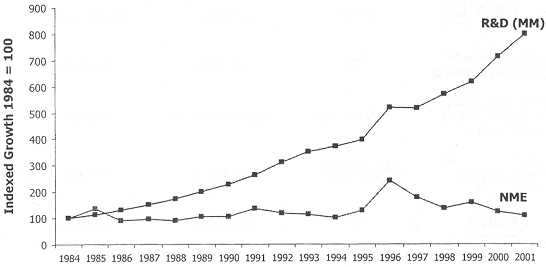
In the last 15-20 years, the pharmaceutical industry has embraced newer drug discovery technologies and focused on shortening discovery timelines in order to meet the intended goal of generating several more NCEs per year. As such, many pharmaceutical companies have discontinued their time- and resource-consuming traditional natural product programs. Instead, they have concentrated on the more high-throughput-friendly area of synthetic chemistry and combinatorial synthesis, and, in a sense, have given up on the unrivalled structural diversity available from nature.
This last drawback can be a major problem when the active compound is too complex to be easily synthesized, as often the case with natural products. With the current emphasis on developing highly efficient discovery processes and on significantly reducing discovery timelines, it is understandable, if somewhat shortsighted, to see why many pharmaceutical companies are discontinuing their in-house natural product programs. Some pharmaceutical companies, however, still maintain efforts in this area, mostly using compounds of microbial origin.
Yet despite this seeming trend away from natural products, there were, in 2001, 143 compounds of natural origin in clinical trials or that had been recently launched. Of these, natural products from plants were by far the dominant source of bioactive compounds (In table below).
Table:. Source of natural product-derived drugs recently launched or in clinical development
MH-B, being one of the only companies that can provide the widest access to natural and novel phytochemodiversity as well as guaranteed, secured and reproducible supply at all scale of bio-active phytochemicals. This is being recognized by the fact that several large pharmaceutical companies have solicited MH-B.
infections, nail deformities, and other problems. Prolonged exposure can lead to eye, skin and lung irritation, abnormal liver or kidney function, nervous system damage or reproductive problems.
With increasing awareness of harmful synthetic chemicals in cosmetics, consumers are demanding products containing safer natural ingredients. In fact, 35% of cosmetic products are plant-based, and this market segment is growing at a rate of 10% per year. For example, most products from Yves Rocher (annual sales: €2B), Pierre Fabre (cosmetics approximately €0.8B) and Clarins (€0.2B), are of plant origin. On the other hand, as one of the pioneers of chemical-based cosmetics, L’Oreal’s market position (€15B) could be considerably affected by this trend.
2- Increasing product regulation
Regulatory agencies are pressing all suppliers to better characterize biologically and chemically the active ingredients, whether of synthetic or natural origin, contained in consumer products and to ensure high levels of reproducibility. These issues remain highly problematic for plant-based products that can only be resolved with great difficulties using traditional production technologies.
3- Increasing marketing demand for new products
The average life cycle of a cosmetic product is 3-5 years, which means that the demand for new products is enormous, with development times, from discovery to commercialization, reduced to less than 12-18 months. For example, L’Oréal markets 5,000 products and, consequently, its R&D team needs to discover and develop 1,000 to 3,000 new products per year.
4- Speeding up the rate of product discovery and development
In an effort to better meet market demand and speed up the rate of product discovery, large cosmetic companies (L’Oréal, Estée Lauder, Shiseido etc.) have adopted, in recent years, modern and faster approaches to speed-up their R&D activities similar to those used in the pharmaceutical industry, for the discovery of new cosmetic ingredients. These involve molecular targets and cellular assays and HTS systems to screen natural product extracts and for extensive toxicity testing prior to human trials. Apparently, this undertaking has not meet with expected success (L’Oréal, personal communications) for the same reasons discussed in section 3.1.1 that led the pharmaceutical industry to shy away from natural product drug discovery using traditional extracts during the last 15-20 years. Therefore, there is an urgent need for companies that have adopted modern discovery technologies to access expertise and plant compound libraries that are compatible with HTS systems such as MH-B’s Phytomics bioprocesses, phytochemical library and Plant Product Discovery Platform (L’Oréal, personal communication).
5- High demand for innovation
An aging population with high levels of disposable income is an important business opportunity for the cosmetic industry. Its is always eagerly seeking novel ingredients both for current product lines and for important unmet market needs such as new anti-aging, natural coloring, skin whitening and natural conservative agents. These innovative ingredients can be crude extracts, partly purified natural products or pure compounds obtained from natural sources.
These trends underline the dynamics of discovery and commercialization in the cosmetic industry. Although the market for individual products may be limited, their large number, high turnover and the insatiable appetite for novelty as well as the demand by regulatory authorities to develop and market safer and better-characterized products represent a tremendous business opportunity. The continuous, reproducible, secured and scalable supply of natural and novel phytochemodiversity compatible with today’s product discovery technologies is a strategic issue for the cosmetic industry. In this context, MH-B is uniquely positioned and has significant competitive advantages to meet this significant and strategic demand of the cosmetic industry.
The Case of the Nutraceutical Industry
The Dietary Supplement Health & Education Act adopted in 1994 in the US has created a regulatory environment that remains difficult to enforce, especially with regards to claims of specific health benefits and prevention. Nutraceuticals and functional foods of natural origin are in great demand, especially from plants. However, discovery, development and production of new products involve mostly conventional approaches in the context of the conservative food market. For example, the flavour industry screens only limited number of compounds annually and focuses mainly on combinations and modifications of known ingredients.
Few companies, however, are developing more systematic and efficient approaches in this area. For example, Senomyx (San Diego, CA) has identified and patented (72 issued patents and 212 pending patent applications) many of the chemokine (GPCR) receptors and ion channels present on the human tongue that detect yumami (savory), sweet, bitter, salt, fat and sour tastes as well as cold and olfactory sensing. This company is a world leader in the exploitation of molecular targets, cellular assays and HTS systems, similar to those used in the pharmaceutical industry, for the discovery and development of novel nutraceuticals. Furthermore, Senomyx has developed a cost effective and efficient process for acceptance of its products by regulatory agencies that yields full approval within 12-24 months from discovery. Senomyx is currently collaborating with Cadbury-Schweppes, Campbell’s Soup Co., Coca-Cola, Kraft and Nestlé.
29
To date, Senomyx has screened 200,000 compounds, mostly synthetic chemicals, using its HTS systems. Following market trends and requests by its collaborators, Senomyx has undertaken limited natural product screening with little success mostly because of reproducibility and supply issues similar to those experienced by the pharmaceutical and cosmetic industries (sections 3.1.1 and 3.1.4).
MARKET SUPPLY FROM THE PHYTOCHEMICAL LIBRARIES
Four types of phytochemical library are commercialized for the discovery and development of new products:
| | |
| 1. | Traditional plant extracts containing 1000’s of phytochemicals (and undesirable impurities) of different polarities, from water soluble to highly lipophilic compounds, present at different concentrations; |
| | |
| 2. | Purified natural phytochemical fractions, obtained from natural extracts, of different polarities, each containing usually 2 to 50 compounds at different concentrations; |
| | |
| 3. | Purified natural phytochemicals (and semi-synthetic derivatives) obtained from separation and purification of higher yield compounds of natural extracts; and |
| | |
| 4. | MH-B’s highly purified natural and novel phytochemical fractions, obtained from cleaner cell culture extracts, of different but more focused polarities, each containing usually 2 to 30 compounds at different concentrations. |
Obviously, all plant compound libraries obtained from natural sources suffer from serious drawbacks, including lack of reproducibility and problematic access of active compounds, especially those of lowest yields. The Phytomics bioprocesses offer MH-B a significant competitive advantage in this area by successfully solving all these technological challenges.
Historically, libraries of crude plant extracts have had limited success, for obvious reasons, in drug discovery environments. Consequently, the value of traditional plant extract libraries has been relatively low. Since purified plant products are not readily available in large numbers, it is difficult to gauge the value that such a library would command, especially when coupled to the breakthrough technologies developed by MH-B. Recently, however, Amgen entered into a three-year, non-exclusive collaboration with Infinity Pharmaceuticals for access to the latter’s “natural product-like” synthetic libraries. The groundbreaking synthetic chemistry-based technologies developed by Infinity Pharmaceuticals mirrors MH-B’s pioneering technological contributions in developing actual plant product biosynthetic libraries for drug discovery. Although most of the deal’s terms were not disclosed, Amgen did make a $25 million equity investment in Infinity.
PATENTS AND INTELLECTUAL PROPERTY
The Company holds one granted US patent ( 6,069,009) with foreign counterpart (Europe, Japan, Australia, Canada and Mexico) on Phytomics Technologies. The process has since been fully integrated into the Phytomics Technologies, to accelerate the growth phase of plant cell cultures and significantly improve the yield of secondary metabolites as well as the economics and reproducibility of the overall production process.
In order to better serve its interest, the Company’s IP strategy will primarily involve aggressively pursuing the filing of patent applications resulting from its product discovery activities including
| | | |
| | • | Proper patent coverage for new plant-based products, that represent the key valuation basis of the Phytomics Technologies and of the Company, and |
| | | |
| | • | Proper process patent coverage for Phytomics bioprocesses used for the production of specific patented plant-based products. |
COMPETITION IN PLANT-BASED PRODUCT DISCOVERY
MH-B’s competitors in plant product discovery include the following.
30
1- Chemistry companies
Companies producing specialty chemicals and those specializing in combinatorial chemistry generally provide large synthetic chemical libraries for discovery programs, that are not as complex as most plant secondary metabolites. Consequently, chemistry companies are not direct competitors of MH-B. On the other hand, MH-B may sub-contract to chemistry companies industrial scale synthesis of promising simple compounds and/or semi-synthesis of promising complex phytochemicals identified through its discovery activities. Consequently, chemistry companies may become valuable partners of MH-B in optimization chemistry and/or bulk synthesis of promising compounds.
2- Companies supplying phytochemical libraries
Currently, few organizations are commercializing phytochemical libraries (In table below). These libraries are composed of crude plant extracts, purified fractions, or pure phytochemicals, with the former being incompatible with modern HTS technologies, are obtained from natural biomass. Therefore, these suppliers cannot guarantee reproducibility, fast supply and large-scale production of active complex compounds. These production issues remain key unresolved difficulties in exploiting plant compound in product discovery projects.
Table: Organizations supplying phytochemical libraries for discovery programs
| | |
Organization | | Characteristics of Phytochemical Library |
| |
|
AnalytiCon Discovery | | 10,000 pure compounds, 25% from plants |
| | |
Albany Molecular | | 170,000 purified fractions, under 10% from plants |
| | |
BioSPECS | | Approximately 2,000 purified compounds, mostly from plants |
| | |
Drug Discovery Ltd. | | Extracts prepared from ~ 9,000 plant species |
| | |
German Natural Product Pool
(Hans-Knöll-Institut) | | Approximately 4,000 purified natural products and derivatives obtained from various sources |
| | |
MerLion | | 500,000 extracts, ~ 25% of from plants |
| | |
Molecular Nature | | 4,000 purified fractions and 1,000 pure compounds obtained from plants |
| | |
National Cancer Institute | | Over 100,000 extracts from plant and marine organisms |
| | |
Sequoia | | 36,000 purified fractions from plants |
COMPETITION IN PLANT COMPOUND PRODUCTION
The competitive landscape in the area of reproducible industrial scale production of specialized complex plant products include the following players.
1- Contract manufacturing organizations (CMO)
Except for DFB Pharmaceuticals/Phytonbiotech (discussed below), all CMO are specializing in either microbial fermentation or mammalian/insect cell cultures, the latter for recombinant protein production. None of them have expertise in plant cell culture bioengineering
31
2- Plant pharming organizations
The number of players in plant pharming is impressive and competition is fierce. Asian, African and South American suppliers offer low cost raw material to American and European extraction companies, which then supply the pharmaceutical, cosmetic and nutraceutical industries with purified plant products. This traditional supply route (i.e., agriculture, harvesting & extraction) has numerous major limitations that can be overcome by industrial scale plant cell culture technologies, which include
| | |
| 1. | Secure and reproducible supply of natural biomass in sufficient quantity on a long term basis; |
| | |
| 2. | Increasing cost of the raw material; |
| | |
| 3. | Proper handling at harvesting of the natural biomass for retained yield, reproducibility and efficient processing; |
| | |
| 4. | Low product yield at processing; |
| | |
| 5. | Efficiency and cost of downstream processing operations to obtain the targeted compound at the desired purity level and in sufficient quantity to meet market demand in a timely fashion. |
This situation is further complicated if the biomass is harvested in the wild and/or when the desired compounds are extracted from plant species that are threatened, grow slowly, are only available in politically unstable areas, are not compatible with greenhouse or large scale culturing, or if harvesting results in the destruction of the plant species.
For cosmetic and nutraceutical products, purification requirements range from partly purified fractions to purified compounds, whilst for pharmaceuticals and specialty chemicals, pure compounds are required. Regulatory agencies, however, are pressuring the nutraceutical and cosmetic industries to increase the level of purification and characterization of commercial plant-based products.
3- Companies involved in industrial scale plant cell culture bioprocessing
Four companies are known to use (or to have used) industrial scale plant cell culture bioprocesses: PhytonBiotech, Inc., Mitsui Chemical, Inc., Samyang Corporation and Protalix Pharmaceuticals Ltd.
PhytonBiotech is the most advanced company in industrial plant cell culture bioprocesses. This company was founded in 1990 to produce Paclitaxel for Brystol-Myers-Squibb, its strategic partner. It succeeded in this undertaking in the late 90’s and its German subsidiary, Phyton GmbH, currently operates the world’s largest commercialcGMP manufacturing facility for the production of extracts containing Paclitaxel and other valuabletaxanes. In 2003, Phyton was acquired by DFB Pharmaceuticals, a CMO, but most of its expert personnel have left the organization.
In the mid 80’s, the Japanese giant Mitsui invested in the development of a first industrial scale plant cell-based bioprocess for the production of shikonin, an anti-inflammatory and coloring agent. This process was successfully scaled up in a 750 L bioreactor and shikonin was produced at high yields. This project, however, was discontinued for marketing reasons. In the late 80’s, Mitsui developed a second plant cell-based bioprocess for the production of ginseng extracts. The production plant was inaugurated in the early 90’s and 1995 sales of the ginseng extract reached US$ 3M. Ginseng extracts were subsequently removed from the market due to severe toxic side effects. Mitsui appears not to have pursued this business.
In the mid 90’s, the Korean company Samyang bought a taxane production technology based on cultured plant cells developed by the American company EsCagenetics. Samyang completed the development of this technology and scaled it up to industrial level for the production of Genexol™, a Paclitaxel-based product currently on the market. This company seems not to have expanded its efforts in this area.
Finally, the Israel start-up company Protalix Biopharmaceutics Ltd (formerly Metabogal, founded in 1994) is now positioning itself as a CMO for the production of recombinant proteins using plant cells cultured in a rudimentary disposable bioreactor system made of 10 L chambers with minimal monitoring and process control.
It appears from our review of the patents published by these companies as well as from personal communications with some of their key executives, that all of them use traditional approaches to plant cell cultures and bioengineering for the production of secondary metabolites. In this context, MH-B is in a good market position and significant competitive advantages in exploiting its highly efficient Phytomics bioprocesses for these business opportunities.
32
BUSINESS MODEL AND STRATEGY
MH-B’s primary business activities will focus on business development (BD). Consequently, the Company will put in place the tools and means to reach out to targeted market stakeholders. Our single most important goal is to identify profitable plant-based product opportunities to develop, as well as to connect with the best companies to partner with, for development.
To carry out its extensive BD activities, MH-B will hire an experienced BD/industrial marketing Vice-President and will develop a network of international BD consultant specialists in the three industries targeted and strategically located, mostly in US and Europe, to complement its business opportunity scouting activities. The latter will actively participate in the identification of clients/partners likely to be interested by the Company’s technologies, expertise, products and services. These consultants work on a back-loaded fee to be paid upon completion of successful transactions.
To support BD activities and identify market opportunities, the Company will create an efficient Data Mining Unit (DMU), under the direction of the VP-BD, that will identify business opportunities and provide the information to drive the Company rationale in elaborating the best-suited and most rewarding strategy for the development of our business activities. Practically, it will be based on a collection of software and procedures to monitor and mine public databases, so as to connect the Company’s technological strengths with stakeholders’ needs.
Market intelligence and public communications will generate a list of prospective partners and clients for the Company. We will adopt a pro-active approach with the market to meet as soon as possible with prospective clients/partners. Recent experience has shown this method to be very productive. Our objective is to establish profitable and value generating commercial partnerships with selected high profile companies in targeted industries.
MH-B istargeting the markets of cosmetic, nutraceutical and pharmaceutical industrial products. More specifically, companies in these markets are either searching for innovative new products of plant origin using modern product discovery technologies and/or struggling to produce efficiently complex plant products. However, they are experiencing serious problems in accessing and producing plant compounds, and currently facing important market gaps and significant unmet needs that MH-B can satisfy efficiently using its unique technologies.
MH-B’s business development activities will be driven by
| | |
| 1. | Needs and requests of prospective customers for plant-based discovery projects and/or production projects targeting promising complex plant products in development and/or already commercialized; and; |
| | |
| 2. | Innovative product concepts identified through market intelligence and DMU research to be developed internally and/or in partnerships |
COMMERCIAL ACTIVITIES
MH-B will focus on two commercial activities.
1- Discovery and development of new plant-based products
The Company exploits the natural and novel chemodiversity, that it can generate using its Phytomics Technologies. This offers the cosmetic, nutraceutical and pharmaceutical industries a new paradigm for the discovery and development of novel products of plant origin, based on its current library of > 160,000 purified phytochemical fractions fully compatible with HTS technologies, and unique expertise and Plant Product Discovery Platform. The strength of this library was shown by partial screening using 11 molecular targets and cellular assays to yield 173 biologically active molecules, of which 59 were new chemical entities. MH-B has the expertise and technologies to expand its purified phytochemical library, including for plant species of interest to partners. Chemical characterization of bioactive compounds can also be conducted in house.
Typicalpartnered product discovery and development projects will usually involve
| | |
| 1. | Selection by the partner of generic or proprietary molecular targets, cellular assays and/or of fraction library to be screened; the latter may be MH-B’s current library or specialized libraries developed by MH-B according to the partner’s requirements; |
| | |
| 2. | Screening by MH-B or the partner fractions using selected targets and assays; |
33
| | |
| 3. | Identification and short term supply of active compounds by MH-B; and |
| | |
| 4. | If the promising plant products are complex, production of pilot (for product development) and commercial quantities of products for the partner and/or final commercializing company. |
These projects will be structured as follows.
| | | |
| 1. | The partner pays |
| | | |
| | • | Licensing fees to access MH-B’s technologies and phytochemical library, |
| | | |
| | • | R&D costs based on a full FTE (Full Time Equivalent) basis that includes profits, or reduced FTE cost (with profits) in exchange for increased milestone and royalty payments to be negotiated, and |
| | | |
| | • | Pre-negotiated milestones (when attained) and royalty (at commercialization) payments. |
| | | |
| 2. | The partner obtains |
| | |
| | • | Co-ownership of product IP, and |
| | | |
| | • | Full commercialization rights with performance clauses. |
| | | |
| 3. | MH-B’s retains |
| | |
| | • | Co-ownership of product IP, and |
| | | |
| | • | Full ownership of production technologies and IP and exclusive industrial scale production rights for complex plant products (2nd commercial activity). |
for which it will retain full product and production IP ownership. Thereafter, MH-B Additionally, MH-B intends to pursue its owninternal product discovery projects will out-license to high profile partners these products for co-development and co-commercialization, which will include license fees, development revenues, milestone and royalty payments as well as exclusive production rights for complex plant products (2nd commercial activity).
