Filed Pursuant to Rule 424(b)(3) and Rule 424(c)
Registration No. 333-198847
Prospectus Supplement No. 15
(To Prospectus filed on July 6, 2015, as supplemented
by Prospectus Supplement No. 1 dated July 14, 2015, Prospectus Supplement No. 2 dated July 28, 2015, Prospectus Supplement No. 3 dated August 4, 2015, Prospectus Supplement No. 4 dated August 12, 2015, Prospectus Supplement No. 5 dated September 17, 2015, Prospectus Supplement No. 6 dated September 18, 2015, Prospectus Supplement No. 7 dated September 24, 2015, Prospectus Supplement No. 8 dated September 25, 2015, Prospectus Supplement No. 9 dated September 30, 2015, Prospectus Supplement No. 10 dated October 2, 2015, Prospectus Supplement No. 11 dated November 3, 2015, Prospectus Supplement No. 12 dated November 10, 2015, Prospectus Supplement No. 13 dated November 18, 2015, and Prospectus Supplement No. 14 dated December 1, 2015)
ENUMERAL BIOMEDICAL HOLDINGS, INC.
This Prospectus Supplement No. 15 supplements the information contained in the Prospectus, dated as of July 6, 2015, as amended by Prospectus Supplement No. 1 dated July 14, 2015, Prospectus Supplement No. 2 dated July 28, 2015, Prospectus Supplement No. 3 dated August 4, 2015, Prospectus Supplement No. 4 dated August 12, 2015, Prospectus Supplement No. 5 dated September 17, 2015, Prospectus Supplement No. 6 dated September 18, 2015, Prospectus Supplement No. 7 dated September 24, 2015, Prospectus Supplement No. 8 dated September 25, 2015, Prospectus Supplement No. 9 dated September 30, 2015, Prospectus Supplement No. 10 dated October 2, 2015, Prospectus Supplement No. 11 dated November 3, 2015, Prospectus Supplement No. 12 dated November 10, 2015, Prospectus Supplement No. 13 dated November 18, 2015, and Prospectus Supplement No. 14 dated December 1, 2015, relating to the resale of up to 52,154,760 shares of our common stock by selling stockholders.
This Prospectus Supplement No. 15 is being filed to include the information set forth in our Current Report on Form 8-K, which was filed with the Securities and Exchange Commission on December 8, 2015.
You should read this Prospectus Supplement No. 15 in conjunction with the Prospectus. This Prospectus Supplement No. 15 is qualified by reference to the Prospectus, except to the extent that the information contained in this Prospectus Supplement No. 15 supersedes the information contained in the Prospectus. This Prospectus Supplement No. 15 is not complete without, and may not be utilized except in connection with, the Prospectus.
You should consider carefully the risks that we have described in “Risk Factors” beginning on page 7 of the Prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this Prospectus Supplement is December 8, 2015
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):December 8, 2015
Enumeral Biomedical Holdings, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 000-55415 | 99-0376434 |
(State or Other Jurisdiction
of Incorporation) | (Commission File Number) | (I.R.S. Employer
Identification Number) |
200 CambridgePark Drive, Suite 2000 Cambridge, Massachusetts (Address of Principal Executive Offices) | | 02140 (Zip Code) |
(617) 945-9146
(Registrant’s telephone number, including area code)
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐ Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐ Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐ Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Item 7.01 Regulation FD Disclosure.
Enumeral Biomedical Holdings, Inc. (the “Company”) may use a slide presentation, in whole or in part, from time to time in presentations to potential partners, investors, analysts and others. A copy of the slide presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and incorporated by reference herein. A copy of the slide presentation is also available on the Company’s website at www.enumeral.com.
The information in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be deemed to be “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that Section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended. The information contained in this Item 7.01 of this Current Report on Form 8-K and in the accompanying Exhibit 99.1 shall not be incorporated by reference into any filing with the U.S. Securities and Exchange Commission made by the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits
| ExhibitNumber | | Description |
| 99.1 | | Enumeral Biomedical Holdings, Inc. Presentation at Oppenheimer 26th Annual Healthcare Conference, dated December 8, 2015 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| | ENUMERAL BIOMEDICAL HOLDINGS, INC. |
| | | |
| Dated: December 8, 2015 | By: | /s/ Kevin G. Sarney |
| | | Name: Kevin G. Sarney |
| | | Title: Vice President of Finance, Chief Accounting Officer and Treasurer |
| | | |
| | | |
EXHIBIT INDEX
Exhibit
No. | | Description |
| | | |
| 99.1 | | Enumeral Biomedical Holdings, Inc. Presentation at Oppenheimer 26th Annual Healthcare Conference, dated December 8, 2015 |
Exhibit 99.1

Oppenheimer 26 th Annual Healthcare Conference December 8, 2015

