QuickLinks -- Click here to rapidly navigate through this document
Exhibit 4

Æterna Zentaris Annual Report 2005 1
COMMITTED TO CURE
2 Æterna Zentaris Annual Report 2005
ÆTERNA ZENTARIS INC. IS A GROWING ONCOLOGY AND ENDOCRINE THERAPY FOCUSED GLOBAL BIOPHARMACEUTICAL COMPANY, WITH PROVEN EXPERTISE IN DRUG DISCOVERY, DEVELOPMENT AND COMMERCIALIZATION.
Æterna Zentaris holds 48.4% of the equity and 64.8% of the voting rights in its subsidiary Atrium Biotechnologies Inc. (TSX: ATB.sv), a leading developer, manufacturer and marketer of science-based products for the cosmetics, pharmaceutical, chemical and nutrition industries. Its products are sold in more than 40 countries, primarily in North America, Europe and Asia.
Æterna Zentaris Inc. (TSX: AEZ, NASDAQ: AEZS)
Æterna Zentaris Annual Report 2005 3
HIGHLIGHTS 05
NOVEMBER
- •
- Completion of patient recruitment forozarelix Phase II trial inbenign prostate hyperplasia (Europe)
SEPTEMBER
- •
- Initiation ofperifosine Phase II trial innon-small cell lung cancer (Europe)
AUGUST
- •
- Completion of patient recruitment forozarelix Phase II trial inprostate cancer (Europe)
MAY
- •
- Initiation ofozarelix Phase I/II trial inprostate cancer (USA)
- •
- Disclosure of positive Phase II trial results withperifosine inprostate cancer
APRIL
- •
- Initiation ofozarelix Phase II trial inbenign prostate hyperplasia (Europe)
- •
- Initiation ofozarelix Phase II trial inprostate cancer (Europe)
- •
- Disclosure of positive Phase I trial results forEP-1572 (growth hormone disorders andcachexia)
- •
- SubsidiaryAtrium IPO
MARCH
- •
- Initiation ofcetrorelix late-stage clinical program inendometriosis (Europe) and Phase II trial inbenign prostate hyperplasia (Japan)
JANUARY
- •
- Initiation ofAN-152 Phase I trial formultiple forms of cancer (Europe)
- •
- Acquisition ofEchelon Biosciences, Salt Lake City, Utah
Note: all amounts throughout this annual report are in US dollars unless otherwise noted.
4 Æterna Zentaris Annual Report 2005

MESSAGE TO SHAREHOLDERS
Steady value creation and increased self-reliance characterized the year 2005 for Æterna Zentaris. With a firm cash position and growing financial leverage, we concentrated our focus on advancing our diversified pipeline in oncology and endocrinology. Further clinical trials brought three key product candidates closer to the marketplace, while drug discovery, pre and early clinical activity created additional potential assets.
The year 2005 was a year for advancing our clinical development pipeline. We launched seven clinical projects involving our lead drug development candidates: cetrorelix, ozarelix, and perifosine. Each of these compounds target well-defined indications for unmet healthcare needs. Together, the progress of the three products demonstrates accelerated value creation, and reflects our long-term goal of emerging as a fully-integrated, global specialty pharmaceutical company.
CETRORELIX:
Following the positive results of a broad 7-Phase II program in partnership with Solvay Pharmaceuticals, we launched a late-stage clinical program for cetrorelix, our lead compound in endocrinology. With endometriosis as the primary indication, this European late-stage clinical program, funded by Solvay, is expected to fully authenticate our many years of research in the luteinizing hormone-releasing hormone (LHRH) antagonist therapeutic approach. Endometriosis afflicts 10% to 20% of child-bearing age women, and involves a market of between $700 million and $1.6 billion.
Additionally, to confirm the efficacy of cetrorelix in benign prostate hyperplasia (BPH), a further Phase II trial was launched in Japan. Our partners, Japanese pharmaceutical companies Shionogi and Nippon Kayaku, will again underwrite the project for BPH which affects more than 50% of men over 60, with a worldwide market of close to $2 billion.
