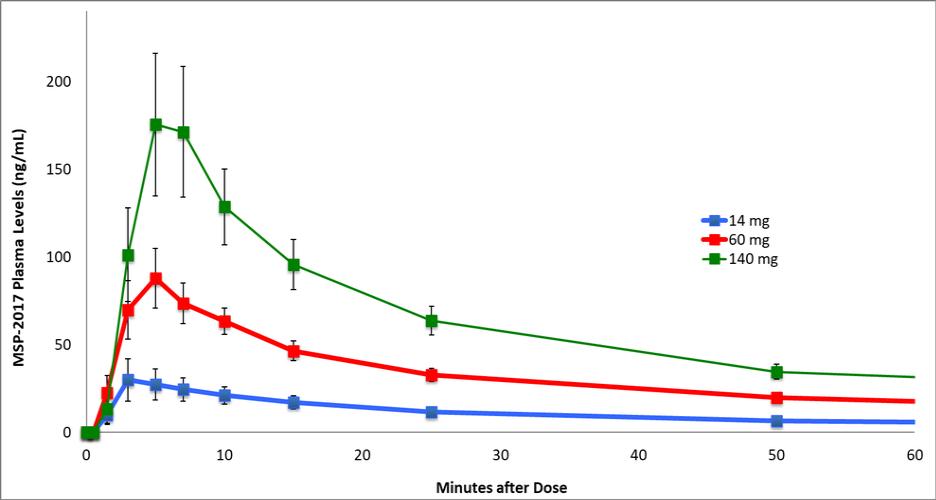
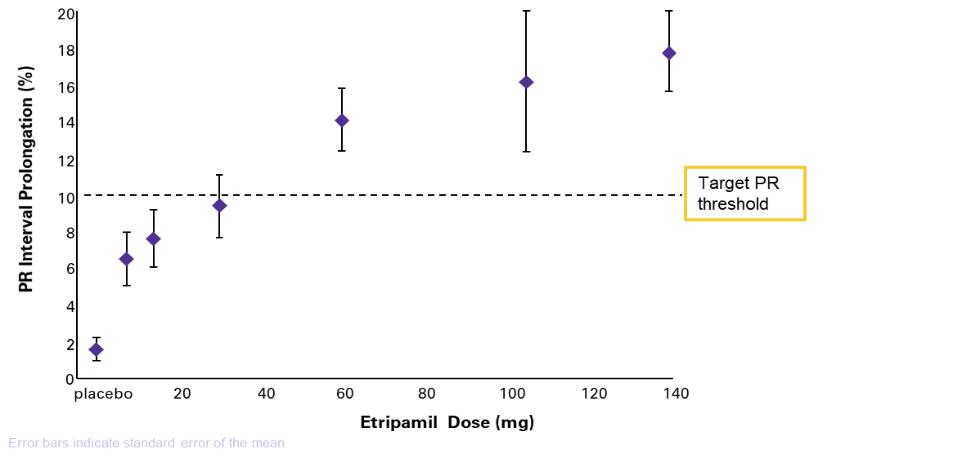
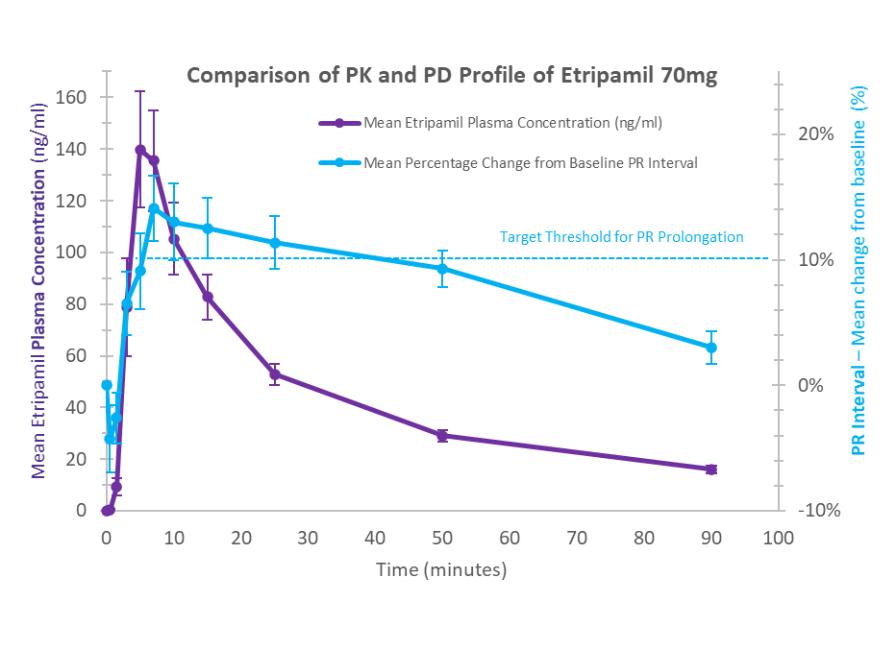
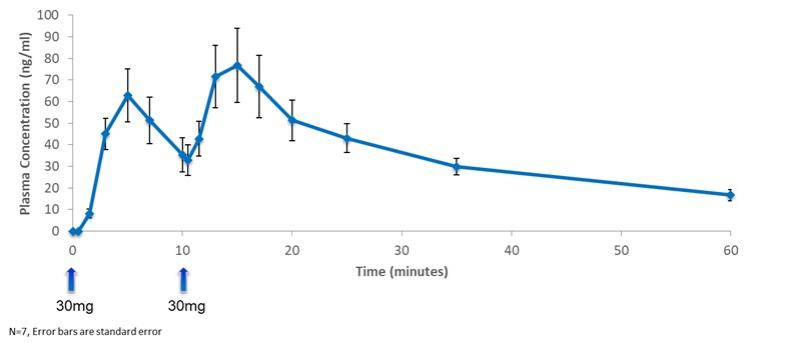
of several drugs or new formulations of existing drugs under development or recently under development for atrial fibrillation, including InRhythm (flecainide), a sodium channel blocker in Phase II from InCarda Therapeutics, Inc., and Gencaro (bucindolol hydrochloride), a beta blocker in Phase 2 from ARCA biopharma, Inc.
Intellectual Property
We have filed numerous patent applications pertaining to etripamil and possible future product candidates, formulations containing etripamil, methods of making such formulations and clinical use. We strive to protect and enhance the proprietary technology, invention and improvements that are commercially important to the development of our business by seeking, maintaining, and defending our intellectual property. We also rely on know-how, continuing technological innovation and potential in-licensing opportunities to develop, strengthen and maintain our position in the field of cardiac arrhythmias, such as PSVT, and immediate rate control in atrial fibrillation, as well as other medical conditions affecting the cardiovascular system. Additionally, we intend to rely on regulatory protection afforded through data exclusivity and market exclusivity, as well as patent term extensions, where available.
As of March 21, 2022, our patent portfolio as it pertains to etripamil included:
• a patent family containing six U.S. patents, projected to expire in 2028, a pending U.S. patent application, which, if granted, is projected to expire in 2028, as well as corresponding patents in Australia, Brazil, Canada, China, Europe, Hong Kong, India, Japan, Mexico, New Zealand and South Korea, directed to etripamil, pharmaceutical compositions including etripamil, and uses of etripamil such as to treat angina or cardiac arrhythmias, including PSVT and atrial fibrillation; and
• a patent family containing one U.S. patent, projected to expire in 2036, a pending U.S. patent application, which, if granted, is projected to expire in 2036, as well as corresponding patents in Australia, China, Europe, Hong Kong, Israel, Japan, Mexico, Russia, South Africa, and Ukraine and corresponding patent applications in Brazil, Canada, China, Europe, Hong Kong, India, New Zealand, South Africa, and South Korea, directed to formulations including etripamil, methods of making such formulations, and uses of such formulations to treat angina or cardiac arrhythmias, such as PSVT and atrial fibrillation.
