
Immatics Corporate Presentation November 18, 2024 Exhibit 99.4

Forward-Looking Statement This presentation (“Presentation”) is provided by Immatics N.V. (“Immatics” or the “Company”) for informational purposes only. The information contained herein does not purport to be all-inclusive and none of Immatics, any of its affiliates, any of its or their respective control persons, officers, directors, employees or representatives makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation. Forward-Looking Statements. Certain statements in this presentation may be considered forward-looking statements. Forward-looking statements generally relate to future events or the Company’s future financial or operating performance. For example, statements concerning timing of data read-outs for product candidates, the timing, outcome and design of clinical trials, the nature of clinical trials (including whether such clinical trials will be registration-enabling), the timing of IND or CTA filing for pre-clinical stage product candidates, the timing of BLA filings for clinical stage product candidates, estimated market opportunities of product candidates, manufacturing timetables, capacity and success rates, the Company’s focus on partnerships to advance its strategy, and other metrics are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may”, “should”, “expect”, “plan”, “target”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by Immatics and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limited to, various factors beyond management's control including general economic conditions and other risks, uncertainties and factors set forth in the Company’s Annual Report on Form 20-F and other filings with the Securities and Exchange Commission (SEC). Nothing in this presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. The Company undertakes no duty to update these forward-looking statements. No Offer or Solicitation. This communication is for informational purposes only and does not constitute, or form a part of, an offer to sell or the solicitation of an offer to sell or an offer to buy or the solicitation of an offer to buy any securities, and there shall be no sale of securities, in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offer of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the Securities Act of 1933, as amended, or in an offering exempt from registration. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and the Company’s own internal estimates and research. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while the Company believes its internal research is reliable, such research has not been verified by any independent source. All the scientific and clinical data presented within this presentation are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification.
Therapeutic Opportunity Potential for addressing large patient populations with high prevalence targets in solid tumors Two Clinical-Stage Modalities Pipeline of TCR-T and TCR Bispecific product candidates in clinical & preclinical development Building a Leading TCR Therapeutics Company Intro Differentiated Platforms Unique technologies to identify true cancer targets and right TCRs Clinical PoC for Cell Therapy High confirmed objective response rate and durable responses in melanoma; registration-enabling Phase 3 trial to commence in December 2024
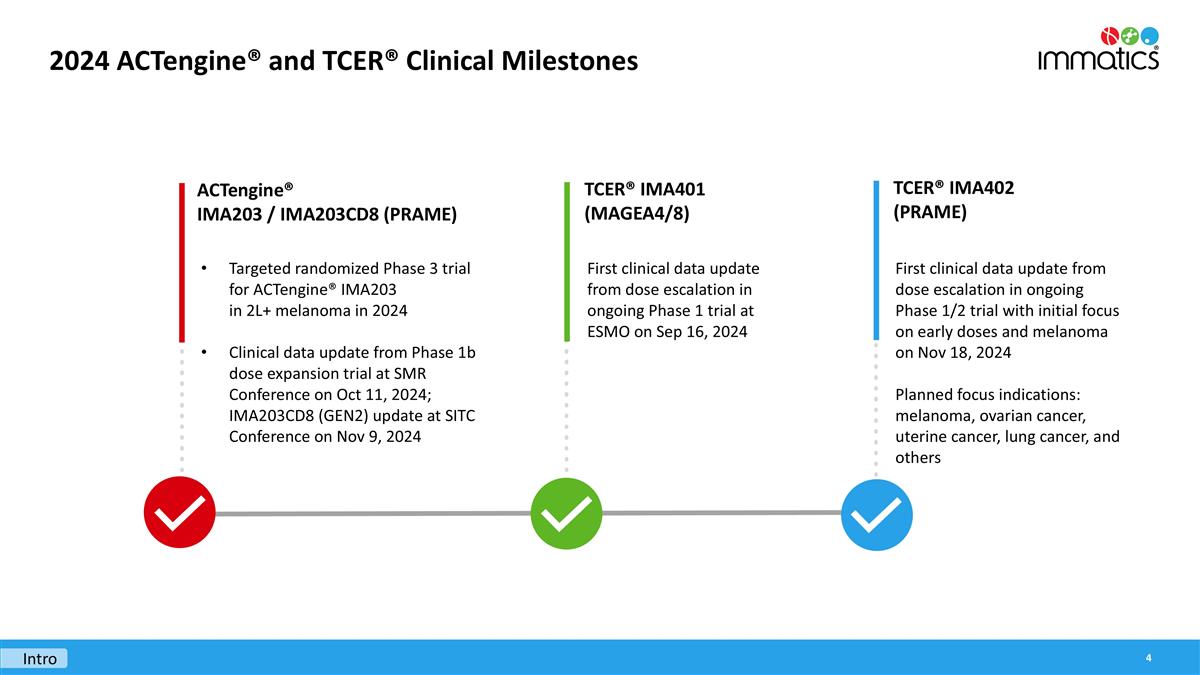
2024 ACTengine® and TCER® Clinical Milestones Targeted randomized Phase 3 trial for ACTengine® IMA203 in 2L+ melanoma in 2024 Clinical data update from Phase 1b dose expansion trial at SMR Conference on Oct 11, 2024; IMA203CD8 (GEN2) update at SITC Conference on Nov 9, 2024 ACTengine® IMA203 / IMA203CD8 (PRAME) First clinical data update from dose escalation in ongoing Phase 1 trial at ESMO on Sep 16, 2024 TCER® IMA401 (MAGEA4/8) First clinical data update from dose escalation in ongoing Phase 1/2 trial with initial focus on early doses and melanoma on Nov 18, 2024 Planned focus indications: melanoma, ovarian cancer, uterine cancer, lung cancer, and others TCER® IMA402 (PRAME) Intro
