
Updated Results from the Phase 1 CARBON Trial of CTX110™ October 12, 2021 ® Exhibit 99.2

The presentation and other related materials may contain a number of “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including statements regarding CRISPR Therapeutics’ expectations about any or all of the following: (i) the safety, efficacy and clinical progress of our various clinical programs, including our CTX110 program; (ii) the status of clinical trials (including, without limitation, the expected timing of data releases, announcement of additional programs and activities at clinical trial sites) and expectations regarding the data that is being presented from our CARBON clinical trial; (iii) the data that will be generated by ongoing and planned clinical trials, and the ability to use that data for the design and initiation of further clinical trials; and (iv) the therapeutic value, development, and commercial potential of CRISPR/Cas9 gene editing technologies and therapies, including as compared to other therapies. Without limiting the foregoing, the words “believes,” “anticipates,” “plans,” “expects” and similar expressions are intended to identify forward-looking statements. You are cautioned that forward-looking statements are inherently uncertain. Although CRISPR Therapeutics believes that such statements are based on reasonable assumptions within the bounds of its knowledge of its business and operations, forward-looking statements are neither promises nor guarantees and they are necessarily subject to a high degree of uncertainty and risk. Actual performance and results may differ materially from those projected or suggested in the forward-looking statements due to various risks and uncertainties. These risks and uncertainties include, among others: the potential for initial and