosine has demonstrated interactions with vital signal transduction mechanisms and induction of programmed cell death (apoptosis).
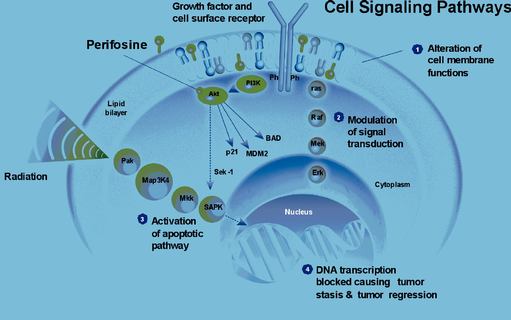
Perifosine exerts a marked cytotoxic effect on animal and human tumor cell lines. The most sensitive cancer cell lines were larynx carcinoma, breast, small cell lung, prostate and colon. Based on thein vitro trials, the mode of action of perifosine appears to be fundamentally different from that of currently available cytotoxics. Pharmacodynamic data have demonstrated that perifosine possesses antitumor activity, including tumor models that are resistant to currently available agents for cancer therapy. This activity is based on a direct and relatively specific action on tumors. A dose-relationship was also shown.
In preclinical and clinical Phase 1 trials (solid tumors), this orally administered agent has been found to have good tolerance. Five Phase 1 trials have been conducted on perifosine, including the trial presented at the June 2004 ASCO meeting.
In four trials, use of perifosine as a single agent in a total of 94 patients provided initial encouraging evidence of anti-tumor activity. In particular, investigators observed two partial responses (>50% reduction) in patients with sarcoma and sixteen stable diseases in patients with breast, prostate, pancreatic and other forms of cancer.
Based on findings in various tumor models, the U.S. National Cancer Institute (NCI), with our North American partner, Keryx Biopharmaceuticals Inc., investigated additional dosage regimens of perifosine in oncology patients. A number of screening Phase 2 studies examine perifosine as a single agent in several tumor types, including prostate, breast, pancreatic, head and neck, sarcoma and melanoma. Encouraging results showing anti-tumor activity were obtained in soft tissue sarcoma, breast and prostate cancers and lead to further development in these indications.
15
A proof-of-concept Phase 1 study of perifosine in combination with radiotherapy conducted by the NCI of the Netherlands was completed in 2004. Results from this trial were presented at ASCO 2004. A total of 21 radiotherapy-naïve patients, 17 of whom had advanced non-small cell lung cancer (NSCLC) and 14 had become refractory to prior chemotherapy, received oral perifosine doses ranging from 50 mg to 200 mg/day concurrently with standard doses of radiotherapy. The trial data demonstrated an acceptable safety and tolerability profile, with 150 mg/day established as the dose recommended for use in subsequent clinical trials. Also demonstrated was preliminary evidence of anti-tumor activity at all dosage levels, including complete or partial responses (complete disappearance and decreased tumor size, respectively), or stable disease, with a median follow-up for responders of eight months. Importantly, in the cohort of 10 patients who were treated with 150 mg/day, the established dose recommended for use in subsequent clinical trials, there were three complete responses, three partial responses, and four patients with stable disease.
On September 22, 2005, we announced the commencement of a Phase 2 clinical study of perifosine in combination with radiotherapy in patients suffering from non-small cell lung cancer. This is a randomized, double-blind, placebo-controlled trial to assess the efficacy and safety of a 150 mg daily dose of perifosine when combined with radiotherapy in 160 patients with inoperable Stage III NSCLC. The trial is being conducted in collaboration with the Netherlands Cancer Institute. The lead investigator is Marcel Verheij, MD PhD, of the Department of Radiation Oncology / Division of Cellular Biochemistry, at The Netherlands Cancer Institute in Amsterdam.
In addition, perifosine is also being evaluated in over 10 clinical trials as a single agent as well as in combination with chemotherapeutic agents and biologic agents by our North American partner, Keryx Biopharmaceuticals.The following are the ongoing trials sponsored by Keryx:
Phase 1 with gemcitabin in multiple cancers
Phase 1 with paclitaxel in multiple cancers
Phase 1 with docetaxel in multiple cancers
Phase 2 in NSCLC
Phase 2 with Herceptin® in breast cancer
Phase 2 all comers
Phase 2 endocrine therapy (with tamoxifene) in breast cancer
Phase 2 sarcoma
Phase 2 with Glivec® in sarcoma
Phase 2 soft tissue sarcoma
Phase 2 refractory leukemia
Phase 1 and 2 refractory multiple myeloma
Partners for Perifosine
A Cooperative Research and Development Agreement (CRADA) was put in place with the NIH/NCI in May 2000. A cooperation and license agreement was signed in September 2002 with the US company, Access Oncology, Inc. (AOI), for the use of perifosine as an anticancer agent covering the United States, Canada and Mexico. In January 2004, AOI was acquired by Keryx Biopharmaceuticals, which is pursuing the clinical development of perifosine under the same conditions as AOI. The agreement, in particular, provides us free access to all data from Keryx Biopharmaceuticals and its partner's studies, as well as milestone payments and scale-up royalties to be paid to us on future net sales of perifosine in North America. We own rest of the world rights of perifosine.
Erucylphosphocholine (ZEN-027)
On January 6, 2005, we announced the initiation of preclinical development of erucylphosphocholine (ZEN-027), an analog of perifosine which is suitable for intravenous administration. Like perifosine, ZEN-027 belongs to a new class of compounds based on alkylphosphocholines. ZEN-027 possesses distinctive reduced hemolytic activity thus allowing for intravenous injection.
16
On January 6, 2005, we also licensed to Keryx Biopharmaceuticals, our current North American partner for perifosine, certain rights to develop and market ZEN-027 in North America, South Africa, Israel, Australia and New Zealand while keeping rights for the rest of the world. According to the agreement with Keryx, the preclinical development costs of ZEN-027 are shared between Keryx and Æterna Zentaris.
Other Signal Transduction Inhibitors
In addition to our activities with alkylphosphocholines, we are seeking for small molecule agonists and antagonists to lipid protein signaling interactions that are new and potentially important therapeutic targets.
On January 6, 2005, we announced the acquisition of Echelon, a biotech company based in Salt Lake City, Utah. Echelon's product pipeline is focused on the rapidly emerging field of transduction signaling technology. Echelon develops small molecule agonists and antagonists to enzyme targets and lipid-protein signaling interactions that are new and potentially important therapeutic targets.
We are focusing our efforts on single and dual inhibitors of Ras-Raf-Mek-Erk and PI3K-Akt pathways. The Ras-Raf-Mek-Erk and the PI3K-Akt pathways are constitutively activated in many cancer types, and influence both tumor development and progression.
Both signaling pathways represent promising therapeutic targets for the treatment of tumors. We have now identified a new compound class with inhibitory activity against both the Erk and PI3K kinases. These small molecules inhibit the kinases at nanomolar concentrations in a dose dependent manner by competing directly at the ATP binding site. In a broad kinase panel, the molecules are very selective against other kinases. In cellular experiments the compounds inhibit the activation of downstream targets Akt and Rsk1, and can stop the proliferation of various human cancer cell lines. We are currently performing firstin vivo studies with the frontrunner compounds. Further optimization of the lead class is ongoing with respect to pharmacokinetic parameters, in order to select a development candidate as soon as possible.
Furthermore, our subsidiary Echelon operates a fine reagents business offering state-of-the-art and novel reagents and assays for the characterization and research of cellular phospholipids which are top-level regulators in critical intra-cellular cell-signaling cascades. Echelon's reagents and kits allow for primary and applied research in these signaling cascades in both academia and in the biopharmaceutical industry.
Miltefosine
Miltefosine, marketed under the brand name Impavido®, is the only oral drug for the treatment of visceral leishmaniasis, which is also known as black fever. Leishmaniasis is a parasitic infection which is prevalent in tropical regions but which also occurs repeatedly and with an increasing tendency in industrialized countries in HIV-infected people. According to the World Health Organization (WHO), 12 million people are affected globally. The number of new cases annually is estimated to be 1 to 1.5 million people. Leishmaniasis is present in more than 88 countries worldwide. Regions most greatly affected are the Indian subcontinent, South America, the Middle East, North Africa and some areas of Central Africa.
Depending on the strain of leishmania, which is transmitted by sandflies, the disorder can be present in the following forms:
Cutaneous Leishmaniasis (CL): In the cutaneous form, this disease occurs most frequently in North and Central Africa, the Middle-East and South America. The skin initially forms protuberances (skin lesions) around the sites of the mosquito bite which can open like ulcers after several weeks or months. Although this form of leishmaniasis is not life-threatening and does not necessarily require medication, drug therapy can accelerate healing and help to prevent formation of scars. However, in about 10% of patients, the infection takes a chronic course and requires drug therapy.