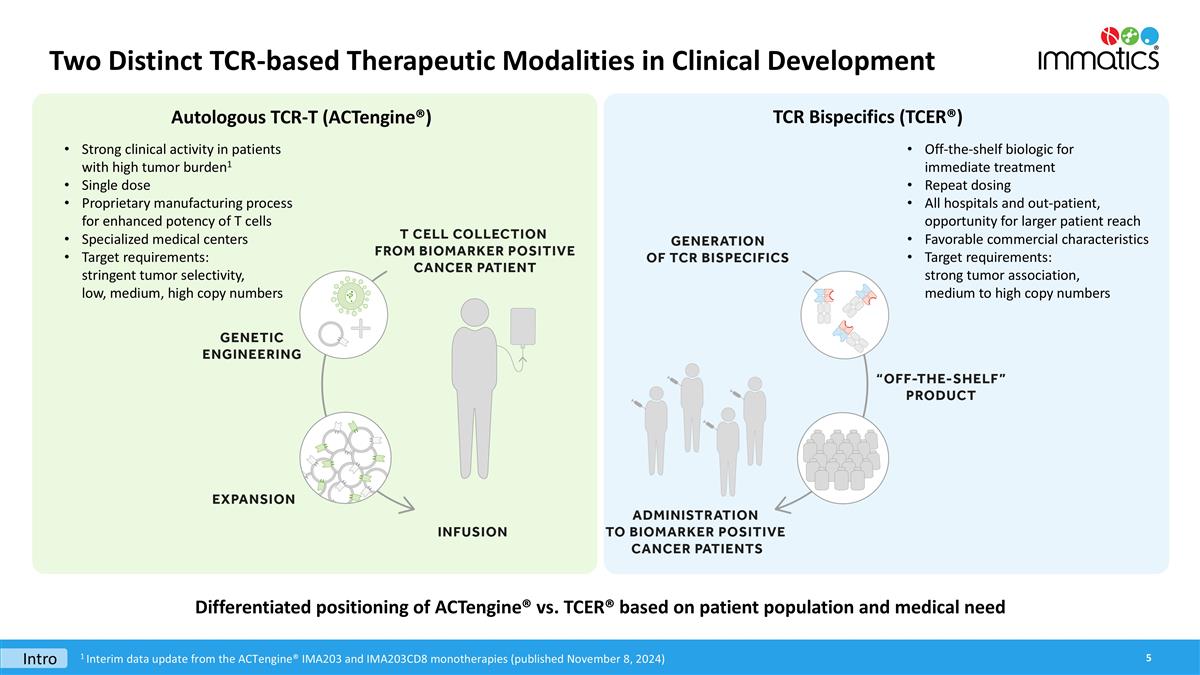
Two Distinct TCR-based Therapeutic Modalities in Clinical Development Differentiated positioning of ACTengine® vs. TCER® based on patient population and medical need Intro 1 Interim data update from the ACTengine® IMA203 and IMA203CD8 monotherapies (published November 8, 2024) Autologous TCR-T (ACTengine®) TCR Bispecifics (TCER®) Strong clinical activity in patients with high tumor burden1 Single dose Proprietary manufacturing process for enhanced potency of T cells Specialized medical centers Target requirements: stringent tumor selectivity, low, medium, high copy numbers Off-the-shelf biologic for immediate treatment Repeat dosing All hospitals and out-patient, opportunity for larger patient reach Favorable commercial characteristics Target requirements: strong tumor association, medium to high copy numbers
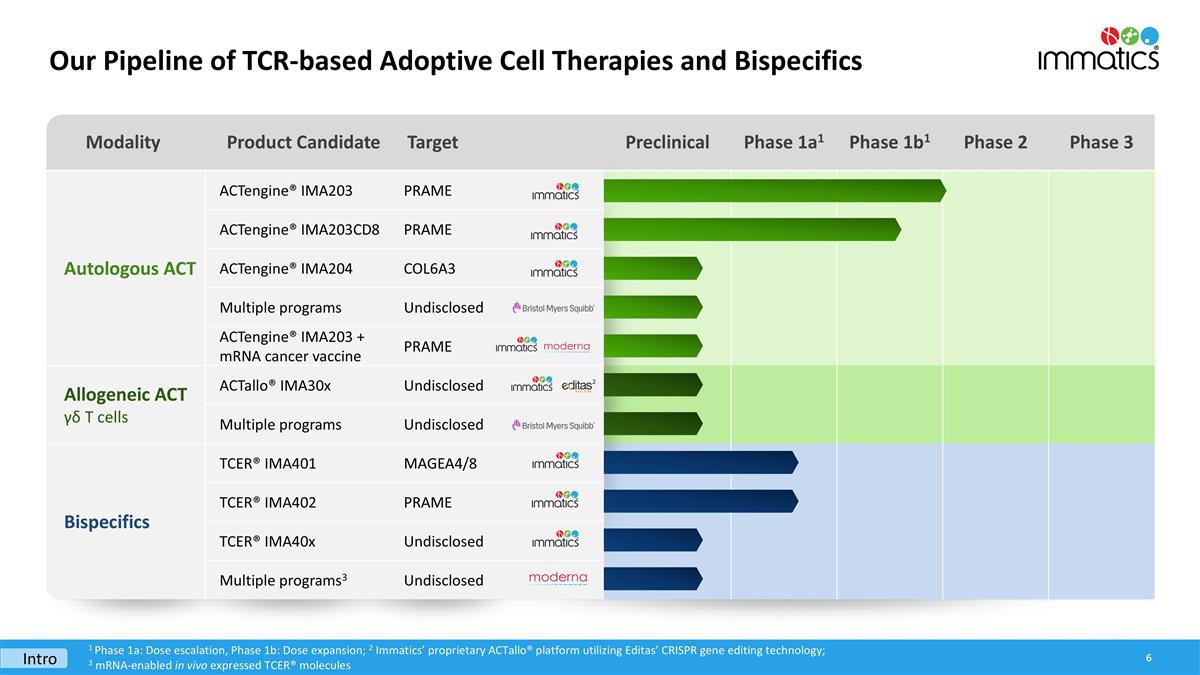
Modality Product Candidate Target Preclinical Phase 1a1 Phase 1b1 Phase 2 Phase 3 Autologous ACT ACTengine® IMA203 PRAME ACTengine® IMA203CD8 PRAME ACTengine® IMA204 COL6A3 Autologous ACT Multiple programs Undisclosed ACTengine® IMA203 + mRNA cancer vaccine PRAME Allogeneic ACT γδ T cells ACTallo® IMA30x Undisclosed Multiple programs Undisclosed Bispecifics TCER® IMA401 MAGEA4/8 TCER® IMA402 PRAME TCER® IMA40x Undisclosed Multiple programs3 Undisclosed Our Pipeline of TCR-based Adoptive Cell Therapies and Bispecifics 1 Phase 1a: Dose escalation, Phase 1b: Dose expansion; 2 Immatics’ proprietary ACTallo® platform utilizing Editas’ CRISPR gene editing technology; 3 mRNA-enabled in vivo expressed TCER® molecules 2 Intro
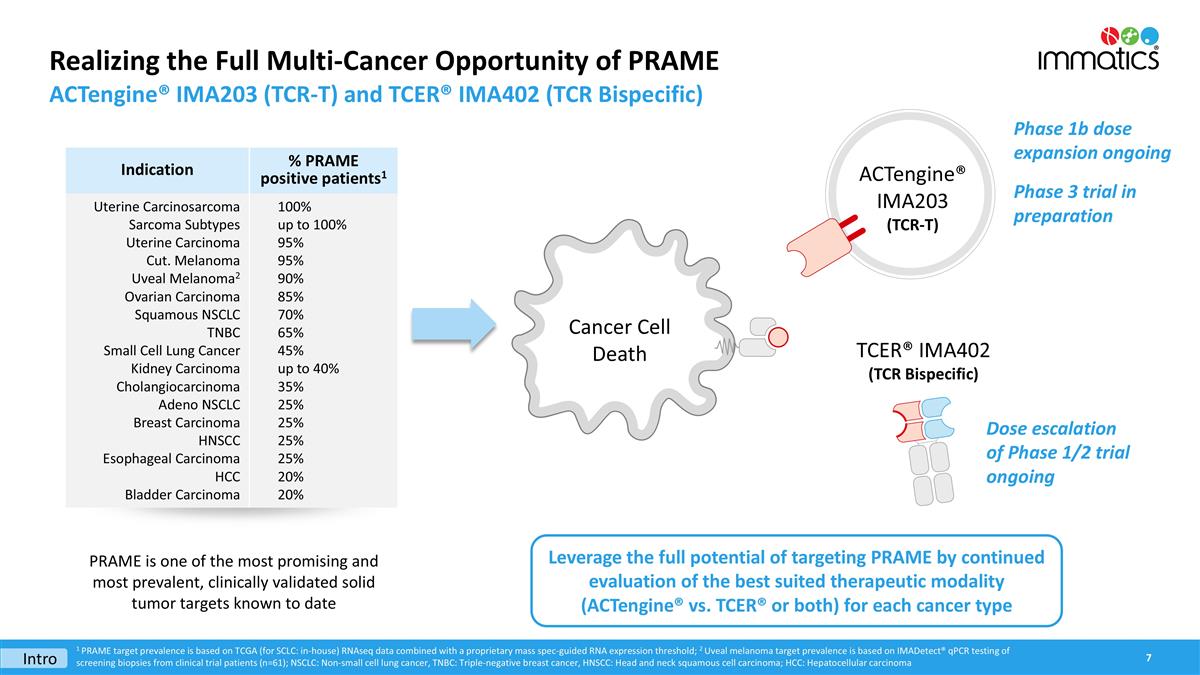
Realizing the Full Multi-Cancer Opportunity of PRAME ACTengine® IMA203 (TCR-T) and TCER® IMA402 (TCR Bispecific) ACTengine® IMA203 (TCR-T) Cancer Cell Death PRAME is one of the most promising and most prevalent, clinically validated solid tumor targets known to date Leverage the full potential of targeting PRAME by continued evaluation of the best suited therapeutic modality (ACTengine® vs. TCER® or both) for each cancer type Intro Phase 1b dose expansion ongoing Phase 3 trial in preparation TCER® IMA402 (TCR Bispecific) 1 PRAME target prevalence is based on TCGA (for SCLC: in-house) RNAseq data combined with a proprietary mass spec-guided RNA expression threshold; 2 Uveal melanoma target prevalence is based on IMADetect® qPCR testing of screening biopsies from clinical trial patients (n=61); NSCLC: Non-small cell lung cancer, TNBC: Triple-negative breast cancer, HNSCC: Head and neck squamous cell carcinoma; HCC: Hepatocellular carcinoma Indication % PRAME positive patients1 Uterine Carcinosarcoma Sarcoma Subtypes Uterine Carcinoma Cut. Melanoma Uveal Melanoma2 Ovarian Carcinoma Squamous NSCLC TNBC Small Cell Lung Cancer Kidney Carcinoma Cholangiocarcinoma Adeno NSCLC Breast Carcinoma HNSCC Esophageal Carcinoma HCC Bladder Carcinoma 100% up to 100% 95% 95% 90% 85% 70% 65% 45% up to 40% 35% 25% 25% 25% 25% 20% 20% Dose escalation of Phase 1/2 trial ongoing

ACTengine® IMA203 – TCR-T Targeting PRAME

The Multi-Cancer Opportunity of PRAME One of the Most Promising Solid Tumor Targets for TCR-based Therapies Known To Date High prevalence High target density Homogeneous expression “Clean” expression profile Clinical proof-of-concept sqNSCLC Ovarian Cancer PRAME fulfills all properties of an ideal target for TCR-based therapies PRAME RNA detection in tumor samples (ISH) ISH: in situ hybridization, sqNSCLC: squamous non-small cell lung cancer IMA203
