As filed with the Securities and Exchange Commission on January 22, 2007 Registration No. 333-
---------------------------------------------------------------------------------------------------------------------
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM SB-2
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
CHINA BIOPHARMA, INC.
(Name of Small Business Issuer in its Charter)
| Delaware | 2834 |
(State or Jurisdiction of Incorporation or Organization) | (Primary Standard Industrial Classification Code Number) |
31 Airpark Road, Princeton, New Jersey 08540
(609) 651-8588
(Address and Telephone Number of Principal Executive Offices
and Principal Place of Business)
Peter Wang, Chief Executive Officer
31 Airpark Road
Princeton, New Jersey 08540
(609) 651-8588
(Name, Address and Telephone Number of Agent for Service)
With a copy to:
Mitchell S. Nussbaum, Esq.
Angela M. Dowd, Esq.
Loeb & Loeb LLP
345 Park Avenue
New York, New York 10154
(212) 407-4000
Approximate date of proposed sale to the public: From time to time after this registration statement becomes effective.
Approximate date of proposed sale to the public: From time to time after this Registration Statement becomes effective.
If this Form is filed to register securities for an offering to be made on a continuous or delayed basis, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If delivery of the prospectus is expected to be made pursuant to Rule 434, check the following box. o
CALCULATION OF REGISTRATION FEE
Title of each class of securities to be registered | | Amount to be registered | | Proposed maximum offering price per unit 1) | | Proposed maximum aggregate offering price(1) | | Amount of registration fee | |
| Common Stock, par value $0.0001 per share | | | 18,000,000 shares | (2) (3) | $ | 0.383 | | $ | 6,894,000 | | $ | 737.66 | |
| Common Stock, par value $0.0001 per share | | | 14,400,000 shares | (3) (4) | $ | 0.383 | | $ | 5,512,200 | | $ | 589.80 | |
| Total | | | 32,400,000 shares | (3) | | | | | | | $ | 1,327.46 | |
1 Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(c) under the Securities Act of 1933, as amended, based on the average of the closing bid and asked prices on January 18, 2007, as reported by the OTC Bulletin Board.
2 The 18,000,000 shares of common stock are being registered (i) for resale by the Selling Stockholders named in this registration statement following the issuance of such shares by the registrant upon the repayment of principal and interest on $3,000,000 aggregate principal amount outstanding of China Biopharma, Inc.’s Secured Convertible Promissory Notes due December 13, 2008 (the “Notes”) and/or (ii) for resale by the Selling Stockholders named in this registration statement, following the issuance of such shares by the registrant upon the conversion of the Notes by the holders thereof at an initial conversion price of $0.25 per share, subject to adjustment in certain circumstances.
3 Pursuant to Rule 416 under the Securities Act of 1933, as amended, the Registrant is also registering such additional indeterminate number of shares as may become necessary to adjust the number of shares as a result of a stock split, stock dividend or similar adjustment of its outstanding common stock.
4 The 14,400,000 shares of common stock are being registered for resale by the Selling Stockholders named in this registration statement, which shares are issuable by the registrant upon the exercise of warrants to purchase the registrant’s Common Stock.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission, acting pursuant to said section 8(a), may determine.
THE INFORMATION IN THIS PROSPECTUS IS NOT COMPLETE AND MAY BE CHANGED. THE SELLING STOCKHOLDERS MAY NOT SELL THESE SECURITIES PUBLICLY UNTIL THE REGISTRATION STATEMENT FILED WITH THE SECURITIES AND EXCHANGE COMMISSION IS EFFECTIVE. THIS PROSPECTUS IS NOT AN OFFER TO SELL THESE SECURITIES AND IT IS NOT SOLICITING AN OFFER TO BUY THESE SECURITIES IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED.
SUBJECT TO COMPLETION, DATED JANUARY 22, 2007
PRELIMINARY PROSPECTUS
32,400,000 SHARES OF COMMON STOCK
CHINA BIOPHARMA, INC.
COMMON STOCK
This prospectus relates to the sale by the Selling Stockholders identified in this prospectus of up to a total of 18,000,000 shares of our common stock following the issuance of such shares by the Company (i) upon the repayment of principal and interest on the outstanding $3,000,000 aggregate principal amount of our Secured Convertible Promissory Notes due December 13, 2008 (the “Notes”) and/or (ii) upon the conversion of the Notes by the holders thereof at an initial conversion price of $0.25 per share subject to adjustment under certain circumstances (collectively, the “Note Shares”).
This prospectus also relates to the sale by the Selling Stockholders identified in this prospectus of up to an aggregate of 14,400,000 shares of our common stock issuable upon the exercise of common stock purchase warrants (collectively, the “Warrant Shares”), which includes:
· 6,000,000 shares of our common stock issuable upon the exercise of Class A Warrants to purchase our common stock. The Class A Warrants were issued in connection with the issuance and sale of the Notes and have an exercise price of $0.30 per share subject to adjustment under certain circumstances; and.
· 6,000,000 shares of our common stock issuable upon the exercise of Class B Warrants to purchase our common stock. The Class B Warrants were issued in connection with the issuance and sale of the Notes and have an exercise price of $0.40 per share subject to adjustment under certain circumstances; and
· 2,400,000 shares of our common stock issuable upon the exercise of Finders Warrants to purchase our common stock. The Finders Warrants were issued in connection with the issuance and sale of the Notes and have an exercise price of $0.30 per share subject to adjustment under certain circumstances.
We will not receive any of the proceeds from the sale of shares by the Selling Stockholders. However, we will receive the proceeds from any exercise of warrants to purchase the Warrant Shares to be sold hereunder to the extent that the Selling Stockholders do not perform cashless exercises. We will also receive the benefit of the reduction in our outstanding indebtedness in consideration for the issuance of the Note Shares to be sold hereunder. See “Use of Proceeds.”
We have agreed to pay the expenses in connection with the registration of these shares.
Our common stock is quoted on the OTC Bulletin Board (“OTCBB”) under the trading symbol “CBPC.” The last reported bid price for our common stock on the OTCBB on January 18, 2007 was $0.37 per share.
Investing in our common stock involves risk. You should carefully consider the risk factors beginning on page 7 of this prospectus before purchasing share of our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2007
TABLE OF CONTENTS
| | Page |
| | |
| SUMMARY | 1 |
| | |
| NOTE REGARDING FORWARD-LOOKING STATEMENTS | 5 |
| | |
| THE OFFERING | 6 |
| | |
| RISK FACTORS | 7 |
| | |
| USE OF PROCEEDS | 19 |
| | |
| SELECTED CONSOLIDATED FINANCIAL DATA | 20 |
| | |
| MANAGEMENT’S DISCUSSION AND ANALYSIS OR PLAN OF OPERATION | 21 |
| | |
| DESCRIPTION OF BUSINESS | 30 |
| | |
| DIRECTORS AND EXECUTIVE OFFICERS | 38 |
| | |
| EXECUTIVE COMPENSATION | 39 |
| | |
| BUSINESS RELATIONSHIPS AND RELATED TRANSACTIONS | 47 |
| | |
| SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT | 48 |
| | |
| SELLING STOCKHOLDERS | 50 |
| | |
| PLAN OF DISTRIBUTION | 53 |
| | |
| DESCRIPTION OF SECURITIES | 55 |
| | |
| DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES | 57 |
| | |
| LEGAL MATTERS | 58 |
| | |
| EXPERTS | 58 |
| | |
| WHERE YOU CAN FIND MORE INFORMATION | 58 |
SUMMARY
This summary highlights material information about us that is described more fully elsewhere in this prospectus. It may not contain all of the information that you find important. You should carefully read this entire document, including the “Risk Factors” section beginning on page 7 of this prospectus and our financial statements and their related notes to those statements appearing elsewhere in this prospectus before making a decision to invest in our common stock. Unless otherwise indicated in this prospectus or the context otherwise requires, references to “we,” “us,” “Techedge,” “the Company” or “our Company” refer to China Biopharma, Inc. and its consolidated subsidiaries.
The Company is a provider of biopharmaceutical products with its focus mainly in human vaccines. Currently, the Company develops its products in China and distributes these products in China and in one other country. The Company has established its distribution and development platform in China through its acquisition of its interest in its subsidiary, Hainan CITIC Bio-pharmaceutical Development Co., Ltd. (“HCBD”) and through its joint venture with Zhejiang Tianyuan Bio-pharmaceutical Co., Ltd.
The emphasis of the Company’s business is on the development of technology and the marketing of products rather than on manufacturing. It is the Company’s goal to operate efficiently and in compliance with applicable regulations and to reduce the risk of any potential factory contaminations with respect to its products. The Company believes that to date, it has been successful in establishing business relationships with a number of local and global manufacturers with the goal of introducing the most advanced technologies and best products to the market in a timely fashion. The Company believes that it has built an experienced and capable management team that will be able to work toward successfully implementing its business plan. As of December 31, 2006, we employed 57 individuals in the United States and China.
Products and Services
The Company’s products are mainly human vaccines for preventing and treating various diseases and illnesses. Currently, the Company provides and distributes its products in China and also exports them to Macedonia.
Preventive Vaccines
The Company currently distributes influenza vaccines manufactured by its joint venture partner, Zhejiang Tianyuan Bio-pharmaceutical Co., Ltd. Flu vaccine is a seasonal product that is used mainly during late the fall and early winter seasons. Every year, the World Health Organization makes recommendations as to what virus strain should be used for the upcoming season and vaccines are manufactured based upon such recommendations.
The Company plans to distribute more preventive vaccines in China once they becoming available, including vaccines for Japanese encephalitis, hepatitis B, allergies, and rabies.
Immunotherapy Products
Immunotherapy (also known as biologic therapy or biotherapy) is a treatment that focuses on certain parts of the immune system to fight various diseases, including cancer. This may be done by either:
| · | Stimulating a patient’s own immune system to work harder or smarter; or |
| · | Giving a patient’s immune system certain natural or man-made components, such as synthetic immune system proteins |
Immunotherapy is sometimes used by itself to treat cancer and other illness, but it is most often used along with or after another type of treatment to add to its effects.
Currently, the Company is working with several international companies on a number of immunotherapy products, including therapeutic vaccines to treat cancers and other illnesses for the Chinese and international markets. In order to bring such product into a country, the local regulations usually require the performance of specific clinical studies and the registration of the subject products.
Other Products and Services
Through its subsidiary, HCBD, the Company may also utilize its distribution platforms and logistics to distribute other medical products and to provide logistic services for other biopharmaceutical companies.
Corporate Summary
The Company was incorporated as Techedge, Inc. (“Techedge”) in Delaware in July 2002 to serve as the successor to the business and interests of BSD Development Partners, LTD. (“BSD”). BSD was a Delaware limited partnership formed in 1997 for the purpose of investing in the intellectual property of emerging and established companies BSD endeavored to continue the business of BSD and sought to enhance the liquidity of the securities owned by its investors merged with Techedge in September 2002. From September 2002 until June 2004, Techedge endeavored to continue the business of BSD and sought to enhance the liquidity of the securities owned by its investors by becoming subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and by seeking to have its common stock quoted on the OTC Bulletin Board, or “OTCBB.”
On June 9, 2004, Techedge acquired all of the issued and outstanding stock of China Quantum Communication Limited (“CQCL”) pursuant to a share exchange agreement, by and among Techedge, certain of its stockholders, CQCL and its stockholders (the “Share Exchange”). Following the Share Exchange, Techedge refocused its business efforts on developing and providing its IP-based Personal Communication Service, a regional mobile VoIP service delivered on unlicensed low power PCS frequencies through IP-enabled local transceiver and IP centric soft-switched networks, operating on an advanced proprietary software centric multi-service global communication service platform and management system. Techedge continued operating CQCL's communications service business through CQCL and CQCL's wholly-owned subsidiaries, China Quantum Communications Inc., a Delaware corporation, and Guang Tong Wang Luo (China) Co. Ltd. (also known as Quantum Communications (China) Co., Ltd.), a Chinese company (“QCCN”).
On January 26, 2006, the Company announced its plans to re-position itself for bio-pharmaceutical and other high growth opportunities in China, while continuing its commercialization of its high potential Mobile Voice over IP solutions.
In conjunction with the Company’s re-positioning plans, on February 27, 2006 the Company entered into an agreement to transfer ownership of its Chinese subsidiary Zheiiang Guang Tong Wang Luo Co., Ltd (ZJQC) to third parties. On January 1, 2006, the Company also entered into an agreement to transfer ownership of its U.S. subsidiary China Quantum Communications, Inc. to a former employee.
During the quarter ended June 30, 2006, the Company entered into a Share Exchange Agreement for the purpose of acquiring 100% of the outstanding capital stock of China BioPharma Limited (“CBL”) a Cayman Islands Company, which has rights to invest in Tianyuan Bio-Pharmaceuticals Company, Ltd. and Zhejiang Tianyuan Biotech Co., Ltd. (“ZTBC”). In exchange for 100% of the outstanding capital of CBL, the Company issued a total of 3,000,000 shares of restricted common stock.
On July 14, 2006, Techedge and China Biopharma, Inc, a Delaware corporation and a wholly-owned subsidiary of Techedge (“CB”) executed and delivered a Plan and Agreement of Merger whereby the parties agreed to merge CB with and into Techedge, with Techedge being the surviving corporation (the “Merger”). By virtue of, and effective upon the consummation of the Merger, the Certificate of Incorporation of the Company was amended to change its name from “Techedge, Inc.” to “China Biopharma, Inc.” The Merger became effective on August 10, 2006.
Subsidiaries
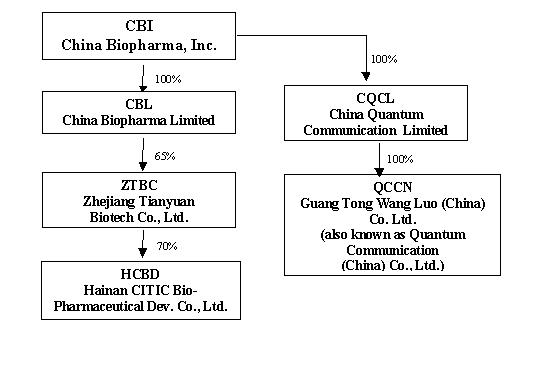
The Company currently has two operational segments. One segment, consisting of CBL and its subsidiaries, provides biopharmaceutical products. The other segment, which consists of CQCL and its subsidiary, was engaged in the business of providing telecommunications services and developing related technology. Following the Company’s re-positioning of its business focus from telecommunications to biopharmaceutical operations, CQCL ceased carrying on any daily business activities and is currently looking for strategic partners in order to continue its business. Set forth below is a graphic representation of the current organizational structure of the Company and its subsidiaries.
RECENT DEVELOPMENTS
Acquisitions and Investments
Zhejiang Tianyuan Biotech Co., Ltd.
On December 22, 2006, the Company paid $1,950,000 as consideration for a 65% interest in ZTBC. ZTBC is a Sino-US joint venture between CBL and Zhejiang Tianyuan Bio-pharmaceutical Co., Ltd. which owns the remaining 35% interest. ZTBC was formed to engage in the business of developing and marketing vaccines. The Company’s joint venture partner, Zhejiang Tianyuan Bio-pharmaceutical Co., Ltd, has agreed to perform all of the manufacturing functions on behalf of ZTBC.
Hainan CITIC Bio-pharmaceutical Development Co., Ltd.
In April 2006, ZTBC acquired 20% of the outstanding stock of HCBD from three individuals in consideration for a payment of $600,000; In August 2006, ZTBC acquired an additional 40% of the outstanding stock of HCBD from CITIC Pharmaceutical and China Biological Engineering Corporation in consideration for a payment of $1,200,000; In December 2006, ZTBC acquired another 10% of the outstanding stock of HCBD from one individual in consideration for a payment of $300,000. The remaining 30% of HCBD is owned by Zhejiang Tianyuan Bio-pharmaceutical Co., Ltd. (20%) and by one of its original owners (10%).
HCBD is a nationwide bio-pharmaceutical distributor in China and has established a distribution platform including “cold-chain” logistics which are the refrigeration logistics in the distribution chain. HCBD distributes the vaccines provided by local vaccine manufacturers, including ZTBC and Zhejiang Tianyuan Bio-pharmaceutical Co., Ltd and other bio-pharmaceutical products made by large global drug manufactures, such as Merck.
Financing Transaction
On December 13, 2006, the Company entered into a Subscription Agreement with respect to the issuance and sale of $3,000,000 aggregate principal amount of its Secured Convertible Promissory Notes due December 13, 2008. The Notes are convertible at the option of the holders at any time into shares of the Company's common stock. Prior to the occurrence of an Event of Default (as defined in the Notes), the Notes are convertible at a per share conversion price equal to $0.25 per share. Following the occurrence of an Event of Default (as defined in the Notes), the Notes are convertible at the lesser of $0.25 per share and 75% of the average of the closing bid prices for the common stock for the five trading days prior to the date of conversion. The Notes bear interest at a rate of eight percent (8%) per annum. Monthly payments, consisting of principal of and accrued interest on the Notes shall commence March 13, 2007. The Company may, at its option pay the monthly payments in the form of either cash or shares of common stock. In the event that the Company elects to pay the monthly amount in cash, the Company shall be obligated to pay 115% of the principal amount component of the monthly amount and 100% of all other components of the monthly amount. In the event that the Company elects to pay the monthly amount in shares of common stock, the stock shall be valued at an applicable conversion rate equal to the lesser of $0.25 per share or seventy five percent (75%) of the average of the closing bid price of the common stock on the principal market on which the common stock is then traded or included for quotation for the five trading days preceding the applicable repayment date. Provided that an Event of Default has not occurred, the Company may, at its option, prepay the outstanding principal amount of the Notes, in whole or in part, at any time upon 30 days written notice to the holders by paying 120% of the principal amount to be repaid together with accrued interest plus any other sums due thereon to the date of redemption. The Notes are secured by a Security Agreement entered into by and among the Company, CQCL, CBL, and QCCN and Barbara R. Mittman, as collateral agent for the purchasers of the Notes. The obligations of the Company under the Subscription Agreement with respect to the Notes and the Notes are guaranteed by the CQCL, CBL and QCCN pursuant to a Guaranty, dated as of December 13, 2006, entered into by the CQCL, CBL and QCCN, for the benefit of the purchasers of the Notes.
In connection with the sale of the Notes, the Company also issued to the purchasers of the Notes, Class A Warrants to purchase up to an aggregate of 6,000,000 shares of common stock and Class B Warrants to purchase up to an aggregate of 6,000,000 shares of common stock (each a “Warrant” and collectively, the “Warrants”). One Class A Warrant and one Class B Warrant were issued for each two shares of common stock that would have been issuable on the closing date assuming the complete conversion of the Notes on such date. The Class A Warrants have an exercise price of $0.30 per share and the Class B Warrants have an exercise price of $0.40.
Melton Management Ltd. acted as the finder with respect to the issuance and sale of the Notes and received a warrant to purchase 2,400,000 shares of our common stock at an exercise price of $0.30 per share.
As January 17, 2007, the Company had 85,520,000 shares of common stock issued and outstanding.
Our principal executive offices are located at 31 Airpark Road, Princeton, New Jersey 08540. Our telephone number at that address is (609) 651-8588. Our corporate website is www.chinabiopharma.net. Information contained on or accessed through our website is not intended to constitute and shall not be deemed to constitute part of this prospectus.
NOTE REGARDING FORWARD-LOOKING STATEMENTS
The statements contained in this Form SB-2 that are not purely historical are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Exchange Act. These include statements about the Company’s expectations, beliefs, intentions or strategies for the future, which are indicated by words or phrases such as “anticipate,” “expect,” “intend,” “plan,” “will,” “the Company believes,” “management believes” and similar words or phrases. The forward-looking statements are based on the Company’s current expectations and are subject to certain risks, uncertainties and assumptions. The Company’s actual results could differ materially from results anticipated in these forward-looking statements. All forward-looking statements included in this document are based on information available to the Company on the date hereof, and the Company assumes no obligation to update any such forward-looking statements.
THE OFFERING
| Common stock being offered by Selling Stockholders | | Up to 32,400,000 shares |
| | | |
| OTCBB Symbol | | CBPC |
| | | |
| Risk Factors | | The securities offered by this prospectus are speculative and involve a high degree of risk and investors purchasing securities should not purchase the securities unless they can afford the loss of their entire investment. See “Risk Factors” beginning on page 7. |
RISK FACTORS
An investment in our common stock is speculative and involves a high degree of risk and uncertainty. You should carefully consider the risks described below, together with the other information contained in this prospectus, including the consolidated financial statements and notes thereto of our company, before deciding to invest in our common stock. The risks described below are not the only ones facing our Company. Additional risks not presently known to us or that we presently consider immaterial may also adversely affect our Company. If any of the following risks occur, our business, financial condition and results of operations and the value of our common stock could be materially and adversely affected.
Risks Related to Our Business
We have reported losses from operations in every year of our operating history.
We have never generated profits from operations in any year. At September 30, 2006, we had an accumulated deficit of $8.78 million. We will need to significantly increase our annual revenue to achieve profitability. We may not be able to do so. Even if we do achieve profitability, we cannot assure you that we will be able to sustain or increase profitability on a quarterly or annual basis in the future.
We have incurred significant expenses in the past. Although we cannot quantify the amount, we expect expenses to continue to increase for the remainder of 2007 and to continue to incur losses.
We may not be able to obtain sufficient funds to grow our business.
We are a development stage company. Due to the nature and the stage of our Company, we require additional capital to fund some or all of the following:
| · | Commercialization of our products and services; |
| · | Marketing and sales expenses targeting market segments; |
| · | Unanticipated opportunities; |
| · | Potential acquisitions; |
| · | Changing business conditions; and |
| · | Unanticipated competitive pressures. |
There can be no assurance that we will be able to raise such capital on favorable terms or at all. If we are unable to obtain such additional capital, we may be required to reduce the scope of our business, which could have a material adverse effect on our business, financial condition and results of operations.
The market in which we compete is highly competitive, fast-paced and fragmented, and we may not be able to maintain market share.
We compete with other companies, many of whom are developing or can be expected to develop products similar to ours. Our market is a large market with many competitors. Many of our competitors are more established than we are, and have significantly greater financial, technical, marketing and other resources than we presently possess. Many of our competitors also have greater name recognition and a larger customer base.
We expect competition to persist and intensify in the future. Our principal competitors are bio-pharmaceutical companies such as Tiantan Bio-Pharmaceutical Co., Ltd. in China and large multi-national corporations such as Merck and GlaxosmithKline. We also compete with a number of other, smaller local distributors including local government owned companies. We face the risk that new competitors with greater resources than ours will enter our market, and that increasing competition will result in lower prices. If we must significantly reduce our prices, the decrease in revenues could adversely affect our profitability. We also face the risks that our competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers, and adopt more aggressive pricing policies.
Our products must keep pace with developments in our industry or they may be displaced by competitors’ products. Our industry is characterized by rapid product development, with significant competitive advantages gained by companies that introduce products that are first to market, deliver constant innovation in products and techniques, offer frequent new product introductions and have competitive prices. Our future growth partially depends on our ability to develop products that are more effective in meeting consumer needs. In addition, we must be able to manufacture and effectively market those products. The sales of our existing products may decline if a competing product is introduced by other companies.
The success of our new product offerings depends upon a number of factors, including our ability to accurately anticipate consumer needs, innovate and develop new products, successfully commercialize new products in a timely manner, price our products competitively, manufacture and deliver our products in sufficient volumes and in a timely manner and differentiate our product offerings from those of our competitors. If we fail to make sufficient investments in research and pay close attention to consumer needs or we focus on technologies that do not lead to more effective products, our current and future products could be surpassed by more effective or advanced products of others.
Establishing and expanding international operations requires significant management attention.
Substantially all of our current revenues are derived from China. We intend to expand our international operations which, if not planned and managed properly, could materially adversely affect our business, financial condition and operating results. Expanding internationally exposes us to legal uncertainties, new regulatory requirements, liability, export and import restrictions, tariffs and other trade barriers, difficulties in managing operations across disparate geographic areas, foreign currency fluctuations, dependence on local distributors and potential disruptions in sales or manufacturing due to military or terrorist acts, as well as longer customer payment cycles and greater difficulties in collecting accounts receivable. We may also face challenges in protecting our intellectual property or avoiding infringement of others’ rights, and in complying with potentially uncertain or adverse tax laws.
Risks related to our strategy and risks related to our inability to carry out such strategy
Our strategy may be based on wrong assumptions, may be seriously flawed and may even damage our performance, competitive position in the market and our ability to survive in the market place. Even if our strategy is correct, we may never be able to successfully implement our strategy or to implement it in the desired fashion.
Risks related to the implementation of our operational and marketing plan
Our operational plan and marketing plan may be seriously flawed and may damage our performance, competitive position in the market and even our ability to survive in the market place. Even if our operational plan and marketing plan are correct, we may never be able to successfully implement these plans or implement our strategy in the desired fashion.
Risks related to our products and services
Our products and services involve direct or indirect impact on human health and life. The drugs, products and services provided may be flawed and cause dangerous side effects and even fatality in certain cases and lead to major business losses and legal and other liabilities and damages to us.
Risks related to product liability claims
We face the risk of loss resulting from, and adverse publicity associated with, product liability lawsuits, whether or not such claims are valid. We may not be able to avoid such claims. In addition, our product liability insurance may not be adequate to cover such claims and we may not be able to obtain adequate insurance coverage in the future at acceptable costs. A successful product liability claim that exceeds our policy limits could require us to pay substantial sums.
Risks related to our technology and our platforms
Our technologies and platforms may be seriously defective and flawed producing wrong and harmful results, exposing us to significant liabilities. Even if they are not defective or flawed, these technologies and platforms may become outdated, losing their value and thus affect our competitive advantages.
Marketing risks
Newly developed drugs and technologies may not be compatible with market needs. Because markets for drugs differentiate geographically inside China, we must develop and manufacture our products to accurately target specific markets to ensure product sales. If we fail to invest in extensive market research to understand the health needs of consumers in different geographic areas, we may face limited market acceptance of our products, which could have material adverse effect on our sales and earning.