In the Pharmaceutical domain, MH-B will actively seek partnered drug discovery projects. In addition, the company has unique expertise in infectious disease and has internally developed promising programs that it will pursue to appropriate levels for subsequent high value partnering with major pharmaceutical companies.
In the cosmetic and nutraceutical domains, the company has already identified a certain number of potential product discovery partnerships (section 4.4.1), one of which has already been signed, that it will pursue actively. MH-B will actively seek new opportunities in these areas. Furthermore, MH-B has identified significant new product opportunities in the cosmetic industry that it intends to pursue internally and/or in partnership.
2- Industrial-scale production of complex plant products of commercial relevance using Phytomics-based bioprocesses
The second commercial activity targets partners that are either selling, intend to conduct or are already conducting product development activities (also with MH-B), including clinical trials on complex plant products that can be obtained using traditional approaches only with great difficulties and at high costs. These products include
| | |
| A. | Partly purified extracts or highly purified phytochemical fractions poorly characterized chemically, whose compounds, or composition cannot be reproduced synthetically, whose supply, using traditional approaches, is problematic, or which contain undesirable components (color, odor, nuisance impurities etc.) difficult to remove at reasonable costs (for example the J&J project discussed in section 4.4.2); and |
| | |
| B. | Purified complex phytochemicals, that cannot be synthesized at industrial scale or produced in sufficient quantities through natural biomass extraction and purification processes, at reasonable costs (Ex.: Taxol™ & Taxotere™). |
Generally, secured, consistent and reproducible supply, in sufficient quantities, of these complex plant products, using traditional approaches, is highly problematic. Uncontrollable uncertainties of supply include political and social issues, poor agricultural and harvesting practices, rare, exotic, endangered, protected and/or slow growing plant species etc. These represent critical issues in conducting reliable product development activities and for subsequent commercialization that remain very difficult to resolve for non-specialist companies.
In the Pharmaceutical domain, MH-B will actively seek production projects. The company has finalized a contact with Pierre Fabre, a French pharmaceutical company, on April 11th 2006.
34
In the cosmetic and nutraceutical domains, the company has already identified a certain number of potential production projects, one of which is being discussed with a cosmetic and consumer multinational. MH-B will actively seek new opportunities in these areas.
A key issue in any cell-based industrial scale production remains the economics of the bioprocess. Traditional approaches to plant cell culture bioengineering have yielded limited industrial success, as underlined in Appendix A (section A.1), in focusing on single products. Still, PhytonBiotech is successful in producing taxane derivatives at industrial scale using a plant-cell based bioprocess. The Phytomics Technologies, which represent significant advances in this field, are solving the limitations previously encountered in plant-cell based bioprocessing. MH-B’s strategy in this area focuses on producing multiple plant products for various customers in the three industries targetedall using the same basic production technologies, including downstream processing operations. This offers important economies of scale in R&D and production and the opportunity for full utilization of planned production facilities that will translate into higher profit margins as well as significant risk reduction. Furthermore, MH-B’s offering of pilot and industrial scale facilities for secured and reproducible production of complex plant products represents an important marketing tool in attracting partners for high value plant product discovery projects.
Typicalproduction projects will usually three phases.Phase I. Feasibility Study involves the development of productive plant cell lines and appropriate culture and downstream processing (extraction, separation & purification) protocols for the production, at high yield and at the target quality level, of the desired product(s). A technico-economical model of this process is also developed to assess the economics of the industrial scale bioprocess. At the end of this work, the partner decides if he wants to undertake further bioprocess development depending on the expected market and production costs of the desired product(s).
DuringPhase II. Pilot Scale Production, the optimized production bioprocess, including downstream processing operations, is developed at pilot level (50 L to 300 L bioreactors; 100 g to kg of product(s)) to validate experimental results obtained at small scale, confirm bioprocess economics and define main scale up parameters. This work also involves production, by MH-B, of pilot quantities (100 g to kg) of desired product(s) at target quality level for partner’s product development activities.
Finally,Phase III. Industrial Scale Production involves the development, by MH-B, of the industrial scale bioprocess for commercial production of desired product(s) and Industrial scale production by MH-B (or subsidiary) of desired product(s) for commercialization.
These projects will be carried out in partnerships and internally, for prospective high potential products. Partnered projects and will be structured as follows.
| | | |
| 1. | The partner pays |
| | |
| | • | Licensing fees to access MH-B’s Phytomics Technologies, |
| | | |
| | • | R&D costs on areduced FTE basis (over US$190,000/FTE) that includes profits, |
| | | |
| | • | Pre-negotiated milestone payments depending on production levels attained and forecasted production costs at Phase I and II of the project, and |
| | | |
| | • | Pilot scale quantities of desired product(s) for development purposes. |
| | | |
| 2. | MH-B’s retains |
| | |
| | • | Full ownership of production technologies and IP and exclusive industrial scale production rights, |
| | | |
| | • | Co-ownership of IP generated from new products discovered during MH-B’s bioprocess development work in the partner’s specific application field, and |
| | | |
| | • | Full ownership of IP generatde from new products discovered during MH-B’s bioprocess development work in application fields other that of the partner’s. |
| | | |
| 3. | MH-B obtains |
| | |
| | • | Royalty payment on final sales of original product(s), |
| | | |
| | • | Exclusive, long term commercial production contract for original product(s) at minimum annual production level and specified and indexed selling price, |
| | | |
| | • | Milestone and royalty payments for new products discovered from MH-B’s bioprocess development work in the partner’s specific application field, |
| | | |
| | • | Exclusive, long term commercial production contract for new products, discovered from MH-B’s bioprocess development work in the partner’s specific application field, at minimum annual production level and specified and indexed selling price, and |
| | | |
| | • | New products discovered from MH-B’s bioprocess development work in application fields other that of the partner’s. |
35
PURSUING THE DEVELOPMENT OF THE PHYTOMICS AND OTHER TECHNOLOGIES
In order to maintain its state-of-the art expertise and increase its competitive advantages in industrial plant biotechnology and phytochemistry, MH-B will pursue the development of its Phytomics Technologies. Within the next 3 years and as illustrated in section 4.6, Phytomics R&D activities will be focused on
| | |
| 1. | Maintaining MH-B’s extensive collection of over 2,200 plant species in culture, which represent an immense source of natural and novel phytochemodiversity immediately available for discovery and production projects as well as a powerful tool in marketing MH-B Phytomics bioprocesses to prospective partners; |
| | |
| 2. | Developing and implementing suitable cryo-preservation protocols and systems for long term, secured conservation of MH-B’s extensive collection of plant species in culture, especially with respect to patent requirements and industrial scale production of high value plant products; |
| | |
| 3. | Pursuing the development of the basic plant cell culture bioprocess, using novel bioengineering and genetic engineering approaches applied to model and commercial biosystems in development, in order to maximize yields and productivity of targeted products; |
| | |
| 4. | Developing and optimizing downstream operations at pilot scale (10-100 g), including those directly interfacing the cell culture process, in order to improve net production yields of purified targeted products; |
| | |
| 5. | Scaling up the Phytomics Technologies from the current 50 L bioreactor to industrial size bioreactors of much larger quantities, including associated downstream processing operations, for the production of purified plant products; this investment project will be undertaken with respect to current and anticipated market demand for discovery and production projects. |
| | |
| 6. | Replenishing and expanding the company’s purified phytochemical library with respect to current and anticipated demand for discovery projects since this expensive undertaking involves long lead times. |
MH-B will also develop a generic technico-economic model that can be applied to a variety of production projects to evaluate their main economic parameters and financial viability.
Co-currently, MH-B also intends to develop cGMP extraction, formulation, encapsulation and packaging facilities that will initially be used for natural source MMH™ products being commercialized by its parent company, Millenia Hope Inc.
These activities will be financed internally and through contribution from product discovery and production projects. In due course, MH-B intends to develop its own industrial scale production facilities to meet market increasing demands.
MARKETS FOR MH-B’S TECHNOLOGIES AND PRODUCT OFFERINGS
For the Pharmaceutical Industry
Pharmaceutical products represent the largest segment of the chemical market with annual sales of US$492B in 2003. The market of plant-based drugs is estimated at more than US$40B per year. The major markets in the pharmaceutical industry are the USA, Japan, Germany, France, UK, Italy and Canada. The R&D budget of the pharmaceutical industry in the US is estimated at US$58B, representing 11.8% of sales of which 60% is spent on clinical trials.
MH-B intends to undertake drug discovery and complex phytochemical production projects for the pharmaceutical industry. The sizes of these market segments are estimated at
| | | |
| | • | Approximately US$4.6B/y for drug discovery excluding preclinical studies (assuming 8% of R&D being spent on drug discovery projects according to (5)), |
| | | |
| | • | Approximately US$47M/y for phytochemical production R&D projects (assuming 1% of R&D spending on plant-based pharmaceuticals ($4.7B) being spent for such projects), and |
| | | |
| | • | Approximately US$0.7B/y for compound sales to pharmaceutical companies (assuming that plant-derived active principles represent 7% of final plant product sales and that 25% of desired phytochemicals cannot be industrially synthesized at industrial scale). |
36
Within the next 3-5 years, MH-B’s objectives are to capture 0.2% of the drug discovery segment ($9M/y), 2% of R&D production projects ($0.9M/y) and 2% of phytochemical-based active principles ($14M/y).
For the Cosmetic Industry
The world market for cosmetics was estimated at US$211B in 2003, of which approximately 35% (US$74B) is plant-based. The R&D spending of this industry is estimated at 3% of total sales or US$6B per year. MH-B intends to undertake product discovery and complex plant-derived product production projects with the cosmetic industry. The sizes of these market segments are estimated at
| | | |
| | • | Approximately US$0.6B/y for product discovery excluding development studies (assuming 10% of R&D spending on product discovery projects), |
| | | |
| | • | Approximately US$22M/y for R&D production projects (assuming 1% of R&D spending on plant-based cosmetic products ($2.2B) being spent on such projects), and |
| | | |
| | • | Approximately US$5.9B/y for plant-based ingredient sales to cosmetic companies (assuming that plant-derived active ingredients represent 8% of final plant-based product sales (the natural origin of products is critical in this market)). |
Within the next 3-5 years, MH-B’s objectives are to capture 1% of the product discovery segment ($6M/y), 5% of R&D production projects ($1M/y) and 0.5% of plant-derived active ingredients ($29M).
Nutraceuticals and functional foods represent the fastest growing segment of the chemical industry with annual growth rates of 20% in some European countries. Global sales in this market were US$138B in 2000 and are expected to increase to US$ 500B by 2010. The largest markets are Japan followed by the US and Europe (mainly the UK, Germany and France). Plant-based products sold for US$ 18.5B in 2000, or 13.5% of this market. The World demand for nutraceutical natural and synthetic chemicals was US$8B in 2005, growing at an annual rate of 6%. The R&D spending of this industry is varies from 3% to 12% of total sales or over US$4B per year.
MH-B intends to undertake product discovery and complex plant-derived product production projects with the nutraceutical industry. The sizes of these market segments are estimated at
| | | |
| | • | Approximately US$24M/y for product discovery excluding development studies (assuming 10% of R&D spending on product discovery projects), |
| | | |
| | • | Approximately US$12M/y for R&D production projects (assuming 5% of R&D spending on plant-based nutraceutical products ($0.5B) being spent on such projects), and |
Within the next 3-5 years, MH-B’s objectives are to capture 6% of the product discovery segment ($1.5M/y), 6% of R&D production projects ($0.7M/y) and 0.2% of ingredient sales ($16M).
In summary, MH-B’s objectives are to generate US$16.5M/y and US$2.6M/y of profitable product discovery and R&D production projects, respectively, in the targeted markets with the next three years excluding milestone and royalty payments. In addition, MH-B expects that the latter projects will translate into US$59M/y profitable production contracts within the next 3-5 years depending on the availability of suitable production facilities.
37
| | | | | | |
Market
(US$/yr) | | Overall
Market Size | | Estimated R&D Spending | | MH-B Target
Market Share |
| |
| |
| |
|
Pharmaceuticals | | $492 Billion
Plant-based products:
$40 Billion (2003) | | $58 Billion (11.8%) | | |
|
1- Drug Discovery | | | | $4.6Billion
(8% of R&D) | | $9 Million (0.2%) |
|
2- R&D production projects | | | | $47Million
(1% of plant-based R&D) | | $0.9Million (2%) |
|
3- Supply of plant-derived | | | | $0.7B (7% *25% of plant-based) | | $14Million (2%) |
|
Active principle | | | | | | |
|
Cosmetics | | $211 Billion
Plant-bases:
$74Billion (2003) | | $6 Billion (3%) | | |
|
1- Product Discovery | | | | $0.6 Billion
(10% of R&D) | | $6Million (1%) |
|
2- R&D production projects | | | | $22 Million
(1% of plant-based R&D) | | $1Million (5%) |
|
3- Sales of plant-derived
ingredients | | | | $5.9 Billion
(8% of plant based) | | $29Million (0.5%) |
|
Nutraceuticals | | $8 Billion (2005) | | $240 Million (3%) | | |
|
1- Product Discovery | | | | $24Million
(10% of R&D) | | $1.5Million (6%) |
|
2- R&D production projects | | | | $12Million
(5% of R&D) | | $0.7Million (6%) |
|
3- Sales of ingredients | | | | $8Billion | | $16Million (0.2%) |
38
EMPLOYEES
As of September 5, 2006, all of our 12 employees are full time employees. The responsibilities of our employees are briefly summarized as follows:
Leonard Stella - - Chief Executive Officer and Director: reviews summarie of its day to day operations, long term strategic planning and operational coordination; head of the sales and marketing teams; handles overall coordination of all scientific testing, etc.
Jean Archambault - President & Chief Operating Officer-Director: Responsible for all day to day operations of MH-B, with support of the CDO and CTO; involved in business development activities with respect to external partners and project coordination, assists in sales and marketing effect;
Bahige Baroudy – Chief Scientific Officer - assists the COO with day to day functions, reviews all scientific data of MH-B heads up all new drug discovery efforts of both MH-B and the parent company
Robert Williams - Chief Development Officer-Director: responsible for supervision of the fulfillment of all contracts; directly involved in management of all scientific teams and projects; assists Plant Biology Group’s scientific endeavors as needed.
Dany Aubry - Chief Technical Officer-Director: responsible for direct operation and management of the application of all contracts; directly involved in management of all functional groups and projects; responsible for plant maintenance and facility expansion;
Martin Gaudette - head of the Biosynthesis and Bioprocess Engineering group; project leader for the L’Oreal Phase 1 project; assists in building maintenance..
Steve Fiset - heads the Bioprocess Scale-up group; responsible for all IT applications at MH-B; assists the Biosynthesis and Bioprocess Engineering group.
Luc Lavoie - head of the Purification group; involved in operation and maintenance of all analytical equipment.
Yvan Chapdelaine – head of the plant biology group; Project Leader for the Pierre Fabre Phase1 project; responsible for all Genetic applications at MH-B.
Chantal Paquin – controller; responsible for all Human Resources; directly involve in financial management at MH-B.
Sophie Roy – works in the Plant Biology group; responsible for maintenance of bank of cell lines.
Francois Harrison – Chemist - works in the Purification group; responsible for the extraction, separation and purification of active compounds assists in the drug discovery efforts;
In addition, we have access to additional clerical services. None of our employees belongs to a union and we believe that our relations with our employees are good. We know of no conflicts of interest between any of our officers and us. We have entered into a work contract with each employee to the exception of Mr. Leonard Stella, our CEO.
DESCRIPTION OF PROPERTY
We lease our corporate offices at 500, Cartier boulevard West, 4th floor, Laval, Quebec at a monthly rate of $50,000 per month rental, excluding office expenses, pursuant to the terms of a month-to-month lease commencing Ferbruary 9, 2006.
39
LEGAL PROCEEDINGS
We are not currently party to any material legal proceedings.
MATERIAL AGREEMENTS
On March 2, 2006, we entered into a consulting agreement with 9111-9081 Quebec Inc. for it to find suitable partners for the financing, licensing or selling of MH-B’s technologies and/or MH-B’s research programs.
We have entered into a number of employment agreements with our employees We have attached the key employment agreements of our 4 officers to the SB-2 Registration Statement as exhibits.
40
MANAGEMENT
DIRECTORS AND EXECUTIVE OFFICERS
The following table sets forth information about our executive officers and directors.
| | | | | |
Name | | Age | | Position | |
| |
| |
| |
| | | | | |
Leonard Stella | | 44 | | Chief Executive Officer and Director
(Principal Financial Officer) | |
| | | | | |
Jean Archambault | | 53 | | President, Chief Operating Officer and Director | |
Bahige Baroudy | | 54 | | Chief Science Officer and Director | |
Dany Aubry | | 35 | | Chief Technical Officer and Director | |
Robert Williams | | 43 | | Senior Science Officer and Director | |
Set forth below is certain biographical information regarding our executive officers and directors:
The above listed officers and directors will serve until the next annual meeting of the shareholders or disqualification, or until their successors have been duly elected and qualified. Vacancies in the existing Board of Directors are filled by majority vote of the remaining Directors. Officers of the Company serve at the will of the Board of Directors. To our knowledge, there are no agreements or understandings for any officer or director to resign at the request of another person nor is any officer or director acting on behalf of or is to act at the direction of any other person other than in his fiduciary capacity of and for the benefit of us and at our direction.
Mr. Leonard Stella, CEO and Director
Mr. Stella has a Bachelor of Arts from McGill University, and received his Graduate Diploma in Administration from Concordia University in 1986. In 1987 Mr. Stella founded and operated a residential and commercial property developer, Dominion City Development and in 1991, he founded Trans-Immobilia, a residential property company. In 1997 he became the principal founding partner and is now the Chief Executive Officer of Millenia Hope Inc., the parent company of MH-B. Mr. Stella’s strategic vision, which guides Millenia Hope Inc., led to the acquisition of MH-B by Millenia in February 2006.
Jean Archambault, Ph.D., MBA- President and COO
Dr. Archambault was the founder, and served as President and CEO, of Avance Pharma (formally also know as Phytobiotech Inc.) from 1997 to 2005, having also served during that period as a Director after the company’s inception in 1997. Dr. Archambault directed the development of Avance’s Phytomics Technologies. Prior to Avance, Dr. Archambault was a professor and head of the Chemical Engineering Department at theUniversité du Québec à Trois-Rivières (1991-1999) and an associate professor at theÉcole Polytechnique de Montréal (1993-1999). From 1987 to 1991, Dr. Archambault was Head of the Cell Culture Group at the National Research Council of Canada’s Biotechnology Research Institute in Montreal, where he developed and directed all mammalian, insect and plant cell culture bioengineering R&D activities. From 1973 to 1983, Dr. Archambault held various engineering and management positions in industry. He received his Ph.D. (1988) from McGill University in Biochemical Engineering. His MBA was also obtained from McGill University in 1981.
