Filed Pursuant to Rule 424(b)(3) and Rule 424(c)
Registration No. 333-198847
Prospectus Supplement No. 14
(To Prospectus filed on July 6, 2015, as supplemented
by Prospectus Supplement No. 1 dated July 14, 2015, Prospectus Supplement No. 2 dated July 28, 2015, Prospectus Supplement No. 3 dated August 4, 2015, Prospectus Supplement No. 4 dated August 12, 2015, Prospectus Supplement No. 5 dated September 17, 2015, Prospectus Supplement No. 6 dated September 18, 2015, Prospectus Supplement No. 7 dated September 24, 2015, Prospectus Supplement No. 8 dated September 25, 2015, Prospectus Supplement No. 9 dated September 30, 2015, Prospectus Supplement No. 10 dated October 2, 2015, Prospectus Supplement No. 11 dated November 3, 2015, Prospectus Supplement No. 12 dated November 10, 2015, and Prospectus Supplement No. 13 dated November 18, 2015)
ENUMERAL BIOMEDICAL HOLDINGS, INC.
This Prospectus Supplement No. 14 supplements the information contained in the Prospectus, dated as of July 6, 2015, as amended by Prospectus Supplement No. 1 dated July 14, 2015, Prospectus Supplement No. 2 dated July 28, 2015, Prospectus Supplement No. 3 dated August 4, 2015, Prospectus Supplement No. 4 dated August 12, 2015, Prospectus Supplement No. 5 dated September 17, 2015, Prospectus Supplement No. 6 dated September 18, 2015, Prospectus Supplement No. 7 dated September 24, 2015, Prospectus Supplement No. 8 dated September 25, 2015, Prospectus Supplement No. 9 dated September 30, 2015, Prospectus Supplement No. 10 dated October 2, 2015, Prospectus Supplement No. 11 dated November 3, 2015, Prospectus Supplement No. 12 dated November 10, 2015, and Prospectus Supplement No. 13 dated November 18, 2015, relating to the resale of up to 52,154,760 shares of our common stock by selling stockholders.
This Prospectus Supplement No. 14 is being filed to include the information set forth in our Current Report on Form 8-K, which was filed with the Securities and Exchange Commission on December 1, 2015.
You should read this Prospectus Supplement No. 14 in conjunction with the Prospectus. This Prospectus Supplement No. 14 is qualified by reference to the Prospectus, except to the extent that the information contained in this Prospectus Supplement No. 14 supersedes the information contained in the Prospectus. This Prospectus Supplement No. 14 is not complete without, and may not be utilized except in connection with, the Prospectus.
You should consider carefully the risks that we have described in “Risk Factors” beginning on page 7 of the Prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this Prospectus Supplement is December 1, 2015
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):December 1, 2015
Enumeral Biomedical Holdings, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 000-55415 | 99-0376434 |
| (State or Other Jurisdiction | (Commission File | (I.R.S. Employer |
| of Incorporation) | Number) | Identification Number) |
200 CambridgePark Drive, Suite 2000 Cambridge, Massachusetts (Address of Principal Executive Offices) | | 02140 (Zip Code) |
(617) 945-9146
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 7.01 Regulation FD Disclosure.
Enumeral Biomedical Holdings, Inc. (the “Company”) may use a slide presentation, in whole or in part, from time to time in presentations to potential partners, investors, analysts and others. A copy of the slide presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein. A copy of the slide presentation is also available on the Company’s website atwww.enumeral.com.
