Filed by Lantheus Holdings, Inc.
Pursuant to Rule 425 of the Securities Act of 1933
and deemed filed pursuant to Rule 14a-12
of the Securities Exchange Act of 1934
Subject Company: Progenics Pharmaceuticals, Inc.
Commission File No.: 000 – 23143
The following is a slide deck relating to the proposed transaction involving Lantheus Holdings, Inc. and Progenics Pharmaceuticals, Inc. available atwww.lantheusprogenics.transactionannouncement.com.


Safe Harbor Statements Important Information For Investors And Stockholders This document does not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote or approval in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to appropriate registration or qualification under the securities laws of such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the U.S. Securities Act of 1933, as amended. In connection with the proposed transaction, Lantheus Holdings intends to file with the Securities and Exchange Commission (“SEC”) a registration statement on Form S-4 that will include a joint proxy statement of Lantheus Holdings and Progenics that also constitutes a prospectus of Lantheus Holdings. Each of Lantheus Holdings and Progenics also plan to file other relevant documents with the SEC regarding the proposed transaction. Any definitive joint proxy statement/prospectus (if and when available) will be mailed to stockholders of Lantheus Holdings and Progenics. INVESTORS AND SECURITY HOLDERS OF LANTHEUS HOLDINGS AND PROGENICS ARE STRONGLY ENCOURAGED TO READ THE JOINT PROXY STATEMENT/PROSPECTUS AND OTHER DOCUMENTS THAT WILL BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. Investors and security holders will be able to obtain free copies of the registration statement and the joint proxy statement/prospectus (if and when available) and other documents filed with the SEC by Lantheus Holdings or Progenics through the website maintained by the SEC at https://www.sec.gov. Copies of the documents filed with the SEC by Lantheus Holdings will also be available free of charge on Lantheus Holdings’ website at https://www.lantheus.com/ or by contacting Lantheus Holdings’ Investor Relations Department by email at ir@lantheus.com or by phone at (978) 671-8001. Copies of the documents filed with the SEC by Progenics will also be available free of charge on Progenics’ internet website at https://www.progenics.com/ or by contacting Progenics’ Investor Relations Department by email at mdowns@progenics.com or by phone at (646) 975-2533. Certain Information Regarding Participants Lantheus Holdings, Progenics, and their respective directors and executive officers may be considered participants in the solicitation of proxies in connection with the proposed transaction. Information about the directors and executive officers of Lantheus Holdings is set forth in its Annual Report on Form 10-K for the year ended December 31, 2018, which was filed with the SEC on February 20, 2019, its definitive proxy statement for its 2019 annual meeting of stockholders, which was filed with the SEC on March 15, 2019, and its Current Report on Form 8-K, which was filed with the SEC on March 25, 2019. Other information regarding the participants of Lantheus Holdings in the proxy solicitation and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the joint proxy statement/prospectus and other relevant materials to be filed with the SEC regarding the proposed transaction when they become available. Information about the directors and executive officers of Progenics is set forth in its Annual Report on Form 10-K for the year ended December 31, 2018, which was filed with the SEC on March 15, 2019 and amended on April 30, 2019, and its definitive proxy statement for its 2019 annual meeting of stockholders, which was filed with the SEC on May 30, 2019. Other information regarding the participants of Progenics in the proxy solicitations and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the joint proxy statement/prospectus and other relevant materials to be filed with the SEC regarding the proposed transaction when they become available. You may obtain these documents (when they become available) free of charge through the website maintained by the SEC at https://www.sec.gov and from Investor Relations at Lantheus Holdings or Progenics as described above.

Safe Harbor Statements Cautionary Statement Regarding Forward-Looking Statements This document contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks and uncertainties and are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Such statements are based upon current plans, estimates and expectations that are subject to various risks and uncertainties. The inclusion of forward-looking statements should not be regarded as a representation that such plans, estimates and expectations will be achieved. Words such as “anticipate,” “expect,” “project,” “intend,” “believe,” “may,” “will,” “should,” “plan,” “could,” “target,” “contemplate,” “estimate,” “predict,” “potential,” “opportunity,” “creates” and words and terms of similar substance used in connection with any discussion of future plans, actions or events identify forward-looking statements. All statements, other than historical facts, including the expected timing of the closing of the merger; the ability of the parties to complete the merger considering the various closing conditions; the expected benefits of the merger, such as efficiencies, cost savings, synergies, revenue growth, creating shareholder value, growth potential, market profile, enhanced competitive position, and financial strength and flexibility; the competitive ability and position of the combined company; and any assumptions underlying any of the foregoing, are forward-looking statements. Important factors that could cause actual results to differ materially from Lantheus Holdings’ and Progenics’ plans, estimates or expectations could include, but are not limited to: (i) Lantheus Holdings or Progenics may be unable to obtain stockholder approval as required for the merger; (ii) conditions to the closing of the merger may not be satisfied; (iii) the merger may involve unexpected costs, liabilities or delays; (iv) the effect of the announcement of the merger on the ability of Lantheus Holdings or Progenics to retain and hire key personnel and maintain relationships with customers, suppliers and others with whom Lantheus Holdings or Progenics does business, or on Lantheus Holdings’ or Progenics’ operating results and business generally; (v) Lantheus Holdings’ or Progenics’ respective businesses may suffer as a result of uncertainty surrounding the merger and disruption of management’s attention due to the merger; (vi) the outcome of any legal proceedings related to the merger; (vii) Lantheus Holdings or Progenics may be adversely affected by other economic, business, and/or competitive factors; (viii) the occurrence of any event, change or other circumstances that could give rise to the termination of the merger agreement; (ix) risks that the merger disrupts current plans and operations and the potential difficulties in employee retention as a result of the merger; (x) the risk that Lantheus Holdings or Progenics may be unable to obtain governmental and regulatory approvals required for the transaction, or that required governmental and regulatory approvals may delay the transaction or result in the imposition of conditions that could reduce the anticipated benefits from the proposed transaction or cause the parties to abandon the proposed transaction; (xi) risks that the anticipated benefits of the merger or other commercial opportunities may otherwise not be fully realized or may take longer to realize than expected; (xii) the impact of legislative, regulatory, competitive and technological changes; (xiii) expectations for future clinical trials, the timing and potential outcomes of clinical studies and interactions with regulatory authorities; and (xiv) other risks to the consummation of the merger, including the risk that the merger will not be consummated within the expected time period or at all. Additional factors that may affect the future results of Lantheus Holdings and Progenics are set forth in their respective filings with the SEC, including each of Lantheus Holdings’ and Progenics’ most recently filed Annual Report on Form 10-K, subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the SEC, which are available on the SEC’s website at www.sec.gov. Readers are urged to consider these factors carefully in evaluating these forward-looking statements, and not to place undue reliance on any forward-looking statements. Readers should also carefully review the risk factors described in other documents that Lantheus Holdings and Progenics file from time to time with the SEC. The forward-looking statements in this document speak only as of the date of these materials. Except as required by law, Lantheus Holdings and Progenics assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.