preliminary data from any clinical trial and initial data from a limited number of patients not to be indicative of final trial results; the potential that clinical trial results may not be favorable; potential impacts due to the coronavirus pandemic, such as the timing and progress of clinical trials; that future competitive or other market factors may adversely affect the commercial potential for CRISPR Therapeutics’ product candidates; uncertainties regarding the intellectual property protection for CRISPR Therapeutics’ technology and intellectual property belonging to third parties, and the outcome of proceedings (such as an interference, an opposition or a similar proceeding) involving all or any portion of such intellectual property; and those risks and uncertainties described under the heading "Risk Factors" in CRISPR Therapeutics’ most recent annual report on Form 10-K, quarterly report on Form 10-Q and in any other subsequent filings made by CRISPR Therapeutics with the U.S. Securities and Exchange Commission, which are available on the SEC's website at www.sec.gov. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date they are made. CRISPR Therapeutics disclaims any obligation or undertaking to update or revise any forward-looking statements contained in this presentation, other than to the extent required by law. Caution should be exercised when interpreting results from separate trials involving separate product candidates. There are differences in the clinical trial design, patient populations, and the product candidates themselves, and the results from the clinical trials of autologous products may have no interpretative value on our existing or future results. CRISPR THERAPEUTICS® standard character mark and design logo, CTX001™, CTX110™, CTX120™, and CTX130™ are trademarks and registered trademarks of CRISPR Therapeutics AG. All other trademarks and registered trademarks are the property of their respective owners. Forward-looking Statements