The information in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| ExhibitNumber | | Description |
| 99.1 | | Enumeral Biomedical Holdings, Inc. Research and Development Program Update Presentation, dated December 1, 2015 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | ENUMERAL BIOMEDICAL HOLDINGS, INC. |
| | |
| | |
| Dated: December 1, 2015 | By: | /s/ Arthur H. Tinkelenberg, Ph.D. |
| | | Name: Arthur H. Tinkelenberg, Ph.D. |
| | | Title: President and Chief Executive Officer |
| | | |
EXHIBIT INDEX
Exhibit
No. | | Description |
| | | |
| 99.1 | | Enumeral Biomedical Holdings, Inc. Research and Development Program Update Presentation, dated December 1, 2015 |
Exhibit 99.1

Research and Development Program Update December 1, 2015

Agenda • Preclinical animal model studies • Ex vivo lung biopsy studies 2
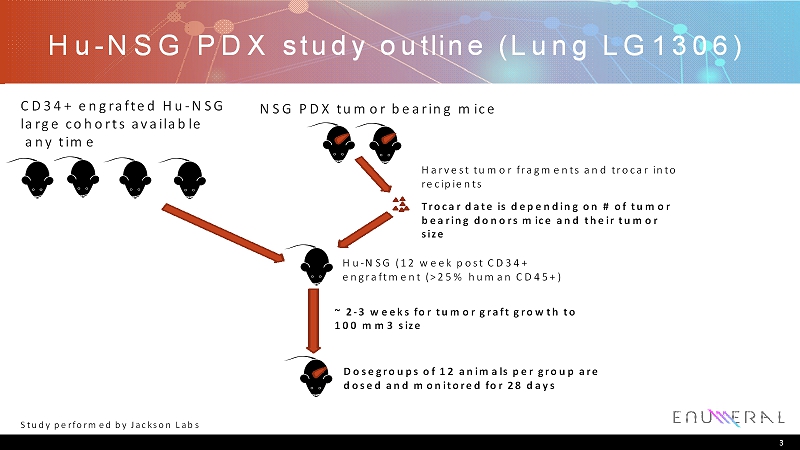
Hu-NSG PDX study outline (Lung LG1306) 3 NSG PDX tumor bearing mice Hu-NSG (12 week post CD34+ engraftment (>25% human CD45+) Harvest tumor fragments and trocar into recipients ~ 2-3 weeks for tumor graft growth to 100 mm3 size Trocar date is depending on # of tumor bearing donors mice and their tumor size Dosegroupsof 12 animals per group are dosed and monitored for 28 days CD34+ engrafted Hu-NSG large cohorts available any time Study performed by Jackson Labs
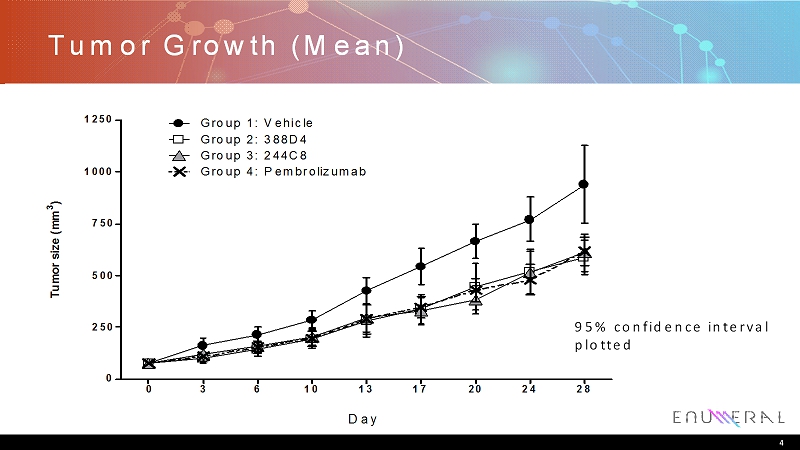