Table of Contents Transaction Rationale, Process, and Due Diligence Conclusions Lantheus Plan for Progenics’ Product Portfolio
Lantheus Merger with Progenics Will Create Value for All Shareholders Lantheus has a clear track record of creating significant shareholder value for its private equity and then public investors over the past 10 years Lantheus long-term success is founded in in-house operational excellence, commercial expertise, financial discipline and robust corporate governance Importantly though, Lantheus, based on extensive due diligence, believes the next 12-18 months for Progenics are absolutely critical to maximizing the value of its portfolio and will require significant investment in capital, market-skilled resources, experienced decision-making and capable execution Lantheus is uniquely capable to deploy its proven management team, accomplished Board of Directors and strong balance sheet to acquire, integrate and execute the Progenics transaction Lantheus enthusiastically shares the view of all Progenics shareholders in the long-term growth potential of the Progenics product portfolio Lantheus’ vision for integrating and executing on the Progenics transaction is value-creating for both Lantheus and Progenics’ shareholders Lantheus Track Record and Capabilities Well Positioned to Create Long-Term Shareholder Value

Transaction Rationale, Process, and Due Diligence Conclusions
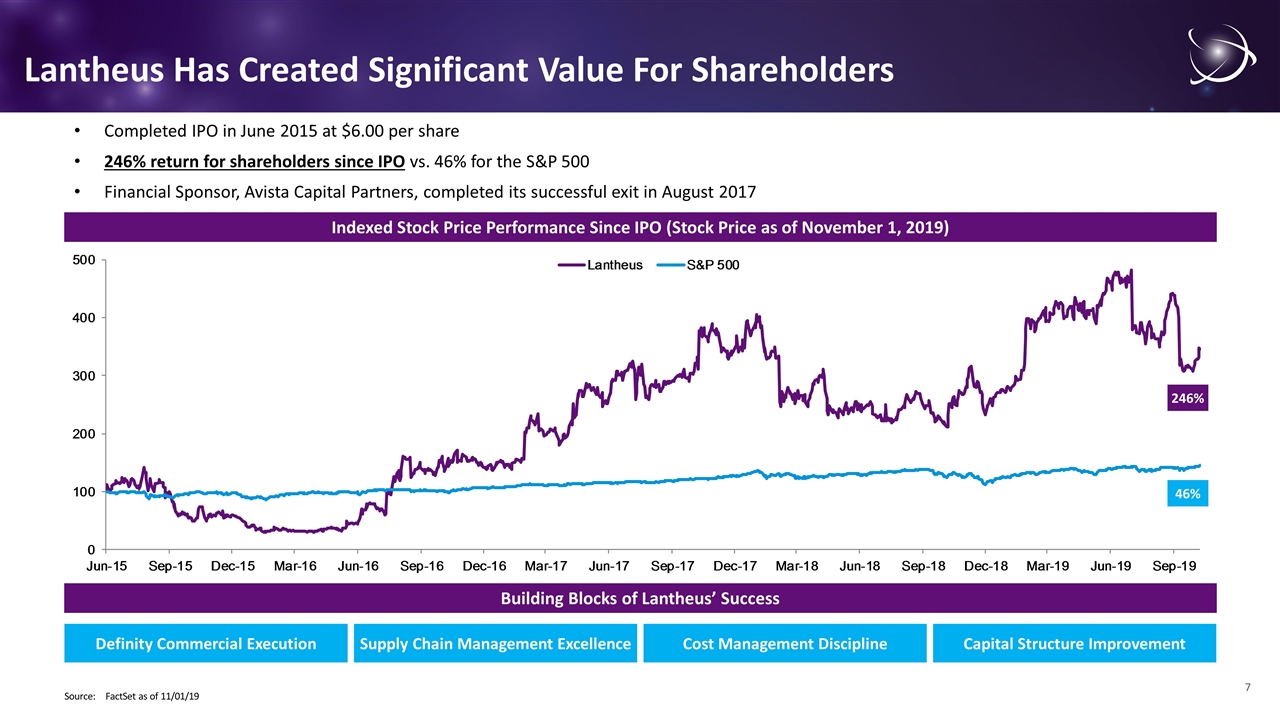
Completed IPO in June 2015 at $6.00 per share 246% return for shareholders since IPO vs. 46% for the S&P 500 Financial Sponsor, Avista Capital Partners, completed its successful exit in August 2017 Indexed Stock Price Performance Since IPO (Stock Price as of November 1, 2019) Source:FactSet as of 11/01/19 Lantheus Has Created Significant Value For Shareholders Building Blocks of Lantheus’ Success Definity Commercial Execution Supply Chain Management Excellence Cost Management Discipline Capital Structure Improvement 246% 46%
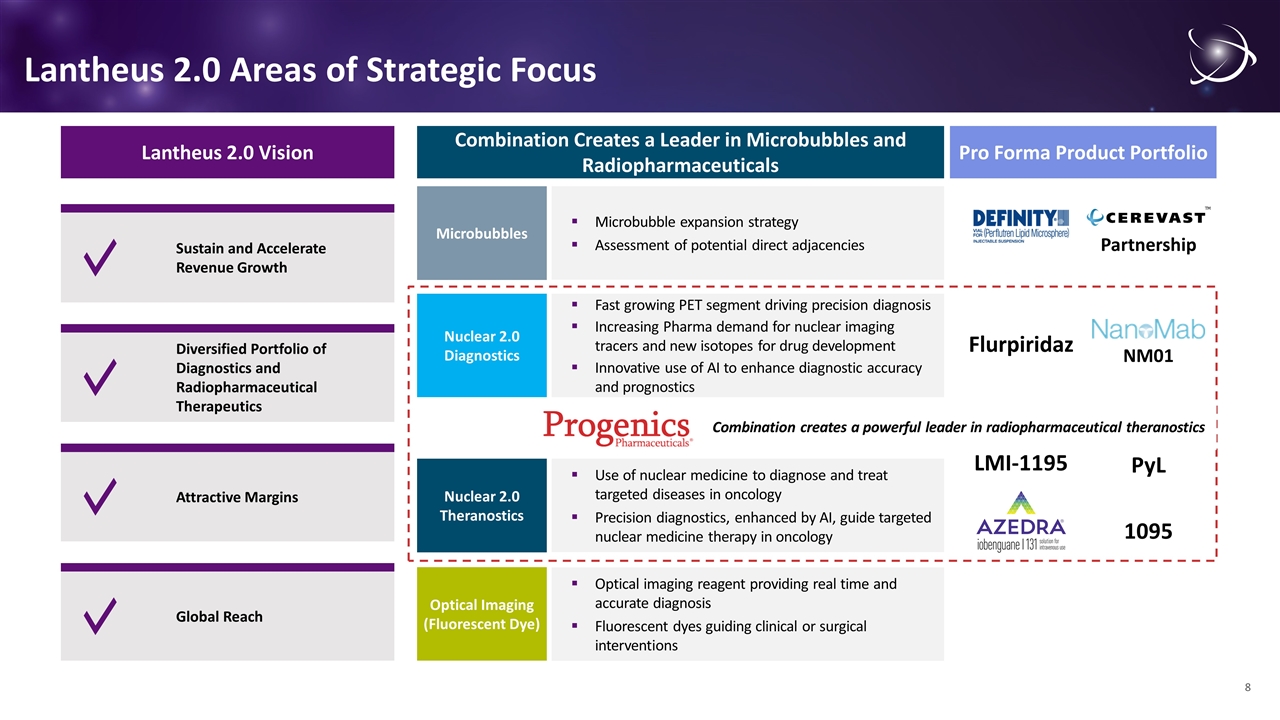
Lantheus 2.0 Areas of Strategic Focus Lantheus 2.0 Vision Sustain and Accelerate Revenue Growth Diversified Portfolio of Diagnostics and Radiopharmaceutical Therapeutics Global Reach Attractive Margins Microbubble expansion strategy Assessment of potential direct adjacencies Microbubbles Fast growing PET segment driving precision diagnosis Increasing Pharma demand for nuclear imaging tracers and new isotopes for drug development Innovative use of AI to enhance diagnostic accuracy and prognostics Nuclear 2.0 Diagnostics Use of nuclear medicine to diagnose and treat targeted diseases in oncology Precision diagnostics, enhanced by AI, guide targeted nuclear medicine therapy in oncology Nuclear 2.0 Theranostics Optical imaging reagent providing real time and accurate diagnosis Fluorescent dyes guiding clinical or surgical interventions Optical Imaging (Fluorescent Dye) Combination Creates a Leader in Microbubbles and Radiopharmaceuticals Pro Forma Product Portfolio 1095 PyL LMI-1195 Flurpiridaz NM01 Partnership Combination creates a powerful leader in radiopharmaceutical theranostics