17
Visceral Leishmaniasis (VL): This infection usually has a subacute or chronic course and particularly affects the liver, spleen, bone marrow and lymph nodes. As a consequence, the patient has a wide variety of general symptoms, e.g. recurrent fever for many weeks, severe enlargement of the spleen and liver, disturbances of the hematopoietic system and blood coagulation, as well as severe emaciation (cachexia). This is the most dangerous form of leishmaniasis which, when untreated, leads to death within six months to two years following the outbreak of the disease. Visceral leishmaniasis occurs in Asia, in particular in India, Bangladesh and Nepal, in Brazil and in Central Africa. There is an emergence of cases in the Mediterranean countries where it usually occurs as a co-infection with HIV. In addition, climate researchers estimate in a recent report a distribution to central Europe because of the climate shift.
In developing countries with poor medical care, miltefosine could significantly reduce hospital treatment. Because it is an oral anti-infective, secondary infections (e.g. co-infection with HIV) associated with the use and possible re-use of syringes can be eliminated.
Miltefosine in Clinical Trials
A clinical development program was initiated under the supervision of the Special Programme for Research and Training in Tropical Diseases (TDR) of the WHO and the United Nations Development Program (UNDP) in visceral leishmaniasis. A dose-ranging and pharmacokinetic Phase 1/2 study and a large Phase 3 trial comparing miltefosine with Amphotericin B were performed in adult patients. In addition, dose-ranging and pharmacokinetic studies, and a confirmatory Phase 3 study, were conducted in children. Across all age groups, miltefosine was found to be equally active in patients with newly diagnosed leishmaniasis and in patients with infections unresponsive to prior standard therapy.
Currently used antimony-based standard therapy may have resistance rates of up to 80%, for instance, in the most affected parts of India, as well as severe side effects such as cardiotoxicity and nephrotoxicity may be encountered. Impavido® was shown to have a cure rate of 95% even in those patients who were resistant to the antimony-based pre-treatment. Impavido® is the first orally applicable medication to treat visceral leishmaniasis. The side effects were generally tolerable and short-lasting (episodes of vomiting, nausea, and diarrhea). Impavido® is even suitable for children, who account for one third of all cases.
In comparison with the side effects of traditional drugs (cardiac arrhythmia, inflammation of the pancreas, fever and blood abnormalities), the side effects of miltefosine are less severe. Other drugs, like liposomal amphotericin B, which are better tolerated, have to be administered via an injection and are virtually unaffordable for patients living in the affected regions. The phenomenon of resistance is increasingly observed even with administration of high doses of conventional drugs to treat infections. Considering the oral route of administration that does not require hospitalization, the treatment with Impavido® is very cost-effective. This is an important issue as 90% of the patients with visceral leishmaniasis live in countries with limited access to medical facilities/treatment: Bangladesh, Brazil, India, Nepal and Sudan. In addition, the oral route prevents HIV co-infection during intravenous treatment for leishmaniasis, which is a significant problem in developing countries. Impavido® has also proven to be effective in cutaneous leishmaniasis and in HIV patients co-infected with visceral leishmaniasis. Thirty-nine (39) cases of HIV co-infected patients in Europe, who were not controlled by state-of-the-art treatment, received miltefosine on a compassionate basis and showed encouraging therapeutic effects.
The results of a Phase 4 study with over 1,100 patients from India were analysed in 2005. In this study, patients were treated under an outpatient setting and analyses show a similar cure rate compared with pre-registration trials in which the drug was tested in hospitalized patients. The report concluded that miltefosine is suitable for use in a public use program with patients receiving treatment on an outpatient basis. This is an important milestone in order to extend the use of Impavido® to the nationwide leishmaniasis control program in India, but also for other territories.
In addition, the international medical humanitarian organization, Médecins Sans Frontières (MSF), has launched a large study of Impavido® in Ethiopia where visceral leishmaniasis with or without HIV co-infection is a major health burden. A study for visceral leishmaniasis in Brazil targeting the efficacy of the product in new world leishmania strains started during 2005.
18
In early 2005, it was found in a Phase 3 trial conducted in Venezuela, Brazil, Colombia and Guatemala that Impavido® accelerates the healing process in cutaneous leishmaniasis. Compared with patients on placebo, the cure rate in patients using Impavido® was significantly (220%) better. A follow-up trial in Bolivia addressed mucosal CL which is a particularly mutilating and difficult-to-treat form of CL occurring in South American countries which can progress to destruction of the entire nose and further parts of the face. Currently, all targeted patients have been treated and they are in the follow-up phase. The NGO HealthNet has started a study in Afghanistan to compare oral Impavido® with other traditionally used modalities in this country where CL recently increased dramatically.
Registration Status
Impavido® is the first oral formulation and has to be administered for 28 days. The Company received approval for Impavido® for the treatment of visceral leishmaniasis in India in 2002 and in Germany in 2004. Furthermore, in 2005, we received approval to market Impavido® in cutaneous and visceral leishmaniasis in Columbia, Argentina, Guatemala, Paraguay and Ecuador. Orphan drug status was granted by the European Agency for the Evaluation of Medicinal Products (EMEA) in 2002.
Partners for Impavido® (Miltefosine)
Impavido® is partnered with German Remedies in India and Bangladesh. It is also partnered with Roche for its distribution in Brazil, and Nimrall in Pakistan and Afghanistan. An agreement was signed for South America (excluding Brazil) with the company Tecnofarma. In Germany, distribution of the registered product will be carried out by our partner Paesel + Lorei. More partnerships are currently being negociated to ensure a fast registration and marketing of this innovative product.
3.1.4 Cytotoxic Conjugates and Cytotoxics
Cytotoxic Conjugates
In view of the non-specific toxicity of most chemotherapeutic agents against normal cells, targeting such drugs to cancerous tissue offers a potential benefit for patients with advanced or metastatic tumors. Targeted cytotoxic peptide conjugates are hybrid molecules composed of a cytotoxic moiety linked to a peptide carrier which binds to receptors on tumors. Cytotoxic conjugates are designed to achieve differential delivery, or targeting, of the cytotoxic agent to cancer vs. normal cells.
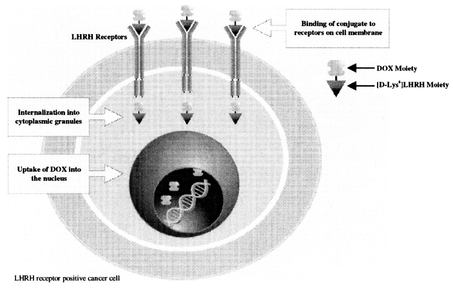
19
Our cytotoxic conjugates represent a novel oncological strategy to control and reduce toxicity and improve the effectiveness of cytotoxic drugs. The development strategy was to create targeted conjugates with high cytotoxic activity based on doxorubicin (DOX), an approved and commercialized product or 2-pyrrolino-DOX which is 500 to 1,000 times more active then the parent compound. We are developing several candidates in which doxorubicin or 2-pyrrolino-DOX were coupled to the peptide carriers targeting LHRH (AN-152 & AN-207), somatostatin (AN-238) or bombesin (AN-215) receptors. These conjugates are less toxic and more effectivein vivo than the respective radicals in inhibiting tumor growth in LHRH receptor-positive models of human ovarian, mammary, or prostatic cancer.
In AN-152, the most advanced of the cytotoxic conjugates, doxorubicin is chemically linked to an LHRH agonist, a modified natural hormone with affinity for the LHRH receptor. This design allows for the specific binding and selective uptake of the cytotoxic conjugate by LHRH receptor positive tumors. Potential benefits of this targeted approach are manifold, and include a more favorable safety profile with lower incidence and severity of side effects, as normal tissues are spared from toxic effects of doxorubicin. In addition, the targeted approach may enable treatment of LHRH receptor positive cancers that have become refractory to doxorubicin given in its non-targeted form.
In preclinical studies conducted to date in several animal models of LHRH receptor positive human cancer cell lines, AN-152's anti-tumor activity and tolerability were shown to be superior to that of doxorubicin. As would be expected, AN 152 was not active or was significantly less active than doxorubicin in LHRH receptor negative cancer cell lines. On January 18, 2005, we announced the initiation of a company-sponsored Phase 1 dose-ranging study with this targeted anti-cancer agent AN-152 and we expect to disclose the Phase 1 results in 2006.
Lobaplatin
Lobaplatin is a platinum derivative that has demonstrated lower toxicity in preclinical studies compared with cisplatinum, specifically renal toxicity, and incomplete cross-resistance with other platinum derivatives suggesting potential therapeutic use even in tumor indications not routinely treated with platinum derivatives.
Clinically, lobaplatin was well tolerated at recommended dosages. Treatment was not associated with typical side effects often seen with cisplatinum, such as nephrotoxocity (impairment of kidney function), otoxicity (loss of hearing capacity), neurotoxicity (effects on sensory function). In addition, vomiting was less severe than published data from both cisplatinum and carboplatinum. Characteristic toxicity of lobaplatin is a short-lasting, spontaneously reversible drop in thrombocyte count (blood platelets).