Æterna Zentaris Annual Report 2005 5
OZARELIX:
Our second value-driving LHRH antagonist, ozarelix, a swiftly moving "break-out" drug candidate for us, entered three clinical trials in 2005:
- •
- We initiated and completed patient recruitment for our European Phase II trial in BPH a full four months ahead of schedule
- •
- We initiated and completed patient recruitment for our European Phase II trial in prostate cancer again ahead of schedule
- •
- We also initiated a Phase I/II monotherapy trial for prostate cancer in the United States
This drug, a fourth generation LHRH antagonist with long-lasting activity, holds powerful promise for Æterna Zentaris and our funding partner Spectrum Pharmaceuticals. We have granted Spectrum a license to develop and market ozarelix for all potential indications in North America and India. We have retained rights to the rest of the world, and will share potential revenues from sales in Japan with Spectrum.
PERIFOSINE:
Our lead compound in oncology, perifosine, derives from our therapeutic approach using signal transduction inhibitors. This year, we began a European multi-centre Phase II trial, in combination with radiotherapy, in non-small cell lung cancer.
Ongoing studies of the efficacy of perifosine are also currently taking place in more than ten clinical trials in the United States with our partner Keryx Biopharmaceuticals to help determine the potential of perifosine as a breakthrough anti-cancer agent. These mono and combination therapy trials conducted in various indications including soft tissue sarcoma, melanoma, breast and prostate cancers, represent our concerted effort to claim a global profile in oncology discovery.
PRECLINICAL AND EARLY CLINICAL:
The Company's objective, on an annual basis, is to take at least one drug candidate to Phase I from our library of 100,000 proprietary compounds. In the past year we advanced compound AN-152, developed from our approach with cytotoxic conjugates, into Phase I trials targeting ovarian and breast cancers.
CORPORATE AFFAIRS:
Early in 2005, we acquired Echelon Biosciences in Utah, to gain a complementary fit for our signal transduction inhibitor therapeutic approach.
Our majority-owned subsidiary, Atrium Biotechnologies, pursued its aggressive growth strategy with two major North American acquisitions as well as a successful Initial Public Offering which raised gross proceeds of CAD$50 million. In January, Atrium expanded its Active Ingredients & Specialty Chemicals Division by acquiring MultiChem in Montreal, while in December, its Health & Nutrition Division profited from the purchase of Pittsburgh-based HVL / Douglas. The acquisitions have broadened Atrium's North American profile and reach, have substantially added to its sales range and product mix, with 2005 pro forma revenues of $266 million.
6 Æterna Zentaris Annual Report 2005
In 2005, we enhanced the strength of our Board of Directors and of our Scientific Advisory Board with four key appointments. Mr. Jürgen Ernst, former worldwide General Manager, Pharmaceutical Sector of Solvay S.A., was named Vice Chairman of our Board of Directors. Gerald J. Martin, former Vice President, Corporate Licensing and Technology Alliances at Abbott Laboratories also joined our Board. Dr. Daniel Douglas Von Hoff, Director of the Translational Drug Development Division of the Translational Genomics Institute in Scottsdale, Arizona, and also a member of President George Bush's National Cancer Advisory Board, joined our Scientific Advisory Board. Dr. Ulf Rapp, Professor of Molecular Cell Biology, Director of the Institute of Medical Radiation and Cell Research at Würzburg University in Germany and Chairman of the German Cancer Society was also appointed to our SAB. Furthermore, Jenene Thomas was appointed Director, Investor Relations. Based in New York, Ms. Thomas' mandate points to our strategy of gaining much greater visibility on the financial markets in the United States.
MILESTONES FOR 2006:
Over the next twelve months, we will pursue our comprehensive development program by continuing to advance product candidates through the pipeline. We will push ahead with cetrorelix's late-stage clinical development program in endometriosis and disclose results from our Japanese confirmatory Phase II BPH trial. Furthermore, after having recently regained worldwide rights for cetrorelix in BPH from our partner Solvay, we expect to initiate a late-stage clinical program in this indication in North America.
As our development of ozarelix proceeds well ahead of schedule, we will disclose our European Phase II prostate cancer results, as well as the data resulting from our European Phase II BPH trials.
In regard to perifosine, we shall complete patient enrolment for our Phase II combination therapy trial in non-small cell lung cancer, and disclose several Phase I/II results in monotherapy and combination therapy.
Complementing our early-stage research, we will bring at least one product from our drug discovery engine to the clinical stage.
STRENGTH AND CONSISTENCY:
With a controlled burn-rate, revenues from two marketed products, and leverage from our stake in Atrium, the Company is financially secure. This security in turn ensures the continued pursuit of our drug development programs, as well as the ongoing introduction of new products. This is the often proven road to success in the biopharmaceutical industry.