Dr. Bahige Baroudy – Chief Science Officer and Director
Dr. Baroudy joined Avance Pharma in early 2004 (of which the equipment and intellectual property were recently acquired by our subsidiary, Millenia Hope Biopharma) and was responsible for leading the company’s drug discovery efforts. He has 25 years of industry/academic experience in basic research, infectious disease drug discovery and virology. Prior to joining Avance, Dr. Baroudy was Group Director, Antiviral and Antimicrobial Therapy at the Schering-Plough Research Institute (Kenilworth, New Jersey). While at the Schering Plough Research Institute, Dr. Baroudy spearheaded the successful development of CCR5 antagonists that are currently in human clinical trials as HIV entry inhibitors. Dr. Baroudy earned a place on “The Scientific American 50” list as the top Research Leader of 2003 in the Medical Treatment category for this contribution. Prior to joining Schering-Plough, Dr. Baroudy was Director, Division of Molecular Virology at the James N. Gamble Institute of Medical Research from 1989 to 1995, where he established research programs in viral hepatitis and liver diseases, HIV/AIDS and vaccinia virus expression and pathogenesis. From 1985 to 1989, Dr. Baroudy was Research Associate Professor in the Division of Molecular Virology and Immunology at Georgetown University. From 1979 to 1985, Dr. Baroudy worked at the NIH in the Laboratory of
41
Infectious Diseases, the Laboratory of Molecular Oncology and the Laboratory of Biology of Viruses. Dr Baroudy received his Ph.D. in Biochemistry from Georgetown University.
Dany Aubry, Chief Technical Officer and Director.
Mr. Aubry received his M.Sc. in bioengineering fromÉcole Polytechnique de Montréal in 1995 and his B.Sc. in Chemical Engineering from theUniversité du Québec à Trois-Rivières (UQTR) in 1993. Mr. Aubry was the Director of Operations at Avance Pharma from 2002 to 2005, overseeing all operational aspects of its Phytomics Technologies including the R&D programs, cell line generation, biosynthesis and purification of phytochemicals, scale-up of bioprocesses, setting up of laboratory facilities, as well as control of the supply chain for drug discovery activities. In total, Mr. Aubry has 13 years of direct experience in developing plant cell culture bioprocesses, having acted as the leader of the biosynthesis group from 2000 to 2002 at Avance, following his tenure as a bioprocess engineer from 1995 to 2000. His expertise in plant cell cultures covers production of secondary metabolites, somatic embryo andEndomycorrhiza production, as well bioreactor design and bioprocess control.
Robert D. Williams, Ph.D.- Chief Development Officer and Director
Dr. Williams received his B.Sc. Honours in Chemistry from Acadia University in 1984 investigating “Inhibitors of ß-galactosidase”. He then went on to obtain his Ph.D. in Biochemistry in 1990 from the University of Guelph, under the direction of Dr. Brian Ellis for his investigations on “Alkaloids from normal and Ri transformed tissues ofPapaver somniferum”. From there he then spent two years at the Biotechnology Research Institute in Montreal working with Dr. Jean Archambault on plant cell culture technology. He subsequently moved toÉcole Polytechnique de Montréal and worked on a variety of projects in the area of plant cell culture on which he has published numerous papers. As one of the cofounders of Avance Pharma, he had been instrumental since 1997 in the development of its cell culture facility and in all aspects of the generation of its proprietary library of purified fractions. Dr. Williams has extensive cell culture expertise for the generation of cell lines from selected plant species.
Committees
We have no standing audit, nominating and compensating committees of the Board of Directors or committees performing similar functions. Under the Sarbanes-Oxley Act of 2002, each public company is required to have an audit committee consisting solely of independent directors and to explain whether or not any independent director is a financial expert. In the event the public company does not have an audit committee, the Board of Directors becomes charged with the duties of the audit committee. We intend to adopt such a committee upon the acceptance of the present Registration by the Securities Exchange Commission. In the event we are successful in the future in obtaining independent directors to serve on the Board of Directors and on a newly formed audit committee, of which there can be no assurances given, the Board of Directors would first adopt a written charter. Such charter would be expected to include, among other things:
- annually reviewing and reassessing the adequacy of the committees formal charter;
- reviewing the annual audited financial statements with the adequacy of its internal accounting controls;
- reviewing analyses prepared by our management and independent auditors concerning significant financial reporting issues and judgments made in connection with the preparation of its financial statements;
- being directly responsible for the appointment, compensation and oversight of the independent auditor, which shall report directly to the Audit Committee, including resolution of disagreements between management and the auditors regarding financial reporting for the purpose of preparing or issuing an audit report or related work;
- reviewing the independence of the independent auditors;
- reviewing our auditing and accounting principles and practices with the independent auditors and reviewing major changes to our auditing and accounting principles and practices as suggested by the independent auditor or its management;
- reviewing all related party transactions on an ongoing basis for potential conflict of interest situations; and
- all responsibilities given to the Audit Committee by virtue of the Sarbanes-Oxley Act of 2002.
42
Code of Ethics
Effective March 3, 2003, the Securities and Exchange Commission requires registrants like us to either adopt a code of ethics that applies to our Chief Executive Officer and Chief Financial Officer or explain why we have not adopted such a code of ethics. For purposes of item 406 of Regulations S-K, the term “code of ethics” means written standards that are reasonably designed to deter wrong doing and to promote:
- Honest and ethical conduct, including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships:
- Full, flair, accurate, timely and understandable disclosure in reports and documents that the company files with, or submits to, the Securities & Exchange Commission and in other public communications made by us;
- Compliance with applicable governmental law, rules and regulations;
- The prompt internal reporting of violations of the code to an appropriate person or persons identified in the code; and
- Accountability for adherence to the code.
Upon acceptance of the present Registration, we will adopt the aforementioned Code of Ethics.
43
EXECUTIVE COMPENSATION
Compensation of Executive Officers
Summary Compensation Table. The following information relates to compensation received by our officers in fiscal years ending November 30, 2005, 2004, 2003 whose salary and compensation exceeded $100,000.
SUMMARY COMPENSATION TABLE
| | | | | | | | | | | | | | | | | | | |
| | Annual Compensation | | Long-Term Compensation | |
| |
| |
| |
Name and Principal Position | | Fiscal Year | | Salary | | Bonus | | Other Annual
Compensation | | Restricted
Stock
Award(s) | | Securities
Underlying
Options | |
Leonard Stella (1) | | | 2005 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
Chief Executive Officer | | | 2004 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
| | | 2003 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
| | | | | | | | | | | | | | | | | | | |
Jean Archambault (2) | | | 2005 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
President | | | 2004 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
| | | 2003 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
| | | | | | | | | | | | | | | | | | | |
Bahige Baroudy (3) | | | 2005 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
Chief Science Officer | | | 2004 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
| | | 2003 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
| | | | | | | | | | | | | | | | | | | |
Dany Aubry (4) | | | 2005 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
Chief Technical Officerr | | | 2004 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
| | | 2003 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
| | | | | | | | | | | | | | | | | | | |
Robert Williams (5) | | | 2005 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
Chief Development Officer | | | 2004 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
| | | 2003 | | | 0 | | | 0 | | | 0 | | | 0 | | | 0 | |
44
For the fiscal year ended November 30, 2006 (December 1, 2005 – May 31, 2006)
| |
(1) | Mr. Stella received $0 in compensation |
| |
(2) | Dr. Archambault received $26,250 in compensation |
| |
(3) | Dr. Baroudy received $35,000 in compensation |
| |
(4) | Mr. Aubry received $19,395 in compensation |
| |
(5) | Dr. Williams received $19,395 in compensation |
Option Grants Table. The following table sets forth information concerning individual grants of stock options to purchase our common stock made to the executive officer named in the Summary Compensation Table during fiscal year ended November 30, 2005.
OPTIONS GRANTS IN PRESENT FISCAL YEAR (Individual Grants)
| | | | | | | | |
Name | | Number of securities
underlying options
granted (#) | | Percent of total
options granted to
employees in last
fiscal year | | Exercise or base
Price ($/Share) | | Expiration Date |
None
AGGREGATED OPTION EXERCISES IN LAST FISCAL YEAR AND FISCAL YEAR-END OPTION VALUES
Aggregated Option Exercises and Fiscal Year-End Option Value Table. The following table sets forth certain information regarding stock options exercised during fiscal year ending November 30, 2005, by the executive officer named in the Summary Compensation Table.
| | | | | | | | |
Name | | Shares acquired on
exercise (#) | | Value realized ($) | | Number of Securities
Underlying Unexercised
Options at Fiscal
Year-End(#) | | Value of Unexercised
In-the-Money Options at
Fiscal Year-
End($)(1) |
| | | | | |
| |
|
| | | | | | Exercisable/ Unexercisable | | Exercisable/
Unexercisable |
None
Employment Contracts
We have entered into employment contracts with our employees and key personnel, with the exception of Mr. Leonard Stella our Chief Executive Officer.
Compensation of Directors
No director receives any compensation for serving on our Board of Directors.
45
PRINCIPAL STOCKHOLDERS
The following table sets forth certain information derived from the named person, or from the transfer agent, concerning the ownership of common stock as of September 5, 2006, of (i) each person who is known to us to be the beneficial owner of more than 5 percent of the common stock; (ii) all directors and executive officers; and (iii) directors and executive officers as a group:
| | | | |
Name and Address of
Beneficial Owner | | Amount and Nature of
Beneficial Ownership | | Percent of
Class (1) |
| | | | |
Millenia Hope Inc. (2)
1250 Rene Levesque W, suite 2200
Montreal, Quebec, CANADA | | 30,008,000 | | 75% |
| | | | |
2632-3345 Quebec Inc.(3)
3400 Souvenir, suite 320
Laval, Quebec Canada | | 7,592,000 | | 19% |
| |
(1) | Based on 40,000,000 issued and outstanding shares as of September 5, 2006. |
| |
(2) | Millenia Hope, Inc. is a 1934 Exchange Act company. For information on its shareholdings, specifically its principal shareholders, please refer to its 1934 Exchange Act filings listed on the SEC’s website atwww.sec.gov. |
| |
(3) | Paolo Mori, President of 2632-3345 Quebec Inc. |
SELLING STOCKHOLDERS
| | | | | | |
Name of Selling
Stockholder | | Shares Beneficially owned
Before offering | | Offering | | After offering |
| | | | | | |
2632-3345 Quebec Inc. | | 7,592,000 | | 7,592,000 | | 0 |
| | | | | | |
Cede& Co. (1) | | 599,910 | | 599,910 | | 0 |
|
(1) Reflects shares held by Cede & Co. in “street name” | | | | | | |
|
GEORGES ADAM | | 439 | | 439 | | 0 |
GEORGES ADAMS | | 3 003 | | 3 003 | | 0 |
CHARLES ALBANO | | 176 | | 176 | | 0 |
FRANCESCA ALBANO | | 9 871 | | 9 871 | | 0 |
AFTAB AL HASSAN | | 5 272 | | 5 272 | | 0 |
46
| | | | | | |
ANNA ALLAN | | 141 | | 141 | | 0 |
|
ALL SAFE LLC | | 4 394 | | 4 394 | | 0 |
|
ISABEL ALVES | | 26 | | 26 | | 0 |
|
AMBROSIA CORP | | 83 740 | | 83 740 | | 0 |
RAYMOND ANDERSON | | 18 | | 18 | | 0 |
|
ARTURO ANIEVAS | | 1 582 | | 1 582 | | 0 |
ARTURO ANIEVAS CUST -ALEXANDER ANIEVAS UNDER | | 176 | | 176 | | 0 |
ANTHONY ARCURI | | 439 | | 439 | | 0 |
|
DOMENIC ARCURI | | 879 | | 879 | | 0 |
ARIKEA INSULUM SOCIEDAD ANONIMA | | 1 757 | | 1 757 | | 0 |
ISABELLE ARNOLD | | 88 | | 88 | | 0 |
ANNE-MARGOT ARSENAULT | | 18 | | 18 | | 0 |
FRANCINE ARSENAULT | | 30 | | 30 | | 0 |
GEORGES ALEXANDRE ARSENAULT | | 18 | | 18 | | 0 |
GILLES ARSENAULT | | 301 | | 301 | | 0 |
LEONARD ARSENAULT | | 282 | | 282 | | 0 |
RAYMOND ARSENAULT | | 60 | | 60 | | 0 |
ROLANDE ARSENAULT | | 36 | | 36 | | 0 |
|
ASPAC BIOMARK | | 68 539 | | 68 539 | | 0 |
|
SALEHI ASSAD | | 264 | | 264 | | 0 |
BARINDER ATHWAL | | 837 | | 837 | | 0 |
|
JACQUES AUBRY | | 35 | | 35 | | 0 |
|
JOHN AXMIT | | 4 | | 4 | | 0 |
ANIL BADABOUDINE | | 60 | | 60 | | 0 |
|
ABDOULAYE BAH | | 2 636 | | 2 636 | | 0 |
BRUNO BALLARANO | | 123 | | 123 | | 0 |
|
RENE BANDET | | 457 | | 457 | | 0 |
47
| | | | | | |
NGUYEN MINH NGOC BAO | | 527 | | 527 | | 0 |
|
LYNE BARRY | | 12 | | 12 | | 0 |
|
JEAN BASTIEN | | 376 | | 376 | | 0 |
JEAN FRANCOIS BASTIEN | | 397 | | 397 | | 0 |
|
JULIE BASTIEN | | 12 | | 12 | | 0 |
MARTINE BASTIEN | | 18 | | 18 | | 0 |
SERGE BEAUDOIN | | 420 | | 420 | | 0 |
ALAIN BEAUREGARD | | 4 | | 4 | | 0 |
MARCEL BEAUDRY | | 53 | | 53 | | 0 |
MARC BEAUREGARD | | 35 | | 35 | | 0 |
STEPHANE BEAUREGARD | | 4 | | 4 | | 0 |