Risks relating to difficulty in defending intellectual property rights from infringement
Our success depends on our ability to protect our current and future technologies and products and to defend our intellectual property rights. If we fail to protect our intellectual property adequately, competitors may manufacture and market products similar to ours. We expect to file patent applications seeking to protect newly developed technologies and products in various countries, including China. Some patent applications in China are maintained in secrecy until the patent is issued. Because the publication of discoveries tends to follow their actual discovery by many months, we may not be the first to invent, or file patent applications on any of our discoveries. Patents may not be issued with respect to any of our patent applications and existing or future patents issued to or licensed by us may not provide competitive advantages for our products. Patents that are issued may be challenged, invalidated or circumvented by our competitors. Furthermore, our patent rights may not prevent our competitors from developing, using or commercializing products that are similar or functionally equivalent to our products.
We also rely on trade secrets, non-patented proprietary expertise and continuing technological innovation that we shall seek to protect, in part, by entering into confidentiality agreements with licensees, suppliers, employees and consultants. These agreements may be breached and there may not be adequate remedies in the event of a breach. Disputes may arise concerning the ownership of intellectual property or the applicability of confidentiality agreements. Moreover, our trade secrets and proprietary technology may otherwise become known or be independently developed by our competitors. If patents are not issued with respect to products arising from research, we may not be able to maintain the confidentiality of information relating to these products.
Risks relating to third parties that may claim that we infringe on their proprietary rights and may prevent us from manufacturing and selling certain of our products
There has been substantial litigation in the pharmaceutical industry with respect to the manufacturing, use and sale of new products. These lawsuits relate to the validity and infringement of patents or proprietary rights of third parties. We may be required to commence or defend against charges relating to the infringement of patent or proprietary rights. Any such litigation could:
| · | require us to incur substantial expense, even if we are insured or successful in the litigation; |
| · | require us to divert significant time and effort of our technical and management personnel; |
| · | result in the loss of our rights to develop or make certain products; and |
| · | require us to pay substantial monetary damages or royalties in order to license proprietary rights from third parties. |
Although patent and intellectual property disputes within the pharmaceutical industry have often been settled through licensing or similar arrangements, costs associated with these arrangements may be substantial and could include the long-term payment of royalties. These arrangements may be investigated by regulatory agencies and, if improper, may be invalidated. Furthermore, the required licenses may not be made available to us on acceptable terms. Accordingly, an adverse determination in a judicial or administrative proceeding or a failure to obtain necessary licenses could prevent us from manufacturing and selling some of our products or increase our costs to market these products.
In addition, when seeking regulatory approval for some of our products, we are required to certify to regulatory authorities, including the PRC State Food and Drug Administration (the “SFDA”), that such products do not infringe upon third party patent rights. Filing a certification against a patent gives the patent holder the right to bring a patent infringement lawsuit against us. Any lawsuit would delay the receipt of regulatory approvals. A claim of infringement and the resulting delay could result in substantial expenses and even prevent us from manufacturing and selling certain of our products.
Our launch of a product prior to a final court decision or the expiration of a patent held by a third party may result in substantial damages to us. If we are found to infringe a patent held by a third party and become subject to such damages, these damages could have a material adverse effect on the results of our operations and financial condition.
Risks related to acquisitions
Part of our strategy involves acquisitions of other companies and products and technologies. We may not be able to complete successfully such acquisitions due to the lack of capital and other factors. Even if we can complete such acquisitions, we may not be able to absorb and integrate the acquired operation and assets successfully into our currently operation. We may even make acquisitions that ultimately do not enhance our business.
Risks related to financial reports and estimates
Our Company is subject to critical accounting policies and actual results may vary from our estimates. Our Company follows generally accepted accounting principles for the United States in preparing its financial statements. As part of this work, we must make many estimates and judgments concerning future events. These affect the value of the assets and liabilities, contingent assets and liabilities, and revenue and expenses reported in our financial statements. We believe that these estimates and judgments are reasonable, and we make them in accordance with our accounting policies based on information available at the time. However, actual results could differ from our estimates, and this could require us to record adjustments to expenses or revenues that could be material to our financial position and results of operations in the future.
Risks related to significant financing needs
We need significant amount of capital to invest in our research and development, in our acquisitions and in our operations. We may not be able to identify and raise sufficient capital in a timely manner to finance our research and development activity, operation, acquisitions, growth and even survival. Even if such financings are available, they may not be timely or sufficient or on the terms desirable, acceptable or not harmful to our existing stockholders.
Risks related to growth and the ability to manage growth
For our Company to survive and to succeed, we have to consistently grow. However, the management and we may not be able to achieve or manage such growth. The inability to achieve and maintain and manage growth will significantly affect our survival and market position.
Risks related to our capital structure
Insiders have substantial control over us, and they could delay or prevent a change in our corporate control even if our other stockholders wanted it to occur.
Our executive officers, directors, and principal stockholders who hold 5% or more of the outstanding common stock and their affiliates beneficially owned as of January 17, 2007, in the aggregate, approximately 77.06% of our outstanding common stock. These stockholders will be able to exercise significant control over all matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This could delay or prevent an outside party from acquiring or merging with us even if our other stockholders wanted it to occur.
Dependence on key personnel
We depend on our key management and technological personnel. The unavailability or departure of such key personnel may seriously disrupt and harm our operations, business and the implementation of our business strategy and plans. Although most of these personnel are founders and stockholders of our Company, there can be no assurance that we can be successful in retaining them.
Risks related to not declaring or paying any dividends to our stockholders
We did not declare any dividends for the years ended December 31, 2005 or 2006. Our board of directors does not intend to distribute dividends in the near future. The declaration, payment and amount of any future dividends will be made at the discretion of the board of directors, and will depend upon, among other things, the results of our operations, cash flows and financial condition, operating and capital requirements, and other factors as the board of directors considers relevant. There is no assurance that future dividends will be paid, and if dividends are paid, there is no assurance with respect to the amount of any such dividend.
Most of our assets are located in China, any dividends of proceeds from liquidation is subject to the approval of the relevant Chinese government agencies.
Our assets are predominantly located inside China. Under the laws governing foreign invested enterprises in China, dividend distribution and liquidation are allowed but subject to special procedures under the relevant laws and rules. Any dividend payment will be subject to the decision of the board of directors and subject to foreign exchange rules governing such repatriation. Any liquidation is subject to both the relevant government agency's approval and supervision as well the foreign exchange control. This may generate additional risk for our investors in case of dividend payment and liquidation.
Risks Associated With Doing Business in China
We are subject to the risks associated with doing business in the People’s Republic of China.
Because most of our operations are conducted in China, we are subject to special considerations and significant risks not typically associated with companies operating in North America and Western Europe. These include risks associated with, among others, the political, economic and legal environments and foreign currency exchange. Our results may be adversely affected by changes in the political and social conditions in China, and by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Although the majority of productive assets in China are owned by the Chinese government, in the past several years the government has implemented economic reform measures that emphasize decentralization and encourage private economic activity. Because these economic reform measures may be inconsistent or ineffectual, there are no assurances that:
| | · | We will be able to capitalize on economic reforms; |
| | · | The Chinese government will continue its pursuit of economic reform policies; |
| | · | The economic policies, even if pursued, will be successful; |
| | · | Economic policies will not be significantly altered from time to time; and |
| | · | Business operations in China will not become subject to the risk of nationalization. |
Economic reform policies or nationalization could result in a total investment loss in our common stock.
Since 1979, the Chinese government has reformed its economic systems. Because many reforms are unprecedented or experimental, they are expected to be refined and improved. Other political, economic and social factors, such as political changes, changes in the rates of economic growth, unemployment or inflation, or in the disparities in per capita wealth between regions within China, could lead to further readjustment of the reform measures. This refining and readjustment process may negatively affect our operations.
Over the last few years, China’s economy has registered a high growth rate. Recently, there have been indications that rates of inflation have increased. In response, the Chinese government has taken measures to curb this excessively expansive economy. These measures include restrictions on the availability of domestic credit, reducing the purchasing capability of certain of its customers, and limited re-centralization of the approval process for purchases of some foreign products. The Chinese government may adopt additional measures to further combat inflation, including the establishment of freezes or restraints on certain projects or markets. These measures may adversely affect our manufacturing operations.
To date, reforms to China’s economic system have not adversely impacted our operations and are not expected to adversely impact operations in the foreseeable future. However, we cannot assure you that the reforms to China’s economic system will continue or that we will not be adversely affected by changes in China’s political, economic, and social conditions and by changes in policies of the Chinese government, such as changes in laws and regulations, measures which may be introduced to control inflation and changes in the rate or method of taxation.
On November 11, 2001, China signed an agreement to become a member of the World Trade Organization (“WTO”), the international body that sets most trade rules, further integrating China into the global economy and significantly reducing the barriers to international commerce. China’s membership in the WTO was effective on December 11, 2001. China has agreed upon its accession to the WTO to reduce tariffs and non-tariff barriers, remove investment restrictions and provide trading and distribution rights for foreign firms. The tariff rate reductions and other enhancements will enable us to develop better investment strategies. In addition, the WTO’s dispute settlement mechanism provides a credible and effective tool to enforce members’ commercial rights. Also, with China’s entry to the WTO, it is believed that the relevant laws on foreign investment in China will be amplified and will follow common practices.
The Chinese legal system is not fully developed and has inherent uncertainties that could limit the legal protections available to investors.
The Chinese legal system is a system based on written statutes and their interpretation by the Supreme People’s Court. Prior court decisions may be cited for reference but have limited legal precedents. Since 1979, the Chinese government has been developing a comprehensive system of commercial laws, and considerable progress has been made in introducing laws and regulations dealing with economic matters such as foreign investment, corporate organization and governance, commerce, taxation and trade. Two examples are the promulgation of the Contract Law of the People’s Republic of China to unify the various economic contract laws into a single code, which went into effect on October 1, 1999, and the Securities Law of the People’s Republic of China, which went into effect on July 1, 1999. However, because these laws and regulations are relatively new, and because of the limited volume of published cases and their non-binding nature, interpretation and enforcement of these laws and regulations involve uncertainties. In addition, as the Chinese legal system develops, changes in such laws and regulations, their interpretation or their enforcement may have a material adverse effect on our business operations.
Enforcement of regulations in China may be inconsistent.
Although the Chinese government has introduced new laws and regulations to modernize its securities and tax systems on January 1, 1994, China does not yet possess a comprehensive body of business law. As a result, the enforcement, interpretation and implementation of regulations may prove to be inconsistent and it may be difficult to enforce contracts.
We may experience lengthy delays in resolution of legal disputes.
As China has not developed a dispute resolution mechanism similar to the Western court system, dispute resolution over Chinese projects and joint ventures can be difficult and we cannot assure you that any dispute involving our business in China can be resolved expeditiously and satisfactorily.
Impact of the United States Foreign Corrupt Practices Act on our business.
We are subject to the United States Foreign Corrupt Practices Act, which generally prohibits United States companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. Foreign companies, including some that may compete with us, are not subject to these prohibitions. Corruption, extortion, bribery, pay-offs, theft and other fraudulent practices occur from time-to-time in mainland China. We have attempted to implement safeguards to prevent and discourage such practices by our employees and agents. We cannot assure you, however, that our employees or other agents will not engage in such conduct for which we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations.
Impact of governmental regulation on our operations.
We may be subjected to liability for product safety that could lead to a product recall. Our operations and properties are subject to regulation by various Chinese government entities and agencies, in particular, the SFDA. Our operations are also subject to production, packaging, quality, labeling and distribution standards. In addition, our facilities are also subject to various local environmental laws and workplace regulations. We believe that our current legal and environmental compliance programs adequately address such concerns and that we are in substantial compliance with applicable laws and regulations. However, compliance with, or any violation of, current and future laws or regulations could require material expenditures or otherwise adversely effect our business and financial results.
We may be liable if the use of any of our products causes injury, illness or death. We may also be required to recall certain of our products that become contaminated or are damaged. Any product liability judgment or a product recall could have a material adverse effect on our business or financial results.
Moreover, the laws and regulations regarding acquisitions of the pharmaceutical industry in the PRC may also change and may significantly impact our ability to grow through acquisitions.
It may be difficult to serve us with legal process or enforce judgments against our management or us.
Most of our assets are located in China. In addition, most of our directors and officers are non-residents of the United States, and all, or substantial portions of the assets of such non-residents, are located outside the United States. As a result, it may not be possible to effect service of process within the United States upon such persons. Moreover, there is doubt as to whether the courts of China would enforce:
| · | Judgments of United States courts against us, our directors or our officers based on the civil liability provisions of the securities laws of the United States or any state; or |
| · | Original actions brought in China relating to liabilities against non-residents or us based upon the securities laws of the United States or any state. |
The Chinese government could change its policies toward private enterprise or even nationalize or expropriate it, which could result in the total loss of your investment.
Our business is subject to significant political and economic uncertainties and may be adversely affected by political, economic and social developments in China. Over the past several years, the Chinese government has pursued economic reform policies including the encouragement of private economic activity and greater economic decentralization. The Chinese government may not continue to pursue these policies or may significantly alter them to our detriment from time to time with little, if any, prior notice. Changes in policies, laws and regulations or in their interpretation or the imposition of confiscatory taxation, restrictions on currency conversion, restrictions or prohibitions on dividend payments to stockholders, devaluations of currency or the nationalization or other expropriation of private enterprises could have a material adverse effect on our business. Nationalization or expropriation could even result in the total loss of our investment in China and in the total loss of your investment.
If political relations between the United States and China worsen, our stock price may decrease and we may have difficulty accessing U.S. capital markets.
At various times during recent years, the United States and China have had significant disagreements over political and economic issues. Controversies may arise in the future between these two countries. Any political or trade controversies between the United States and China, whether or not directly related to our business, could adversely affect the market price of our common stock and our ability to access U.S. capital markets.
Foreign Exchange Control Risks
Fluctuations in the value of the Chinese Renminbi relative to foreign currencies could affect our operating results.
Substantially all our revenues and expenses are currently denominated in the Chinese Renminbi. However, we use denominations in United States dollars for financial reporting purposes. The value of Chinese Renminbi against the United States dollar and other currencies may fluctuate and is affected by, among other things, changes in China’s political and economic conditions. The Chinese government recently announced that it is valuing the exchange rate of the Chinese Renminbi against a number of currencies, rather than just exclusively to the United States dollar. Although the Chinese government has stated its intention to support the value of the Chinese Renminbi, we cannot assure you that the government will not revalue it. As our operations are primarily in China, any significant revaluation of the Chinese Renminbi may materially and adversely affect our cash flows, revenues and financial condition. For example, to the extent that we need to convert United States dollars into Chinese Renminbi for our operations, appreciation of this currency against the United States dollar could have a material adverse effect on our business, financial condition and results of operations. Conversely, if we decide to convert our Chinese Renminbi into United States dollars for other business purposes and the United States dollar appreciates against this currency, the United States dollar equivalent of the Chinese Renminbi we convert would be reduced. To date, we have not engaged in any hedging transactions in connection with our operations.
The PRC government imposes control over the conversion of the Chinese Renminbi into foreign currencies. Under the current unified floating exchange rate system, the People’s Bank of China publishes an exchange rate, which we refer to as the PBOC exchange rate, based on the previous day’s dealings in the inter-bank foreign exchange market. Financial institutions authorized to deal in foreign currency may enter into foreign exchange transactions at exchange rates within an authorized range above or below the PBOC exchange rate according to market conditions.
Pursuant to the Foreign Exchange Control Regulations of the PRC issued by the State Council which came into effect on April 1, 1996, and the Regulations on the Administration of Foreign Exchange Settlement, Sale and Payment of the PRC which came into effect on July 1, 1996, regarding foreign exchange control, conversion of Renminbi into foreign exchange by Foreign Investment Enterprises, or FIEs for use on current account items, including the distribution of dividends and profits to foreign investors, is permissible. FIEs are permitted to convert their after-tax dividends and profits to foreign exchange and remit such foreign exchange to their foreign exchange bank accounts in the PRC.
Conversion of Renminbi into foreign currencies for capital account items, including direct investment, loans, and security investment, is still subject to certain restrictions. On January 14, 1997, the State Council amended the Foreign Exchange Control Regulations and added, among other things, an important provision, which provides that the PRC government shall not impose restrictions on recurring international payments and transfers under current account items.
Enterprises in the PRC (including FIEs) which require foreign exchange for transactions relating to current account items, may, without approval of the State Administration of Foreign Exchange, or SAFE, effect payment from their foreign exchange account or convert and pay at the designated foreign exchange banks by providing valid receipts and proofs.
Convertibility of foreign exchange in respect of capital account items, such as direct investment and capital contribution, is still subject to certain restrictions, and prior approval from the SAFE or its relevant branches must be sought.
Risks Related to Common Stock
Risks of lack of liquidity and volatility
Currently our common stock is quoted in the OTC Bulletin Board market, the liquidity of our common stock may be very limited and affected by its limited trading market. The OTC Bulletin Board market is an inter-dealer market much less regulated than the major exchanges and are subject to abuses and volatilities and shorting. There is currently no broadly followed and established trading market for our common stock. An established trading market may never develop or be maintained. Active trading markets generally result in lower price volatility and more efficient execution of buy and sell orders. Absence of an active trading market reduces the liquidity of the shares traded there.
The trading volume of our common stock may be limited and sporadic. As a result of such trading activity, the quoted price for our common stock on the OTC Bulletin Board may not necessarily be a reliable indicator of its fair market value. Further, if we cease to be quoted, holders would find it more difficult to dispose of, or to obtain accurate quotations as to the market value of our common stock and as a result, the market value of our common stock likely would decline.
Risks related to penny stocks
Our common stock may be subject to regulations prescribed by the Securities and Exchange Commission (the “SEC”) relating to “Penny Stocks.” The SEC has adopted regulations that generally define a penny stock to be any equity security that has a market price (as defined in such regulations) of less than $5.00 per share, subject to certain exceptions. If our common stock meets the definition of a penny stock, it will be subject to these regulations, which impose additional sales practice requirements on broker-dealers who sell such securities to persons other than established customers and accredited investor, generally institutions with assets in excess of $5,000,000 and individuals with a net worth in excess of $1,000,000 or annual income exceeding $200,000 (individually) or $300,000 (jointly with their spouse).
Existing stockholders may experience some dilution
We have issued the Notes and, in conjunction with the Notes, the Class A Warrants, the Class B Warrants and the Finder’s Warrants to purchase, collectively, up to 14,400,000 shares of our common stock, subject to adjustment. We have also previously issued a total of 6,860,616 options and 1,089,118 warrants to purchase our common stock to different investors. Pursuant to the terms of the Notes, we have the right, at our option, to repay the outstanding principal of and interest on the Notes in the form of shares of our common stock. Any such issuance of shares for the purpose of the repayment of the Notes as well as any issuances of shares upon any conversion of the Notes, exercise of the Class A Warrants, the Class B Warrants and the Finder’s Warrants, exercise of outstanding options and exercise of our other outstanding warrants will cause dilution in the interests of our stockholders
Moreover, we may need to raise additional funds in the future to finance new developments or expand existing operations. If we raise additional funds through the issuance of new equity or equity-linked securities, other than on a pro rata basis to our existing stockholders, the percentage ownership of the existing stockholders may be reduced.
Future sales of our common stock may depress our stock price.
Sales of a substantial number of shares of our common stock in the public market could cause a decrease in the market price of our common stock. At January 17, 2007, we had 85,520,000 shares of common stock outstanding. A significant number of our outstanding shares either are eligible for resale to the public without restriction pursuant to Rule 144(k) of the Securities Act of 1933, as amended (the “Securities Act”) or are eligible for resale to the public pursuant to Rule 144 of the Securities Act. At January 17, 2007, options to purchase 6,860,616 shares of our common stock were outstanding of which 5,612,415 were vested, and warrants or other rights to purchase 15,489,118 shares of our common stock (including the 14,400,000 Warrants Shares covered by this prospectus) were outstanding. This prospectus also covers the resale of up to 18,000,000 Note Shares which may be issued by the Company either for the repayment of the outstanding principal of and interest on the Notes or upon conversion of the Notes. We may also issue additional shares of stock in connection with our business and may grant additional stock options to our employees, officers, directors and consultants under our stock option plans or warrants to third parties. If a significant portion of these shares were sold in the public market, the market value of our common stock could be adversely affected.
USE OF PROCEEDS
We will not receive any proceeds from sale of our common stock by our Selling Stockholders. The proceeds from the sale of each Selling Stockholder’s shares of common stock will belong to that Selling Stockholder.
We will not receive any proceeds from the issuance of our common stock to the Selling Stockholders other than the exercise price of any warrants that are exercised by the Selling Stockholders who do not conduct cashless exercises, the proceeds of which we expect to use for working capital. If all 14,400,000 of the Class A Warrants, the Class B Warrants and the Finder’s Warrants were exercised in full for cash, the proceeds to the Company would be approximately $4,920,000.
We will receive the benefit of the reduction in our outstanding indebtedness in consideration for the issuance of shares of our Common Stock to repay outstanding principal and interest on the Notes or upon conversion of the Notes.
SELECTED CONSOLIDATED FINANCIAL DATA
The selected consolidated financial data presented below as of the years ended December 31, 2004 and 2005 and for the nine month periods ended September 30, 2005 and 2006 have been derived from our unaudited consolidated financial statements, which include, in the opinion of management, all adjustments necessary to present fairly the data for such periods. The historical results are not necessarily indicative of results to be expected in any future period. See the notes to the audited consolidated financial statements and to the unaudited consolidated financial statements included elsewhere in the prospectus for more information.
| | | Nine Months Ended
September 30, | | Years Ended December 31, | |
| | | 2006 | | 2005 | | 2005 | | 2004 | |
| | | (in thousands, of US dollars except per share data) | | (in thousands, of US dollars except per share data) | |
STATEMENT OF OPERATIONS DATA | | | | | |
| Revenue | | | - | | $ | 186.9 | | $ | 380.5 | | $ | 312.9 | |
| Net (loss) attributable to common stockholders | | | ($529.6 | ) | | ($2,079.1 | ) | | ($2,841.3 | ) | | ($1,445.6 | ) |
| Net (Loss) per share, basic and diluted | | | ($0.01 | ) | | ($0.03 | ) | | ($0.03 | ) | | ($0.02 | ) |
| | | | | | | | | | | | | | |
BALANCE SHEET DATA | | | | | | | | | | | | | |
| Working Capital (Deficit) | | | ($2,105.7 | ) | | ($1,442.8 | ) | | ($1,818.8 | ) | | ($1,094.4 | ) |
| Total Current Assets | | $ | 404.1 | | $ | 528.4 | | $ | 452.4 | | $ | 324.2 | |
| Total Current Liabilities | | $ | 2,509.8 | | $ | 1,971.2 | | $ | 2,271.2 | | $ | 1,418.6 | |
| Total Stockholders’ Equity (Deficit) | | | ($2,052.3 | ) | | ($956.8 | ) | | ($1,663.3 | ) | | ($547.8 | ) |
MANAGEMENT’S DISCUSSION AND ANALYSIS OR PLAN OF OPERATION
Except for the historical information contained herein, the matters discussed in this “MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS,” and elsewhere in this report are forward-looking statements that involve risks and uncertainties. The factors listed in the section captioned “RISK FACTORS,” as well as any cautionary language in this report, provide examples of risks, uncertainties and events that may cause our actual results to differ materially from those projected. Except as may be required by law, we undertake no obligation to update any forward-looking statement to reflect events after the date of this report. The following discussion should be read in conjunction with our consolidated financial statements and the notes thereto included elsewhere in this prospectus and in conjunction with our “SELECTED CONSOLIDATED FINANCIAL DATA”:
NOTE REGARDING FORWARD-LOOKING STATEMENTS
You should read the following discussion together with the more detailed business information and consolidated financial statements and related notes that appear elsewhere in this prospectus. This prospectus may contain certain “forward-looking” information within the meaning of the Private Securities Litigation Reform Act of 1995. In some cases, you can identify forward-looking statements by our use of words such as “may,” “will,” “should,” “could,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative or other variations of these words, or other comparable words or phrases. This information involves risks and uncertainties. Our actual results may differ materially from the results discussed in the forward-looking statements. Factors that might cause such a difference include, but are not limited to, those discussed in “RISK FACTORS”.
Unless the context requires otherwise, references to "we," "us," "our," "China Biopharma" and the "Company" refer to China Biopharma, Inc. and its consolidated subsidiaries.
CRITICAL ACCOUNTING POLICIES
Set forth below is a summary description of certain of our critical accounting policies. See “Summary of Significant Accounting Policies” in the Notes to the Company’s Consolidated Financial Statements for the year ended December 31, 2005, included elsewhere in this prospectus, for a full description of our critical accounting policies.
Consolidation
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. Significant intercompany accounts and transactions have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Income Taxes
The Company accounts for income taxes in accordance with Statement of Financial Accounting Standards No. 109, Accounting for Income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Because the realization of tax benefits related to the Company’s net deferred tax asset is uncertain, a valuation allowance has been provided against the net deferred tax asset.
Financial Instruments
The carrying amounts reported in the consolidated balance sheets for the Company’s cash, accounts receivable, accounts payable, and accrued expenses approximate their fair values due to the short maturities of these financial instruments.
The carrying amounts reported in the consolidated balance sheets for the Company’s amounts recorded as other liabilities and due to officers approximate their values based on current rates at which the Company could borrow funds with similar maturities.
Comprehensive Income (Loss)
The Company adopted SFAS No. 130, Reporting Comprehensive Income, which establishes rules for the reporting of comprehensive income and its components. In addition to net loss, comprehensive income (loss) includes all changes in equity during a period, except those resulting from investments by and distributions to owners. Items of comprehensive income include foreign currency exchanges.
Research and Development Costs
Research and development costs are charged to operations as incurred. These costs consist primarily of salaries and related costs of employees engaged in research, design and development activities, the cost of parts for prototypes and equipment depreciation.