Presenters on Today’s Call Samarth Kulkarni, PhD Chief Executive Officer Tony Ho, MD Executive Vice President, Head of Research & Development Ewelina Morawa, MD Vice President, Clinical Development

4 clinical programs; >450 employees; ~$2.5B cash balance Over 50 sickle cell and beta-thalassemia patients treated with CTX001™ showing a consistent, functionally curative profile Regulatory filings possible in the next 18-24 months with 30,000+ patients suitable for treatment in the U.S. and Europe if approved Proof of concept achieved with CTX110, paving the way for our CAR-T pipeline Expect to complete construction of state-of-the-art internal manufacturing facility in 2021 and bring facility on-line in 2022 On track to initiate clinical trial of our allogeneic stem cell-derived therapy for T1D in 2021 with our partner ViaCyte Expect to move multiple programs utilizing in vivo approaches into the clinic in the next 18-24 months Building the Leading CRISPR Company
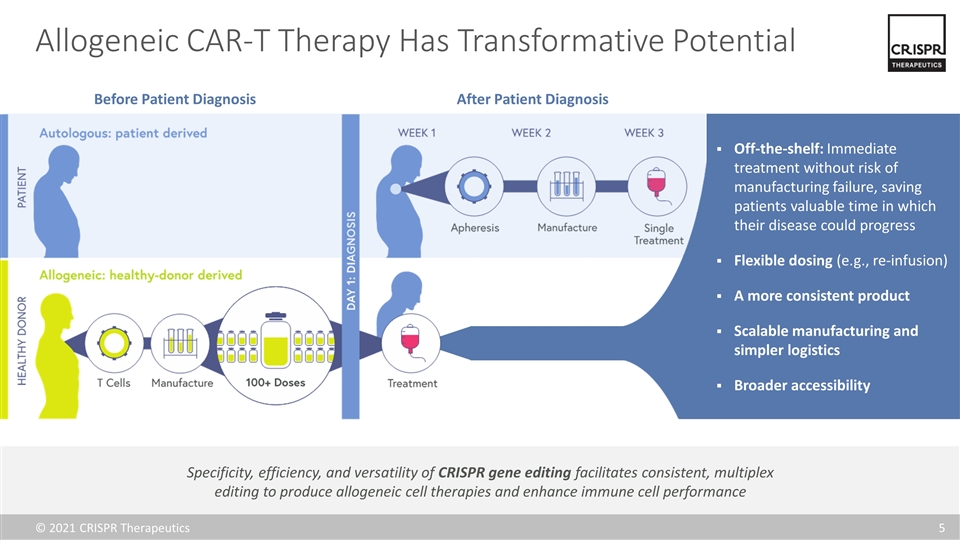
Allogeneic CAR-T Therapy Has Transformative Potential Specificity, efficiency, and versatility of CRISPR gene editing facilitates consistent, multiplex editing to produce allogeneic cell therapies and enhance immune cell performance Before Patient Diagnosis After Patient Diagnosis Off-the-shelf: Immediate treatment without risk of manufacturing failure, saving patients valuable time in which their disease could progress Flexible dosing (e.g., re-infusion) A more consistent product Scalable manufacturing and simpler logistics Broader accessibility
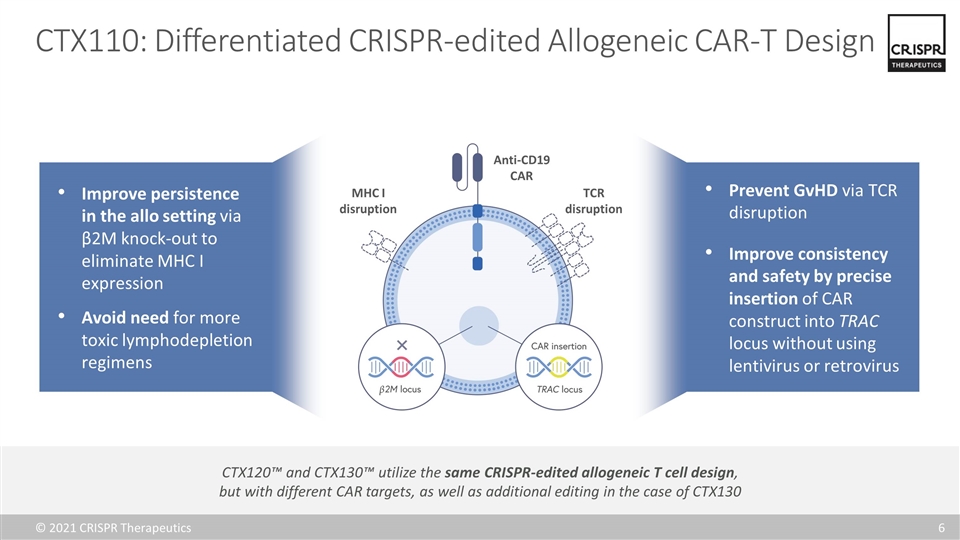