Forward Looking Statements OTC QB: ENUM THIS PRESENTATIONCONTAINS FORWARD-LOOKING STATEMENTSTHATARE BASED ON THECOMPANY’SCURRENTEXPECTATIONS,ASSUMPTIONS,ESTIMATESANDPROJECTIONS ABOUTTHECOMPANYANDTHEPHARMACEUTICAL INDUSTRY.THECOMPANYMAKESNO REPRESENTATIONS ABOUT THE ACCURACY OF SUCH STATEMENTS ESTIMATES OR PROJECTIONS. FORWARD-LOOKING STATEMENTSARE INDICATED BY WORDS SUCH AS: MAY,WILL,SHOULD,PREDICT,CONTINUE,PLAN,EXPECT,ANTICIPATE,ESTIMATE,INTEND, BELIEVE, COULD, GOAL OBJECTIVES AND SIMILAR EXPRESSIONS. FORWARD-LOOKING STATEMENTSMAYINCLUDE, BUT ARE NOT LIMITED TO, STATEMENTSCONCERNING THE COMPANY’S ANTICIPATED PERFORMANCE, INCLUDING REVENUE AND PROFIT EXPECTATIONS; DEVELOPMENT AND IMPLEMENTATION OF OUR COLLABORATIONS; DURATION; SIZE; SCOPE AND REVENUE ASSOCIATED WITH COLLABORATION PARTNERSHIPS; BENEFITS PROVIDED TO COLLABORATION PARTNERS BY OUR TECHNOLOGY;BUSINESSMIX;REVENUESANDGROWTHINOURPARTNERBASE;MARKET OPPORTUNITIES;COMPETINGTECHNOLOGIES,INDUSTRYCONDITIONSANDTRENDS;AND REGULATORY DEVELOPMENTS. ACTUAL RESULTS MAY DIFFER MATERIALLY FROM THE ANTICIPATEDRESULTSDUETOSUBSTANTIALRISKSANDUNCERTAINTIESRELATEDTOTHE COMPANYANDTHEBIOPHARMACEUTICALINDUSTRYINWHICHTHECOMPANYOPERATES. 2

Investment Highlights • The immunotherapy revolution has begun and best-in-class treatments have yet to be elucidated • Enumeral’svalidated “immune system on a chip” platform leverages translational human biology insights to unlock potential best-in-class rational antibody combinations for immunotherapy • Enumeral’sdifferentiated lead anti-PD-1 antibody program has potential to be best-in-class • Rapidly growing market with room for new entrants where many large oncology franchises have no product candidates • Significant progress to date on minimal capital and well-positioned in market segment that can generate value quickly 3
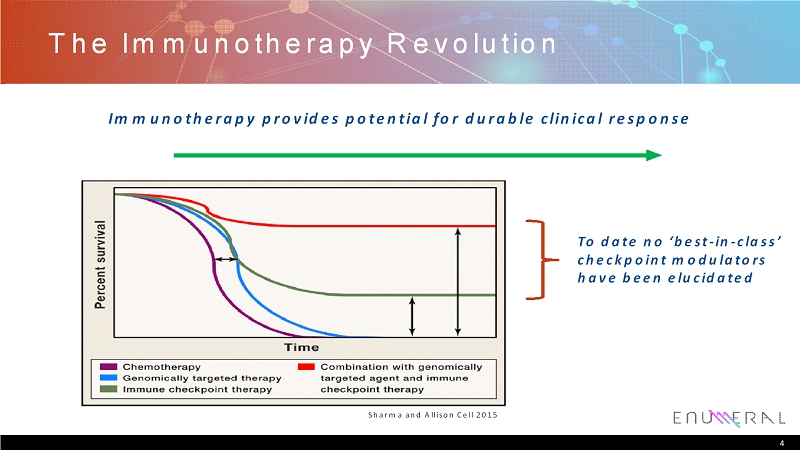
The Immunotherapy Revolution 4 Sharma and Allison Cell 2015 To date no ‘best-in-class’ checkpoint modulators have been elucidated Immunotherapy provides potential for durable clinical response
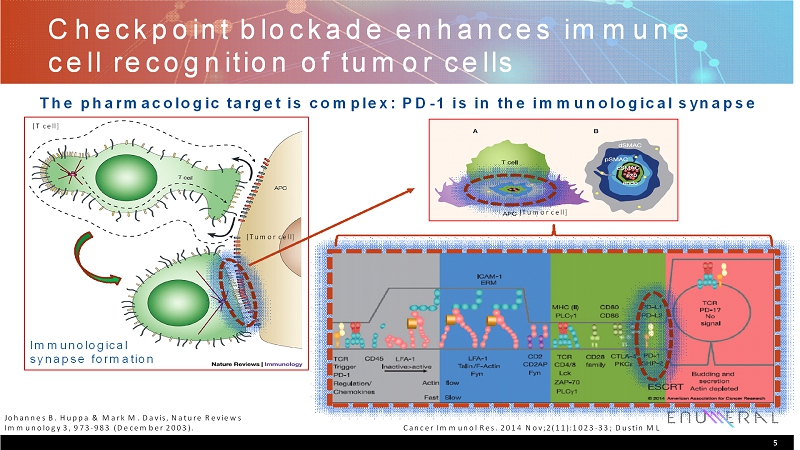
Checkpoint blockade enhances immune cell recognition of tumor cells 5 Johannes B. Huppa& Mark M. Davis, Nature Reviews Immunology 3, 973-983 (December 2003). The pharmacologic target is complex: PD-1 is in the immunological synapse Cancer ImmunolRes. 2014 Nov;2(11):1023-33; Dustin ML [Tumor cell] [T cell] [Tumor cell] Immunological synapse formation

We are just beginning to unlock new discoveries in the immunotherapy space 6 “Highly multiplexed, single-cell technologies may be critical for identifying correlates of disease or immunological interventions as well as for elucidating the underlying mechanisms of immunity.”* *PratipK Chattopadhyay, Todd M Gierahn, Mario Roederer, J Christopher Love. Nature Immunology. 2014;15(2):128-135. Keytruda® Yervoy® Opdivo® Best in class antibodies Combination therapies Novel modalities New undiscovered targets

EnumeralPlatform Advantages 7 84,672 50µm microwells • Proprietary broadly enabling “Immune system on a chip” platform • Immuno-oncology still a “black box” – Requires mechanistically differentiated assets for entry – Requires translational biology insights to gain competitive advantage Measures key parameters of response of individual immune cells from patient samples to drug candidates Uniquelyidentifies rare immune cellscritical to responses for mechanistic differentiation Recovercells of interest and gene sequences encoding natural antibodies and T cell receptors
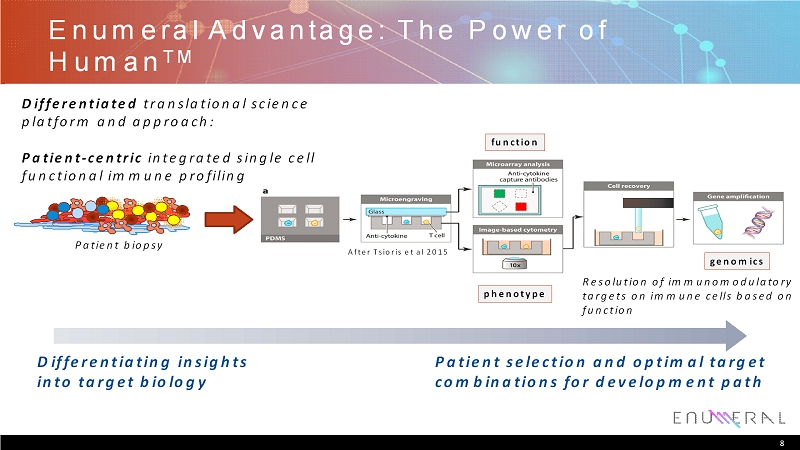
Enumeral Advantage: The Power of Human TM 8 function phenotype genomics Resolution of immunomodulatory targets on immune cells based on function Patient biopsy Differentiating insights into target biology Patient selection and optimal target combinations for development path After Tsioriset al 2015 Differentiatedtranslational science platform and approach: Patient-centricintegrated single cell functional immune profiling
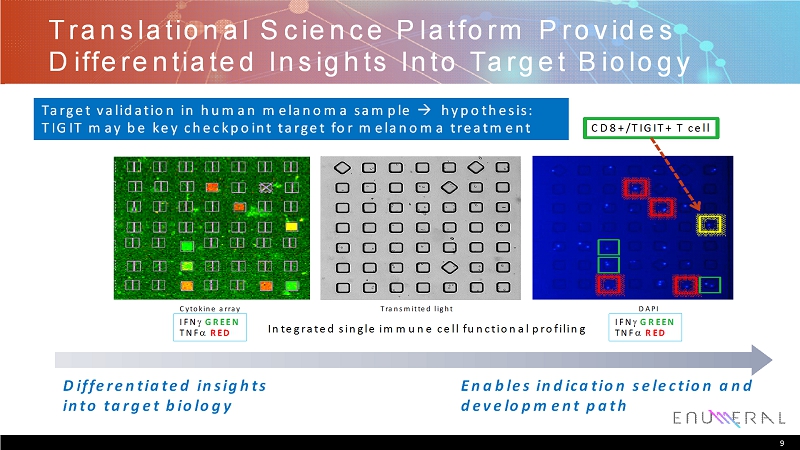
Translational Science Platform Provides Differentiated Insights Into Target Biology 9 Target validation in human melanoma sample hypothesis: TIGIT may be key checkpoint target for melanoma treatment Differentiated insights into target biology Enables indication selection and development path Cytokine array Transmitted light DAPI IFNGREEN TNFRED IFNGREEN TNFRED CD8+/TIGIT+ T cell Integrated single immune cell functional profiling
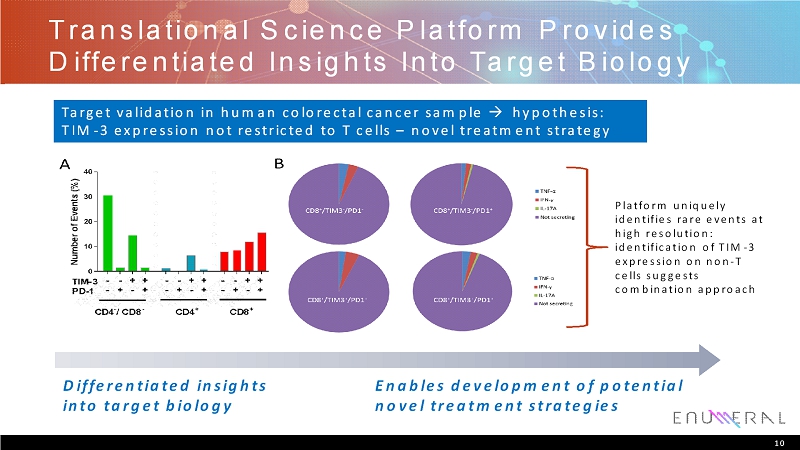
Translational Science Platform Provides Differentiated Insights Into Target Biology 10 Platform uniquely identifies rare events at high resolution: identification of TIM-3 expression on non-T cells suggests combination approach Differentiated insights into target biology Enables development of potential novel treatment strategies Target validation in human colorectal cancer sample hypothesis: TIM-3 expression not restricted to T cells –novel treatment strategy

Validation of Technology Platform* 11 *Publications from laboratory of scientific founder, J. Christopher Love, Ph.D.

