Filed Pursuant to Rule 424(b)(3) and Rule 424(c)
Registration No. 333-198847
Prospectus Supplement No. 13
(To Prospectus filed on July 6, 2015, as supplemented
by Prospectus Supplement No. 1 dated July 14, 2015, Prospectus Supplement No. 2 dated July 28, 2015, Prospectus Supplement No. 3 dated August 4, 2015, Prospectus Supplement No. 4 dated August 12, 2015, Prospectus Supplement No. 5 dated September 17, 2015, Prospectus Supplement No. 6 dated September 18, 2015, Prospectus Supplement No. 7 dated September 24, 2015, Prospectus Supplement No. 8 dated September 25, 2015, Prospectus Supplement No. 9 dated September 30, 2015, Prospectus Supplement No. 10 dated October 2, 2015, Prospectus Supplement No. 11 dated November 3, 2015, and Prospectus Supplement No. 12 dated November 10, 2015)
ENUMERAL BIOMEDICAL HOLDINGS, INC.
This Prospectus Supplement No. 13 supplements the information contained in the Prospectus, dated as of July 6, 2015, as amended by Prospectus Supplement No. 1 dated July 14, 2015, Prospectus Supplement No. 2 dated July 28, 2015, Prospectus Supplement No. 3 dated August 4, 2015, Prospectus Supplement No. 4 dated August 12, 2015, Prospectus Supplement No. 5 dated September 17, 2015, Prospectus Supplement No. 6 dated September 18, 2015, Prospectus Supplement No. 7 dated September 24, 2015, Prospectus Supplement No. 8 dated September 25, 2015, Prospectus Supplement No. 9 dated September 30, 2015, Prospectus Supplement No. 10 dated October 2, 2015, Prospectus Supplement No. 11 dated November 3, 2015, and Prospectus Supplement No. 12 dated November 10, 2015, relating to the resale of up to 52,154,760 shares of our common stock by selling stockholders.
This Prospectus Supplement No. 13 is being filed to include the information set forth in our Current Report on Form 8-K, which was filed with the Securities and Exchange Commission on November 18, 2015.
You should read this Prospectus Supplement No. 13 in conjunction with the Prospectus. This Prospectus Supplement No. 13 is qualified by reference to the Prospectus, except to the extent that the information contained in this Prospectus Supplement No. 13 supersedes the information contained in the Prospectus. This Prospectus Supplement No. 13 is not complete without, and may not be utilized except in connection with, the Prospectus.
You should consider carefully the risks that we have described in “Risk Factors” beginning on page 7 of the Prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this Prospectus Supplement is November 18, 2015
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):November 18, 2015
Enumeral Biomedical Holdings, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 000-55415 | 99-0376434 |
(State or Other Jurisdiction of Incorporation) | (Commission
File Number) | (I.R.S. Employer
Identification Number) |
200 CambridgePark Drive, Suite 2000 Cambridge, Massachusetts (Address of Principal Executive Offices) | | 02140 (Zip Code) |
(617) 945-9146
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 7.01 Regulation FD Disclosure.
Enumeral Biomedical Holdings, Inc. (the “Company”) may use a slide presentation, in whole or in part, from time to time in presentations to potential partners, investors, analysts and others. A copy of the slide presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein. A copy of the slide presentation is also available on the Company’s website at www.enumeral.com.