The Combination Of Lantheus And Progenics Accelerates Growth and Fuels Value Creation Diversify and further accelerate revenue growth while augmenting long-term margin and cash flows Achieve significant synergies and leverage established Lantheus infrastructure in nuclear medicine Achieve enhanced leadership position in radiopharmaceutical marketplace; leverage longstanding channel, customer and society relationships Profitable Double-Digit Revenue Growth Combined company will have the pre-eminent diagnostic and radiopharmaceutical therapeutic for patients with norepinephrine-positive neuroendocrine tumors Potential enhanced clinical value through improved diagnostic accuracy; potential for better patient identification and management Synergy Between AZEDRA and 1195 in the Diagnosis and Treatment of Neuroendocrine Tumors 1095 Phase 2 study underway with cost of >$30 million over the next two years Interim data readout from subset of patients could enable Phase 3 study initiation Progenics is targeting earlier stage prostate cancer patients for 1095 (versus Novartis’ PSMA-617 therapeutic asset), which could create a differentiated position within a broader market Create competitive edge with the only fully-integrated radiopharmaceutical prostate portfolio: PyL, 1095, & PSMA AI AZEDRA and PyL1 can achieve ~$150 million in combined 2023 net sales under Lantheus management Lantheus’ established capabilities with radiopharmacy networks will accelerate PyL time to peak sales Two Near-Term Growth Drivers in AZEDRA and PyL Accretive to EBITDA in 2021 and EPS in 2022 $20 million run rate G&A cost-savings starting in 2020 plus significant future cost avoidance Continued disciplined approach to spending across the business Well capitalized balance sheet enables investment in pipeline Attractive Near- and Medium-Term Financial Profile 1.If Pyl is approved by the FDA in mid-2021 and assuming clinical study success and FDA approval for AZEDRA label expansion High-Potential Phase 2 Asset in 1095 Could Create Value in Near-Term
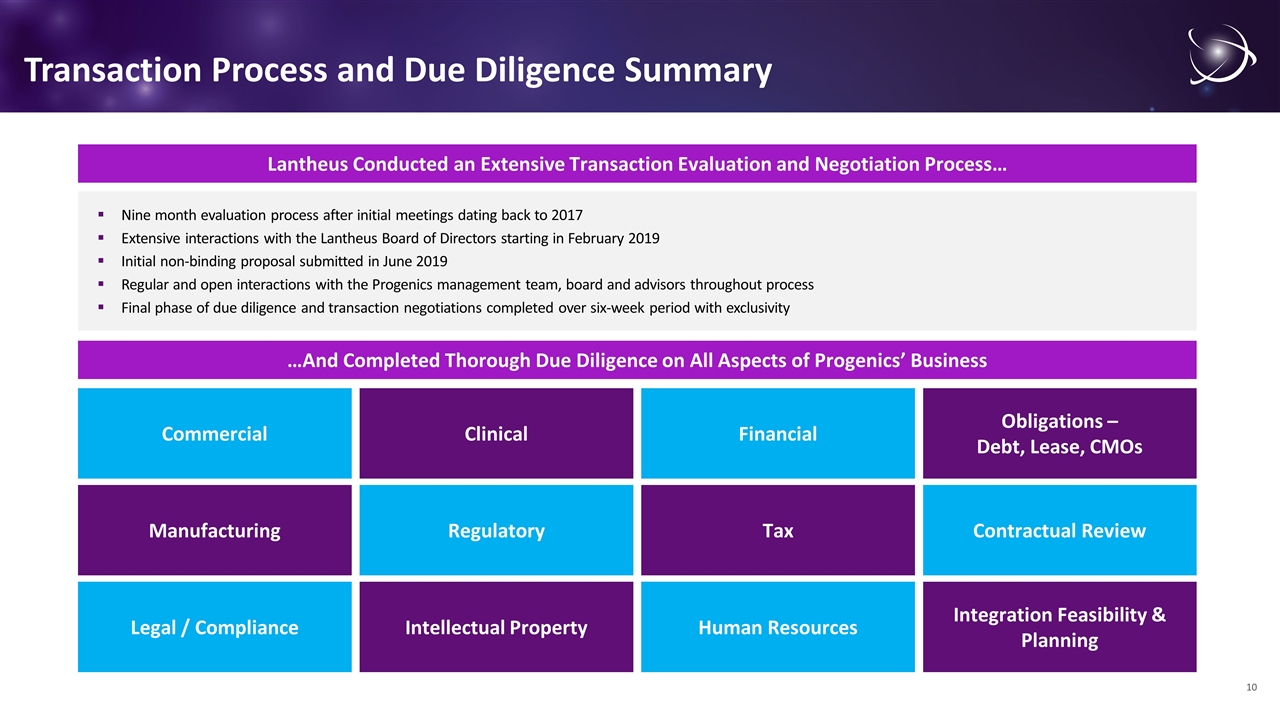
Transaction Process and Due Diligence Summary Lantheus Conducted an Extensive Transaction Evaluation and Negotiation Process… Nine month evaluation process after initial meetings dating back to 2017 Extensive interactions with the Lantheus Board of Directors starting in February 2019 Initial non-binding proposal submitted in June 2019 Regular and open interactions with the Progenics management team, board and advisors throughout process Final phase of due diligence and transaction negotiations completed over six-week period with exclusivity …And Completed Thorough Due Diligence on All Aspects of Progenics’ Business Commercial Legal / Compliance Manufacturing Clinical Intellectual Property Regulatory Financial Human Resources Tax Obligations – Debt, Lease, CMOs Integration Feasibility & Planning Contractual Review
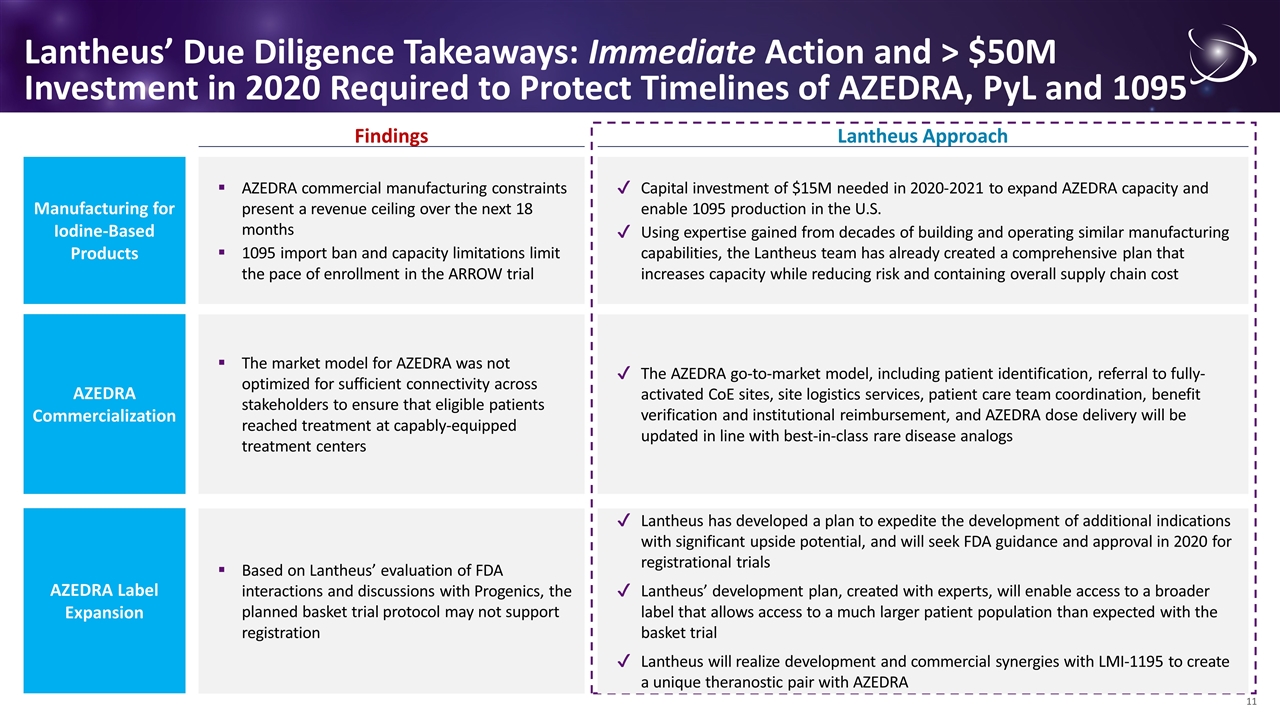
Lantheus’ Due Diligence Takeaways: Immediate Action and > $50M Investment in 2020 Required to Protect Timelines of AZEDRA, PyL and 1095 Manufacturing for Iodine-Based Products Findings Lantheus Approach AZEDRA commercial manufacturing constraints present a revenue ceiling over the next 18 months 1095 import ban and capacity limitations limit the pace of enrollment in the ARROW trial Capital investment of $15M needed in 2020-2021 to expand AZEDRA capacity and enable 1095 production in the U.S. Using expertise gained from decades of building and operating similar manufacturing capabilities, the Lantheus team has already created a comprehensive plan that increases capacity while reducing risk and containing overall