In a Phase 2 study conducted in China that included 284 patients with a broad range of solid and non-solid tumors, safety and particularly good therapeutic efficacy were demonstrated in patients with breast cancer, small cell lung cancer (SCLC), and chronic myeloid leukemia (CML) (a cancer of the hematopoietic system). The primary endpoint in solid tumor patients was the remission rate according to WHO criteria, while response in CML was assessed according to the disease-specific criteria of Talpaz. The favorable results of this study were the basis for approval of the product in China including all three indications: breast cancer, SCLC, and CML.
In China, lobaplatin has been approved by the Chinese health authorities for the treatment of inoperable, advanced breast cancer, SCLC and CML. In December 2002, we signed a contract with Hainan Chang An Pharmaceuticals Ltd. for the marketing in China of lobaplatin. The contract includes the worldwide manufacturing rights of lobaplatin by Hainan Chang An Pharmaceuticals. The technology transfer agreement provided for a first payment to us upon signature and a later manufacturing-related payment.
Disorazol compounds
Disorazol compounds are being developed for the treatment of cancer. They are aromatic polyketides isolated from the bacteriumSorangium cellulosum. Their mechanism of action is still being elucidated, although they have demonstrated a potent activity at picomolar concentrations. The Disorazol compounds are being investigated as single agents and their development is currently at the preclinical stage.
20
3.1.5 Tubulin Inhibitors / Vascular Targeting Agents
Development of a Low Molecular Weight Tubulin Inhibitor
Tubulin is a protein found in all cells that plays an important role during cell division, in that it helps to transmit genetic information to the daughter cells. Inhibition of this process leads to death of the affected cell. The anti-tumor agents taxol and vincristine, which are widely used in cancer therapy, are based on this principle. Both compounds are expensive natural substances and cause severe side effects when used in humans.
We are currently identifying and developing novel tubulin inhibitors which, compared with currently used products, exhibit in animal models improved efficacy, have a more acceptable side effect profile, an incomplete or no cross-resistance and are administered orally.
ZEN-012 and ZEN-017 are drug development candidates with an excellent tolerability profile showing excellentin vivo activity in various tumor models including mammary, colon, melanoma and leukemia cancers after per os administration. This compound expresses different modes of action. Strong anticancer activity is combined with pro-apoptotic and anti-angiogenic properties. ZEN-012/017 inhibits the polymerization of cancer tubulin rather than bovine brain tubulin, and it destroys the mitotic spindel of the cancer cells. ZEN-012/017 arrests the cancer cells in the G2M phase at a nanomolar concentration and induced apoptosis. ZEN-012/017 is not cross-resistant to cisplatin, vincristine and doxorubicine in cell lines resistant to these drugs. With this profile of activity, ZEN-012/017 is a promising candidate for further preclinical development.
We have discovered a novel pyrazole derivative, ZEN-014, that inhibits tubulin polymerization. It represents a new class of small molecule tubulin binders with antiangiogenic properties which are assumed to be novel, highly-potent anticancer drugs. The treatment with non-toxic concentrations of ZEN-014 inhibits endothelial cell sprouting and vessel formation. Cancer cells were arrested completely in the G2M phase of mitosis at nanomolar concentrations and subsequently underwent apoptosis. Several apoptotic parameters such as cell membrane alterations, increase of caspase 3 and 7 activity, DNA fragmentation and inactivation of the Bcl-2 protein are detectable in U937 cancer cells after treatment with nanomolar concentrations of ZEN-014. The compound shows an excellent antitumor activity profile in a broad panel of tumor cell lines including paclitaxel and vincristine resistant cells. ZEN-014 exhibits promisingin vivo activity in a renal cell carcinoma model at a dose of 50 mg/kg after oral application.
Æ-941 (Neovastat®)
Æ-941 (Neovastat®) is an oral antiangiogenic product with multiple mechanisms of action. Studies have presented evidence supporting the antiangiogenic activity at different stages of the angiogenesis process, such as selectively inhibiting matrix metalloproteinases (MMPs 2, 9 and 12), blocking the action of VEGF to its receptor, inducing apoptosis (cellular death) of the endothelial cells, and inducing the production of tissue type Plasminogen activator (TPa) by endothelial cell located within the tumor area.
Phase 3 Clinical Trial in Lung Cancer Sponsored by the U.S. NCI
In September 1998, Æ-941 (Neovastat®) was selected by the NCI as a drug candidate to assess the potential of a blocker of angiogenesis in the treatment of lung cancer. The agreement with the NCI includes the realization of a double-blind, randomized, placebo-controlled Phase 3 trial. This study is sponsored by the National Cancer Institute (NCI) of the United States. According to the terms of this agreement, we are responsible for supplying Æ-941 (Neovastat®) for the entire duration of the study, while the data will be provided to us by the NCI for a registration dossier.
21
This Phase 3 trial is being conducted in hospitals and research centers in the United States and Canada under the supervision of the MD Anderson Collaborative Community Oncology Program. 760 patients (approximately 380 were recruited as of December 2005) with newly diagnosed non-metastatic non-small-cell lung cancer ("NSCLC") need to be enrolled in this trial. All patients receive standard chemotherapy and radiotherapy treatments. Patients are randomly assigned to oral Æ-941 (Neovastat®) or placebo. The primary endpoint is improvement of the median survival time. On February 17, 2006, we announced an update on the NCI sponsored Phase 3 trial with Neovastat® in NSCLC. Following a Data and Safety Monitoring Board recommendation, based solely on slow patient recruitment rate, the NCI has decided to interrupt patient recruitment for the ongoing Phase 3 trial while awaiting an interim efficacy data analysis planned at 320 events.
RC-3095
RC-3095 is an antagonist to a growth factor, Bombesin, present in various tumors, in particular in small-cell lung cancer (SCLC), but also in pancreatic carcinoma, breast cancer and tumors of the gastrointestinal tract. It appears to play a significant part in the regulation of epidermal growth factor (EGF) and gastrin receptor expression. The blockade of the bombesin receptor may therefore be an effective way to control the growth of certain tumors. RC-3095 is a hormone-like peptide that is being developed for multiple types of cancers. As a gastrin-releasing peptide inhibitor, the compound has proven angiogenesis inhibitionin vivo and down regulation of HER-2 receptor. RC 3095 was tested in several cancers such as small cell lung, pancreatic, colorectal, breast and prostate.
In a Phase 1 trial in patients with various solid tumors, the subcutaneous injection of RC-3095 up to the highest dose level tested was tolerated without clinically relevant side effects; systemic tolerability of RC-3095 was very good. Although tumor response was not a primary endpoint in Phase 1, patients with different tumor types showed clinical response. Based on these Phase 1 data, additional studies are exploring the activity of RC-3095 as a monotherapy in SCLC and prostate cancer. Additional preclinical data may be needed to guide the optimum integration of RC-3095 into drug combination regimens.
3.1.6 GH-RH Modulators
Development of a Growth Hormone Secretagogue
Growth hormone secretagogues (GHS) represent a new class of pharmacological agents which directly stimulate growth hormone (GH) secretion from the pituitary gland without the involvement of growth hormone-releasing hormone (GH-RH) or somatostatin. There is no GHS on the market yet. Since GH is a potent regulator of lipid, sugar and protein metabolism, the potential clinical uses of GHS are numerous. They include growth retardation in children and treatment of cachexia in AIDS patients, which are currently the only approved uses of therapy of GH. The administration of GH, which has to be injected every day, is cumbersome. Therefore, there is a need for new orally active drugs like GHS.
As part of our university collaboration, we accessed new peptidomimetic compounds with GH secretagogue properties. The lead development candidate, EP-1572, is a novel peptidomimetic GH secretagogue (GHS) with potent and selective GH-releasing activity in humans. EP-1572 underwent limited clinical pharmacology tests which demonstrated a potent stimulation of the GH secretion after oral administration in human volunteers. This product has been licensed to Ardana, which initiated an open, randomized, placebo-controlled Phase 1 dose ranging study in April 2004. Thirty-six (36) healthy subjects were included in this study to receive either the reference hormone GH-RH by I.V. route or one of the following dose levels of EP-1572: 0.005, 0.05 or 0.5 mg/kg by oral route. EP-1572 at the dose of 0.5 mg/kg orally caused an increase in growth hormone release equivalent to that induced by GH-RH intravenously. The compound was well tolerated and no other hormones showed a significant modification after any dose of EP-1572.
22
Ghrelin Receptor Antagonists
Ghrelin is a natural peptide hormone, a peptidic linear molecule of 28 amino acids, and the stomach is recognized as the major source of circulating ghrelin. It is mainly expressed from the neck to the base of the oxyntic gland of the stomach and its levels progressively decline along the gastroinstestinal tract. The expression is not confined to the gastrointestinal system, but is variably present in different tissues.
Ghrelin appears to be under physiological control and acts on the central nervous system (CNS) to stimulate food intake, induces accumulation of fat tissue and its controlled reduction may be a valid therapeutic option. Antagonists of ghrelin receptor binding are therefore seen as a potential treatment of obesity through the modulation of CNS control of gastric function. The use of ghrelin antagonists as appetite suppressants could open up new opportunities for the treatment of obesity. In addition to the field of obesity, ghrelin could have therapeutic benefits for other potential indications, such as metabolic and cardiovascular diseases, as well as cancer.
