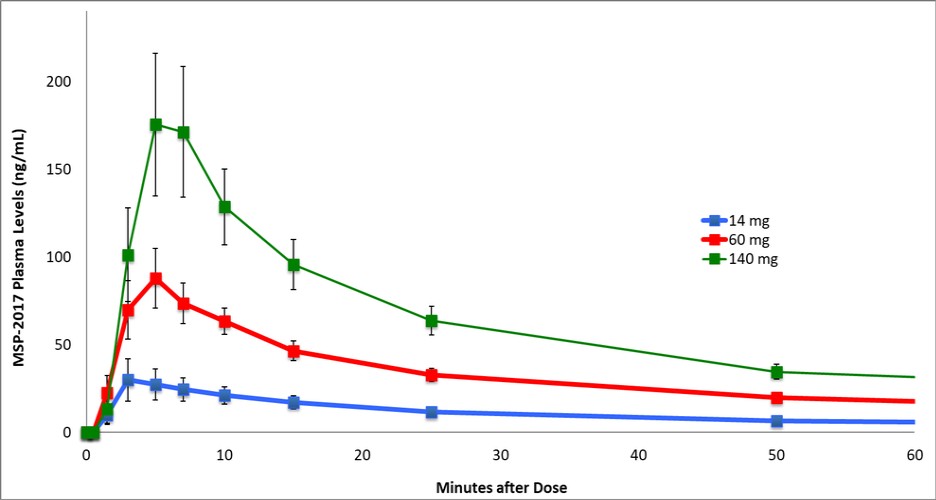
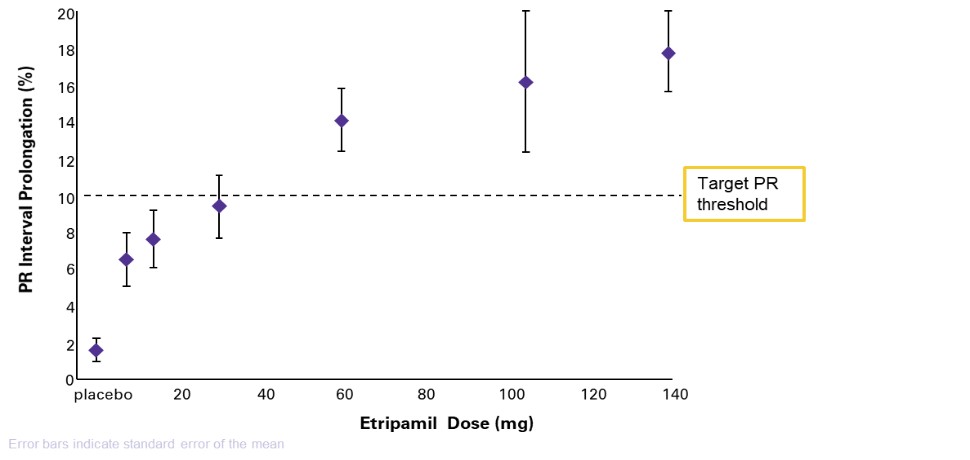
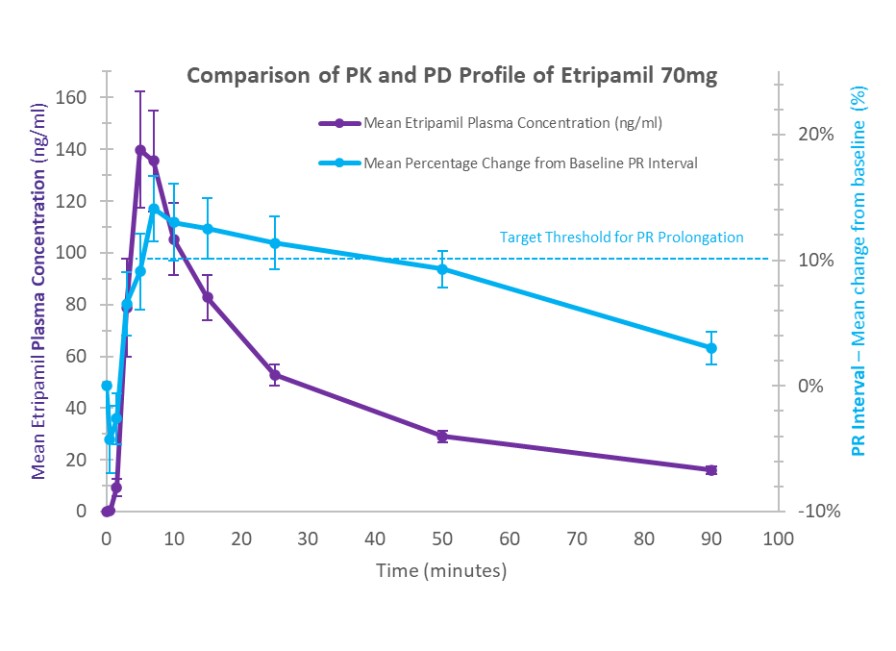
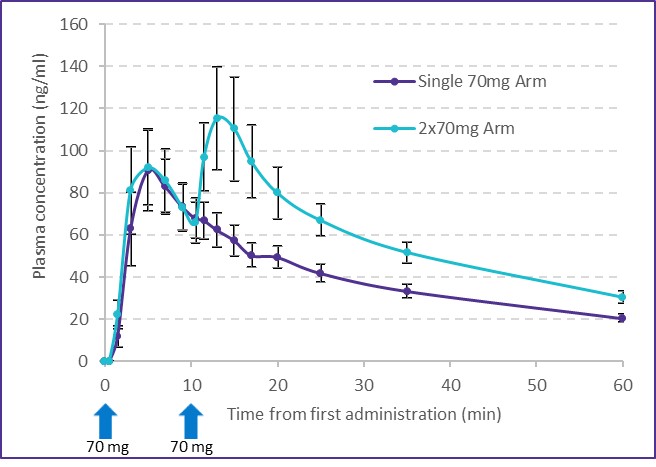
United States and in foreign countries. It is also possible that a competitor may develop a cure or more effective treatment method for the diseases we are targeting, which could render our current or future product candidates non-competitive or obsolete, or reduce the demand for our product candidates before we can recover our development and commercialization expenses.
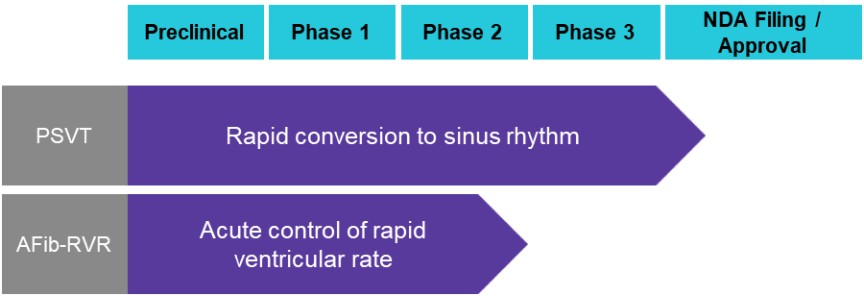
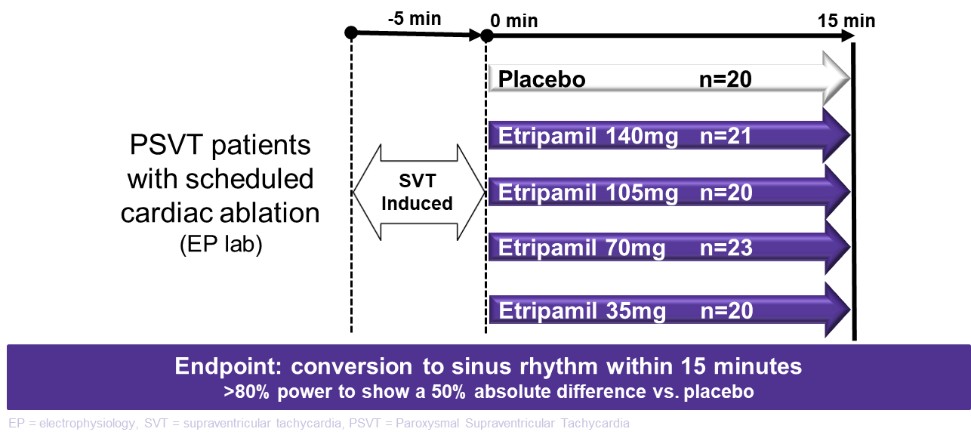
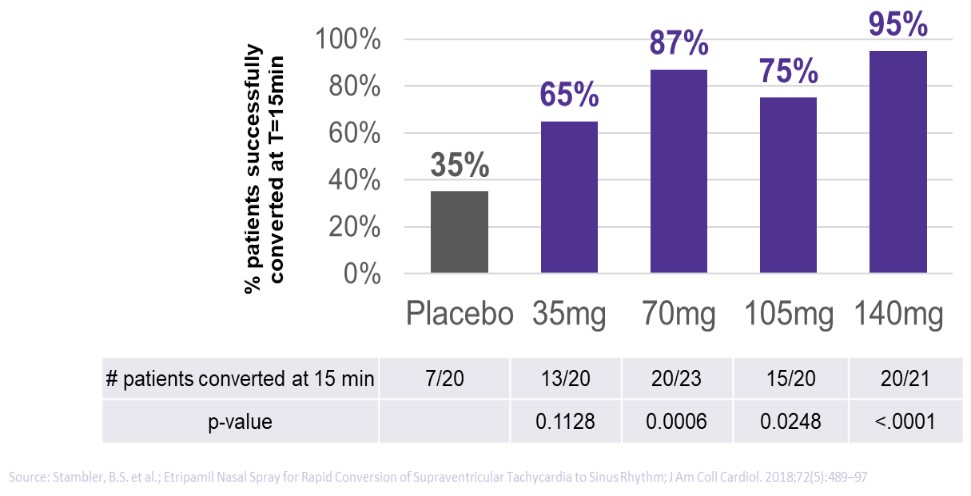
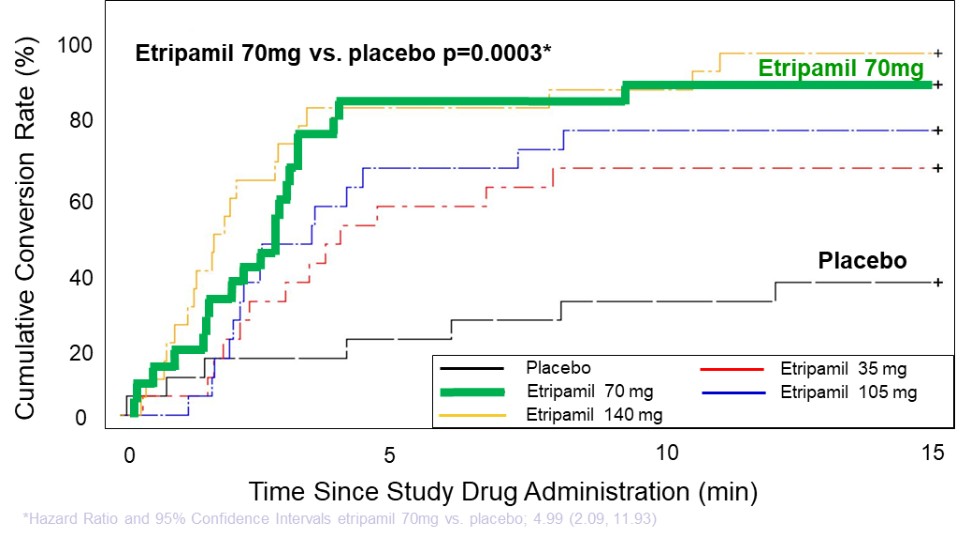
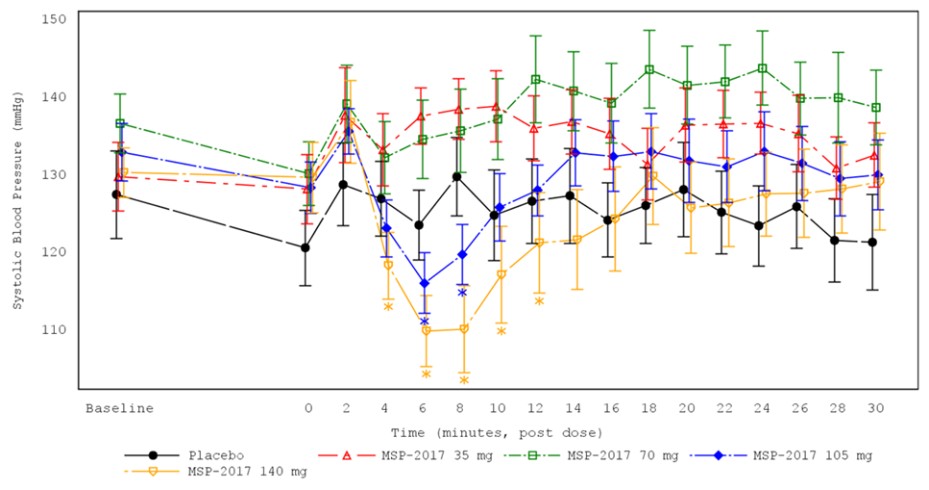
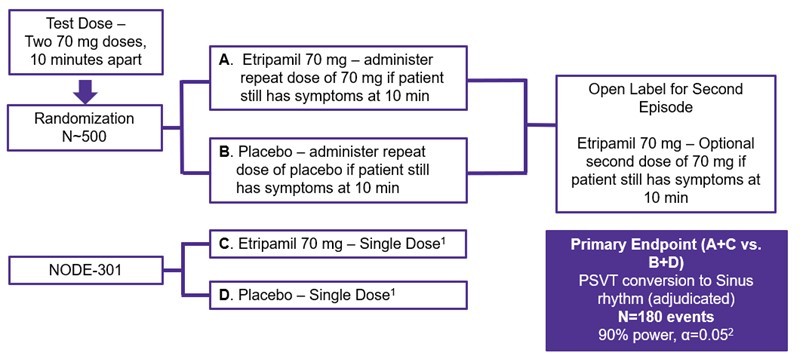
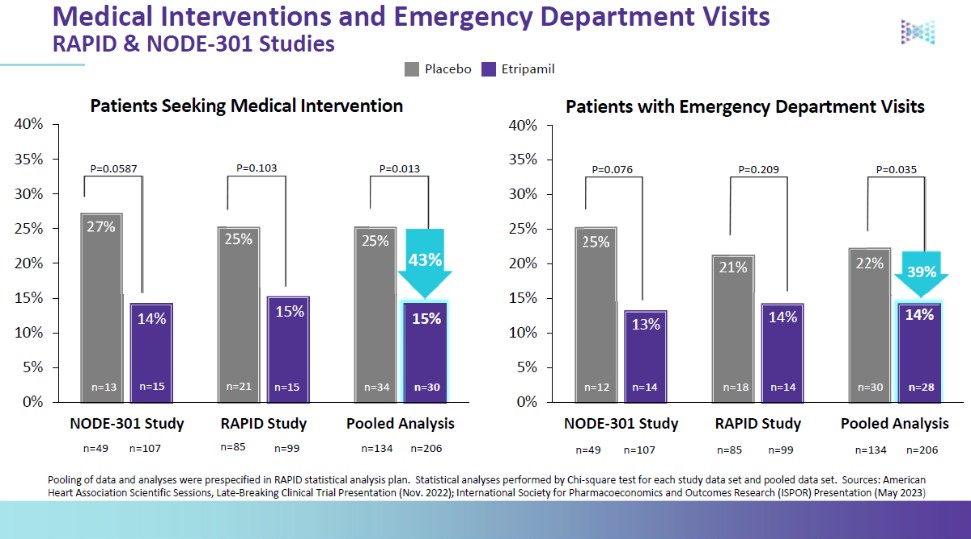
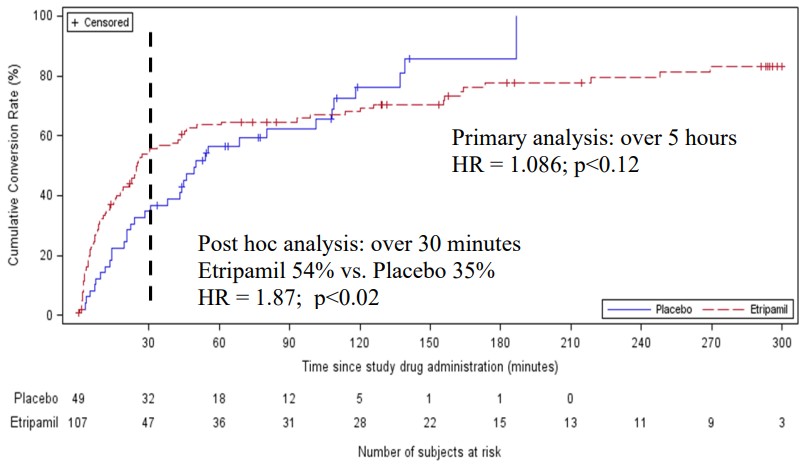
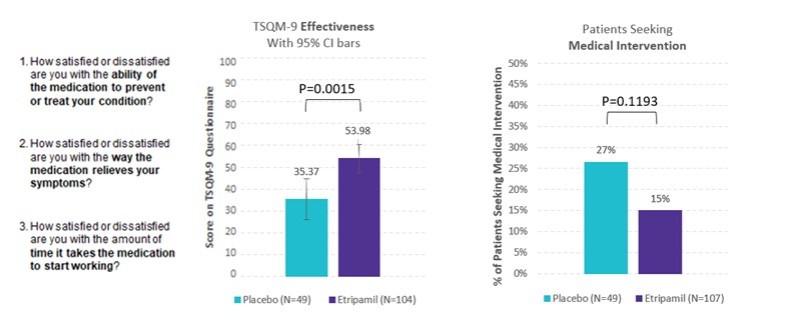
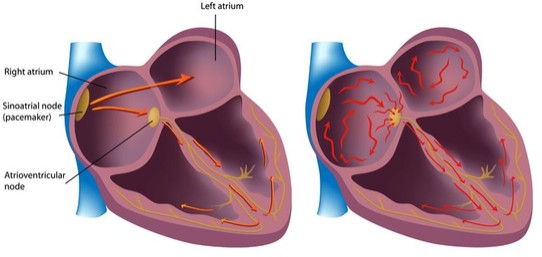