|
ANDRE BEAULIEU | | 289 | | 289 | | 0 |
MARC BEAUSEJOUR | | 313 | | 313 | | 0 |
|
PATRICK BEDARD | | 5 859 | | 5 859 | | 0 |
ALICE BEDROSSIAN | | 88 | | 88 | | 0 |
MICHEL BEDROSSIAN | | 53 | | 53 | | 0 |
JEAN PHILIPPE BEGIN | | 35 | | 35 | | 0 |
FLORIAN BELHUMEUR | | 1 757 | | 1 757 | | 0 |
|
ENZO BELLANCA | | 70 | | 70 | | 0 |
MESSOD BENDAYAN | | 18 | | 18 | | 0 |
DANIEL BENJAFIELD | | 18 | | 18 | | 0 |
|
CLEMENT BENOIT | | 301 | | 301 | | 0 |
GILLES BENOIT | | 120 | | 120 | | 0 |
|
VIOLETTA BENOIT | | 241 | | 241 | | 0 |
CHRISTIAN BERGERON | | 879 | | 879 | | 0 |
NELSON GERGERON | | 241 | | 241 | | 0 |
|
GILLES BERNARD | | 145 | | 145 | | 0 |
|
JOEL BERNIER | | 169 | | 169 | | 0 |
THERESE BERNIER | | 24 | | 24 | | 0 |
48
| | | | | | |
FREDERIC BERTOLDI | | 351 | | 351 | | 0 |
AMIR ALI BHOJANI | | 30 | | 30 | | 0 |
|
GEORGE BINET | | 4 921 | | 4 921 | | 0 |
|
BIOMED PHARMA | | 5 805 | | 5 805 | | 0 |
BIO-T CONSULTANTS INC | | 93 028 | | 93 028 | | 0 |
ANDRE BISSONNETTE | | 281 | | 281 | | 0 |
|
JOHANNE BLAIS | | 67 | | 67 | | 0 |
|
RICHARD BLAIS | | 296 | | 296 | | 0 |
DAVID WAYNE BLANCHETTE | | 20 | | 20 | | 0 |
DENIS BLAQUIERE | | 1 648 | | 1 648 | | 0 |
|
FRANCE BONIN | | 96 | | 96 | | 0 |
LAURETTE BORDUAS | | 36 | | 36 | | 0 |
|
LOUISE BORYS | | 95 | | 95 | | 0 |
|
WALTER BORYS | | 60 | | 60 | | 0 |
|
CLAUDE BOSSE | | 5 272 | | 5 272 | | 0 |
|
GINO BOSSIO | | 203 | | 203 | | 0 |
JOCELYNE BOUCHER | | 120 | | 120 | | 0 |
STEPHANE BOUCHER | | 12 | | 12 | | 0 |
BEVERLY BOUDREAULT | | 88 | | 88 | | 0 |
BOB BOUDREAULT | | 88 | | 88 | | 0 |
|
DENIS BOUDRIAS | | 24 | | 24 | | 0 |
|
SERGE BOULAIS | | 234 | | 234 | | 0 |
FRANCINE BOULERICE | | 24 | | 24 | | 0 |
JACQUES BOURBONNAIS | | 1 054 | | 1 054 | | 0 |
GERALD BOURDON | | 398 | | 398 | | 0 |
ROLANDE BOURDON-CARBONNEAU | | 24 | | 24 | | 0 |
ROLLANDECARBONEAU-BOURDON | | 24 | | 24 | | 0 |
DAVID BOURNE | | 18 | | 18 | | 0 |
49
| | | | | | |
PATRICIA BOURNE | | 18 | | 18 | | 0 |
FRANCOIS BOURRET | | 602 | | 602 | | 0 |
MICHEL BOUTIN &CHRISTIANE NOLET JT TEN | | 123 | | 123 | | 0 |
|
RAOUL BOUVIN | | 125 | | 125 | | 0 |
|
ALAIN BOYER | | 12 | | 12 | | 0 |
JOCELYNE BRASSARD | | 5 | | 5 | | 0 |
|
LUCIE BRASSARD | | 30 | | 30 | | 0 |
|
BRIGITTE BRIE | | 60 | | 60 | | 0 |
|
BENOIT BRIERE | | 481 | | 481 | | 0 |
|
SYLVAIN BRIERE | | 823 | | 823 | | 0 |
RICHARD BRODMAN | | 1 098 | | 1 098 | | 0 |
|
LORNE BROTMAN | | 176 | | 176 | | 0 |
MAXWELL BROTMAN | | 619 | | 619 | | 0 |
STACEY BROTMAN | | 176 | | 176 | | 0 |
PIERRE BRUNELLE | | 60 | | 60 | | 0 |
JOCELYNE BRUNET | | 74 | | 74 | | 0 |
RINO BUFFONE & ANTONIO DI VINCENZO JTTEN | | 239 | | 239 | | 0 |
|
JOE T BUSBY | | 35 | | 35 | | 0 |
|
DORIS BUSQUE | | 181 | | 181 | | 0 |
JACINTHE BUSQUE | | 60 | | 60 | | 0 |
MARGARET BYWATERS | | 2 636 | | 2 636 | | 0 |
M JOSE CABELLOS | | 70 | | 70 | | 0 |
CLAUDE CADORETTE | | 12 | | 12 | | 0 |
|
RICHARD CAHILL | | 18 927 | | 18 927 | | 0 |
|
PIETRO CALCARA | | 49 | | 49 | | 0 |
128413 CANADA LTD | | 176 | | 176 | | 0 |
4049870 CANADA INC | | 2 636 | | 2 636 | | 0 |
50
| | | | | | |
4170059 CANADA INC | | 17 802 | | 17 802 | | 0 |
4220-1116 CANADA INC | | 3 515 | | 3 515 | | 0 |
4291034 CANADA INC | | 29 876 | | 29 876 | | 0 |
ANGELA CAPURSO | | 3 368 | | 3 368 | | 0 |
CHRISTIAN CARDINAL | | 12 | | 12 | | 0 |
ANTONIO CARNEVALE | | 1 757 | | 1 757 | | 0 |
MARCEL CARPENTIER | | 176 | | 176 | | 0 |
|
PETER CARR | | 18 | | 18 | | 0 |
|
JOE CARUSO | | 62 | | 62 | | 0 |
|
GAIL CASEY | | 4 | | 4 | | 0 |
LEONARDO CASTIELLO | | 1 494 | | 1 494 | | 0 |
ANTONIO CASTILIANO | | 365 | | 365 | | 0 |
GIUSEPPE CASTIGLIONE | | 19 106 | | 19 106 | | 0 |
|
SOFIA CATALINI | | 165 | | 165 | | 0 |
ROSS CATION | | 4 | | 4 | | 0 |
|
GEORGE CATTAN | | 120 | | 120 | | 0 |
DARREN CAVANAGH | | 176 | | 176 | | 0 |
|
FRANK CAVANAGH | | 176 | | 176 | | 0 |
JACQUES CAYER | | 53 | | 53 | | 0 |
GEORGES CHALMERS | | 2 | | 2 | | 0 |
FREDERICK CHAMPAGNE | | 808 | | 808 | | 0 |
NICOLAS CHAMPAGNE | | 808 | | 808 | | 0 |
ALEXANDRE CHAPDELAINE | | 72 | | 72 | | 0 |
CARINE CHAPDELAINE | | 72 | | 72 | | 0 |
JULES CHAPDELAINE | | 120 | | 120 | | 0 |
SUZANNE CHAPDELAINE | | 241 | | 241 | | 0 |
|
ANDRE CHAREST | | 72 | | 72 | | 0 |
MARCH CHARRETTE | | 86 | | 86 | | 0 |
LANGIS CHARRON | | 241 | | 241 | | 0 |
51
| | | | | | |
ROBERT CHELHOT | | 120 | | 120 | | 0 |
|
JOHN CHESNUTT | | 4 | | 4 | | 0 |
STEVEN CHESNUTT | | 4 | | 4 | | 0 |
JACQUES CHIASSON | | 181 | | 181 | | 0 |
|
DENIS CHICOINE | | 202 | | 202 | | 0 |
JEAN PIERRE CHICOINE | | 120 | | 120 | | 0 |
|
JULES CHICOINE | | 602 | | 602 | | 0 |
|
DAVID CHITYAL | | 2 197 | | 2 197 | | 0 |
PERRY CHOINIERE | | 13 181 | | 13 181 | | 0 |
NBCN CLEARING INC FBO -PERRY CHOINIERE 1AVFGYA | | 703 | | 703 | | 0 |
SYLVAIN CHOINIERE | | 293 | | 293 | | 0 |
JUSTINE CHOLETTE | | 313 | | 313 | | 0 |
MICHEL CHOLETTE | | 1 493 | | 1 493 | | 0 |
PHILIPPE CHOLETTE | | 60 | | 60 | | 0 |
MICHEL CHOQUETTE | | 11 | | 11 | | 0 |
CHRISTOS CHRISTOU | | 780 | | 780 | | 0 |
STEPHANE CHRETIEN | | 176 | | 176 | | 0 |
|
SORIBA CISSE | | 6 063 | | 6 063 | | 0 |
|
CLOMAX INC | | 59 | | 59 | | 0 |
|
LINDA COALLIER | | 18 | | 18 | | 0 |
RAFFAEL COBUZZI | | 36 | | 36 | | 0 |
|
THOMAS COFFEY | | 98 | | 98 | | 0 |
|
RONIN COHEN | | 26 | | 26 | | 0 |
|
RENE COMPTOIS | | 120 | | 120 | | 0 |
|
DENISE COMTOIS | | 60 | | 60 | | 0 |
ROBERT COMTOIS | | 84 | | 84 | | 0 |
RODNEY COOMER | | 2 | | 2 | | 0 |
CHANTAL CORBEIL | | 18 | | 18 | | 0 |
52
| | | | | | |
MYLENE CORBEIL | | 18 | | 18 | | 0 |
SUZANNE CORMIER | | 32 | | 32 | | 0 |
ANDRE CORRIVEAU | | 96 | | 96 | | 0 |
ADRIEN CORRIVEAU | | 602 | | 602 | | 0 |
GISELLE CORRIVEAU | | 87 | | 87 | | 0 |
|
BRIAN COSTELLO | | 1 757 | | 1 757 | | 0 |
BRIAN COSTELLO JR | | 1 757 | | 1 757 | | 0 |
BERTHE BLANCHETTE COTE | | 61 | | 61 | | 0 |
|
CLAIRE COTE | | 60 | | 60 | | 0 |
|
JEAN MARC COTE | | 189 | | 189 | | 0 |
|
DAVID COULL | | 148 | | 148 | | 0 |
|
JOAN COULL | | 148 | | 148 | | 0 |
CELINE COULOMBE | | 181 | | 181 | | 0 |
SOLANGE COULOMBE | | 60 | | 60 | | 0 |
ALAIN COUSINEAU | | 527 | | 527 | | 0 |
|
LYNE COUTURE | | 176 | | 176 | | 0 |
CREATION PUBLICITAIRES INC | | 120 | | 120 | | 0 |
SYLVANO CRIVELLO | | 89 | | 89 | | 0 |
|
DIANE CULLEN | | 293 | | 293 | | 0 |
|
FRANK CURTI | | 26 | | 26 | | 0 |
|
EDWARD CUTLER | | 967 | | 967 | | 0 |
|
YVES CYR | | 120 | | 120 | | 0 |
PIERRE DAGENAIS | | 264 | | 264 | | 0 |
BERNARD DALLAIRE | | 18 | | 18 | | 0 |
FILOMENA D’ANDREA | | 239 | | 239 | | 0 |
|
JOSEPH DANIELE | | 12 571 | | 12 571 | | 0 |
GIUSEPPE DANIELLE | | 17 574 | | 17 574 | | 0 |
CHANTAL DANSEREAU | | 60 | | 60 | | 0 |
53
| | | | | | |
NGUYEN THI DAO | | 8 787 | | 8 787 | | 0 |
|
BENOIT DAOUST | | 24 | | 24 | | 0 |
|
DIANE DAOUST | | 60 | | 60 | | 0 |
CHRISTIAN DAVIAU | | 1 757 | | 1 757 | | 0 |
|
LAILA DEBS | | 60 | | 60 | | 0 |
ANNA MARIA DEL BELLO | | 527 | | 527 | | 0 |
FRANK DEL BELLO | | 4 394 | | 4 394 | | 0 |
|
NINO DEL BELLO | | 4 394 | | 4 394 | | 0 |
|
PASCAL DELCY | | 264 | | 264 | | 0 |
|
MANON DELISLE | | 12 | | 12 | | 0 |
|
BRUNO DELORME | | 24 | | 24 | | 0 |
JACQUES DELORME | | 351 | | 351 | | 0 |
FILOMENA DELUCA | | 105 | | 105 | | 0 |
ANGELO DE LUCIA | | 105 | | 105 | | 0 |
JACQUES DENONCOURT | | 18 | | 18 | | 0 |
|
JOHANNE DEPOT | | 60 | | 60 | | 0 |
JEAN GERALD DESIRE | | 2 724 | | 2 724 | | 0 |
MARIO DESJARDINS | | 62 | | 62 | | 0 |
MANON DESROSIERS | | 120 | | 120 | | 0 |
|
FRANK DIACO | | 207 | | 207 | | 0 |
|
J DIAVATIS | | 70 | | 70 | | 0 |
KENNETH DI BREWIN | | 228 | | 228 | | 0 |
LUCIANO DI MARCO | | 615 | | 615 | | 0 |
ROBERTO DI MARCO | | 615 | | 615 | | 0 |
|
BICH VAN DINH | | 5 272 | | 5 272 | | 0 |
|
LOISEL DIOGENE | | 12 | | 12 | | 0 |
CHRISTIAN DIONNE | | 24 | | 24 | | 0 |
AURORA DI PAOLA | | 18 | | 18 | | 0 |
ANTONIO DI VINCENZO | | 214 | | 214 | | 0 |
54
| | | | | | |
DM INVESTMENTS | | 35 148 | | 35 148 | | 0 |
MOHAMMED DOCRAT | | 45 | | 45 | | 0 |
DIETER DOEDERLINE | | 4 | | 4 | | 0 |
CHRISTIAN DONATO | | 15 | | 15 | | 0 |
|
DANIEL DONAVAN | | 351 | | 351 | | 0 |
|
TOM DOUGLAS | | 72 | | 72 | | 0 |
|
SID DRAPKIN | | 88 | | 88 | | 0 |
|
ERIC DROUIN | | 181 | | 181 | | 0 |
|
FANNY DUBUC | | 12 | | 12 | | 0 |
|
ROBERT DUCA | | 176 | | 176 | | 0 |
GREGORY DUCOS | | 28 | | 28 | | 0 |
|
SYLVIE DUFAUX | | 24 | | 24 | | 0 |
JEREMIE DUFOUR | | 3 515 | | 3 515 | | 0 |
|
REAL DUFOUR | | 879 | | 879 | | 0 |
JACQUELINE DUHAMEL | | 24 | | 24 | | 0 |
|
MARIO DUMAIS | | 1 054 | | 1 054 | | 0 |
JEAN-MARIE DUMESNIL | | 248 | | 248 | | 0 |
|
GIZELLE DUMONT | | 120 | | 120 | | 0 |
|
JESSICA DUMONT | | 88 | | 88 | | 0 |
JOSIANE DUMONT | | 88 | | 88 | | 0 |
|
LOUISE DUMONT | | 12 | | 12 | | 0 |
MARC ANDRE DUMONT | | 30 | | 30 | | 0 |
TRUONG VAN DUNG | | 176 | | 176 | | 0 |
|
ANDRE EMOND | | 60 | | 60 | | 0 |
|
DENIS EMOND | | 5 861 | | 5 861 | | 0 |
|
GIULIANO ERCOLI | | 25 | | 25 | | 0 |
|
SYLVAIN ETHIER | | 241 | | 241 | | 0 |
GUY FAFARD &MICHEL FAFARD JT TEN | | 72 | | 72 | | 0 |
55
| | | | | | |
FENCO | | 1 757 | | 1 757 | | 0 |
FENCO ASSOCIATES | | 2 074 | | 2 074 | | 0 |
JEAN-PIERRE FERLAND | | 1 318 | | 1 318 | | 0 |
ROBERT FERRARO | | 176 | | 176 | | 0 |
|
FIDUCIE S.A.M. | | 14 059 | | 14 059 | | 0 |
|
COLLETTE FILION | | 53 | | 53 | | 0 |
|
COLETTE FILLION | | 16 | | 16 | | 0 |
ROBERT FITZGERALD | | 2 | | 2 | | 0 |
LEDO. RAFAEL COLON FLORES | | 88 | | 88 | | 0 |
FONDATION JEUNESSE IN’AFU | | 249 | | 249 | | 0 |
|
LES FORD | | 2 | | 2 | | 0 |
ALEXANDRE FORTIER | | 60 | | 60 | | 0 |
|
DANY FORTIER | | 30 | | 30 | | 0 |
GUY FORTIN | | 989 | | 989 | | 0 |
|
MARIO FORTIER | | 241 | | 241 | | 0 |
|
CHANTAL FORTIN | | 60 | | 60 | | 0 |
|
FRANCE FORTIN | | 12 | | 12 | | 0 |
|
GUY FORTIN | | 717 | | 717 | | 0 |
DONALD FRANCES | | 2 | | 2 | | 0 |
MARCO FRAPPIER | | 5 887 | | 5 887 | | 0 |
BRIGITTE FREGAULT | | 1 172 | | 1 172 | | 0 |
NBCN CLEARING INC FBO- DOMINIQUE FREGAULT | | 908 | | 908 | | 0 |
FRANCOIS FREGAULT | | 60 | | 60 | | 0 |
GINETTE FREGAULT | | 908 | | 908 | | 0 |
MARC-ANDRE FREGAULT | | 908 | | 908 | | 0 |
PATRICIA FRONTEIRA | | 70 | | 70 | | 0 |
|
JOHN FUOCO | | 204 | | 204 | | 0 |
|
MICHEL GAGNE | | 24 | | 24 | | 0 |
56
| | | | | | |
ANDRE GAGNON | | 176 | | 176 | | 0 |
GEORGES GAGNON | | 60 | | 60 | | 0 |
|
RICHER GAGNON | | 60 | | 60 | | 0 |
FRANCES H GAJANO | | 124 | | 124 | | 0 |
PATRICK GALLAME | | 84 | | 84 | | 0 |
GARDERIE LA BASCULE INC | | 301 | | 301 | | 0 |
|
DAVID GAUDETTE | | 1 054 | | 1 054 | | 0 |
GILLES GAUDETTE | | 785 | | 785 | | 0 |
STEPHANIE GAUDETTE | | 12 | | 12 | | 0 |
FRANCE GAUTHIER | | 4 394 | | 4 394 | | 0 |
MAURICE GAUTHIER | | 589 | | 589 | | 0 |
GDCI CAPITAL INC | | 7 820 | | 7 820 | | 0 |
CLAIRE GEOFFRION | | 70 | | 70 | | 0 |
GESTION & CONULTATION MP BEAUDOIN | | 24 | | 24 | | 0 |
GESTION LA SOLUTION LTD | | 9 666 | | 9 666 | | 0 |
GESTION SERBEAU INC | | 96 | | 96 | | 0 |
GESTION SYLVAIN TREMBLAY (1998) INC | | 289 | | 289 | | 0 |
|
CRISTINE GHAKIS | | 1 757 | | 1 757 | | 0 |
|
MIKE GHAKIS | | 5 272 | | 5 272 | | 0 |
|
PATRICK GHAKIS | | 1 757 | | 1 757 | | 0 |
|
CLAIRE GIARD | | 72 | | 72 | | 0 |
SELONKOUE FEIBONAZOUI GILBERT | | 879 | | 879 | | 0 |
MARTINE GINGRAS | | 14 | | 14 | | 0 |
|
DANIEL GINGUES | | 351 | | 351 | | 0 |
|
GABRIELE GIOBI | | 35 | | 35 | | 0 |
|
MAURIZIO GIOBI | | 118 | | 118 | | 0 |
|
HENRI GIRARD | | 35 | | 35 | | 0 |
57
| | | | | | |
CONSULTANT JEAN PIERRE GIRARD | | 35 | | 35 | | 0 |
|
PIERRE GIROUX | | 72 | | 72 | | 0 |
|
SYLVIE GIROUX | | 120 | | 120 | | 0 |
|
CECILE GODIN | | 18 | | 18 | | 0 |
MARY GOLDSMITH | | 9 | | 9 | | 0 |
MARIO GONCALVES | | 60 | | 60 | | 0 |
|
JACQUES GOYET | | 120 | | 120 | | 0 |
|
K C GRAINGER | | 4 077 | | 4 077 | | 0 |
KENNETH C GRAINGER | | 70 | | 70 | | 0 |
MARION GRAINGER | | 381 | | 381 | | 0 |
|
CLAUDE GRAVEL | | 351 | | 351 | | 0 |
|
LOUIS GRECO | | 6 151 | | 6 151 | | 0 |
GREEN PERFORMANCE S.A. | | 8 787 | | 8 787 | | 0 |
|
GIANNI GRIMALDI | | 167 | | 167 | | 0 |
VINCENZA GRIVAS | | 18 | | 18 | | 0 |
|
SABINE GROULX | | 12 | | 12 | | 0 |
|
VANESSA GUAY | | 439 | | 439 | | 0 |
|
MELISSA GUAY | | 1 318 | | 1 318 | | 0 |
CLEMENT GUERARD | | 120 | | 120 | | 0 |
ANGELE GUILBERT | | 24 | | 24 | | 0 |
LOUISE GUILBERT | | 12 | | 12 | | 0 |
RAYMOND GUITARD | | 12 | | 12 | | 0 |
GUYLAINE GRAVEL | | 60 | | 60 | | 0 |
|
J HAMMEL | | 879 | | 879 | | 0 |
|
JOHN K HAMMEL | | 351 | | 351 | | 0 |
MARIE FRANCE HARBEC | | 18 | | 18 | | 0 |
|
SERGE HARBEC | | 290 | | 290 | | 0 |
CHRISTINE HARVEY | | 602 | | 602 | | 0 |
58
| | | | | | |
COULOMBE HAYES | | 28 | | 28 | | 0 |
|
PHILIPPE HAYES | | 60 | | 60 | | 0 |
HERAN CAPITAL INC | | 439 | | 439 | | 0 |
|
TODD HESKELL | | 30 | | 30 | | 0 |
|
ANDRE HETU | | 30 | | 30 | | 0 |
JOHN HIGBINSON SR | | 2 | | 2 | | 0 |
|
LE LE HOANG | | 8 787 | | 8 787 | | 0 |
|
MEIKEN HOHMAN | | 4 | | 4 | | 0 |
|
RORY HOPE | | 2 | | 2 | | 0 |
|
TA NGOC HO | | 1 406 | | 1 406 | | 0 |
|
DENIS HOULD | | 7 | | 7 | | 0 |
|
WILLIAM HUM | | 60 | | 60 | | 0 |
HELEN HURTUBISE | | 53 | | 53 | | 0 |
THE HONG HUYNH | | 1 054 | | 1 054 | | 0 |
|
THE LANG HUYNH | | 1 054 | | 1 054 | | 0 |
|
THE LAY HUYNH | | 492 | | 492 | | 0 |
|
THE IEM HUYNH | | 12 302 | | 12 302 | | 0 |
THE NGHIEM HUYNH | | 4 657 | | 4 657 | | 0 |
|
TRI MINH HUYNH | | 8 523 | | 8 523 | | 0 |
|
LA HY | | 180 | | 180 | | 0 |
SALVATORE IAVARONE | | 235 | | 235 | | 0 |
ANTHONY IERFINO | | 8 787 | | 8 787 | | 0 |
ANTHONY IERFINO & LILLA MAGRI JT TEN | | 3 515 | | 3 515 | | 0 |
|
YAEL IFERGAN | | 22 | | 22 | | 0 |
|
AHMED IKBAL | | 60 | | 60 | | 0 |
INCHRAY INVESTMENTS LIMITED | | 1 582 | | 1 582 | | 0 |
INTERNATIONAL GLOBAL INVESTMENT | | 110 | | 110 | | 0 |
PATRICIA IOANNONI | | 58 | | 58 | | 0 |
59
| | | | | | |
FRANCE IPOTESI | | 132 | | 132 | | 0 |
|
NAILA IQBAL | | 15 | | 15 | | 0 |
LISETTE JACQUES | | 264 | | 264 | | 0 |
RVD. OMAR JAHEN | | 88 | | 88 | | 0 |
MARCELLE JARRY | | 120 | | 120 | | 0 |
SYLVAIN JOANNETTE | | 18 | | 18 | | 0 |
|
NAILA JOBAL | | 15 | | 15 | | 0 |
|
DYLAN JOFFRE | | 117 | | 117 | | 0 |
|
HAROLD JOFFRE | | 937 | | 937 | | 0 |
|
DAVID JONES | | 2 | | 2 | | 0 |