Tumor Growth (Mean) 4 Tumor size (mm 3 ) 0 3 6 10 13 17 20 24 28 0 250 500 750 1000 1250 Group 1: Vehicle Group 2: 388D4 Group 3: 244C8 Group 4: Pembrolizumab Day 95% confidence interval plotted

Study Observations • End of study tumor biopsies collected and analyzed • Low TIL % in tumors – <1% TIL in tumors – Suggests narrow dynamic range for immuno-modulation • Unclear if myeloid functionality in hu-NSG model – Kinetics of engraftment may not recapitulate biology of myeloid cells – Newer NSG-SGM3 model thought to rectify this 5
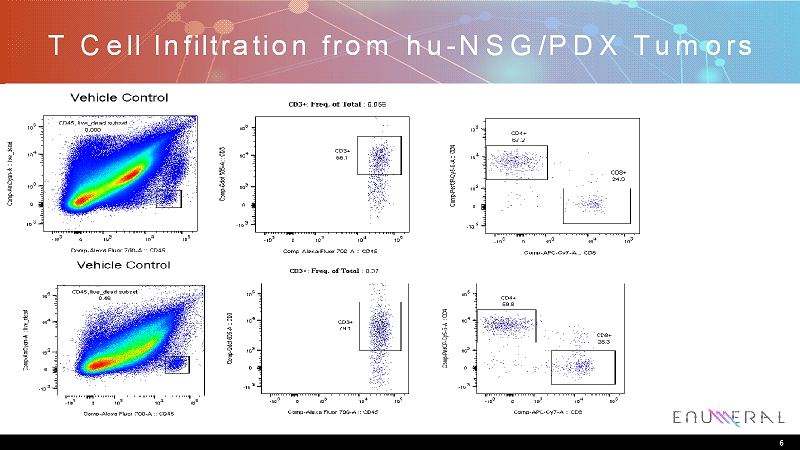
T Cell Infiltration from hu-NSG/PDX Tumors 6

Summary • Enumeralanti-PD1 antibodies 388D4 and 244C8 demonstrate activity in an accepted in vivo model of immunomodulation – huNSGlung PDxmodel used – Tumor growth inhibition similar to that observed with pembrolizumab • Initial characterization of tumor biopsy suggests low levels of TIL infiltration – Model has a narrow dynamic range for measuring activity of novel immunomodulatorsand may not reflect effects on non-T cell compartments 7
PD-1 Blockade in NSCLC Tumor Samples Complex IMR biology 8

Overview: Data Annotation • The following slides show data obtained from two different lung biopsies obtained from patients undergoing staging surgeries • Characterization of baseline IMR expression on TILs: – TIM-3 lo /TIGIT lo -WD36444 – TIM-3 hi /TIGIT hi -WD36988 9
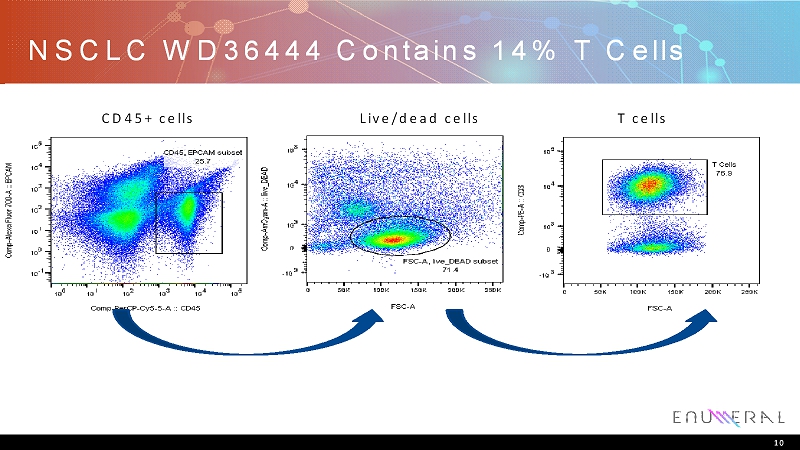
NSCLC WD36444 Contains 14% T Cells 10 CD45+ cells Live/dead cells T cells