CTX110: Differentiated CRISPR-edited Allogeneic CAR-T Design Anti-CD19 CAR TCR disruption MHC I disruption Prevent GvHD via TCR disruption Improve consistency and safety by precise insertion of CAR construct into TRAC locus without using lentivirus or retrovirus Improve persistence in the allo setting via β2M knock-out to eliminate MHC I expression Avoid need for more toxic lymphodepletion regimens CTX120™ and CTX130™ utilize the same CRISPR-edited allogeneic T cell design, but with different CAR targets, as well as additional editing in the case of CTX130
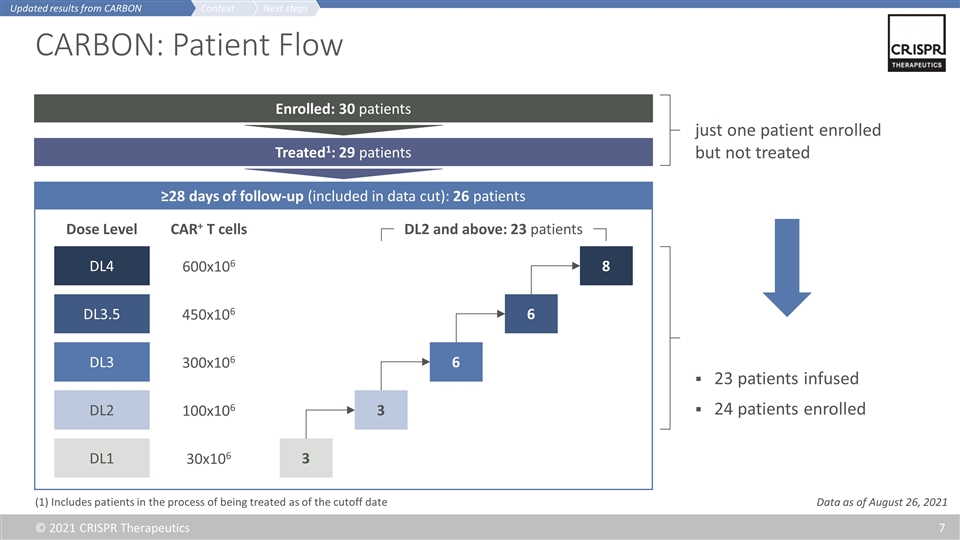
CARBON: Patient Flow ≥28 days of follow-up (included in data cut): 26 patients Enrolled: 30 patients Treated1: 29 patients just one patient enrolled but not treated Data as of August 26, 2021 23 patients infused 24 patients enrolled (1) Includes patients in the process of being treated as of the cutoff date 3 3 6 8 6 300x106 100x106 30x106 600x106 450x106 DL1 DL2 DL3 DL4 DL3.5 CAR+ T cells Dose Level DL2 and above: 23 patients Next steps Context Updated results from CARBON
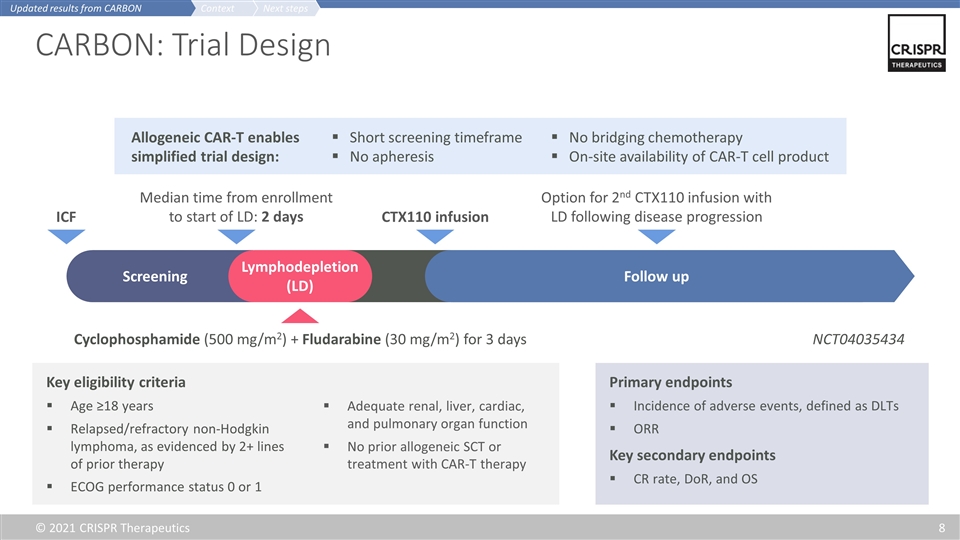
CARBON: Trial Design Key eligibility criteria Age ≥18 years Relapsed/refractory non-Hodgkin lymphoma, as evidenced by 2+ lines of prior therapy ECOG performance status 0 or 1 Adequate renal, liver, cardiac, and pulmonary organ function No prior allogeneic SCT or treatment with CAR-T therapy Primary endpoints Incidence of adverse events, defined as DLTs ORR Key secondary endpoints CR rate, DoR, and OS Median time from enrollment to start of LD: 2 days Cyclophosphamide (500 mg/m2) + Fludarabine (30 mg/m2) for 3 days CTX110 infusion Screening Lymphodepletion (LD) Follow up ICF Option for 2nd CTX110 infusion with LD following disease progression NCT04035434 Allogeneic CAR-T enables simplified trial design: No bridging chemotherapy On-site availability of CAR-T cell product Short screening timeframe No apheresis Next steps Context Updated results from CARBON