Foreign Currency Translation
Operations of certain of the Company’s subsidiaries are conducted in China and the financial statements of those subsidiaries are translated from China’s Renminbi, the functional currency, into U.S. Dollars in accordance with SFAS No. 52, “Foreign Currency Translation.” Accordingly, all foreign currency assets and liabilities are translated at the period-end exchange rate and all revenues and expenses are translated at the average exchange rate for the period. The effects of translating the financial statements of foreign subsidiaries into U.S. Dollars are reported as a cumulative translation adjustment, a separate component of comprehensive income in stockholder’s equity
Loss Per Common Share, Basic and Diluted
The Company. accounts for net loss per common share in accordance with the provisions of SFAS No. 128, “Earnings per Share” (“EPS”). SFAS No. 128 requires the disclosure of the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock or resulted in the issuance of common stock that then shared in the earnings of the entity. Common equivalent shares have been excluded from the computation of diluted EPS since their effect would be anti-dilutive.
Concentrations of Business and Credit Risk
Financial Risks
At times throughout the year, the Company may maintain certain bank accounts in excess of FDIC insured limits.
Geographical Risks
Approximately 80% of the Company’s assets and approximately 20% of the Company’s operations are in China. Therefore, the Company’s business, financial condition and results of operations may be adversely affected by significant political, economical and social uncertainties in China.
Reclassifications
Certain amounts in the 2004 financial statements have been reclassified for comparative purpose to conform to presentation in the 2005 financial statements.
ADOPTION OF NEW ACCOUNTING STANDARDS
On January 1, 2006, the Company adopted the provisions of Statement of Financial Accounting Standards (SFAS) No. 123(R) “Share-Based Payment” using the modified prospective application. The Company has been expensing share based awards granted after January 1, 2003 under the provisions of SFAS No. 123 “Accounting for Stock-Based Compensation”. For the nine months ended September 30, 2006 and 2005, included in net loss are expenses of $168,542 and $148,300 after tax, respectively, of stock based compensation related to stock options granted. If the Company had followed the fair value recognition provisions of SFAS 123(R) for all outstanding and unvested stock options and other stock-based compensation for the nine months ended September 30, 2005, there would have been no material impact on the Company’s financial statements.
RESULTS OF OPERATIONS
Nine Months Ended September 30, 2006 Compared to Nine Months Ended September 30, 2005
Revenues
As a result of the Company’s re-positioning for bio-pharmaceutical opportunities in China and its exit from value-added communications services in the U.S., there was no revenue in the third quarter of 2006.
Revenue was $59,307 in the third quarter of 2005. No customer represented more than 10% of our total revenues for the third quarter of 2006 or 2005.
Comprehensive Loss
Comprehensive loss decreased to $(166,365) in the third quarter of 2006 from $(792,950) in the third quarter of 2005. The decrease in loss is due to a decrease in general and administrative, and research and development expenses.
Cost of Revenues
There was no cost of revenue incurred in the third quarter of 2006 as the Company had no revenue in the period.
The cost of revenues for the third quarter of 2005 was $33,476. The cost of 2005 service revenues consisted of costs primarily associated with network operations and related personnel, telephony origination and termination services provided by third-party carriers, and indirect costs associated with purchasing, scheduling and quality assurance.
Research and Development Expenses
The Company incurred no research and development (“R&D”) expenses in the third quarter of 2006.
R&D expenses for 2005 consisted primarily of compensation paid to personnel involved in system design, implementation, and testing, and equipment costs associated with IP-PCS systems and solutions development. R&D expenses were $161,214 in the third quarter of 2005. R&D costs, including software development costs and system integration costs, were expensed as they incurred.
General and Administrative Expenses
General and administrative (“G&A”) expenses consist primarily of compensation paid to personnel and related overhead costs for sales, marketing, finance, legal, human resources and general management. Such costs also include sales commissions, trade show, advertising and other marketing and promotional expenses.
G&A expenses decreased to $160,090 in the third quarter of 2006 from $637,296 in the third quarter of 2005. The Company experienced significant decrease in marketing, sales, and personnel expenses, as it exited from value-added communications services in the United States. The Company continues to incur significant expenses in legal, accounting, finance, and SEC filing fees associated with being a publicly traded company. The Company anticipates that G&A expenses to increase as it carries out its re-positioning plan for bio-pharmaceutical opportunities in China.
Other Income (Expense)
There was no other income or expenses in the third quarter of 2006 compared to other income of $102 in the third quarter of 2005.
Income Taxes
No income taxes were payable and no tax provision has been recorded for 2006 or 2005 as a result of the cumulative operating losses we have generated.
RESULTS OF OPERATIONS
Year Ended December 31, 2005 Compared to Year Ended December 31, 2004
Revenues
Revenues during the fiscal years ended December 31, 2005 and 2004 respectively are from limited sales of VoIP and value added communications services to business and residential customers. Such revenues are not significant as we continue to focus on the commercialization of our initial IP-PCS product and development of additional IP-PCS systems and solutions.
Revenue increased to $380,519 in 2005 from $312,899 in 2004, as a result of increase of IP-PCS sales, offset by a decrease in VoIP service revenues due to reduced marketing and sales activities and significant rate cuts in response to continued price reductions among competitors.
One customer, Alosat Communications Inc, represented more than 10% of our total revenues for 2005. No customer represented more than 10% of our total revenues for 2004.
Comprehensive Loss
Comprehensive loss increased to $(2,841,316) in 2005 from $(1,448,884) in 2004. The increase in loss is largely due to increase in general and administrative expenses, cost of sales, and research and development expenses.
Cost of Revenues and Gross Margin
The cost of service revenues consists of costs primarily associated with network operations and related personnel, telephony origination and termination services provided by third-party carriers, and indirect costs associated with purchasing, scheduling and quality assurance.
The cost of revenues increased to $176,817 in 2005 from $156,676 in 2004, primarily due to increase in IP-PCS solution component cost.
Research and Development Expenses
Research and development expenses consist primarily of compensation paid to personnel involved with system design, implementation, and testing, and equipment costs associated with IP-PCS systems and solutions development. R&D expenses were $611,362 in 2005 and $432,144 in 2004. R&D costs, including software development and system integration costs, are expensed as incurred.
General and Administrative Expenses
General and administrative expenses consist primarily of compensation paid to personnel and related overhead costs for sales, marketing, finance, legal, human resources and general management. Such costs also include sales commissions, trade show, advertising and other marketing and promotional expenses.
G&A expenses increased to $2,230,956 in 2005 from $1,081,465 in 2004. We experienced a significant increase in marketing and sales expenses related to the commercialization of IP-PCs solutions, and continued increase in legal, accounting, and SEC filing fees associated with being a publicly traded Company.
Other Income (Expense)
Other income increased to $216,667 in 2005 from $(12,291) in 2004, primarily due to the sale of net operating loss carryforwards.
Income Taxes
No income taxes were payable and no tax provision has been recorded for 2005 or 2004 as a result of the cumulative operating losses we have generated.
The Company accounts for income taxes in accordance with Statement of Financial Accounting Standards No. 109, Accounting for Income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. During 2005, the Company received proceeds of $216,247 as a result of the sale of net operating loss carryforwards resulting from cumulative losses through the year ended December 31, 2004. At December 31, 2005 the Company had net deferred income tax assets of approximately $772,000 comprised principally of the future tax benefit of net operating loss carryforwards, beginning to expire in the year 2007 through 2025. Because the realization of tax benefits related to the Company’s net deferred tax asset is uncertain, a full valuation allowance has been provided against the net deferred tax asset. As a result of the sale of net operating loss carryforwards and cumulative losses through the year ended December 31, 2005, the net change in the valuation allowance amounted to a decrease of $756,000 for the year ended December 31, 2005.
LIQUIDITY AND CAPITAL RESOURCES
Cash and cash equivalents; working capital deficit.
As of September 30, 2006, we had cash and cash equivalents of $10,623 and a working capital deficit of $(2,052,332), as compared to $2,769 and $(1,906,790), respectively, at June 30, 2006. The increase in our working capital deficit reflects a decrease in current assets and an increase in current liabilities. Our current liabilities of $2,509,815 include $874,442 in non-secured loans from and deferred compensation due to the officers of the Company which are payable on demand.
Net cash provided by operating activities.
Net cash provided by operating activities was ($15,096) for the nine months ended September 30, 2006, as compared to ($1,882,397) net cash provided by operating activities for the nine months ended September 30, 2005.
Net cash used in investing activities.
Net cash used in investing activities was ($5,876) for the nine months ended September 30, 2006, as compared to $0 for the nine months ended September 30, 2005.
Net cash provided by financing activities.
Net cash provided by financing activities was $1,568 for the nine months ended September 30, 2006, compared to $1,851,915, including $1,355,000 through issuance of common stock, and $496,915 from officers’ advances for the nine months ended September 30, 2005.
Capital Stock Transactions.
In February 2005, the Company completed a private placement of 260,000 shares of common stock at a price of $1.00 per share, or gross proceeds of $260,000.
During the quarter ended, March 31, 2005, the Company granted 402,000 fully vested, non-forfeitable warrants to purchase shares of common stock to two consultants for services in addition to cash payments. Also during the quarter ended, March 31, 2005, the Company granted 100,000 fully vested, non-forfeitable shares of common stock to a consultant for services.
In April 2005, the Company completed a private placement of 95,000 shares of common stock at a purchase price of $1.00 per share, or gross proceeds of $95,000, and, for no additional consideration, a cashless 2-year warrant to purchase an additional 95,000 shares at an exercise price of $1.50 per share. A value of $36,770 of the proceeds has been allocated to the warrant.
In May 2005, the Company completed a private placement of 500,000 shares of common stock at a purchase price of $0.50 per share, or gross proceeds of $250,000, and for no additional consideration, a cashless 5-year warrant to purchase an additional 147,059 shares at an exercise price of $0.75 per share. A value of $71,470 of the proceeds has been allocated to the warrant.
Also in May 2005, the Company completed a private placement of 500,000 shares of common stock at a purchase price of $0.50 per share, or gross proceeds of $250,000, and for no additional consideration, a cashless 5-year warrant to purchase an additional 147,059 shares at an exercise price of $0.75 per share. A value of $68,240 of the proceeds has been allocated to the warrant.
In July 2005, the Company completed a private placement of 1,000,000 of common stock at a purchase price of $0.50 per share, or gross proceeds of $500,000 and, for no additional consideration, a cashless 5-year warrant to purchase an additional 400,000 shares at an exercise price of $0.75 per share. A value of $168,000 of the proceeds has been allocated to the warrant.
Also in July 2005, the Company entered into a service agreement pursuant to which the Company agreed to issue warrants to purchase up to an aggregate of 200,000 shares (the “Service Warrant Shares”) of the Company’s common stock in exchange for investor relations services. The Company had the right to terminate the service agreement at any time on or after October 5, 2005, upon 30 days prior written notice. The Service Warrant Shares were scheduled to vest in accordance with the following schedule and are purchasable at the following exercise prices:
| · | 50,000 Service Warrant Shares were immediately vested and may be purchased at an exercise price of $0.90 per share; |
| · | 50,000 Service Warrant Shares were scheduled to vest on the 91st day following the date of the service agreement and were purchasable at an exercise price of $1.10 per share; |
| · | 50,000 Service Warrant Shares were scheduled to vest on the 181st day following the date of the service agreement and were purchasable at an exercise price of $1.30 per share; |
| · | 50,000 Service Warrant Shares were scheduled to vest on the 271st day following the date of the service agreement and were purchasable at an exercise price of $1.50 per share. |
The warrants shall terminate on the 24-month anniversary of the effective date of a registration statement filed by the Company to register the resale of the Service Warrant Shares; provided, however, in the event that the Company elects to terminate the service agreement early as described above, the warrants will immediately terminate as to any Service Warrant Shares that are not then vested. By October 5, 2005, the Company terminated the service agreement, resulting in only 50,000 Service Warrant Shares vested with an exercise price of $0.90 per share.
On January 24, 2006, the Company granted 2,701,000 options, of which 1,901,000 are fully vested, to purchase shares of common stock at an exercise price of $0.52, to officers, employees and consultants of the Company.
On January 26, 2006, the Company announced its plans to re-position itself for bio-pharmaceutical and other high growth opportunities in China, while continuing its commercialization of its high potential Mobile Voice over IP solutions.
In conjunction with the Company’s re-positioning plans, on February 27, 2006 the Company entered into an agreement to transfer ownership of its Chinese subsidiary Zheiiang Guang Tong Wang Luo Co., Ltd (ZJQC) to third parties. On January 1, 2006, the Company also entered into an agreement to transfer ownership of its U.S. subsidiary China Quantum Communications, Inc. to a former employee.
During the quarter ended June 30, 2006, the Company entered into a Share Exchange Agreement for the purpose of acquiring 100% of the outstanding capital stock of China BioPharma Limited (“CBL”) a Cayman Islands Company, which has rights to invest in Tianyuan Bio-Pharmaceuticals Company, Ltd. and Zhejiang Tianyuan Biotech Co., Ltd. (“ZTBC”). In exchange for 100% of the outstanding capital of CBL, the Company issued a total of 3,000,000 shares of restricted common stock.
In December 2006 the Company amended its Certificate of Incorporation to increase the number authorized shares of its common stock from 100,000,000 to 200,000,000.
On December 13, 2006, the Company entered into a Subscription Agreement with respect to the issuance and sale of $3,000,000 aggregate principal amount of its Secured Convertible Promissory Notes due December 13, 2008. The Notes are convertible at the option of the holders at any time into shares of the Company's common stock. Prior to the occurrence of an Event of Default (as defined in the Notes), the Notes are convertible at a per share conversion price equal to $0.25 per share. Following the occurrence of an Event of Default (as defined in the Notes), the Notes are convertible at the lesser of $0.25 per share and 75% of the average of the closing bid prices for the common stock for the five trading days prior to the date of conversion. The Notes bear interest at a rate of eight percent (8%) per annum. Monthly payments, consisting of principal of and accrued interest on the Notes shall commence March 13, 2007. The Company may, at its option pay the monthly payments in the form of either cash or shares of common stock. In the event that the Company elects to pay the monthly amount in cash, the Company shall be obligated to pay 115% of the principal amount component of the monthly amount and 100% of all other components of the monthly amount. In the event that the Company elects to pay the monthly amount in shares of common stock, the stock shall be valued at an applicable conversion rate equal to the lesser of $0.25 per share or seventy five percent (75%) of the average of the closing bid price of the common stock on the principal market on which the common stock is then traded or included for quotation for the five trading days preceding the applicable repayment date. Provided that an Event of Default has not occurred, the Company may, at its option, prepay the outstanding principal amount of the Notes, in whole or in part, at any time upon 30 days written notice to the holders by paying 120% of the principal amount to be repaid, together with accrued interest thereon plus any other sums due to the date of redemption. The Notes are secured by a Security Agreement entered into by and among the Company, CQCL, CBL, and QCCN and Barbara R. Mittman, as collateral agent for the purchasers of the Notes. The obligations of the Company under the Subscription Agreement with respect to the Notes and the Notes are guaranteed by the CQCL, CBL and QCCN pursuant to a Guaranty, dated as of December 13, 2006, entered into by the CQCL, CBL and QCCN, for the benefit of the purchasers of the Notes.
In connection with the sale of the Notes, the Company also issued to the purchasers of the Notes, Class A Warrants to purchase up to an aggregate of 6,000,000 shares of common stock and Class B Warrants to purchase up to an aggregate of 6,000,000 shares of common stock. One Class A Warrant and one Class B Warrant were issued for each two shares of common stock that would have been issuable on the closing date assuming the complete conversion of the Notes on such date. The Class A Warrants have an exercise price of $0.30 per share and the Class B Warrants have an exercise price of $0.40.
Melton Management Ltd. acted as the finder with respect to the issuance and sale of the Notes and received a warrant to purchase 2,400,000 shares of our common stock at an exercise price of $0.30 per share.
Currency exchange fluctuations.
For the purpose of funding operations of our Chinese subsidiary, we have implemented simple currency hedging against fluctuations in the Chinese Renminbi to United States dollar exchange rate.
Need for current financing.
Our ability to continue as a going concern is dependent upon our ability to raise capital in the near term to: (1) satisfy our current obligations, and (2) continue our planned re-positioning for bio-pharmaceutical opportunities in China. We do not have sufficient capital to fund our operations at the current level unless we receive additional capital either through external independent or related party funding, revenues from sales, further expense reductions or some combination thereof.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
DESCRIPTION OF BUSINESS
BUSINESS OVERVIEW
The Company is a provider of biopharmaceutical products with its focus mainly on the development and sale of human vaccines. In 2006, the Company re-focused its business from telecommunications to biopharmaceuticals. Currently, the Company develops its products in China and distributes these products in China and in one other country. The Company has established its distribution and development platform in China and as a result of its acquisition of its interest in its subsidiary, Hainan CITIC Bio-pharmaceutical Development Co., Ltd. and, as a result of its joint venture with Zhejiang Tianyuan Bio-pharmaceutical Co., Ltd.
The emphasis of the Company’s business is on the development of technology and the marketing of products rather than on manufacturing. It is the Company’s goal to operate efficiently and in compliance with applicable regulations and to reduce the risk of any potential factory contaminations with respect to its products. The Company believes that to date, it has been successful in establishing business relationships with a number of local and global manufacturers with the goal of introducing the most advanced technologies and best products to the market in a timely fashion. The Company believes that it has built an experienced and capable management team that will be able to work toward successfully implementing its business plan.
Description of Company
The Company was incorporated as Techedge, Inc. in Delaware in July 2002 to serve as the successor to the business and interests of BSD Development Partners, LTD. BSD was a Delaware limited partnership formed in 1997 for the purpose of investing in the intellectual property of emerging and established companies BSD merged with Techedge in September 2002. From September 2002 until June 2004, Techedge endeavored to continue the business of BSD and sought to enhance the liquidity of the securities owned by its investors by becoming subject to the reporting requirements of the Exchange Act and by seeking to have its common stock quoted on the OTC Bulletin Board, or OTCBB.
On June 9, 2004, Techedge acquired all of the issued and outstanding stock of China Quantum Communication Limited pursuant to a share exchange agreement, by and among Techedge, certain of its stockholders, CQCL and its stockholders. In connection with the Share Exchange, Techedge's then existing directors and officers resigned as directors and officers of Techedge and were replaced by directors and officers designated by CQCL.
Following the Share Exchange, Techedge refocused its business efforts on developing and providing its IP-based Personal Communication Service, a regional mobile VoIP service delivered on unlicensed low power PCS frequencies through IP-enabled local transceiver and IP centric soft-switched networks, operating on an advanced proprietary software centric multi-service global communication service platform and management system. Techedge continued operating CQCL's communications service business through CQCL and CQCL's wholly-owned subsidiaries, China Quantum Communications Inc., a Delaware corporation, and Guang Tong Wang Luo (China) Co. Ltd. (also known as Quantum Communications (China) Co., Ltd.), a Chinese company.
On January 26, 2006, the Company announced its plans to re-position itself for bio-pharmaceutical and other high growth opportunities in China, while continuing its commercialization of its high potential Mobile Voice over IP solutions.
In conjunction with the Company’s re-positioning plans, on February 27, 2006 the Company entered into an agreement to transfer ownership of its Chinese subsidiary Zheiiang Guang Tong Wang Luo Co., Ltd to third parties. On January 1, 2006, the Company also entered into an agreement to transfer ownership of its U.S. subsidiary China Quantum Communications, Inc. to a former employee.
During the quarter ended June 30, 2006, the Company entered into a Share Exchange Agreement for the purpose of acquiring 100% of the outstanding capital stock of China BioPharma Limited (“CBL”) a Cayman Islands Company, which has rights to invest in Tianyuan Bio-Pharmaceuticals Company, Ltd. and Zhejiang Tianyuan Biotech Co., Ltd. (“ZTBC”). In exchange for 100% of the outstanding capital of CBL, the Company issued a total of 3,000,000 shares of restricted common stock.
On July 14, 2006, Techedge and China Biopharma, Inc, a Delaware corporation and a wholly-owned subsidiary of Techedge executed and delivered a Plan and Agreement of Merger whereby the parties agreed to merge CB with and into Techedge, with Techedge being the surviving corporation. By virtue of, and effective upon the consummation of the Merger, the Certificate of Incorporation of the Company was amended to change its name from “Techedge, Inc.” to “China Biopharma, Inc.” The Merger became effective on August 10, 2006.
Products and Services
The Company’s products consist of primarily vaccines for preventing and treating various diseases and illnesses in humans. Currently, the Company provides and distributes its products in China and also exports them internationally.
Preventive Vaccines
The Company currently distributes flu vaccines manufactured by its joint venture partner, Zhejiang Tianyuan Bio-pharmaceutical Co., Ltd. Flu vaccine is a seasonal product that is mostly administered during late the fall and early winter seasons. For over ten years, the World Health Organization has made recommendations as to what virus strain should be used in the upcoming season and, as a result, vaccines are manufactured based upon such recommendations.
The Company plans to distribute more preventive vaccines in China to the Chinese Center for Disease Control and Prevention (“CDCs” ) once they become available, including vaccines for Japanese encephalitis, hepatitis B, allergy, and rabies.
Immunotherapy Products
Immunotherapy (also known as biologic therapy or biotherapy) is a treatment that focuses on certain parts of the immune system to fight various diseases. This may be done by either:
- Stimulating a patient’s own immune system to work harder or smarter; or
- Giving a patient’s immune system certain natural or man-made components, including synthetic system proteins
Currently the Company is working with several international companies on a number of immunotherapy products, including therapeutic vaccines which are intended to treat cancers and other illnesses. In order to export a product, the local regulations usually require various clinical studies and product registrations.
Other Products and Services
Through its subsidiary, HCBD, the Company may also utilize its distribution platforms and logistics to distribute other medical products and to provide logistic services for other biopharmaceutical companies.
Market Overview
The global market for vaccines has steadily increased for the past ten years. In China, the vaccine market has increased four fold during the past decade. The Company estimates that the total vaccine market in China reached approximately $500 million in revenue in 2006.
Subsidiaries
The Company currently has two operational segments. One segment, consisting of China Biopharma Limited and its subsidiaries provides biopharmaceutical products. The other segment, which consists of CQCL and its subsidiary is engaged in the business of providing telecommunications services and developing related technology. However, following the Company’s re-positioning of its business focus from telecommunications to biopharmaceutical operations, CQCL ceased carrying on any daily business activities and is currently looking for strategic partners in order to continue its business. Set forth below is a graphic representation of the current organizational structure of the Company and its subsidiaries.
China Biopharma Limited
China Biopharma Limited (“CBL”) is Cayman Islands company and a wholly-owned subsidiary of the Company. CBL manages and operations all the Company’s biopharmaceutical business in China.
Zhejiang Tianyuan Biotech Co., Ltd.
Zhejiang Tianyuan Biotech Co., Ltd.(“ZTBC”) is a Sino-US joint Venture between China Biopharma Limited and Zhejiang Tianyuan Bio-pharmaceutical Co., Ltd. (“Zhenjiang Tianyuan”). The Company owns 65% of ZTBC and Zhejiang Tianyuan owns 35% of ZTBC. ZTBC was formed on June 24, 2006 and was funded on December 22, 2006. Of the total $3,000,000 initial capitalization of ZTBC, CBL invested $1,950,000 and Zhejiang invested $1,050,000 in cash.
Zhejiang Tianyuan has agreed to perform all of the manufacturing functions on behalf of ZTBC and ZTBC will handle all future vaccine development and marketing. All the marketing and sales personnel of Zhejiang Tianyuan have been transferred to ZTBC.
Hainan CITIC Bio-pharmaceutical Development Co., Ltd.
In April 2006, ZTBC acquired 20% of the outstanding stock of Hainan CITIC Bio-pharmaceutical Development Co., Ltd. (HCBD) from three individuals in consideration for a payment of $600,000; In August 2006, ZTBC acquired an additional 40% of the outstanding stock of HCBD from CITIC Pharmaceutical and China Biological Engineering Corporation in consideration for a payment of $1,200,000; In December 2006, ZTBC acquired another 10% of the outstanding stock of HCBD from one individual in consideration for a payment of $300,000. The remaining 30% of HCBD is owned by Zhejiang Tianyuan Bio-pharmaceutical Co., Ltd. (20%) and by one of its original owners (10%).
HCBD is a nationwide bio-pharmaceutical distributor in China and has established a distribution platform including “cold-chain” logistics which are the refrigeration logistics in the distribution chain. HCBD distributes the vaccines provided by local vaccine manufacturers, including ZTBC and Zhejiang Tianyuan Bio-pharmaceutical Co., Ltd and other bio-pharmaceutical products made by large global drug manufactures, such as Merck.
China Quantum Communication Limited
China Quantum Communication Limited is a Cayman Islands company and a wholly-owned subsidiary of the Company. CQCL holds all of the Company’s telecommunications business interests and owns all the intellectual property of “mobile voice over IP” technologies. Following the Company’s decision to reposition its business focus from telecommunications to biopharmaceuticals, CQCL is no longer engaged in daily business operation activities. CQCL is currently seeking a strategic partner in order to be able to continue its telecommunications business operations.
Guang Tong Wang Luo Ke Ji (China) Co., Ltd.
Guang Tong Wang Luo Ke Ji (China) Co., Ltd. (also known as Quantum Communication (China) Co., Ltd (“QCCN”)) is a wholly owned subsidiary of CQCL in China. QCCN has acted as the Company’s Chinese business center in order to support all of its administrative activities. In addition, QCCN operated a communication service operations center and a technology development center on behalf of CQCL. Following the Company’s decision to reposition its business focus from telecommunications to biopharmaceuticals, QCCN ceased its telecommunications related business operations.
Management
The Company has an experienced management team. Many of the senior managers have international operational experiences. Members of the management team has operational experience, marketing and sales experience, research and development experience, and financial experience. The Company believes the existing management team shall be able to carry out the execution of the existing business plan.
As a result of acquiring HCBD, one of the largest independent biopharmaceutical product distributors in China, the Company has established a distribution foundation in China. HCBD provides the Company with what management believes are strategic and operative advantages over other global companies.
Management has an ongoing desire to develop global partnerships with small and mid-sized biopharmaceutical companies in U.S. and Europe to introduce matured yet not currently available products into Chinese markets.