In 2004, we established a research collaboration agreement with the Centre National de Recherche Scientifique (CNRS) and the University of Montpellier (France) pursuant to which new chemical entities with potential ghrelin receptor antagonist properties are expected to be synthetized. According to the agreement, we have the rights to develop and exploit worldwide the new compounds for any indication. Compounds with the most potent affinity for the ghrelin receptor will be investigated further through an international network of academic investigators with expertise in the field of endocrinology in order to identify clinical development candidates.
In August 2005, a first patent application was filed by Æterna Zentaris to protect a series of new chemical entities characterized as ghrelin receptor ligands.
GH-RH Antagonists
GH-RH is a hormone secreted in the brain by the hypothalamus that acts on the pituitary gland to stimulate the synthesis and the release of growth hormone (GH). Many tumor types are potentially dependent on levels of GH and insulin-like growth factors, IGF-I and IGF-II, which stimulate cell proliferation while inhibiting programmed cell death (apoptosis).
GH-RH antagonists represent a potential novel class of promising anti-cancer agents that may offer distinct advantages compared to other classes of anti-tumor agents, with utility in a variety of tumor types. GH-RH antagonists possess the ability to exert both direct (by blocking GH-RH receptors on tumor cells) and indirect (by blocking the secretion of GH from the pituitary and thereby suppressing the production of IGF-I in the liver) anti-proliferative effect. Early evidence for the anti-tumor activity of GH-RH antagonists was provided by research conducted at Tulane University, which demonstrated that GH-RH antagonists inhibit the growth of a broad range of cancer cell lines, including pancreatic, colorectal, prostate, breast, renal, small-cell/non small-cell lung cancer, osteosarcoma and glioblastoma. Importantly, GH-RH antagonists were shown to have a direct anti-proliferative effectin vitro on certain cancer cell types, an action that is thought to be mediated by the presence of locally-produced GH-RH, which may act as an autocrine growth factor, and its receptors in the respective cancer cell lines. GH-RH antagonists also inhibit indirectly the production of IGF-I and IGF-II in tumors.
3.1.7 Immunotherapy / Vaccines
Cellular proteins expressed by oncogenes have been recognized as a major cause of tumor development. One of the central oncoproteins involved in cancer formation are the Raf proteins. Based on these proteins, new unique therapeutic strategies, new predictive animal models and new development products have been generated to efficiently combat cancer. These consist of virulence attenuated, gene modified bacteria expressing oncoproteins or enzymes. Such bacteria are used for vaccination as well as tumor targeting and delivery of antitumoral compounds towards the tumor tissues. This new vaccine approach therefore exploits the ability of bacteria to induce potent immune responses as well as direct these responses against malignancies. The immunogenicity of the vaccine will be further enhanced by the capacity of bacteria to colonize tumor tissues. This property will be used to transport substances, e.g. proteins, into the tumor tissue, which are capable of converting non-toxic pro-drugs into active drugs. The use of bacterial carriers for therapeutic vaccination against tumors and the concept of bacterial tumor targeting will be further developed with the Julius-Maximilians-University including the highly recognized researchers Prof. Dr. Ulf R. Rapp, who is member of our Scientific Advisory Board, and Prof. Dr. Werner Goebel. Prof. Rapp is a known expert in the field of cell and tumor biology and Prof. Goebel is a pioneer in the field of vaccines based on recombinant bacteria.
23
The preclinical proof of principle has already been shown in a transgenic animal model and is supported by several patent applications. The first expected targets for this research project would be the development of vaccines against prostate cancer and melanoma.
3.1.8 Drug Discovery
There is an increasing demand on the world market for active substances. Our internal drug discovery unit provides an important prerequisite for the provision of new patented active substances, which can then be developed further or licensed to third parties.
The drug discovery unit concentrates on the search for active substances for innovative targets which open the door to the introduction of new therapeutic approaches. Furthermore, this unit searches for new active substances having improved properties for clinically validated targets for which drugs are already being used in humans and which produce inadequate effects, cause severe side effects, are not economical or are not available in a patient-friendly form.
To this end, we possess an original substance library for the discovery of active compounds with a comprehensive range of promising natural substances which can serve as models for the construction of synthetic molecules. The initial tests involve 120,000 samples from our internal substance library in the form of high-throughput screening. The hits, i.e. the first active compounds found in the library, are tested further and built up specifically into potential lead structures. Based on two to three lead structures, they are then optimized in a further step to potential development candidates.
As a complement to these activities, our acquisition of Echelon has provided novel biological targets in the lipid signaling pathway. In addition, Echelon has developed numerous biological assays that will permit complementary and synergistic testing of our library of compounds.
3.1.9 Strategic Alliances
Cetrorelix
Ares Trading S.A. (Serono International S.A.), Vaumarcus, Switzerland: Serono holds an exclusive worldwide license (except Japan) to commercialize Cetrotide® (cetrorelix in the indication IVF/COS/ART). This agreement provides the Company, amongst other things, with manufacturing income, royalties on worldwide (except Japan) net sales as well as fixed annual lump sum payments until 2010. After 2010, these fixed annual lump sum payments will become high double digit royalties on the net worldwide sales of Cetrotide® (except Japan) and the remainder of the agreement will continue as is.
Solvay Pharmaceuticals Bv., Weesp, Netherlands: Since September 2002, Solvay obtained an exclusive license to develop, use, commercialize and manufacture cetrorelix worldwide with the exception of Japan and for all indications except for IVF/COS/ART and, as announced in January 2006, for BPH. Solvay undertakes, at its own cost, all activities necessary to obtain regulatory and marketing approvals for cetrorelix. Additionally, the agreement provides milestones payments and low double digit royalties on future worldwide (except Japan, as well as sales in IVF/COS/ART and BPH) net sales of cetrorelix.
24
Shionogi & Co. Ltd. and Nippon Kayaku Co. Ltd. of Japan signed two license and distribution agreements. They were granted a semi-exclusive license for Japan to commercialize cetrorelix. Shionogi & Co. Ltd. and Nippon Kayaku Co. Ltd. also obtained a semi-exclusive license for Japan for the development of cetrorelix for human use.
Ozarelix (D-63153)
Spectrum Pharmaceuticals Inc., Irvine CA, USA: On August 12, 2004, we entered into a licensing and collaboration agreement with Spectrum Pharmaceuticals for the LHRH antagonist ozarelix. Under the terms of the agreement, we granted Spectrum an exclusive license to develop and commercialize ozarelix for all potential indications in North America (including Canada and Mexico) and India. We received an upfront payment which included cash and Spectrum's equity, at signing, and we are eligible to receive payments upon achievement of certain development and regulatory milestones, in addition to royalties (scale-up royalties from high single to low double digit) on potential net sales. We have retained exclusive rights for the rest of world and will share with Spectrum upfront and milestone payments, royalties or profits from potential sales in Japan.
Teverelix
Ardana Bioscience Ltd., Edinburgh, Scotland: In 2002, Zentaris granted an exclusive license to Ardana to develop and commercialize teverelix for all therapeutic uses worldwide with the exception of Japan, Korea and Taiwan. On April 2, 2004, Ardana acquired full worldwide rights and was assigned the intellectual property rights relating to teverelix and the underlying microcrystalline suspension technology for the use of teverelix and LHRH antagonists. The agreement provides, amongst other things, signature payment, annual guaranteed payments until December 2006 and royalties (low single digit) on future worldwide net sales.
A license and cooperation agreement withTeikoku Hormone, Japan, granting an exclusive license to develop and commercialize teverelix for certain indications (excluding the IVF/COS/ART indication) for Japan, Korea and Taiwan was terminated on October 14, 2003.
LHRH Peptidomimetics
Solvay Pharmaceuticals Bv., Weesp. Netherlands: In December 2005, we reached an agreement with Solvay Pharmaceuticals whereby we regained exclusive worldwide rights for all endocrinological indications that had been granted to Solvay in January 2004. We now have worldwide rights for all indications in all therapeutic areas for this class of compound.
Perifosine
Following the acquisition of AOI Pharma, Inc. in January 2004 byKeryx Biopharmaceuticals, New York, USA, Keryx Biopharmaceuticals has taken over the license and co-operation agreement signed withAOI Pharma, Inc., New York, USA. Keryx Biopharmaceuticals will undertake, at its own cost, all clinical activities necessary to obtain regulatory and marketing approvals of perifosine for all uses in the United States, Canada and Mexico. The agreement provides, amongst other things, availability of data generated by all parties free of charge, milestones and scale-up royalties (from high single to low double digit) on future net sales in the United States, Canada and Mexico.
Miltefosine (Impavido®)
Impavido® is partnered withGerman Remedies in India and Bangladesh. It is also partnered withRoche for distribution in Brazil andNimrall in Pakistan and Afghanistan. An agreement was signed for South America excluding Brazil with the companyTecnofarma. In Germany, distribution of the registered product will be effected by our partnerPaesel + Lorei. More partnerships are currently under negotiations to ensure a fast registration and marketing of this innovative product.