CARBON Only Enrolled Patients with Aggressive Disease including DLBCL NOS, high-grade lymphoma (e.g., triple hit), and transformed follicular lymphoma with significant baseline tumor volume Both relapsed and refractory patients, including – 31% of patients had progressed through 2 or more lines of therapy and received CTX110 within 9 months of their first lymphoma treatment Next steps Context Updated results from CARBON
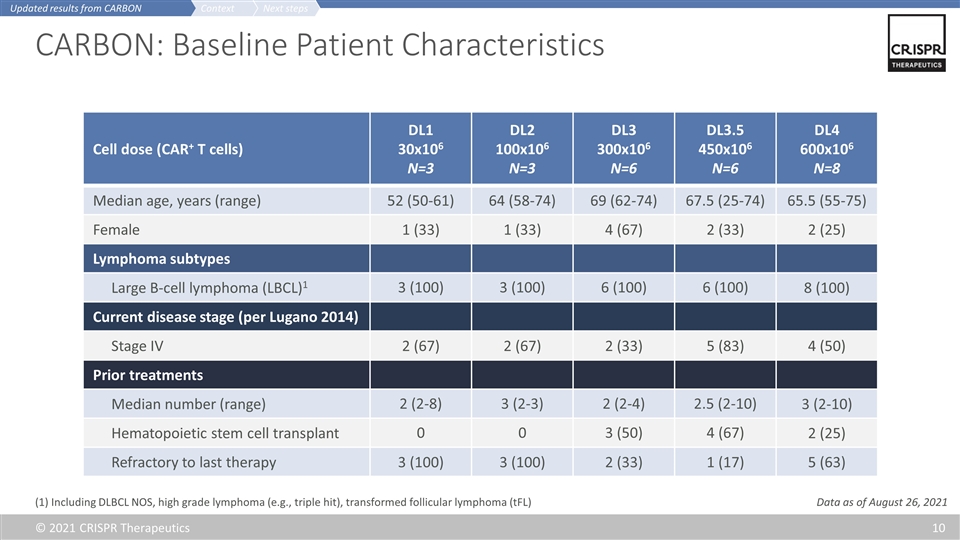
CARBON: Baseline Patient Characteristics Cell dose (CAR+ T cells) DL1 30x106 N=3 DL2 100x106 N=3 DL3 300x106 N=6 DL3.5 450x106 N=6 DL4 600x106 N=8 Median age, years (range) 52 (50-61) 64 (58-74) 69 (62-74) 67.5 (25-74) 65.5 (55-75) Female 1 (33) 1 (33) 4 (67) 2 (33) 2 (25) Lymphoma subtypes Large B-cell lymphoma (LBCL)1 3 (100) 3 (100) 6 (100) 6 (100) 8 (100) Current disease stage (per Lugano 2014) Stage IV 2 (67) 2 (67) 2 (33) 5 (83) 4 (50) Prior treatments Median number (range) 2 (2-8) 3 (2-3) 2 (2-4) 2.5 (2-10) 3 (2-10) Hematopoietic stem cell transplant 0 0 3 (50) 4 (67) 2 (25) Refractory to last therapy 3 (100) 3 (100) 2 (33) 1 (17) 5 (63) (1) Including DLBCL NOS, high grade lymphoma (e.g., triple hit), transformed follicular lymphoma (tFL) Next steps Context Updated results from CARBON Data as of August 26, 2021
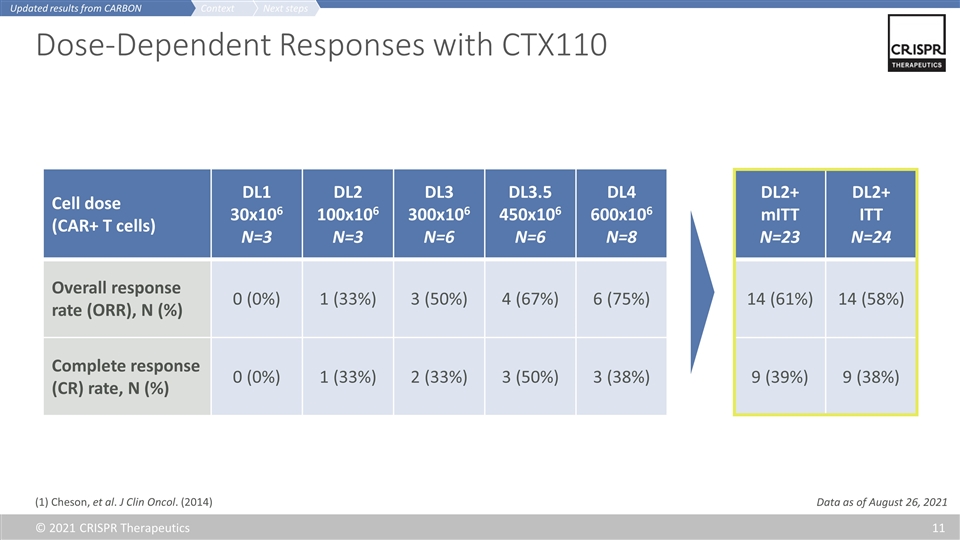
Cell dose (CAR+ T cells) DL1 30x106 N=3 DL2 100x106 N=3 DL3 300x106 N=6 DL3.5 450x106 N=6 DL4 600x106 N=8 Overall response rate (ORR), N (%) 0 (0%) 1 (33%) 3 (50%) 4 (67%) 6 (75%) Complete response (CR) rate, N (%) 0 (0%) 1 (33%) 2 (33%) 3 (50%) 3 (38%) Dose-Dependent Responses with CTX110 DL2+ mITT N=23 DL2+ ITT N=24 14 (61%) 14 (58%) 9 (39%) 9 (38%) (1) Cheson, et al. J Clin Oncol. (2014) Next steps Context Updated results from CARBON Data as of August 26, 2021