In a turbulent environment for biopharmaceutical companies worldwide in 2005, we made no short-term moves that would put at risk our long-term strategy of becoming a solid global biopharmaceutical company. Accordingly, we focused on what is at the core of our business; moving drugs efficiently through our innovative and deep pipeline which resulted in bringing our three lead compounds into late-stage clinical development. We look forward to achieving many more exciting milestones in the upcoming year, and in so doing, we are convinced that we will continue to gradually build value for the Company and you, the shareholders.
We would like to take this opportunity to thank all the employees and stakeholders of Æterna Zentaris for their continued commitment and confidence.
 | |  |
Gilles Gagnon, MSc,
President & CEO | | Eric Dupont, PhD MBA
Executive Chairman |
Æterna Zentaris Annual Report 2005 7
BUILDING VALUE THROUGH DRUG DEVELOPMENT
Æterna Zentaris' pipeline encompasses exciting and promising novel products focused on oncology and endocrine disorders. The Company aims at building value through a comprehensive development strategy of those products.
Some development programs are strongly supported by pharma partners, providing Æterna Zentaris with milestone payments and double-digit royalties. For other programs, mainly in oncology, Æterna Zentaris assumes full development costs with the goal of building its own sales force and becoming a fully-integrated global biopharmaceutical company.
8 Æterna Zentaris Annual Report 2005
DEEP AND FOCUSED PIPELINE
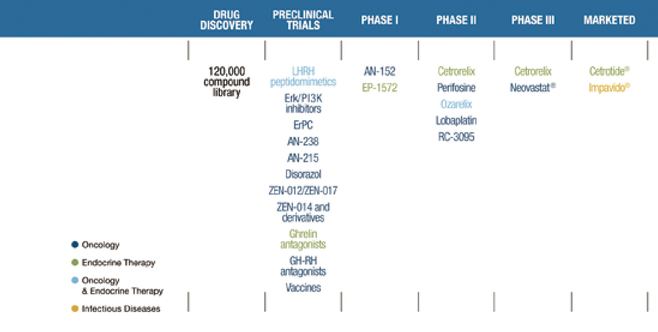
From drug discovery…
…to market
Æterna Zentaris Annual Report 2005 9
LARGE MARKET OPPORTUNITIES:
Endometriosis(Benign abnormal growth of tissue outside the uterus)
- •
- About 26 million* cases (U.S., Europe, Japan)
- •
- 10% to 20% of women of child-bearing age
- •
- Under diagnosed
- •
- $700M to $1.6B market size in 2003
*Source: Datamonitor
Benign Prostatic Hyperplasia(Non-cancerous enlargement of the prostate gland)
Prostate Cancer
Non-Small Cell Lung Cancer
10 Æterna Zentaris Annual Report 2005
3VALUE DRIVERS
ÆTERNA ZENTARIS' PIPELINE ENCOMPASSES THREE VALUE DRIVING PRODUCTS IN ADVANCED CLINICAL DEVELOPMENT FOR BOTH BENIGN AND CANCEROUS TUMORS.
1: CETRORELIX
BREAKING NEW GROUND IN TREATING BENIGN TUMORS WHILE REDUCING SERIOUS SIDE-EFFECTS
Cetrorelix is part of our Luteinizing Hormone Releasing Hormone (LHRH) antagonist therapeutic approach. This peptide-based active substance was developed by the Company in cooperation with Nobel-prize winner Professor Andrew Schally of Tulane University in New Orleans.
For the treatment of endometriosis, reducing the estrogen level using cetrorelix produces a rapid regression of tissue growth and associated complaints. Cetrorelix allows for targeted control of the hormone level to give fast effects, while avoiding menopause-like symptoms and potential risks associated with an otherwise complete and long-term withdrawal of hormones.
For the treatment of benign prostate hyperplasia, cetrorelix has been shown to adequately suppress the formation of the male sex hormone testosterone, which plays a principal role in cell growth of the prostate. Since cell growth is stopped, surgical removal of the prostate might be avoided.
All studies performed so far with cetrorelix in patients with symptomatic BPH, revealed an improvement in symptoms as assessed primarily by the I-PSS (International Prostate Symptom Score), an increase in urinary peak flow rate as well as a reduction in prostate volume. Studies have also shown the excellent safety and tolerability profile of cetrorelix. |
|
Current clinical status:
• Late-stage clinical program for endometriosis in Europe
• Phase II trial for benign prostate hyperplasia in Japan
Partnerships:
Solvay for Europe / Shionogi – Nippon Kayaku for Japan
Clinical development strategy for 2006:
• Report Japanese Phase II results for BPH
• Initiate BPH late-stage clinical program in the U.S.