25
Erucylphosphocholine (ZEN-027)
On August 26, 2004, we licensed certain rights toKeryx Biopharmaceuticals, New York, USA to develop and market ZEN-027 in North America, South Africa, Israel, Australia and New Zealand while keeping rights for the rest of the world. The agreement provides, amongst other things, availability of all data generated by all parties free of charge, milestones and scale-up royalties (from high single to low double digit) on future net sales in the United States, Canada, Israel, New Zeland, Australia and South Africa.
Lobaplatin
On January 8, 2003, the Company announced that Hainan Tianwang International Pharmaceutical (now Hainan Chang An Pharmaceuticals) signed a contract for the manufacture and marketing in China of lobaplatin. The transfer agreement provided us with a one-time payment. The Company owns the worldwide rights (except China) on lobaplatin.
Æ-941 (Neovastat®)
On December 22, 2004, We announced an agreement with our subsidiaryAtrium Biotechologies Inc. whereby we transferred worldwide rights to Atrium's Health and Nutrition division, excluding North America, to market and distribute our antiangiogenic product Neovastat®. The existing marketing partnerships for Neovastat® withGrupo Ferrer, LG Life Sciences and Mayne Pharma remain in place and are managed by Atrium.
Growth Hormone Secratogogue (GHS)
Ardana Bioscience Ltd., Edinburgh, Scotland: In 2002, Ardana was granted an exclusive worldwide license to develop and commercialize the growth hormone secretagogue EP-1572. Ardana undertakes, at its own cost, all activities necessary to obtain regulatory and marketing approvals for the substance. Furthermore, the agreement provides, amongst other things, milestone payments as well as low double digit royalties on future worldwide net sales of EP-1572.
In addition, we have entered into the following collaborative agreements:
We signed license agreements dated September 17, 2002 with the Tulane Educational Fund (Tulane University, New Orleans, Louisiana, USA) with regard to the substances AN-152, AN-201, AN-238 and AN-215 and to bombesin antagonists. Under the agreements, Zentaris obtained exclusive worldwide licenses to use Tulane's patents to develop, manufacture, market and distribute these substances.
On April 21, 2005, we announced a new research collaboration with Würzburg/Germany-based Julius-Maximilians-University on the development of tumor vaccines based on attenuated bacterial carriers. We also acquired patent rights from the university and the inventors covering several aspects of both immunotherapeutic approaches against cancer as well as bacterial tumor targeting. The goal of this collaboration would be the development of vaccines against prostate cancer and melanoma.
Two agreements, one with the Laboratory of Aminoacids, Peptides and Proteins of the University of Montpellier, France, and another with the Department of Experimental and Environmental Medicine of the University of Milan, Italy, deal with the development of ghrelin antagonists.
Pursuant to another agreement signed in the field of oncology with the Institute for Molecular Biotechnology of Jena, and a research group at the University of Münster, both in Germany, we have gained access to specific university know-how and screening technologies in the field of proteins of the cytoskeleton.
26
Under all these agreements, we are obligated to support some of the research expenses incurred by the university laboratories and pay royalties on future sales of the products. In turn, we have retained exclusive rights for the worldwide exploitation of results generated during the collaborations.
On October 27, 2004, we announced that we had entered into a license and collaboration agreement with Tulane University, in New Orleans, for the development of growth hormone-releasing hormone (GH-RH) antagonists, a novel class of potential anti-cancer agents. Under the terms of the agreement, we obtained worldwide exclusive rights to develop and commercialize GH-RH antagonists for all potential indications, including cancer and endocrine disorders.
3.1.10 Growth Strategy
Æterna Zentaris is a growing oncology and endocrine therapy-focused global biopharmaceutical company with proven expertise in drug discovery, pharmaceutical development and commercialization. We are committed to using our resources, our management expertise and depth and leveraging our Atrium subsidiary as well as our strategic alliances with a view to aggressively advancing our product pipeline with the goal of becoming a late-stage development company in the near-term. Ultimately, our objective is to become a fully-integrated specialty pharmaceutical company with a strategic focus on oncology, primarily targeting the North American and European markets.
3.2 ATRIUM BIOTECHNOLOGIES INC.
3.2.1 Company Overview
Atrium is a leading developer, manufacturer and marketer of value-added products for the cosmetics, pharmaceutical, chemical and nutrition industries. Atrium's head office is located in Quebec City, Quebec. Its offices, facilities and warehouses are strategically located in Canada, the United States and France. As of February 1, 2006, Atrium had 489 employees, including 24 involved in business and product development, 267 in production and logistics, and 130 in sales and marketing. Many of its sales and marketing employees have a scientific background in order to support its sophisticated customers.
To better address the needs of its customers, Atrium, together with its subsidiaries, including Unipex S.A.S. ("Unipex), Chimiray S.A.S. ("Chimiray), MultiChem, Pure Encapsulations and Douglas Laboratories, are organized in two business divisions: (i) Active Ingredients & Specialty Chemicals Division; and (ii) Health & Nutrition Division. The Active Ingredients & Specialty Chemicals Division offers value-added products that include high-value proprietary active ingredients developed, acquired or in-licensed by Atrium. Through the Health & Nutrition Division, Atrium develops, manufactures and markets proprietary health and nutrition finished products.
Active Ingredients & Specialty Chemicals Division
The Active Ingredients & Specialty Chemicals Division offers more than 1,500 value-added products, 44 of which are high-value proprietary active ingredients developed, acquired or in-licensed by Atrium. The balance is sourced from third-party manufacturers, including major multinational companies such as Ajinomoto, Ciba and Dow Chemical. Atrium is the sole marketer for a majority of these third-party products in key markets in which it has a direct sales force. Its product portfolio includes active ingredients, specialty lipids, chemical synthesis intermediates, functional chemicals, innovative additives, preservatives and excipients.
Its products enhance customers' end products by improving performance, providing essential product attributes, lowering costs and simplifying manufacturing processes. In particular, Atrium's 44 proprietary active ingredients, mostly derived from biotechnologies, have proven biological activities and are key value drivers of its customers' finished products. Atrium's non-proprietary value-added products complement its product portfolio, help it achieve industry diversification to maximize the potential of its products, and build critical mass with its strategic customers. These non-proprietary products have diverse applications. They are used, among other things, in the manufacturing of drugs and value-added foods and in numerous industrial applications.
27
To efficiently sell its products, Atrium also offers to customers the scientific, technical and regulatory support needed to better understand the potential uses of its products and to reduce the development time of its finished products. This is essential to the success in marketing scientific value-added products. Its experts share application ideas, help resolve formulation or application challenges and support customers' new product development efforts and regulatory compliance.
Atrium sells to approximately 2,000 manufacturers in the cosmetics, pharmaceutical, chemical and nutrition industries. Major customers of the Active Ingredients & Specialty Chemicals Division include Akzo Nobel, Clarins, DuPont, Estée Lauder, Guerbet, L'Oréal, L.V.M.H., Nestlé, Novartis, Olymel, Patheon, Pfizer, Pierre Fabre and Servier. In North America and Europe, Atrium sells its products through its own sales and marketing organization. The proprietary active ingredients are also marketed through a network of more than 35 specialized distributors in over 40 countries. Atrium's sophisticated logistics systems enable it to service its customers on a timely basis. The proprietary active ingredients are either manufactured in-house or outsourced to reliable contract manufacturers.
Health & Nutrition Division
Through the Health & Nutrition Division, Atrium develops, manufactures and markets more than 1,300 proprietary health and nutrition finished products. These products are generated primarily from natural sources and include vitamins, minerals and specialized products. Innovative and high-end, these products are not suited for mass market channels. They are sold primarily through healthcare practitioners, such as physicians, chiropractors and naturopaths, and are based on scientifically supported formulas to deliver the expected health benefits. Some of the products are manufactured using molecular separation biotechnology.
In the United States, Atrium sells its products through more than 40,000 healthcare practitioners. In addition, certain of its products are offered in more than 25 countries through a network of more than 45 distributors targeting niche markets. Virtually all of Atrium's health and nutrition products are manufactured in its state-of-the-art facilities in Quebec City, Quebec, Sudbury, Massachusetts and Pittsburgh, Pennsylvania.
3.2.2 Products
Atrium offers a comprehensive product line consisting of more than 1,500 active ingredients and specialty chemicals and 1,300 health and nutrition finished products. These include 1,368 proprietary products, of which 49 were developed internally, 1,309 were acquired and ten were in-licensed from third parties. This broad product portfolio plays an important role in providing the differentiating factors required by its customers to compete in their markets. In order to increase the breadth and innovative character of its product offering, Atrium intends to continue to acquire, in-license and develop new proprietary products.
Atrium has built a solid reputation as a reliable provider of quality products, which contributes to long-term repeat business. The efficacy and safety of its proprietary products have been thoroughly documented. Quality control of all of its proprietary products includes testing by independent laboratories.
Active Ingredients & Specialty Chemicals Division
Atrium commercializes active ingredients and specialty chemicals in the cosmetics, pharmaceutical, nutrition and chemical industries, as described below.