|
JPCV CAPITAL | | 35 148 | | 35 148 | | 0 |
|
ANDRE JULIEN | | 24 | | 24 | | 0 |
|
JOHN JURICHKO | | 141 | | 141 | | 0 |
|
HELENE JUTEAU | | 44 | | 44 | | 0 |
|
ROBERT JUTRAS | | 120 | | 120 | | 0 |
ZACHARY KARABATSOS | | 35 | | 35 | | 0 |
ANTHONY KAUFMAN | | 18 | | 18 | | 0 |
|
JOAN KAUFMAN | | 18 | | 18 | | 0 |
|
K-BRO LLC | | 4 394 | | 4 394 | | 0 |
RICHARD J KESSELL | | 9 | | 9 | | 0 |
MANA KEUSHGUERIAN | | 18 | | 18 | | 0 |
KEYS FAMILY PARTNERS LTD | | 65 024 | | 65 024 | | 0 |
TRUONG NHU KHANG | | 351 | | 351 | | 0 |
TRUONG NHU KHANH | | 2 460 | | 2 460 | | 0 |
KNOTTY PINE HOLDINGS INC | | 439 | | 439 | | 0 |
|
CAROLINE KOCH | | 176 | | 176 | | 0 |
|
BRANDON KOPS | | 474 | | 474 | | 0 |
|
YEHUDA KOPS | | 98 668 | | 98 668 | | 0 |
60
| | | | | | |
KENNETH KWAN | | 176 | | 176 | | 0 |
JOCELYN L’ABBEE | | 88 | | 88 | | 0 |
|
REAL L’ABBEE | | 18 | | 18 | | 0 |
FRANCOIS PIERRE LABERGE | | 1 406 | | 1 406 | | 0 |
|
BRUNO LABODIE | | 88 | | 88 | | 0 |
CAROLE LABRECQUE | | 235 | | 235 | | 0 |
GHISLAIN LABRECQUE | | 235 | | 235 | | 0 |
JEAN MARIE LABRECQUE | | 235 | | 235 | | 0 |
JEAN GUY LABRECQUE | | 7 618 | | 7 618 | | 0 |
|
LISE LABRECQUE | | 235 | | 235 | | 0 |
MARIUS LABRECQUE | | 235 | | 235 | | 0 |
MARIELLE LABRECQUE | | 235 | | 235 | | 0 |
MONIQUE LABRECQUE | | 235 | | 235 | | 0 |
NATHALIE LABRECQUE | | 120 | | 120 | | 0 |
YOLANDE LABRECQUE | | 235 | | 235 | | 0 |
JEREMIE LACASSE | | 12 | | 12 | | 0 |
DANIELLE LACROIX | | 42 | | 42 | | 0 |
|
DENIS LAFLAMME | | 48 | | 48 | | 0 |
FERNAND LAFLAMME | | 24 | | 24 | | 0 |
JEAN CLAUDE LAFLAMME | | 12 | | 12 | | 0 |
|
RENE LAFLAMME | | 351 | | 351 | | 0 |
SYLVAIN LAFLAMME | | 12 | | 12 | | 0 |
THERESE LAFLAMME | | 12 | | 12 | | 0 |
MICHELE LAFRANCOIS | | 88 | | 88 | | 0 |
DENNIS LAFRENIERE | | 2 | | 2 | | 0 |
|
PETER LAI | | 176 | | 176 | | 0 |
|
KARINE LALONDE | | 12 | | 12 | | 0 |
|
LIETTE LALONDE | | 12 | | 12 | | 0 |
ROBERT LAMARRE | | 48 | | 48 | | 0 |
61
| | | | | | |
YVON LAMBERT | | 24 | | 24 | | 0 |
MICHELE LAMOUREUX | | 60 | | 60 | | 0 |
|
FULLER LANDAU | | 32 | | 32 | | 0 |
|
BENOIT LANDRY | | 24 | | 24 | | 0 |
LANDRY PEPIN CONSTRUCTION INC | | 48 | | 48 | | 0 |
|
DANIEL LANDRY | | 821 | | 821 | | 0 |
|
DANIEL LANDRY | | 700 | | 700 | | 0 |
STEPHANE LANDRY | | 48 | | 48 | | 0 |
|
CLAUDE LAVIGNE | | 17 574 | | 17 574 | | 0 |
MARIE-JOSEE LANGEVIN | | 18 | | 18 | | 0 |
JEAN PIERRE LANGLADE | | 37 | | 37 | | 0 |
|
GILLES LANGLOIS | | 84 | | 84 | | 0 |
GREGORY LANGNER | | 9 | | 9 | | 0 |
JEFFREY LANGNER | | 9 | | 9 | | 0 |
|
WILLI LANGNER | | 18 | | 18 | | 0 |
DANIELLE LAPIERRE | | 146 | | 146 | | 0 |
|
RENEE LAPIERRE | | 151 | | 151 | | 0 |
EVELINE LAPORTE | | 148 | | 148 | | 0 |
|
GILLES LAROSE | | 849 | | 849 | | 0 |
DENISE LAROCHELLE | | 123 | | 123 | | 0 |
SEBASTIEN LAROCHELLE | | 53 | | 53 | | 0 |
ROBERT D LATTAS | | 439 | | 439 | | 0 |
VINCENZO LAURELLI | | 1 142 | | 1 142 | | 0 |
|
GILLES LAVALLEE | | 120 | | 120 | | 0 |
ROBERT H LAVERS | | 4 | | 4 | | 0 |
|
CLAUDE LAVIGNE | | 268 | | 268 | | 0 |
|
GARY LAVOIE | | 9 | | 9 | | 0 |
|
KATHY LAVOIE | | 9 | | 9 | | 0 |
62
| | | | | | |
PAUL LAVOIE | | 48 | | 48 | | 0 |
|
JOHN LEADER | | 351 | | 351 | | 0 |
|
SHAUN LEARY | | 351 | | 351 | | 0 |
|
FRED LEBEDOFF | | 88 | | 88 | | 0 |
FRED LEBEDOFF &BEVERLY LEBEDOFF JT TEN | | 88 | | 88 | | 0 |
|
GILLES LEBEL | | 879 | | 879 | | 0 |
|
ALLEN LEBLANC | | 30 | | 30 | | 0 |
|
ROGER LEBLANC | | 60 | | 60 | | 0 |
|
ANDRE LEBLOND | | 120 | | 120 | | 0 |
JEAN PIERRE LECLERC | | 18 | | 18 | | 0 |
YOLANDE L’ECUYER | | 60 | | 60 | | 0 |
|
LAURENT LEDUC | | 334 | | 334 | | 0 |
|
JOHN LEEDHAM | | 2 | | 2 | | 0 |
PIERRE LEFEBVRE | | 5 448 | | 5 448 | | 0 |
|
DAVID LEGAULT | | 261 | | 261 | | 0 |
SUZANNE LEGAULT | | 3 367 | | 3 367 | | 0 |
|
WILLIAM LEHR | | 703 | | 703 | | 0 |
|
GUY LEMIEUX | | 120 | | 120 | | 0 |
MICHEL LEONARD | | 726 | | 726 | | 0 |
CHARLES LEPAGE | | 11 775 | | 11 775 | | 0 |
LES HABITATION MAGRIBEC INC | | 2 636 | | 2 636 | | 0 |
|
CARL LESSARD | | 60 | | 60 | | 0 |
LES SVCS INFORMATIQUES JEAN MORIN INC | | 105 | | 105 | | 0 |
|
LEO LEVEILLE | | 351 | | 351 | | 0 |
|
YVAN LEVESQUE | | 144 | | 144 | | 0 |
|
ANTIGONI LIAPIS | | 3 515 | | 3 515 | | 0 |
M R S SECURITIES SERVICES INC ITF GEORGE LIAPIS A/C | | 1 951 | | 1 951 | | 0 |
63
| | | | | | |
KOSTA LIAPIS | | 1 757 | | 1 757 | | 0 |
|
MARK LIDSTONE | | 1 757 | | 1 757 | | 0 |
|
HUYNH GIA LONG | | 1 757 | | 1 757 | | 0 |
HUYNH QUY LONG | | 1 757 | | 1 757 | | 0 |
|
AURELLA B LORD | | 120 | | 120 | | 0 |
|
ORELLA B LORD | | 351 | | 351 | | 0 |
GABRIEL LO RUSSO | | 35 | | 35 | | 0 |
|
JOSEPH LOUIS | | 88 | | 88 | | 0 |
PIERRE-FELIX LOUIS | | 18 | | 18 | | 0 |
|
MARIO LOYER | | 176 | | 176 | | 0 |
BERTRAND LUSSIER | | 7 564 | | 7 564 | | 0 |
DANIELLE LUSSIER | | 193 | | 193 | | 0 |
GENEVIEVE LUSSIER | | 132 | | 132 | | 0 |
MARIELLE LUSSIER | | 88 | | 88 | | 0 |
MELANIE LUSSIER | | 4 | | 4 | | 0 |
|
ROBERT LUSSIER | | 673 | | 673 | | 0 |
VERONIQUE LUSSIER | | 4 | | 4 | | 0 |
ALAN MAC DONALD | | 9 | | 9 | | 0 |
ANNE MAC DONALD | | 4 | | 4 | | 0 |
GONTRAND MADORE | | 120 | | 120 | | 0 |
|
LILLA C MAGRI | | 176 | | 176 | | 0 |
|
ROSANNA MAGRI | | 63 | | 63 | | 0 |
GHISLAIN MAILLOUX | | 602 | | 602 | | 0 |
JACQUES MAILLOUX | | 123 | | 123 | | 0 |
ROBERT MALUQORINI | | 179 | | 179 | | 0 |
MARIO MARCHAND | | 126 | | 126 | | 0 |
CARMINE MARINO | | 780 | | 780 | | 0 |
|
PIERRE MAROIS | | 4 745 | | 4 745 | | 0 |
64
| | | | | | |
STEPHANE MARSAN | | 185 | | 185 | | 0 |
|
MATHIEU MARTEL | | 12 | | 12 | | 0 |
|
MICHEL MARTIAL | | 48 | | 48 | | 0 |
|
ROCCI MASELLA | | 439 | | 439 | | 0 |
RICHARD MASSON | | 88 | | 88 | | 0 |
JIMMY MAURADIAN | | 145 | | 145 | | 0 |
ATHENA MAVRODIS | | 105 | | 105 | | 0 |
|
ANNA MAZOLA | | 105 | | 105 | | 0 |
ARTHUR MERCANTE | | 299 | | 299 | | 0 |
DIANE PIETTE MERCIER | | 60 | | 60 | | 0 |
|
MICHEL MERCIER | | 351 | | 351 | | 0 |
|
JEAN MESSIER | | 24 | | 24 | | 0 |
JOHANNE MESSIER | | 120 | | 120 | | 0 |
MET P FORTIER INC | | 60 | | 60 | | 0 |
|
TOMASSO MIELE | | 527 | | 527 | | 0 |
ANIELLO DANIEL MILELE | | 148 | | 148 | | 0 |
|
ANDRE MILLETTE | | 1 757 | | 1 757 | | 0 |
|
RUSSELL MILLS | | 9 | | 9 | | 0 |
JOSEPH MINCIOTTI | | 360 | | 360 | | 0 |
|
JEAN MIREAULT | | 501 | | 501 | | 0 |
ROBERTO MOCELLA | | 50 | | 50 | | 0 |
REV VINCENT MONACO | | 176 | | 176 | | 0 |
|
JOHN MOORE | | 9 | | 9 | | 0 |
NATALIE PAGUETTE MOORE | | 84 | | 84 | | 0 |
|
DANY MOREAU | | 11 775 | | 11 775 | | 0 |
|
RENE MOREL | | 9 666 | | 9 666 | | 0 |
|
NADIA MORI | | 1 318 | | 1 318 | | 0 |
|
PAOLO MORI | | 23 725 | | 23 725 | | 0 |
65
| | | | | | |
CLOTILDE MARCOTTE MORIN | | 439 | | 439 | | 0 |
|
JEAN MORIN | | 88 | | 88 | | 0 |
|
LISE MORIN | | 176 | | 176 | | 0 |
|
WILLIE MORIN | | 9 | | 9 | | 0 |
|
ALAN MORISHITA | | 4 | | 4 | | 0 |
DOMINIQUE MORISOT | | 2 636 | | 2 636 | | 0 |
|
PAOLO MORI | | 6 451 | | 6 451 | | 0 |
GIUSEPPE MUCCARI | | 1 757 | | 1 757 | | 0 |
|
GAIL MULLINS | | 12 | | 12 | | 0 |
ANDREW MURRAY | | 527 | | 527 | | 0 |
DORYS F MURRAY | | 120 | | 120 | | 0 |
|
BILL NAGY | | 88 | | 88 | | 0 |
|
NUYNH AL NHI | | 1 757 | | 1 757 | | 0 |
LINDA NICOLACOPOULOS | | 141 | | 141 | | 0 |
|
ANTHONY NINI | | 18 | | 18 | | 0 |
GILLES NOISEAUX | | 879 | | 879 | | 0 |
|
FERNAND NOLET | | 41 | | 41 | | 0 |
ANDREW NOWOSTAWSKY | | 48 | | 48 | | 0 |
|
SYBIL OGILVIE | | 9 | | 9 | | 0 |
|
PETER O’NESI | | 4 | | 4 | | 0 |
|
MARC ST. ONGE | | 545 | | 545 | | 0 |
|
ONTARIO | | 60 | | 60 | | 0 |
OPEN FACE INTERNET | | 527 | | 527 | | 0 |
JACQUES OUELLET | | 88 | | 88 | | 0 |
|
PIERRE OUELLET | | 120 | | 120 | | 0 |
RICHARD OUELLETTE | | 4 701 | | 4 701 | | 0 |
|
YVAN OUELLET | | 301 | | 301 | | 0 |
|
MICHEAL OWENS | | 9 | | 9 | | 0 |
66
| | | | | | |
RICHARD PANBRUN | | 36 | | 36 | | 0 |
CHRISTINE PANGALO | | 70 | | 70 | | 0 |
|
REA PANGALO | | 105 | | 105 | | 0 |
MAURICE PAQUETTE | | 1 013 | | 1 013 | | 0 |
SIMON PAQUETTE | | 12 | | 12 | | 0 |
|
GAETAN PARADIS | | 1 757 | | 1 757 | | 0 |
SUZANNE PARADIS | | 96 | | 96 | | 0 |
MICHAEL PARNIAK | | 1 757 | | 1 757 | | 0 |
ANTONIO PARZIALE | | 119 | | 119 | | 0 |
ANTONIO PARZIALLE | | 378 | | 378 | | 0 |
|
TONY PARZIALE | | 732 | | 732 | | 0 |
ARMAND PATENAUDE | | 1 144 | | 1 144 | | 0 |
LOUIS-PHILLIPPE PAULET | | 54 | | 54 | | 0 |
|
FRANCINE PAUZE | | 203 | | 203 | | 0 |
CAROLE PELLETIER &RENE MARCOTTE JT TEN | | 88 | | 88 | | 0 |
NANCY PELLETIER | | 12 | | 12 | | 0 |
|
PAUL PELOQUIN | | 26 | | 26 | | 0 |
PENSON FINANCIAL SERVICES OF CANADA INC | | 12 741 | | 12 741 | | 0 |
ANDRE PEPIN &JULIE PEPIN JT TEN | | 6 | | 6 | | 0 |
|
ANDRE PEPIN | | 1 798 | | 1 798 | | 0 |
|
DAVID PEPIN | | 439 | | 439 | | 0 |
|
PERATECH INC | | 79 | | 79 | | 0 |
COLLEEN PETERSON | | 88 | | 88 | | 0 |
GERALD PETERSON | | 88 | | 88 | | 0 |
|
BIOMED PHARMA | | 17 574 | | 17 574 | | 0 |
|
LIEU THI PHAN | | 4 130 | | 4 130 | | 0 |
67
| | | | | | |
OLIVIER PHARAND | | 88 | | 88 | | 0 |
|
DANIEL PICARD | | 351 | | 351 | | 0 |
TONY PIETRATONIO | | 123 | | 123 | | 0 |
NICK PIETROCUPA | | 83 | | 83 | | 0 |
NICOLA PIETRACUPA | | 83 | | 83 | | 0 |
|
ANDRE PIETTE | | 30 | | 30 | | 0 |
|
FRANCE PIETTE | | 1 172 | | 1 172 | | 0 |
NORMAND PINARD | | 241 | | 241 | | 0 |
PIRILINI HOLDINGS | | 1 142 | | 1 142 | | 0 |
|
JOSEPH PIRILLO | | 569 | | 569 | | 0 |
|
GUY PLANEUF | | 879 | | 879 | | 0 |
FRANCOIS PLANTE | | 200 | | 200 | | 0 |
ISABELLE PLANTE | | 60 | | 60 | | 0 |
|
JOHN PLESA | | 439 | | 439 | | 0 |
|
PHILLIPPE PLOUF | | 562 | | 562 | | 0 |
JEAN-YVES POIRIER | | 351 | | 351 | | 0 |
MICHELINE LALONDE POIRIER | | 120 | | 120 | | 0 |
RICHARD POIRIER | | 141 | | 141 | | 0 |
CLAUDE POISSON | | 351 | | 351 | | 0 |
ROBERT POISSANT | | 18 | | 18 | | 0 |
|
UGO POLETTA | | 2 988 | | 2 988 | | 0 |
|
UGO POLLETA | | 1 757 | | 1 757 | | 0 |
|
UGO POLLETTA | | 176 | | 176 | | 0 |
DANIELLE PONTBRIAND | | 24 | | 24 | | 0 |
JOHNNY PORRECA | | 3 585 | | 3 585 | | 0 |
|
NICOLE POIRIER | | 123 | | 123 | | 0 |
RICHARD PORTAS | | 123 | | 123 | | 0 |
ROYSTON PORTER | | 62 | | 62 | | 0 |
68
| | | | | | |
DALE PORTERFIELD & | | 2 001 | | 2 001 | | 0 |
PORTERWAY HOLDING | | 149 | | 149 | | 0 |
DANIELL PORTERFIELD &DUANE PORTERFIELD JT TEN | | 1 757 | | 1 757 | | 0 |
PORTER-WELL HOLDING | | 149 | | 149 | | 0 |
|
PIERRE POULIN | | 181 | | 181 | | 0 |
|
DAVID PRESIMAN | | 4 | | 4 | | 0 |
JEAN-PIERRE PREVOST | | 48 | | 48 | | 0 |
|
MICHEL PREVOST | | 220 | | 220 | | 0 |
|
HARVEY PRIEST | | 4 | | 4 | | 0 |
JACQUELINE PRINCE | | 9 | | 9 | | 0 |
JACQUELINE MARIE PRINCE | | 142 | | 142 | | 0 |
PRO SPECK CAPITAL | | 123 | | 123 | | 0 |
|
PRO SPORT | | 351 | | 351 | | 0 |
FRANCOIS PROVOST | | 48 | | 48 | | 0 |
ALPHONSO PUCCIO | | 44 | | 44 | | 0 |
THINH TUONG QUAN | | 13 181 | | 13 181 | | 0 |
THINH THUONG QUAN | | 3 515 | | 3 515 | | 0 |
2961 1589 QUEBEC INC | | 176 | | 176 | | 0 |
9006-3785 QUEBEC INC | | 70 | | 70 | | 0 |
9026 6206 QUEBEC INC | | 36 | | 36 | | 0 |
9033-0176 QUEBEC INC | | 17 635 | | 17 635 | | 0 |
9093-9232 QUEBEC INC | | 888 | | 888 | | 0 |
9137-1534 QUEBEC INC | | 26 800 | | 26 800 | | 0 |
|
QUEBECON | | 202 | | 202 | | 0 |
9142-1438 QUEBEC INC | | 46 571 | | 46 571 | | 0 |
RAYNALD RACICOT | | 120 | | 120 | | 0 |
NBCN CLEARING INC FBO ANDRE RACINE | | 351 | | 351 | | 0 |
69
| | | | | | |
NBCN CLEARING INC FBO GENEVIEIVE RACINE | | 176 | | 176 | | 0 |
JOSIANE RAKOTOMANGA | | 4 394 | | 4 394 | | 0 |
|
DENNIS RAU | | 4 | | 4 | | 0 |
MICHEL RAYMOND | | 192 | | 192 | | 0 |
ARTHUR RECHTEN | | 630 | | 630 | | 0 |
STEVEN REDMOND | | 562 | | 562 | | 0 |
CLAUDE REMILLARD | | 120 | | 120 | | 0 |
|
GAETAN RENAUD | | 69 | | 69 | | 0 |
RESA INVESTMENT LTD | | 2 033 | | 2 033 | | 0 |
CHRISTIAN RICHARD | | 270 | | 270 | | 0 |
|
GUY RICHARD | | 1 502 | | 1 502 | | 0 |
MARYSE RICHARD | | 36 | | 36 | | 0 |
SUZANNE RICHARD | | 60 | | 60 | | 0 |
|
PATRICIA RIMOK | | 1 634 | | 1 634 | | 0 |
CLAUDETTE RINFRET | | 265 | | 265 | | 0 |
|
ROCH RIVARD | | 703 | | 703 | | 0 |
|
YANNICK RIVARD | | 703 | | 703 | | 0 |
|
CAROLE ROBERT | | 1 757 | | 1 757 | | 0 |
|
ONIL ROBERT | | 241 | | 241 | | 0 |
JEAN PIERRE ROBICHAUD | | 439 | | 439 | | 0 |
JEAN-MARIE ROBILLARD | | 53 | | 53 | | 0 |
JACQUES ROCHEFORT | | 53 | | 53 | | 0 |
JEZABEL ROCHELEAU | | 24 | | 24 | | 0 |
FRANCOIS ROCHON | | 105 | | 105 | | 0 |
|
JACQUES ROGER | | 60 | | 60 | | 0 |
|
JACQUES ROGER | | 60 | | 60 | | 0 |
|
MARTIN ROMPRE | | 18 | | 18 | | 0 |
ROSEDALE HOLDINGS | | 14 938 | | 14 938 | | 0 |
70
| | | | | | |
GINETTE ROSS | | 8 | | 8 | | 0 |
JEAN-MICHEL ROSS | | 184 | | 184 | | 0 |
|
MARIO ROSSI | | 1 757 | | 1 757 | | 0 |
|
MAURO ROSSI | | 1 617 | | 1 617 | | 0 |
PETER R ROTHSCHILD | | 176 | | 176 | | 0 |
HELENE ROTONDO | | 264 | | 264 | | 0 |
VERONIQUE ROUVEYROLLIS &ROMAIN ROUVEYROLLIS JTTEN | | 60 | | 60 | | 0 |
|
YVES ROUX | | 264 | | 264 | | 0 |
|
BRIAN ROWE | | 4 | | 4 | | 0 |
|
HUGUETTE ROY | | 879 | | 879 | | 0 |
JEAN-PIERRE ROY | | 27 | | 27 | | 0 |
|
|
MATHIEU ROY | | 44 | | 44 | | 0 |
|
ROBERT ROY | | 24 | | 24 | | 0 |
|
VERONIQUE ROY | | 60 | | 60 | | 0 |
|
ANDRE RUELLE | | 88 | | 88 | | 0 |
|
SERGIO SAGARIA | | 545 | | 545 | | 0 |
ROLANDE SAINDON | | 35 | | 35 | | 0 |
DOMINIC SALERNO | | 105 | | 105 | | 0 |
|
NICOLE SAMSON | | 60 | | 60 | | 0 |
RYAN SAMVELYAN | | 126 | | 126 | | 0 |
|
ROBERT SANTINI | | 53 | | 53 | | 0 |
|
ERIC SARDANO | | 88 | | 88 | | 0 |
|
JOSE SARDANO | | 2 777 | | 2 777 | | 0 |
M J ANNY SARDANO | | 32 | | 32 | | 0 |
|
EMIL SARIC | | 264 | | 264 | | 0 |
|
JASMINE SARIC | | 264 | | 264 | | 0 |
JACQUES SARRAZIN | | 145 | | 145 | | 0 |
SEBASTIEN SAUVE | | 1 307 | | 1 307 | | 0 |
71
| | | | | | |
ROBERTO SAVOCA | | 703 | | 703 | | 0 |
MICHELINE SAWYER | | 12 | | 12 | | 0 |
ANNUZIATO SCARAMELLA | | 3 515 | | 3 515 | | 0 |
BRENDA E SCHOENBERGER &JEFFREY L SCHOENBERGER | | 42 | | 42 | | 0 |
FRANCESCO SCUCCIMARI | | 49 | | 49 | | 0 |
|
PHOK HONG SE | | 703 | | 703 | | 0 |
|
JULIA SHAAR | | 120 | | 120 | | 0 |
HOWARD J SHAPIRO &EDWARD M SHAPIRO JTWROS | | 799 | | 799 | | 0 |
JACQUES IP FOOK SHING | | 351 | | 351 | | 0 |