• Advance late-stage clinical program in endometriosis
Advantages of LHRH antagonists over LHRH agonists in treating endometriosis and BPH:
• No flare-up effect
• Fast onset of action / shorter treatment period
• Intermittent / chronic therapy possible
• Rapid and durable responses
• Reduced side-effects such as hot flashes, chemical castration and loss of libido
• Diminished risk of osteoporosis |
Cetrotide®
Cetrorelix is also marketed under the brand name Cetrotidethe®, the first LHRH antagonist approved for therapeutic use as part ofin vitro fertilization programs (controlled ovulation stimulation /assisted reproductive technologies) in Europe and the U.S.. It was launched on the market through Serono S.A. in the U.A., Europe and in several other countries. Shionogi and Nippon Kayaku have the rights for Japan where approval is pending.
• Marketed forin vitro fertilization (U.S. & Europe)
• Partnered with Serono (world ex. Japan)
• Generates more than $19M revenue per year | |  |
Æterna Zentaris Annual Report 2005 11
2: OZARELIX
THE NEXT GENERATION OF LHRH ANTAGONISTS IN TUMOR TREATMENT
Ozarelix is the newest product in our LHRH antagonist therapeutic approach. It is a promising fourth generation LHRH antagonist aiming for extended suppression of testosterone levels that does not require a sophisticated depot technology for long-lasting activity. Ozarelix is the result of ongoing research activities for additional compounds within cetrorelix's class that are particularly suitable for both benign and malign tumor therapy.
Advantages over existing therapies for BPH and prostate cancer:
• No flare-up effect
• Rapid and durable responses
• Intermittent / chronic therapy possible
• Reduced side-effects
• No loss of libido | | Current clinical status:
• Phase II for benign prostate hyperplasia in Europe (recruitment completed)
• Phase II for prostate cancer in Europe (recruitment completed)
• Phase I/II for prostate cancer in the U.S.
Partnerships:
Spectrum for North America and India
Clinical development strategy for 2006:
• Report European Phase II results for BPH and prostate cancer |
12 Æterna Zentaris Annual Report 2005
3: PERIFOSINE
A NOVEL ORAL ANTI-CANCER AGENT WITH LARGE MARKET POTENTIAL
Perifosine is a novel, first-in-class, oral anti-cancer agent developed through our signal transduction inhibitor therapeutic approach. In tumor cells, perifosine demonstrated interactions with vital signal transduction mechanisms and showed induction of programmed cell death (apoptosis). Studies involving about 350 patients have shown no dose-limiting toxicity at various doses and treatment schedules.
Advantages over existing cancer therapies:
• Novel mechanism of action compared to available cytotoxics
• Direct and relatively specific action on tumor cells, including tumor models resistant to currently available anti-cancer agents
• Does not weaken the immune system
• Well suited for use in combination therapies |
|
Current clinical status:
• Phase II combination therapy for non-small cell lung cancer in Europe
• Phase II combination therapy for breast cancer in the U.S.
• Phase II monotherapy for soft tissue sarcoma in the U.S.
• Phase II monotherapy and combination therapy for multiple myeloma in the U.S.
• Phase I/II monotherapy for non-small cell lung cancer in the U.S.
• Phase I combination therapy for multiple cancers in the U.S.
• Screening monotherapy trials for multiple cancers in the U.S.
Partnership:
Keryx for North America
Clinical development strategy for 2006:
• Report monotherapy and combination therapy Phase I/II results for multiple cancers in the U.S.
• Complete patient enrolment for combination therapy Phase II for non-small cell lung cancer in Europe |
IMPAVIDO®
Impavido®, the first marketed oral therapy for visceral (also known as black fever) and cutaneous leishmaniasis, a severe parasitic infection, is also part of our signal transduction inhibitor therapeutic approach. According to the World Health Organization, leishmaniasis is endemic in 88 countries with nearly 350 million people at risk. An estimated 12 million people, mainly in the Middle East and South America, suffer from this condition with 1 million to 1.5 million new cases reported annually. If untreated, the visceral form will be lethal. The cutaneous form leads to painful bodily and disfiguring facial lesions.
Impavido® is marketed in India and Colombia. It has been approved in Germany and is under registration in several countries of the sub-Indian continent as well as in South America.