supply chain cost AZEDRA Commercialization The market model for AZEDRA was not optimized for sufficient connectivity across stakeholders to ensure that eligible patients reached treatment at capably-equipped treatment centers The AZEDRA go-to-market model, including patient identification, referral to fully-activated CoE sites, site logistics services, patient care team coordination, benefit verification and institutional reimbursement, and AZEDRA dose delivery will be updated in line with best-in-class rare disease analogs AZEDRA Label Expansion Based on Lantheus’ evaluation of FDA interactions and discussions with Progenics, the planned basket trial protocol may not support registration Lantheus has developed a plan to expedite the development of additional indications with significant upside potential, and will seek FDA guidance and approval in 2020 for registrational trials Lantheus’ development plan, created with experts, will enable access to a broader label that allows access to a much larger patient population than expected with the basket trial Lantheus will realize development and commercial synergies with LMI-1195 to create a unique theranostic pair with AZEDRA
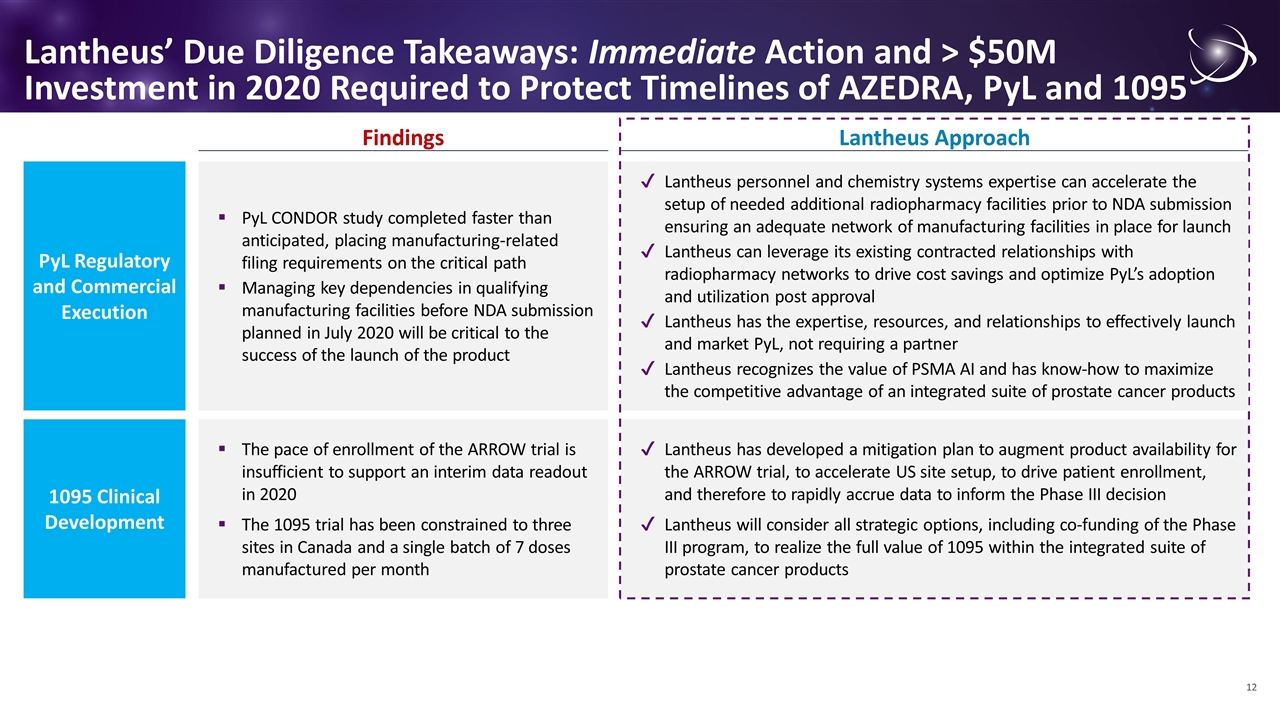
Lantheus’ Due Diligence Takeaways: Immediate Action and > $50M Investment in 2020 Required to Protect Timelines of AZEDRA, PyL and 1095 1095 Clinical Development Findings Lantheus Approach The pace of enrollment of the ARROW trial is insufficient to support an interim data readout in 2020 The 1095 trial has been constrained to three sites in Canada and a single batch of 7 doses manufactured per month Lantheus has developed a mitigation plan to augment product availability for the ARROW trial, to accelerate US site setup, to drive patient enrollment, and therefore to rapidly accrue data to inform the Phase III decision Lantheus will consider all strategic options, including co-funding of the Phase III program, to realize the full value of 1095 within the integrated suite of prostate cancer products PyL Regulatory and Commercial Execution PyL CONDOR study completed faster than anticipated, placing manufacturing-related filing requirements on the critical path Managing key dependencies in qualifying manufacturing facilities before NDA submission planned in July 2020 will be critical to the success of the launch of the product Lantheus personnel and chemistry systems expertise can accelerate the setup of needed additional radiopharmacy facilities prior to NDA submission ensuring an adequate network of manufacturing facilities in place for launch Lantheus can leverage its existing contracted relationships with radiopharmacy networks to drive cost savings and optimize PyL’s adoption and utilization post approval Lantheus has the expertise, resources, and relationships to effectively launch and market PyL, not requiring a partner Lantheus recognizes the value of PSMA AI and has know-how to maximize the competitive advantage of an integrated suite of prostate cancer products

Lantheus Plan for Progenics’ Product Portfolio

Lantheus’ Plan for Realizing the Value of the Progenics Portfolio Expand Manufacturing Capabilities to Support Future Growth 1 Execute on AZEDRA Growth Plan 2 Invest in AZEDRA Label Expansion 3 Lantheus Commercial Launch Will Maximize Value of PyL 4 Transform Relistor Royalties Into Meaningful EPS Contributor for Shareholders 5 Execute on Pro Forma Financial Plan to Benefit ALL Shareholders 6 Lantheus Management Team Has the Experience to Integrate and Execute on an Acquisition of Progenics 7 Lantheus Board Offers Diverse and Impressive Experience Base and Leadership Credentials in Commercial, Operations, R&D, M&A and Finance 8