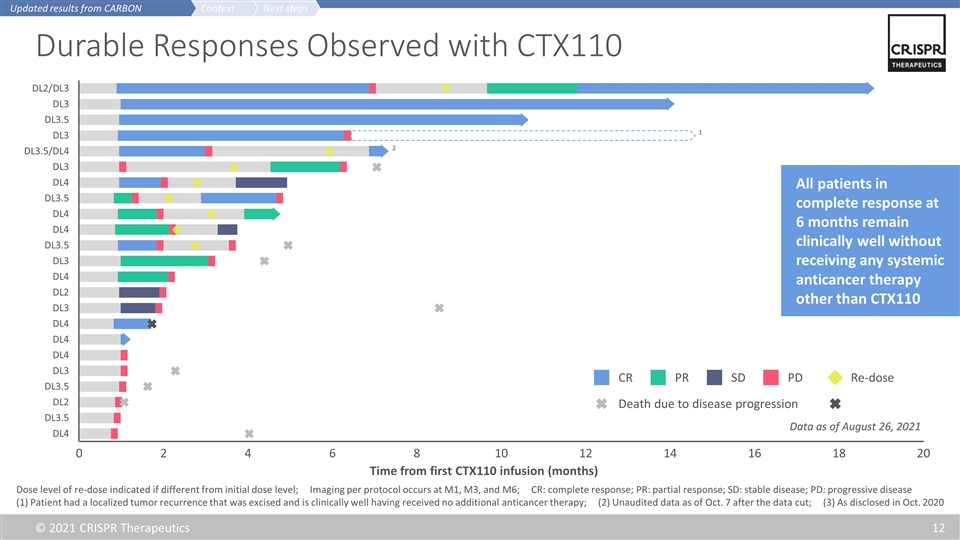
Durable Responses Observed with CTX110 Dose level of re-dose indicated if different from initial dose level; Imaging per protocol occurs at M1, M3, and M6; CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease (1) Patient had a localized tumor recurrence that was excised and is clinically well having received no additional anticancer therapy; (2) Unaudited data as of Oct. 7 after the data cut; (3) As disclosed in Oct. 2020 CR SD PD PR Re-dose Death due to disease progression 1 All patients in complete response at 6 months remain clinically well without receiving any systemic anticancer therapy other than CTX110 2 Time from first CTX110 infusion (months) 0 20 18 16 14 12 10 8 6 4 2 Next steps Context Updated results from CARBON Data as of August 26, 2021
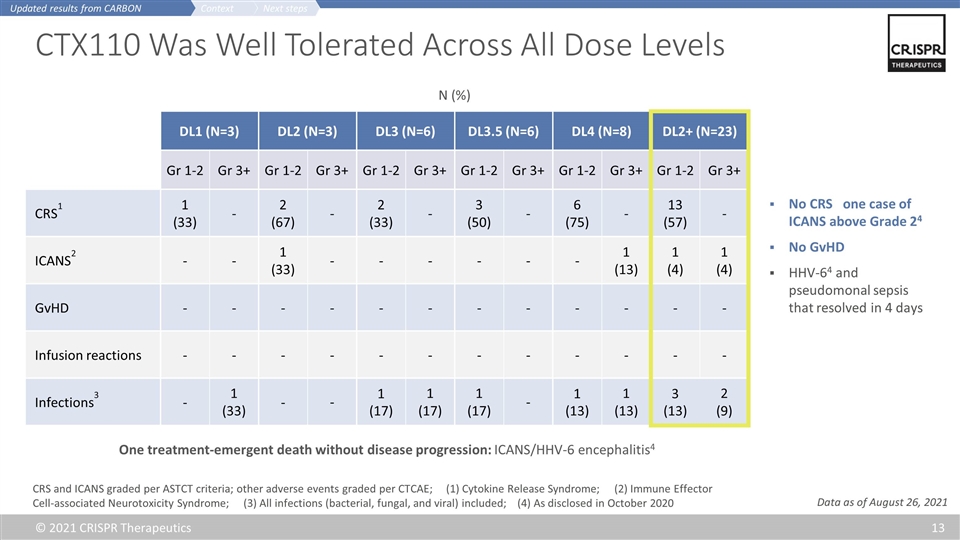
CTX110 Was Well Tolerated Across All Dose Levels DL1 (N=3) DL2 (N=3) DL3 (N=6) DL3.5 (N=6) DL4 (N=8) DL2+ (N=23) Gr 1-2 Gr 3+ Gr 1-2 Gr 3+ Gr 1-2 Gr 3+ Gr 1-2 Gr 3+ Gr 1-2 Gr 3+ Gr 1-2 Gr 3+ CRS1 1 (33) - 2 (67) - 2 (33) - 3 (50) - 6 (75) - 13 (57) - ICANS2 - - 1 (33) - - - - - - 1 (13) 1 (4) 1 (4) GvHD - - - - - - - - - - - - Infusion reactions - - - - - - - - - - - - Infections3 - 1 (33) - - 1 (17) 1 (17) 1 (17) - 1 (13) 1 (13) 3 (13) 2 (9) CRS and ICANS graded per ASTCT criteria; other adverse events graded per CTCAE; (1) Cytokine Release Syndrome; (2) Immune Effector Cell-associated Neurotoxicity Syndrome; (3) All infections (bacterial, fungal, and viral) included; (4) As disclosed in October 2020 No CRS one case of ICANS above Grade 24 No GvHD HHV-64 and pseudomonal sepsis that resolved in 4 days One treatment-emergent death without disease progression: ICANS/HHV-6 encephalitis4 N (%) Next steps Context Updated results from CARBON Data as of August 26, 2021
