Exhibit 99.1
CRISPR Therapeutics Reports Positive Results from its Phase 1 CARBON Trial of CTX110™ in Relapsed
or Refractory CD19+ B-cell malignancies
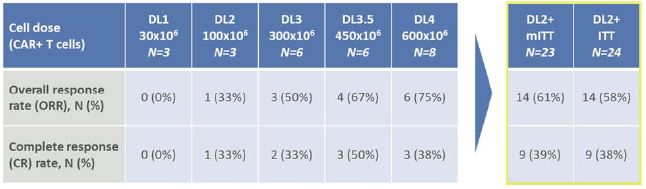
-58% overall response rate (ORR) and 38% complete response (CR) rate in large B-cell lymphoma (LBCL)
with a single dose of CTX110 at Dose Level 2 (DL2) and above on an intent-to-treat (ITT) basis-
-Durable responses in LBCL achieved with six-month CR rate of 21% and longest response on-going at
over 18 months after initial infusion-
-Response rates and durability are similar to approved autologous CD19 CAR-T therapies on an ITT basis-
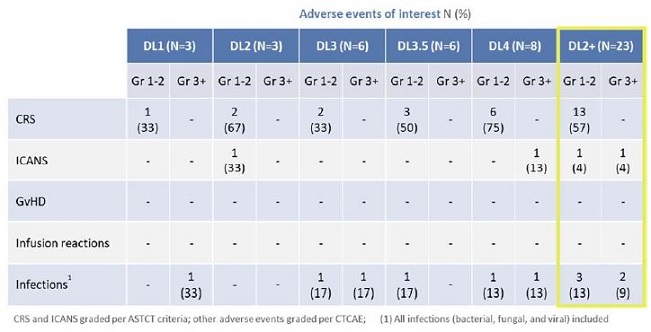
-Positively differentiated safety profile; no Grade 3 or higher cytokine release syndrome (CRS) and low
rates of infection and Immune Effector Cell-Associated Neurotoxicity Syndrome (ICANS)-
-Expanding CARBON into a potentially registrational trial in 1Q 2022-
-Management to host webcast and conference call today at 4:30 PM ET-
ZUG, Switzerland and CAMBRIDGE, Mass., October 12, 2021 — CRISPR Therapeutics (Nasdaq: CRSP), a biopharmaceutical company focused on creating transformative gene-based medicines for serious diseases, today announced updated results from the Company’s ongoing Phase 1 CARBON trial evaluating the safety and efficacy of CTX110™, its wholly-owned allogeneic CAR-T cell therapy targeting CD19+ B-cell malignancies.
“We are excited to share positive data from our CARBON trial, which show that CTX110 could offer patients with large B-cell lymphomas an immediately available ‘off-the-shelf’ therapy with efficacy similar to autologous CAR-T and a differentiated safety profile,” said Samarth Kulkarni, Ph.D., Chief Executive Officer of CRISPR Therapeutics. “Furthermore, we have the potential to improve upon already observed efficacy with a consolidation dosing strategy. Based on these encouraging results, we are planning to expand CARBON into a potentially registrational trial in the first quarter of 2022.”
CARBON Trial Overview
The Phase 1 CARBON trial is an open-label, multicenter clinical trial evaluating the safety and efficacy of CTX110 in adult patients with relapsed or refractory B-cell CD19+ malignancies who have received at least two prior lines of therapy. To date, enrollment has been focused on patients with the most aggressive disease presentations, including diffuse large B-cell lymphoma (DLBCL), not otherwise specified (NOS), high-grade double- or triple-hit lymphomas, and transformed follicular lymphoma. The majority of patients had Stage IV lymphoma and were refractory to their last line of therapy before entering the trial. Nine patients received prior autologous stem cell transplant. Patients who received prior autologous CAR-T therapy were not eligible.
As of the August 26, 2021 data cutoff, 30 patients with large B-cell lymphoma (LBCL) had been enrolled, of which 26 patients had received CTX110 with at least 28 days of follow-up and are included in the analysis. Only one enrolled patient did not receive CTX110. Three patients at the time of the data cut had less than 28 days of follow-up and were not evaluable for this analysis.
Patients were infused with a single CTX110 infusion following three days of a standard lymphodepletion regimen consisting of fludarabine (30mg/m2/day) and cyclophosphamide (500mg/m2/day). Patients could