• 28-day oral treatment
• 95% cure rate
• Mild side effects / less toxic than current therapies
• No hospitalization required | |  |
Æterna Zentaris Annual Report 2005 13
ATRIUMAN ÆTERNA ZENTARIS SUBSIDIARY
LEADING THE WAY IN THE HEALTH AND PERSONAL CARE SECTOR
Æterna Zentaris holds 48.4% of the equity and 64.8% of the voting rights in its subsidiary Atrium Biotechnologies Inc. (TSX: ATB.sv), a leading developer, manufacturer and marketer of science-based products for the cosmetics, pharmaceutical, chemical and nutrition industries. Its products are sold in more than 40 countries, primarily in North America, Europe and Asia.
HIGHLIGHTS05
DECEMBER
- •
- Acquisition of Pittsburgh-based HVL ("Douglas")
APRIL
- •
- Atrium IPO (TSX: ATB.sv)
JANUARY
- •
- Acquisition of MultiChem in Canada
AWARDS
Profit 100 Award (Canada's Fastest Growing Companies)
- •
- Canada 50 Best Award
- •
- Mérite Commercial Desjardins Awards – Company of the month, September
- •
- Innovation Awards – Export Prize
- •
- Trophées Vision Awards – Visionary Prize in healthcare services, health and personal care
14 Æterna Zentaris Annual Report 2005
OPERATIONAL OVERVIEW
Atrium has facilities in Canada, the United States and Europe, selling its products worldwide through a network of more than 55 distributors. The Company's activities are organized in two business divisions in order to effectively address the different needs of the clientele.
ACTIVE INGREDIENTS & SPECIALTY CHEMICALS DIVISION
Atrium develops, manufactures and markets over 1,500 value-added active ingredients and specialty chemicals for the cosmetic, pharmaceutical, nutrition and chemical sectors, while also providing scientific, technical and regulatory support to some 2,000 customers including leading international companies such as Estée Lauder, L'Oréal and Nestlé. This division's product portfolio includes additives, preservatives, excipients, specialty lipids, polymers and antioxidants geared to a market that yields above average growth annually.
HEALTH & NUTRITION DIVISION
Atrium also develops, manufactures and markets more than 1,300 high quality vitamins, minerals, specialized nutrition and health products to a network of over 40,000 healthcare professionals in the United States. According to the Nutrition Business Journal, between 2000 and 2003, this market segment in the United States had an annual growth rate of more than 10%. In addition to the strong growth associated with the trends towards healthy living and the ageing of the population, the increasing awareness of governmental authorities on economic benefits linked to a healthier society is another environmental factor on which the Company can capitalize on for future growth.
FINANCIAL OVERVIEW
BUILDING VALUE THROUGH STRONG INTERNAL GROWTH AND ACCRETIVE ACQUISITIONS
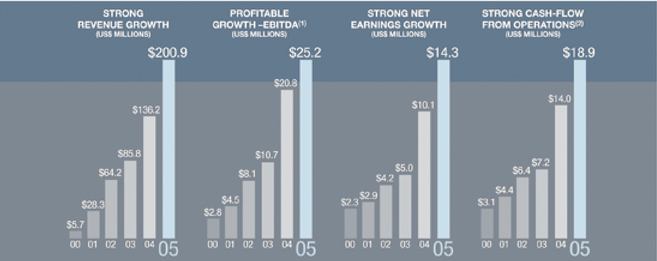
OVER LAST 5 YEARS
• 8 acquisitions totalling more than $190M
• From 18 to 2,800 products
• From 100 customers to more than 2,000 corporate customers and 40,000 health care professionals
• From 20 to over 500 employees
(1) Earnings before interest, taxes, depreciation and amortization
(2) Change in non-cash operating working capital items
Æterna Zentaris Annual Report 2005 15
ÆTERNA ZENTARIS:
POISED FOR GROWTH
The Company has all the key fundamentals to emerge as a fully-integrated global specialty pharmaceutical company with a strategic focus on oncology.
TEAM
- •
- Pharma expertise with proven track record
FINANCE
- •
- Strong financial position
PARTNERS
- •
- Development and financial support
PORTFOLIO
- •
- Focus on oncology and endocrine therapy
- •
- 3 lead products in advanced-stage clinical trials with large market opportunities
- •
- Over 90 patent families
16 Æterna Zentaris Annual Report 2005
QuickLinks
Exhibit 4