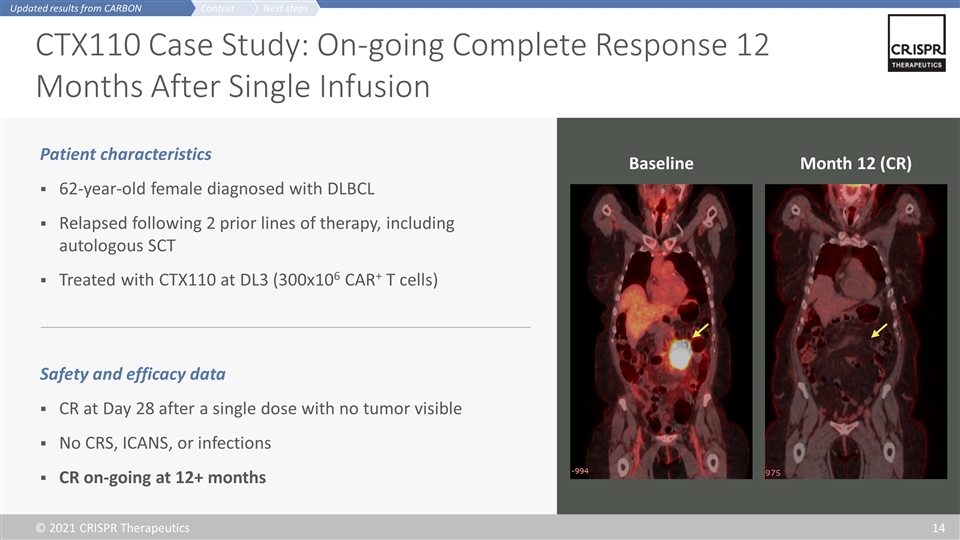
CTX110 Case Study: On-going Complete Response 12 Months After Single Infusion Patient characteristics 62-year-old female diagnosed with DLBCL Relapsed following 2 prior lines of therapy, including autologous SCT Treated with CTX110 at DL3 (300x106 CAR+ T cells) Safety and efficacy data CR at Day 28 after a single dose with no tumor visible No CRS, ICANS, or infections CR on-going at 12+ months Baseline Month 12 (CR) Next steps Context Updated results from CARBON
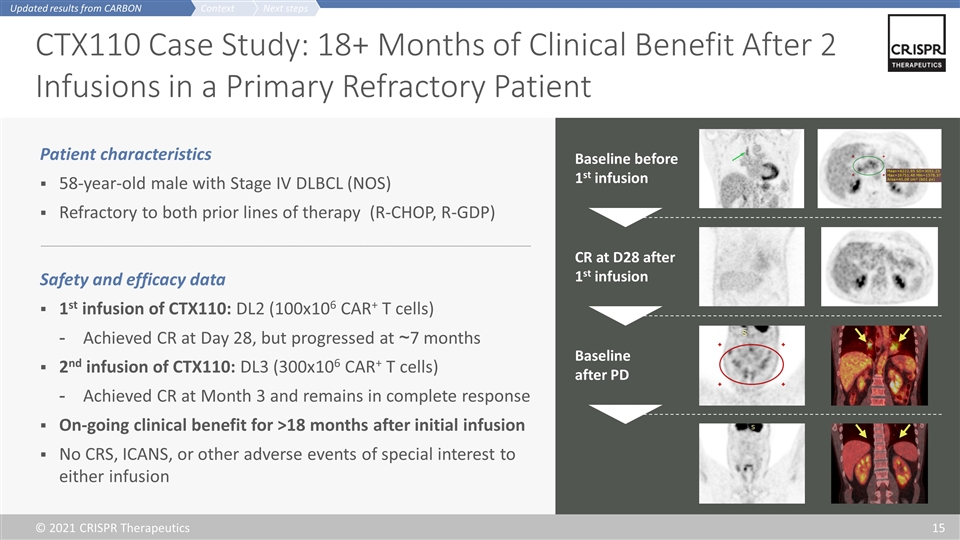
CTX110 Case Study: 18+ Months of Clinical Benefit After 2 Infusions in a Primary Refractory Patient Patient characteristics 58-year-old male with Stage IV DLBCL (NOS) Refractory to both prior lines of therapy (R-CHOP, R-GDP) Safety and efficacy data 1st infusion of CTX110: DL2 (100x106 CAR+ T cells) Achieved CR at Day 28, but progressed at ~7 months 2nd infusion of CTX110: DL3 (300x106 CAR+ T cells) Achieved CR at Month 3 and remains in complete response On-going clinical benefit for >18 months after initial infusion No CRS, ICANS, or other adverse events of special interest to either infusion Baseline before 1st infusion Baseline after PD CR at D28 after 1st infusion Next steps Context Updated results from CARBON
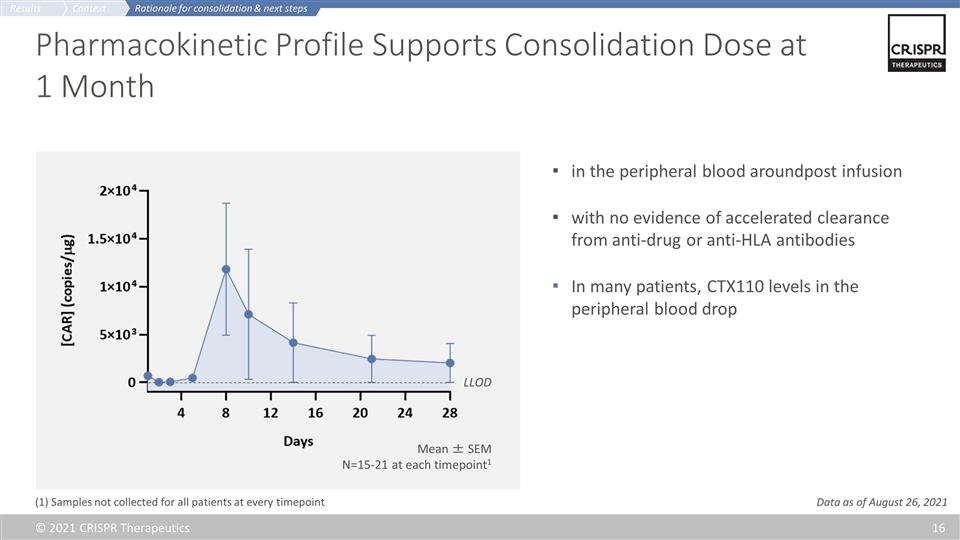
Pharmacokinetic Profile Supports Consolidation Dose at 1 Month in the peripheral blood aroundpost infusion with no evidence of accelerated clearance from anti-drug or anti-HLA antibodies In many patients, CTX110 levels in the peripheral blood drop LLOD Mean ± SEM N=15-21 at each timepoint1 (1) Samples not collected for all patients at every timepoint Rationale for consolidation & next steps Context Results Data as of August 26, 2021