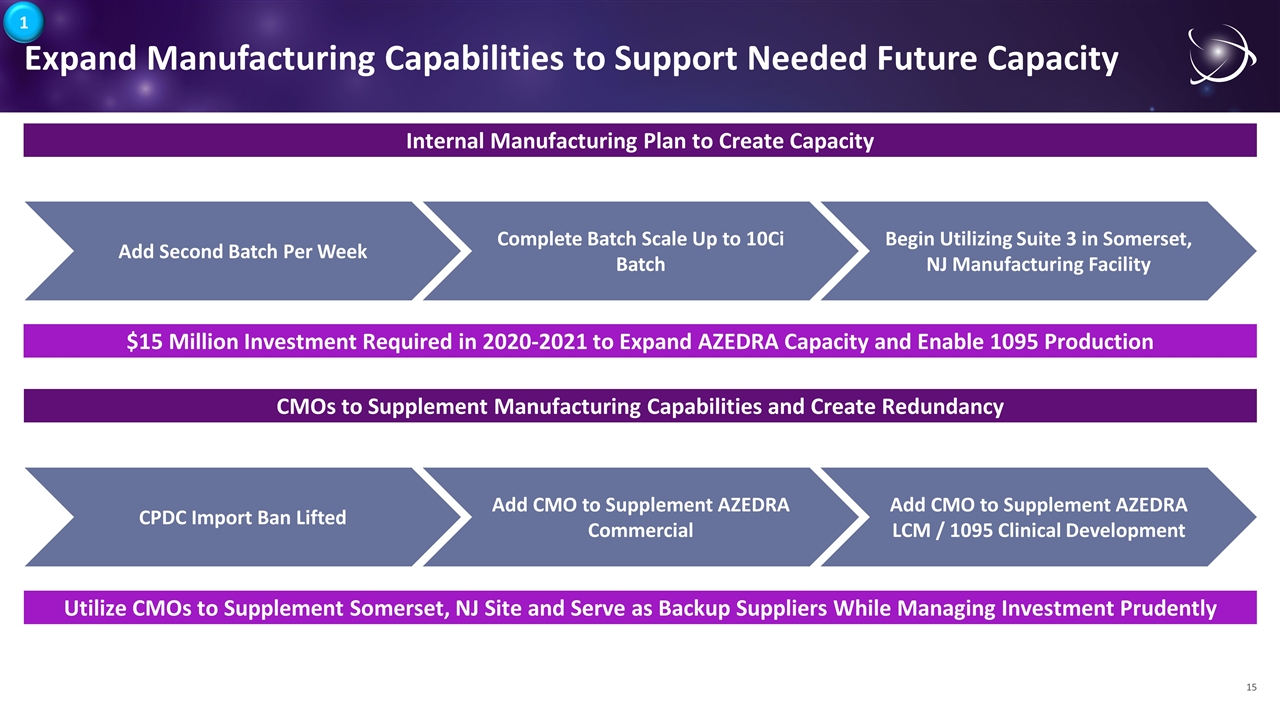
Expand Manufacturing Capabilities to Support Needed Future Capacity $15 Million Investment Required in 2020-2021 to Expand AZEDRA Capacity and Enable 1095 Production Internal Manufacturing Plan to Create Capacity Add Second Batch Per Week Complete Batch Scale Up to 10Ci Batch Begin Utilizing Suite 3 in Somerset, NJ Manufacturing Facility Utilize CMOs to Supplement Somerset, NJ Site and Serve as Backup Suppliers While Managing Investment Prudently CMOs to Supplement Manufacturing Capabilities and Create Redundancy CPDC Import Ban Lifted Add CMO to Supplement AZEDRA Commercial Add CMO to Supplement AZEDRA LCM / 1095 Clinical Development 1

Optimize AZEDRA Go-to-Market Plan Comprehensive Center of Excellence Operational Setup Use Lantheus customer service to improve the complex multi-stakeholder management process encompassing all logistics from scheduling of patients, facility, and staffing; just-in-time manufacturing and delivery of the dosimetry dose to the radiopharmacy Provide Lantheus services to support efficient dosimetry dose preparation; dosimetry imaging and therapeutic dose calculation, including cost-efficient access to qualified software systems Facilitate Patient Scheduling and Dosimetry Reset the customer-facing model, deploying medical, advocacy and promotional resources to ensure timely patient referral to nuclear medicine within the stakeholder network of centers of excellence Foster patient-centric and multi-disciplinary connections within identified centers of excellence to bridge silos, drive streamlined processes, and ensure timely and optimal clinical outcomes for patients Implement site-finder system and tools for patients, advocacy groups, and providers to find AZEDRA-ready centers for treatment and improve connections across the treatment network Tune hub-services model to ensure patients are expeditiously qualified and referred for treatment Fund a patient registry in partnership with centers of excellence to track patient outcomes and recurrence; leverage data to demonstrate value and drive pull-through Drive Patient Identification and Pull Through Expand LASH group capabilities to ensure timely follow-up and appropriate patient and clinician support for therapeutic doses and to enhance the patient experience Use Lantheus customer service and best-in-class operations to provide logistics support for just-in-time delivery of therapeutic doses Improve Timely and Optimal Patient Treatment Invest in expanded Lantheus operations efficiency toolkit to support centers of excellence for more rapid setup and efficient operations. Operations efficiency toolkit comprises a comprehensive training program for staffing, dosimetry calculations tools, nuclear licensing, patient scheduling, room and mobile shielding and process management Leverage Lantheus’ long-term operations within nuclear medicine to support hospital investment decisions for required lead-lined room, shielding and systems Execute an educational program to establish the superiority of AZEDRA to the standard of care through peer-to-peer interaction, working closely with ASCO, NCCN, SNMMI and NANETs Ensure appropriate coverage policies are in place with commercial payers surrounding each center of excellence and provide best practices on case rate negotiation from centers actively treating patients Integrate guidance on optimal reimbursement via the new technology add-on payment and other applicable CMS mechanisms (e.g. outlier payments) into reimbursement toolkit 2