Marketing and Sales
The Company is targeting customers in the Chinese vaccine market seeking vaccines treating common diseases and illnesses. The Company avoids carrying vaccines that are mostly administrated by the government on free-of-charge basis because the government typically gives priority to government owned or operated companies when deciding from whom to purchase vaccines for such programs.
The Company plans to focus its primary marketing and sales on hospitals and clinics. The Company intends to team up with local and international vaccine manufacturers to jointly promote their products in China.
The Company also plans to develop and promote new products on the market carrying its own brand name.
Distribution
The Company distributes vaccines and other biopharmaceutical products through its subsidiary, HCBD. HCBD has built a system of refrigerated or “cold-chain” distribution logistics. HCBD has five regional distribution centers covering about 236 major cities in China. In the event of an outbreak of a contagious disease, HCBD could deliver needed vaccines from local manufactures to any part of these cities within 24 hours.
HCBD distributes vaccines in China with two different arrangements, representative arrangements and buy-and-sell arrangements. With representative arrangements, HCBD sells and distributes the products on the behalf of original manufacturers, and is paid with sales commission after the sales close. With buy-and-sell arrangements, HCBD actually takes title to the products and sell them as its own products.
In most cases, the proceeds from sales of products are collected after the products actually administered to patients instead of being paid on delivery. In most cases, the time from delivery of products to actual collection of proceeds may vary anywhere from one to three months.
Key Suppliers
The Company sources its products from local and international companies. The Company has formed a strategic alliance with its joint venture partner, Zhejiang Tianyuan to market and distribute its vaccine products in China and overseas. Currently, more than 50% of the Company’s total sales are based on flu vaccine product supplied by Zhejiang Tianyuan Bio-pharmaceutical Co., Ltd.
Principal Customers
The Company distributes its products directly or indirectly to local hospitals, clinics, pension fund health programs, and Center for Disease Control and Prevention (“CDCs” ) in China. Currently, the Company’s main customers are local CDCs that buy, store, and administer the vaccinations throughout China.
Competition
The Chinese market for human vaccine is very competitive. There are many local and global vaccine suppliers in China selling various human vaccines. The Company competes with large established global drug companies such as Merck, Glaxosmith Kline, Sanofi Pasteur, and Novartis. These companies offer a wide range of range of vaccine products that use similare formulations and competing technologies.
The Company also faces significant competition from traditional local small vaccines providers, as well as local government owned companies. The Company competes for customers based principally on product offerings, price and customer service.
Most of the market for EPI (Extended Program Initiative) vaccine products, that are purchased by government and administered free-of-charge to the Chinese citizens, are dominated by Chinese government owned institutions and local producers with favorable selection criteria toward government owned and local companies.
Research and Development
The Company conducts research and development activities both in the United States and China. The Company’s employees or consultants are assigned on each research and development project as a working group. The working group evaluates, formulates, conducts, and manages the activities and progress of each project. The Company out-sources certain research and development activities to third parties.
The Company’s future success will depend, in part, on its ability to improve its existing product mix and product lines, to retain the services of talented employees and to develop new products and services that incorporate the leading market positions.
Government Regulations
Due to its nature, vaccine business is highly regulated in the most of countries. Various government agents regulate product registrations, production certifications, distribution licenses, application control, and other factors beyond general business operation.
When a company imports a vaccine or related product into a country, regulators normally requires registration and local clinical studies to approve its safety and efficacy before the product could be distributed and sold in the country regardless of its maturity and approval status in somewhere else.
The Company distributes its products in China through its subsidiary, HCBD using its biopharmaceutical distribution license.
Intellectual Property
Management regards copyrights, trademarks, trade secrets, patents, patent applications, and similar intellectual property as critical to the Company’s success and the Company relies on trademark and copyright law, trade secret protection and confidentiality and/or license agreements with our employees, customers, suppliers, and others to protect our proprietary rights.
Employees
As of January 17, 2007, we employed 56 individuals in the United States and China. Most employees are located in China because the Company conducts a significant part of its business operation in China. The Company’s employees are not represented by a labor union and management considers its employee relations to be good.
Revenues and Assets by Geographic Location
Substantially all of the Company’s revenues have been generated in China and a significant majority of our long-lived assets are located within China.
Description of Property
Neither the Company nor any of its subsidiaries owns any real property. The following is a summary of the material leased facilities where we currently conduct our business operations:
| Locations | | Sq. Ft. | | Description | | Lease Expiration Date |
| Princeton, New Jersey | | shared space | | Rep. Office | | Month to month |
| Hangzhou, China | | 1,000 | | Head Office | | Year to Year |
| Hangzhou, China | | 2,000 | | CBL/ZTBC Office | | Year to Year |
| Beijing, China | | 2,500 | | HCBD Office | | Year to Year |
| Haikou, China | | 1,000 | | HCBC Office | | Year to Year |
In addition to the facilities listed above, HCBD, one of our subsidiaries, has entered into leases for five regional distribution centers with refrigerated warehouses on favorable “as needed” lease terms. We believe that our facilities are suitable and adequate for our current business needs and that suitable additional or alternative space will be available in the future at commercially reasonable terms.
Legal Proceedings
The Company has no pending legal proceedings.
DIRECTORS AND EXECUTIVE OFFICERS
NAME | | AGE | | TITLE |
| Peter Wang | | 53 | | Chairman, Chief Executive Officer |
| Ya Li | | 36 | | Chief Financial Officer, Director, Secretary |
| Charles Xue | | 54 | | Director |
| Wind Chen | | 49 | | Chief Operating Officer |
Peter Wang, 53, has served as the Chairman and Chief Executive Officer since the Company’s acquisition of Quantum Communications Ltd. (“CQCL”) in June 2004. Since March 2002, Mr. Wang had served in the same capacities with CQCL. Prior to joining CQCL, Mr. Wang co-founded and successfully built Unitech Telecom (renamed UTStarcom) as well as several other technology and service ventures. Mr. Wang has more than 20 years of experience in the telecommunication equipment and services industry and has held management, operations, and research and development positions in companies such as AT&T Bell Labs and Racal-Milgo Information System.
Ya Li, 36, has served as our Chief Financial Officer and as a Director since our acquisition of CQCL in June 2004, and had been our Chief Operating Officer from June 2004 to March 2005. From March 2002 until November 2005, he served as Chief Operating Officer and a Director of CQCL. From August 1998 to March 2000, Mr. Li was the Chairman and Chief Executive Officer of Global Villager Inc., which he founded and which was acquired by Startec Global Communications Inc., a telecommunications carrier focused on ethnic markets, in March 2000. Mr. Li has a B.S. in engineering from the University of Science & Technology of China, a M.S. in computer science from Temple University, and completed the two-year Management Program from the University of Pennsylvania's Wharton School of Business. From 1994 to 1999, Mr. Li worked in the information, telecommunications, and financial industries for Bell Atlantic, Donaldson Lufkin and Jenrette, Lehman Brothers, and Morgan Stanley. Mr. Li has served as a Director for the Chinese Finance Society, Council on U.S.-China Affairs, and China Chamber of Commerce in the U.S.
Charles Xue, 54, has served as a Director of CQCL since May 2002, and one of our Directors since our acquisition of CQCL in June 2004. Since 2001, Mr. Xue has served as Chairman of PRCEDU.com, one of the largest online education companies in China. Mr. Xue co-founded Unitech Telecom, which was renamed UTStarcom, Inc., and served as its Chairman from 1990 to 1996 and as its Vice-Chairman from 1996 to 2002. Mr. Xue founded 8848.net, a leading e-commerce site in China, and has served as its Chairman since 1998.
Wind Chen, 49, has served as our Chief Operating Officer since November 28, 2005. Previously, he was a Senior Vice President and Chief Strategy Officer of China CQCL, from 2001 to 2004. Prior to joining CQCL, he was a Vice President of business strategy of Japan Telecom, in charge of international corporate strategy & planning. Prior to Japan Telecom, he was the Managing Director of Suppliers Market of Bellcore (Telcordia), in charge of business development in Asian Pacific and northern Europe (Scandinavia).
EXECUTIVE COMPENSATION
SUMMARY COMPENSATION TABLE
The following table sets forth all cash compensation paid or to be paid by the Company, as well as certain other compensation paid or accrued, during each of the Company's last three fiscal years to each named executive officer.
| | | Year | | Salary | | Bonus | | Option Awards | | All other compensation (1) | | Total | |
| | | | | ($) | | ($) | | | | ($) | | ($) | |
| Peter Wang, CEO (2) | | | 2006 | | | 50,000 (3 | ) | | 0 | | | (4 | ) | | 0 | | | 50,000 | |
| | | | 2005 | | | 75,000 (5 | ) | | 0 | | | - | | | 0 | | | 75,000 | |
| | | | 2004 | | | 0 (6 | ) | | 0 | | | - | | | 0 | | | 0 | |
| Ya Li, CFO (2) | | | 2006 | | | 0 (7 | ) | | 0 | | | (8 | ) | | 0 | | | 0 | |
| | | | 2005 | | | 140,000 (9 | ) | | 0 | | | - | | | 0 | | | 140,000 | |
| | | | 2004 | | | 81,667 (10 | ) | | 0 | | | - | | | 0 | | | 81,667 | |
| Eugene Chen, Former COO (11) | | | 2006 | | | 0 | | | 0 | | | (12 | ) | | 0 | | | 0 | |
| | | | 2005 | | | 120,000 (13 | ) | | 0 | | | - | | | 0 | | | 120,000 | |
| | | | 2004 | | | 0 | | | 0 | | | - | | | 0 | | | 0 | |
| Wind Chen, COO (14) | | | 2006 | | | 0 | | | 0 | | | (13 | ) | | 0 | | | 0 | |
| | | | 2005 | | | 0 | | | 0 | | | - | | | 0 | | | 0 | |
| | | | 2004 | | | 0 | | | 0 | | | - | | | 0 | | | 0 | |
(1) We have concluded that the aggregate amounts of perquisites and other personal benefits paid to the named individuals do not exceed the lesser of $25,000 or 10% of the compensation reported in the table for such individuals.
(2) Began employment with the Company after its acquisition of CQCL in June 2004.
(3) Includes $50,000 of salary, the payment of which has been deferred.
(4) Mr. Peter Wang received a stock option grant of 300,000 shares in January 2006 at an exercise price of $0.52 per share, all of which have vested and are currently exercisable.
(5) Includes $69,167 of salary, the payment of which has been deferred.
(6) Prior to the commencement of employment with the Company, Mr. Wang earned $20,000 in salary from CQCL during 2004, the payment of which has been deferred. Mr. Wang earned $80,000 in salary from CQCL during 2003, the payment of which has been deferred, and $120,000 in salary from CQCL during 2002, the payment of which has been deferred.
(7) Mr. Ya Li did not receive any salary compensation during 2006.
(8) Mr. Ya Li received a stock option grant of 250,000 shares in January 2006 at an exercise price of $0.52 per share, all of which have vested and are currently exercisable.
(9) Includes $32,817 of salary, the payment of which has been deferred.
(10) Includes $71,667 of salary, the payment of which has been deferred. Prior to the commencement of employment with the Company, Mr. Li earned $58,333 in salary from CQCL during 2004, the payment of which has been deferred. Mr. Li earned $146,667 in salary from CQCL during 2003, the payment of $81,278 of which has been deferred, and $126,155 in salary from CQCL during 2002, the payment of $47,308 of which has been deferred.
(11) Mr. Eugene Chen served as our Chief Operating Officer between March and November 2005.
(12) Mr. Eugene Chen received a stock option grant of 100,000 shares in January 2006 at an exercise price of $0.52 per share, all of which was immediately vested and are currently exercisable.
(13) Includes $109,500 of salary, the payment of which has been deferred.
(14) Mr. Wind Chen served as our Chief Operating Officer since November 29, 2005. Mr. Wind Chen did not receive any salary compensation during 2005 and 2006.
(15) Mr. Wind Chen received a stock option grant of 150,000 shares in January 2006 at an exercise price of $0.52 per share, all of which have vested and are currently exercisable.
OPTION/SAR GRANTS DURING 2006 FISCAL YEAR (INDIVIDUAL GRANTS)
The following table contains information regarding the grant of stock options to the Named Executive Officers during the year ended December 31, 2006.
Name | | Grant Date | | Approval Date | | All Other Option Awards: Number of Securities Underlying Options (#) | | Exercise or Base Price of Option Awards ($/Sh) | | Grant Date Fair Value of Stock and Option Awards ($) | | Grant Date Fair Market Value of a Share ($/Sh) | |
| Peter Wang, CEO | | | 1/24/06 | | | 1/24/06 | | | 300,000 | | $ | .52 | | | 0 | | $ | .52 | |
| Ya Li, CFO | | | 1/24/06 | | | 1/24/06 | | | 250,000 | | $ | .52 | | | 0 | | $ | .52 | |
| Wind Chen, COO | | | 1/24/06 | | | 1/24/06 | | | 150,000 | | $ | .52 | | | 0 | | $ | .52 | |
Charles Xue, the Company’s only independent director, received a stock option grant of 250,000 shares on January 24, 2006 at an exercise price of $0.52 per share, all of which have vested and are immediately exercisable.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
| | | Option Awards | |
Name | | Number of Securities Underlying Unexercised Options (#) Exercisable | | Number of Securities Underlying Unexercised Options (#) Unexercisable | | Equity Incentive Plan Awards; Number of Securities Underlying Unexercised Unearned Options (#) | | Option Exercise Price ($) | | Option Expiration Date | |
| Peter Wang, CEO | | | 300,000 | | | 0 | | | 0 | | $ | .52 | | | 1/23/11 | |
| Ya Li, CFO | | | 250,000 | | | 0 | | | 0 | | $ | .52 | | | 1/23/16 | |
| Wind Chen, COO | | | 150,000 | | | 0 | | | 0 | | $ | .52 | | | 1/23/16 | |
OPTION EXERCISES AND STOCK VESTED
The following table summarizes stock option exercises and realization of stock awards by our named executive officers in 2006.
| | | Option Awards | | Stock Awards | |
Name | | Number of Shares Acquired on Exercise (#) | | Value Realized on Exercise ($) | | Number of Shares Acquired on Vesting (#) | | Value Realized on Vesting ($) | |
| Peter Wang, CEO | | | 0 | | | - | | | - | | | - | |
| Ya Li, CFO | | | 0 | | | - | | | - | | | - | |
| Wind Chen, COO | | | 0 | | | - | | | - | | | - | |
EMPLOYMENT CONTRACTS AND TERMINATION OF EMPLOYMENT, AND CHANGE-IN-CONTROL
On November 29, 2005, the Board of Directors of the Company appointed Wind Chen as its new Chief Operating Officer. There is no employment contract for Wind Chen’s appointment.
Pension Benefits
We do not sponsor any qualified or non-qualified defined benefit plans.
Nonqualified Deferred Compensation
We do not maintain any non-qualified defined contribution or deferred compensation plans. The compensation committee, which is comprised solely of “outside directors” as defined for purposes of Section 162(m) of the Code, may elect to provide our officers and other employees with non-qualified defined contribution or deferred compensation benefits if the compensation committee determines that doing so is in our best interests.
Summary of Director Compensation
We do not provide cash compensation to our directors for their services as members of the Board or for attendance at Board or committee meetings. However, our directors will be reimbursed for reasonable travel and other expenses incurred in connection with attending meetings of the Board and its committees. Our directors are eligible to receive stock option grants in consideration for their service on our board.
Charles Xue, the Company’s only independent director, received a stock option grant of 250,000 shares in January 2006 at an exercise price of $0.52 per share, all of which have vested and are currently exercisable.
EQUITY COMPENSATION PLAN INFORMATION
The following table gives information as of December 31, 2006, about the Company’s common stock that may be issued upon the exercise of options and rights under the Company’s, the CQCL 2001 Stock Plan, and the Company’s 2005 Equity Compensation Plan. These plans were the Company’s only equity compensation plans in existence as of December 31, 2006.
Equity Compensation Plan Table
Plan Category | | (a) Number Of Securities To Be Issued Upon Exercise Of Outstanding Options, Outstanding Options, Warrants and Rights | | (b) Weighted Average Exercise Price Of Outstanding Options, Outstanding Options, Warrants and Rights | | (c) Number of Securities Remaining Available For Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected In Column (a)) | |
Equity Compensation Plans Approved by Stockholders | | | 2,701,000 | (1) | $ | 0.52 | | | 5,799,000 | (2) |
Equity Compensation Plans Not Approved by Stockholders | | | 3,157,546 | (3) | $ | 0.20 | | | — | (4) |
| TOTAL | | | 5,858,546 | | $ | 0.35 | | | 5,799,000 | |
(1) On January 24, 2006, the Company granted 2,701,000 options under the Company’s 2005 Equity Compensation Plan, which are fully vested, to purchase shares of common stock at an exercise price of $0.52, to officers, employees and consultants of the Company.
(2) Represents shares of our common stock that remain available for future issuance under the Company’s 2005 Equity Compensation Plan.
(3) Represents shares of our common stock issuable upon exercise of options to purchase CQCL ordinary shares under the CQCL 2001 Stock Plan that we assumed as a result of our acquisition of CQCL in June 2004.
(4) After the Company’s 2005 Equity Compensation Plan was approved by stockholders, no additional options to purchase shares of common stock will be granted under the CQCL 2001 Stock Plan.
Description of the CQCL 2001 Stock Plan
Adoption and Shares Reserved. Our board of directors approved the assumption of the CQCL 2001 Stock Plan in August 2004 in connection with our acquisition of CQCL. The CQCL 2001 Stock Plan provides for the grant of incentive stock options to our employees, and for the grant of nonstatutory stock options to our employees, directors and consultants.
The CQCL 2001 Stock Plan provides that the maximum aggregate number of shares that may be subject to option and sold pursuant to the plan is 11,557,488 shares. We are required to reserve and keep available such number of shares to satisfy the requirements of the plan.
Administration. Our board of directors or a committee of our board administers the CQCL 2001 Stock Plan. The administrator has the power to determine the fair market value of the shares, select the employees, directors or consultants to whom options are to be granted, the terms of the options granted, including the exercise price, the number of shares covered by each option, form of consideration, terms of exercisability of the options and vesting acceleration or waiver of forfeiture restrictions.
Exercise Price. The administrator determines the exercise price of options granted under the CQCL 2001 Stock Plan, subject to the following requirements: (i) the exercise price of incentive stock options shall be no less than 100% of the fair market value per share, and for incentive stock options granted to employees who own greater than 10% of the voting power of all classes of our stock, the exercise price shall be no less than 110% of the fair market value per share; and (ii) the exercise price of nonstatutory stock options shall be no less than 85% of the fair market value per share, and for nonstatutory stock options granted to employees, directors or consultants who own greater than 10% of the voting power of all classes of stock, the exercise price shall be no less than 110% of the fair market value per share. The exercise price may differ from the above requirements on options issued pursuant to a merger or other corporate transaction. The term of an option may not exceed 10 years from the date of grant, except in the case of incentive stock options granted to employees owning more than 10% of the voting power of all of our classes of stock, in which case the term shall be no more than 5 years.
Termination of Employment. After termination of one of our employees, directors or consultants, that person may exercise an option for the period of time stated in the option agreement. In the case of termination of one of our employees, directors or consultants due to death or disability, the option will remain exercisable for 6 months following the date of termination. In all other cases, in the absence of a period of time in the option agreement, to the extent the option is vested the option will remain exercisable for 30 days following the date of termination. To the extent that an option is not exercised within the applicable time period, the unexercised option is reverted to the plan. If on the date of termination, the option is not fully vested, the unvested portion of the option is reverted to the plan.
Non-Transferability of Options. Our 2001 plan generally does not allow for the transfer of options, except by will or the laws of descent, and only the holder of an option may exercise the option during the holder’s lifetime.
Adjustments upon Merger or Asset Sale. Our 2001 plan provides that the Administrator may allow holders to exercise options in the event of a proposed dissolution or liquidation of the company. The plan also provides that if we merge with another corporation, sell all or substantially all of our assets, the successor corporation will assume or provide a substitute for each option. If the outstanding options are not assumed or substituted, the options shall terminate as of the date of the merger or asset sale.
Amendment and Termination. Our 2001 plan will automatically terminate ten years from the effective date of the plan or the latest Board approval of an increase in the number of shares reserved for issuance under the plan, unless we terminate it sooner. Our Board of Directors has the authority to amend, suspend or terminate the plan provided it does not adversely affect any option previously granted under it. After the Company’s 2005 Equity Compensation Plan was approved by stockholders, it was determined that no additional options to purchase shares of common stock will be granted under the CQCL 2001 Stock Plan.
Description of the 2005 Equity Compensation Plan
Administration. The 2005 Plan will be administered by a duly authorized committee appointed by the Board of Directors and charged with administration of the 2005 Plan. The Board may grant options to purchase shares of the Company’s common stock, stock purchase rights and restricted or unrestricted stock awards (“awards”) of shares of common stock to eligible employees, directors and consultants, determine the terms and conditions of each option, stock purchase right or award and adopt, amend and rescind rules and regulations for the administration of the 2005 Plan. No options, stock purchase rights or awards may be made under the Plan after April 14, 2015, but the 2005 Plan shall continue thereafter while previously granted options, stock purchase rights or awards remain subject to the 2005 Plan.
Employees, Directors and Consultants Eligible to Receive Options or Awards Under the 2005 Plan. Persons eligible to receive options, stock purchase rights or awards under the 2005 Plan are those employees, directors and consultants of the Company and its subsidiaries who, in the opinion of the Board, are in a position to make a significant contribution to our success.
Shares Subject to the 2005 Plan. Subject to adjustments set forth in the 2005 Plan, the aggregate number of shares of common stock available for issuance in connection with options granted under the 2005 Plan will be 8,500,000, subject to customary adjustments for stock splits, stock dividends or similar transactions. If any option granted under the 2005 Plan terminates without having been exercised in full or if any award is forfeited, the number of shares of common stock as to which such option or award was forfeited shall be available for future grants within certain limits under the 2005 Plan. No director, employee or consultant may receive awards of or relating to more than 4,000,000 shares of the Company’s common stock in the aggregate in any year.
Terms and Conditions of Options. The Board determines the exercise price of options granted under the 2005 Plan. The exercise price of incentive stock options, however, must be at least equal to the fair market value per share of common stock (or 110% of fair market value in the case of incentive options granted to a ten-percent stockholder) issuable upon exercise of the option at the time the incentive option was granted. No option may be exercisable for more than ten years (five years in the case of an incentive option granted to a ten-percent stockholder) from the date of grant. Options issued under the 2005 Plan will be exercisable at such time or times as the Board prescribes at the time of grant. Unless otherwise determined by the Board, options will generally be exercisable as to 12.5% of the shares of common stock underlying such option six months after the date of grant and as to 1/42 of the remaining shares subject to the option each month thereafter.
Generally, the option price may be paid (a) in cash or by certified check, bank draft or money order, (b) through delivery of shares of common stock having a fair market value equal to the purchase price, or (c) a combination of these methods. The Board is also authorized to establish a cashless exercise program.
No option may be transferred other than by will or by the laws of descent and distribution, and during a recipient’s lifetime an option may be exercised only by the recipient. Unless otherwise determined by the Board, options that are exercisable at the time of a recipient’s termination of service with the Company will continue to be exercisable for three months (twelve months if the optionee terminates service due to death or disability).
Terms and Conditions of Stock Purchase Rights. Stock purchase rights may be issued either alone, or in tandem with, options or other awards under the 2005 Plan. A stock purchase right allows a recipient to purchase a share of common stock at a price determined by the Board. The Company will have the right to repurchase the shares of common stock that are the subject to the award upon the recipient’s termination of service. Unless otherwise determined by the Board, the Company’s right of repurchase will lapse as to 12.5% of the purchased shares 6 months after the date of grant and will lapse as to 1/42 of the remaining purchased shares each month thereafter.
Terms and Conditions of Restricted Stock Awards. The Board may also grant a restricted stock award to any eligible employee, director or consultant. Under a restricted stock award, shares of common stock that are the subject of the award are generally subject to forfeiture to the extent that the recipient terminates service with the Company prior to the award having vested. Unless otherwise determined by the Board, 12.5% of the shares subject to a restricted stock award will vest 6 months after the date of grant and as to 1/42 of the remaining shares each month thereafter. Unless otherwise determined by the Board, holders of restricted shares will have the right to vote such shares and to receive any cash dividends with respect thereto during the restriction period. Any stock dividends will be subject to the same restrictions as the underlying shares of restricted stock.
Terms and Conditions of Unrestricted Stock Awards. The Board may grant unrestricted stock awards to any eligible employee, director or consultant. Unrestricted shares do not require any payment by the recipient and are not subject to forfeiture.
In the event of a consolidation or merger in which the Company is not the surviving corporation or which results in the acquisition of substantially all of the Company’s outstanding stock by a single person or entity or by a group of persons and/or entities acting in concert, or in the event of the sale or transfer of substantially all of the Company’s assets, the 2005 Plan provides that all outstanding options will become exercisable, unless the successor entity assumes such options, and that the Company’s right of repurchase with respect to shares covered by all outstanding stock purchase rights and all restrictions with respect to restricted stock awards will lapse.
The Board may at any time amend the 2005 Plan for the purpose of satisfying the requirements of the Internal Revenue Code of 1986, as amended (the “Code”), or other applicable law or regulation or for any other legal purpose, provided that, without the consent of our stockholders, the Board may not (a) increase the number of shares of common stock available under the 2005 Plan, (b) change the group of individuals eligible to receive options and/or purchase grants, or (c) extend the term of the 2005 Plan.