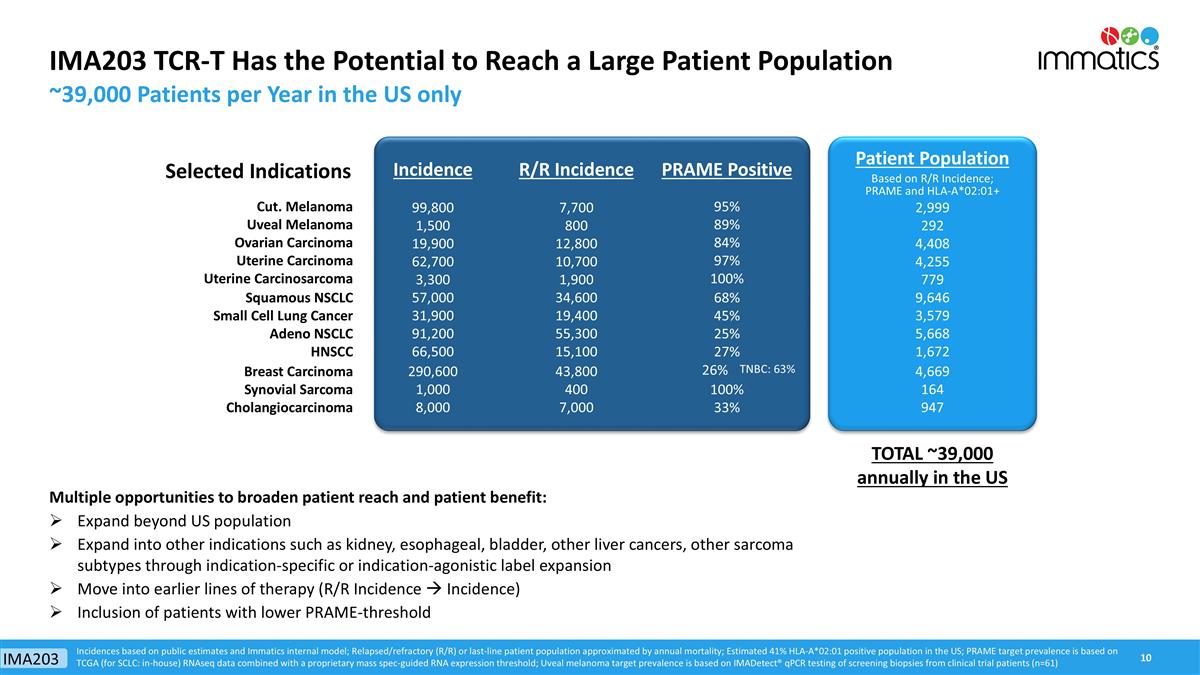
Selected Indications Incidence R/R Incidence PRAME Positive Patient Population Based on R/R Incidence; PRAME and HLA-A*02:01+ Cut. Melanoma 99,800 7,700 95% 2,999 Uveal Melanoma 1,500 800 89% 292 Ovarian Carcinoma 19,900 12,800 84% 4,408 Uterine Carcinoma 62,700 10,700 97% 4,255 Uterine Carcinosarcoma 3,300 1,900 100% 779 Squamous NSCLC 57,000 34,600 68% 9,646 Small Cell Lung Cancer 31,900 19,400 45% 3,579 Adeno NSCLC 91,200 55,300 25% 5,668 HNSCC 66,500 15,100 27% 1,672 Breast Carcinoma 290,600 43,800 26% TNBC: 63% 4,669 Synovial Sarcoma 1,000 400 100% 164 Cholangiocarcinoma 8,000 7,000 33% 947 IMA203 TCR-T Has the Potential to Reach a Large Patient Population ~39,000 Patients per Year in the US only Incidences based on public estimates and Immatics internal model; Relapsed/refractory (R/R) or last-line patient population approximated by annual mortality; Estimated 41% HLA-A*02:01 positive population in the US; PRAME target prevalence is based on TCGA (for SCLC: in-house) RNAseq data combined with a proprietary mass spec-guided RNA expression threshold; Uveal melanoma target prevalence is based on IMADetect® qPCR testing of screening biopsies from clinical trial patients (n=61) Multiple opportunities to broaden patient reach and patient benefit: Expand beyond US population Expand into other indications such as kidney, esophageal, bladder, other liver cancers, other sarcoma subtypes through indication-specific or indication-agonistic label expansion Move into earlier lines of therapy (R/R Incidence à Incidence) Inclusion of patients with lower PRAME-threshold TOTAL ~39,000 annually in the US IMA203
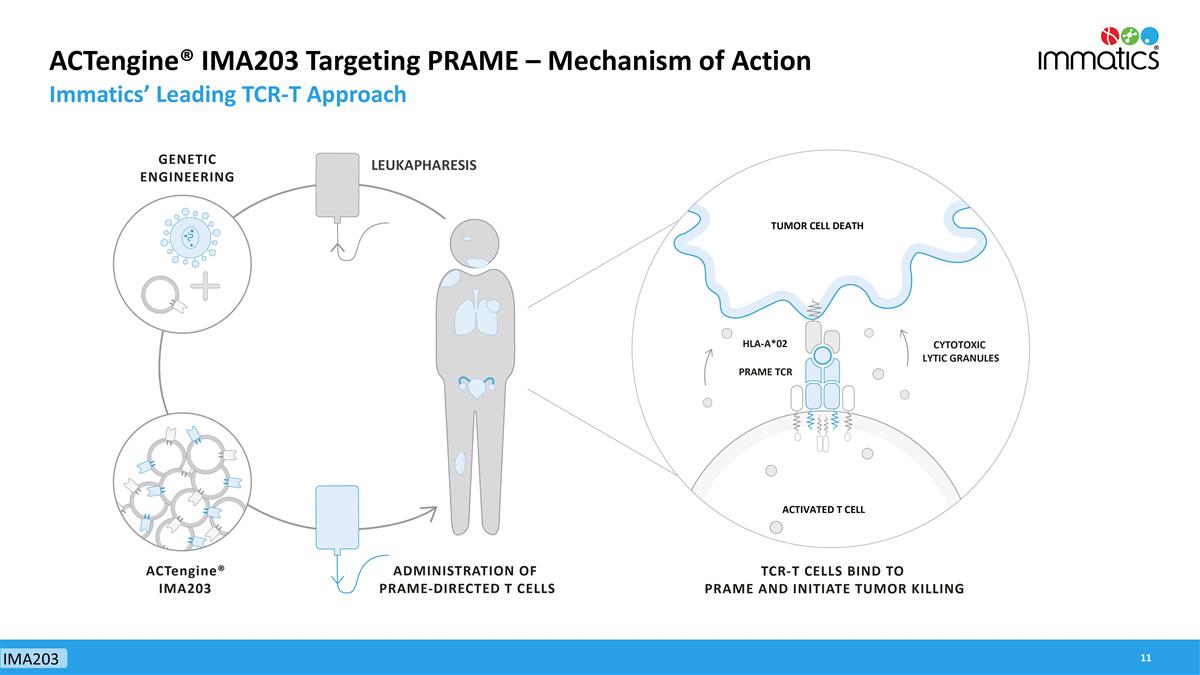
ACTengine® IMA203 Targeting PRAME – Mechanism of Action Immatics’ Leading TCR-T Approach IMA203 LEUKAPHARESIS
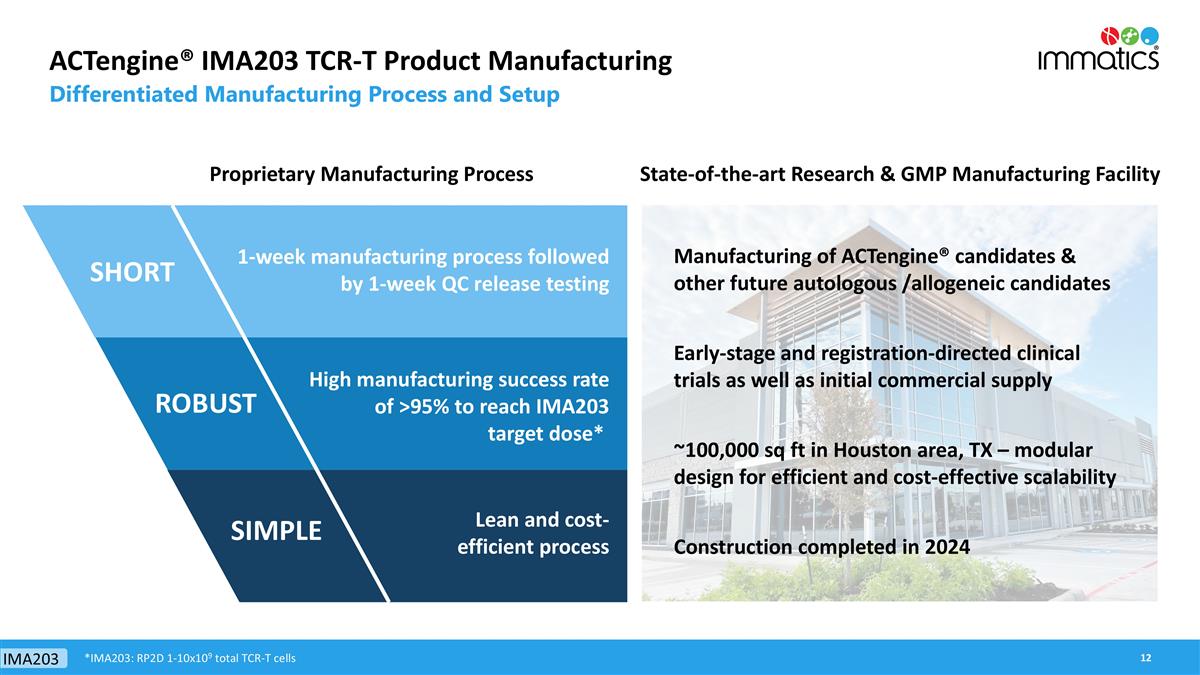
ACTengine® IMA203 TCR-T Product Manufacturing Differentiated Manufacturing Process and Setup 1-week manufacturing process followed by 1-week QC release testing High manufacturing success rate of >95% to reach IMA203 target dose* Lean and cost-efficient process Proprietary Manufacturing Process IMA203 SHORT SIMPLE ROBUST *IMA203: RP2D 1-10x109 total TCR-T cells Manufacturing of ACTengine® candidates & other future autologous /allogeneic candidates Construction completed in 2024 ~100,000 sq ft in Houston area, TX – modular design for efficient and cost-effective scalability Early-stage and registration-directed clinical trials as well as initial commercial supply State-of-the-art Research & GMP Manufacturing Facility
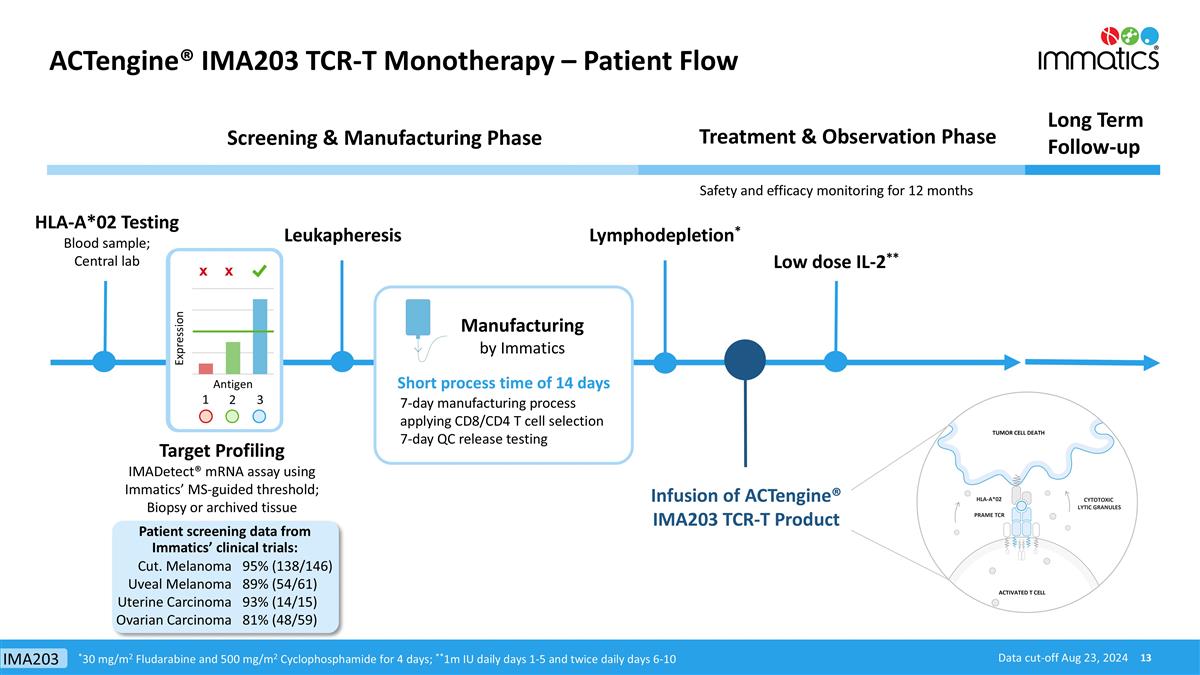
ACTengine® IMA203 TCR-T Monotherapy – Patient Flow HLA-A*02 Testing Blood sample; Central lab Treatment & Observation Phase Long Term Follow-up Screening & Manufacturing Phase Manufacturing by Immatics Infusion of ACTengine® IMA203 TCR-T Product Lymphodepletion* Target Profiling IMADetect® mRNA assay using Immatics’ MS-guided threshold; Biopsy or archived tissue Low dose IL-2** Safety and efficacy monitoring for 12 months Leukapheresis x x 1 3 2 Short process time of 14 days *30 mg/m2 Fludarabine and 500 mg/m2 Cyclophosphamide for 4 days; **1m IU daily days 1-5 and twice daily days 6-10 7-day manufacturing process applying CD8/CD4 T cell selection 7-day QC release testing Cut. Melanoma Uveal Melanoma Uterine Carcinoma Ovarian Carcinoma 95% (138/146) 89% (54/61) 93% (14/15) 81% (48/59) Patient screening data from Immatics’ clinical trials: Data cut-off Aug 23, 2024 IMA203
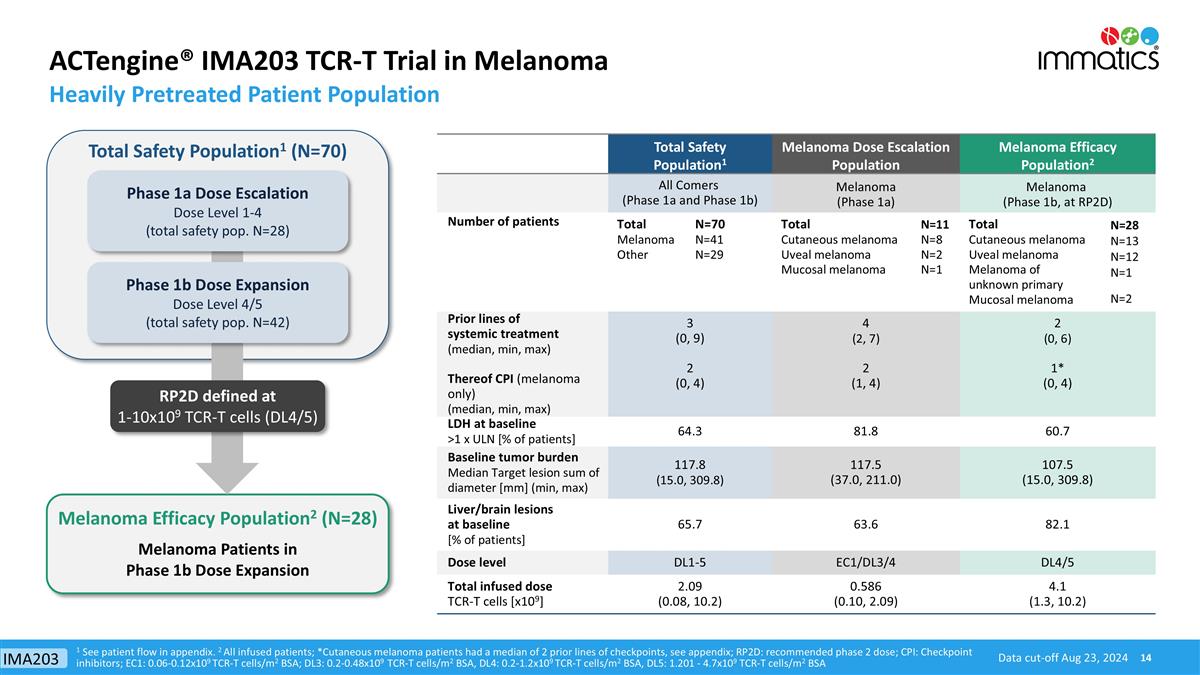