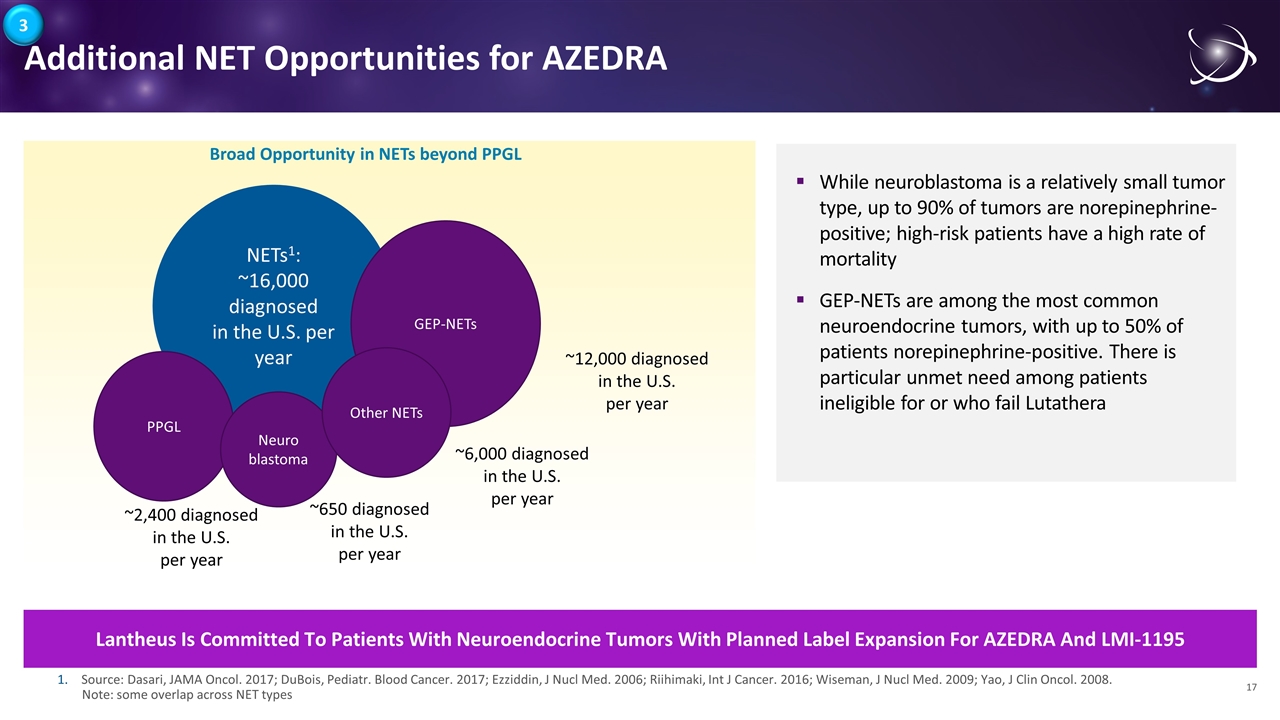
While neuroblastoma is a relatively small tumor type, up to 90% of tumors are norepinephrine-positive; high-risk patients have a high rate of mortality GEP-NETs are among the most common neuroendocrine tumors, with up to 50% of patients norepinephrine-positive. There is particular unmet need among patients ineligible for or who fail Lutathera Additional NET Opportunities for AZEDRA Lantheus Is Committed To Patients With Neuroendocrine Tumors With Planned Label Expansion For AZEDRA And LMI-1195 NETs1: ~16,000 diagnosed in the U.S. per year PPGL ~2,400 diagnosed in the U.S. per year Broad Opportunity in NETs beyond PPGL Neuro blastoma ~650 diagnosed in the U.S. per year GEP-NETs ~12,000 diagnosed in the U.S. per year Source: Dasari, JAMA Oncol. 2017; DuBois, Pediatr. Blood Cancer. 2017; Ezziddin, J Nucl Med. 2006; Riihimaki, Int J Cancer. 2016; Wiseman, J Nucl Med. 2009; Yao, J Clin Oncol. 2008. Note: some overlap across NET types Other NETs ~6,000 diagnosed in the U.S. per year 3
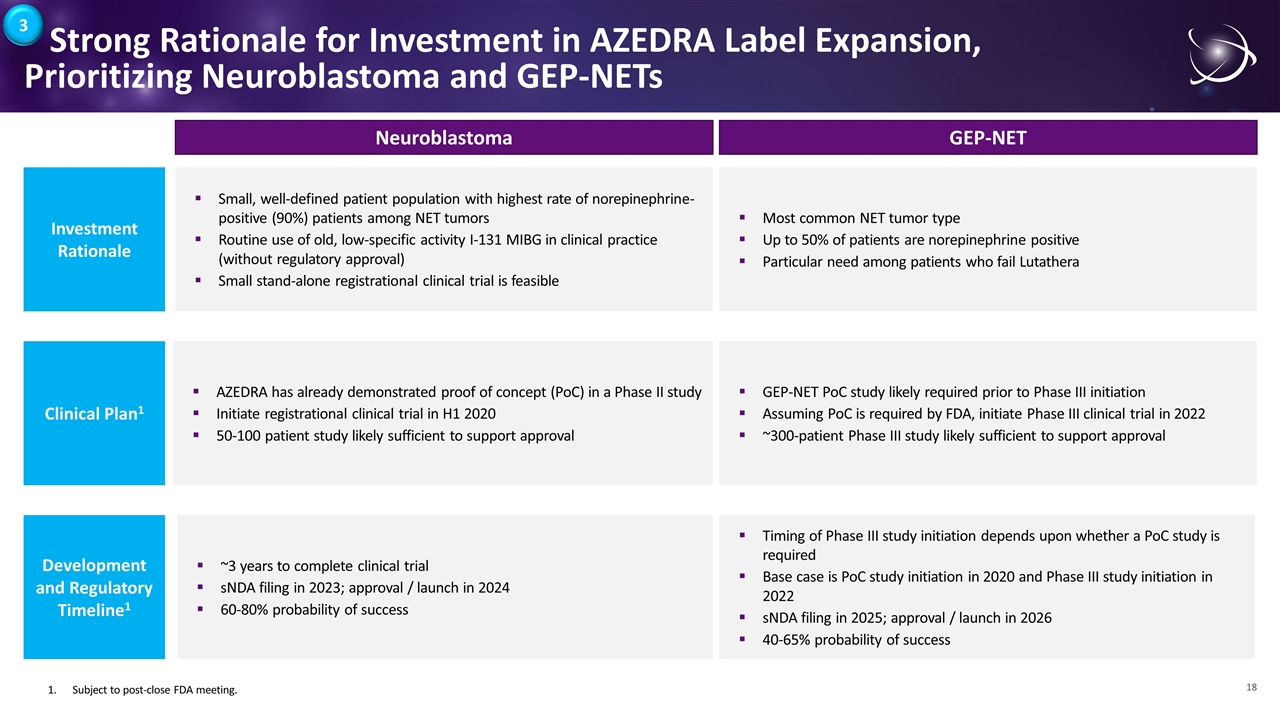
Strong Rationale for Investment in AZEDRA Label Expansion, Prioritizing Neuroblastoma and GEP-NETs Investment Rationale Small, well-defined patient population with highest rate of norepinephrine-positive (90%) patients among NET tumors Routine use of old, low-specific activity I-131 MIBG in clinical practice (without regulatory approval) Small stand-alone registrational clinical trial is feasible Clinical Plan1 ~3 years to complete clinical trial sNDA filing in 2023; approval / launch in 2024 60-80% probability of success Development and Regulatory Timeline1 AZEDRA has already demonstrated proof of concept (PoC) in a Phase II study Initiate registrational clinical trial in H1 2020 50-100 patient study likely sufficient to support approval Neuroblastoma GEP-NET Most common NET tumor type Up to 50% of patients are norepinephrine positive Particular need among patients who fail Lutathera GEP-NET PoC study likely required prior to Phase III initiation Assuming PoC is required by FDA, initiate Phase III clinical trial in 2022 ~300-patient Phase III study likely sufficient to support approval Timing of Phase III study initiation depends upon whether a PoC study is required Base case is PoC study initiation in 2020 and Phase III study initiation in 2022 sNDA filing in 2025; approval / launch in 2026 40-65% probability of success 1.Subject to post-close FDA meeting. 3