• a patent family containing two pending U.S. provisional patent applications and a pending Canadian patent application, which, if granted, is projected to expire in 2041, directed to uses of formulations including etripamil to treat angina, cardiac arrhythmias, such as PSVT and atrial fibrillation, or migraines.
The terms of individual patents may vary based on the countries in which they are obtained. Generally, patents issued for applications filed in the United States are effective for 20 years from the earliest effective non-provisional filing date in the absence, for example, of a terminal disclaimer shortening the term of the patent or patent term adjustment increasing the term of the patent. In addition, in certain instances, a patent term can be extended to recapture a portion of the term effectively lost as a results of FDA regulatory review periods. The restoration period cannot be longer than five years and the total term, including the restoration period, must not exceed 14 years following FDA approval. The duration of patents outside the United States varies in accordance with provisions of applicable local law, but typically is also 20 years from the earliest non-provisional filing date.
In addition to patents and patent applications that we own, we rely on know-how to develop and maintain our competitive position. We seek to protect our proprietary technology and processes, and obtain and maintain ownership of certain technologies, in part, through confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors and commercial partners.
Our future commercial success depends, in part, on our ability to obtain and maintain patent and other proprietary protection for commercially important technology, inventions and know-how related to our business; defend and enforce our patents; and operate without infringing valid enforceable patents and proprietary rights of third parties. Our ability to stop third parties from making, using, selling, offering to sell or importing our products may depend on the extent to which