ACTengine® IMA203 TCR-T Trial in Melanoma Heavily Pretreated Patient Population Data cut-off Aug 23, 2024 1 See patient flow in appendix. 2 All infused patients; *Cutaneous melanoma patients had a median of 2 prior lines of checkpoints, see appendix; RP2D: recommended phase 2 dose; CPI: Checkpoint inhibitors; EC1: 0.06-0.12x109 TCR-T cells/m2 BSA; DL3: 0.2-0.48x109 TCR-T cells/m2 BSA, DL4: 0.2-1.2x109 TCR-T cells/m2 BSA, DL5: 1.201 - 4.7x109 TCR-T cells/m2 BSA Total Safety Population1 Melanoma Dose Escalation Population Melanoma Efficacy Population2 All Comers (Phase 1a and Phase 1b) Melanoma (Phase 1a) Melanoma (Phase 1b, at RP2D) Number of patients Total Melanoma Other N=70 N=41 N=29 Total Cutaneous melanoma Uveal melanoma Mucosal melanoma N=11 N=8 N=2 N=1 Total Cutaneous melanoma Uveal melanoma Melanoma of unknown primary Mucosal melanoma N=28 N=13 N=12 N=1 N=2 Prior lines of systemic treatment (median, min, max) Thereof CPI (melanoma only) (median, min, max) 3 (0, 9) 2 (0, 4) 4 (2, 7) 2 (1, 4) 2 (0, 6) 1* (0, 4) LDH at baseline >1 x ULN [% of patients] 64.3 81.8 60.7 Baseline tumor burden Median Target lesion sum of diameter [mm] (min, max) 117.8 (15.0, 309.8) 117.5 (37.0, 211.0) 107.5 (15.0, 309.8) Liver/brain lesions at baseline [% of patients] 65.7 63.6 82.1 Dose level DL1-5 EC1/DL3/4 DL4/5 Total infused dose TCR-T cells [x109] 2.09 (0.08, 10.2) 0.586 (0.10, 2.09) 4.1 (1.3, 10.2) Melanoma Efficacy Population2 (N=28) Total Safety Population1 (N=70) Melanoma Patients in Phase 1b Dose Expansion RP2D defined at 1-10x109 TCR-T cells (DL4/5) Phase 1b Dose Expansion Dose Level 4/5 (total safety pop. N=42) Phase 1a Dose Escalation Dose Level 1-4 (total safety pop. N=28) IMA203
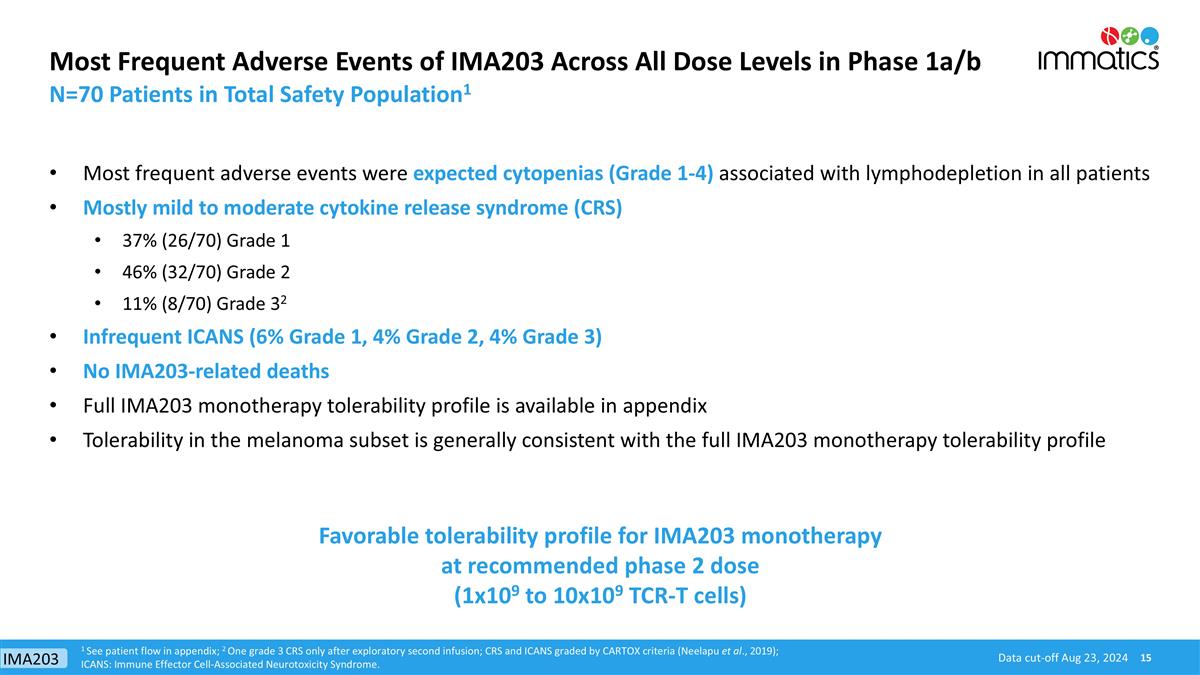
Data cut-off Aug 23, 2024 Most Frequent Adverse Events of IMA203 Across All Dose Levels in Phase 1a/b Most frequent adverse events were expected cytopenias (Grade 1-4) associated with lymphodepletion in all patients Mostly mild to moderate cytokine release syndrome (CRS) 37% (26/70) Grade 1 46% (32/70) Grade 2 11% (8/70) Grade 32 Infrequent ICANS (6% Grade 1, 4% Grade 2, 4% Grade 3) No IMA203-related deaths Full IMA203 monotherapy tolerability profile is available in appendix Tolerability in the melanoma subset is generally consistent with the full IMA203 monotherapy tolerability profile 1 See patient flow in appendix; 2 One grade 3 CRS only after exploratory second infusion; CRS and ICANS graded by CARTOX criteria (Neelapu et al., 2019); ICANS: Immune Effector Cell-Associated Neurotoxicity Syndrome. N=70 Patients in Total Safety Population1 Favorable tolerability profile for IMA203 monotherapy at recommended phase 2 dose (1x109 to 10x109 TCR-T cells) IMA203
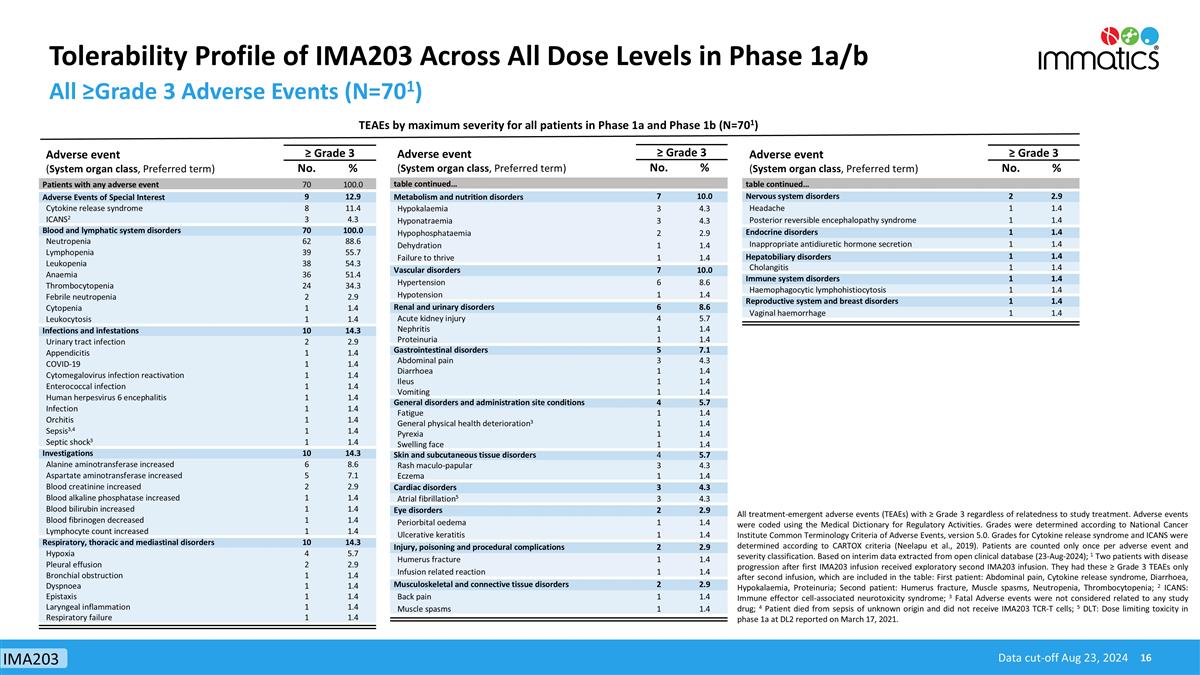
Tolerability Profile of IMA203 Across All Dose Levels in Phase 1a/b All ≥Grade 3 Adverse Events (N=701) All treatment-emergent adverse events (TEAEs) with ≥ Grade 3 regardless of relatedness to study treatment. Adverse events were coded using the Medical Dictionary for Regulatory Activities. Grades were determined according to National Cancer Institute Common Terminology Criteria of Adverse Events, version 5.0. Grades for Cytokine release syndrome and ICANS were determined according to CARTOX criteria (Neelapu et al., 2019). Patients are counted only once per adverse event and severity classification. Based on interim data extracted from open clinical database (23-Aug-2024); 1 Two patients with disease progression after first IMA203 infusion received exploratory second IMA203 infusion. They had these ≥ Grade 3 TEAEs only after second infusion, which are included in the table: First patient: Abdominal pain, Cytokine release syndrome, Diarrhoea, Hypokalaemia, Proteinuria; Second patient: Humerus fracture, Muscle spasms, Neutropenia, Thrombocytopenia; 2 ICANS: Immune effector cell-associated neurotoxicity syndrome; 3 Fatal Adverse events were not considered related to any study drug; 4 Patient died from sepsis of unknown origin and did not receive IMA203 TCR-T cells; 5 DLT: Dose limiting toxicity in