Recognition of Differentiated Approach • MERCK: collaboration with a leading immuno-oncology pharmaceutical company – Focused on using Enumeral'splatform to interrogate the tumor microenvironment in colorectal cancer tissues to identify functional cellular responses to therapies being developed by Merck – R&D funding and undisclosed milestone payments – Merck has exclusive rights to data related to its proprietary compounds – ENUM recently achieved first milestone in the collaboration • NCI:awarded Phase 2 contract for ~$1 million over two years – Automation of human tissue immuno-oncology profiling – Opens door to broader pipeline and potentially accelerated development – Collaboration with leading scientists: 12 – Jedd Wolchok’sgroup at MSKCC - genetic basis for response to checkpoint inhibitors and novel immunotherapeutics – Doug Kwon’sgroup at MGH/RagonInstitute - pioneering techniques for single cell immune cell analysis in biopsy
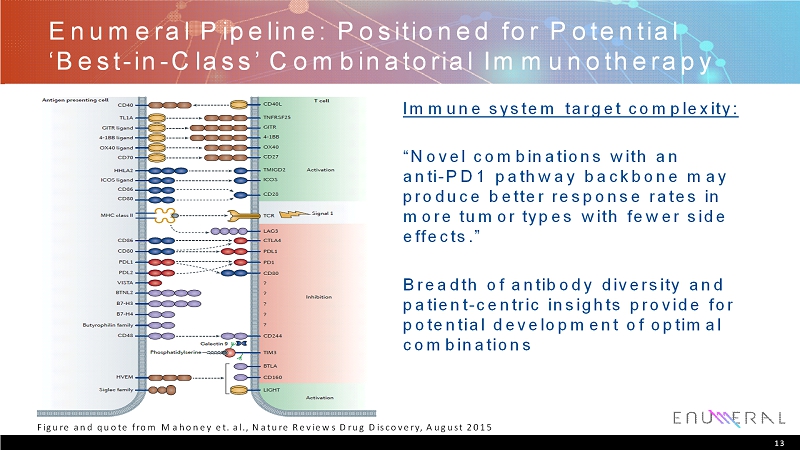
EnumeralPipeline: Positioned for Potential ‘Best-in-Class’ Combinatorial Immunotherapy Immune system target complexity: “Novel combinations with an anti-PD1 pathway backbone may produce better response rates in more tumor types with fewer side effects.” Breadth of antibody diversity and patient-centric insights provide for potential development of optimal combinations 13 Figure and quote from Mahoney et. al., Nature Reviews Drug Discovery, August 2015
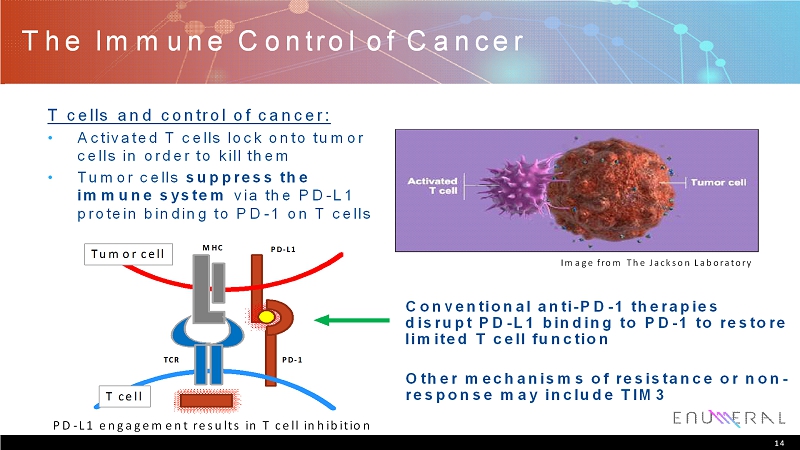
The Immune Control of Cancer T cells and control of cancer: • Activated T cells lock onto tumor cells in order to kill them • Tumor cells suppress the immune system via the PD-L1 protein binding to PD-1 on T cells 14 Conventional anti-PD-1 therapies disrupt PD-L1 binding to PD-1 to restore limited T cell function Other mechanisms of resistance or non- response may include TIM3 Image from The Jackson Laboratory PD-L1 engagement results in T cell inhibition Tumor cell T cell MHC TCR PD-1 PD-L1
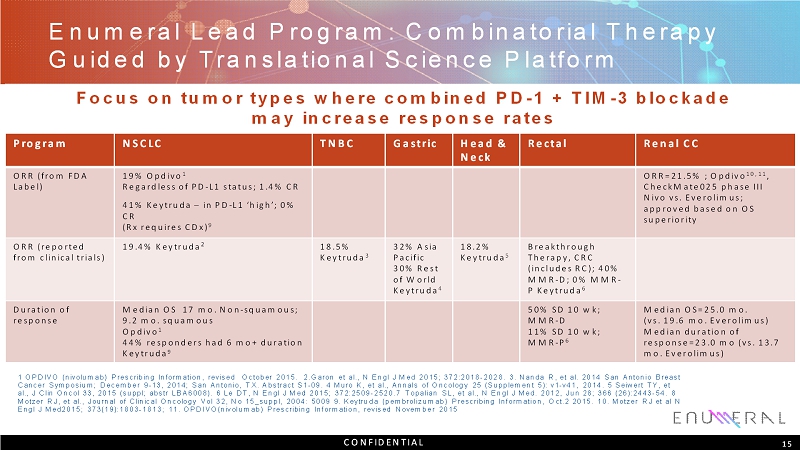
EnumeralLead Program: Combinatorial Therapy Guided by Translational Science Platform Program NSCLC TNBC Gastric Head & Neck Rectal Renal CC ORR (from FDA Label) 19% Opdivo 1 Regardless of PD-L1 status; 1.4% CR 41% Keytruda–in PD-L1 ‘high’; 0% CR (Rx requires CDx) 9 ORR=21.5% ; Opdivo 10, 11 , CheckMate025 phase III Nivovs. Everolimus; approved based on OS superiority ORR (reported from clinical trials) 19.4% Keytruda 2 18.5% Keytruda 3 32% Asia Pacific 30%Rest of World Keytruda 4 18.2% Keytruda 5 Breakthrough Therapy,CRC (includes RC);40% MMR-D;0% MMR- P Keytruda 6 Duration of response Median OS 17 mo. Non-squamous; 9.2 mo. squamous Opdivo 1 44% responders had 6 mo+ duration Keytruda 9 50% SD 10 wk; MMR-D 11% SD 10 wk; MMR-P 6 Median OS=25.0 mo. (vs. 19.6 mo. Everolimus) Median duration of response=23.0 mo (vs. 13.7 mo. Everolimus) 15 Focus on tumor types where combined PD-1 + TIM-3 blockade may increase response rates 1 OPDIVO (nivolumab) Prescribing Information, revised October 2015. 