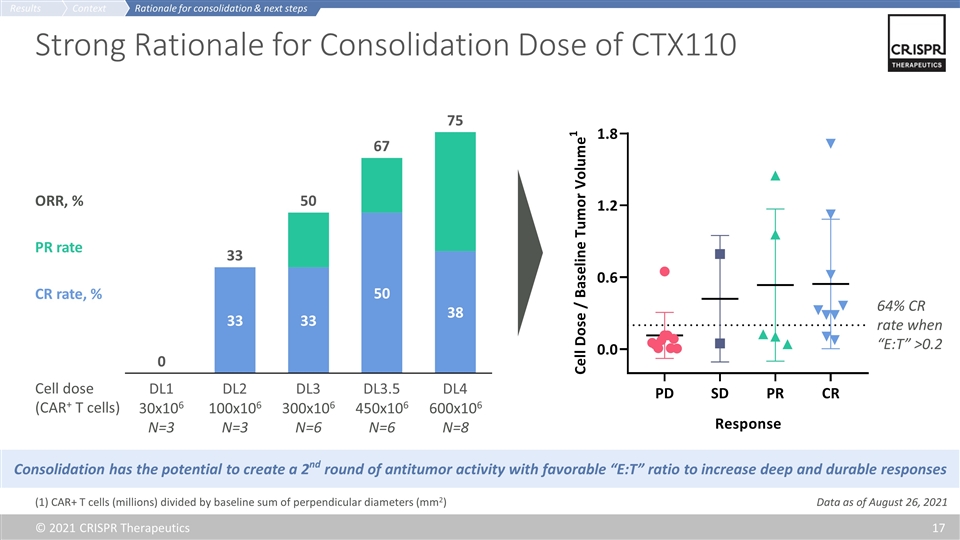
Strong Rationale for Consolidation Dose of CTX110 30x106 N=3 100x106 N=3 300x106 N=6 450x106 N=6 600x106 N=8 Cell dose (CAR+ T cells) ORR, % CR rate, % Consolidation has the potential to create a 2nd round of antitumor activity with favorable “E:T” ratio to increase deep and durable responses (1) CAR+ T cells (millions) divided by baseline sum of perpendicular diameters (mm2) PR rate 64% CR rate when “E:T” >0.2 Rationale for consolidation & next steps Context Results Data as of August 26, 2021

Expand CARBON into a in Q1 2022 Broaden into Further scale manufacturing in our by advancing additional gene-edited allogeneic CAR-T programs to the clinic, including novel edits for increased potency Conclusions Rationale for consolidation & next steps Context Results with approved autologous CAR-T therapies Ability to achieve Positively Potential to CTX110 is a potentially best-in-class allogeneic cell therapy in r/r LBCL with a profile that can compete with approved autologous CAR-T therapies
Thank You to Patients and Their Families Thank you to and their families, investigators, and site staff CTX110 sites United States Emory University Atlanta, GA Mayo Clinic Jacksonville, FL Oregon Health and Science University Portland, OR Sarah Cannon Research Institute Nashville, TN Texas Transplant Institute San Antonio, TX University of Minnesota Minneapolis, MN University of Chicago Chicago, IL University of Kansas Westwood, KS UT Southwestern Medical Center Dallas, TX Washington University St. Louis, MO Europe Clínica Universidad de Navarra Navarra, Spain University of Hamburg Hamburg, Germany Australia Peter MacCallum Cancer Centre Melbourne Royal Prince Alfred Hospital Sydney




