phase 1a at DL2 reported on March 17, 2021. TEAEs by maximum severity for all patients in Phase 1a and Phase 1b (N=701) Data cut-off Aug 23, 2024 Adverse event (System organ class, Preferred term) ≥ Grade 3 No. % Patients with any adverse event 70 100.0 Adverse Events of Special Interest 9 12.9 Cytokine release syndrome 8 11.4 ICANS2 3 4.3 Blood and lymphatic system disorders 70 100.0 Neutropenia 62 88.6 Lymphopenia 39 55.7 Leukopenia 38 54.3 Anaemia 36 51.4 Thrombocytopenia 24 34.3 Febrile neutropenia 2 2.9 Cytopenia 1 1.4 Leukocytosis 1 1.4 Infections and infestations 10 14.3 Urinary tract infection 2 2.9 Appendicitis 1 1.4 COVID-19 1 1.4 Cytomegalovirus infection reactivation 1 1.4 Enterococcal infection 1 1.4 Human herpesvirus 6 encephalitis 1 1.4 Infection 1 1.4 Orchitis 1 1.4 Sepsis3,4 1 1.4 Septic shock3 1 1.4 Investigations 10 14.3 Alanine aminotransferase increased 6 8.6 Aspartate aminotransferase increased 5 7.1 Blood creatinine increased 2 2.9 Blood alkaline phosphatase increased 1 1.4 Blood bilirubin increased 1 1.4 Blood fibrinogen decreased 1 1.4 Lymphocyte count increased 1 1.4 Respiratory, thoracic and mediastinal disorders 10 14.3 Hypoxia 4 5.7 Pleural effusion 2 2.9 Bronchial obstruction 1 1.4 Dyspnoea 1 1.4 Epistaxis 1 1.4 Laryngeal inflammation 1 1.4 Respiratory failure 1 1.4 Adverse event (System organ class, Preferred term) ≥ Grade 3 No. % table continued… Metabolism and nutrition disorders 7 10.0 Hypokalaemia 3 4.3 Hyponatraemia 3 4.3 Hypophosphataemia 2 2.9 Dehydration 1 1.4 Failure to thrive 1 1.4 Vascular disorders 7 10.0 Hypertension 6 8.6 Hypotension 1 1.4 Renal and urinary disorders 6 8.6 Acute kidney injury 4 5.7 Nephritis 1 1.4 Proteinuria 1 1.4 Gastrointestinal disorders 5 7.1 Abdominal pain 3 4.3 Diarrhoea 1 1.4 Ileus 1 1.4 Vomiting 1 1.4 General disorders and administration site conditions 4 5.7 Fatigue 1 1.4 General physical health deterioration3 1 1.4 Pyrexia 1 1.4 Swelling face 1 1.4 Skin and subcutaneous tissue disorders 4 5.7 Rash maculo-papular 3 4.3 Eczema 1 1.4 Cardiac disorders 3 4.3 Atrial fibrillation5 3 4.3 Eye disorders 2 2.9 Periorbital oedema 1 1.4 Ulcerative keratitis 1 1.4 Injury, poisoning and procedural complications 2 2.9 Humerus fracture 1 1.4 Infusion related reaction 1 1.4 Musculoskeletal and connective tissue disorders 2 2.9 Back pain 1 1.4 Muscle spasms 1 1.4 Adverse event (System organ class, Preferred term) ≥ Grade 3 No. % table continued… Nervous system disorders 2 2.9 Headache 1 1.4 Posterior reversible encephalopathy syndrome 1 1.4 Endocrine disorders 1 1.4 Inappropriate antidiuretic hormone secretion 1 1.4 Hepatobiliary disorders 1 1.4 Cholangitis 1 1.4 Immune system disorders 1 1.4 Haemophagocytic lymphohistiocytosis 1 1.4 Reproductive system and breast disorders 1 1.4 Vaginal haemorrhage 1 1.4 IMA203
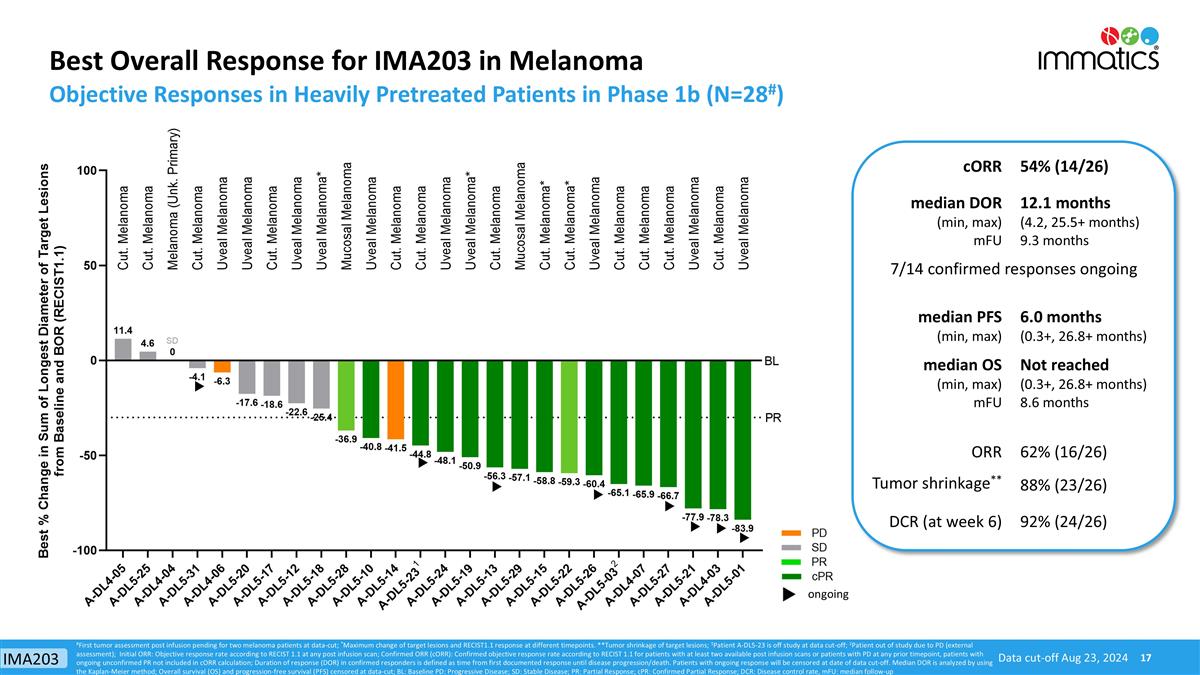
Best Overall Response for IMA203 in Melanoma Objective Responses in Heavily Pretreated Patients in Phase 1b (N=28#) Data cut-off Aug 23, 2024 ongoing #First tumor assessment post infusion pending for two melanoma patients at data-cut; *Maximum change of target lesions and RECIST1.1 response at different timepoints. **Tumor shrinkage of target lesions; 1Patient A-DL5-23 is off study at data cut-off; 2Patient out of study due to PD (external assessment); Initial ORR: Objective response rate according to RECIST 1.1 at any post infusion scan; Confirmed ORR (cORR): Confirmed objective response rate according to RECIST 1.1 for patients with at least two available post infusion scans or patients with PD at any prior timepoint, patients with ongoing unconfirmed PR not included in cORR calculation; Duration of response (DOR) in confirmed responders is defined as time from first documented response until disease progression/death. Patients with ongoing response