2.Garon et al., N EnglJ Med 2015; 372:2018-2028. 3. Nanda R, et al. 2014 San Antonio Breast Cancer Symposium; December 9 -13, 2014; San Antonio, TX. Abstract S1 -09. 4 MuroK, et al., Annals of Oncology 25 (Supplement 5): v1 -v41, 2014. 5 SeiwertTY, et al., J ClinOncol33, 2015 (suppl; abstrLBA6008). 6 Le DT, N EnglJ Med 2015; 372:2509-2520.7 Topalian SL, et al., N EnglJ Med. 2012, Jun 28; 366 (26):2443-54. 8 MotzerRJ, et al., Journal of Clinical Oncology Vol 32, No 15_suppl, 2004: 5009 9. Keytruda (pembrolizumab) Prescribing Information, Oct.2 2015. 10. MotzerRJ et al N EnglJ Med2015; 373(19):1803-1813; 11. OPDIVO(nivolumab) Prescribing Information, revised November 2015 CONFIDENTIAL
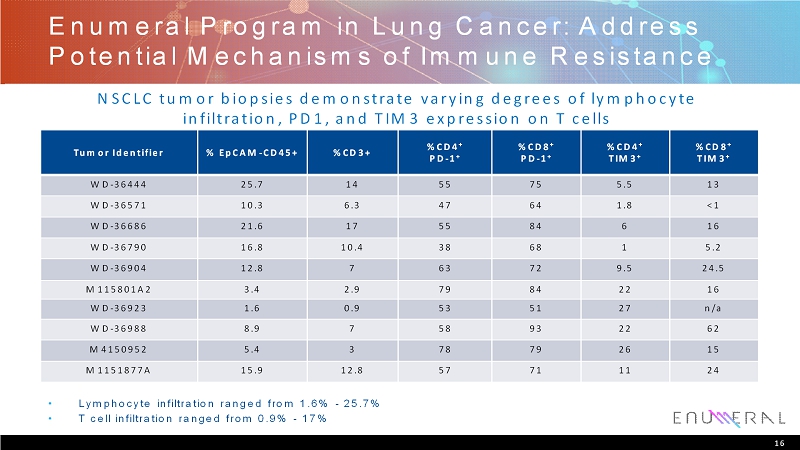
EnumeralProgram in Lung Cancer: Address Potential Mechanisms of Immune Resistance Tumor Identifier % EpCAM-CD45+ %CD3+ %CD4 + PD-1 + %CD8 + PD-1 + %CD4 + TIM3 + %CD8 + TIM3 + WD-36444 25.7 14 55 75 5.5 13 WD-36571 10.3 6.3 47 64 1.8 <1 WD-36686 21.6 17 55 84 6 16 WD-36790 16.8 10.4 38 68 1 5.2 WD-36904 12.8 7 63 72 9.5 24.5 M115801A2 3.4 2.9 79 84 22 16 WD-36923 1.6 0.9 53 51 27 n/a WD-36988 8.9 7 58 93 22 62 M4150952 5.4 3 78 79 26 15 M1151877A 15.9 12.8 57 71 11 24 • Lymphocyte infiltration ranged from 1.6% -25.7% • T cell infiltration ranged from 0.9% -17% NSCLC tumor biopsies demonstrate varying degrees of lymphocyte infiltration, PD1, and TIM3 expression on T cells 16
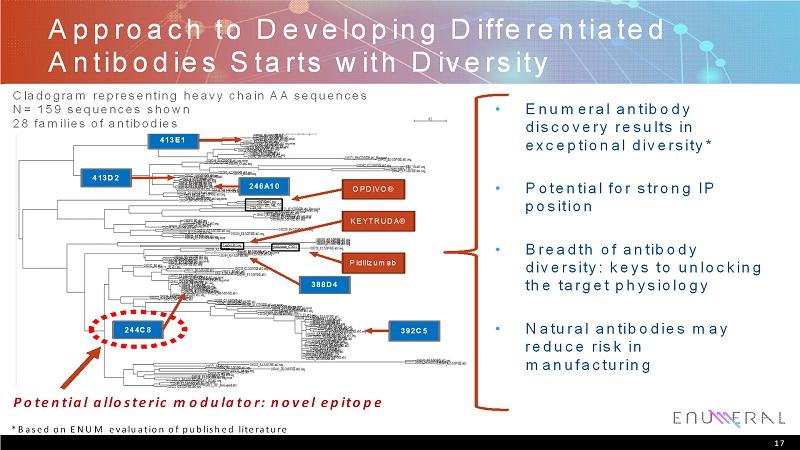
Approach to Developing Differentiated Antibodies Starts with Diversity • Enumeralantibody discovery results in exceptional diversity* • Potential for strong IP position • Breadth of antibody diversity: keys to unlocking the target physiology • Natural antibodies may reduce risk in manufacturing OPDIVO® KEYTRUDA® Pidilizumab 392C5 246A10 413D2 413E1 244C8 388D4 *Based on ENUM evaluation of published literature Cladogram representing heavy chain AA sequences N= 159 sequences shown 28 families of antibodies Potential allosteric modulator: novel epitope 17
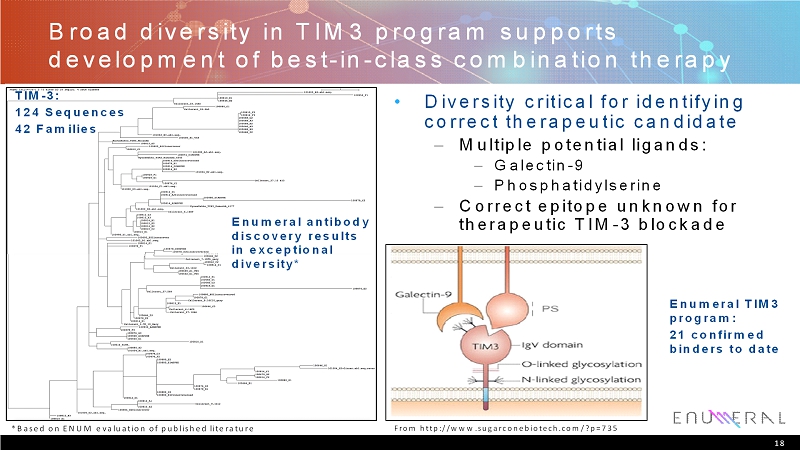
Broad diversity in TIM3 program supports development of best-in-class combination therapy • Diversity critical for identifying correct therapeutic candidate – Multiple potential ligands: –Galectin-9 –Phosphatidylserine – Correct epitope unknown for therapeutic TIM-3 blockade 18 TIM-3: 124 Sequences 42 Families *Based on ENUM evaluation of published literature From http://www.sugarconebiotech.com/?p=735 Enumeralantibody discovery results in exceptional diversity* EnumeralTIM3 program: 21 confirmed binders to date
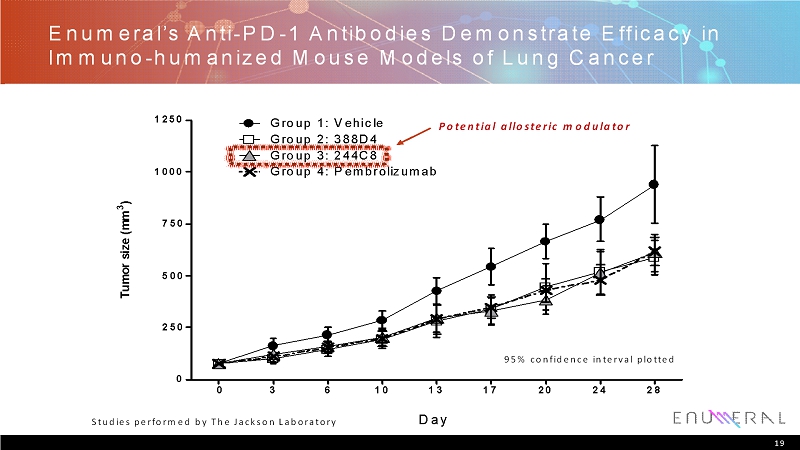
Enumeral’sAnti-PD-1 Antibodies Demonstrate Efficacy in Immuno-humanized Mouse Models of Lung Cancer 19 Tumor size (mm 3 ) 0 3 6 10 13 17 20 24 28 0 250 500 750 1000 1250 Group 1: Vehicle Group 2: 388D4 Group 3: 244C8 Group 4: Pembrolizumab Day 95% confidence interval plotted Studies performed by The Jackson Laboratory Potential allosteric modulator
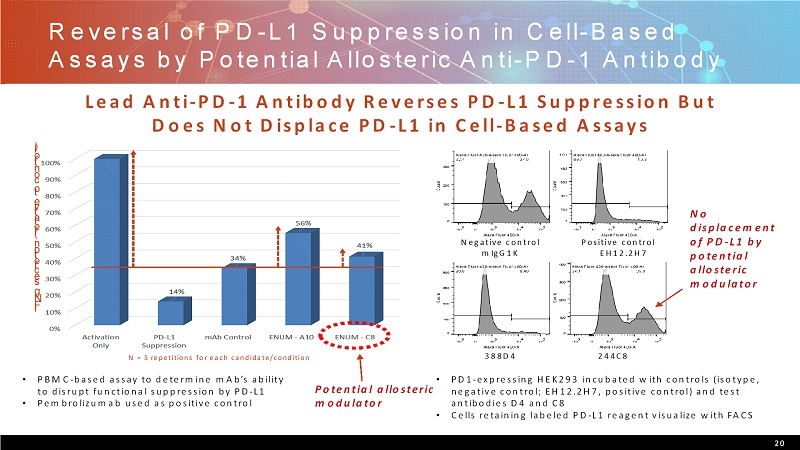