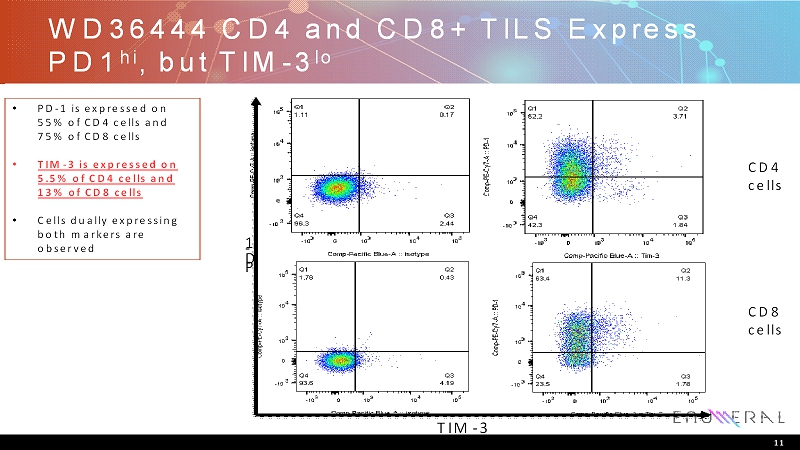
WD36444 CD4 and CD8+ TILS Express PD1 hi , but TIM-3 lo CD4 cells CD8 cells P D - 1 TIM-3 • PD-1 is expressed on 55% of CD4 cells and 75% of CD8 cells • TIM-3 is expressed on 5.5% of CD4 cells and 13% of CD8 cells • Cells dually expressing both markers are observed 11
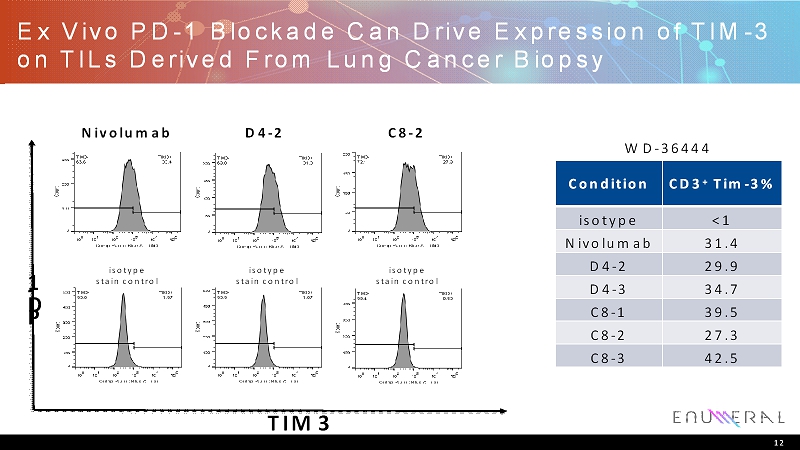
Ex Vivo PD-1 Blockade Can Drive Expression of TIM-3 on TILs Derived From Lung Cancer Biopsy Nivolumab P D - 1 TIM3 D4-2 C8-2 isotype stain control isotype stain control isotype stain control WD-36444 Condition CD3 + Tim-3% isotype <1 Nivolumab 31.4 D4-2 29.9 D4-3 34.7 C8-1 39.5 C8-2 27.3 C8-3 42.5 12
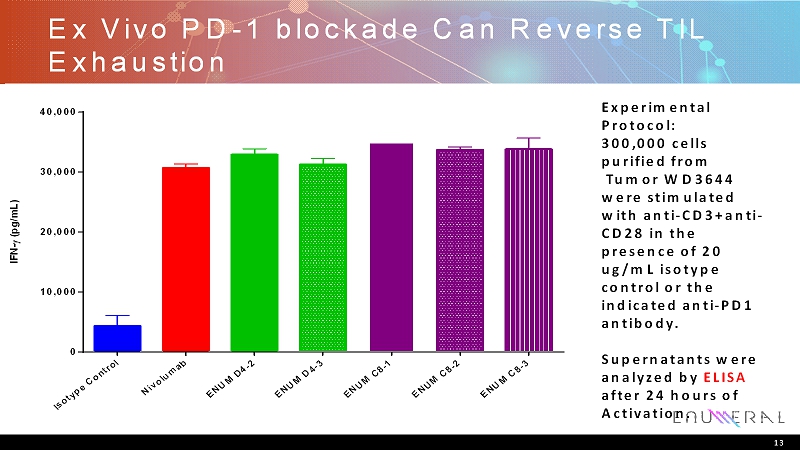
Ex Vivo PD-1 blockade Can Reverse TIL Exhaustion 13 Experimental Protocol: 300,000 cells purified from Tumor WD3644 were stimulated with anti-CD3+anti- CD28 in the presence of 20 ug/mL isotype control or the indicated anti-PD1 antibody. Supernatants were analyzed by ELISA after 24 hours of Activation. I s o t y p e C o n t r o l N i v o l u m a b E N U M D 4 - 2 E N U M D 4 - 3 E N U M C 8 - 1 E N U M C 8 - 2 E N U M C 8 - 3 0 10,000 20,000 30,000 40,000 I F N - ( p g / m L )
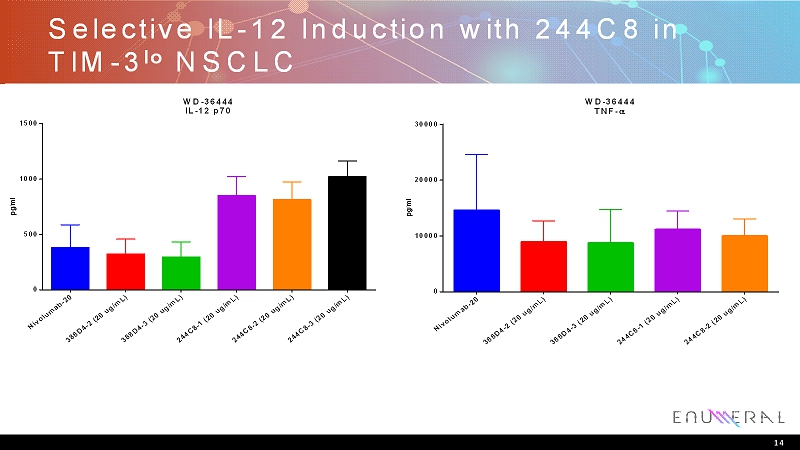
Selective IL-12 Induction with 244C8 in TIM-3 lo NSCLC N i v o l u m a b - 2 0 3 8 8 D 4 - 2 ( 2 0 u g / m L ) 3 8 8 D 4 - 3 ( 2 0 u g / m L ) 2 4 4 C 8 - 1 ( 2 0 u g / m L ) 2 4 4 C 8 - 2 ( 2 0 u g / m L ) 2 4 4 C 8 - 3 ( 2 0 u g / m L ) 