The information in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| Exhibit Number | | Description |
| 99.1 | | Enumeral Biomedical Holdings, Inc. PD-1 Program Update Presentation, dated November 18, 2015 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | ENUMERAL BIOMEDICAL HOLDINGS, INC. |
| | | |
| Dated: November 18, 2015 | By: | /s/ Kevin G. Sarney |
| | | Name: Kevin G. Sarney |
| | | Title: Vice President of Finance, Chief Accounting Officer and Treasurer |
EXHIBIT INDEX
Exhibit
No. | | Description |
| | | |
| 99.1 | | Enumeral Biomedical Holdings, Inc. PD-1 Program Update Presentation, dated November 18, 2015 |
Exhibit 99.1

EnumeralPD-1 Program Update: Differentiated Anti-PD-1 Antibody Functional Characterization in Ex VivoHuman Lung Biopsy Assays November 18, 2015
Background • Enumeraluses a unique single cell technology platform and approach to identify functionally differentiated antibody candidates – Enumeralhas identified two classes of anti-PD-1 antibodies with distinct modes of binding to PD-1 – Both classes demonstrate enhancement of T cell activation via reversal of PD-1-dependent immunosuppression 2

Enumeral’sApproach to Developing Differentiated Antibodies Starts with Diversity • Enumeralantibody discovery results in exceptional diversity* • Potential for strong IP position • Breadth of diversity: keys to unlocking the target physiology • Multiple potential program opportunities OPDIVO® KEYTRUDA® Pidilizumab 392C5 246A10 413D2 413E1 244C8 388D4 *Based on ENUM evaluation of published literature Cladogram representing heavy chain AA sequences N= 159 sequences shown 28 families of antibodies 3

EnumeralPD-1 Program • Enumeralhas identified a novel potentially allosteric anti-PD-1 antagonist (ENUM 244C8) displaying the following properties: – Reversal of PD-L1-dependent immunosuppression – Binding to PD-1 via a novel epitope – Increased levels of T cell activation in cell-based assays – Binding to PD-1 independent of PD-L1 • ENUM 244C8 antibody and a currently-marketed anti-PD-1 antibody were tested for their ability to reverse tumor infiltrating lymphocyte (TIL) exhaustion using lymphocytes derived from human lung biopsy – ENUM 244C8 observed restoring T cell function to a higher level than the positive control nivolumab 4