Reversal of PD-L1 Suppression in Cell-Based Assays by Potential Allosteric Anti-PD-1 Antibody 20 Lead Anti-PD-1 Antibody Reverses PD-L1 Suppression But Does Not Displace PD-L1 in Cell-Based Assays I F N g s e c r e t i o n ( r e l a t i v e t o c o n t r o l ) N = 5 repetitions for each candidate/condition • PBMC-based assay to determine mAb’sability to disrupt functional suppression by PD-L1 • Pembrolizumabused as positive control Positive control EH12.2H7 Negative control mIgG1K 388D4 244C8 No displacement of PD-L1 by potential allosteric modulator • PD1-expressing HEK293 incubated with controls (isotype, negative control; EH12.2H7, positive control) and test antibodies D4 and C8 • Cells retaining labeled PD-L1 reagent visualize with FACS Potential allosteric modulator
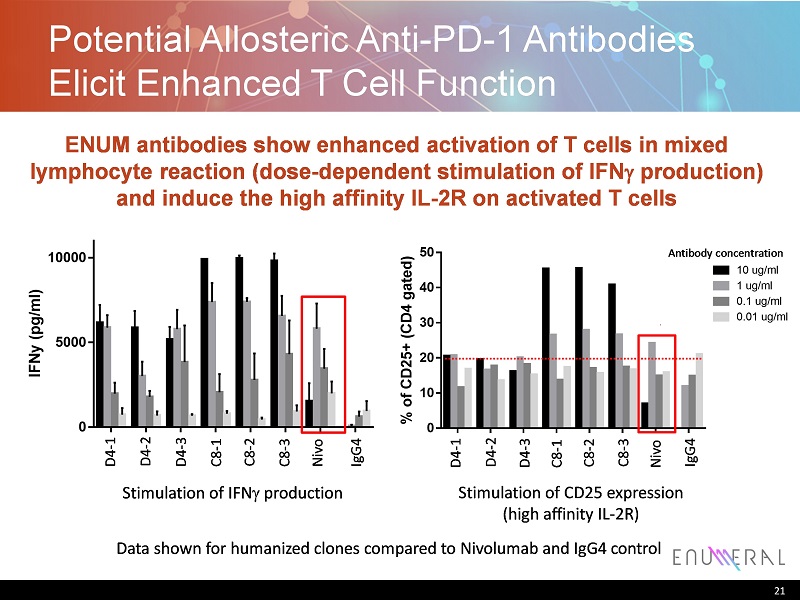
D 4 - H C 3 + L C 1 D 4 - H C 1 + L C 3 D 4 - H C 3 + L C 3 C 8 - H C 1 + L C 1 C 8 - H C 1 + L C 3 C 8 - H C 2 + L C 1 N i v o - I g G 4 h I g G 4 - H C A 2 4 7 0 5000 10000 MLR Donor 337+23 (IFNy) I F N y ( p g / m l ) 10 ug/ml 1 ug/ml 0.1 ug/ml 0.01 ug/ml Antibody concentration Antibody concentration D 4 - H C 3 + L C 1 D 4 - H C 1 + L C 3 D 4 - H C 3 + L C 3 C 8 - H C 1 + L C 1 C 8 - H C 1 + L C 3 C 8 - H C 2 + L C 1 N i v o - I g G 4 h I g G 4 - H C A 2 4 7 0 10 20 30 40 50 % o f C D 2 5 + ( C D 4 g a t e d ) 1 ug/ml 10 ug/ml 0.1 ug/ml 0.01 ug/ml MLR Donor 337+23 (T cell activation analysis by FACS) Stimulation of IFNproduction Antibody concentration D 4 - H C 3 + L C 1 D 4 - H C 1 + L C 3 D 4 - H C 3 + L C 3 C 8 - H C 1 + L C 1 C 8 - H C 1 + L C 3 C 8 - H C 2 + L C 1 N i v o - I g G 4 h I g G 4 - H C A 2 4 7 0 10 20 30 40 50 % o f C D 2 5 + ( C D 4 g a t e d ) 1 ug/ml 10 ug/ml 0.1 ug/ml 0.01 ug/ml MLR Donor 337+23 (T cell activation analysis by FACS) Stimulation of CD25 expression (high affinity IL-2R) N i v o I g G 4 D 4 - 1 D 4 - 2 D 4 - 3 C 8 - 1 C 8 - 2 C 8 - 3 N i v o I g G 4 D 4 - 1 D 4 - 2 D 4 - 3 C 8 - 1 C 8 - 2 C 8 - 3 Potential Allosteric Anti-PD-1 Antibodies Elicit Enhanced T Cell Function 21 ENUM antibodies show enhanced activation of T cells in mixed lymphocyte reaction (dose-dependent stimulation ofIFNproduction) and induce the high affinity IL-2R on activated T cells Data shown for humanized clones compared to Nivolumaband IgG4 control

Lead Anti-PD-1 Antibodies Enhance Ex Vivo Reversal of Tumor-infiltrating Lymphocyte Exhaustion Functional response of tumor infiltrating lymphocytes following PD-1 blockade. 3e5 WD36571 tumor cells containing 10% lymphocytes were activated in the presence of anti-CD3+anti-CD28 and the indicated antibodies. NSCLC WD36571 I s o t y p e C o n t r o l N i v o l u m a b ( 1 0 u g / m L ) E N U M D 4 - 2 ( 1 0 u g / m L ) E N U M C 8 - 2 ( 1 0 u g / m L ) 0 500 1,000 1,500 2,000 I F N - ( p g / m L ) p<0.05 p<0.05 22 ENUM antibodies increased interferon-secretion from TILs derived from human lung biopsy
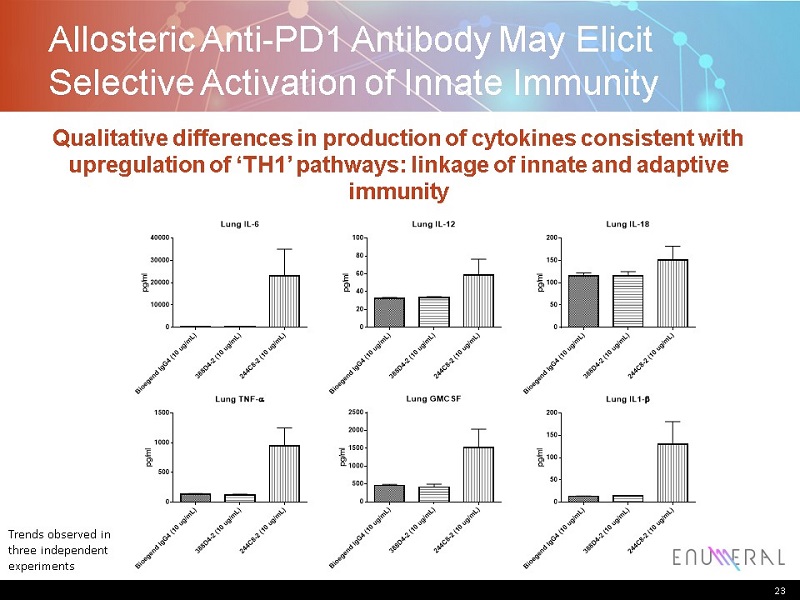