Maximize the Value of PyL Via Commercial Launch by Lantheus > $8M in Investment and Skilled Execution Required Over the Next 12-18 Months to Successfully Launch Pyl, With Critical Path Steps Required in the First Half of 2020 Set up the Radiopharmacy Manufacturing Facilities Prepare the Market Prepare the NDA Set contracting structure with radiopharmacy manufacturing facilities that optimizes margin for PyL, leveraging Lantheus’ long-term relationship with radiopharmacy networks Initiate communications via scientific exchange to inform guidelines and establish superiority to standard of care in the next 12-18 months Prepare nuclear medicine departments for camera setup, image capture, and reading of PyL images before launch Register, launch and utilize PSMA AI to prepare the market regarding PSMA value proposition before PyL launch Prepare submission to CMS within the next 18 months to support code availability at launch; develop materials to support hospital decision making and commercial payers Develop a positioning, messaging and promotional plan aligned with the target stakeholders identified via early engagement and market research Optimize chemistry system to ensure production yield for driving maximal margin at launch and develop capacity plan Set up sufficient commercial-ready radiopharmacy manufacturing facilities for guaranteeing reach into full target market for prostate cancer Ensure all sites are referenced in the CMC file by June 2020, with over $5M direct investment required to enable launch readiness at approval Hire and train four dedicated headcount to oversee the management of all radiopharmacy manufacturing facilities to ensure systems are established to optimize patient scheduling aligned with dose production By June 2020, manage critical dependencies leading up to the NDA in Q3 2020, including CMC reference to sufficient radiopharmacy manufacturing facilities in order to be able to supply PyL across the US for launch Before end of May 2020, qualify, set up and update the CMC file for new precursor manufacturer In Q1 2020, fulfill additional FDA-requirements necessary for approvability of the NDA data package 4
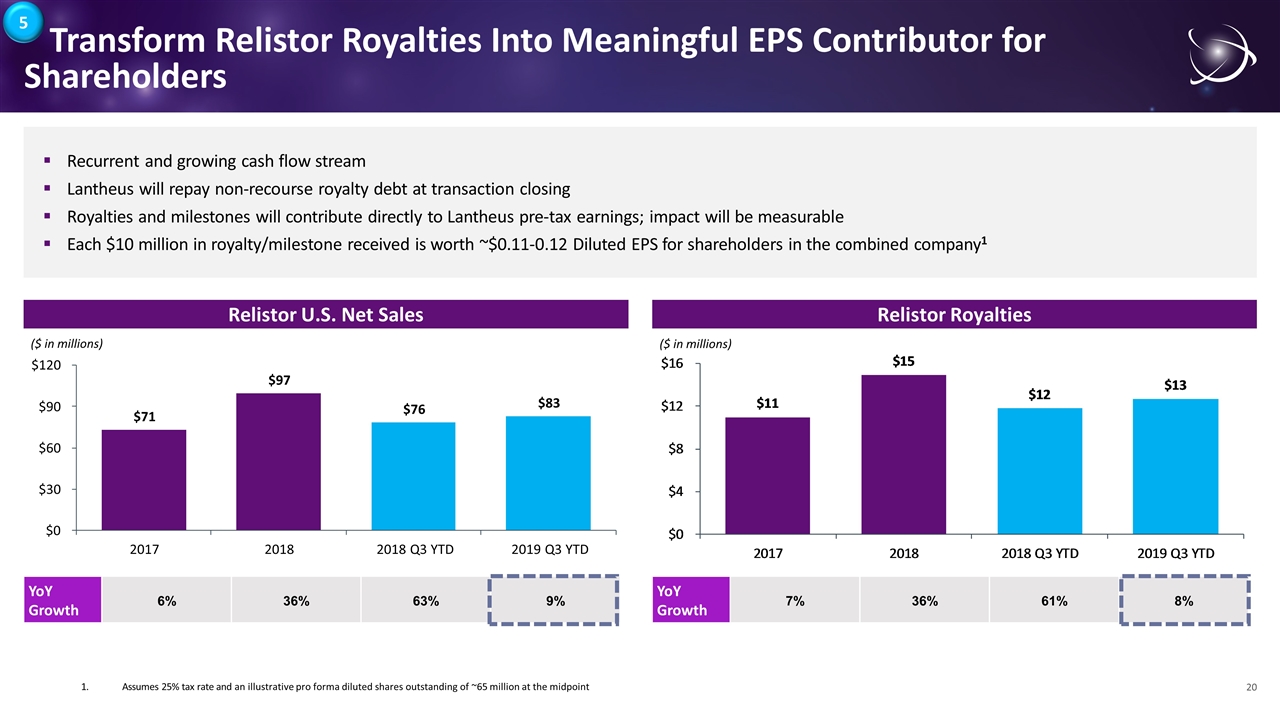
Transform Relistor Royalties Into Meaningful EPS Contributor for Shareholders Recurrent and growing cash flow stream Lantheus will repay non-recourse royalty debt at transaction closing Royalties and milestones will contribute directly to Lantheus pre-tax earnings; impact will be measurable Each $10 million in royalty/milestone received is worth ~$0.11-0.12 Diluted EPS for shareholders in the combined company1 Relistor U.S. Net Sales Relistor Royalties YoY Growth 6% 36% 63% 9% YoY Growth 7% 36% 61% 8% 1.Assumes 25% tax rate and an illustrative pro forma diluted shares outstanding of ~65 million at the midpoint 5 $71 $97 $76 $83 $0 $30 $60 $90 $120 2017 2018 2018 Q3 YTD 2019 Q3 YTD ($ in millions) ($ in millions)
Lantheus’ Plan Yields Compelling Value for All Shareholders – Creating Sustainable and Profitable, Double-Digit Growth All Shareholders Will Participate in Near-term Value Creation While Maintaining the Ability to Realize Future Upside Transaction Structure Benefitting All Shareholders Lantheus’ acquisition transaction represents a tax-free, all-stock transaction Strong pro forma balance sheet well-positioned to invest in organic and inorganic growth Progenics shareholders will own a substantial 35% stake of the combined company Compelling Financial Rationale Combined company with profitable, double-digit topline growth in a high-valuation sector ~$370 million in pro forma diversified revenue streams due to additional marketed products Deal allows combined ownership group to access a portion of NOLs not currently realizable on a standalone basis No new debt or equity financing required to fund operations Impact of Lantheus P&L Accelerates and diversifies revenue growth Rapid expansion of gross and EBITDA margins in 2021 and beyond Incremental R&D and SG&A expenses of <$50mm in 2021 and <$40mm in 2022 and beyond Accretive to 2021 EBITDA and 2022 Adj. EPS 6