Ex Vivo Reversal of TIL Exhaustion: Methods • NSCLC samples from staging surgeries were analyzed within 24 hours of collection • Flow cytometry analyzed extent of T cell infiltration and co- expression of immunomodulatory receptors (PD-1 and TIM-3) • Cells were incubated with anti-CD3/anti-CD28 antibodies for 24 hours and either negative control (isotype, Biolegend), nivolumab (Invivogen), or humanized derivatives of ENUM 388D4 and ENUM 244C8 (designated D4-1, D4-2, D4-3, C8-1, C8-2, C8-3) • Interferon gamma production was measured (ELISA) and data is expressed as pg/mL IFN- 5
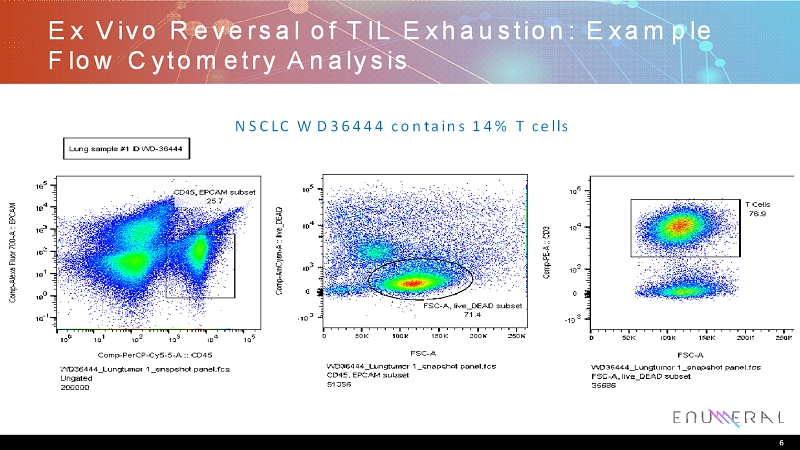
Ex Vivo Reversal of TIL Exhaustion: Example Flow Cytometry Analysis 6 NSCLC WD36444 contains 14% T cells
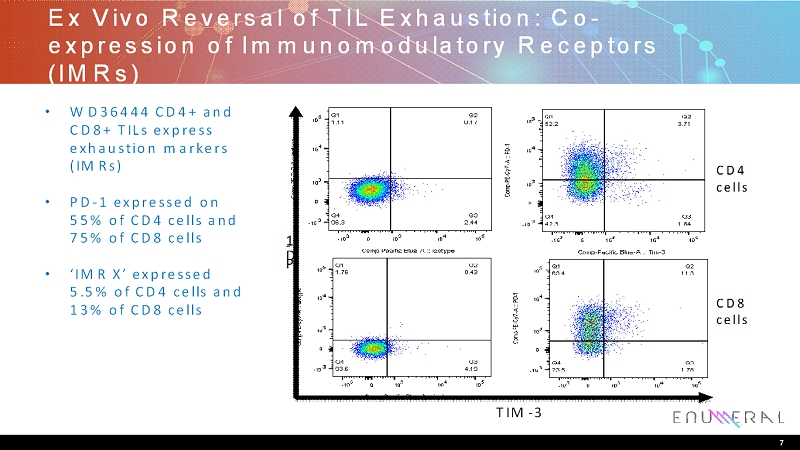
Ex Vivo Reversal of TIL Exhaustion: Co- expression of Immunomodulatory Receptors (IMRs) • WD36444 CD4+ and CD8+ TILs express exhaustion markers (IMRs) • PD-1 expressed on 55% of CD4 cells and 75% of CD8 cells • ‘IMR X’ expressed 5.5% of CD4 cells and 13% of CD8 cells 7 CD4 cells CD8 cells P D - 1 Immunomodulatory Receptor (IMR) X TIM-3

Ex Vivo Reversal of TIL Exhaustion: Variability Across Patients 8 Tumor Identifier % EpCAM-CD45+ %CD3+ %CD4 + PD-1 + %CD8 + PD-1 + %CD4 + TIM3 + %CD8 + TIM3 + WD-36444* 25.7 14 55 75 5.5 13 WD-36571* 10.3 6.3 47 64 1.8 <1 WD-36686* 21.6 17 55 84 6 16 WD-36790* 16.8 10.4 38 68 1 5.2 WD-36904* 12.8 7 63 72 9.5 24.5 M115801A2* 3.4 2.9 79 84 22 16 WD-36923 1.6 0.9 53 51 27 n/a WD-36988* 8.9 7 58 93 22 62 M4150952 5.4 3 78 79 26 15 M1151877A 15.9 12.8 57 71 11 24 • Data from flow cytometry analysis – Lymphocyte infiltration ranged from 1.6% -25.7% – T cell infiltration ranged from 0.9% -17% NSCLC tumor biopsies demonstrate varying degrees of lymphocyte infiltration and PD-1 expression on T cells *Data on functional reversal of exhaustion reported on following slides