NICHOLAS SKAFIDAS | | 351 | | 351 | | 0 |
SKYLINE RESOURCES | | 107 705 | | 107 705 | | 0 |
SKYLINE INVESTMENTS | | 2 162 | | 2 162 | | 0 |
|
GUY SMITH | | 1 318 | | 1 318 | | 0 |
JOSEPH SOLOMITA | | 488 | | 488 | | 0 |
MICHAEL SOLOMITA | | 683 | | 683 | | 0 |
|
ERIC SONIGO | | 4 569 | | 4 569 | | 0 |
|
NICOLE SOULARD | | 1 648 | | 1 648 | | 0 |
NICOLE SOULARD &DENIS BLAQUIERE JT TEN | | 868 | | 868 | | 0 |
|
NICK SPAGNOLO | | 105 | | 105 | | 0 |
JOHN A SPAULDING | | 141 | | 141 | | 0 |
|
URSULA STARK | | 9 | | 9 | | 0 |
|
DANNY STEIDL | | 35 | | 35 | | 0 |
|
BIANCA J STELLA | | 88 | | 88 | | 0 |
JOANNA B STELLA | | 88 | | 88 | | 0 |
|
LEONARD STELLA | | 160 368 | | 160 368 | | 0 |
72
| | | | | | |
DENIS ST-MARTIN | | 60 | | 60 | | 0 |
|
MARK ST-ONGE | | 413 | | 413 | | 0 |
|
CELINE ST-ONGE | | 6 | | 6 | | 0 |
|
MARTIN ST-ONGE | | 36 | | 36 | | 0 |
|
TONY ST-ONGE | | 492 | | 492 | | 0 |
RYAN STUCKLESS | | 88 | | 88 | | 0 |
|
GUY TAILLEFER | | 4 077 | | 4 077 | | 0 |
|
DORIS TALBOT | | 35 | | 35 | | 0 |
VIPADA TANGKITJAVISUTH | | 56 | | 56 | | 0 |
|
ALAIN TANGUAY | | 2 784 | | 2 784 | | 0 |
|
GUY TAPIN | | 84 | | 84 | | 0 |
|
JACQUES TAPIN | | 181 | | 181 | | 0 |
TEASDALE INVESTMENT LTD | | 2 636 | | 2 636 | | 0 |
GISELE L TETRAULT | | 132 | | 132 | | 0 |
GISELE TETREAULT | | 60 | | 60 | | 0 |
LORRAINE TETRAULT | | 4 | | 4 | | 0 |
|
MARIO TETRAULT | | 4 | | 4 | | 0 |
|
SONIA TETRAULT | | 4 | | 4 | | 0 |
SYLVAIN TETRAULT | | 4 | | 4 | | 0 |
DU TUONG PHUONG THAO | | 176 | | 176 | | 0 |
CLAUDE THEBERGE | | 120 | | 120 | | 0 |
ROBERTO THEBERGE &LINDA NOLET JT TEN | | 120 | | 120 | | 0 |
|
JEAN THELLEN | | 36 | | 36 | | 0 |
JEAN MARC THERRIEN | | 120 | | 120 | | 0 |
MICHEL THERRIEN | | 351 | | 351 | | 0 |
|
XYSTE THERRIEN | | 120 | | 120 | | 0 |
HUGUES TETREAULT | | 18 | | 18 | | 0 |
DAVID THEVENAZ & BRENDA THEVENAZ JT TEN | | 176 | | 176 | | 0 |
73
| | | | | | |
BRENDA THEVENAZ | | 44 | | 44 | | 0 |
|
DAVID THEVENAZ | | 44 | | 44 | | 0 |
VALERIE THEVENAZ | | 88 | | 88 | | 0 |
FREDERICOS TORRES | | 281 | | 281 | | 0 |
|
JOHN TOTERA | | 105 | | 105 | | 0 |
SIV KHEANG TRAN | | 3 734 | | 3 734 | | 0 |
THOMAS TREICHLER | | 1 098 | | 1 098 | | 0 |
CHRISTINE TREMBLAY | | 241 | | 241 | | 0 |
JEAN MARC TREMBLAY | | 24 | | 24 | | 0 |
|
MARC TREMBLAY | | 984 | | 984 | | 0 |
|
LISE TREMBLAY | | 60 | | 60 | | 0 |
SYLVAIN TREMBLAY | | 3 905 | | 3 905 | | 0 |
|
REAL TREMBLAY | | 323 | | 323 | | 0 |
|
YVES TREMBLAY | | 36 | | 36 | | 0 |
PIERRE TREPANIER | | 176 | | 176 | | 0 |
MARISE TURCOTTE | | 62 | | 62 | | 0 |
JOANNE TURGEON | | 60 | | 60 | | 0 |
ALEXANDRE VADNAIS | | 1 177 | | 1 177 | | 0 |
|
LISE VALCOURT | | 88 | | 88 | | 0 |
|
HUGO VALENTE | | 38 053 | | 38 053 | | 0 |
|
LEA VALENTE | | 1 757 | | 1 757 | | 0 |
LAURINA VALENTE | | 1 757 | | 1 757 | | 0 |
LEONARDO VALENTE | | 5 272 | | 5 272 | | 0 |
ANTOINE VANDERZON | | 176 | | 176 | | 0 |
FRANCOIS VARNEY | | 18 | | 18 | | 0 |
|
JOHN VAZQUEZ | | 60 | | 60 | | 0 |
|
NOEL VEILLEUX | | 351 | | 351 | | 0 |
74
| | | | | | |
CAROLL VEILLEUX | | 24 | | 24 | | 0 |
RAYMONDE VEILLEUX | | 12 | | 12 | | 0 |
JEAN-LOUIS VERDIER | | 241 | | 241 | | 0 |
MARIE-CLAUDE VIAU | | 18 | | 18 | | 0 |
|
GILLES VIGNEAU | | 5 096 | | 5 096 | | 0 |
MARC ANDRE VIGNEAULT | | 1 294 | | 1 294 | | 0 |
MARYSE VIGNEAULT | | 385 | | 385 | | 0 |
CLAUDE VILLENEUVE | | 176 | | 176 | | 0 |
MICHELLE VINCELLETTE | | 234 | | 234 | | 0 |
VIRAL RESEARCH ASSOCIATES | | 113 352 | | 113 352 | | 0 |
|
FILIPPO VITA | | 125 | | 125 | | 0 |
|
MARIO VOLPE | | 5 500 | | 5 500 | | 0 |
VON DER WELT DISTRIBUTIONS INC | | 35 | | 35 | | 0 |
|
HUYNH AL VY | | 1 757 | | 1 757 | | 0 |
|
STEVEN WALKER | | 12 | | 12 | | 0 |
|
BRUCE WATSON | | 9 | | 9 | | 0 |
|
KEN WEBB | | 2 | | 2 | | 0 |
MARILYN WEIGHILL | | 9 | | 9 | | 0 |
ROBERTA WEINER | | 18 | | 18 | | 0 |
BEVERLY WHITEAR | | 18 | | 18 | | 0 |
MICHAEL WHITEAR | | 18 | | 18 | | 0 |
|
CHRISTIAN WILER | | 2 | | 2 | | 0 |
|
ELIANE WILER | | 15 | | 15 | | 0 |
|
ELIANNE WILER | | 12 | | 12 | | 0 |
|
EVELYNE WILER | | 9 | | 9 | | 0 |
|
JUDITH WONG | | 119 | | 119 | | 0 |
KIRSTIE YERGENS | | 88 | | 88 | | 0 |
|
LOGAN YERGENS | | 88 | | 88 | | 0 |
75
| | | | | | |
MICHELE YERGENS | | 1 757 | | 1 757 | | 0 |
|
BRIAN ZAKO | | 879 | | 879 | | 0 |
JOHN A ZAMOS &JOANNE L ZAMOS JT TEN | | 211 | | 211 | | 0 |
|
ELAINE ZAKO | | 879 | | 879 | | 0 |
ANGELO ZAPPITELLI &RITA CASTIELLO JT TEN | | 1 494 | | 1 494 | | 0 |
|
XU HONG ZHANG | | 176 | | 176 | | 0 |
HOWARD ZINGBOIM | | 176 | | 176 | | 0 |
76
DESCRIPTION OF CAPITAL STOCK
The following description of our capital stock does not purport to be complete and is subject to, and qualified in its entirety by, our certificate of incorporation and bylaws, which we have included as exhibits to the registration statement of which this prospectus forms a part.
Upon the closing of this offering, our authorized capital stock will consist of 280 million shares of common stock, par value $0.0001 per share, and 50 million shares of undesignated preferred stock, par value $0.0001 per share. As of the date of this registration statement, an aggregate of 40,000,000 shares of common stock are issued and outstanding and 829,554 shares of preferred stock are issued and outstanding.
Common Stock
We are authorized to issue 280,000,000 shares of Common Stock, $0.0001 par value. As of September 5, 2006 there were 40,000,000 shares of common stock outstanding, held of record by one majority stockholder, Millenia Hope Inc. our parent corporation, by one shareholder of more than 10% of our common stock and, after the issuance of thestock dividend of 6% of the issued shares of Millenia Hope Pharmaceuticals by Millenia Hope Inc. to its shareholders of record of July 3, 2006, over 2,000 minority shareholders
The holders of our common stock are entitled to one vote per share on any matter to be voted upon by stockholders. The holders of our common stock are entitled to dividends as our board of directors may declare from time to time from legally available funds subject to the preferential rights of the holders of any shares of our preferred stock that we may issue in the future.
Our certificate of incorporation does not provide for cumulative voting in connection with the election of directors. Accordingly, directors will be elected by a plurality of the shares voting once a quorum is present. No holder of our common stock will have any preemptive right to subscribe for any shares of capital stock issued in the future.
Upon any voluntary or involuntary liquidation, dissolution or winding up of our affairs, the holders of our common stock are entitled to share, on a pro rata basis, all assets remaining after payment to creditors and subject to prior distribution rights of any shares of preferred stock that we may issue in the future. All of the outstanding shares of common stock are fully paid and non-assessable. Holders of our common stock do not have cumulative voting rights, so the holders of more than 50% of the combined shares voting for the election of directors may elect all of the directors if they choose to do so, and, in that event, the holders of the remaining shares will not be able to elect any members to the Board of Directors.
Preferred Stock
We are presently authorized to issue 50,000,000 shares of Preferred Stock, $.0001 par value. As of September 5, 2006, there were 829,554 shares of Preferred Stock issued and outstanding.
The characteristics of our Preferred Stock have not been determined by our Board of Directors.
PLAN OF DISTRIBUTION
All of the stock owned by the selling security holders will be registered by the registration statement of which this prospectus is a part. The selling security holders may sell some or all of their shares immediately after they are registered. The selling security holders shares may be sold or distributed from time to time by the selling stockholders or by pledgees, donees or transferees of, or successors in interest to, the selling stockholders, directly to one or more purchasers (including pledgees) or through brokers, dealers or underwriters who may act solely as agents or may acquire shares as principals, at market prices prevailing at the time of sale, at prices related to such prevailing market prices, at negotiated prices or at fixed prices, which may be changed. The distribution of the shares may be effected in one or more of the following methods:
| | |
| * | ordinary brokers transactions, which may include long or short sales, |
77
| | |
| * | transactions involving cross or block trades on any securities exchange or market where our common stock is trading, |
| | |
| * | Purchases by brokers, dealers or underwriters as principal and resale by such purchasers for their own accounts pursuant to this prospectus, “at the market” to or through market makers or into an existing market for the common stock, |
| | |
| * | in other ways not involving market makers or established trading markets, including direct sales to purchasers or sales effected through agents, |
| | |
| * | through transactions in options, swaps or other derivatives (whether exchange listed or otherwise), or |
| | |
| * | any combination of the foregoing, or by any other legally available means. |
In addition, the selling stockholders may enter into hedging transactions with broker-dealers who may engage in short sales, if short sales were permitted, of shares in the course of hedging the positions they assume with the selling stockholders. The selling stockholders may also enter into option or other transactions with broker-dealers that require the delivery by such broker-dealers of the shares, which shares may be resold thereafter pursuant to this prospectus.
Brokers, dealers, underwriters or agents participating in the distribution of the shares may receive compensation in the form of discounts, concessions or commissions from the selling stockholders and/or the purchasers of shares for whom such broker-dealers may act as agent or to whom they may sell as principal, or both (which compensation as to a particular broker-dealer may be in excess of customary commissions). The selling stockholders and any broker-dealers acting in connection with the sale of the shares hereunder may be deemed to be underwriters within the meaning of Section 2(11) of the Securities Act of 1933, and any commissions received by them and any profit realized by them on the resale of shares as principals may be deemed underwriting compensation under the Securities Act of 1933. Neither the selling stockholders nor we can presently estimate the amount of such compensation. We know of no existing arrangements between the selling stockholders and any other stockholder, broker, dealer, underwriter or agent relating to the sale or distribution of the shares.
We will not receive any proceeds from the sale of the shares of the selling security holders pursuant to this prospectus. We have agreed to bear the expenses of the registration of the shares, including legal and accounting fees, and such expenses are estimated to be approximately $25,000.
We have informed the selling stockholders that certain anti-manipulative rules contained in Regulation M under the Securities Exchange Act of 1934 may apply to their sales in the market and have furnished the selling stockholders with a copy of such rules and have informed them of the need for delivery of copies of this prospectus. The selling stockholders may also use Rule 144 under the Securities Act of 1933 to sell the shares if they meet the criteria and conform to the requirements of such rule.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Millenia Hope Inc, majority shareholder of Millenia Hope Pharmaceuticals, undertook to issue a 6% stock dividend, to all Millenia Hope Shareholders of record July 3, 2006, constituting in aggregate 2,400,000 common shares of Millenia Hope Pharmaceuticals Inc. to its shareholders, numbering an excess of 2,000.
Other than as noted above, none of the directors, executive officers or any member of the immediate family of any director or executive officer has been indebted to us since its inception. We have not and do not intend to enter into any additional transactions with our management or any nominees for such positions. We have not and do not intend to enter into any transactions with our beneficial owners, with the exception of our parent company, Millenia Hope Inc.
78
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON
ACCOUNTING AND FINANCIAL DISCLOSURE
There have been no disagreements with Stark Winter Schenkein & Co., LLP, our independent auditors, on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure.
TRANSFER AGENT
Our transfer agent will be Intercontinental Registrar & Transfer Agency, Inc. 900 Buchanan Blvd suite 1, Boulder City, Nevada, 89005. Their telephone number is (702) 293-6717.
EXPERTS
The financial statements included in this prospectus have been audited by Stark Winter Schenkein & Co., LLP, independent auditors, as stated in their report appearing herein and elsewhere in the registration statement, which report expresses an unqualified opinion and has been so included in reliance upon the reports of such firm given upon their authority as experts in accounting and auditing.
LEGAL MATTERS
The validity of our common shares offered will be passed upon for us by Anslow & Jaclin, LLP, Manalapan, New Jersey 07726.
79
FINANCIAL STATEMENTS
We have attached to this prospectus, copies of our audited financial statements for the years ended November 30, 2004 and November 30, 2005 and unaudited financial statements for the six month period ended May 31, 2006.
80
PROSPECTUS
YOU SHOULD RELY ONLY ON THE INFORMATION CONTAINED IN THIS DOCUMENT OR THAT WE HAVE REFERRED YOU TO. WE HAVE NOT AUTHORIZED ANYONE TO PROVIDE YOU WITH INFORMATION THAT IS DIFFERENT. THIS PROSPECTUS IS NOT AN OFFER TO SELL COMMON STOCK AND IS NOT SOLICITING AN OFFER TO BUY COMMON STOCK IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED.