Allosteric Anti-PD1 AntibodyMay Elicit Selective Activation of Innate Immunity 23 Qualitative differences in production of cytokines consistent with upregulation of ‘TH1’ pathways: linkage of innate and adaptive immunity B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 10000 20000 30000 40000 Lung IL-6 p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 20 40 60 80 100 Lung IL-12 p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 50 100 150 200 Lung IL-18 p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 500 1000 1500 Lung TNF- p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 500 1000 1500 2000 2500 Lung GMCSF p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 50 100 150 200 Lung IL1- p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 10000 20000 30000 40000 Lung IL-6 p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 20 40 60 80 100 Lung IL-12 p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 50 100 150 200 Lung IL-18 p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 500 1000 1500 Lung TNF- p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 500 1000 1500 2000 2500 Lung GMCSF p g / m l B i o e g e n d I g G 4 ( 1 0 u g / m L ) 3 8 8 D 4 - 2 ( 1 0 u g / m L ) 2 4 4 C 8 - 2 ( 1 0 u g / m L ) 0 50 100 150 200 Lung IL1- p g / m l Trends observed in three independent experiments
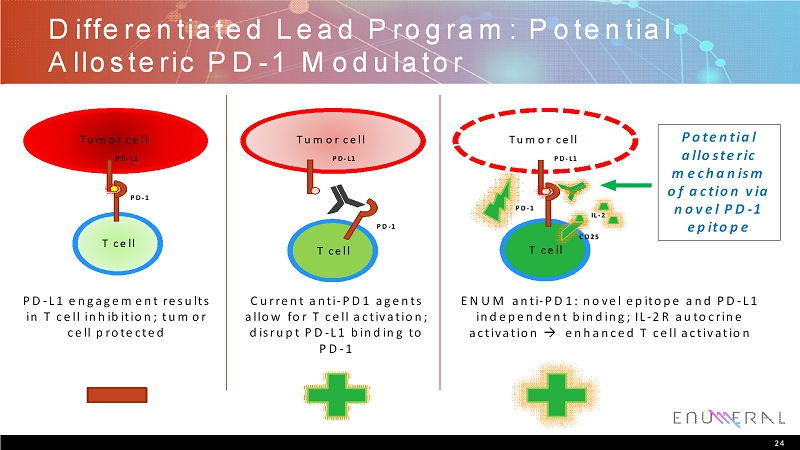
Differentiated Lead Program: Potential Allosteric PD-1 Modulator 24 Current anti-PD1 agents allow for T cell activation; disrupt PD-L1 binding to PD-1 PD-L1 engagement results in T cell inhibition; tumor cell protected ENUM anti-PD1: novel epitope and PD-L1 independent binding; IL-2R autocrine activation enhanced T cell activation PD-1 T cell Tumor cell PD-L1 Tumor cell PD-L1 PD-1 T cell Tumor cell PD-L1 T cell PD-1 CD25 IL-2 Potential allosteric mechanism of action via novel PD-1 epitope
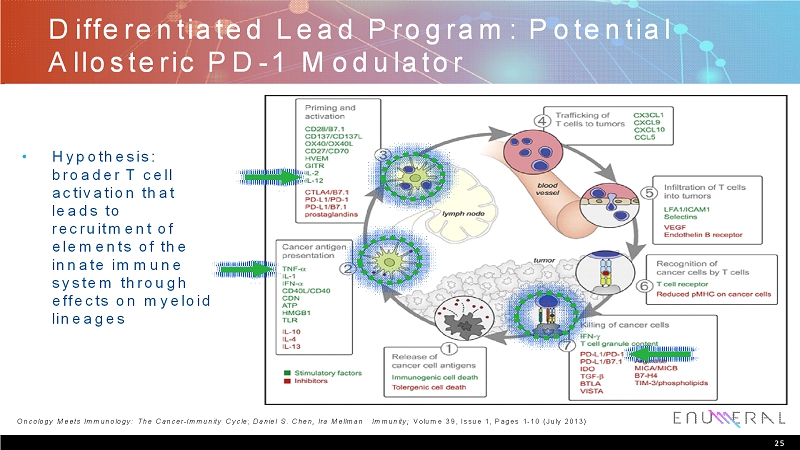
Differentiated Lead Program: Potential Allosteric PD-1 Modulator • Hypothesis: broader T cell activation that leads to recruitment of elements of the innate immune system through effects on myeloid lineages 25 Oncology Meets Immunology: The Cancer -Immunity Cycle; DanielS. Chen, Ira Mellman Immunity; Volume 39, Issue 1, Pages 1-10 (July 2013)
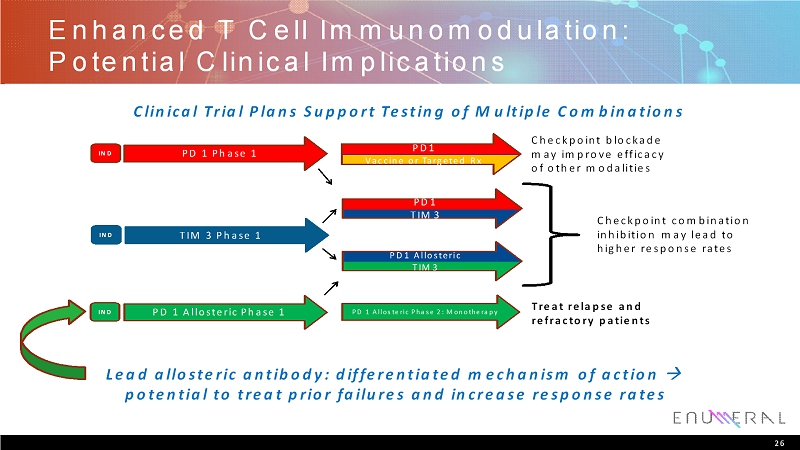
Enhanced T Cell Immunomodulation: Potential Clinical Implications 26 Checkpoint blockade may improve efficacy of other modalities Treat relapse and refractory patients Checkpoint combination inhibition may lead to higher response rates Clinical Trial Plans Support Testing of Multiple Combinations IND TIM 3 Phase 1 PD 1 Phase 1 IND PD1 Allosteric TIM3 PD1 Vaccine or Targeted Rx PD1 TIM3 PD 1 Allosteric Phase 2: MonotherapyIND PD 1 Allosteric Phase 1 Lead allosteric antibody: differentiated mechanism of action potential to treat prior failures and increase response rates
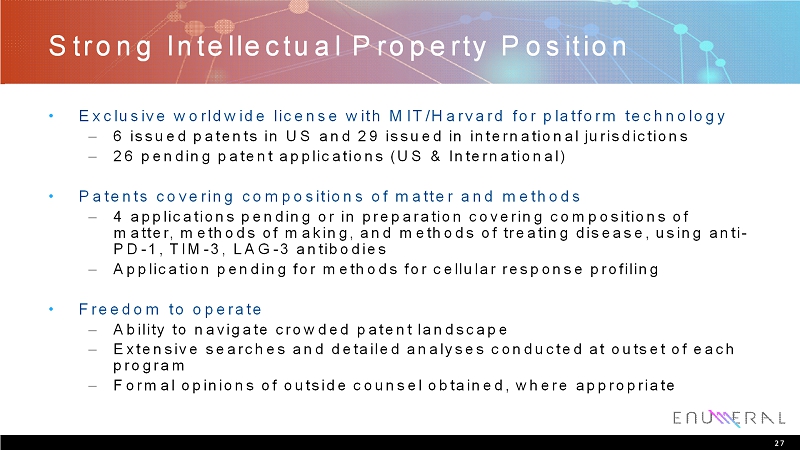