28
Cosmetics Industry
In the cosmetics industry, the product portfolio is comprised of active ingredients, specialty additives, excipients, surfactants, preservatives, sunscreens, pigments and lacquers. They include performance enhancers for skin care, hair care and makeup products, designed to improve the safety, efficacy, texture and stability of the customers' finished products.
The main proprietary products consist of cosmetic active ingredients targeting primarily the fast growing anti-ageing and skin care market segments. Certain of these products were developed in-house, while the majority were acquired or in-licensed by Atrium. Most of Atrium's key proprietary active ingredients are subject to clinical studies, some of which are conducted in collaboration with industry leaders.
Pharmaceutical Industry
In the pharmaceutical industry, Atrium commercializes excipients, preservatives, flavouring agents and active pharmaceutical ingredients ("APIs") such as peptides, nucleotides, amino acids, antibiotics and sulfamides. APIs are marketed to both ethical and generic drug manufacturers. For generic drugs, Atrium often provides clients with both the ingredients and their complete registration file; it may adapt the latter to comply with regulatory requirements.
Some of the APIs which Atrium commercializes are: (i) articaine, an anesthetic used in dentistry; (ii) progesterone, used in menopause-discomfort drugs; (iii) quinine, an anti-paludic used in the treatment and prevention of malaria; and (iv) polyvinylpyrrolidone iodine, an antiseptic used in applications such as operating field disinfection.
The following are certain of Atrium's formulation additives: (i) amino acids used in parenteral nutrition; (ii) vitamin E-TPGS, an exclusive form of vitamin E used to facilitate the oral absorption of anti-cancer drugs; and (iii) sodium benzoate, an excipient used as a key component in various drugs.
Nutrition Industry
In the nutrition industry, Atrium commercializes processing aids, antioxidants, vitamins, minerals, preservatives and flavouring and texturing agents for manufacturers of dietary supplements, food and animal feed. These ingredients are used to enhance product formulation, nutritional value and taste, for better acceptance by consumers.
The following are certain of the products: (i) inuline, known for its bifidogenic prebiotic action, used in transformed nutrition products for diabetics, newborns and children, and in healthy foodstuffs; and (ii) lactoserum protein hydrolyzates, used in hypoallergenic nutrition for athletes and children, and in geriatric and hospital nutrition.
Chemical Industry
In the chemical industry, Atrium markets specialty chemicals which are used in a wide variety of industries such as coatings, construction, plastics, rubber, textile, ink, automotive, photography, paint, electronics and adhesives. Atrium also commercializes chemical synthesis intermediates and building blocks which are primarily used in the manufacturing of pharmaceutical products.
Some of the products that Atrium markets to the chemical industry include: (i) L-Norvaline, a chemical synthesis intermediate used to produce a drug for the treatment of hypertension and heart failure; (ii) Benzoflex 9-88SG, a safe plasticizer used in polyurethane ink roll coatings as a substitute for phthalates, some of which are considered carcinogenic by the FDA; (iii) Ajicure MY-24, an innovative additive incorporated in a product which is used in the automotive industry as a replacement for bitumen-based protection in a car's lower body, as a sound insulator and anti-vibration component; and (iv) interferential pigments, which are the most technically advanced pigments today, and are used in bank notes for protection against counterfeiting and in other security applications.
29
Health & Nutrition Division
The following describes Atrium's main health and nutrition product lines, all of which are proprietary:
CarTCell Product Line
CarTCell is a complex of natural molecules obtained from marine biomass using molecular separation biotechnology. The product line is comprised of eight different products. Comitris, a more potent version of the initial product, was launched in North America and Europe in January 2005 and helps maintain healthy angiogenic balance and blood parameters. It is typically used by people having critical or debilitating conditions, to help improve their quality of life. The CarTCell product line has been successfully commercialized internationally since 1992.
NatCell Product Line
The NatCell line of products is obtained from various biomasses using molecular separation biotechnology. It consists of more than 10 different products, the most popular being NatCell Thymus, Cytofactors, Zepatix and CF Support. They have different functions depending on the mix of peptides and molecules. Some help maintain a healthy immune function while others help maintain energy levels or contribute to healthy ageing. The NatCell product line has also been successfully commercialized internationally since 1992.
Pure Encapsulations Product Line
The Pure Encapsulations product line is comprised of more than 300 products offered in various formats to satisfy the needs of healthcare practitioners. Pure Encapsulations' products have been offered to healthcare practitioners since 1991. All products contain quantities of vitamins, minerals, nutrients, amino acids or herbal extracts with scientifically-proven health benefits. Pure Encapsulations uses only premium natural hypoallergenic products in the manufacturing of its supplements. All capsules are vegetable-based. Key products include highly potent and natural multi-vitamins for adults and children, specialized nutrition products such as UltraNutrient, Nutrient 950 and PediaNutrients, high-end antioxidants such as CoQ10, and condition-specific products such as the Macular Support Formula, designed to protect and support the central area of the retina, responsible for sharp vision.
Douglas Laboratories Product Line
Douglas Laboratories offers a broad selection of approximately 960 branded products offered in various formats to satisfy the needs of healthcare practitioners. Many products are unique formulations that are only available at Douglas Laboratories, including the Ultra Preventive and Basic Preventive lines — the most widely recommended professional grade multiple vitamin and mineral formulas in the marketplace. Douglas Laboratories also offers an extensive array of herbal supplements including Ayurvedic herbs, herbal combinations and the Max-V exclusive line of standardized herbs in vegetarian capsules. In addition to these and our other fine supplements, Douglas Laboratories is continually developing new products based upon the latest scientific and clinical research. Douglas Laboratories relies on a solid team of sales representatives that covers the entire United States. Douglas Laboratories has been marketing health and nutritional products through healthcare practitioners for over 50 years and is recognized across the industry for its quality and innovation.
3.2.3 Competition
The competition faced by these two divisions is as follows:
30
Active Ingredients & Specialty Chemicals Division
The markets for active ingredients are highly fragmented. The majority of Atrium's competitors in this segment are privately owned while others are part of larger specialty chemicals or commodity groups such as Arch Chemicals, Cognis, Croda, DSM, Lonza and Symrise. Smaller competitors include Codif, Coletica, Pentapharm, Secma and Silab. While some of Atrium's competitors offer active ingredients coming strictly from botanical or marine sources, Atrium offers a comprehensive portfolio derived from diverse sources and using various biotechnologies.
There are numerous specialty chemicals producers around the world, resulting in a very fragmented market. Certain segments of the specialty chemicals industry are dominated by large multinational groups such as BASF, Clariant, Degussa, Dow Chemical, DSM and Lonza. For specialty chemicals developed by companies such as Ajinomoto, Ciba and Dow Chemical, Atrium acts as a channel partner, commercializing selected products on an exclusive basis to its wide base of customers in Europe. The competition in this industry consists primarily of manufacturers of specialty chemicals similar to those which Atrium markets for third parties.
Health & Nutrition Division
The health and nutrition industry is vast and competitive. Product quality and distribution channels vary widely; the latter include retail chains, multi-level marketing organizations and web-based retailers. In retail and mass market channels, there are a great number of brands and price points are generally low. To avoid competing on such grounds, Atrium markets primarily to healthcare practitioners, who in turn sell Atrium's products to their patients.
There are a multitude of competitors in the United States, which is Atrium's primary market. Atrium's main competition in sales to healthcare practitioners comes from privately-owned businesses such as Metagenics, Thorne Research and Standard Process. The European and Asian markets are even more fragmented. They are characterized by a much greater number of smaller privately-owned businesses, often operating as part or a spin-off of treatment clinics. Atrium believes that it distinguishes itself from competitors with the consistency and quality of its products, which are all supported by scientific literature or evidence. Atrium is also among the very few companies to provide full disclosure of all ingredients in its formulations and to offer an open plant policy to healthcare practitioners who want to inspect facilities.
3.2.4 Manufacturing and Supply
Atrium operates three state-of-the-art manufacturing facilities, where it manufactures virtually all of its proprietary products. The first is in Quebec City, Quebec, where Atrium produces health and nutrition finished products and cosmetic active ingredients using molecular separation biotechnology equipment. The second is in Sudbury, Massachusetts, where Atrium blends, encapsulates and bottles health and nutrition finished products. The third is in Pittsburgh, Pennsylvania, where Atrium blends, encapsulates and bottles health and nutrition finished products. Based on its expected growth rate, Atrium believes that its manufacturing capacity will be sufficient to meet its requirements for at least the next three years without having to incur significant capital expenditures. The policy is to limit investment in manufacturing assets, except when deemed strategic in terms of know-how or consistency of supply.
For the limited number of proprietary products that Atrium does not manufacture in-house, Atrium relies on a solid network of contract manufacturers located in North America and Europe. All production is rigorously controlled by its scientific and technical team. Production outsourcing minimizes investment in capital equipment. In order to meet its volume requirements over the next several years, Atrium has developed relationships with selected contract manufacturers. Atrium is not dependent on any such contract manufacturer. Atrium is of the view that, if necessary, its current selected contract manufacturers could be replaced with minimal disruption to its operations.