BUSINESS RELATIONSHIPS AND RELATED TRANSACTIONS
Since January 1, 2005, there has not been nor is there proposed any transaction or series of similar transactions to which we were or are to be a party in which:
| | · | The amount involved exceeded one percent (1%) of the average of the Company’s total assets at year end for the Company’s past three completed fiscal years; and |
| · | In which any director, executive officer, holder of more than 5% of our common stock or any member of the immediate family of any of these persons had or will have a direct or indirect material interest other than: |
| - | compensation agreements and other arrangements, that are described where required under “Executive Compensation;” |
| - | the transactions described below: |
On April 26, 2005, the Company completed a private placement with Pacific Century Fund LLC of 95,000 shares of common stock at a purchase price of $1.00 per share, or gross proceeds of $95,000, and, for no additional consideration, a cashless 2-year warrant to purchase additional 95,000 shares at an exercise price of $1.50 per share. Under the agreement, the Company granted Pacific Century the right to include the shares that it purchased in any registration statement that the Company might subsequently file (other than a registration statement on Form S-4, S-8 or other limited purpose form), subject to cutback in the case of an underwritten offering.
Peter Wang, the Company’s Chief Executive Officer and Chairman of the Board of Directors, Ya Li, the Company’s Chief Financial Officer, and Wind Chen, the Company’s Chief Operating Officer, are each members of Pacific Century Fund LLC owning 28.38%, 30.38% and 3.77%, respectively, of the ownership interests of Pacific Century.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding beneficial ownership of the Company’s common stock as of January 17, 2007 by (i) each person (or group of affiliated persons) who is known by us to own more than five percent of the outstanding shares of our common stock, (ii) each director and executive officer, and (iii) all of our directors and executive officers as a group.
Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. We believe that all persons named in the table have sole voting and investment power with respect to shares beneficially owned by them. All share ownership figures include shares issuable upon exercise of options or warrants exercisable within 60 days of January 17, 2007, which are deemed outstanding and beneficially owned by such person for purposes of computing his or her percentage ownership, but not for purposes of computing the percentage ownership of any other person.
| Name and Address of Beneficial Owner (1) | | Number of Shares Beneficially Owned** | | % of Common Stock Beneficially Owned** | |
| SB China Holdings PTE Ltd.(2) | | | 11,928,935 | | | 13.95 | % |
| UTStarcom Inc.(3) | | | 11,928,935 | | | 13.95 | % |
| Pacific Century Fund LLC(4) | | | 15,836,112 | | | 18.52 | % |
| PZW Family LLP(5) | | | 18,556,209 | | | 21.69 | % |
| Peter Wang(6) | | | 27,318,230 | | | 31.59 | % |
| Ya Li(7) | | | 1,050,200 | | | * | |
| Wind Chen(8) | | | 660,120 | | | * | |
| Charles Xue (9) | | | 387,500 | | | * | |
| All Directors and Executive Officers as a Group (4 persons) | | | 28,328,550 | | | 33.13 | % |
* Indicates less than one percent.
** Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to the shares shown. Except as indicated by footnote and subject to community property laws where applicable, to our knowledge, the stockholders named in the table have sole voting and investment power with respect to all common stock shares shown as beneficially owned by them. A person is deemed to be the beneficial owner of securities that can be acquired by such person within 60 days upon the exercise of options, warrants or convertible securities (in any case, the “Currently Exercisable Options”). Each beneficial owner’s percentage ownership is determined by assuming that the Currently Exercisable Options that are held by such person (but not those held by any other person) have been exercised and converted.
(1) The address for those persons for whom an address is not otherwise indicated is c/o China Biopharma, Inc, 31 Airpark Road, Princeton, New Jersey 08540.
(2) The address for SB China Holdings PTE Ltd. Is 28F-A Zhao Feng World Trade Building, 369 Jiang Su Road, Shanghai 200050, PRC China.
(3) The address for UTStarcom, Inc., is 1275 Harbor Bay Parkway, Alameda, California 94502.
(4) Peter Wang, China Biopharma’s Chief Executive Officer and Chairman of the Board of Directors, Ya Li, China Biopharma’s Chief Financial Officer, and Wind Chen, China Biopharma’s Chief Operating Officer, are each members of Pacific Century Fund LLC owning 28.38%, 30.38% and 3.77%, respectively, of the ownership interests of Pacific Century. The address for Pacific Century Fund LLC is 68 Cottonwood Court, Monmouth Junction, New Jersey 08852.
(5) PZW Family LLP is 20% owned by Peter Wang. The address for PZW Family LLP is 58261 Melton Road, Hillard, Florida 32046.
(6) Includes 3,976,336 shares held by MAC Wireless/PW LLC which is 80% owned by Mr. Wang, 1,325,469 shares held by Hangzhou Joray Electronics CO Ltd. Which is 50% owned by Mr. Wang, 18,556,209 shares held by PZW Family LLP which is 20% owned by Mr. Wang, and 812,700 shares issuable upon exercise of Currently Exercisable Options. As the owner of 50% of the equity interests in Hangzhou Joray Electronics, Mr. Wang shares voting and investment power over the shares of China Biopharma common stock held by Hangzhou Joray Electronics. As one of the general partners of PZW Family LLP, Mr. Wang shares voting and investment power over the shares of China Biopharma common stock held by PZW Family LLP. Mr. Wang disclaims beneficial ownership of the shares held by MAC Wireless/PW LLC, Hangzhou Joray Electronics, and PZW Family LLP except to the extent of his pecuniary interest in the shares. Includes options to purchase 300,000 shares at an exercise price of $0.52 per share, 100,000 of such shares vested upon grant, all of which have fully vested and are currently exercisable.
(7) Includes 800,200 shares issuable upon exercise of Currently Exercisable Options. Also Includes options to purchase 250,000 shares at an exercise price of $0.52 per share, all of which have fully vested and are currently exercisable.
(8) Includes 510,120 shares issuable upon exercise of Currently Exercisable Options. Also Includes options to purchase 150,000 shares at an exercise price of $0.52 per share, all of which have fully vested and are currently exercisable.
(9) Includes options to purchase 250,000 shares at an exercise price of $0.52 per share, all of which have fully vested and are currently exercisable.
SELLING STOCKHOLDERS
The following table sets forth as of January 17, 2007, information regarding the current beneficial ownership of our common stock by the persons identified, based on information provided to us by them, which we have not independently verified. Although we have assumed for purposes of the table that the Selling Stockholders will sell all of the shares offered by this prospectus, because they may from time to time offer all or some of their shares under this prospectus or in another manner, no assurance can be given as to the actual number of shares that will be resold by the Selling Stockholders (or any of them), or that will be held after completion of the resales. In addition, a Selling Stockholder may have sold or otherwise disposed of shares in transactions exempt from the registration requirements of the Securities Act or otherwise since the date he or she provided information to us. The Selling Stockholders are not making any representation that the shares covered by this prospectus will be offered for sale. Except as set forth below, no Selling Stockholder has held any position nor had any material relationship with us or our affiliates during the past three years.
Name of Selling Stockholder | | Warrant Shares Beneficially Owned Prior to Offering | | Note Shares Beneficially Owned Prior to the Offering (1) | | Maximum Number of Shares to be Sold | | Shares Beneficially Owned After Offering | | Percentage Ownership After Offering (%) | |
| Chestnut Ridge Partners, LP (2) | | | 1,000,000 | (3) | | 1,000,000 | | | 2,000,000 | | | 0 | | | 0 | |
| Nite Capital LP (4) | | | 1,000,000 | (5) | | 1,000,000 | | | 2,000,000 | | | 0 | | | 0 | |
| Professional Offshore Opportunity Fund, Ltd. (6) | | | 1,200,000 | (7) | | 1,200,000 | | | 2,400,000 | | | 0 | | | 0 | |
| Marvin Mermelstein (8) | | | 1,000,000 | (9) | | 1,000,000 | | | 2,000,000 | | | 0 | | | 0 | |
| Brio Capital L.P. (10) | | | 600,000 | (11) | | 600,000 | | | 1,200,000 | | | 0 | | | 0 | |
| Vision Opportunity Master Fund, Ltd. (12) | | | 2,800,000 | (13) | | 2,800,000 | | | 5,600,000 | | | 0 | | | 0 | |
| Double U Master Fund L.P. (14) | | | 400,000 | (15) | | 400,000 | | | 800,000 | | | 0 | | | 0 | |
| Monarch Capital Fund Ltd. (16) | | | 1,000,000 | (17) | | 1,000,000 | | | 2,000,000 | | | 0 | | | 0 | |
| Anthony Heller (18) | | | 600,000 | (19) | | 600,000 | | | 1,200,000 | | | 0 | | | 0 | |
| First Mirage, Inc. (20) | | | 500,000 | (21) | | 500,000 | | | 1,000,000 | | | 0 | | | 0 | |
| Generation Capital Associates (22) | | | 500,000 | (23) | | 500,000 | | | 1,000,000 | | | 0 | | | 0 | |
| Centurion Microcap, LP (24) | | | 1,000,000 | (25) | | 1,000,000 | | | 2,000,000 | | | 0 | | | 0 | |
| Burstein & Lindsay Sec. Corp. (26) | | | 400,000 | (27) | | 400,000 | | | 800,000 | | | 0 | | | 0 | |
| Melton Management Ltd. (28) | | | 2,400,000 | (29) | | 0 | | | 2,400,000 | | | 0 | | | 0 | |
| (1) | Share numbers based on the number of shares issuable upon conversion of the Notes at an initial conversion price of $0.25 per share. |
| | |
| (2) | The Selling Stockholder has an address at 50 Tice Boulevard, Woodcliff Lake, NJ 07677. Mr. Kenneth Pasternak is deemed to have voting and dispositive power over the shares of our common stock owned by Chestnut Ridge Partners, LP, however, Mr. Pasternak disclaims beneficial ownership of the shares. The Selling Stockholder has contractually agreed that it will not convert debt or exercise warrants to the extent that such conversion or exercise would result in it, together with its affiliates, beneficially owning more than 4.99% of the number of shares of our common stock outstanding at the time of conversion or exercise. |
| | |
| (3) | Represents 500,000 shares of Common stock initially issuable upon exercise of Class A Warrants and 500,000 shares of common stock initially issuable upon exercise of Class B Warrants. |
| | |
| (4) | The Selling Stockholder has an address at 100 E. Cook Avenue, Suite 201, Libertyville, IL 60048. Mr. Keith Goodman is deemed to have voting and dispositive power over the shares of our common stock owned by the Selling Stockholder, however, Mr. Goodman disclaims beneficial ownership of the shares. The Selling Stockholder has contractually agreed that it will not convert debt or exercise warrants to the extent that such conversion or exercise would result in it, together with its affiliates, beneficially owning more than 4.99% of the number of shares of our common stock outstanding at the time of conversion or exercise. |
| (5) | Represents 500,000 shares of common stock initially issuable upon exercise of Class A Warrants and 500,000 shares of common stock initially issuable upon exercise of Class B Warrants. |
| | |
| (6) | The Selling Stockholder has an address at 1400 Old Country Road, Suite 206, Westbury, NY 11590. Mr. Mark K. Swickle is deemed to have voting and dispositive power over the shares of our common stock owned by the Selling Stockholder, however, Mr. Swickle disclaims beneficial ownership of the shares. The Selling Stockholder has contractually agreed that it will not convert debt or exercise warrants to the extent that such conversion or exercise would result in it, together with its affiliates, beneficially owning more than 4.99% of the number of shares of our common stock outstanding at the time of conversion or exercise. |
| | |
| (7) | Represents 600,000 shares of common stock initially issuable upon exercise of Class A Warrants and 600,000 shares of common stock initially issuable upon exercise of Class B Warrants. |
| | |
| (8) | The Selling Stockholder is an individual with an address at 6500 N. Hamlin, Lincolnwood, IL 60712. The Selling Stockholder has contractually agreed that it will not convert debt or exercise warrants to the extent that such conversion or exercise would result in it, together with its affiliates, beneficially owning more than 4.99% of the number of shares of our common stock outstanding at the time of conversion or exercise. |
| | |
| (9) | Represents 500,000 shares of Common Stock initially issuable upon exercise of Class A Warrants and 500,000 shares of common stock initially issuable upon exercise of Class B Warrants. |
| | |
| (10) | The Selling Stockholder has an address at 401 East 34th Street, Suite South 33-C, New York, NY 10016. Mr. Shaye Hirsch is deemed to have voting and dispositive power over the shares of our Common stock owned by the Selling Stockholder, however, Mr. Hirsch disclaims beneficial ownership of the shares. The Selling Stockholder has contractually agreed that it will not convert debt or exercise warrants to the extent that such conversion or exercise would result in it, together with its affiliates, beneficially owning more than 4.99% of the number of shares of our common stock outstanding at the time of conversion or exercise. |
| | |
| (11) | Represents 300,000 shares of common stock initially issuable upon exercise of Class A Warrants and 300,000 shares of common stock initially issuable upon exercise of Class B Warrants. |
| | |
| (12) | The Selling Stockholder has an address at 20 West 55th Street, 5th Floor, New York, NY 10019. Mr. Adam Benwitz is deemed to have voting and dispositive power over the shares of our Common Stock owned by the Selling Stockholder, however, Mr. Benwitz disclaims beneficial ownership of the shares. The Selling Stockholder has contractually agreed that it will not convert debt or exercise warrants to the extent that such conversion or exercise would result in it, together with its affiliates, beneficially owning more than 4.99% of the number of shares of our common stock outstanding at the time of conversion or exercise. |
| | |
| (13) | Represents 1,400,000 shares of common stock initially issuable upon exercise of Class A Warrants and 1,400,000 shares of common stock initially issuable upon exercise of Class B Warrants. |
| | |
| (14) | The Selling Stockholder has an address at P.O. Box 972, Harbour House, Road Town, Tortola BVI. The Selling Stockholder is a master fund in a master-feeder structure with B&W Equities, LLC, as its general partner. Mr. Isaac Winehouse is the manager of B&W Equities, LLC and Mr. Winehouse has ultimate responsibility of trading with respect to shares held by the Selling Stockholder. Mr. Winehouse disclaims beneficial ownership of the shares. The Selling Stockholder has contractually agreed that it will not convert debt or exercise warrants to the extent that such conversion or exercise would result in it, together with its affiliates, beneficially owning more than 4.99% of the number of shares of our common stock outstanding at the time of conversion or exercise. |
| | |
| (15) | Represents 200,000 shares of common stock initially issuable upon exercise of Class A Warrants and 200,000 shares of common stock initially issuable upon exercise of Class B Warrants. |
| | |
| (16) | The Selling Stockholder has an address at Harbour House, 2nd Floor, Waterfront Drive, Road Town, Tortola BVI. Navigator Management Ltd. is the manager of the Selling Stockholder. Mr. David Sims has ultimate responsibility of trading with respect to shares held by the Selling Stockholder. The Selling Stockholder has contractually agreed that it will not convert debt or exercise warrants to the extent that such conversion or exercise would result in it, together with its affiliates, beneficially owning more than 4.99% of the number of shares of our common stock outstanding at the time of conversion or exercise. |
| | |
| (17) | Represents 500,000 shares of common stock initially issuable upon exercise of Class A Warrants and 500,000 shares of common stock initially issuable upon exercise of Class B Warrants. |
| | |
| (18) | The Selling Stockholder has an address at c/o Plazacorp Investments Limited, 10 Wanless Avenue, Suite 201, Toronto, Ontario M4N 1V6 Canada. The Selling Stockholder has contractually agreed that it will not convert debt or exercise warrants to the extent that such conversion or exercise would result in it, together with its affiliates, beneficially owning more than 4.99% of the number of shares of our common stock outstanding at the time of conversion or exercise. |
| | |
| (19) | Represents 300,000 shares of common stock initially issuable upon exercise of Class A Warrants and 300,000 shares of common stock initially issuable upon exercise of Class B Warrants. |
| | |
| (20) | The Selling Stockholder has an address at 333 Sandy Springs Circle, Suite 230, Atlanta, GA 30328. The Selling Stockholder is a wholly owned subsidiary of High Capital Funding, LLC. David A. Rappaport, Fred A. Brasch and Frank E. Hart, each individuals, have responsibility of trading with respect to shares held by the Selling Stockholder. The Selling Stockholder has contractually agreed that it will not convert debt or exercise warrants to the extent that such conversion or exercise would result in it, together with its affiliates, beneficially owning more than 4.99% of the number of shares of our common stock outstanding at the time of conversion or exercise. |
| (21) | Represents 250,000 shares of Common Stock initially issuable upon exercise of Class A Warrants and 250,000 shares of common stock initially issuable upon exercise of Class B Warrants. |
| | |
| (22) | The Selling Stockholder has an address at 1085 Riverside Trace, Atlanta, GA 30328. The Selling Stockholder is a wholly owned subsidiary of High Capital Funding, LLC. David A. Rappaport, Fred A. Brasch and Frank E. Hart, each individuals, have responsibility of trading with respect to shares held by the Selling Stockholder. The Selling Stockholder has contractually agreed that it will not convert debt or exercise warrants to the extent that such conversion or exercise would result in it, together with its affiliates, beneficially owning more than 4.99% of the number of shares of our common stock outstanding at the time of conversion or exercise. |
| | |
| (23) | Represents 250,000 shares of common stock initially issuable upon exercise of Class A Warrants and 250,000 shares of common stock initially issuable upon exercise of Class B Warrants. |
| | |
| (24) | The Selling Stockholder has an address at 3014 Avenue L, Brooklyn, NY 11210. Mr. Abraham Schwartz is the General Partner of the Selling Stockholder. Mr. Schwartz has voting and dispositive power with respect to the shares held by the Selling Stockholder. The Selling Stockholder has contractually agreed that it will not convert debt or exercise warrants to the extent that such conversion or exercise would result in it, together with its affiliates, beneficially owning more than 4.99% of the number of shares of our common stock outstanding at the time of conversion or exercise. |
| | |
| (25) | Represents 500,000 shares of common stock initially issuable upon exercise of Class A Warrants and 500,000 shares of common stock initially issuable upon exercise of Class B Warrants. |
| | |
| (26) | The Selling Stockholder has an address at 140 Birmensdorfer Str., CH 8003, Zurich, Switzerland. Mr. Mosi Krans has power of attorney to act on behalf of the Selling Stockholder. Mr. Krans has voting and dispositive power with respect to the shares held by the Selling Stockholder. The Selling Stockholder has contractually agreed that it will not convert debt or exercise warrants to the extent that such conversion or exercise would result in it, together with its affiliates, beneficially owning more than 4.99% of the number of shares of our common stock outstanding at the time of conversion or exercise. |
| | |
| (27) | Represents 200,000 shares of common stock initially issuable upon exercise of Class A Warrants and 200,000 shares of common stock initially issuable upon exercise of Class B Warrants. |
| | |
| (28) | Melton Management Ltd., with an address at 12 Agasi Street, Har Nof, Jerusalem, 93877, Israel, acted as the finder with respect to the issuance and sale of the Notes. Yehuda Breitkope, Director of the Selling Stockholder, has voting and dispositive power with respect to the share held by Melton Management Ltd. |
| | |
| (29) | Represents 2,400,000 shares of common stock initially issuable upon exercise of warrants at an exercise price of $0.30 per share. |
PLAN OF DISTRIBUTION
The Selling Stockholders of our common stock and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These sales may be at fixed or negotiated prices. The Selling Stockholders may use any one or more of the following methods when selling shares:
| · | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| · | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| · | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| · | an exchange distribution in accordance with the rules of the applicable exchange; |
| · | privately negotiated transactions; |
| · | settlement of short sales entered into after the date of this prospectus; |
| · | broker-dealers may agree with the Selling Stockholders to sell a specified number of such shares at a stipulated price per share; |
| · | a combination of any such methods of sale; |
| · | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; or |
| · | any other method permitted pursuant to applicable law. |
The Selling Stockholders may also sell shares under Rule 144 under the Securities Act of 1933, as amended, if available, rather than under this prospectus.
Broker-dealers engaged by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. Each Selling Stockholder does not expect these commissions and discounts relating to its sales of shares to exceed what is customary in the types of transactions involved.
In connection with the sale of our common stock or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the common stock in the course of hedging the positions they assume. The Selling Stockholders may also sell shares of our common stock short and deliver these securities to close out their short positions, or loan or pledge the common stock to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Stockholders and any broker-dealers or agents that are involved in selling the shares may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each Selling Stockholder has informed the Company that it does not have any agreement or understanding, directly or indirectly, with any person to distribute the common stock.
The Company is required to pay certain fees and expenses incurred by the Company incident to the registration of the shares. The Company has agreed to indemnify the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
Because the Selling Stockholders may be deemed to be “underwriters” within the meaning of the Securities Act, they will be subject to the prospectus delivery requirements of the Securities Act. In addition, any securities covered by this prospectus which qualify for sale pursuant to Rule 144 under the Securities Act may be sold under Rule 144 rather than under this prospectus. Each Selling Stockholder has advised us that they have not entered into any agreements, understandings or arrangements with any underwriter or broker-dealer regarding the sale of the resale shares. There is no underwriter or coordinating broker acting in connection with the proposed sale of the resale shares by the Selling Stockholders.
We agreed to keep this prospectus effective until the earlier of (i) two years from the effective date of the registration statement of which this prospectus forms a part or (ii) the date on which the Selling Stockholders have disposed of all resale shares in accordance with the methods of distribution contemplated hereby. The resale shares will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale shares may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale shares may not simultaneously engage in market making activities with respect to our common stock for a period of two business days prior to the commencement of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of shares of our common stock by the Selling Stockholders or any other person. We will make copies of this prospectus available to the Selling Stockholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale.
DESCRIPTION OF SECURITIES
Our current authorized capital stock consists of 200,000,000 shares of common stock, par value $.0001 per share, of which 85,520,000 shares were issued and outstanding as of January 17, 2007, and 1,000,000 shares of preferred stock, par value $.001 per share, of which no shares are issued or outstanding.
Common Stock
Each share of common stock entitles the holder to one vote on all matters submitted to a vote of the Company’s stockholders. When a dividend is declared by the Board, all stockholders are entitled to receive a fixed dividend. To date, no dividends have been declared. All shares issued in the company are of the same class, and have equal liquidation, preference, and adjustment rights.
Holders of our common stock have no conversion, preemptive or other subscription rights, and there are no redemption provisions for our common stock. The rights of the holders of common stock are subject to any rights that may be fixed for holders of preferred stock, when and if any additional preferred stock is authorized and issued. All outstanding shares of our common stock are, and the shares underlying all options and warrants and convertible securities will be, duly authorized, validly issued, fully paid and non-assessable upon our issuance of these shares.
Transfer agent and registrar
The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company, 17 Battery Place, 8th Floor, New York, New York 10004, telephone number 212-509-4000.
Market Information
Our common stock price is quoted on the OTC Bulletin Board, or OTCBB, under the symbol “CBPC”. The following table sets forth for the periods indicated the high and low prices per share traded for our common stock as reported on the OTCBB.
| Year Ended December 31, 2007 | | High | | Low | |
| First Quarter (through January 17, 2007) | | $ | 0.43 | | $ | 0.36 | |
| Year Ended December 31, 2006 | | High | | Low | |
| First Quarter | | $ | 0.75 | | $ | 0.41 | |
| Second Quarter | | $ | 0.67 | | $ | 0.32 | |
| Third Quarter | | $ | 0.45 | | $ | 0.27 | |
| Fourth Quarter | | $ | 0.50 | | $ | 0.27 | |
| Year Ended December 31, 2005 | | High | | Low | |
| First Quarter | | $ | 1.15 | | $ | 0.75 | |
| Second Quarter | | $ | 1.04 | | $ | 0.41 | |
| Third Quarter | | $ | 0.95 | | $ | 0.60 | |
| Fourth Quarter | | $ | 0.75 | | $ | 0.38 | |
The quotations shown reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not represent actual transactions.
Holders
As of December 31, 2006, there were approximately 373 stockholders of record of our common stock and no stockholders of record of our preferred stock, par value $.0001 per share.
Dividends
We have not paid any cash dividends in the past and do not intend to pay cash dividends on our capital stock for the foreseeable future. Instead, we intend to retain all earnings, if any, for use in the operation and expansion of our business. The payment of any dividends in the future will be at the sole discretion our Board of Directors.
DISCLOSURE OF COMMISSION POSITION
ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Our certificate of incorporation provides that none of our directors will be personally liable to the Company or any of our stockholders for monetary damages arising from the director’s breach of fiduciary duty as a director, with certain limited exceptions.
Pursuant to Delaware corporation law, every Delaware corporation has the power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than an action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation or is or was serving in such a capacity at the request of the corporation for another corporation, partnership, joint venture, trust or other enterprise, against any and all expenses, judgments, fines and amounts paid in settlement and reasonably incurred in connection with such action, suit or proceeding. The power to indemnify applies only if such person acted in good faith and in a manner such person reasonably believed to be in the best interests, or not opposed to the best interests, of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful.
The power to indemnify applies to actions brought by or in the right of the corporation as well, but only to the extent of defense and settlement expenses and not to any satisfaction of a judgment or settlement of the claim itself, and with the further limitation that in such actions no indemnification shall be made in the event of any adjudication of negligence or misconduct unless the court, in its discretion, believes that in light of all the circumstances indemnification should apply. Our articles of incorporation contain provisions authorizing it to indemnify our officers and directors to the fullest extent permitted by Delaware corporation law.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers or controlling persons pursuant to the foregoing provisions or otherwise, the Company has been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by us of expenses incurred or paid by a director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by one of our directors, officers, or controlling persons in connection with the securities being registered, we will, unless in the opinion of our legal counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
LEGAL MATTERS
The validity of the securities offered hereby has been passed upon for us by Loeb & Loeb LLP, New York, New York.
EXPERTS
Our audited financial statements for the period ended December 31, 2005 have been included in this prospectus in reliance upon the report of Patrizio & Zhao, LLC, independent auditors (such report being reliant on the report of Rosenberg Rich Baker Berman & Company dated February 28, 2005, relating to our audited financial statements for the period ended December 31, 2004), appearing in this registration statement, and their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We are a public company and file annual, quarterly and special reports, proxy statements and other information with the SEC. You may read and copy any document we file at the SEC’s public reference room at 100 F Street, N.E., Washington, D.C. 20549. You can request copies of these documents by writing to the SEC and paying a fee for the copying cost. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the public reference room. Our SEC filings are also available, at no charge, to the public at the SEC’s web site at http://www.sec.gov.