Ex Vivo Reversal of TIL Exhaustion: Experimental Questions • Tumor biopsy from n=10 patients found to harbor TILs that express exhaustion markers including PD-1. 1. What is the activity of the T cells following activation? – If cells do not produce IFN-in response to TCR triggering (anti-CD3 + anti- CD28), cells are “exhausted”. 2. Is cellular activity modified by the addition of an anti-PD-1 antibody? 3. Do nivolumaband ENUM antibodies behave differently in this experiment? 4. Do different classes of anti-PD-1 antibody exhibit additive effects on reversal of TIL exhaustion? 9
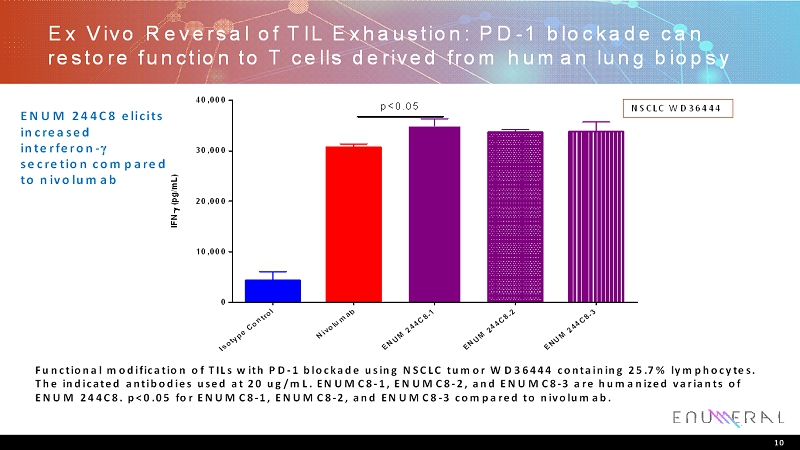
Ex Vivo Reversal of TIL Exhaustion: PD-1 blockade can restore function to T cells derived from human lung biopsy Functional modification of TILs with PD-1 blockade using NSCLC tumor WD36444 containing 25.7% lymphocytes. The indicated antibodies used at 20 ug/mL.ENUMC8-1, ENUMC8-2, and ENUMC8-3 are humanized variants of ENUM 244C8. p<0.05 for ENUMC8-1, ENUMC8-2, and ENUMC8-3 compared to nivolumab. 10 NSCLC WD36444 ENUM 244C8 elicits increased interferon- secretion compared to nivolumab I s o t y p e C o n t r o l N i v o l u m a b E N U M 2 4 4 C 8 - 1 E N U M 2 4 4 C 8 - 2 E N U M 2 4 4 C 8 - 3 0 10,000 20,000 30,000 40,000 I F N - ( p g / m L ) p<0.05
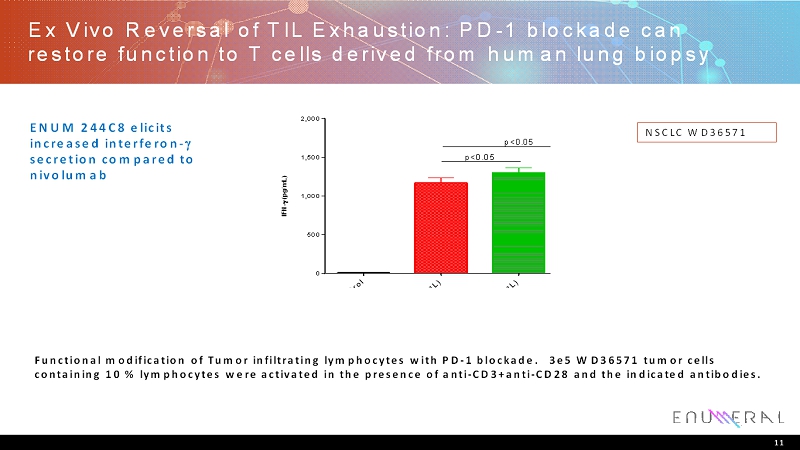
Ex Vivo Reversal of TIL Exhaustion: PD-1 blockade can restore function to T cells derived from human lung biopsy 11 NSCLC WD36571 Functional modification of Tumor infiltrating lymphocytes with PD-1 blockade. 3e5 WD36571 tumor cells containing 10 % lymphocytes were activated in the presence of anti-CD3+anti-CD28 and the indicated antibodies. I s o t y p e C o n t r o l N i v o l u m a b ( 1 0 u g / m L ) E N U M D 4 - 2 ( 1 0 u g / m L ) E N U M C 8 - 2 ( 1 0 u g / m L ) 0 500 1,000 1,500 2,000 I F N - ( p g / m L ) p<0.05 p<0.05 ENUM 244C8 elicits increased interferon- secretioncompared to nivolumab
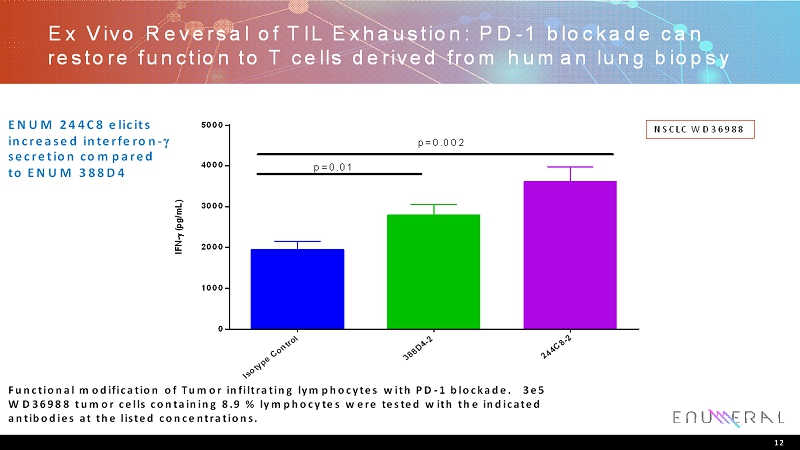