0 500 1000 1500 WD-36444 IL-12 p70 p g / m l N i v o l u m a b - 2 0 3 8 8 D 4 - 2 ( 2 0 u g / m L ) 3 8 8 D 4 - 3 ( 2 0 u g / m L ) 2 4 4 C 8 - 1 ( 2 0 u g / m L ) 2 4 4 C 8 - 2 ( 2 0 u g / m L ) 0 10000 20000 30000 WD-36444 TNF- p g / m l 14
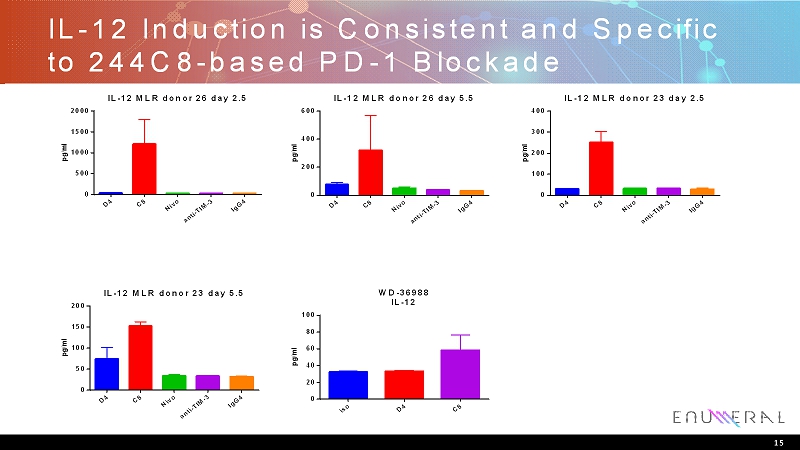
IL-12 Induction is Consistent and Specific to 244C8-based PD-1 Blockade D 4 C 8 N i v o a n t i - T I M - 3 I g G 4 0 500 1000 1500 2000 IL-12 MLR donor 26 day 2.5 p g / m l D 4 C 8 N i v o a n t i - T I M - 3 I g G 4 0 200 400 600 IL-12 MLR donor 26 day 5.5 p g / m l D 4 C 8 N i v o a n t i - T I M - 3 I g G 4 0 100 200 300 400 IL-12 MLR donor 23 day 2.5 p g / m l D 4 C 8 N i v o a n t i - T I M - 3 I g G 4 0 50 100 150 200 IL-12 MLR donor 23 day 5.5 p g / m l i s o D 4 C 8 0 20 40 60 80 100 WD-36988 IL-12 p g / m l 15
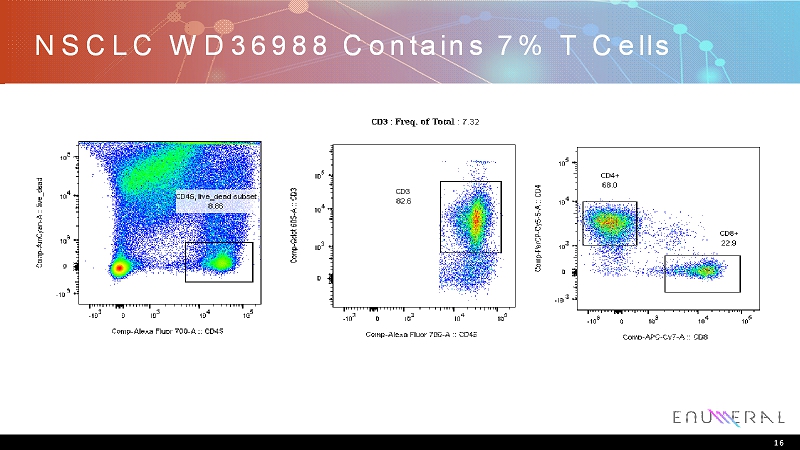
NSCLC WD36988 Contains 7% T Cells 16
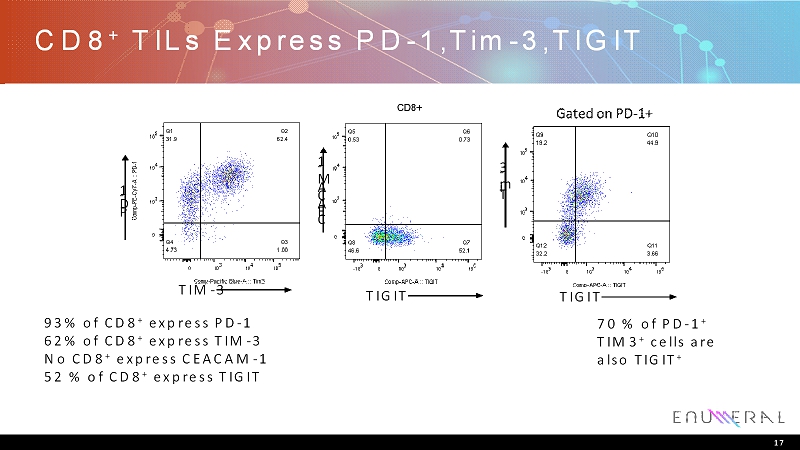
CD8 + TILs Express PD-1,Tim-3,TIGIT 17 93% of CD8 + express PD-1 62% of CD8 + express TIM-3 No CD8 + express CEACAM-1 52 % of CD8 + express TIGIT 70 % of PD-1 + TIM3 + cells are also TIGIT + TIM-3 P D - 1 TIGIT TIGIT C E A C A M - 1 T i m - 3

Ex Vivo PD-1 Blockade can still increase IFNproduction in TIM-3 hi /TIGIT hi T cells B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 1000 2000 3000 4000 IFN- p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 500 1000 1500 2000 Lung IL-2 p g / m l 18