UNTIL __________, ALL DEALERS THAT EFFECT TRANSACTIONS IN THESE SECURITIES, WHETHER OR NOT PARTICIPATING IN THIS OFFERING, MAY BE REQUIRED TO DELIVER A PROSPECTUS. THIS IS IN ADDITION TO THE DEALERS’ OBLIGATION TO DELIVER A PROSPECTUS WHEN ACTING AS UNDERWRITERS AND WITH RESPECT TO THEIR UNSOLD ALLOTMENTS OR SUBSCRIPTIONS.
81
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| |
ITEM 24. | INDEMNIFICATION OF DIRECTORS, OFFICERS, EMPLOYEES AND AGENTS. |
Our Certificate of Incorporation and By-laws provide that we shall indemnify to the fullest extent permitted by Canadian law any person whom we may indemnify thereunder, including our directors, officers, employees and agents. Such indemnification (other than as ordered by a court) shall be made by us only upon a determination that indemnification is proper in the circumstances because the individual met the applicable standard of conduct i.e., such person acted in good faith and in a manner he reasonably believed to be in or not opposed to our best interest. Advances for such indemnification may be made pending such determination. Such determination shall be made by a majority vote of a quorum consisting of disinterested directors, or by independent legal counsel or by the stockholders. In addition, our Certificate of Incorporation provides for the elimination, to the extent permitted by Canada, of personal liability of our directors and our stockholders for monetary damages for breach of fiduciary duty as directors.
We have agreed to indemnify each of our directors and certain officers against certain liabilities, including liabilities under the Securities Act of 1933. Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers and controlling persons pursuant to the provisions described above, or otherwise, we have been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than our payment of expenses incurred or paid by our director, officer or controlling person in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
| |
ITEM 25. | OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION. |
The following table sets forth the expenses in connection with the issuance and distribution of the securities being registered hereby. All such expenses will be borne by the registrant; none shall be borne by any selling stockholders.
| | | | |
SEC registration fee | | $ | 1,000 | |
| | | | |
Legal fees and expenses (1) | | $ | 8,000 | |
| | | | |
Accounting fees and expenses (1) | | $ | 5,000 | |
| | | | |
Miscellaneous and Printing fees (1) | | $ | 11,000 | |
| | | | |
Total (1) | | $ | 25,000 | |
| |
ITEM 26. | RECENT SALES OF UNREGISTERED SECURITIES. |
On September 1, 2000 the Company entered into a subscription agreement and issued 10,000 common shares to Millenia Hope Inc., at its par value of $0.0001.
On February 14, 2002 the Company entered into a subscription agreement and issued 39,990,000 common shares to Millenia Hope Inc., as its par value of $0.0001.
82
All of the above issuances of shares of our common stock qualified for exemption under Section 4(2) of the Securities Act of 1933 since the issuance of such shares by us did not involve a public offering. Each of these shareholders was a sophisticated investor and had access to information regarding us. The offering was not a “public offering” as defined in Section 4(2) due to the insubstantial number of persons involved in the deal, size of the offering, manner of the offering and number of shares offered. We did not undertake an offering in which we sold a high number of shares to a high number of investors. In addition, these shareholders had the necessary investment intent as required by Section 4(2) since they agreed to and received a share certificate bearing a legend stating that such shares are restricted pursuant to Rule 144 of the 1933 Securities Act. These restrictions ensure that these shares would not be immediately redistributed into the market and therefore not be part of a “public offering.” Based on an analysis of the above factors, we have met the requirements to qualify for exemption under Section 4(2) of the Securities Act of 1933 for the above transactions.
| |
ITEM 27. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES. |
(a) Exhibits:
The following exhibits are filed as part of this registration statement:
| | |
EXHIBIT | | DESCRIPTION |
| | |
3.1 | | Articles of Incorporation |
| | |
3.2 | | By-Laws |
| | |
5.1 | | Opinion and Consent of Anslow & Jaclin, LLP |
| | |
10.1 | | Agreement with 9111-9080 Quebec Inc. |
| | |
10.2. | | Employment Agreements with Dr. Jean Archambault, Dr. Bahige Baroudy, Dr. Robert Williams and Mr. Dany Aubry. |
| | |
23.1 | | Consent of Stark Winter Schenkein & Co., LLP, Independent Registered Public Accounting Firm |
| | |
24.1 | | Power of Attorney (included on signature page of Registration Statement) |
83
| |
(A) The undersigned Registrant hereby undertakes: |
|
| |
(1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement to: |
| | |
(i) | | Include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| | |
(ii) | | Reflect in the prospectus any facts or events which, individually or together, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| | |
(iii) | | Include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| |
(2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| |
(3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
(B) Undertaking Required by Regulation S-B, Item 512(e).
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or controlling persons pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel that the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue.
(C) Undertaking Required by Regulation S-B, Item 512(f)
The undersigned Registrant hereby undertakes that, for purposes of determining any liability under the Securities Act of 1933, each filing of the Registrant’s annual report pursuant to Section 13(a) or 15(d) of the Exchange Act of 1934 that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at the time shall be deemed to be the initial bona fide offering thereof.
84
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Montreal, Province of Quebec, Country of Canada, on the 6th day of September, 2006.
| | |
| MILLENIA HOPE PHARMACEUTICALS INC. |
| | |
| By: | /S/ Leonard Stella |
| |
|
| | Leonard Stella
CEO
and Director
(Principal Accounting Officer) |
POWER OF ATTORNEY
The undersigned directors and officers of Millenia Hope Pharmaceuticals Inc. hereby constitute and appoint Leonard Stella, with full power to act without the other and with full power of substitution and resubstitution, our true and lawful attorneys-in-fact with full power to execute in our name and behalf in the capacities indicated below any and all amendments (including post-effective amendments and amendments thereto) to this registration statement under the Securities Act of 1933 and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission and hereby ratify and confirm each and every act and thing that such attorneys- in-fact, or any them, or their substitutes, shall lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| | |
SIGNATURE | TITLE | DATE |
| | |
/S/ LEONARD STELLA. | CEO and Director | SEPTEMBER 6, 2006 |
| | |
LEONARD STELLA | | |
| | |
/S/ BAHIGE BAROUDY. | Chief Science Officer and Director | SEPTEMBER 6, 2006 |
| | |
BAHIGE BAROUDY | | |
| | |
/S/ JEAN ARCHABAULT | President, Chief Operating Officer and Director | SEPTEMBER 6, 2006 |
| | |
JEAN ARCHAMBAULT | | |
| | |
/S/ DANY AUBRY | Chief Technical Officer and Director | SEPTEMBER 6, 2006 |
| | |
DANY AUBRY | | |
| | |
/S/ ROBERT WILLIAMS | Chief Development Officer and Director | SEPTEMBER 6, 2006 |
| | |
ROBERT WILLIAMS | | |
85
Report of Independent Registered Public Accounting Firm
To the Shareholder
Millenia Hope Pharmaceuticals, Inc.
We have audited the accompanying balance sheet of Millenia Hope Pharmaceuticals, Inc. as of November 30, 2004, and the related statements of operations, stockholder’s equity and cash flows for the year ended November 30, 2004. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The financial statements show the Company has no revenues and relies upon its parent Company to fund its operations. This factor raises substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Millenia Hope Pharmaceuticals, Inc. as of November 30, 2004, and the results of its operations and cash flows for the years ended November 30, 2004, in conformity with accounting principles generally accepted in the United States of America.
Denver, Colorado
September 5, 2006
Millenia Hope Pharmaceuticals Inc.
Balance Sheet
November 30, 2004
| | | | |
Assets | |
| | | | |
Current Assets | | | | |
Cash | | $ | — | |
| |
|
| |
| | | | |
Liabilities & Stockholders’ Equity | |
| | | | |
Current Liabilities | | | | |
Accounts Payable and Accrued Liabilities | | $ | 244 | |
| | | | |
| |
|
| |
Total Current Liabilities | | | 244 | |
| |
|
| |
| | | | |
Stockholders’ Equity | | | | |
Preferred Stock, $.0001 par value; 50,000,000 shares authorized, none issued or outstanding | | | — | |
Common Stock, $.0001 par value; 280,000,000 shares authorized, 40,000,000 Issued and outstanding | | | 4,000 | |
Stock Subscriptions Receivable | | | (4,000 | ) |
Paid in Capital | | | — | |
Deficit | | | (244 | ) |
| |
|
| |
| | | (244 | ) |
| |
|
| |
| | | | |
| | $ | — | |
| |
|
| |
See the accompanying notes to the financial statements.
Millenia Hope Pharmaceuticals Inc.
Statements of Operations
Year ended November 30, 2004 and November 30, 2003, and
the Period From Inception (January 13, 2000) to November 30, 2004
| | | | | | | | | | |
| | Year
ended
November 30,
2004 | | Year
ended
November 30,
2003 | | Inception to
November 30,
2004 | |
| |
| |
| |
| |
Revenues | | $ | — | | $ | — | | $ | — | |
| | | | | | | | | | |
Cost of Sales | | | — | | | — | | | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Gross Profit | | | — | | | — | | | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Operating Expenses | | | | | | | | | | |
Selling, general and administrative | | | 122 | | | 122 | | | 244 | |
| |
|
| |
|
| |
|
| |
| | | 122 | | | 122 | | | 244 | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Operating (Loss) | | | (122 | ) | | (122 | ) | | (244 | ) |
| | | | | | | | | | |
Other income (expense) | | | — | | | — | | | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Net (Loss) | | $ | (122 | ) | $ | (122 | ) | $ | (244 | ) |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Per share information - basic and diluted: | | | | | | | | | | |
Weighted Average Number of Common Shares Outstanding | | | 40,000,000 | | | 40,000,000 | | | | |
| |
|
| |
|
| | | | |
| | | | | | | | | | |
Net (Loss) Per Common Share | | $ | (0.00 | ) | $ | (0.00 | ) | | | |
| |
|
| |
|
| | | | |
See the accompanying notes to the financial statements.
Millenia Hope Pharmaceuticals, Inc.
(A Company in the Development Stage)
Statement of Stockholder’s Equity (Deficit)
January 13, 2000 (inception) to November 30, 2004
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | Common Stock
Subscription | | Additional
Paid-in
Capital | | (Deficit)
Accumulated
During the
Development
Stage | | Total
Stockholder’s
Equity (Deficit) | |
| | Common Stock | | Preferred Stock | | | | |
| |
| |
| | | | |
| | Shares | | Amount | | Shares | | Amount | | | | | |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, January 13, 2000 | | | — | | $ | — | | | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Stock issued for subscription | | | 10,000 | | | 1 | | | — | | | — | | | (1 | ) | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Net (loss) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at November 30, 2000 | | | 10,000 | | | 1 | | | — | | | — | | | (1 | ) | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Net (loss) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at November 30, 2001 | | | 10,000 | | | 1 | | | — | | | — | | | (1 | ) | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Stock issued for subscription | | | 39,990,000 | | | 3,999 | | | — | | | — | | | (3,999 | ) | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Net (loss) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at November 30, 2002 | | | 40,000,000 | | | 4,000 | | | — | | | — | | | (4,000 | ) | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Net (loss) | | | — | | | — | | | — | | | — | | | — | | | — | | | (122 | ) | | (122 | ) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at November 30, 2003 | | | 40,000,000 | | | 4,000 | | | — | | | — | | | (4,000 | ) | | — | | | (122 | ) | | (122 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Net (loss) | | | — | | | — | | | — | | | — | | | — | | | — | | | (122 | ) | | (122 | ) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at November 30, 2004 | | | 40,000,000 | | | 4,000 | | | — | | | — | | | (4,000 | ) | | — | | | (244 | ) | | (244 | ) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Millenia Hope Pharmaceuticals Inc.
Statements of Cash Flows
Year ended November 30, 2004 and November 30, 2003, and
the Period From Inception (January 13, 2000) to November 30, 2004
| | | | | | | | | | |
| | Year
ended
November 30,
2004 | | Year
ended
November 30,
2003 | | Inception to
November 30,
2004 | |
| |
| |
| |
| |
Operating Activities | | | | | | | | | | |
Net (loss) | | $ | (122 | ) | $ | (122 | ) | $ | (244 | ) |
Changes in: | | | | | | | | | | |
Accounts payable | | | 122 | | | 122 | | | 244 | |
| |
|
| |
|
| |
|
| |
Cash (used in) operating activities | | | — | | | — | | | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Financing Activities | | | | | | | | | | |
Cash provided by financing activities | | | — | | | — | | | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Investing activities | | | | | | | | | | |
Cash provided by investing activities | | | — | | | — | | | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Increase (decrease) in cash | | | — | | | — | | | — | |
| | | | | | | | | | |
Cash and cash equivalents | | | | | | | | | | |
Beginning of year | | | — | | | — | | | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
End of year | | $ | — | | $ | — | | $ | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Cash paid for interest | | $ | — | | $ | — | | $ | — | |
| |
|
| |
|
| |
|
| |
Cash paid for taxes | | $ | — | | $ | — | | $ | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Supplemental schedule of noncash investing and financing activities: | | | | | | | | | | |
| | | | | | | | | | |
Stock subscription receivable | | $ | — | | $ | — | | $ | 4,000 | |
| |
|
| |
|
| |
|
| |
Report of Independent Registered Public Accounting Firm
To the Shareholder
Millenia Hope Pharmaceuticals, Inc.
We have audited the accompanying balance sheet of Millenia Hope Pharmaceuticals, Inc. as of November 30, 2005, and the related statements of operations, stockholder’s equity and cash flows for the years ended November 30, 2005 and 2004. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The financial statements show the Company has no revenues and relies upon its parent Company to fund its operations. This factor raises substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Millenia Hope Pharmaceuticals, Inc. as of November 30, 2005, and the results of its operations and cash flows for the years ended November 30, 2005 and 2004, in conformity with accounting principles generally accepted in the United States of America.
Denver, Colorado
September 5, 2006
Millenia Hope Pharmaceuticals Inc.
(A Company in the Development Stage)
Balance Sheet
November 30, 2005
Assets
| | | | |
Current Assets | | | | |
Cash | | $ | — | |
| |
|
| |
| | | | |
Liabilities & Stockholder’s Equity | | | | |
| | | | |
Current Liabilities | | $ | — | |
| |
|
| |
| | | | |
Stockholder’s Equity | | | | |
Preferred Stock, $.0001 par value; 50,000,000 shares authorized, none issued or outstanding | | | — | |
Common Stock, $.0001 par value; 280,000,000 shares authorized, 40,000,000 issued and outstanding | | | 4,000 | |
Common Stock Subscribed | | | (3,458 | ) |
(Deficit) accumulated during the development stage | | | (542 | ) |
| |
|
| |
| | | — | |
| |
|
| |
| | | | |
| | $ | — | |
| |
|
| |
See the accompanying notes to the financial statements.
3
Millenia Hope Pharmaceuticals Inc.
(A Company in the Development Stage)
Statements of Operations
| | | | | | | | | | |
| | Year
ended
November 30,
2005 | | Year
ended
November 30,
2004 | | Inception
(January 13,
2000) to
November 30,
2005 | |
| |
| |
| |
| |
| | | | | | | | | | |
Revenues | | $ | — | | $ | — | | $ | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Operating Expenses | | | | | | | | | | |
Selling, General and Administrative | | | 298 | | | 122 | | | 542 | |
| |
|
| |
|
| |
|
| |
| | | 298 | | | 122 | | | 542 | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Operating (Loss) | | | (298 | ) | | (122 | ) | | (542 | ) |
| | | | | | | | | | |
Other Income (Expense) | | | — | | | — | | | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Net (Loss) | | $ | (298 | ) | $ | (122 | ) | $ | (542 | ) |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Per Share Information - Basic and Diluted: | | | | | | | | | | |
Weighted Average Number of Common Shares Outstanding | | | 40,000,000 | | | 40,000,000 | | | | |
| |
|
| |
|
| | | | |
| | | | | | | | | | |
(Loss) Per Common Share | | $ | (0.00 | ) | $ | (0.00 | ) | | | |
| |
|
| |
|
| | | | |
See the accompanying notes to the financial statements.
4
Millenia Hope Pharmaceuticals, Inc.
(A Company in the Development Stage)
Statement of Stockholder’s Equity (Deficit)
January 13, 2000 (inception) to November 30, 2005
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | Common Stock
Subscription | | | | | (Deficit)
Accumulated
during the
Development
Stage | | | | |
| | | | | | | | | | | | | | | Additional
Paid-in
Capital | | | | | |
| | Common Stock | | Preferred Stock | | | | | Total
Stockholder’s
Equity (Deficit) | |
| |
| |
| | | | | |
| | Shares | | Amount | | Shares | | Amount | | | | | |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, January 13, 2000 | | | — | | $ | — | | | — | | $ | — | | $ | — | | $ | — | | $ | — | | $ | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Stock issued for subscription | | | 10,000 | | | 1 | | | — | | | — | | | (1 | ) | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Net (loss) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at November 30, 2000 | | | 10,000 | | | 1 | | | — | | | — | | | (1 | ) | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Net (loss) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at November 30, 2001 | | | 10,000 | | | 1 | | | — | | | — | | | (1 | ) | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Stock issued for subscription | | | 39,990,000 | | | 3,999 | | | — | | | — | | | (3,999 | ) | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Net (loss) | | | — | | | — | | | — | | | — | | | — | | | — | | | — | | | — | |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at November 30, 2002 | | | 40,000,000 | | | 4,000 | | | — | | | — | | | (4,000 | ) | | — | | | — | | | — | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Net (loss) | | | — | | | — | | | — | | | — | | | — | | | — | | | (122 | ) | | (122 | ) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at November 30, 2003 | | | 40,000,000 | | | 4,000 | | | — | | | — | | | (4,000 | ) | | — | | | (122 | ) | | (122 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Net (loss) | | | — | | | — | | | — | | | — | | | — | | | — | | | (122 | ) | | (122 | ) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at November 30, 2004 | | | 40,000,000 | | | 4,000 | | | — | | | — | | | (4,000 | ) | | — | | | (244 | ) | | (244 | ) |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Operating expenses paid for by parent | | | — | | | — | | | — | | | — | | | 542 | | | — | | | — | | | 542 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
Net (loss) | | | — | | | — | | | — | | | — | | | — | | | — | | | (298 | ) | | (298 | ) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at November 30, 2005 | | | 40,000,000 | | $ | 4,000 | | $ | — | | $ | — | | $ | (3,458 | ) | $ | — | | $ | (542 | ) | $ | — | |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
See accompanying notes to the financial statements.
5
Millenia Hope Pharmaceuticals Inc.
(A Company in the Development Stage)
Statements of Cash Flows
| | | | | | | | | | |
| | Year
ended
November 30,
2005 | | Year
ended
November 30,
2004 | | Inception
(January 13,
2000) to
November 30,
2005 | |
| |
| |
| |
| |
Operating Activities | | | | | | | | | | |
Net (loss) | | $ | (298 | ) | $ | (122 | ) | $ | (542 | ) |
Expenses paid for by parent | | | 542 | | | | | | 542 | |
Changes in: | | | | | | | | | | |
Accounts payable | | | (244 | ) | | 122 | | | — | |
| |
|
| |
|
| |
|
| |
Cash (used in) operating activities | | | — | | | — | | | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Financing Activities | | | | | | | | | | |
Cash provided by financing activities | | | — | | | — | | | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Investing activities | | | | | | | | | | |
Cash provided by investing activities | | | — | | | — | | | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Increase (decrease) in cash | | | — | | | — | | | — | |
| | | | | | | | | | |
Cash and cash equivalents | | | | | | | | | | |
Beginning of period | | | — | | | — | | | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
End of period | | $ | — | | $ | — | | $ | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Cash paid for interest | | $ | — | | $ | — | | $ | — | |
| |
|
| |
|
| |
|
| |
Cash paid for taxes | | $ | — | | $ | — | | $ | — | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
See the accompanying notes to the financial statements.