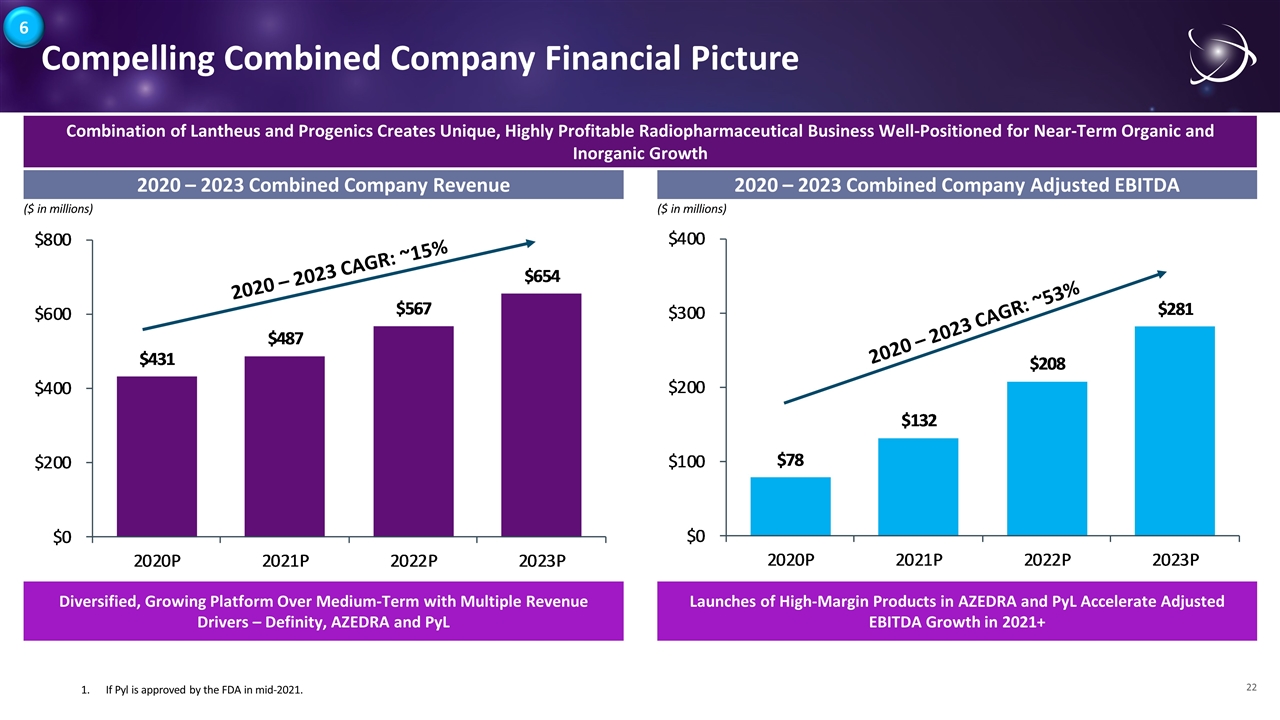
Compelling Combined Company Financial Picture 2020 – 2023 Combined Company Revenue 2020 – 2023 Combined Company Adjusted EBITDA 2020 – 2023 CAGR: ~15% 2020 – 2023 CAGR: ~53% 1.If Pyl is approved by the FDA in mid-2021. ($ in millions) ($ in millions) Combination of Lantheus and Progenics Creates Unique, Highly Profitable Radiopharmaceutical Business Well-Positioned for Near-Term Organic and Inorganic Growth Diversified, Growing Platform Over Medium-Term with Multiple Revenue Drivers – Definity, AZEDRA and PyL Launches of High-Margin Products in AZEDRA and PyL Accelerate Adjusted EBITDA Growth in 2021+ 6

Lantheus’ Management Team Has the Experience to Integrate and Execute on the Acquisition of Progenics Joined Lantheus in April 2013 as Chief Commercial Officer and was promoted to Chief Operating Officer in March 2015, before becoming President and Chief Executive Officer in August 2015 Fmr. President and SVP of World Wide Sales and Marketing at Angelini Labopharm LLC and Labopharm USA Fmr. VP of Strategic Planning and Competitive Intelligence and VP of Sales at Centocor, Inc., a Johnson & Johnson Company Mary Anne Heino President, CEO and Director Lantheus Holdings Strong Leadership Joined Lantheus in September 2018 as Chief Financial Officer and Treasurer Fmr. VP of Americas Finance and Corporate Treasurer of Zimmer Biomet Holdings Fmr. VP of Investor Relations and Treasurer of Zimmer Biomet Holdings Robert Marshall Chief Financial Officer and Treasurer Joined Lantheus in May 2018 as SVP of Technical Operations and was promoted to Chief Operating Officer in March 2019 Fmr. VP of Supply Chain, North America at GlaxoSmithKline Fmr. VP and Global Head of External Supply and Global Contract Manufacturing at GlaxoSmithKline John Bolla Chief Operations Officer 7
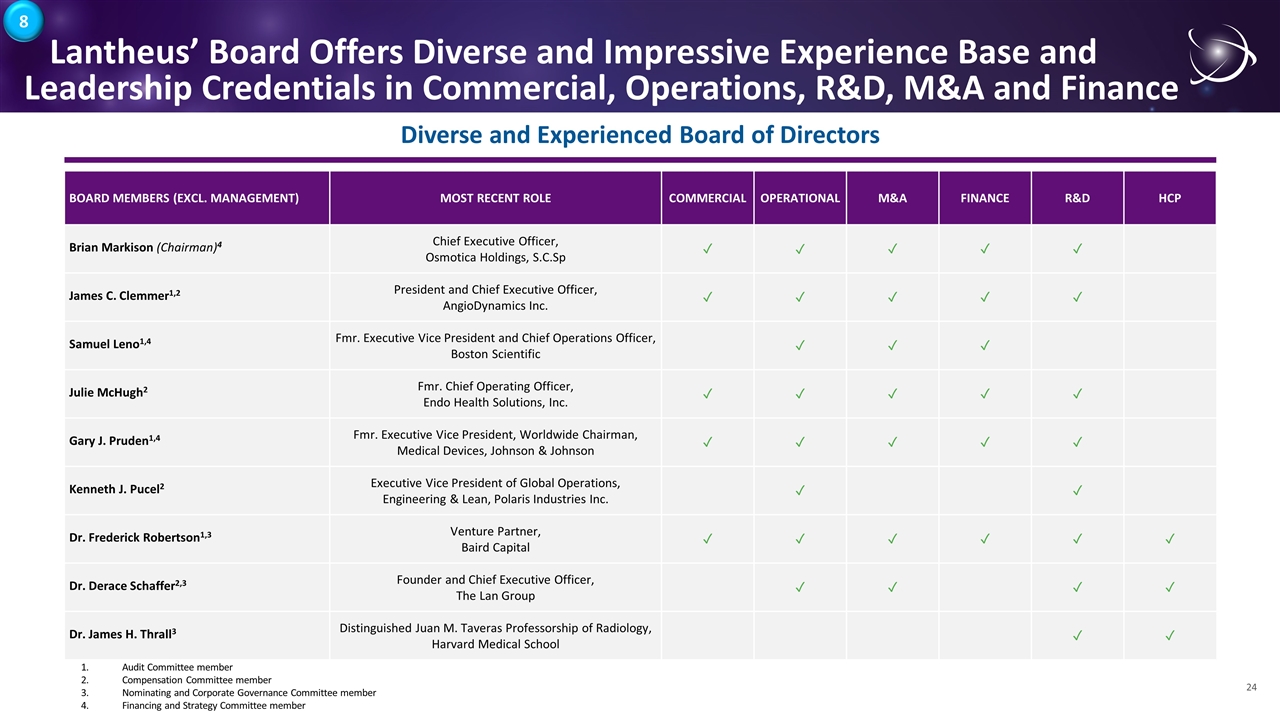
Lantheus’ Board Offers Diverse and Impressive Experience Base and Leadership Credentials in Commercial, Operations, R&D, M&A and Finance Diverse and Experienced Board of Directors BOARD MEMBERS (EXCL. MANAGEMENT) MOST RECENT ROLE COMMERCIAL OPERATIONAL M&A FINANCE R&D HCP Brian Markison (Chairman)4 Chief Executive Officer, Osmotica Holdings, S.C.Sp ✓ ✓ ✓ ✓ ✓ James C. Clemmer1,2 President and Chief Executive Officer, AngioDynamics Inc. ✓ ✓ ✓ ✓ ✓ Samuel Leno1,4 Fmr. Executive Vice President and Chief Operations Officer, Boston Scientific ✓ ✓ ✓ Julie McHugh2 Fmr. Chief Operating Officer, Endo Health Solutions, Inc. ✓ ✓ ✓ ✓ ✓ Gary J. Pruden1,4 Fmr. Executive Vice President, Worldwide Chairman, Medical Devices, Johnson & Johnson ✓ ✓ ✓ ✓ ✓ Kenneth J. Pucel2 Executive Vice President of Global Operations, Engineering & Lean, Polaris Industries Inc. ✓ ✓ Dr. Frederick Robertson1,3 Venture Partner, Baird Capital ✓ ✓ ✓ ✓ ✓ ✓ Dr. Derace Schaffer2,3 Founder and Chief Executive Officer, The Lan Group ✓ ✓ ✓ ✓ Dr. James H. Thrall3 Distinguished Juan M. Taveras Professorship of Radiology, Harvard Medical School ✓ ✓ 1.Audit Committee member 2.Compensation Committee member 3.Nominating and Corporate Governance Committee member 4.Financing and Strategy Committee member 8

101394330 v1
