will be censored at date of data cut-off. Median DOR is analyzed by using the Kaplan-Meier method; Overall survival (OS) and progression-free survival (PFS) censored at data-cut; BL: Baseline PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response; DCR: Disease control rate, mFU: median follow-up cORR 54% (14/26) median DOR (min, max) mFU 12.1 months (4.2, 25.5+ months) 9.3 months 7/14 confirmed responses ongoing median PFS (min, max) 6.0 months (0.3+, 26.8+ months) median OS (min, max) mFU Not reached (0.3+, 26.8+ months) 8.6 months ORR 62% (16/26) Tumor shrinkage** 88% (23/26) DCR (at week 6) 92% (24/26) IMA203
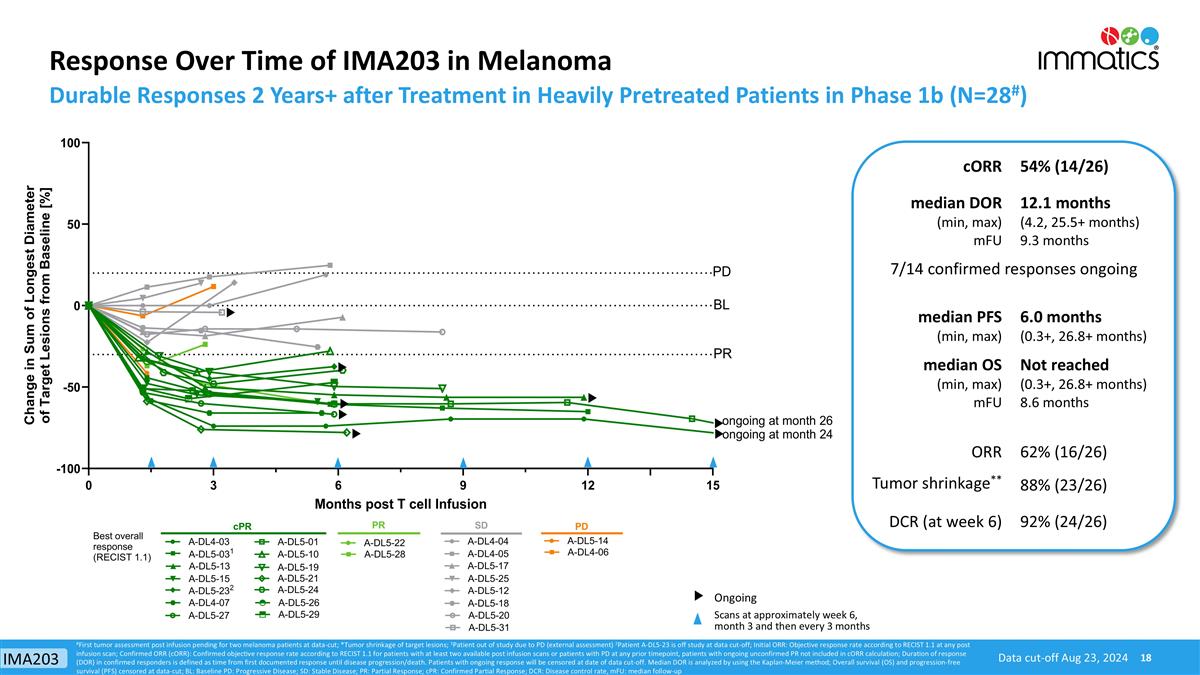
Response Over Time of IMA203 in Melanoma Durable Responses 2 Years+ after Treatment in Heavily Pretreated Patients in Phase 1b (N=28#) Scans at approximately week 6, month 3 and then every 3 months Ongoing Data cut-off Aug 23, 2024 #First tumor assessment post infusion pending for two melanoma patients at data-cut; *Tumor shrinkage of target lesions; 1Patient out of study due to PD (external assessment) 2Patient A-DL5-23 is off study at data cut-off; Initial ORR: Objective response rate according to RECIST 1.1 at any post infusion scan; Confirmed ORR (cORR): Confirmed objective response rate according to RECIST 1.1 for patients with at least two available post infusion scans or patients with PD at any prior timepoint, patients with ongoing unconfirmed PR not included in cORR calculation; Duration of response (DOR) in confirmed responders is defined as time from first documented response until disease progression/death. Patients with ongoing response will be censored at date of data cut-off. Median DOR is analyzed by using the Kaplan-Meier method; Overall survival (OS) and progression-free survival (PFS) censored at data-cut; BL: Baseline PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response; DCR: Disease control rate, mFU: median follow-up cORR 54% (14/26) median DOR (min, max) mFU 12.1 months (4.2, 25.5+ months) 9.3 months 7/14 confirmed responses ongoing median PFS (min, max) 6.0 months (0.3+, 26.8+ months) median OS (min, max) mFU Not reached (0.3+, 26.8+ months) 8.6 months ORR 62% (16/26) Tumor shrinkage** 88% (23/26) DCR (at week 6) 92% (24/26) IMA203
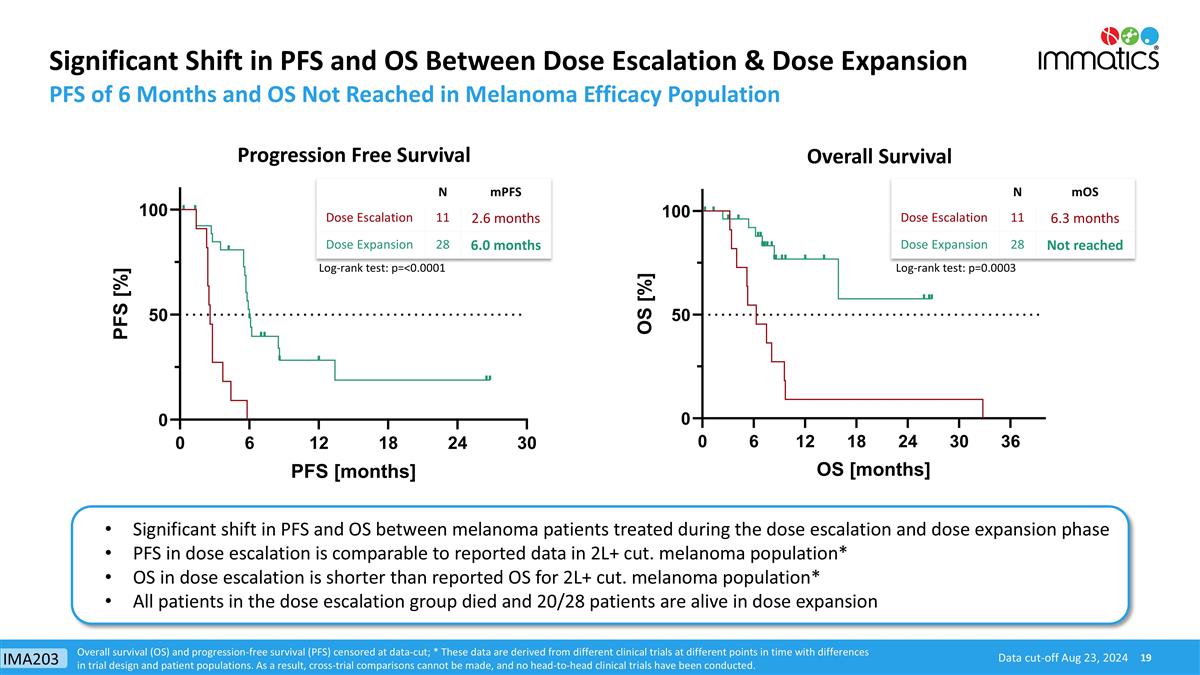
Significant Shift in PFS and OS Between Dose Escalation & Dose Expansion PFS of 6 Months and OS Not Reached in Melanoma Efficacy Population Progression Free Survival Data cut-off Aug 23, 2024 Overall Survival Overall survival (OS) and progression-free survival (PFS) censored at data-cut; * These data are derived from different clinical trials at different points in time with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. N mPFS Dose Escalation 11 2.6 months Dose Expansion 28 6.0 months N mOS Dose Escalation 11 6.3 months Dose Expansion 28 Not reached Log-rank test: p=<0.0001 Log-rank test: p=0.0003 Significant shift in PFS and OS between melanoma patients treated during the dose escalation and dose expansion phase PFS in dose escalation is comparable to reported data in 2L+ cut. melanoma population* OS in dose escalation is shorter than reported OS for 2L+ cut. melanoma population* All patients in the dose escalation group died and 20/28 patients are alive in dose expansion IMA203
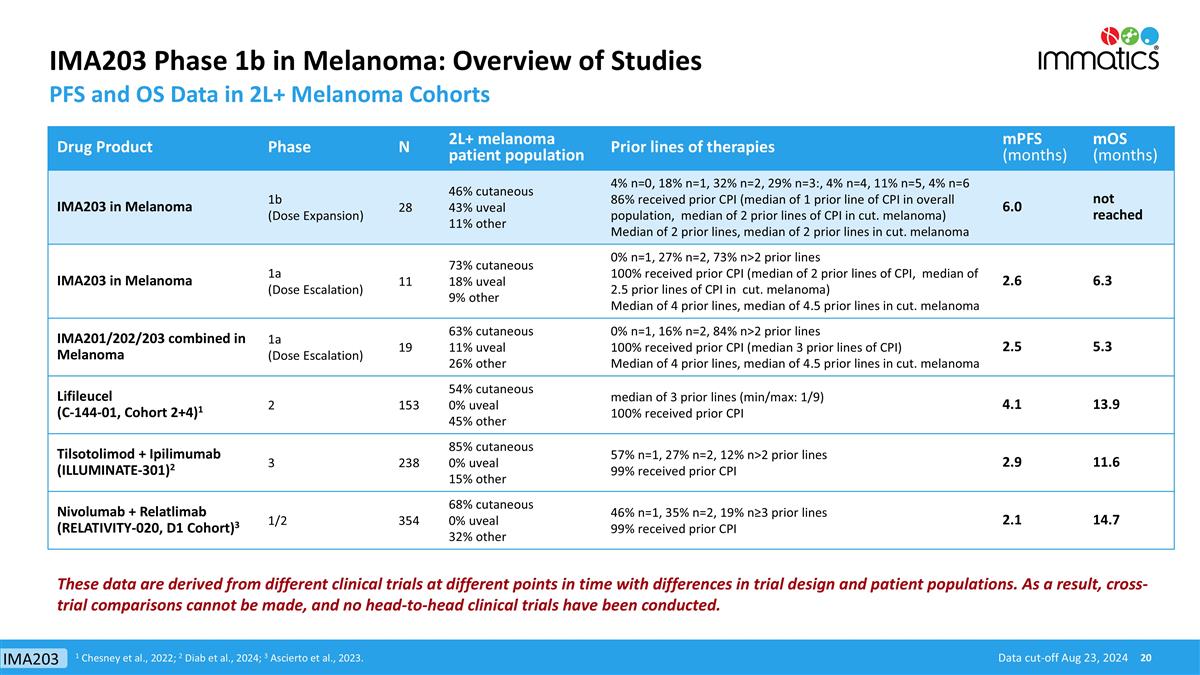