Report of Independent Registered Public Accounting Firm
To the Board of Directors
China Biopharma Inc. and Subsidiaries:
We have reviewed the accompanying consolidated balance sheet of China Biopharma Inc. and Subsidiaries (a Delaware corporation in the development stage) as of September 30, 2006, and the related consolidated statements of operations and cash flows for the six-month period ended September 30, 2006. These financial statements are the responsibility of the company’s management.
We conducted our review in accordance with the standards of the Public Company Accounting Oversight Board (United States). A review of interim financial information consists principally of applying analytical procedures and making inquiries of persons responsible for financial and accounting matters. It is substantially less in scope than an audit conducted in accordance with the standards of the Public Company Accounting Oversight Board (United States), the objective of which is the expression of an opinion regarding the financial statements taken as a whole. Accordingly, we do not express such an opinion.
Based on our review, we are not aware of any material modifications that should be made to the accompanying financial statements for them to be in conformity with accounting principles generally accepted in the United States of America.
| | | |
| Patrizio & Zhao, LLC | |
| | |
| Lodi, New Jersey | | |
| November 6, 2006 | |
| | |
CHINA BIOPHARMA INC. AND SUBSIDIARIES
(FORMERLY TECHEDGE INC)
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED BALANCE SHEET
SEPTEMBER 30, 2006
(UNAUDITED)
ASSETS
CURRENT ASSETS | | | | |
Cash and cash equivalents | | $ | 10,623 | |
Accounts receivable, net of bad debt reserve of $39,294 | | | 55,094 | |
Due from related parties | | | 329,208 | |
Prepaid expenses and other current assets | | | 9,133 | |
| | | | | |
Total Current Assets | | | 404,058 | |
| | | | | |
PROPERTY AND EQUIPMENT, NET | | | 48,213 | |
| | | | | |
OTHER ASSETS | | | 5,212 | |
| | | | | |
Total Assets | | $ | 457,483 | |
| | | | | |
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | | | | |
| | | | | |
CURRENT LIABILITIES | | | | |
Accounts payable and accrued expenses | | $ | 1,528,691 | |
Other Liabilities | | | 106,682 | |
Due to officers | | | 874,442 | |
| | | | | |
Total Current Liabilities | | | 2,509,815 | |
| | | | | |
STOCKHOLDERS’ EQUITY (DEFICIT) | | | | |
Common stock, stated value $.0001, 100,000,000 | | | | |
shares authorized; 8,8520,000 shares issued and | | | | |
Outstanding | | | 8,852 | |
Additional paid-in capital | | | 6,699,073 | |
Deficit accumulated during development stage | | | (8,777,135 | ) |
Accumulated other comprehensive income | | | 16,878 | |
| | | | | |
Total Stockholders' Equity (Deficit) | | | (2,052,332 | ) |
| | | | | |
| | | | | |
Total Liabilities and Stockholders' Equity (Deficit) | | $ | 457,483 | |
See notes to consolidated financial statements.
CHINA BIOPHARMA INC. AND SUBSIDIARIES
(FORMERLY TECHEDGE INC)
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF OPERATIONS
(UNAUDITED)
| | | | | For the Period From | |
| | | For the three Months Ended | | September 13, 2000 | |
| | | September 30, | | (Date of Inception) to | |
| | | 2006 | | 2005 | | September 30, 2006 | |
| | | | | | | | |
REVENUE | | $ | - | | $ | 59,307 | | $ | 1,537,958 | |
| | | | | | | | | | | |
COSTS AND EXPENSES | | | | | | | | | | |
Cost of sales | | | - | | | 33,476 | | | 764,741 | |
Research and development | | | - | | | 161,214 | | | 2,274,698 | |
General and administrative (including share-based | | | | | | | | | | |
payment of $-, $148,300 and $182,153, respectively) | | | 160,090 | | | 637,296 | | | 6,727,462 | |
Depreciation and amortization | | | 6,275 | | | 20,373 | | | 366,955 | |
| | | | | | | | | | | |
Total Costs and Expenses | | | 166,365 | | | 852,359 | | | 10,133,856 | |
| | | | | | | | | | | |
(LOSS) FROM OPERATIONS | | | (166,365 | ) | | (793,052 | ) | | (8,595,898 | ) |
| | | | | | | | | | | |
OTHER INCOME (EXPENSE) | | | | | | | | | | |
Loss from unconsolidated subsidiary | | | - | | | - | | | (60,134 | ) |
| Loss from disposal of subsidiary, net of tax | | | - | | | - | | | (48,142 | ) |
| Sale of subsidiary’s net operating losses | | | - | | | - | | | 216,247 | |
Gain (loss) on foreign currency | | | - | | | - | | | 660 | |
Interest income (expense), net | | | - | | | 102 | | | 34,299 | |
| | | | | | | | | | | |
Total Other Income (Expense) | | | - | | | 102 | | | 142,930 | |
| | | | | | | | | | | |
(LOSS) BEFORE CUMULATIVE EFFECT OF CHANGE IN ACCOUNTING PRINCIPLE | | | (166,365 | ) | | (792,950 | ) | | (8,452,968 | ) |
| | | | | | | | | | | |
CUMULATIVE EFFECT OF CHANGE IN ACCOUNTING PRINCIPLE, NET OF TAX | | | - | | | - | | | (324,167 | ) |
| | | | | | | | | | | |
NET (LOSS) | | | (166,365 | ) | | (792,950 | ) | | (8,777,135 | ) |
| | | | | | | | | | | |
UNREALIZED GAIN (LOSS) ON FOREIGN CURRENCY TRANSLATION, NET OF TAX | | | - | | | - | | | (3,526 | ) |
| | | | | | | | | | | |
COMPREHENSIVE (LOSS) | | | ($166,365 | ) | | ($792,950 | ) | | ($8,780,661 | ) |
| | | | | | | | | | | |
LOSS PER COMMON SHARE, BASIC AND DILUTED | | | ($ 0.00 | ) | | ($ 0.01 | ) | | | |
| | | | | | | | | | | |
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING, BASIC AND DILUTED | | | 85,520,000 | | | 82,411,044 | | | | |
See notes to consolidated financial statements.
CHINA BIOPHARMA INC. AND SUBSIDIARIES
(FORMERLY TECHEDGE INC)
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF OPERATIONS
(UNAUDITED)
| | | | | For the Period From | |
| | | For the Nine Months Ended | | September 13, 2000 | |
| | | September 30, | | (Date of Inception) to | |
| | | 2006 | | 2005 | | September 30, 2006 | |
| | | | | | | | |
REVENUE | | $ | - | | $ | 186,921 | | $ | 1,537,958 | |
| | | | | | | | | | | |
COSTS AND EXPENSES | | | | | | | | | | |
Cost of sales | | | - | | | 101,697 | | | 764,741 | |
Research and development | | | - | | | 503,034 | | | 2,274,698 | |
General and administrative (including share-based payment of $168,542, $148,300 and $182,153, respectively) | | | 462,382 | | | 1,607,821 | | | 6,727,462 | |
Depreciation and amortization | | | 19,043 | | | 54,386 | | | 366,955 | |
| | | | | | | | | | | |
Total Costs and Expenses | | | 481,425 | | | 2,266,938 | | | 10,133,856 | |
| | | | | | | | | | | |
(LOSS) FROM OPERATIONS | | | (481,425 | ) | | (2,080,017 | ) | | (8,595,898 | ) |
| | | | | | | | | | | |
OTHER INCOME (EXPENSE) | | | | | | | | | | |
Loss from unconsolidated subsidiary | | | - | | | - | | | (60,134 | ) |
| Loss from disposal of subsidiary, net of tax | | | (48,142 | ) | | - | | | (48,142 | ) |
| Sale of subsidiary’s net operating losses | | | - | | | - | | | 216,247 | |
Gain (loss) on foreign currency | | | - | | | 594 | | | 660 | |
Interest income (expense), net | | | - | | | 341 | | | 34,299 | |
| | | | | | | | | | | |
Total Other Income (Expense) | | | (48,142 | ) | | 935 | | | 142,930 | |
| | | | | | | | | | | |
(LOSS) BEFORE CUMULATIVE EFFECT OF CHANGE IN ACCOUNTING PRINCIPLE | | | (529,567 | ) | | (2,079,082 | ) | | (8,452,968 | ) |
| | | | | | | | | | | |
CUMULATIVE EFFECT OF CHANGE IN ACCOUNTING PRINCIPLE, NET OF TAX | | | - | | | - | | | (324,167 | ) |
| | | | | | | | | | | |
NET (LOSS) | | | (529,567 | ) | | (2,079,082 | | | (8,777,135 | ) |
| | | | | | | | | | | |
UNREALIZED GAIN (LOSS) ON FOREIGN CURRENCY TRANSLATION, NET OF TAX | | | - | | | - | | | (3,526 | ) |
| | | | | | | | | | | |
COMPREHENSIVE (LOSS) | | | ($529,567 | ) | | ($2,079,082 | | | ($8,780,661 | ) |
| | | | | | | | | | | |
LOSS PER COMMON SHARE, BASIC AND DILUTED | | | ($ 0.01 | ) | | ($ 0.03 | ) | | | |
| | | | | | | | | | | |
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING, BASIC AND DILUTED | | | 85,455,000 | | | 81,215,952 | | | | |
See notes to consolidated financial statements.
CHINA BIOPHARMA INC. AND SUBSIDIARIES
(FORMERLY TECHEDGE INC)
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
| | | | | For the Period From | |
| | | For the Nine Months Ended | | September 13, 2000 | |
| | | September 30, | | (Date of Inception) to | |
| | | 2006 | | 2005 | | September 30, 2006 | |
CASH FLOWS FROM OPERATING ACTIVITIES | | | | | | | | | | |
| Net loss | | | ($529,567 | ) | | ($2,079,082 | ) | | ($ 8,777,135 | ) |
| Adjustments to reconcile net loss to net cash used in | | | | | | | | | | |
| operating activities: | | | | | | | | | | |
| Depreciation and amortization | | | 19,043 | | | 60,570 | | | 336,671 | |
| Provision for doubtful accounts | | | - | | | - | | | 14,326 | |
| Loss on unconsolidated subsidiary | | | - | | | - | | | 60,134 | |
| Loss on foreign currency translation | | | - | | | - | | | (3,526 | ) |
| Loss on sale of subsidiaries, net of tax | | | 48,142 | | | - | | | 48,142 | |
| Stock-based compensation | | | 173,542 | | | 281,300 | | | 468,453 | |
| Changes in assets and liabilities: | | | | | | | | | | |
Accounts receivable | | | 25,731 | | | (14,869 | ) | | (55,094 | ) |
Due from related parties | | | (69,465 | ) | | (366,127 | ) | | (329,208 | ) |
Prepaid expenses and other current assets | | | 39,074 | | | 180,236 | | | (9,133 | ) |
Other assets | | | 39,816 | | | - | | | (5,212 | ) |
Accounts payable and accrued expenses | | | 374,755 | | | 315,575 | | | 1,528,691 | |
Other liabilities | | | (136,167 | ) | | (260,000 | ) | | 106,682 | |
Net Cash Used In Operating Activities | | | (15,096 | ) | | (1,882,397 | ) | | (6,616,209 | ) |
| | | | | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES | | | | | | | | | | |
Investment in unconsolidated subsidiary | | | - | | | - | | | (409,832 | ) |
Purchase of property and equipment | | | (5,876 | ) | | - | | | (249,639 | ) |
Net Cash Used In Investing Activities | | | (5,876 | ) | | - | | | (659,471 | ) |
| | | | | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | | | | | |
Net proceeds from private placement of common stock | | | - | | | 1,355,000 | | | 1,897,651 | |
| Purchase of treasury stock | | | (432 | ) | | - | | | (432 | ) |
| Net proceeds from private placement of preferred stock | | | 2,000 | | | - | | | 4,002,000 | |
Net repayments for officers advances | | | - | | | 496,915 | | | 1,371,357 | |
Net Cash Provided By Financing Activities | | | 1,568 | | | 1,851,915 | | | 7,270,576 | |
| | | | | | | | | | | |
EFFECT OF FOREIGN CURRENCY CONVERSION ON CASH | | | (33,580 | ) | | - | | | 15,727 | |
| | | | | | | | | | | |
NET INCEASE (DECREASE) IN CASH | | | (52,985 | ) | | (30,482 | ) | | 10,623 | |
| | | | | | | | | | | |
CASH AND CASH EQUIVALENTS - BEGINNING | | | 63,608 | | | 54,876 | | | - | |
| | | | | | | | | | | |
CASH AND CASH EQUIVALENTS - ENDING | | $ | 10,623 | | $ | 24,394 | | $ | 10,623 | |
See notes to consolidated financial statements.
CHINA BIOPHARMA INC. AND SUBSIDIARIES
(FORMERLY TECHEDGE INC)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2006 AND 2005
NOTE 1 - ORGANIZATION AND NATURE OF BUSINESS
The financial statements should be read in conjunction with a reading of the Company’s annual Report on Form 10-KSB for the fiscal year ended December 31, 2005.
The accompanying unaudited consolidated financial statements have been prepared in accordance with generally accounting principles applicable to interim financial information and with the requirements of Form 10-QSB and Item 310 of Regulation S-B of the Securities and Exchange Commission. Accordingly, they do not include all of the information and footnotes required by accounting principles generally accepted in the United States of America for complete financial statements. Interim results are not necessarily indicative of results for a full year. In the opinion of management, all adjustments considered necessary for a fair presentation of the financial position and the results of operations and cash flows for the interim periods have been included.
NOTE 2 - ADOPTION OF NEW ACCOUNTING STANDARDS
On January 1, 2006, the Company adopted the provisions of Statement of Financial Accounting Standards (SFAS) No. 123(R) “Share-Based Payment” using the modified prospective application. The Company has been expensing share based awards granted after January 1, 2003 under the provisions of SFAS No. 123 “Accounting for Stock-Based Compensation”. For the nine months ended September 30, 2006 and 2005, included in net loss is expense of $168,542 and $148,300 after tax, respectively, of stock based compensation related to stock options granted. If the Company had followed the fair value recognition provisions of SFAS 123(R) for all outstanding and unvested stock options and other stock-based compensation for the nine months ended September 30, 2005, there would have been no material impact on the Company’s financial statements.
NOTE 3 - LOSSES DURING THE DEVELOPMENT STAGE AND MANAGEMENT’S PLANS
Through September 30, 2006 the Company had incurred development stage losses totaling $8,777,135, and net cash used in operating activities of $15,096. At September 30, 2006, the Company had $10,623 of cash and cash equivalents and $55,094 of net trade receivables to fund short-term working capital requirements.
The Company’s ability to continue as a going concern and its future success is dependent upon its ability to raise capital in the near term to: (1) satisfy its current obligations, (2) continue its business efforts, and (3) successfully deploy and market its products on a wide scale.
CHINA BIOPHARMA INC. AND SUBSIDIARIES
(FORMERLY TECHEDGE INC)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2006 AND 2005
NOTE 4 - STOCKHOLDERS’ EQUITY
During the quarter ended June 30, 2006, the Company entered into a consulting agreement whereby Ludlow Capital Inc. would provide corporate Investor Relations advisory services. In exchange for these services, the Company issued 50,000 restricted shares of the Company’s common stock.
During the quarter ended June 30, 2006, the Company entered into a Share Exchange Agreement for the purpose of acquiring 100% of the outstanding capital stock of China BioPharma Limited (CBL) a Cayman Islands Company, which has rights to invest in Tianyuan Bio-Pharmaceuticals Company, Ltd. and Zhejiang Tianyuan Biotech Co., Ltd. (“ZTBC”). The transaction is subject to the final approval by the government of China.
In exchange for 100% of the outstanding capital of CBL, the Company issued a total of 3,000,000 shares of restricted common stock.
NOTE 5 - RELATED PARTY TRANSACTIONS
The Company records material related party transactions. Those charges are included in general and administrative expenses.
The Company occasionally engages in advances to and advances from related parties. The advances have no stated terms of repayment and carry no interest.
Following is a summary of transactions and balances with affiliated entities and related parties 2006 and 2005:
| | | Nine Months Ended | |
| | | September 30, | |
| | | 2006 | | 2005 | |
| | | | | | |
| Revenues from related parties | | $ | - | | $ | - | |
| | | | | | | | |
| Purchases and expenses from related parties | | $ | 1,200 | | $ | 32,670 | |
| | | | | | | | |
| Due from related parties | | $ | 329,208 | | $ | 414,531 | |
| | | | | | | | |
| Due to officers | | $ | 874,442 | | $ | 978,792 | |
Amounts due to officers consist of advances from the Company’s CEO to fund the Company’s operations. It also includes compensation deferred by the Company’s CEO, CFO, CTO and COO. No written repayment agreements exist with either officer. Amounts are unsecured, non-interest bearing and due upon demand.
CHINA BIOPHARMA INC. AND SUBSIDIARIES
(FORMERLY TECHEDGE INC)
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
SEPTEMBER 30, 2006 AND 2005
NOTE 6 - SUBSEQUENT EVENTS
On November 2, 2006, the board of directors unanimously approved to amend the Company’s Certificate of Incorporation to increase the number of common stocks from 100,000,000 to 200,000,000.
Techedge Inc. and Subsidiaries
(A Delaware Corporation in
the Development Stage)
Financial Statements
December 31, 2005 and 2004
Index to Consolidated Financial Statements
| | | Page | |
| | | | | |
| Report of Independent Registered Public Accounting Firm | | | F-10 | |
| | | | | |
| Financial Statements | | | | |
| | | | | |
| Consolidated Balance Sheet as of December 31, 2005 | | | F-11 | |
| | | | | |
| Consolidated Statements of Operations for the years ended December 31, 2005 and 2004 | | | | |
| and for the period from September 13, 2000 (date of inception) through December 31, 2005 | | | F-12 | |
| | | | | |
| Consolidated Statements of Changes in Stockholders' Equity (Deficit) for the period from | | | | |
| September 13, 2000 (date of inception) to December 31, 2005 | | | F-13 | |
| | | | | |
| Consolidated Statements of Cash Flows for the years ended December 31, 2005 and 2004 | | | | |
| and for the period from September 13, 2000 (date of inception) through December 31, 2005 | | | F-14 | |
| | | | | |
| Notes to Consolidated Financial Statements | | | F-15 - F-29 | |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders
Techedge Inc. and Subsidiaries:
We have audited the accompanying consolidated balance sheet of Techedge Inc. and Subsidiaries (a Delaware corporation in the development stage) for the year ended December 31, 2005, and the related consolidated statements of operations, changes in stockholders’ equity (deficit), and cash flows for the year then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit. The financial statements of Techedge Inc. and Subsidiaries as of December 31, 2004, were audited by other auditors whose report dated February 28, 2005, on those statements included an explanatory paragraph that described conditions that raised substantial doubt about the Company’s ability to continue as a going concern discussed in Note 2 to the financial statements.
We conducted our audit in accordance with auditing standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the 2005 financial statements referred to above present fairly, in all material respects, the financial position of Techedge inc. and Subsidiaries as of December 31, 2005, and the results of their operations and cash flows for the year then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has suffered recurring losses from operations and is in a working capital deficit position that raises substantial doubt about its ability to continue as a going concern. Management’s plans concerning these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Patrizio & Zhao, LLC
Lodi, New Jersey
February 22, 2006
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED BALANCE SHEET
DECEMBER 31, 2005
ASSETS
CURRENT ASSETS | | | | |
Cash and cash equivalents | | $ | 63,608 | |
Accounts receivable, net of bad debt reserve of $14,326 | | | 80,825 | |
Due from related parties | | | 259,743 | |
Prepaid expenses and other current assets | | | 48,207 | |
| | | | | |
Total Current Assets | | | 452,383 | |
| | | | | |
PROPERTY AND EQUIPMENT, NET | | | 110,531 | |
| | | | | |
OTHER ASSETS | | | 45,028 | |
| | | | | |
Total Assets | | $ | 607,942 | |
| | | | | |
LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | |
| | | | | |
CURRENT LIABILITIES | | | | |
Accounts payable and accrued expenses | | $ | 1,153,936 | |
Other Liabilities | | | 242,849 | |
Due to officers | | | 874,442 | |
| | | | | |
Total Current Liabilities | | | 2,271,227 | |
| | | | | |
STOCKHOLDERS’ EQUITY (DEFICIT) | | | | |
Common stock, stated value $.0001, 100,000,000 | | | | |
shares authorized; 82,455,000 shares issued and | | | | |
outstanding | | | 8,246 | |
Additional paid-in capital | | | 6,523,837 | |
Deficit accumulated during development stage | | | (8,247,569 | ) |
Accumulated other comprehensive income | | | 52,201 | |
| | | | | |
Total Stockholders' Equity (Deficit) | | | (1,663,285 | ) |
| | | | | |
Total Liabilities And Stockholders' Equity | | $ | 607,942 | |
See notes to consolidated financial statements.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF OPERATIONS
| | | | | For the Period From | |
| | | For the Years Ended | | September 13, 2000 | |
| | | December 31, | | (Date of Inception) to | |
| | | 2005 | | 2004 | | December 31, 2005 | |
| | | | | | | | |
REVENUE | | $ | 380,519 | | $ | 312,899 | | $ | 1,537,958 | |
| | | | | | | | | | | |
COSTS AND EXPENSES | | | | | | | | | | |
Cost of sales | | | 176,817 | | | 156,676 | | | 764,741 | |
Research and development | | | 611,362 | | | 432,144 | | | 2,274,698 | |
General and administrative (including stock-based compensation of $-0-, $13,611 and $13,611, respectively) | | | 2,230,956 | | | 1,081,465 | | | 6,270,058 | |
Depreciation and amortization | | | 95,200 | | | 75,945 | | | 341,373 | |
| | | | | | | | | | | |
Total Costs and Expenses | | | 3,114,335 | | | 1,746,230 | | | 9,650,870 | |
| | | | | | | | | | | |
(LOSS) FROM OPERATIONS | | | (2,733,816 | ) | | (1,433,331 | ) | | (8,112,912 | ) |
| | | | | | | | | | | |
OTHER INCOME (EXPENSE) | | | | | | | | | | |
Loss from unconsolidated subsidiary | | | - | | | (20,796 | ) | | (60,134 | ) |
Sale of net operating loss carryforwards | | | 216,247 | | | - | | | 216,247 | |
Gain on foreign currency | | | - | | | 4,810 | | | 660 | |
Interest income (expense), net | | | 420 | | | 3,695 | | | 34,299 | |
| | | | | | | | | | | |
Total Other Income (Expense) | | | 216,667 | | | (12,291 | ) | | 191,072 | |
| | | | | | | | | | | |
(LOSS) BEFORE CUMULATIVE EFFECT OF CHANGE IN ACCOUNTING PRINCIPLE | | | (2,517,149 | ) | | (1,445,622 | ) | | (7,921,840 | ) |
| | | | | | | | | | | |
CUMULATIVE EFFECT OF CHANGE IN ACCOUNTING PRINCIPLE, NET OF TAX | | | (324,167 | ) | | - | | | (324,167 | ) |
| | | | | | | | | | | |
NET (LOSS) | | | (2,841,316 | ) | | (1,445,622 | ) | | (8,246,007 | ) |
| | | | | | | | | | | |
UNREALIZED LOSS ON FOREIGN CURRENCY TRANSLATION, NET OF TAX | | | - | | | (3,262 | ) | | (3,526 | ) |
| | | | | | | | | | | |
COMPREHENSIVE (LOSS) | | | ($2,841,316 | ) | | ($1,448,884 | ) | | ($8,249,533 | ) |
| | | | | | | | | | | |
LOSS PER COMMON SHARE, BASIC AND DILUTED | | | | | | | | | | |
| | | | ($ 0.03 | ) | | ($ 0.02 | ) | | | |
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING, BASIC AND DILUTED | | | 81,528,260 | | | 74,120,075 | | | | |
See notes to consolidated financial statements.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE PERIOD FROM SEPTEMBER 13, 2000 (DATE OF INCEPTION) TO December 31, 2005
| | | Preferred Series A Stock | | Common Stock | | | | (Deficit) | | | | Accumulated | |
| | | | | $.0001 | | | | $.0001 | | Additional | | During | | Other | | Stockholders’ | |
| | | | | Stated | | | | Stated | | Paid-In | | Development | | Comprehensive | | Equity | |
| | | Shares | | Value | | Shares | | Value | | Capital | | Stage | | Income | | (Deficit) | |
BALANCE - September 13, 2000 | | | | | | | | | | | | | | | | | | | | | | | | | |
(date of inception) | | | - | | $ | - | | | - | | $ | - | | $ | - | | $ | - | | $ | - | | $ | - | |
Common stock issued in private placement | | | - | | | - | | | 63,619,200 | | | 6,362 | | | - | | | (1,562 | ) | | - | | | 4,800 | |
Net loss | | | - | | | - | | | - | | | - | | | - | | | (93,837 | ) | | - | | | (93,837 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
BALANCE - DECEMBER 31, 2000 | | | - | | $ | - | | | 63,619,200 | | | 6,362 | | | - | | | (95,399 | ) | | - | | | (89,037 | ) |
Preferred stock issued in private placement | | | 5,301,600 | | | 530 | | | - | | | - | | | 3,999,470 | | | - | | | - | | | 4,000,000 | |
Foreign currency translation | | | | | | - | | | - | | | - | | | - | | | - | | | (825 | ) | | (825 | ) |
Net loss | | | - | | | - | | | - | | | - | | | - | | | (1,251,210 | ) | | - | | | (1,251,210 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
BALANCE - DECEMBER 31, 2001 | | | 5,301,600 | | $ | 530 | | | 63,619,200 | | | 6,362 | | | 3,999,470 | | | (1,346,609 | ) | | (825 | ) | | 2,658,928 | |
Issuance of common stock in consideration | | | | | | | | | | | | | | | | | | | | | | | | | |
for all of the assets of WCG | | | | | | | | | | | | | | | | | | | | | | | | | |
Communications LLC | | | - | | | - | | | 3,976,200 | | | 398 | | | 111,465 | | | - | | | - | | | 111,863 | |
Issuance of preferred stock in consideration for 100% ownership of Zhejiang VSAT Satellite Communication Co., Ltd. | | | 1,325,400 | | | 133 | | | - | | | - | | | 226,395 | | | - | | | - | | | 226,528 | |
Foreign currency translation | | | - | | | - | | | - | | | - | | | - | | | - | | | 3,716 | | | 3,716 | |
Net loss | | | - | | | - | | | - | | | - | | | - | | | (1,550,180 | ) | | - | | | (1,550,180 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
BALANCE - DECEMBER 31, 2002 | | | 6,627,000 | | $ | 663 | | | 67,595,400 | | $ | 6,760 | | | 4,337,330 | | | (2,896,789 | ) | | 2,891 | | | 1,450,855 | |
Stock issued for services | | | 18,025 | | | 2 | | | 144,204 | | | 14 | | | 13,595 | | | - | | | - | | | 13,611 | |
Foreign currency translation | | | - | | | - | | | - | | | - | | | (3,155 | ) | | (3,155 | ) | | - | | | - | |
Net loss | | | - | | | - | | | - | | | - | | | - | | | (1,063,842 | ) | | - | | | (1,063,842 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
BALANCE - DECEMBER 31, 2003 | | | 6,645,025 | | | (665 | ) | | 67,739,604 | | | 6,774 | | | 4,350,925 | | | (3,960,631 | ) | | (264 | ) | | 397,469 | |
Repurchase and cancellation of common stock | | | - | | | - | | | (5,725,728 | ) | | (573 | ) | | 141 | | | - | | | - | | | (432 | ) |
Common stock issued in private placement | | | - | | | - | | | 3,340,008 | | | 334 | | | 503,666 | | | - | | | - | | | 504,000 | |
Effect of merger and recapitalization | | | (6,645,025 | ) | | (665 | ) | | 14,646,116 | | | 1,465 | | | (800 | ) | | - | | | - | | | - | |
Foreign currency translation | | | - | | | - | | | - | | | - | | | - | | | - | | | (3,262 | ) | | (3,262 | ) |
Net loss | | | - | | | - | | | - | | | - | | | - | | | (1,445,622 | ) | | - | | | (1,445,622 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
BALANCE - DECEMBER 31, 2004 | | | - | | $ | - | | | 80,000,000 | | $ | 8,000 | | $ | 4,853,932 | | | ($5,406,253 | ) | | ($ 3,526 | ) | | ($ 547,847 | ) |
Common stock issued in private placement | | | - | | | - | | | 2,455,000 | | | 246 | | | 1,669,905 | | | - | | | - | | | 1,670,151 | |
Foreign currency translation | | | - | | | - | | | - | | | - | | | - | | | - | | | 55,727 | | | 55,727 | |
Net loss | | | - | | | - | | | - | | | - | | | - | | | (2,841,316 | ) | | - | | | (2,841,316 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | | |
BALANCE - DECEMBER 31, 2005 | | | - | | $ | - | | | 82,455,000 | | $ | 8,246 | | $ | 6,523,837 | | | ($8,247,569 | ) | $ | 52,201 | | | ($1,663,285 | ) |
See notes to consolidated financial statements.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | | | For the Period From | |
| | | For the Years Ended | | September 13, 2000 | |
| | | December 31, | | (Date of Inception) to | |
| | | 2005 | | 2004 | | December 31, 2005 | |
CASH FLOWS FROM OPERATING ACTIVITIES | | | | | | | | | | |
| Net loss | | | ($2,841,316 | ) | | ($1,445,622 | ) | | ($8,246,007 | ) |
| Adjustments to reconcile net loss to net cash | | | | | | | | | | |
| used in operating activities: | | | | | | | | | | |
| Depreciation and amortization | | | 136,000 | | | 108,190 | | | 487,373 | |
| Cumulative effect of change in accounting principle | | | 324,167 | | | - | | | 324,167 | |
| Loss on unconsolidated subsidiary | | | - | | | 20,796 | | | 60,134 | |
| Provision for doubtful accounts | | | - | | | - | | | 14,326 | |
| Loss on foreign currency translation | | | - | | | (3,262 | ) | | (3,526 | ) |
| Stock-based compensation | | | 281,300 | | | - | | | 294,911 | |
| Changes in assets and liabilities: | | | | | | | | | | |
Accounts receivable | | | (45,886 | ) | | (11,371 | ) | | (66,499 | ) |
Due from related parties | | | (41,485 | ) | | (176,676 | ) | | (259,743 | ) |
Prepaid expenses and other current assets | | | (32,103 | ) | | 43,562 | | | (48,207 | ) |
Other assets | | | (14,791 | ) | | 1,093 | | | (48,148 | ) |
Accounts payable and accrued expenses | | | 869,731 | | | (174,129 | ) | | 1,153,936 | |
Other liabilities | | | (17,151 | ) | | 260,000 | | | 242,849 | |
Net Cash Used In Operating Activities | | | (1,381,534 | ) | | (1,377,419 | ) | | (6,094,434 | ) |
| | | | | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES | | | | | | | | | | |
Investment in unconsolidated subsidiary | | | - | | | - | | | (409,832 | ) |
Purchase of property and equipment | | | (50,786 | ) | | (12,420 | ) | | (255,988 | ) |
Net Cash Used In Investing Activities | | | (50,786 | ) | | (12,420 | ) | | (665,820 | ) |
| | | | | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | | | | | |
Net proceeds from private placement of common stock | | | 1,388,851 | | | 504,000 | | | 1,897,651 | |
Repurchase of treasury stock | | | - | | | (432 | ) | | (432 | ) |
Net proceeds from private placement of preferred stock | | | - | | | - | | | 4,000,000 | |
Proceeds from officers’ advances | | | - | | | 230,453 | | | 874,442 | |
Net Cash Provided By Financing Activities | | | 1,388,851 | | | 734,021 | | | 7,052,961 | |
| | | | | | | | | | | |
EFFECT OF FOREIGN CURRENCY CONVERSION | | | | | | | | | | |
ON CASH | | | 52,201 | | | - | | | 52,201 | |
| | | | | | | | | | | |
NET INCEASE (DECREASE) IN CASH | | | 8,732 | | | (655,818 | ) | | 63,608 | |
| | | | | | | | | | | |
CASH AND CASH EQUIVALENTS - BEGINNING | | | 54,876 | | | 710,694 | | | - | |
| | | | | | | | | | | |
CASH AND CASH EQUIVALENTS - ENDING | | $ | 63,608 | | $ | 54,876 | | $ | 63,608 | |
See notes to consolidated financial statements.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 1 - ORGANIZATION AND NATURE OF BUSINESS
China Quantum Communications, Ltd. (“CQ”) was organized on October 4, 2000. The primary business of China Quantum Communications, Ltd. is to provide wireless, VoIP, and value-added communication services to commercial and residential users in the U.S. and China.