Strong Intellectual Property Position • Exclusive worldwide license with MIT/Harvard for platform technology – 6issued patents in US and 29 issued in international jurisdictions – 26 pending patent applications (US & International) • Patents covering compositions of matter and methods – 4applications pending or in preparation covering compositions of matter, methods of making, and methods of treating disease, using anti- PD-1, TIM-3, LAG-3 antibodies – Application pending for methods for cellular response profiling • Freedom to operate – Ability to navigate crowded patent landscape – Extensive searches and detailed analyses conducted at outset of each program – Formal opinions of outside counsel obtained, where appropriate 27
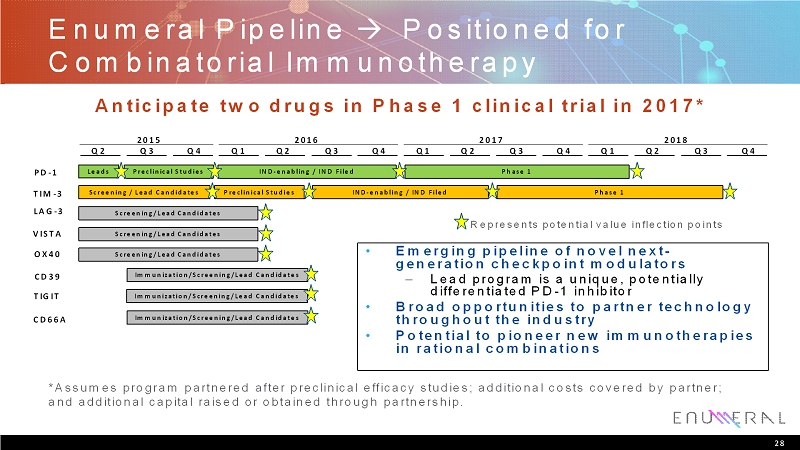
EnumeralPipeline Positioned for Combinatorial Immunotherapy 28 Represents potential value inflection points *Assumes program partnered after preclinical efficacy studies; additional costs covered by partner; and additional capital raised or obtained through partnership. Leads Preclinical Studies IND-enabling / IND Filed Phase 1 Screening / Lead Candidates Preclinical Studies IND-enabling / IND Filed Phase 1 Screening/Lead Candidates PD-1 TIM-3 LAG-3 VISTA OX40 CD39 TIGIT CD66A Q1 Q2 Q3 Q4 2018 Q1 Q2 Q3 Q4 2017 Q1 Q2 Q3 Q4 2016 Q2 Q3 Q4 2015 Screening/Lead Candidates Screening/Lead Candidates Immunization/Screening/Lead Candidates Immunization/Screening/Lead Candidates Immunization/Screening/Lead Candidates Anticipate two drugs in Phase 1 clinical trial in 2017* • Emerging pipeline of novel next- generation checkpoint modulators – Lead program is a unique, potentially differentiated PD-1 inhibitor • Broad opportunities to partner technology throughout the industry • Potential to pioneer new immunotherapies in rational combinations
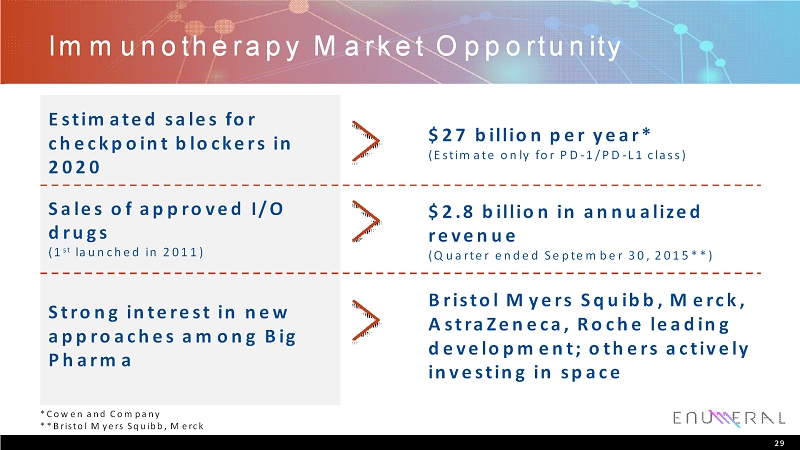
Immunotherapy Market Opportunity 29 *Cowen and Company **Bristol Myers Squibb, Merck Estimated sales for checkpoint blockers in 2020 $27 billion per year* (Estimateonly for PD-1/PD-L1 class) Sales of approved I/O drugs (1 st launched in 2011) $2.8 billion in annualized revenue (Quarter ended September 30, 2015**) Strong interest in new approaches among Big Pharma Bristol Myers Squibb, Merck, AstraZeneca, Roche leading development; others actively investing in space
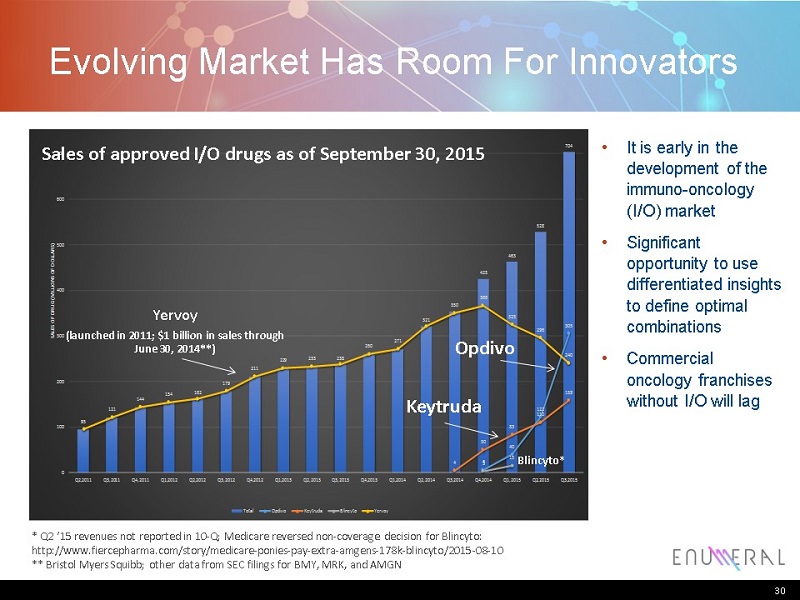
Evolving Market Has Room For Innovators * Q2 ‘15 revenues not reported in 10-Q; Medicare reversed non-coverage decision for Blincyto: http://www.fiercepharma.com/story/medicare-ponies-pay-extra-amgens-178k-blincyto/2015-08-10 ** Bristol Myers Squibb; other data from SEC filings for BMY, MRK, and AMGN • Itis early in the development of the immuno-oncology (I/O) market • Significant opportunity to use differentiated insights to define optimal combinations • Commercial oncology franchises without I/O will lag 30 162 238 321 425 463 528 704 6 40 122 305 4 50 83 110 159 3 15 95 121 144 154 179 211 229 233 260 271 350 366 325 296 240 0 100 200 300 400 500 600 700 800 Q2,2011 Q3,2011 Q4,2011 Q1,2012 Q2,2012 Q3,2012 Q4,2012 Q1,2013 Q2,2013 Q3,2013 Q4,2013 Q1,2014 Q2,2014 Q3,2014 Q4,2014 Q1,2015 Q2,2015 Q3,2015 S A L E S O F D R U G ( M I L L I O N S O F D O L L A R S ) Sales Trends of FDA Approved Immuno-oncology Drugs Total Opdivo Keytruda Blincyto Yervoy Yervoy (launched in 2011; $1 billion in sales through June 30, 2014**) Keytruda Opdivo Blincyto* Sales of approved I/O drugs as of September 30, 2015
