TCER® IMA401 (MAGEA4/8)
On September 16, 2024, Immatics announced the proof-of-concept clinical data for the first candidate of its next-generation, half-life extended TCR Bispecifics platform, TCER® IMA401 (MAGEA4/8), during an oral presentation at the European Society for Medical Oncology (ESMO) Congress 2024.
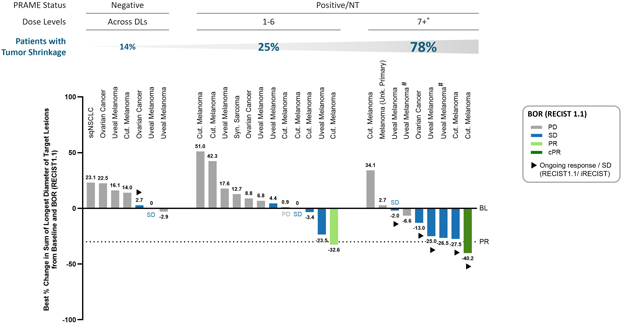
As of data cut-off on July 23, 2024, 35 heavily pretreated patients with recurrent and/or refractory solid tumors were treated with IMA401 monotherapy across nine escalating dose levels. The treated patient population was composed of patients with 16 different solid tumor indications who were both HLA-A*02:01 and MAGEA4/8-positive, had received a median of four and up to eight lines of prior systemic treatments and the majority had an ECOG performance status of ≥ 1.
Proof-of-concept clinical data from the Phase 1a first-in-human dose escalation basket trial showed initial anti-tumor activity in multiple tumor types, durable objective responses, including confirmed responses ongoing at 13+ months, a manageable tolerability profile and an half-life of 14+ days.
Treatment with IMA401 monotherapy in patients with relevant IMA401 doses and MAGEA4/8high levels (N=17) demonstrated:
| | • | | Objective response rate of 29% with confirmed responses observed in 25% of patients |
| | • | | Disease control rate of 53% and tumor shrinkage of 53% |
As the clinical trial progresses, the Company aims to further leverage the potential of IMA401 by focusing on the enrollment of indications with high MAGEA4/8 target expression, such as lung and head and neck cancer patients, seeking to optimize the treatment schedule and also exploring the incremental clinical benefit available to patients through combining IMA401 with a checkpoint inhibitor. The next data update on IMA401 is expected in 2025.
ACTengine® Cell Therapy Program
ACTengine® IMA203
On November 8, 2024, Immatics announced an expanded clinical dataset that included all infused patients in the Phase 1b dose expansion part of the trial (N=41), consisting of the 28 melanoma patients reported on October 10, 2024, and 13 non-melanoma patients, of which 10 non-melanoma patients were reported on November 8, 2023.
| | |
| Immatics Press Release November 18, 2024 | | 5 | 15 |