Ex Vivo Reversal of TIL Exhaustion: PD-1 blockade can restore function to T cells derived from human lung biopsy 12 NSCLC WD36988 ENUM 244C8 elicits increased interferon- secretioncompared to ENUM 388D4 Functional modification of Tumor infiltrating lymphocytes with PD-1 blockade. 3e5 WD36988 tumor cells containing 8.9 % lymphocytes were tested with the indicated antibodiesat the listed concentrations. I s o t y p e C o n t r o l 3 8 8 D 4 - 2 2 4 4 C 8 - 2 0 1000 2000 3000 4000 5000 Lung 9-WD36988 I F N - ( p g / m L ) p=0.01 p=0.002
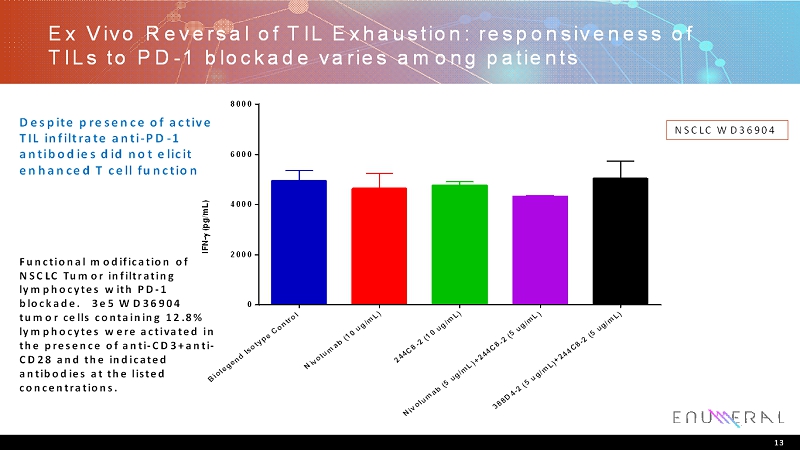
Ex Vivo Reversal of TIL Exhaustion: responsiveness of TILs to PD-1 blockade varies among patients 13 NSCLC WD36904 Despite presence of active TIL infiltrate anti-PD-1 antibodies did not elicit enhanced T cell function Functional modification of NSCLC Tumor infiltrating lymphocytes with PD-1 blockade. 3e5 WD36904 tumor cells containing 12.8% lymphocytes were activated in the presence of anti-CD3+anti- CD28 and the indicated antibodies at the listed concentrations. B i o l e g e n d I s o t y p e C o n t r o l N i v o l u m a b ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) N i v o l u m a b ( 5 u g / m L ) + 2 4 4 C 8 - 2 ( 5 u g / m L ) 3 8 8 D 4 - 2 ( 5 u g / m L ) + 2 4 4 C 8 - 2 ( 5 u g / m L ) 0 2000 4000 6000 8000 I F N - ( p g / m L )
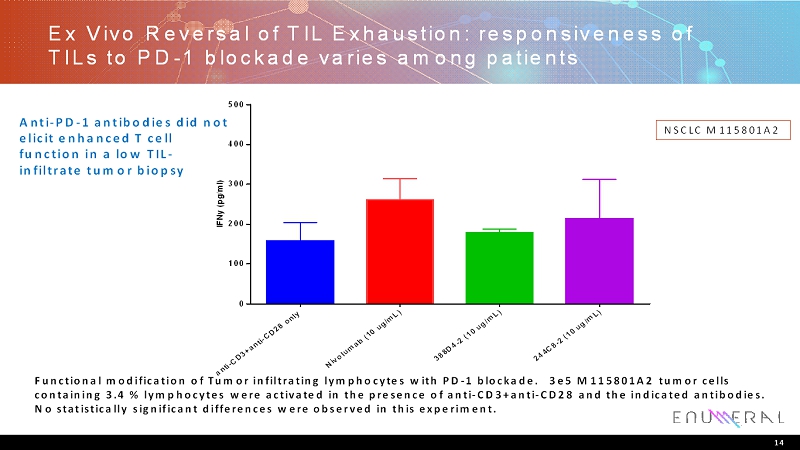
Ex Vivo Reversal of TIL Exhaustion: responsiveness of TILs to PD-1 blockade varies among patients 14 NSCLC M115801A2 Functional modification of Tumor infiltrating lymphocytes with PD-1 blockade. 3e5 M115801A2 tumor cells containing 3.4 % lymphocytes were activated in the presence of anti-CD3+anti-CD28 and the indicated antibodies. No statistically significant differences were observed in this experiment. Anti-PD-1 antibodies did not elicit enhanced T cell function in a low TIL- infiltrate tumor biopsy a n t i - C D 3 + a n t i - C D 2 8 o n l y N i v o l u m a b ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 100 200 300 400 500 I F N y ( p g / m l )

15 Functional modification of Tumor infiltrating lymphocytes with PD-1 blockade. 3e5 WD36790 tumor cells containing 16.8 % lymphocytes were tested with the indicated antibodiesat the listed concentrations. NSCLC WD36790 Ex Vivo Reversal of TIL Exhaustion: additive effects of two anti-PD-1 antibodies ENUM 244C8 elicits increased interferon- secretion and can augment nivolumabactivity