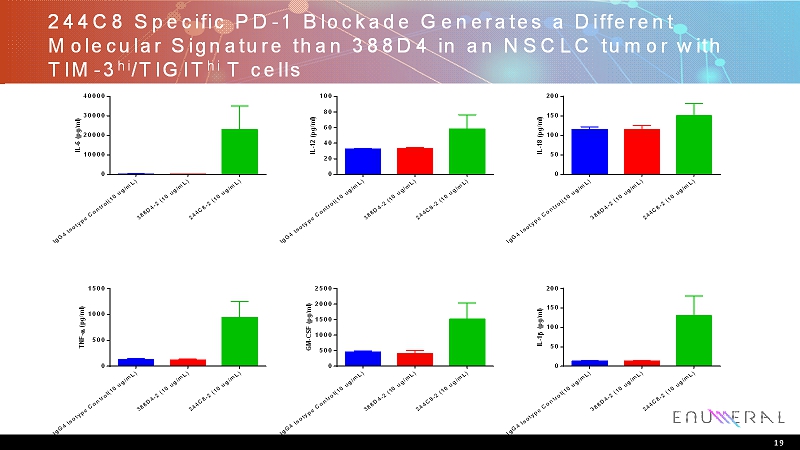
244C8 Specific PD-1 Blockade Generates a Different Molecular Signature than 388D4 in an NSCLC tumor with TIM-3 hi /TIGIT hi T cells I g G 4 I s o t y p e C o n t r o l ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 10000 20000 30000 40000 I L - 6 ( p g / m l ) I g G 4 I s o t y p e C o n t r o l ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 20 40 60 80 100 I L - 1 2 ( p g / m l ) I g G 4 I s o t y p e C o n t r o l ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 50 100 150 200 I L - 1 8 ( p g / m l ) I g G 4 I s o t y p e C o n t r o l ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 500 1000 1500 T N F - ( p g / m l ) I g G 4 I s o t y p e C o n t r o l ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 500 1000 1500 2000 2500 G M - C S F ( p g / m l ) I g G 4 I s o t y p e C o n t r o l ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 50 100 150 200 I L - 1 ( p g / m l ) 19
Summary • NSCLC tumors generally have PD-1 hi T cell infiltrates, however the level of additional checkpoint markers varies considerably • PD-1 biology appears to be linked to TIM-3 receptor expression and both markers appear to be important in T cell exhaustion in NSCLC tumors • PD-1 blockade can be biologically distinct between anti-PD-1 antibodies – Enumeral244C8 is an anti-PD-1 antibody that may promote release of non T cell (myeloid) derived anti-tumor cytokines 20





