To supply products to customers in a timely manner, Atrium has developed an expertise in international logistics. Atrium uses advanced information technology (IT) systems and detailed procedures to optimize the logistics operations. Relying on a network of warehouses strategically located in North America and Europe, Atrium is able to supply all of its customers within very short delays. In France, the main warehouse for the Active Ingredients & Specialty Chemicals Division is fully computerized with a wireless network linking fork-lifts with computer systems for on-time and accurate control of inventory and shipping. In Sudbury, Massachusetts and Pittsburgh, Pennsylvania, the sophisticated computer systems support the customer service and shipping teams, enabling them to meet Atrium's 48-hour delivery policy for all health and nutrition products.
31
3.3 RISK FACTORS
Our business entails significant risks. In addition to the usual risks associated with a business, you will find on pages 20 to 22 of our annual Management's Discussion and Analysis ("MD&A") dated February 28, 2006, for the financial year ended December 31, 2005, a general description of certain significant risk factors which are applicable to our business, which pages are incorporated by reference into this Annual Information Form.
ITEM 4. DIVIDENDS
4.1 Dividends
Since our incorporation, we have not paid any dividends and we do not anticipate paying any dividends in the foreseeable future.
ITEM 5. GENERAL DESCRIPTION OF CAPITAL STRUCTURE
5.1 General Description of Capital Structure
Our authorized share capital consists of an unlimited number of shares of the following classes:
- •
- Common Shares: The holders of the Common Shares are entitled to one (1) vote for each Common Share held by them at all meetings of shareholders, except meetings at which only shareholders of a specified class of shares are entitled to vote. In addition, the holders are entitled to receive dividends if, as and when declared by our Board of Directors on the Common Shares. Finally, the holders of the Common Shares are entitled to receive the remaining property of the Company upon any liquidation, dissolution or winding-up of the affairs of the Company, whether voluntary or involuntary.
- •
- Preferred Shares: The First and Second Preferred Shares are issuable in series with rights and privileges specific to each class. The holders of Preferred Shares are not entitled to receive notice of or to attend or vote at meetings of shareholders.
All classes are without nominal or par value. As at March 24, 2006, there were 53,133,681 Common Shares and no Preferred Shares issued and outstanding.
ITEM 6. MARKET FOR SECURITIES
6.1 Trading Price and Volume
Our Common Shares are listed and posted for trading on the Toronto Stock Exchange (TSX) and are quoted on the NASDAQ National Market (NASDAQ).
The following table sets forth, for the periods indicated, the reported high, low, and closing sale prices (in Canadian dollars) and the volume of our Common Shares traded on the TSX.
32
TSX
(in Canadian dollars)
|
|---|
Month
| | High
| | Low
| | Close
| | Volume
|
|---|
| January 2005 | | 7.89 | | 6.52 | | 6.54 | | 1,198,699 |
| February 2005 | | 7.64 | | 6.55 | | 7.04 | | 907,517 |
| March 2005 | | 7.45 | | 6.15 | | 6.19 | | 1,307,519 |
| April 2005 | | 6.75 | | 5.96 | | 6.09 | | 713,115 |
| May 2005 | | 6.75 | | 5.77 | | 6.07 | | 766,822 |
| June 2005 | | 6.20 | | 5.50 | | 5.65 | | 943,500 |
| July 2005 | | 6.05 | | 5.28 | | 5.90 | | 915,339 |
| August 2005 | | 6.26 | | 5.56 | | 5.83 | | 1,051,876 |
| September 2005 | | 6.60 | | 5.50 | | 5.60 | | 3,268,562 |
| October 2005 | | 5.69 | | 5.36 | | 5.56 | | 1,024,179 |
| November 2005 | | 6.04 | | 4.85 | | 5.18 | | 1,412,480 |
| December 2005 | | 5.97 | | 4.97 | | 5.90 | | 2,507,815 |
The following table sets forth, for the periods indicated, the reported high, low, and closing sale prices (in U.S. dollars) and the volume of our Common Shares traded on the NASDAQ.
NASDAQ
(in U.S. dollars)
|
|---|
Month
| | High
| | Low
| | Close
| | Volume
|
|---|
| January 2005 | | 6.47 | | 4.67 | | 5.26 | | 963,356 |
| February 2005 | | 6.23 | | 5.05 | | 5.72 | | 782,051 |
| March 2005 | | 6.10 | | 5.10 | | 5.12 | | 728,991 |
| April 2005 | | 5.75 | | 4.55 | | 4.88 | | 410,760 |
| May 2005 | | 5.88 | | 4.56 | | 4.83 | | 531,481 |
| June 2005 | | 5.00 | | 4.00 | | 4.61 | | 604,810 |
| July 2005 | | 4.99 | | 4.21 | | 4.85 | | 685,332 |
| August 2005 | | 5.75 | | 4.55 | | 4.86 | | 1,149,332 |
| September 2005 | | 5.70 | | 4.69 | | 4.80 | | 1,301,442 |
| October 2005 | | 4,89 | | 4.53 | | 4.81 | | 938,169 |
| November 2005 | | 5.16 | | 4.10 | | 4.47 | | 1,201,848 |
| December 2005 | | 5.15 | | 4.25 | | 5.10 | | 621,042 |
ITEM 7. DIRECTORS AND OFFICERS
7.1 Directors
Our Board of Directors currently consists of ten directors. Each director remains in office until the following annual shareholders' meeting or until the election of his or her successor, unless he or she resigns or his or her office becomes vacant as a result of his or her death, removal or any other cause.
The following table sets forth, for each director, the name, place of residence, principal occupation, security holdings, and the period during which he or she has acted as a director:
33
Name and Place
of Residence
| | Principal Occupation
| | Director
Since
| | Number of Common
Shares Held in the
Company and %
| | Number of
Subordinate
Voting Shares
Held in Atrium
and %
|
|---|
Marcel Aubut
Quebec, Canada | | Managing Partner
Heenan Blaikie Aubut
(law firm) | | 1996 | | 45,000 | | — | | 56,500 | | — |
|
Stormy Byorum(1)
New York, USA | | Senior Managing Director
Stephens Cori Capital Advisors, LLC
(strategic and financial advisory services company) | | 2001 | | 12,000 | | — | | — | | — |
|
Éric Dupont, PhD(2)
Quebec, Canada | | Executive Chairman of the Board
Æterna Zentaris Inc. | | 1991 | | 3,767,413 | | 7.1% | | 40,000 | | — |
|
Prof. Dr. Jürgen Engel
Frankfurt, Germany | | Executive Vice President, Global R&D and Chief Operating Officer
Æterna Zentaris Inc. | | 2003 | | 31,279 | | — | | 1,000 | | — |
|
Jürgen Ernst(2)(3)
Brussels, Belgium | | Vice Chairman of the Board
Æterna Zentaris Inc.
Former Managing Director
Pharmaceutical Sector of Solvay S.A.
(international chemical and pharmaceutical group) | | 2005 | | 8,850 | | — | | 2,222 | | — |
|
Gilles Gagnon
Quebec, Canada | | President and Chief Executive Officer
Æterna Zentaris Inc. | | 2002 | | 70,617 | | 0.13% | | — | | — |
|
Pierre Laurin, PhD(2)
Quebec, Canada | | Executive in Residence
HEC Montréal
(management faculty of university) | | 1998 | | 11,200 | | — | | 180,000 | | 0.6% |
|
Gérard Limoges, FCA(1)
Quebec, Canada | | Corporate Director | | 2004 | | 1,000 | | — | | — | | — |
|
Pierre MacDonald(1)(2)
Quebec, Canada | | Chairman of the Board
Eurocopter Canada Ltd.
(a designer/manufacturer of civil helicopters) | | 2000 | | 11,500 | | — | | — | | — |
|
Gerald J. Martin(4)
California, USA | | Corporate Director
Former Vice President, Corporate Licensing and Technology Alliances at Abbott Laboratories Inc. | | 2006 | | — | | — | | — | | — |
- (1)
- Member of the Audit Committee.
- (2)
- Member of the Corporate Governance, Nominating and Human Resources Committee.
- (3)
- Mr. Ernst was appointed Vice Chairman of the Board on November 14, 2005.
- (4)
- Mr. Martin was appointed director in order to fill a vacancy on the Company's Board of Directors on January 25, 2006.
Notes:
Mr. Marcel Aubut served as a director of Albums DF Ltée, a privately held company based in Longueuil, Quebec, from September 5, 1997 to September 16, 2003, which company became bankrupt on December 6, 2003.
Mr. Pierre Laurin served as a director of Microcell Telecommunications Inc. ("Microcell") from May 1999 until May 2003. Microcell entered into a Plan of Reorganization and of Compromise and Arrangement with its creditors and shareholders effective May 1, 2003 pursuant to theCompanies' Creditors Arrangement Act (Canada). Mr. Laurin was a member of the Special Committee of the Board of Directors of Microcell created in connection with the foregoing restructuring.