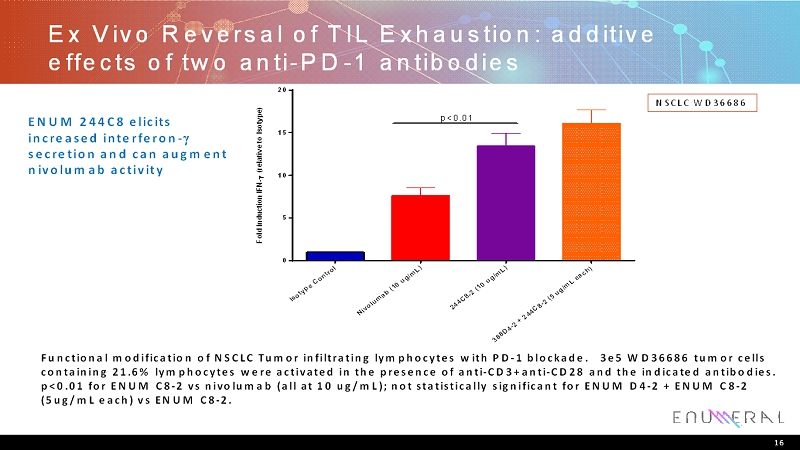
Ex Vivo Reversal of TIL Exhaustion: additive effects of two anti-PD-1 antibodies 16 Functional modification of NSCLC Tumor infiltrating lymphocytes with PD-1 blockade. 3e5 WD36686 tumor cells containing 21.6% lymphocytes were activated in the presence of anti-CD3+anti-CD28 and the indicated antibodies. p<0.01 for ENUM C8-2 vs nivolumab(all at 10 ug/mL); not statistically significant for ENUM D4-2 + ENUM C8-2 (5ug/mL each) vs ENUM C8-2. NSCLC WD36686 ENUM 244C8 elicits increased interferon- secretion and can augment nivolumabactivity I s o t y p e C o n t r o l N i v o l u m a b ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 + 2 4 4 C 8 - 2 ( 5 u g / m L e a c h ) 0 5 10 15 20 F o l d I n d u c t i o n I F N - ( r e l a t i v e t o I s o t y p e ) p<0.01

Ex Vivo Reversal of TIL Exhaustion: Summary of Experimental Observations • Tumor biopsy from n=10 patients were found to harbor TILs that express exhaustion markers including PD-1. • IFN-production was measured as an indicator of reversal of T cell exhaustion following incubation with anti-CD3/anti-CD28 and various anti-PD-1 antibodies. – Example data shown on slides 10-16 1. T cell activity following CD3/CD28 stimulation varied from 200 to 30,000 pg/mL across the 10 samples; 2. 50% of biopsies assayed were found to harbor TILs responsive to anti-PD-1 antibody using IFN-production as a readout 3. ENUM 244C8 elicited a higher level of IFN-production from responsive TILs than Nivolumabor ENUM 388D4; 4. ENUM 244C8 used in combination with other antibodies had an additive effect on production of IFN-from TILs in 3 of 5 responsive samples 17
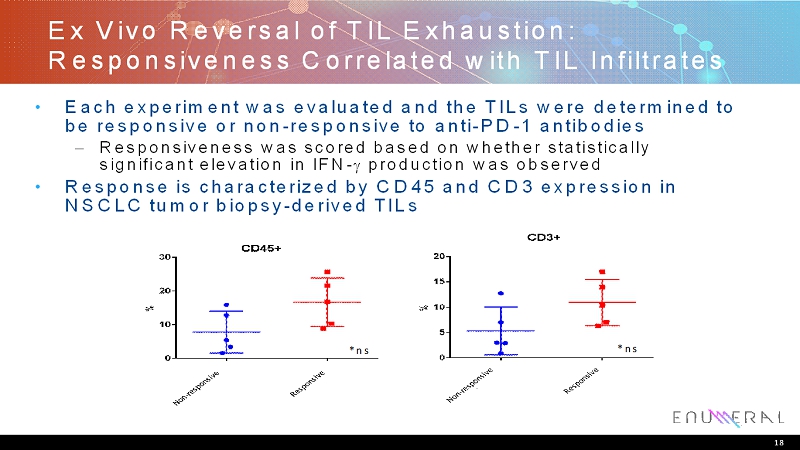
Ex Vivo Reversal of TIL Exhaustion: Responsiveness Correlated with TIL Infiltrates • Each experiment was evaluated and the TILs were determined to be responsive or non-responsive to anti-PD-1 antibodies – Responsiveness was scored based on whether statistically significant elevation in IFN-production was observed • Response is characterized by CD45 and CD3 expression in NSCLC tumor biopsy-derived TILs 18 *ns *ns