On December 29, 2000, CQ purchased 100% of the common stock of China Quantum Communications, Inc., which was formed on September 13, 2000, and China Quantum Communications, Inc. became a wholly owned subsidiary. Based on its controlling interest in China Quantum Communications, Inc., the operating results of China Quantum Communications, Inc. are included in the consolidated results of the Company since December 29, 2000.
On January 21, 2001, CQ formed China Quantum Communications, Ltd. (China), a wholly owned subsidiary. Based on its controlling interest in China Quantum Communications Ltd. (China), the operating results of China Quantum Communications, Ltd. (China) are included in the consolidated results of the Company since January 21, 2001.
In January 2001, CQ purchased 100% ownership of Zhejiang VSAT Satellite Communications Co., Ltd., owned in the majority by the Company’s CEO. In September 2002, the Board of Directors authorized the issuance of 1,325,400 shares of Series A preferred stock as final consideration for the transaction. This transaction was accounted for as a purchase pursuant to SFAS Statement No. 141, “Business Combinations”. The total purchase price of approximately $226,528, which was based on the fair market value of the assets purchased, was allocated among the various assets purchased in the acquisition.
On June 9, 2004, Techedge, Inc., a Delaware company (the “Company”), acquired all of the issued and outstanding stock of China Quantum Communications, Ltd., a Cayman Islands company (“CQ”), pursuant to a Share Exchange Agreement (the “Exchange Agreement”), by and among the Company, the stockholders of the Company, CQ and the stockholders of CQ.
Pursuant to the Exchange Agreement, CQ became a wholly-owned subsidiary of the Company, and in exchange for the CQ shares, the Company issued 72,000,000 shares of its common stock to the stockholders of CQ, representing approximately 90% of the Company’s outstanding stock at the time.
For accounting purposes, because the Company had become a shell company prior to the share exchange, the share exchange was treated as a recapitalization of the Company. As such, the historical financial information prior to the share exchange is that of CQ and its subsidiaries, Historical share amounts have been restated to reflect the effect of the share exchange.
Currently the Company focuses its activities on providing low cost mobile Voice over IP (VoIP) solutions to service providers. It offers an “IP-PCS solution” that utilizes PHS mobile technology on an unlicensed PCS frequency for low-cost VoIP services. It has developed proprietary technologies in mobile IP call processing and owns key intellectual properties. It has substantially completed development of the IP-PCS product and is engaged in efforts to commercialize this product.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 2 - LOSSES DURING THE DEVELOPMENT STAGE AND MANAGEMENT’S PLANS
Through December 31, 2005 the Company had incurred development stage losses totaling $8,246,007, a working capital deficit of $1,818,844 and net cash used in operation activities of $1,662,834. At December 31, 2005, the Company had $63,608 of cash and cash equivalents and $80,825 of net trade receivables to fund short-term working capital requirements.
The Company’s ability to continue as a going concern and its future success is dependent upon its ability to raise capital in the near term to: (1) satisfy its current obligations, (2) continue its research and development efforts, and (3) successfully develop, deploy and market its products on a wide scale.
The Company believes that it will be able to complete the necessary steps in order to meet its cash flow requirements throughout fiscal 2005 and continue its development and commercialization efforts. Management’s plans in this regard include, but are not limited to, the following:
Management estimates that the Company will need additional minimum capital of $1,900,000 by December 31, 2006 to continue its operations either through revenues from sales, external independent or related party funding, further expense reductions or some combination thereof.
The Company presently has ongoing discussions and negotiations with a number of additional financing alternatives, one or more of which it believes will be able to successfully close to provide necessary working capital, while maintaining sensitivity to stockholder dilution issues. However, the Company has no definitive agreements to provide funding at this time.
Management believes that actions presently being taken to complete the Company’s development stage through the commercial roll-out of its IP based Personal Communication Service (PCS) platform will be successful. However, there can be no assurance that Techedge Inc. will generate sufficient revenues to provide positive cash flows from operations or that sufficient capital will be available, when required, to permit the Company to realize its plans. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty.
NOTE 3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
PRINCIPLES OF CONSOLIDATION
The consolidated financial statements include the accounts of Techedge Inc., and it’s wholly owned subsidiaries. Significant intercompany accounts and transactions have been eliminated in consolidation.
CHANGE IN ACCOUNTING PRINCIPLE
On September 13, 2000, CQ purchased 100% ownership of Zhejiang Guang Tong Wang Luo Co., Ltd (ZJQC), a Chinese telecommunications company, and was owned through a trust. As of December 31, 2004, in accordance with Document 17 as issued by the Ministry of Information Industry, People’s Republic of China, foreign groups and/or individuals were prohibited from directly conducting telecommunications activities, either in the form of investment, joint venture of partnership. Additionally, telecommunication companies were prohibited from soliciting foreign investment or allowing foreign stockholders or other form of ownership. Accordingly the Company recognized its ownership in accordance with SFAS No. 94, “Consolidation of All Majority-Owned Subsidiaries”, and ARB 51, “Consolidated Financial Statements”, and recorded its investment using the equity investment method through December 31, 2004, since Techedge Inc. could not exercise control or influence over management and/or Board of Directors of the foreign subsidiary. However, during 2005 the Chinese government changed the ruling to allow foreign investment in Chinese companies. As a result of this change, for the year ended December 31, 2005, the operating results of ZJQC were included in the consolidated results of the Company which resulted in a charge to earnings for the cumulative effect of change in accounting principle of $324,167 for the year ended December 31, 2005.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
Following is a summary of financial position and results of operations of ZJQC as of December 31, 2004:
| Current assets | | $ | 117,527 | |
| Property, plant and equipment | | | 34,826 | |
| Other assets | | | 14,395 | |
| | | | | |
Total assets | | $ | 166,748 | |
| | | | | |
| Current liabilities | | | 347,678 | |
| | | | | |
| Owner’s equity (deficit) | | | (180,930 | ) |
| | | | | |
Total liabilities and owner’s equity | | $ | 166,748 | |
| | | | | |
| Sales | | $ | 32,866 | |
| | | | | |
| Net loss | | $ | (20,796 | ) |
REVENUE RECOGNITION
The Company recognizes revenue for its telecommunications services at the time the services are provided to the end user.
CASH AND CASH EQUIVALENTS
For the purposes of the statements of cash flows, the Company considers cash and cash equivalents to include cash on hand, deposits in banks, and all highly liquid investments with a maturity of three months or less.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
ACCOUNTS RECEIVABLE AND BAD DEBT RESERVES
Substantially all of the Company’s accounts receivable are due from commercial and residential end users of telephone and communication services. The Company provides credit in the normal course of business. The Company continuously performs credit evaluations of its customers, considering numerous inputs including past payment history, financial condition, and other information. While the Company believes that adequate allowances for doubtful accounts have been provided in the financial statements, it is possible that the Company could experience unexpected credit losses.
The Company provides for an allowance for doubtful accounts equal to the estimated losses that will be incurred in the collection of all receivables. Estimated losses are based on a review of the current status of the existing receivables. The bad debt reserve was $14,326 at December 31, 2005.
PROPERTY AND EQUIPMENT
Property and equipment is recorded at cost. Depreciation is provided on the straight-line method over the estimated useful lives of five years. Repairs and maintenance expenditures, which do not extend the useful lives of the related assets, are expensed as incurred.
Under SFAS No. 144 “Accounting for the Impairment or Disposal of Long-Lived Assets”, the Company’s long-lived assets are evaluated for impairment when events or changes in business circumstances indicate that the carrying amount of the assets may not be fully recoverable. The Company also assesses these assets for impairment based on their estimated future cash flows. The Company has not incurred any losses in connection with the adoption of this statement.
USE OF ESTIMATES
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
INCOME TAXES
The Company accounts for income taxes in accordance with Statement of Financial Accounting Standards No. 109, Accounting for Income taxes. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Because the realization of tax benefits related to the Company’s net deferred tax asset is uncertain, a valuation allowance has been provided against the net deferred tax asset. During 2005, the Company received proceeds of $216,247 as a result of the sale of net operating loss carryforwards resulting from cumulative losses through the year ended December 31, 2004. At December 31, 2005 the Company had net deferred income tax assets of approximately $772,000 comprised principally of the future tax benefit of net operating loss carryforwards, beginning to expire in the year 2007 through 2025. Because the realization of tax benefits related to the Company’s net deferred tax asset is uncertain, a full valuation allowance has been provided against the net deferred tax asset. As a result of the sale of net operating loss carryforwards and cumulative losses through the year ended December 31, 2005, the net change in the valuation allowance amounted to a decrease of $756,000 for the year ended December 31, 2005.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
FINANCIAL INSTRUMENTS
The carrying amounts reported in the consolidated balance sheets for Techedge Inc.’s cash, accounts receivable, accounts payable, and accrued expenses approximate their fair values due to the short maturities of these financial instruments.
The carrying amounts reported in the consolidated balance sheets for Techedge Inc.’s amounts recorded as other liabilities and due to officers approximate their values based on current rates at which the Company could borrow funds with similar maturities.
STOCK BASED COMPENSATION
Financial Accounting Statement No. 123, Accounting for Stock Based Compensation, encourages, but does not require companies to record compensation cost for stock-based employee compensation plans at fair value. The Company has chosen to continue to account for stock-based compensation using the intrinsic value method prescribed in Accounting Principles Board Opinion No. 25, Accounting for Stock Issued to Employees, and related interpretations. Accordingly, compensation cost for stock options is measured as the excess, if any, of the quoted market price of the Company’s stock at the date of the grant over the amount an employee must pay to acquire the stock. The Company has adopted the “disclosure only” alternative described in SFAS 123 and SFAS 148, which require pro forma disclosures of net income and earnings per share as if the fair value method of accounting had been applied.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
STOCK BASED COMPENSATION (continued)
Had compensation expense for stock options granted under the Plan and certain warrants granted to employees in 2005 and 2004, been determined based on fair value at the grant dates, Techedge’s net loss for 2005 and 2004 would have been increased to the pro forma amounts shown below.
| | | December 31, | |
| | | 2005 | | 2004 | |
| Net Loss: | | | | | |
| As reported | | | ($2,841,316 | ) | | ($1,445,622 | ) |
| | | | | | | | |
| Pro forma | | | ($2,841,316 | ) | | ($1,472,828 | ) |
| | | | | | | | |
| Net Loss Per Share: | | | | | | | |
| As reported | | | ($ 0.03 | ) | | ($ 0.02 | ) |
| | | | | | | | |
| Pro forma | | | ($ 0.03 | ) | | ($ 0.02 | ) |
ADVERTISING COSTS
Advertising costs are expensed as incurred. Advertising expense was $7,684, $2,639 and $159,731 for the years ended December 31, 2005 and 2004 and for the period from September 13, 2000 (date of inception) to December 31, 2005, respectively.
COMPREHENSIVE INCOME (LOSS)
The Company adopted SFAS No. 130, Reporting Comprehensive Income, which establishes rules for the reporting of comprehensive income and its components. In addition to net loss, comprehensive income (loss) includes all changes in equity during a period, except those resulting from investments by and distributions to owners. Items of comprehensive income include foreign currency exchanges.
RESEARCH AND DEVELOPMENT COSTS
Research and development costs are charged to operations as incurred and amounted to $611,362, $432,144, and $2,274,698 for the years ended December 31, 2005 and 2004 and for the period from September 13, 2000 (date of inception) to December 31, 2005, respectively. Costs consist primarily of salaries and related costs of employees engaged in research, design and development activities, the cost of parts for prototypes and equipment depreciation.
FOREIGN CURRENCY TRANSLATION
Operations of two of the Company’s subsidiaries are conducted in China and the financial statements of those subsidiaries are translated from China’s Renminbi, the functional currency, into U.S. Dollars in accordance with SFAS No. 52, “Foreign Currency Translation.” Accordingly, all foreign currency assets and liabilities are translated at the period-end exchange rate and all revenues and expenses are translated at the average exchange rate for the period. The effects of translating the financial statements of foreign subsidiaries into U.S. Dollars are reported as a cumulative translation adjustment, a separate component of comprehensive income in stockholder’s equity. Foreign currency transaction gains and losses are reported in earnings and consisted of $-0- of gains in 2005, $4,810 of losses in 2004 and $660 of gains for the period from September 13, 2000 (date of inception) to December 31, 2005.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
LOSS PER COMMON SHARE, BASIC AND DILUTED
Techedge Inc. accounts for net loss per common share in accordance with the provisions of SFAS No. 128, “Earnings per Share” (“EPS”). SFAS No. 128 requires the disclosure of the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock or resulted in the issuance of common stock that then shared in the earnings of the entity. Common equivalent shares have been excluded from the computation of diluted EPS since their effect would be anti-dilutive.
CONCENTRATIONS OF BUSINESS AND CREDIT RISK
FINANCIAL RISKS
At times throughout the year, the Company may maintain certain bank accounts in excess of FDIC insured limits.
GEOGRAPHICAL RISKS
For the year ended December 31, 2005, approximately 80% of the Company’s assets and approximately 20% of the Company’s operations were in China. Therefore, the Company’s business, financial condition and results of operations may be adversely affected by significant political, economical and social uncertainties in China.
SEGMENT REPORTING
The Company operates in a single segment, offering telephony services while focusing its activities on providing low cost mobile Voice over IP (VoIP) solutions to service providers.
RECLASSIFICATIONS
Certain amounts in the 2004 financial statements have been reclassified for comparative purpose to conform to presentation in the 2005 financial statements.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 3 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (continued)
RECENT ACCOUNTING PRONOUNCEMENTS
In December 2004, the FASB issued SFAS Statement No. 123(R) on Share-Based Payments which is a revision of FASB Statement No. 123, Accounting for Stock-Based Compensation. This Statement establishes standards for the accounting for transactions in which an entity exchanges its equity instruments for goods or services that are based on the fair value of the entity’s equity instruments or that may be settled by the issuance of those equity instruments. This statement focuses primarily on accounting for transactions in which an entity obtains employee services in share-based payment transactions. This statement is effective for public entities that file as small business issuers as of the beginning of the first interim or annual reporting period that begins after December 15, 2005.
NOTE 4 - SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION
| | | | | For the period from | |
| | | | | September 13, 2000 | |
| | | December 31, | | (date of inception) to December 31, | |
| | | 2005 | | 2004 | | 2005 | |
| | | | | | | | |
| Interest paid | | $ | - | | $ | - | | $ | - | |
| Income taxes paid | | $ | 800 | | $ | 500 | | $ | 2,973 | |
| | | | | | | | | | | |
Schedules of Non-Cash Investing and Financing Activities: | | | | | | | | | | |
| | | | | | | | | | |
| Issuance of 72,000,000 shares of common stock in exchange for 100% of the outstanding preferred and ordinary shares of China Quantum Communications, Ltd | | $ | - | | $ | 2,388 | | $ | - | |
NOTE 5 - PROPERTY AND EQUIPMENT
Property and equipment, at cost, consists of the following at December 31, 2005:
| Equipment | | $ | 658,986 | |
| Office furniture and equipment | | | 15,067 | |
| | | | 674,053 | |
| | | | | |
| Less: Accumulated depreciation | | | (563,522 | ) |
| | | | | |
| | | $ | 110,531 | |
Depreciation expense for the years ended December 31, 2005 and 2004 and for the period from September 13, 2000 (date of inception) to December 31, 2005, was $136,000, $108,,190 and $487,373, respectively, of which $, $32,245 and $146,000 respectively, is included in research and development expense.
NOTE 6 - STOCKHOLDERS’ EQUITY
On October 4, 2000, in connection with its incorporation, China Quantum Communications, Ltd. (“CQ”) had authorized capital of 50,000 ordinary shares with a par value of $0.0001.
On January 2, 2001, CQ sold 48,000,000 ordinary shares (which were exchanged for 63,619,200 shares of Techedge common stock as part of the Share Exchange (as defined below) in a private placement with proceeds of $4,800.
On January 10, 2001, CQ increased its authorized capital to 60,000,000 ordinary shares, par value $0.0001 per share, by subdividing its existing authorized shares.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 6 - STOCKHOLDERS’ EQUITY (continued)
On May 2, 2001, CQ sold 4,000,000 Series A preferred shares (which were exchanged for 5,301,600 shares of Techedge common stock as part of the Share Exchange) in a private placement for proceeds of $4,000,000.
On May 2, 2001, CQ increased its authorized capital to provide for 75,000,000 ordinary shares, par value $0.0001 per share, and 6,250,000 Series A preferred shares.
On September 18, 2002, CQ issued 3,000,000 ordinary shares (which were exchanged for 3,976,200 shares of Techedge common stock as part of the Share Exchange) shares for all of the assets of WCG Communications, LLC, a company owned in the majority by the Company’s CEO for $111,863, the fair market value.
On September 18, 2002, CQ issued 1,000,000 Series A preferred shares (which were exchanged for 1,325,400 shares of Techedge common stock as part of the Share Exchange) for 100% ownership of Zhejiang VSAT Satellite Communication Co., Ltd., a company owned in the majority by the Company’s CEO for $226,528, the fair market value.
During the year ended December 31, 2003, CQ issued 108,800 ordinary shares (which were exchanged for 144,204 shares of Techedge Common Stock in the Share Exchange) and 13,600 Series A preferred shares (which were exchanged for 18,025 shares of Techedge common stock in the Share Exchange) to consultants for services performed. The Company recognized a charge of operations of $13,611, based upon the fair market value of the services provided.
On May 6, 2004, the CQ board of directors approved the purchase and cancellation of 4,320,000 ordinary shares from a related party for an aggregate price of $432.
On June 4, 2004, CQ sold 2,520,000 ordinary shares (which were exchanged for 3,340,008 shares of Techedge common stock in the Share Exchange) in a private placement for proceeds of $504,000.
On June 9, 2004, the Company completed the merger with CQ, Pursuant to the Exchange agreement, the stockholders of CQ exchanged all of their outstanding preferred and common shares (5,013,600 and 49,308,800, respectively) for 72,000,000 shares of the Company’s common stock, representing approximately 90% of the Company’s common stock at the time.
In February 2005, the company completed a private placement of 260,000 shares of common stock at a purchase price of $1.00 per share, or gross proceeds of $260,000.
During the quarter ended, March 31, 2005, the Company granted 402,000 fully vested, nonforfeitable warrants to purchase shares of common stock to two consultants for services in addition to cash payments.
During the quarter ended, March 31, 2005, the Company granted 100,000 fully vested, nonforfeitable shares of common stock to a consultants for services
In April 2005, the company completed a private placement of 95,000 shares of common stock at a purchase price of $1.00 per share, or gross proceeds of $95,000, and, for no additional consideration, a cashless 2-year warrant to purchase additional 95,000 shares at an exercise price of $1.50 per share. A value of $36,770 of the proceeds has been allocated to the warrant.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 6 - STOCKHOLDERS’ EQUITY (continued)
In May 2005, the Company completed a private placement of 500,000 shares of common stock at a purchase price of $0.50 per share, or gross proceeds of $250,000, and for no additional consideration, a cashless 5-year warrant to purchase an additional 147,059 shares at an exercise price of $0.75 per share. A value of $71,470 of the proceeds has been allocated to the warrant.
Also in May 2005 the Company completed a private placement of 500,000 shares of common stock at a purchase price of $0.50 per share, or gross proceeds of $250,000, and for no additional consideration, a cashless 5-year warrant to purchase an additional 147,059 shares at an exercise price of $0.75 per share. A value of $68,240 of the proceeds has been allocated to the warrant.