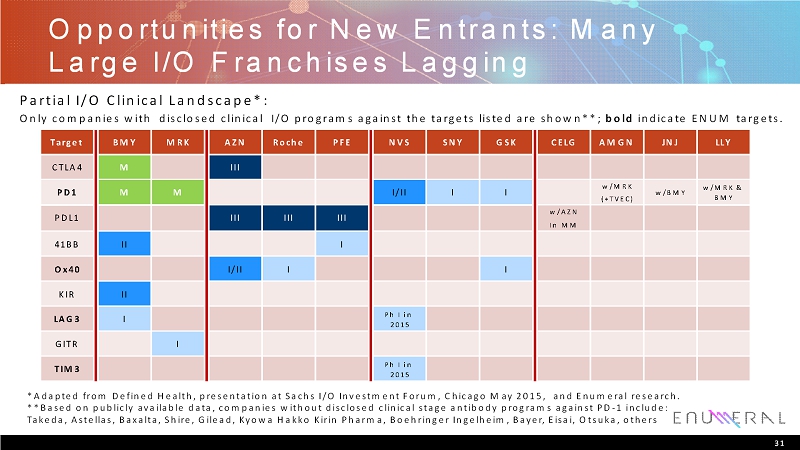
Opportunities for New Entrants: Many Large I/O Franchises Lagging 31 Only companies with disclosed clinical I/O programs against the targets listed are shown**; boldindicate ENUM targets. *Adapted from Defined Health, presentation at Sachs I/O Investment Forum, Chicago May 2015, and Enumeralresearch. **Based on publicly available data, companies without disclosed clinical stage antibody programs against PD-1 include: Takeda, Astellas, Baxalta, Shire, Gilead, Kyowa Hakko Kirin Pharma, BoehringerIngelheim, Bayer, Eisai, Otsuka, others Partial I/O Clinical Landscape*: Target BMY MRK AZN Roche PFE NVS SNY GSK CELG AMGN JNJ LLY CTLA4 M III w/MRK (+TVEC) w/AZN In MM 41BB II I Ox40 I/II I I KIR II LAG3 I Ph I in 2015 GITR I TIM3 Ph I in 2015 PDL1 IIIIII III I I w/BMY w/MRK & BMY PD1 M M I/II

Experienced Leadership Team 32 John J. Rydzewski ExecutiveChairman, Co-Founder, Director Arthur H. Tinkelenberg, Ph.D. President & CEO, Co-Founder, Director CokeyNguyen, Ph.D. Vice President,Research&Development Isabel Chiu, Ph.D. Vice President, Translational& Clinical Sciences Kevin G. Sarney Vice President, Finance, &Chief Accounting Officer Derek Brand Vice President, Business Development Matthew A. Ebert General Counsel Gary L. Creason, Ph.D. Vice President, Intellectual Property
Accomplished Board of Directors 33 John J. Rydzewski ExecutiveChairman, Co-Founder, Director Arthur H. Tinkelenberg, Ph.D. President & CEO, Co-Founder, Director BarryBuckland, Ph.D. Co-Founder, Chairman, Scientific Advisory Board Robert J. Easton Director Allan Rothstein Director PaulJ. Sekhri Director Robert L. Van Nostrand Director

Financials & Capitalization Common Shares Outstanding 51,732,571 Warrants Outstanding* 24,803,409 Options Outstanding** 5,990,360 Burn Rate, Q3 ’15 ($2.4M) Total Debt $0.0M Cash, Cash Equivalents and Marketable Securities** $5.9M 34 OTC QB: ENUM Price: $0.26 Market Cap (12/04/2015) $13.45M Enumeralis uniquely positioned to discover and develop novel ‘best-in-class’ immunotherapies * 21,549,510 are exercisable at $2.00 ** As of September 30, 2015

Summary: Investment Highlights 35 • Enumeral’slead program has potential to be ‘best-in-class’ checkpoint blocker • Platform-driven pipeline and translational approach positions Enumeralfor leadership in combinatorial immunotherapy – Broad diversity in anti-TIM3 program positions first combination opportunity in multiple potential tumor types • Early days in a rapidly growing market where many oncology franchises lag or have missed the immunotherapy revolution – Market has grown from ~$1B as of 6/30/14 to $2.8B as of 9/30/15 • Significant progress to-date on minimal capital and well- positioned in market segment that can generate value quickly

THE POWER ofHUMAN™



