IMA203 Phase 1b in Melanoma: Overview of Studies PFS and OS Data in 2L+ Melanoma Cohorts Drug Product Phase N 2L+ melanoma patient population Prior lines of therapies mPFS (months) mOS (months) IMA203 in Melanoma 1b (Dose Expansion) 28 46% cutaneous 43% uveal 11% other 4% n=0, 18% n=1, 32% n=2, 29% n=3:, 4% n=4, 11% n=5, 4% n=6 86% received prior CPI (median of 1 prior line of CPI in overall population, median of 2 prior lines of CPI in cut. melanoma) Median of 2 prior lines, median of 2 prior lines in cut. melanoma 6.0 not reached IMA203 in Melanoma 1a (Dose Escalation) 11 73% cutaneous 18% uveal 9% other 0% n=1, 27% n=2, 73% n>2 prior lines 100% received prior CPI (median of 2 prior lines of CPI, median of 2.5 prior lines of CPI in cut. melanoma) Median of 4 prior lines, median of 4.5 prior lines in cut. melanoma 2.6 6.3 IMA201/202/203 combined in Melanoma 1a (Dose Escalation) 19 63% cutaneous 11% uveal 26% other 0% n=1, 16% n=2, 84% n>2 prior lines 100% received prior CPI (median 3 prior lines of CPI) Median of 4 prior lines, median of 4.5 prior lines in cut. melanoma 2.5 5.3 Lifileucel (C-144-01, Cohort 2+4)1 2 153 54% cutaneous 0% uveal 45% other median of 3 prior lines (min/max: 1/9) 100% received prior CPI 4.1 13.9 Tilsotolimod + Ipilimumab (ILLUMINATE-301)2 3 238 85% cutaneous 0% uveal 15% other 57% n=1, 27% n=2, 12% n>2 prior lines 99% received prior CPI 2.9 11.6 Nivolumab + Relatlimab (RELATIVITY-020, D1 Cohort)3 1/2 354 68% cutaneous 0% uveal 32% other 46% n=1, 35% n=2, 19% n≥3 prior lines 99% received prior CPI 2.1 14.7 Data cut-off Aug 23, 2024 1 Chesney et al., 2022; 2 Diab et al., 2024; 3 Ascierto et al., 2023. These data are derived from different clinical trials at different points in time with differences in trial design and patient populations. As a result, cross-trial comparisons cannot be made, and no head-to-head clinical trials have been conducted. IMA203
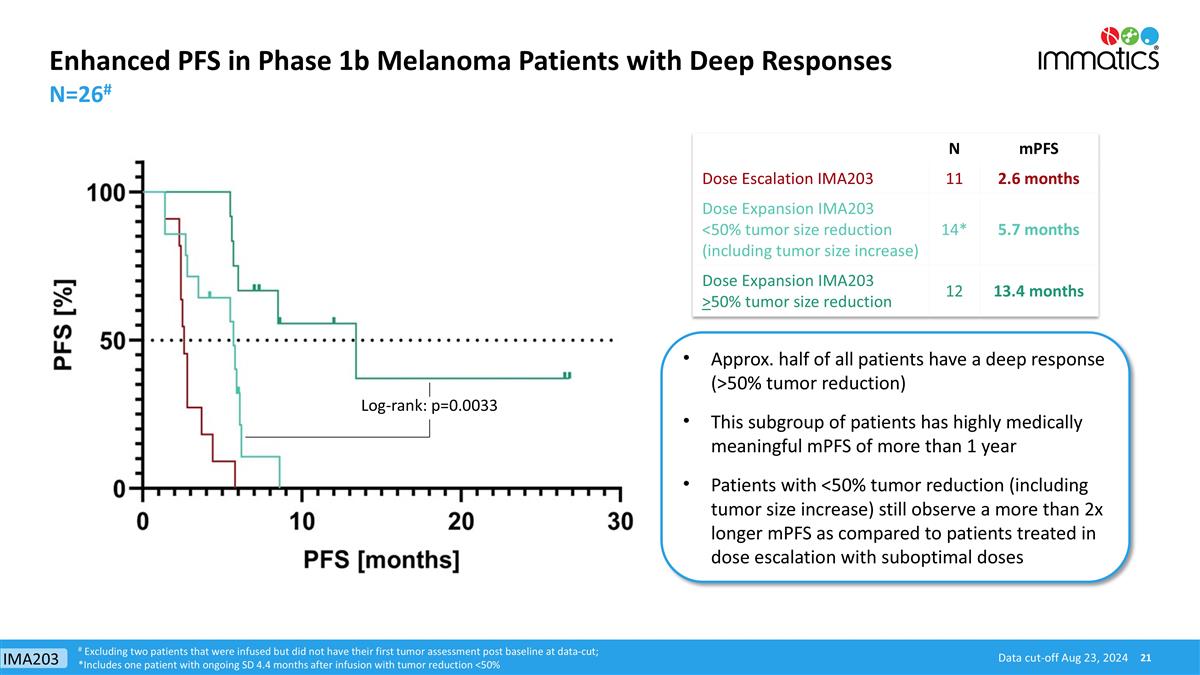
Enhanced PFS in Phase 1b Melanoma Patients with Deep Responses N=26# Approx. half of all patients have a deep response (>50% tumor reduction) This subgroup of patients has highly medically meaningful mPFS of more than 1 year Patients with <50% tumor reduction (including tumor size increase) still observe a more than 2x longer mPFS as compared to patients treated in dose escalation with suboptimal doses Data cut-off Aug 23, 2024 N mPFS Dose Escalation IMA203 11 2.6 months Dose Expansion IMA203 <50% tumor size reduction (including tumor size increase) 14* 5.7 months Dose Expansion IMA203 >50% tumor size reduction 12 13.4 months # Excluding two patients that were infused but did not have their first tumor assessment post baseline at data-cut; *Includes one patient with ongoing SD 4.4 months after infusion with tumor reduction <50% Log-rank: p=0.0033 IMA203
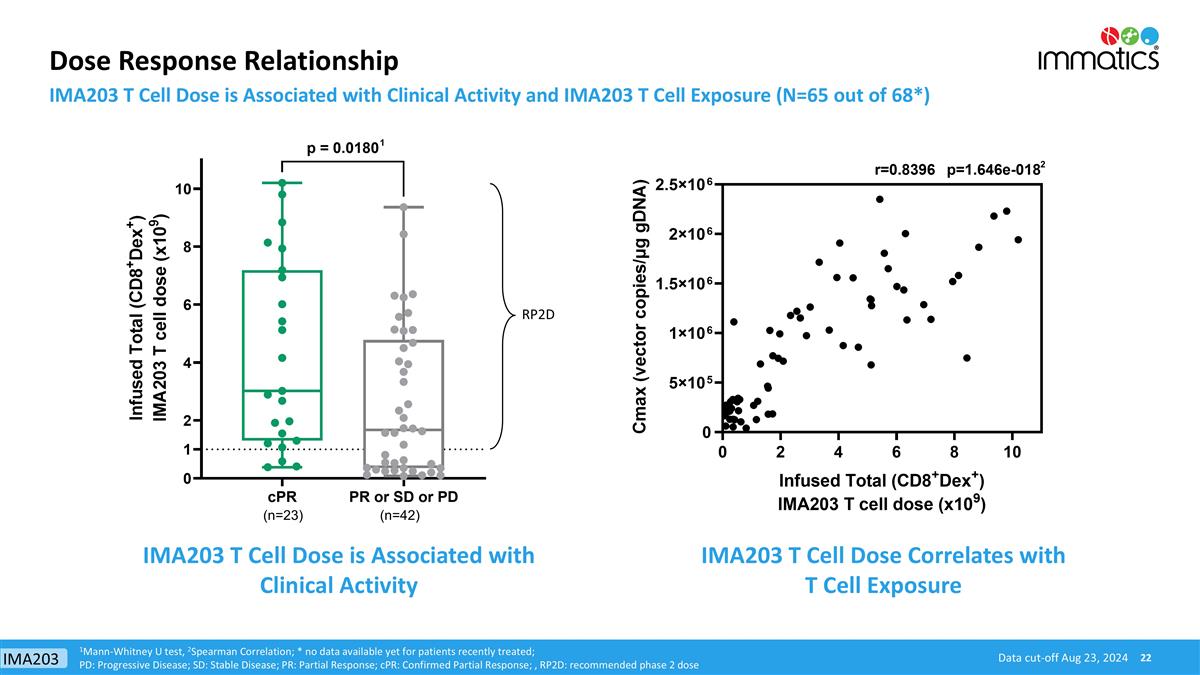
Dose Response Relationship IMA203 T Cell Dose is Associated with Clinical Activity and IMA203 T Cell Exposure (N=65 out of 68*) RP2D (n=23) (n=42) IMA203 T Cell Dose is Associated with Clinical Activity IMA203 T Cell Dose Correlates with T Cell Exposure 1Mann-Whitney U test, 2Spearman Correlation; * no data available yet for patients recently treated; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response; , RP2D: recommended phase 2 dose 1 2 Data cut-off Aug 23, 2024 IMA203
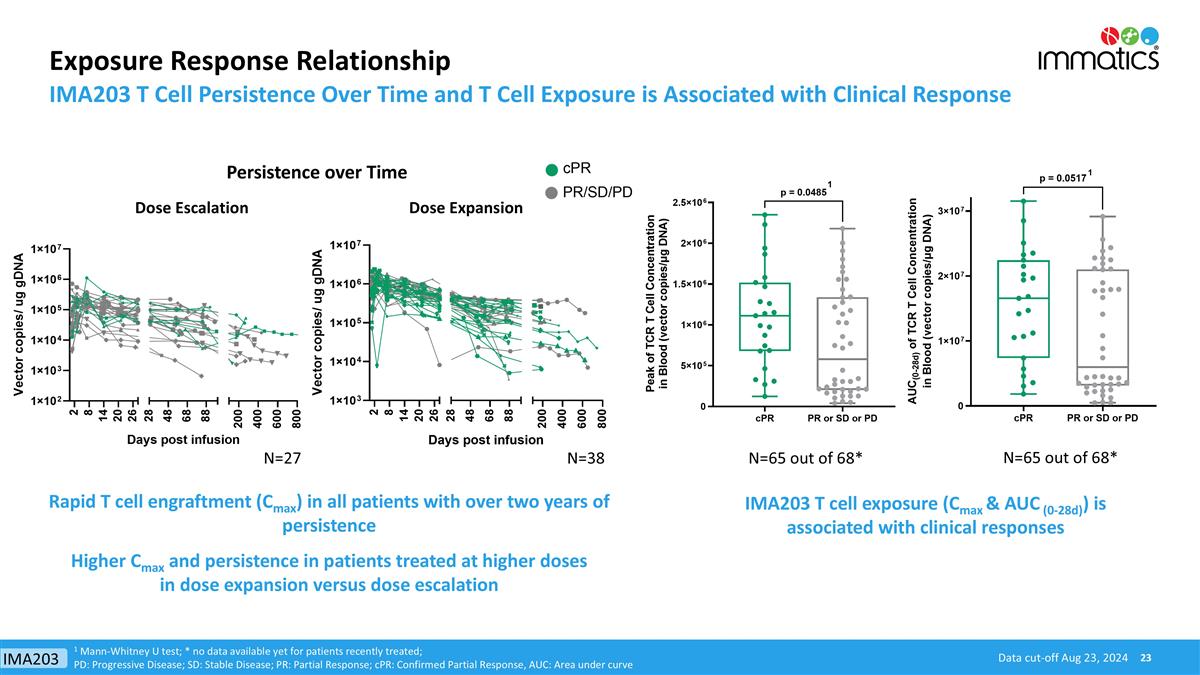
Exposure Response Relationship IMA203 T Cell Persistence Over Time and T Cell Exposure is Associated with Clinical Response IMA203 T cell exposure (Cmax & AUC (0-28d)) is associated with clinical responses Rapid T cell engraftment (Cmax) in all patients with over two years of persistence Higher Cmax and persistence in patients treated at higher doses in dose expansion versus dose escalation 1 Mann-Whitney U test; * no data available yet for patients recently treated; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response, AUC: Area under curve Data cut-off Aug 23, 2024 1 1 N=65 out of 68* N=38 N=27 Persistence over Time Dose Escalation Dose Expansion N=65 out of 68* IMA203
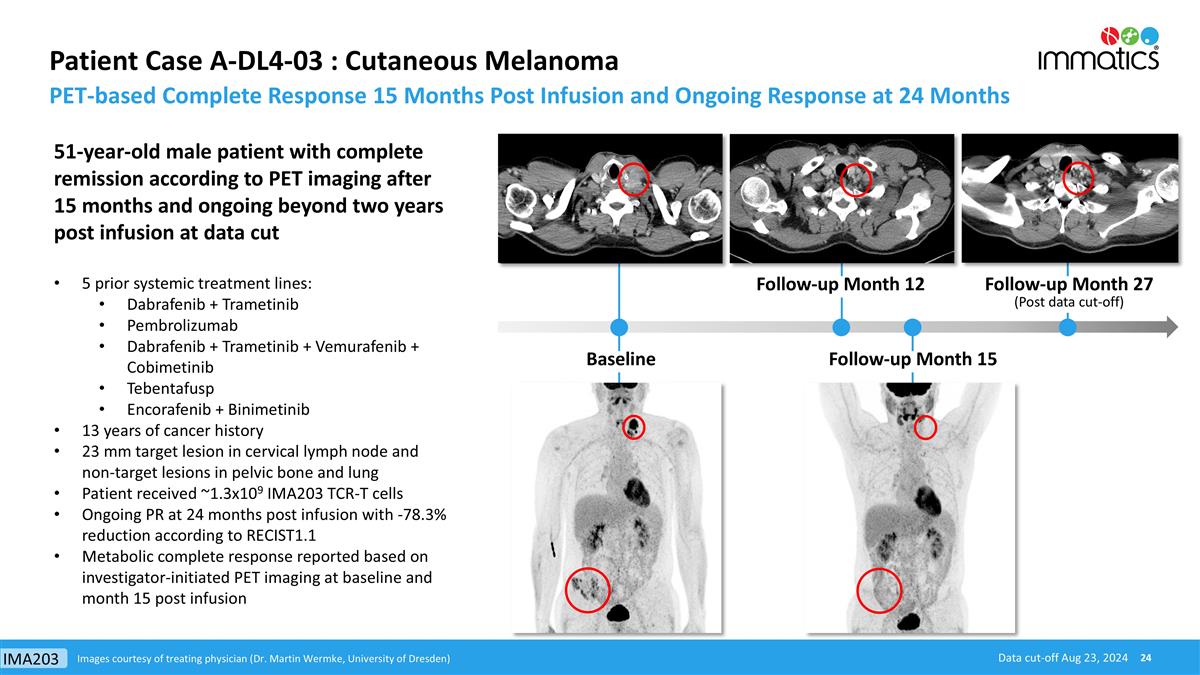