We are not aware of any approved drug or any drug candidate in clinical development for a patient with PSVT to self-administer treatment to terminate SVT episodes. In the acute setting, IV treatments of generic drugs such as adenosine, verapamil and diltiazem, are routinely given. Additionally, some practitioners prescribe oral medications, such as calcium channel blockers, beta blockers and antiarrhythmics to be taken at the onset of an episode. However, these interventions are not acutely effective and are not approved by the FDA or other regulatory agencies for this use.
For atrial fibrillation, there are a number of marketed generic antiarrhythmic drugs that are used for chronic and/or acute rate control, such as metoprolol, propranolol, esmolol, pindolol, atenolol, nadolol, verapamil and diltiazem. We are aware of several drugs or new formulations of existing drugs under development or recently under development for atrial fibrillation, including InRhythm (flecainide), a sodium channel blocker in Phase 3 from InCarda Therapeutics, Inc., and an IV and potentially oral small-molecule SK channel inhibitor being developed by Acesion Pharma for acute conversion and chronic maintenance of sinus rhythm, respectively, in patients with AFib. Acesion’s lead asset, AAP30663, is the IV-formulated short acting AFib conversion therapy for hospital use that has successfully completed a Phase 2 trial.
Intellectual Property
We have filed numerous patent applications pertaining to etripamil and possible future product candidates, formulations containing etripamil, methods of making such formulations and clinical use. We strive to protect and enhance the proprietary technology, invention and improvements that are commercially important to the development of our business by seeking, maintaining, and defending our intellectual property. We also rely on know-how, continuing technological innovation and potential in-licensing opportunities to develop, strengthen and maintain our position in the field of cardiac arrhythmias, such as PSVT, and immediate rate control in atrial fibrillation, as well as other medical conditions affecting the cardiovascular system. Additionally, we intend to rely on regulatory protection afforded through data exclusivity and market exclusivity, as well as patent term extensions, where available.