6
Millenia Hope Pharmaceuticals Inc.
(A Company in the Development Stage)
Notes to Financial Statements
November 30, 2005
| |
1. | Organization and Basis of Presentation |
| |
| Millenia Hope Pharmaceuticals Inc. (the “Company”) was incorporated in the Canada under the Canadian Business Corporations Act on January 13, 2000. Through its patented proprietary Phytomics Technologies, the Company focuses on exploiting major advancements in accessing one of the most productive sources of biologically active compounds, plants, to discover, develop and produce new products for human health and well being. The Company commenced its commercial activity on February 14, 2006. |
| |
2. | Summary of Significant Accounting Policies |
| |
| Use of Estimates |
| |
| The preparation of financial statements in conformity with generally accepted accounting principles in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Certain amounts included in the financial statements are estimated based on currently available information and management’s judgement as to the outcome of future conditions and circumstances. |
| |
| Changes in the status of certain facts or circumstances could result in material changes to the estimates used in the preparation of financial statements and actual results could differ from the estimates and assumptions. |
| |
| Fair Value of Financial Instruments |
| |
| Fair value estimates discussed herein are based upon certain market assumptions and pertinent information available to management as of November 30, 2005. The respective carrying value of certain on-balance-sheet financial instruments approximated their fair values. These financial instruments include cash, and accounts payable. Fair values were assumed to approximate carrying values for financial instruments because they are short term in nature, their carrying amounts approximate fair values, and they are payable on demand. |
| |
| Cash and Cash Equivalents |
| |
| The Company considers all highly liquid investments with original maturities of ninety days or less and bank indebtedness to be cash and cash equivalents. |
| |
| Revenue Recognition |
| |
| The Company records revenue when persuasive evidence of an arrangement exists, services have been rendered or product delivery has occurred, the sales price to the customer is fixed or determinable, and collectibility is reasonably assured. |
-1-
Millenia Hope Pharmaceuticals Inc.
(A Company in the Development Stage)
Notes to Financial Statements
November 30, 2005
| | | |
| Earnings (Loss) Per Share |
| | | |
| The Company follows Statement of Financial Accounting Standards (“SFAS”) 128, “Earnings Per Share.” Basic earnings (loss) per common share (“EPS”) calculations are determined by dividing net income (loss) by the weighted average number of shares of common stock outstanding during the year. Diluted earnings per common share calculations are determined by dividing net income (loss) by the weighted average number of common shares and dilutive common share equivalents outstanding. During the periods presented common stock equivalents were not considered, as their effect would be anti-dilutive. |
| | | |
| Recent Accounting Pronouncements |
| | | |
| In March 2006, Financial Accounting Standards Board (FASB) issued Statement of Financial Accounting Standards (SFAS) No. 156, “Accounting for Servicing of Financial Assets—an amendment of FASB Statement No. 140,” with respect to the accounting for separately recognized servicing assets and servicing liabilities. This Statement: |
| | | |
| | 1. | Requires an entity to recognize a servicing asset or servicing liability each time it undertakes an obligation to service a financial asset by entering into a servicing contract in certain situations. |
| | | |
| | 2. | Requires all separately recognized servicing assets and servicing liabilities to be initially measured at fair value, if practicable. |
| | | |
| | 3. | Permits an entity to choose either the amortization method or the fair value measurement method for each class of separately recognized servicing assets and servicing liabilities. |
| | | |
| | 4. | At its initial adoption, permits a one-time reclassification of available-for-sale securities to trading securities by entities with recognized servicing rights, without calling into question the treatment of other available-for-sale securities under SFAS No. 115, provided that the available-for-sale securities are identified in some manner as offsetting the entity’s exposure to changes in fair value of servicing assets or servicing liabilities that a servicer elects to subsequently measure at fair value. |
| | | |
| | 5. | Requires separate presentation of servicing assets and servicing liabilities subsequently measured at fair value in the statement of financial position and additional disclosures for all separately recognized servicing assets and servicing liabilities. |
| | | |
| Adoption of this Statement is required as of the beginning of the first fiscal year that begins after September 15, 2006. The adoption of this statement is not expected to have a material impact on the Company’s financial statements. |
| | | |
| In February 2006, the FASB issues SFAS No. 155, “Accounting for Certain Hybrid Financial Instruments—an amendment of FASB Statements No. 133 and 140.” This amends SFAS No. 133, “Accounting for Derivative Instruments and Hedging Activities,” and No. 140, “Accounting for Transfers and Servicing of Financial Assets and Extinguishments of Liabilities.” This Statement resolves issues addressed in SFAS No. 133 Implementation Issue No. D1, “Application of Statement 133 to Beneficial Interests in Securitized Financial Assets.” |
-2-
Millenia Hope Pharmaceuticals Inc.
(A Company in the Development Stage)
Notes to Financial Statements
November 30, 2005
| | | |
| This Statement: |
| | | |
| | a. | Permits fair value remeasurement for any hybrid financial instrument that contains an embedded derivative that otherwise would require bifurcation |
| | | |
| | b. | Clarifies which interest-only strips and principal-only strips are not subject to the requirements of SFAS No. 133 |
| | | |
| | c. | Establishes a requirement to evaluate interests in securitized financial assets to identify interests that are freestanding derivatives or that are hybrid financial instruments that contain an embedded derivative requiring bifurcation |
| | | |
| | d. | Clarifies that concentrations of credit risk in the form of subordination are not embedded derivatives |
| | | |
| | e. | Amends SFAS No. 140 to eliminate the prohibition on a qualifying special-purpose entity from holding a derivative financial instrument that pertains to a beneficial interest other than another derivative financial instrument. |
| | | |
| This Statement is effective for all financial instruments acquired or issued after the first fiscal year that begins after September 15, 2006. |
| | | |
| The fair value election provided for in paragraph 4(c) of this Statement may also be applied upon adoption of this Statement for hybrid financial instruments that had been bifurcated under paragraph 12 of SFAS No. 133 prior to the adoption of this Statement. Earlier adoption is permitted as of the beginning of our fiscal year, provided we have not yet issued financial statements, including financial statements for any interim period, for that fiscal year. Provisions of this Statement may be applied to instruments that we hold at the date of adoption on an instrument-by-instrument basis. |
| | | |
| The Company is currently reviewing the effects of adoption of this statement but it is not expected to have a material impact on its financial statements. |
| | | |
| In May 2005, the FASB issued SFAS No. 154, “Accounting Changes and Error Corrections--a replacement of APB Opinion No. 20 and FASB Statement No. 3.” This Statement replaces APB Opinion No. 20, “Accounting Changes,” and SFAS No. 3, “Reporting Accounting Changes in Interim Financial Statements,” and changes the requirements for the accounting for, and reporting of, a change in accounting principle. This Statement applies to all voluntary changes in accounting principle. It also applies to changes required by an accounting pronouncement in the unusual instance that the pronouncement does not include specific transition provisions. When a pronouncement includes specific transition provisions, those provisions should be followed. |
| | | |
| SFAS No. 154 is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. It will only affect the financial statements of the Company if there is a change any accounting principle. At this time, no such changes are contemplated or anticipated. |
| | | |
| In December 2004, the FASB issued SFAS No. 123(R), “Share-Based Payments.” SFAS No. 123 (R) requires all entities to recognize compensation expense in an amount equal to the fair value of share-based payments such as stock options granted to employees. SFAS No. 123 (R) is effective for the first reporting period beginning after December 15, 2005. The adoption of FAS 123 (R) did not have a material impact on the financial statements. |
| | | |
-3-
Millenia Hope Pharmaceuticals Inc.
(A Company in the Development Stage)
Notes to Financial Statements
November 30, 2005
| |
| The Company accounts for income taxes under SFAS 109, which requires use of the liability method. SFAS 109 provides that deferred tax assets and liabilities are recorded based on the differences between the tax bases of assets and liabilities and their carrying amounts for financial reporting purposes, referred to as temporary differences. Deferred tax assets and liabilities at the end of each period are determined using the currently enacted tax rates applied to taxable income in the periods in which the deferred tax assets and liabilities are expected to be settled or realized. |
| |
4. | Stockholders’ Equity |
| |
| Common Stock |
| |
| On September 1, 2000 the Company entered into a subscription agreement and issued 10,000 shares of its $0.0001 par value common stock at $.0001 per share. |
| |
| On February 14, 2002 the Company entered into a subscription agreement and issued 39,990,000 shares of its $0.0001 par value common stock at $.0001 per share. |
| |
| All of the shares were issued to the parent company, Millenia Hope, Inc. |
| |
5. | Related Party Transactions |
| |
| During the years ended November 30, 2005 and 2004, the sole shareholder paid expenses of the Company in the amounts of $298 and $122, respectively. The Company reflected these payments as reductions in the Common Stock Subscribed account. For the period, inception (January 13, 2000) to November 30, 2005, the shareholder paid a total of $542 of such expenses. |
| |
6. | Subsequent Events |
| |
| On February 14, 2006, the Company obtained a cash advance from their parent company, Millenia Hope, Inc., and purchased research equipment and intellectual property from Avance Pharma, an unrelated third party, for $526,070. |
| |
| On June 5, 2006, the Board of Directors of the parent company authorized the payment of a dividend to all shareholders of the parent company of record on July 3, 2006. The dividend consists of 6% of the issued shares of the Company, or a total of 2,400,000 shares, of the shares held by the parent. The effect of this transaction is to decrease the ownership by the parent company by 2,400,000 shares and increase the number of shareholders to approximately 2,000. |
| |
| On April 11, 2006, the Company entered into an agreement with Pierre Fabre, a French pharmaceutical company. During the period ended May 31, 2006, the Company received an initial revenue payment from Pierre Fabre of $150,325. |
-4-
Millenia Hope Pharmaceuticals Inc.
(A Development Stage Enterprise)
Balance Sheet
May 31, 2006
(Unaudited)
| | | | |
Assets | | | | |
Current Assets | | | | |
Cash | | $ | 3 | |
Deposits | | | 900 | |
Consumption taxes receivable | | | 67,155 | |
| |
|
| |
Total Current Assets | | | 68,058 | |
| |
|
| |
| | | | |
Property and equipment, net | | | 499,957 | |
| |
|
| |
| | | | |
| | $ | 568,015 | |
| |
|
| |
| | | | |
Liabilities & Stockholders’ Equity | | | | |
Current Liabilities | | | | |
Accounts payable and accrued liabilities | | $ | 41,513 | |
Due to parent company | | | 12,985 | |
| |
|
| |
Total Current Liabilities | | | 54,498 | |
| |
|
| |
| | | | |
| | | | |
Stockholders’ Equity | | | | |
Preferred Stock, 6% cumulative, $.0001 par value; 50,000,000 shares authorized, 829,554 issued and outstanding | | | 829,554 | |
Common Stock, $.0001 par value; 280,000,000 shares authorized, 40,000,000 issued and outstanding | | | 4,000 | |
(Deficit) accumulated during the development stage | | | (320,037 | ) |
| |
|
| |
| | | 513,517 | |
| |
|
| |
| | | | |
| | $ | 568,015 | |
| |
|
| |
See the accompanying notes to the financial statements.
Millenia Hope Pharmaceuticals Inc.
(A Development Stage Enterprise)
Statements of Operations
(Unaudited)
| | | | | | | | | | | | | | | | |
| | Three months
ended
May 31,
2006 | | Three months
ended
May 31,
2005 | | Six months
ended
May 31,
2006 | | Six months
ended
May 31,
2005 | | Inception
(January 13, 2000)
to May 31,
2006 | |
| |
| |
| |
| |
| |
| |
| | | | | | | | | | | | | | | | |
Revenues | | $ | 150,325 | | $ | — | | $ | 231,785 | | $ | — | | $ | 231,785 | |
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| | | | | | | | | | | | | | | | |
Operating Expenses | | | | | | | | | | | | | | | | |
Biotech wages | | | 143,926 | | | — | | | 165,902 | | | — | | | 165,902 | |
Administrative salaries | | | — | | | — | | | — | | | — | | | — | |
Marketing | | | 10,836 | | | — | | | 10,836 | | | — | | | 10,836 | |
Research and development | | | 55,080 | | | — | | | 55,080 | | | — | | | 55,080 | |
Selling, general and administrative | | | 291,993 | | | — | | | 307,019 | | | — | | | 307,561 | |
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| | | 501,835 | | | — | | | 538,837 | | | — | | | 539,379 | |
| |
|
| |
|
| |
|
| |
|
| |
|
| |
Operating (loss) | | | (351,510 | ) | | — | | | (307,052 | ) | | — | | | (307,594 | ) |
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| | | | | | | | | | | | | | | | |
Other income (expense) | | | | | | | | | | | | | | | | |
Interest expense | | | — | | | — | | | (12,443 | ) | | — | | | (12,443 | ) |
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| | | — | | | — | | | (12,443 | ) | | — | | | (12,443 | ) |
| |
|
| |
|
| |
|
| |
|
| |
|
| |
Net (loss) | | $ | (351,510 | ) | $ | — | | $ | (319,495 | ) | $ | — | | $ | (320,037 | ) |
| |
|
| |
|
| |
|
| |
|
| |
|
| |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Per share information - basic and diluted: | | | | | | | | | | | | | | | | |
Weighted Average Number of Common shares outstanding | | | 40,000,000 | | | 40,000,000 | | | 40,000,000 | | | 40,000,000 | | | | |
| |
|
| |
|
| |
|
| |
|
| | | | |
| | | | | | | | | | | | | | | | |
Net (Loss) Per Common Share | | $ | — | | $ | — | | $ | — | | $ | — | | | | |
| |
|
| |
|
| |
|
| |
|
| | | | |
See the accompanying notes to the financial statements.
Millenia Hope Pharmaceuticlas Inc.
(A Development Stage Enterprise)
Statements of Cash Flows
(Unaudited)
| | | | | | | | | | |
| | Six months
ended
May 31,
2006 | | Six months
ended
May 31,
2005 | | Inception
(January 13, 2000)
to May 31,
2006 | |
| |
| |
| |
| |
Operating Activities | | | | | | | | | | |
Cash (used in) operating activities | | $ | (319,724 | ) | $ | — | | $ | (320,266 | ) |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Financing Activities | | | | | | | | | | |
Related party payable, net | | | 12,985 | | | — | | | 12,985 | |
Proceeds from stock subscription receivable | | | 3,458 | | | — | | | 4,000 | |
Issuance of preferred stock for cash | | | 829,554 | | | — | | | 829,554 | |
| |
|
| |
|
| |
|
| |
Cash provided by financing activities | | | 845,997 | | | — | | | 846,539 | |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Investing Activities | | | | | | | | | | |
Purchase of Avance assets | | | (526,270 | ) | | — | | | (526,270 | ) |
| |
|
| |
|
| |
|
| |
Cash flows (used in) investing activities | | | (526,270 | ) | | — | | | (526,270 | ) |
| |
|
| |
|
| |
|
| |
| | | | | | | | | | |
Increase in cash | | | 3 | | | — | | | 3 | |
| | | | | | | | | | |
Cash and cash equivalents | | | | | | | | | | |
Beginning of period | | | — | | | — | | | — | |
| |
|
| |
|
| |
|
| |
End of period | | $ | 3 | | $ | — | | $ | 3 | |
| |
|
| |
|
| |
|
| |
See the accompanying notes to the financial statements.
MILLENIA HOPE PHARMACEUTICALS INC.
NOTES TO THE INTERIM FINANCIAL STATEMENTS
MAY 31, 2006
(UNAUDITED)
NOTE 1. DESCRIPTION OF BUSINESS
Millenia Hope Pharmaceuticals Inc. (the “Company”) was incorporated in Canada on January 13, 2000 under the Canada Business Corporations Act, or CBCA. The Company does business as Millenia Hope Biopharma (MH-B) and is a Montreal-based biotechnology company focused on exploiting major advancements in accessing a source of biologically active compounds, plants, to discover, develop and produce new products for human health and well being. The Company commenced commercial activity on February 14, 2006, upon completion of the acquisition of assets from a third party. The Company is a majority owned subsidiary of Millenia Hope Inc.
The Company has developed patented proprietary enabling Phytomics Technologies that allow access to the potential of the plant kingdom’s untapped diversity of chemicals found therein. To exploit its unique chemical capabilities, the Company has developed a distinctive Plant Product Discovery Platform combining today’s biological target-based discovery processes and Phytomics to discover novel products for the health industry.
MH-B is leveraging its Phytomics and Plant Product Discovery Platforms to build a high-value proprietary and partnered pipeline of important plant-derived products for the pharmaceutical, cosmetic and nutraceutical industries and to develop proprietary Phytomics-based bioprocesses for the industrial scale production of complex phytoproducts of commercial relevance.
NOTE 2. BASIS OF PRESENTATION
The accompanying unaudited financial statements of Millenia Hope Pharmaceuticals Inc. have been prepared in accordance with generally accepted accounting principles for interim financial information and with the instructions to Form 10-QSB and Rule 10-01 of Regulation S-X. The financial statements reflect all adjustments consisting of normal recurring adjustments which, in the opinion of management, are necessary for a fair presentation of the results for the periods shown. Accordingly, they do not include all of the information and footnotes required by generally accepted accounting principles for complete financial statements. These financial statements should be read in conjunction with the audited financial statements and footnotes thereto of the Company as of November 30, 2005.
NOTE 3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Certain amounts included in the financial statements are estimated based on currently available information and management’s judgment as to the outcome of future conditions and circumstances. Changes in the status of certain facts or circumstances could result in material changes to the estimates used in the preparation of financial statements and actual results could differ from the estimates and assumptions.
Earnings (Loss) Per Share
The Company follows Statement of Financial Accounting Standards (“SFAS”) 128, “Earnings Per Share.” Basic earnings (loss) per common share (“EPS”) calculations are determined by dividing net income (loss) by the weighted average number of shares of common stock outstanding during the year. Diluted earnings per common share calculations are determined by dividing net income (loss) by the weighted average number of common shares and dilutive common share equivalents outstanding. During the periods presented common stock equivalents were not considered, as their effect would be anti-dilutive.
Property and Equipment
Property and equipment is recorded at cost and depreciated using the straight-line method over the estimated useful life of the asset, generally 3-5 years.
Repairs and maintenance are charged to operations as incurred and expenditures for significant improvements are capitalized. The cost of property and equipment retired or sold, together with the related accumulated depreciation, are removed from the appropriate asset and depreciation accounts, and the resulting gain or loss is included in operations.
NOTE 4. STOCKHOLDERS’ EQUITY
During the quarter ended May 31, 2006, the Company issued 829,554 shares of 6% Cumulative Preferred Stock at $1.00 per share, in payment of advances from shareholder of $829,554.
NOTE 5. PROPERTY AND EQUIPMENT
On February 14, 2006, the Company purchased intellectual property and research equipment from Avance Pharma, an unrelated party, for $526,270 in cash. During the quarter ended May 31, 2006, the Company recorded depreciation expense of $26,313.
NOTE 6. SUBSEQUENT EVENTS
On June 5, 2006, the Board of Directors of the parent company authorized the payment of a dividend to all shareholders of the parent company of record on July 3, 2006. The dividend consists of 6% of the issued shares, or a total of 2,400,000 shares, of the shares held by the parent. The effect of this transaction is to decrease the ownership by the parent company by 2,400,000 shares and increase the number of shareholders to approximately 2,000.