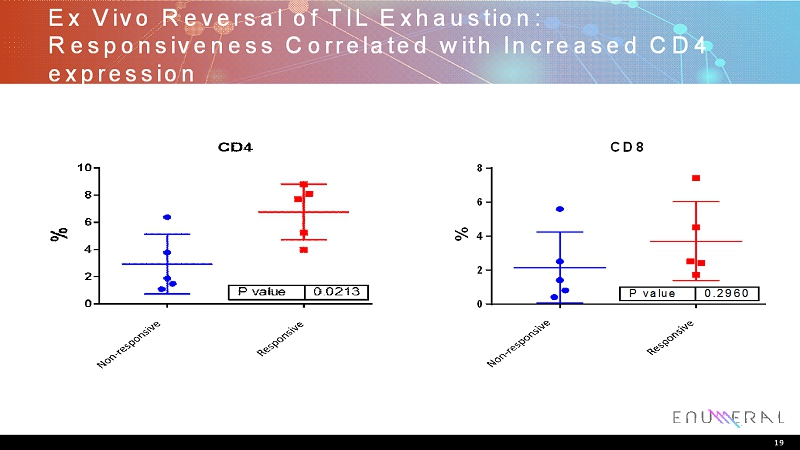
Ex Vivo Reversal of TIL Exhaustion: Responsiveness Correlated with Increased CD4 expression 19 N o n r e s p o n d e r R e s p o n d e r 0 2 4 6 8 CD8 % P value 0.2960
Ex Vivo Reversal of TIL Exhaustion: hypo- responsiv

eness associated with increased CD4 + PD-1 + and CD4 + TIM-3 + levels 20 N o n r e s p o n d e r R e s p o n d e r 0 20 40 60 80 100 %CD4+ PD-1+ % P value 0.0445 N o n r e s p o n d e r R e s p o n d e r -10 0 10 20 30 %CD4+ TIM-3+ % P value 0.0569 N o n r e s p o n d e r R e s p o n d e r 0 20 40 60 80 100 %CD8+ PD-1+ % N o n r e s p o n d e r R e s p o n d e r -20 0 20 40 60 80 % %CD8+ TIM-3+ *ns *ns
Summary of NSCLC findings to date • NSCLC tumors contain varying levels of TILs that are heterogeneous in expression levels of lineage markers (CD4, CD8) and immunomodulatory receptors (PD-1, TIM-3) • Experimental Summary: 50% of samples analyzed found to harbor TILs responsive to PD-1 blockade ex vivo. – Samples were found to respond to nivolumab, ENUM 388D4 and ENUM 244C8, a pharmacologically distinct potentially allosteric PD-1 antagonist antibody. – ENUM 244C8 elicited a higher level of IFN-production from TILs than Nivolumabor ENUM 388D4, and these effects were found to be additive certain samples. • IFN-production in response to anti-PD-1 antibodies correlated with higher levels of TIL infiltration and lower levels of PD-1, and TIM-3 on CD4 cells. 21

Conclusions • ENUM 244C8 is a pharmacologically distinct humanized anti-PD- 1 antibody that binds PD-1 non-competitively with PD-L1 • Incubation with anti-CD3/anti-CD28 antibodies results in limited reversal of exhaustion of NSCLC tumor-derived T cells; PD-1 blockade is required to enhance IFN-secretion. • ENUM 244C8 can augment ex vivo IFN-secretion by TILs to a greater level than nivolumaband may display additive effects. • Enumeral’splatform enables generation of broad antibody diversity that can translate into functionally distinct and potentially improved therapeutic candidates. 22

THE POWER ofHUMAN™






