As of December 31, 2023, our patent portfolio as it pertains to etripamil included:
• a patent family containing six U.S. patents, projected to expire in 2028, a pending U.S. patent application, which, if granted, is projected to expire in 2028, as well as corresponding patents in Australia, Brazil, Canada, China, Europe, Hong Kong, India, Japan, Mexico, New Zealand and South Korea, directed to etripamil, pharmaceutical compositions including etripamil, and uses of etripamil such as to treat cardiac arrhythmias, including PSVT and atrial fibrillation; and
• a patent family containing one U.S. patent, projected to expire in 2036, a pending U.S. patent application, which, if granted, is projected to expire in 2036, as well as corresponding patents in Australia, Brazil, Canada, China, Europe, Hong Kong, Israel, Japan, Mexico, Russia, South Africa, and Ukraine and corresponding patent applications in China, Europe, Hong Kong, India, New Zealand, South Africa, and South Korea, directed to formulations including etripamil, methods of making such formulations, and uses of such formulations to treat cardiac arrhythmias, such as PSVT and atrial fibrillation.
• a patent family containing pending applications in the United States, Canada, and Europe, which, if granted, is projected to expire in 2041, directed to uses of formulations including etripamil to treat cardiac arrhythmias, such as PSVT and atrial fibrillation, or migraines.
The terms of individual patents may vary based on the countries in which they are obtained. Generally, patents issued for applications filed in the United States are effective for 20 years from the earliest effective non-provisional filing date in the absence, for example, of a terminal disclaimer shortening the term of the patent or patent term adjustment increasing the term of the patent. In addition, in certain instances, a patent term can be extended to recapture a portion of the term