Mr. MacDonald served as a director of Slater Steel Inc. ("SSI"), a manufacturer of specialty steel products from February 1998 until August 2004. SSI and its subsidiaries filed for creditor protection under theCompany's Creditors Arrangement Act (Canada) and under Chapter 11 of the US Bankruptcy Code on June 2, 2003, and they have conducted an orderly wind-down.
34
7.2 EXECUTIVE OFFICERS
The table below sets forth the name, place of residence and the position with Æterna Zentaris of each of its executive officers on the date hereof.
Name and Place
of Residence
| | Principal Occupation
|
|---|
Éric Dupont, PhD
Quebec, Canada | | Executive Chairman of the Board |
Gilles Gagnon
Quebec, Canada | | President and Chief Executive Officer |
Prof. Dr. Jürgen Engel
Frankfurt, Germany | | Executive Vice President, Global Research and Development and Chief Operating Officer |
Dr. Eckhard Günther
Frankfurt, Germany | | Vice President, Drug Discovery |
Mario Paradis, CA
Quebec, Canada | | Senior Finance Director and Corporate Secretary |
Dr. Matthias Rischer
Frankfurt, Germany | | Vice President, Pharmaceutical Development |
Dr. Manfred Peukert
Frankfurt, Germany | | Vice President, Medical Affairs |
Dennis Turpin, CA
Quebec, Canada | | Vice President and Chief Financial Officer |
During the past five years, the directors and executive officers mentioned above have held their present principal occupations, except as indicated below.
Prof. Dr. Engel has been Chairman and Managing Director of Zentaris GmbH since January 2003. Previously, he was Chief Executive Officer of Zentaris AG after having been head of Corporate Research and Development, including drug discovery, at Asta Medica AG in Frankfurt, Germany.
Head of drug discovery at Zentaris AG since January 2001, Dr. Günther has more than 15 years of experience in the biotechnology and biopharmaceutical industries, as a researcher as well as a manager. At Asta Medica, he was Group Leader Planning & Controlling, Research Coordination and Head of Research Coordination, before becoming Head of Medicinal Chemistry Oncology.
Mario Paradis was named Corporate Secretary on February 27, 2004. He joined the Company as Finance Director in June 1999 and was named Senior Finance Director in 2001.
In addition to his current function as Medical Director at our subsidiary in Germany, Dr. Manfred Peukert, was appointed Vice President, Medical Affairs. Dr. Peukert is a seasoned executive who spent more than 30 years in the pharmaceutical industry. He joined Asta Medica in 1976 where he held several executive positions up to Global Head of Medical Research activities. He has a broad experience in many therapeutic areas with a specific expertise in the management of medical research projects in oncology and endocrinology.
35
Since January 2001, Dr. Matthias Rischer has been Head of the Pharmaceutical Development at Zentaris. From 1992 until 1999, he was a top executive at the multinational Asta Medica, as head of two analytical labs in the Department of Pharmaceutical Development before becoming Head of the Department of Pharmaceutical Development Analytics. He had overall analytical responsibility for new projects for the treatment of several diseases such as cancer, diabetes, Parkinson's and infertility.
As of March 24, 2006, our directors and executive officers, as a group, beneficially owned or exercised control or direction over, directly or indirectly, approximately 3,964,329 Common Shares, representing 7.5% of our issued and outstanding Common Shares. Collectively, our directors and executive officers beneficially owned or exercised control or direction over, directly or indirectly, less than 1% of Atrium's voting securities as of March 24, 2006.
ITEM 8. LEGAL PROCEEDINGS
8.1 Legal Proceedings
There are no outstanding material legal proceedings to which Æterna Zentaris or any of our subsidiaries is a party, nor, to our knowledge, are any such proceedings contemplated.
ITEM 9. INTEREST OF MANAGEMENT AND OTHERS IN MATERIAL TRANSACTIONS
To the best of our knowledge, as of March 24, 2006, (i) none of our directors or executive officers, (ii) no person or company that is the direct or indirect beneficial owner of, or who exercises control or direction over, more than 10 percent of our Common Shares, and (iii) no associate or affiliate of any of the persons or companies referred to in (i) and (ii) above, has had any material interest, direct or indirect, in any transaction within the three most recently completed financial years or during the current financial year that has materially affected or will materially affect us, except as set forth below.
In February 2006, Solidarity Fund (QFL) and SGF Santé Inc., each of whom holds more than 10% of the outstanding Common Shares of the Company, exercised its right to convert its portion of a convertible loan under a loan agreement originally entered into among the Company, Solidarity Fund (QFL) and SGF Santé Inc. in 2003, pursuant to which each of the foregoing shareholders loaned the Company a principal amount of CAN$12.5 million. Upon conversion by Solidarity Fund (QFL) and SGF Santé Inc. of both all principal and interest due under the convertible loan agreement, the Company issued to each of them 3,477,544 Common Shares in accordance with the provisions of the agreement and additional arrangements. Following the conversion and share issuance described above, there remains no amount of indebtedness outstanding under the loan agreement.
On February 28, 2006, the Board of Directors of the Company approved the acquisition by it of a patent held by Prof. Dr. Jürgen Engel for a total consideration of US$175,000 (CAN$199,150) paid by the Company issuing 28,779 Common Shares from treasury. Prof. Dr. Engel is the Executive Vice President, Global Research and Development and Chief Operating Officer and he is also a director of the Company. This transaction was duly approved at a meeting of all six of the Company's independent directors, at which meeting Prof. Dr. Engel was not present during the discussions on this transaction. The aforementioned 28,779 Common Shares were issued to Prof. Dr. Engel on March 8, 2006.
ITEM 10. Transfer Agent and Registrar
10.1 TRANSFER AGENT AND REGISTRAR
The name and principal address of the registrar and transfer agent for the Common Shares, being the only class of our publicly listed securities, is indicated below:
National Bank Trust Inc.
1100 University Street, 12th Floor
Montreal, Quebec H3B 2G7
36
ITEM 11. Material Contracts
11.1 MATERIAL CONTRACTS
Except for contracts entered into the ordinary course of business, the only material contract entered into by us or by any of our subsidiaries during the financial year ended December 31, 2005 is the agreement dated December 8, 2005, with respect to the acquisition by Atrium of all of the outstanding shares of HVL Parent Incorporated, which has been filed on SEDAR at www.sedar.com.
ITEM 12. INTERESTS OF EXPERTS AND AUDIT COMMITTEE DISCLOSURE
12.1 Names and Interest of Experts and Audit Committee Disclosure
The Company's auditors are PricewaterhouseCoopers LLP, Chartered Accountants. They have prepared an independent auditors' report dated February 28, 2006 in respect of the Company's consolidated financial statements as at December 31, 2005 and 2004, with accompanying notes as at and for each of the year in the three-year period ended December 31, 2005. PricewaterhouseCoopers LLP has advised that they are independent with respect to the Company within the meaning of the Rules of Professional Conduct of the Institute of Chartered Accountants of Quebec and the rules of the US Securities and Exchange Commission.
All information related to external auditor service fees and the disclosure required in respect of audit committees under Multilateral Instrument 52-110 — Audit Committees is set forth at pages 14 to 16 of our Management Proxy Circular dated March 15, 2006 under the Section "Audit Committee Disclosure", which pages are incorporated herein by reference. Our Management Proxy Circular is available on SEDAR at www.sedar.com.
ITEM 13. ADDITIONAL INFORMATION
13.1 Additional Information
Additional information, including directors' and officers' remuneration and indebtedness, the principal securityholders of the Company and securities authorized for issuance under equity compensation plans, is contained in our Management Proxy Circular dated March 15, 2006, available on SEDAR at www.sedar.com. Additional financial information is provided in the Company's consolidated financial statements and Management's Discussion and Analysis for the financial year ended December 31, 2005.
All information incorporated by reference into this Annual Information Form is contained or included in one of our continuous disclosure documents filed with the Canadian securities regulatory authorities which may be viewed on SEDAR at www.sedar.com. Where a section of this Annual Information Form incorporates by reference information from one of our other continuous disclosure documents, such section makes specific reference to the document in which such information is originally contained or included, as well as to the relevant page and/or section.
ITEM 14. FORWARD-LOOKING STATEMENTS
14.1 Forward-Looking Statements
Certain statements in this document are forward-looking and prospective. Forward-looking statements generally can be identified by the use of forward-looking terminology such as "may," "will," "expect," "intend," "estimate," "anticipate," "plan," "foresee," "believe" or "continue" or the negatives of these terms or variations of them or similar terminology. Forward-looking statements involve known and unknown risks and uncertainties, which may cause our actual results in future periods to differ materially from forecasted results. Those risks include, among others, business conditions in the pharmaceutical and related industries, as well as the general economy, changes in governmental regulation, changes in the healthcare industry, competitive factors such as those influencing expenditures for research and development, or the availability of markets for the Company's products. The Company disclaims any intention, and assumes no obligation, to update these forward-looking statements.
37
QuickLinks
Exhibit 1ANNUAL INFORMATION FORM FOR THE FINANCIAL YEAR ENDED DECEMBER 31, 2005