In July 2005, the Company completed a private placement of 1,000,00,000 shares of common stock at a purchase price of $0.50 per share, or gross proceeds of $500,000, and for no additional consideration, a cashless 5-year warrant to purchase an additional 400,000 shares at an exercise price of $0.75 per share. A value of $168,000 of the proceeds has been allocated to the warrant.
In July 2005, the Company entered into a service agreement pursuant to which the Company agreed to issue warrants to purchase up to an aggregate of 200,000 shares (the Warrant Shares) of the Company’s common stock in exchange for investor relations services. Techedge has the right to terminate the service agreement at any time on or after October 5, 2005, upon 30 days prior written notice. The Warrant Shares shall vest in accordance with the following schedule and are purchasable at the following exercise prices:
50,000 Warrant Shares are immediately vested and may be purchased at an exercise price of $0.90 per share;
50,000 Warrant Shares will vest on the 91st day following the date of service agreement and may be purchased at an exercise price of $1.10 per share;
50,000 Warrant Shares will vest on the 181st day following the date of service agreement and may be purchased at an exercise price of $1.30 per share;
50,000 Warrant Shares will vest on the 271st day following the date of service agreement and may be purchased at an exercise price of $1.50 per share;
The warrants shall terminate on the 24-month anniversary of the effective date of a registration statement filed by the Company to register the resale of the Warrant Shares; provided, however, in the event that Techedge elects to terminate the service agreement early as described above, the Warrants will terminate as to any Warrant Shares that are not then vested. By October 5, 2005, the Company terminated such service, resulting in only 50,000 Warrant Shares vested with an exercise price of $0.90 per share.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 6 - STOCKHOLDERS’ EQUITY (continued)
On November 29, 2005, the Company made a modification to the exercise price of the warrants in conjunction with a private placement completed in May and July, 2005 from the original exercise price of $1.10 per share to an amended exercise price of $0.40 per share.
PREFERRED STOCK
The CQ preferred stock was redeemable, cumulative and was convertible into shares of common stock by a majority vote of the holders or automatically upon the closing of a firm commitment underwritten public offering pursuant to an effective registration statement under the Securities Act of 1933 of the United States of America.
Preferred shares were convertible into common at any time, without the payment of additional consideration, into such number of fully paid ordinary shares per preference share as would have been determined by dividing the original purchase price by the conversion price (initially the original price). Dividends could have been declared for preferred stockholders at the discretion of the Board of Directors. The Board of Directors declared no dividends on preferred stock for the period from September 13, 2000 (date of inception) to June 9, 2004, the date of the Share Exchange.
EQUITY COMPENSATION PLAN
On December 29, 2000, China Quantum Communications, Ltd. established its Stock Option Plan (the “Plan”), in which incentive stock options and nonqualified stock options may be granted to officers, employees and consultants of the Company. The vesting of such options is four years and the options expire in ten years.
On August 4, 2004, Techedge Inc. adopted the 2001 Stock Option Plan established by China Quantum Communications, Ltd. under an Option Exchange agreement approved by the board of directors. Pursuant to the agreement, the Company exchanged an option to purchase 1.3254 shares of Techedge common stock for each option to purchase one ordinary share of China Quantum Communications, Ltd. All other terms and conditions of existing stock option agreements remain unchanged as to exercise price and vesting. The amounts presented in the table below have been restated to reflect the change.
On May 20, 2005 the Company’s stockholders approved the 2005 Equity Compensation Plan (the 2005 Plan) and no additional options to purchase shares of common stock will be granted under the 2001 Stock Option Plan. Under the 2005 Plan, the Company may grant options to purchase shares of the Company’s common stock, stock purchase rights and restricted or unrestricted stock awards of shares of common stock to eligible employees, directors and consultants, determine the terms and conditions of each option, stock purchase right or award and adopt, amend and rescind rules and regulations for the administration of the 2005 Plan.
The 2005 Plan will be administered by a duly authorized committee appointed by the Board of Directors. The aggregate number of shares of common stock available for issuance in connection with options granted under the 2005 Plan will be 8,500,000, subject to customary adjustments for stock splits, stock dividends or similar transactions.
The Committee determines the exercise price of options granted under the 2005 Plan, however the exercise price must be at least equal to the fair market value per share of common stock (or 110% of fair market value in the case of incentive options granted to a ten-percent stockholder) issuable upon exercise of the option at the time the incentive option was granted. No options may be exercisable for more than ten years (five years in the case of an incentive option granted to a ten-percent stockholder) from the date of the grant.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 6 - STOCKHOLDERS’ EQUITY (continued)
EQUITY COMPENSATION PLAN (continued)
A summary of the stock option activity for the years ended December 31, 2005 and 2004 pursuant to the terms of the Plan, which include incentive stock options and non-qualified stock options, is set forth below:
| | | | | Weighted | |
| | | Number of | | Average | |
| | | Options | | Exercise Price | |
| | | | | | |
| Outstanding at December 31, 2003 | | | 4,266,685 | | $ | 0.20 | |
| | | | | | | | |
| Granted | | | - | | | - | |
| | | | | | | | |
| Exercised | | | - | | | - | |
| Canceled / Expired | | | - | | | - | |
| | | | | | | | |
| Outstanding at December 31, 2004 | | | 4,266,685 | | $ | 0.20 | |
| | | | | | | | |
| Granted | | | - | | | - | |
| | | | | | | | |
| Exercised | | | - | | | - | |
| Canceled / Expired | | | - | | | - | |
| | | | | | | | |
| Outstanding at December 31, 2005 | | | 4,266,685 | | $ | 0.20 | |
| | | | | | | | |
| Exercisable at December 31, 2005 | | | 4,203,617 | | $ | 0.20 | |
The per share weighted average remaining life of the options outstanding at December 31, 2005 and 2004 is 5.10 and 6.10 years, respectively.
NOTE 7 - RELATED PARTY TRANSACTIONS
The Company records material related party transactions. The Company incurs costs for certain administrative expenses from a company owned 100% by the Company’s CEO. Those charges are included in general and administrative expenses. The Company also provides services to a related party and those amounts are included in revenue.
The Company also purchases equipment used in Research and Development from a company owned 100% by the Company’s CEO.
The Company also engages in advances to and advances from related parties. The advances have no stated terms of repayment and carry no interest.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 7 - RELATED PARTY TRANSACTIONS (continued)
Following is a summary of transactions and balances with affiliated entities and related parties for 2005 and 2004:
| | | | | For the period from | |
| | | | | September 13, 2000 | |
| | | December 31, | | (date of inception) to | |
| | | 2005 | | 2004 | | December 31, 2005 | |
| | | | | | | | |
| Revenues from related parties | | $ | 44,626 | | $ | 24,100 | | $ | 69,446 | |
| Purchases and expenses to | | | | | | | | | | |
| related parties | | $ | 47,635 | | $ | 74,765 | | $ | 139,776 | |
| | | | | | | | | | | |
| Due from related parties | | $ | 259,743 | | $ | 218,258 | | $ | 259,743 | |
Amounts due to officers consist of advances from the Company’s CEO to fund the Company’s operations. It also includes compensation deferred by the Company’s CEO and CFO. No written repayment agreements exist with either officer. Amounts are unsecured, non-interest bearing and due upon demand.
NOTE 8 - COMMITMENTS AND CONTINGENCIES
OPERATING LEASE COMMITMENTS
The Company leases office equipment and certain office space in New Jersey, New York and the Peoples’ Republic of China under operating leases. Lease agreements vary from one to four-year lease agreements with a renewal option for New Jersey for two additional years.
The following is a schedule of future minimum rental payments (exclusive of common area charges) required under operating leases that have initial or remaining non-cancelable lease terms in excess of one year as of December 31, 2005.
| Year ending December 31, | | | |
| 2006 | | $ | 43,200 | |
| 2007 | | | 43,200 | |
| 2008 | | | 14,400 | |
| | | | | |
| Total minimum payments required | | $ | 100,800 | |
The leases also contain provisions for contingent rental payments based upon increases in taxes and common area maintenance expense.
TECHEDGE INC. AND SUBSIDIARIES
(A DEVELOPMENT STAGE COMPANY)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2005 AND 2004
NOTE 8 - COMMITMENTS AND CONTINGENCIES (continued)
Following is a summary of rental expenses under all operating leases:
| | | December 31, | |
| | | 2005 | | 2004 | |
| | | | | | |
| Minimum rentals | | $ | 146,186 | | $ | 133,000 | |
| Contingent rentals | | | 2,352 | | | 2,352 | |
| | | | | | | | |
Total rent expense | | $ | 148,538 | | $ | 135,352 | |
NOTE 9- SEGMENT REPORTING
The company intends to provide low cost mobile Voice over IP (VoIP) solutions to service providers. The Company has operations in two geographic areas, China and the United States. The chief operating decision makers evaluate performance, make operating decisions, and allocate resources based on consolidated financial data. Gross profit, operating income, income from operations, and income taxes are not allocated to specific individual departments within the organization. In accordance with SFAS No. 131 “Disclosures about Segments of an Enterprises and Related Information”, the Company is considered a single reportable segment. The Company is required to disclose certain information about revenues, information about geographic areas, information about major customers, and information about long-lived assets.
| | | Year Ended December 31, 2005 | |
| | | United States | | China | | Total | |
| | | | | | | | |
| Revenues | | $ | 244,604 | | $ | 5,091 | | $ | 249,695 | |
| | | | | | | | | | | |
| Long-lived assets | | $ | 159,019 | | $ | 515,034 | | $ | 674,053 | |
| | | Year Ended December 31, 2004 | |
| | | United States | | China | | Total | |
| | | | | | | | |
| Revenues | | $ | 312,898 | | $ | - | | $ | 312,898 | |
| | | | | | | | | | | |
| Long-lived assets | | $ | 159,019 | | $ | 387,604 | | $ | 546,623 | |
For the years ended December 31, 2005 and 2004, the Company did not have any major customers.
NOTE 10 - SUBSEQUENT EVENTS
On January 24, 2006, the Company granted 2,701,000 options, of which 1,901,000 are fully vested, to purchase shares of common stock at an exercise price of $0.52, to officers, employees and consultants of the Company.
On January 26, 2006, the Company announced its plans to re-position itself for bio-pharmaceutical and other high growth opportunities in China, while looking for strategic partners to carry out the commercialization of its unique and high potential Mobile Voice over IP solutions.
In conjunction with the Company’s re-positioning plans, On January 1, 2006, the Company entered into an agreement to transfer ownership and assets of China Quantum Communications, Inc. to a former employee. Under the agreement, the Company will assume all the debts and liabilities that China Quantum Communications, Inc has incurred through December 31, 2005. On February 13, 2006, the Company signed a letter of intent to acquire China BioPharma Limited, a Cayman island Company, which has the rights to have majority ownership in one of the largest non-governmental owned vaccine manufacturers in China. On February 27, 2006 the Company entered into an agreement to transfer ownership of its Chinese subsidiary Zhejiang Guang Tong Wang Luo Co., Ltd (ZJQC) to third parties.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
INDEMNIFICATION OF DIRECTORS AND OFFICERS
Our certificate of incorporation provides that to the fullest extent permitted by the Delaware General Corporation Law, directors of the registrant shall not be liable to it or its stockholders for monetary damages for breach of fiduciary duty as a director. The Company is also subject to Section 145 of the Delaware General Corporation Law, set forth below.
“Section 145. Indemnification of officers, directors, employees and agents; insurance.
“(a) A corporation shall have power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of the corporation) by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with such action, suit or proceeding if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe the person’s conduct was unlawful. The termination of any action, suit or proceeding by judgment, order, settlement, conviction, or upon a plea of nolo contendere or its equivalent, shall not, of itself, create a presumption that the person did not act in good faith and in a manner which the person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had reasonable cause to believe that the person’s conduct was unlawful.
“(b) A corporation shall have power to indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses (including attorneys’ fees) actually and reasonably incurred by the person in connection with the defense or settlement of such action or suit if the person acted in good faith and in a manner the person reasonably believed to be in or not opposed to the best interests of the corporation and except that no indemnification shall be made in respect of any claim, issue or matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or the court in which such action or suit was brought shall determine upon application that, despite the adjudication of liability but in view of all the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
“(c) To the extent that a present or former director or officer of a corporation has been successful on the merits or otherwise in defense of any action, suit or proceeding referred to in subsections (a) and (b) of this section, or in defense of any claim, issue or matter therein, such person shall be indemnified against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection therewith.
“(d) Any indemnification under subsections (a) and (b) of this section (unless ordered by a court) shall be made by the corporation only as authorized in the specific case upon a determination that indemnification of the present or former director, officer, employee or agent is proper in the circumstances because the person has met the applicable standard of conduct set forth in subsections (a) and (b) of this section. Such determination shall be made, with respect to a person who is a director or officer at the time of such determination, (1) by a majority vote of the directors who are not parties to such action, suit or proceeding, even though less than a quorum, or (2) by a committee of such directors designated by majority vote of such directors, even though less than a quorum, or (3) if there are no such directors, or if such directors so direct, by independent legal counsel in a written opinion, or (4) by the stockholders.
“(e) Expenses (including attorneys’ fees) incurred by an officer or director in defending any civil, criminal, administrative or investigative action, suit or proceeding may be paid by the corporation in advance of the final disposition of such action, suit or proceeding upon receipt of an undertaking by or on behalf of such director or officer to repay such amount if it shall ultimately be determined that such person is not entitled to be indemnified by the corporation as authorized in this section. Such expenses (including attorneys’ fees) incurred by former directors and officers or other employees and agents may be so paid upon such terms and conditions, if any, as the corporation deems appropriate.
“(f) The indemnification and advancement of expenses provided by, or granted pursuant to, the other subsections of this section shall not be deemed exclusive of any other rights to which those seeking indemnification or advancement of expenses may be entitled under any bylaw, agreement, vote of stockholders or disinterested directors or otherwise, both as to action in such person’s official capacity and as to action in another capacity while holding such office.
“(g) A corporation shall have power to purchase and maintain insurance on behalf of any person who is or was director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against any liability asserted against such person and incurred by such person in any such capacity, or arising out of such person’s status as such, whether or not the corporation would have the power to indemnify such person against such liability under this section.
“(h) For purposes of this section, references to (the corporation) shall include, in addition to the resulting corporation, any constituent corporation (including any constituent of a constituent) absorbed in a consolidation or merger which, if its separate existence had continued, would have had power and authority to indemnify its directors, officers, and employees or agents, so that any person who is or was a director, officer, employee or agent of such constituent corporation, or is or was serving at the request of such constituent corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, shall stand in the same position under this section with respect to the resulting or surviving corporation as such person would have with respect to such constituent corporation if its separate existence had continued.
“(i) For purposes of this section, references to (other enterprises) shall include employee benefit plans; references to “fines” shall include any excise taxes assessed on a person with respect to any employee benefit plan; and references to “serving at the request of the corporation” shall include any service as a director, officer, employee or agent of the corporation which imposes duties on, or involves services by, such director, officer, employee or agent with respect to an employee benefit plan, its participants or beneficiaries; and a person who acted in good faith and in a manner such person reasonably believed to be in the interest of the participants and beneficiaries of an employee benefit plan shall be deemed to have acted in a manner “not opposed to the best interests of the corporation” as referred to in this section.
“(j) The indemnification and advancement of expenses provided by, or granted pursuant to, this section shall, unless otherwise provided when authorized or ratified, continue as to a person who has ceased to be a director, officer, employee or agent and shall inure to the benefit of the heirs, executors and administrators of such a person.
“(k) The Court of Chancery is hereby vested with exclusive jurisdiction to hear and determine all actions for advancement of expenses or indemnification brought under this section or under any bylaw, agreement, vote of stockholders or disinterested directors, or otherwise. The Court of Chancery may summarily determine a corporation’s obligation to advance expenses (including attorneys’ fees).”
OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
The estimated expenses payable by the Registrant in connection with the issuance and distribution of the securities being registered are as follows:
SEC Registration Fee | | $ | 1,327.46 | |
Printing Expenses * | | $ | 2,000.00 | |
| Legal Fees and Expenses * | | $ | 55,000.00 | |
| Accounting Fees and Expenses * | | $ | 5,000.00 | |
Total | | $ | 63,327.46 | |
* Estimated
RECENT SALES OF UNREGISTERED SECURITIES
The following represent sales of unregistered securities of China Biopharma, Inc. (the “Company”) in the last three years:
In February 2005, the Company completed a private placement of 260,000 shares of common stock at a price of $1.00 per share, or gross proceeds of $260,000. No underwriting discounts or commissions were paid in connection with this transaction. The Company issued the foregoing securities in reliance on Section 4(2) of the Securities Act, based on the identity and number of investors.
During the quarter ended, March 31, 2005, the Company granted 402,000 fully vested, non-forfeitable warrants to purchase shares of common stock to two consultants for services in addition to cash payments. No fees were paid in connection with this transaction. The Company issued the foregoing securities in reliance on Section 4(2) of the Securities Act, based on the identity and number of investors.
Also during the quarter ended, March 31, 2005, the Company granted 100,000 fully vested, non-forfeitable shares of common stock to a consultant for services. No fees were paid in connection with this transaction. The Company issued the foregoing securities in reliance on Section 4(2) of the Securities Act, based on the identity and number of investors.
In April 2005, the Company completed a private placement of 95,000 shares of common stock at a purchase price of $1.00 per share, or gross proceeds of $95,000, and, for no additional consideration, a cashless 2-year warrant to purchase an additional 95,000 shares at an exercise price of $1.50 per share. A value of $36,770 of the proceeds has been allocated to the warrant. No commissions were paid in connection with this transaction. The Company issued the foregoing securities in reliance on Section 4(2) of the Securities Act, based on the identity and number of investors.
In May 2005, the Company completed a private placement of 500,000 shares of common stock at a purchase price of $0.50 per share, or gross proceeds of $250,000, and for no additional consideration, a cashless 5-year warrant to purchase an additional 147,059 shares at an exercise price of $0.75 per share. A value of $71,470 of the proceeds has been allocated to the warrant. No commissions were paid in connection with this transaction. The Company issued the foregoing securities in reliance on Section 4(2) of the Securities Act, based on the identity and number of investors.
Also in May 2005, the Company completed a private placement of 500,000 shares of common stock at a purchase price of $0.50 per share, or gross proceeds of $250,000, and for no additional consideration, a cashless 5-year warrant to purchase an additional 147,059 shares at an exercise price of $0.75 per share. A value of $68,240 of the proceeds has been allocated to the warrant. No commissions were paid in connection with this transaction. The Company issued the foregoing securities in reliance on Section 4(2) of the Securities Act, based on the identity and number of investors.
In July 2005, the Company completed a private placement of 1,000,000 of common stock at a purchase price of $0.50 per share, or gross proceeds of $500,000 and, for no additional consideration, a cashless 5-year warrant to purchase an additional 400,000 shares at an exercise price of $0.75 per share. A value of $168,000 of the proceeds has been allocated to the warrant. No commissions were paid in connection with this transaction. The Company issued the foregoing securities in reliance on Section 4(2) of the Securities Act, based on the identity and number of investors.
Also in July 2005, the Company entered into a service agreement pursuant to which the Company agreed to issue warrants to purchase up to an aggregate of 200,000 shares (the “Service Warrant Shares”) of the Company’s common stock in exchange for investor relations services. The Company had the right to terminate the service agreement at any time on or after October 5, 2005, upon 30 days prior written notice. The Service Warrant Shares were scheduled to vest in accordance with the following schedule and are purchasable at the following exercise prices:
| · | 50,000 Service Warrant Shares were immediately vested and may be purchased at an exercise price of $0.90 per share; |
| · | 50,000 Service Warrant Shares were scheduled to vest on the 91st day following the date of the service agreement and were purchasable at an exercise price of $1.10 per share; |
| · | 50,000 Service Warrant Shares were scheduled to vest on the 181st day following the date of the service agreement and were purchasable at an exercise price of $1.30 per share; |
| · | 50,000 Service Warrant Shares were scheduled to vest on the 271st day following the date of the service agreement and were purchasable at an exercise price of $1.50 per share. |
The warrants shall terminate on the 24-month anniversary of the effective date of a registration statement filed by the Company to register the resale of the Service Warrant Shares; provided, however, in the event that the Company elects to terminate the service agreement early as described above, the warrants will immediately terminate as to any Service Warrant Shares that are not then vested. By October 5, 2005, the Company terminated the service agreement, resulting in only 50,000 Service Warrant Shares vested with an exercise price of $0.90 per share.
Pursuant to a Subscription Agreement (the “Subscription Agreement”) dated December 13, 2006, by and among the Company and the subscribers (the “Subscribers”) identified on the signature page thereto, the Company sold to the Subscribers $3,000,000 of principal amount of secured convertible promissory notes of the Company (the “Notes”). The Notes are convertible at the option of the Subscribers at any time into shares of the Company’s common stock. Prior to the occurrence of an Event of Default (as defined in the Notes) the Notes are convertible at a per share conversion price equal to $0.25 per share. Following an Event of Default, the Notes are convertible at the lesser of $0.25 per share and 75% of the average of the closing bid prices for the Common Stock for the five trading days prior to the date of conversion. The Notes bear interest at a rate of eight percent (8%) per annum. Monthly payments on the Notes shall commence March 13, 2007, three months from the date the Notes were issued. Provided that an Event of Default has not occurred, the Company may, at its option, prepay the outstanding principal amount of the Notes, in whole or in part, by paying 120% of the principal amount to be repaid, together with accrued interest plus any other sums due thereon to the date of redemption.
The Notes are secured by a Security Agreement (the “Security Agreement”) entered into by and among the Company, China Quantum Communications Ltd., a Cayman Islands corporation (“Quantum”), China Biopharma Ltd., a Cayman Islands corporation (“Biopharma Ltd”), and Guang Tong Wang Luo (China) Co. Ltd., a corporation incorporated in the People’s Republic of China (“Guang” and together with Quantum and Biopharma Ltd., the “Subsidiaries”) and Barbara R. Mittman, as collateral agent for the Subscribers. The obligations of the Company under the Subscription Agreement and the Notes are guaranteed by that certain Guaranty (the “Guaranty”), dated as of December 13, 2006, entered into by the Subsidiaries, for the benefit of the Subscribers.
In connection with the sale of the Notes, the Company also issued to the Subscribers, Class A Warrants to purchase 6,000,000 shares of common stock and Class B Warrants to purchase 6,000,000 shares of common stock. One Class A Warrant and one Class B Warrant were issued for each two shares of common stock that would have been issuable on such date upon the complete conversion of the Notes. The Class A Warrants have an exercise price of $0.30 per share and the Class B Warrants have an exercise price of $0.40. Melton Management Ltd. acted as the finder and received a warrant to purchase 2,400,000 shares of common stock at an exercise price of $0.30 per share. The issuance of the Notes, the Class A Warrants, the Class B Warrants and the warrant to the finder were in reliance on Section 4(2) of the Securities Act, based on the number and sophistication of the investors.
EXHIBITS
| 4.1 | Form of Secured Convertible Promissory Note* |
| | |
| 4.2 | Form of Class A Warrant* |
| | |
| 4.3 | Form of Class B Warrant* |
| | |
| 4.4 | Form of Finder Warrant* |
| | |
| 5.1 | Opinion of Loeb & Loeb LLP |
| | |
| 10.1 | Subscription Agreement, dated December 13, 2006, by and among the Company and the subscribers identified on the signature page thereto* |
| | |
| 10.2 | Security Agreement, dated December 13, 2006, by and between the Company, China Quantum Communications Ltd., China Biopharma Ltd., Guang Tong Wang Luo (China) Co. Ltd., and Barbara R. Mittman, as collateral agent for the Subscribers* |
| | |
| 10.3 | Guaranty, dated as of December 13, 2006, entered into by the Subsidiaries, for the benefit of the Subscribers* |
| | |
| 23.1 | Consent of Patrizio & Zhao, LLC** |
| | |
| 23.2 | Consent of Rosenberg Rich Baker Berman & Company** |
| | |
| 23.3 | Consent of Loeb & Loeb LLP (included in its opinion filed as Exhibit 5.1) |
* Incorporated by reference to the Company’s Form 8-K filed with the SEC on December 13, 2006.
** Filed herewith.
UNDERTAKINGS.
Undertaking Required by Item 512 of Regulation S-B.
(a) The undersigned registrant will:
(1) File, during any period in which it offers or sells securities, a post-effective amendment to this registration statement to:
(i) include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) reflect in the prospectus any facts or events which, individually or together, represent a fundamental change in the information in the registration statement; and notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) include any additional or changed material information on the plan of distribution.
(2) For determining liability under the Securities Act, treat each post-effective amendment as a new registration statement of the securities offered, and the offering of the securities at that time to be the initial bona fide offering.
(3) File a post-effective amendment to remove from registration any of the securities that remain unsold at the end of the offering.
(b) For determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the registrant undertakes that in a primary offering of securities of the registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the registrant or used or referred to by the registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the registrant or its securities provided by or on behalf of the registrant; and
(iv) Any other communication that is an offer in the offering made by the registrant to the purchaser.
(c) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
SIGNATURES
In accordance with the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements of filing on Form SB-2 and has authorized this registration statement to be signed on its behalf by the undersigned in Princeton, New Jersey, on January 19, 2007.
| | | | |
| | CHINA BIOPHARMA, INC. | |
| | | |
| By: | /s/ Peter Wang | |
| |
Name: Peter Wang | |
| | Title: Chief Executive Officer, President and Director | |
| | | | |
| By: | /s/ Ya Li | |
| |
Name: Ya Li | |
| | Title: Chief Financial Officer and Director | |
| | | | |
| By: | /s/ Charles Xue | |
| |
Name: Charles Xue | |
| | Title: Director | |
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints each of Peter Wang and Ya Li as his true and lawful attorney-in-fact, with full power of substitution and resubstitution for him and in his name, place and stead, in any and all capacities to sign any and all amendments including post-effective amendments to this Registration Statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Commission, hereby ratifying and confirming all that said attorney-in-fact or his substitute, each acting alone, may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons and in the capacities and on the dates indicated.
| | | | |
| Dated: January 19, 2007 | By: | /s/ Charles Xue | |
| |
Name: Charles Xue | |
| Title: Director | |