Patient Case A-DL4-03 : Cutaneous Melanoma PET-based Complete Response 15 Months Post Infusion and Ongoing Response at 24 Months Images courtesy of treating physician (Dr. Martin Wermke, University of Dresden) 51-year-old male patient with complete remission according to PET imaging after 15 months and ongoing beyond two years post infusion at data cut 5 prior systemic treatment lines: Dabrafenib + Trametinib Pembrolizumab Dabrafenib + Trametinib + Vemurafenib + Cobimetinib Tebentafusp Encorafenib + Binimetinib 13 years of cancer history 23 mm target lesion in cervical lymph node and non-target lesions in pelvic bone and lung Patient received ~1.3x109 IMA203 TCR-T cells Ongoing PR at 24 months post infusion with -78.3% reduction according to RECIST1.1 Metabolic complete response reported based on investigator-initiated PET imaging at baseline and month 15 post infusion Follow-up Month 15 Follow-up Month 27 (Post data cut-off) Baseline Follow-up Month 12 Data cut-off Aug 23, 2024 IMA203
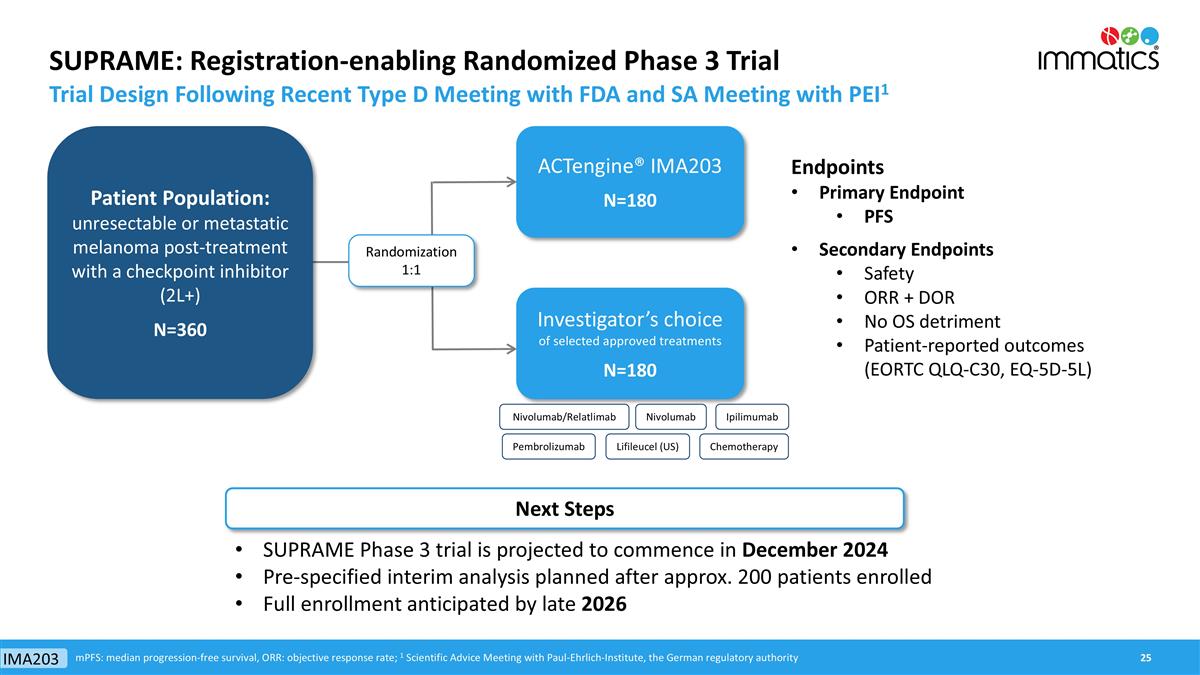
SUPRAME: Registration-enabling Randomized Phase 3 Trial Trial Design Following Recent Type D Meeting with FDA and SA Meeting with PEI1 ACTengine® IMA203 N=180 Investigator’s choice of selected approved treatments N=180 Endpoints Primary Endpoint PFS Secondary Endpoints Safety ORR + DOR No OS detriment Patient-reported outcomes (EORTC QLQ-C30, EQ-5D-5L) mPFS: median progression-free survival, ORR: objective response rate; 1 Scientific Advice Meeting with Paul-Ehrlich-Institute, the German regulatory authority Randomization 1:1 SUPRAME Phase 3 trial is projected to commence in December 2024 Pre-specified interim analysis planned after approx. 200 patients enrolled Full enrollment anticipated by late 2026 Next Steps Patient Population: unresectable or metastatic melanoma post-treatment with a checkpoint inhibitor (2L+) N=360 IMA203 Nivolumab/Relatlimab Nivolumab Pembrolizumab Ipilimumab Chemotherapy Lifileucel (US)
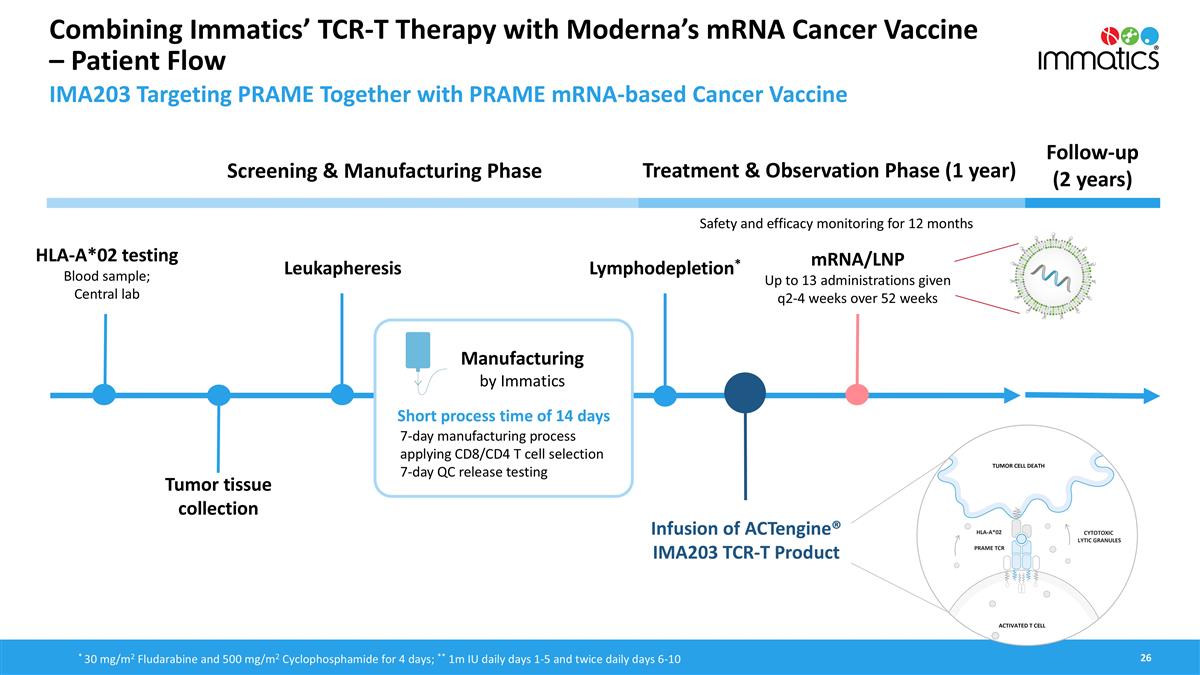
Combining Immatics’ TCR-T Therapy with Moderna’s mRNA Cancer Vaccine – Patient Flow HLA-A*02 testing Blood sample; Central lab Treatment & Observation Phase (1 year) Follow-up (2 years) Screening & Manufacturing Phase Manufacturing by Immatics Infusion of ACTengine® IMA203 TCR-T Product Lymphodepletion* mRNA/LNP Up to 13 administrations given q2-4 weeks over 52 weeks Safety and efficacy monitoring for 12 months Leukapheresis Short process time of 14 days * 30 mg/m2 Fludarabine and 500 mg/m2 Cyclophosphamide for 4 days; ** 1m IU daily days 1-5 and twice daily days 6-10 7-day manufacturing process applying CD8/CD4 T cell selection 7-day QC release testing IMA203 Targeting PRAME Together with PRAME mRNA-based Cancer Vaccine Tumor tissue collection
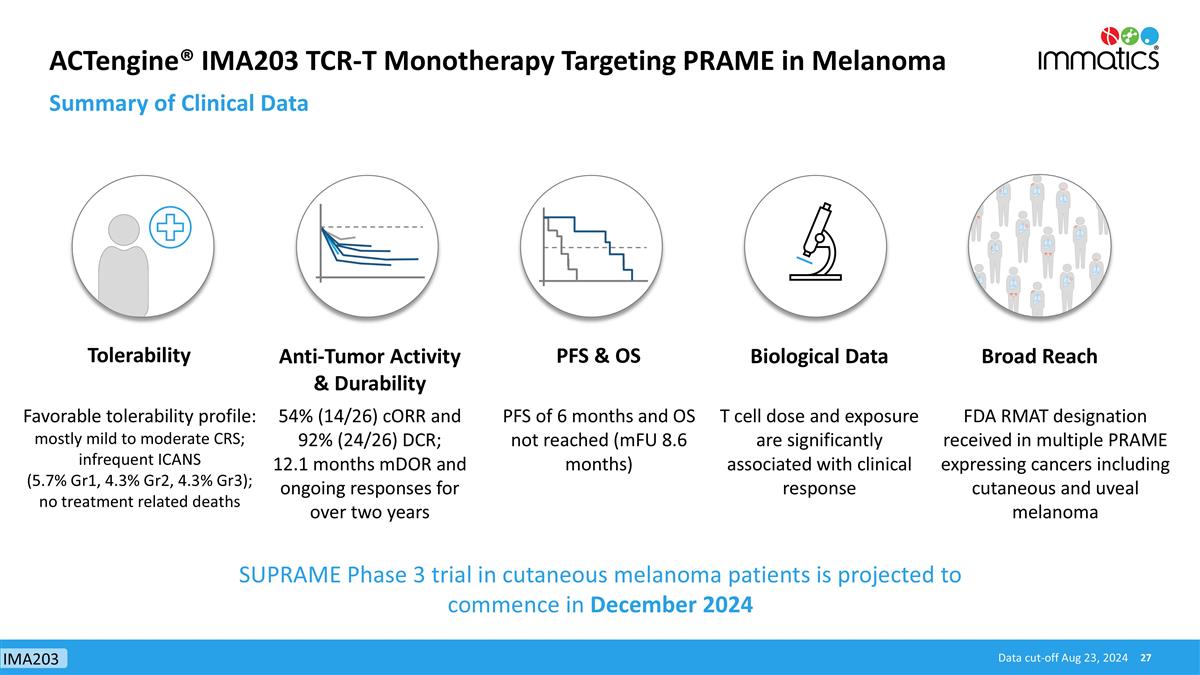
ACTengine® IMA203 TCR-T Monotherapy Targeting PRAME in Melanoma Summary of Clinical Data Tolerability Anti-Tumor Activity & Durability PFS & OS Biological Data Broad Reach Favorable tolerability profile: mostly mild to moderate CRS; infrequent ICANS (5.7% Gr1, 4.3% Gr2, 4.3% Gr3); no treatment related deaths 54% (14/26) cORR and 92% (24/26) DCR; 12.1 months mDOR and ongoing responses for over two years PFS of 6 months and OS not reached (mFU 8.6 months) T cell dose and exposure are significantly associated with clinical response FDA RMAT designation received in multiple PRAME expressing cancers including cutaneous and uveal melanoma Data cut-off Aug 23, 2024 SUPRAME Phase 3 trial in cutaneous melanoma patients is projected to commence in December 2024 IMA203
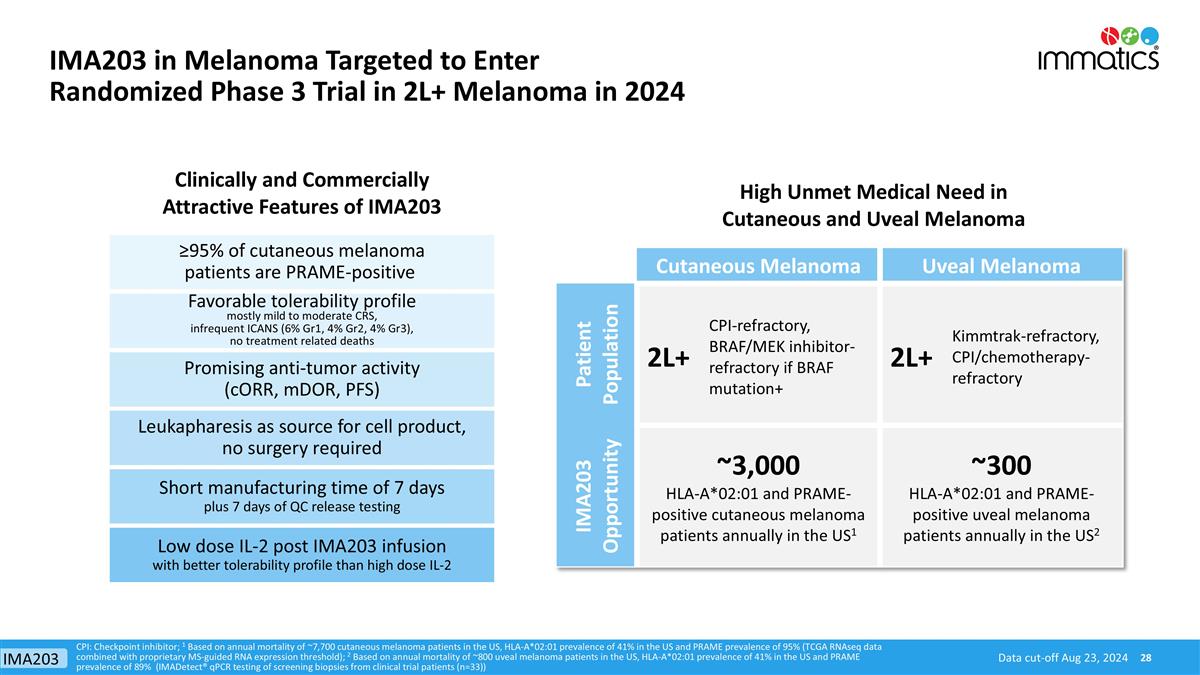
High Unmet Medical Need in Cutaneous and Uveal Melanoma Clinically and Commercially Attractive Features of IMA203 Cutaneous Melanoma Uveal Melanoma Patient Population 2L+ CPI-refractory, BRAF/MEK inhibitor-refractory if BRAF mutation+ 2L+ Kimmtrak-refractory, CPI/chemotherapy-refractory IMA203 Opportunity ~3,000 HLA-A*02:01 and PRAME-positive cutaneous melanoma patients annually in the US1 ~300 HLA-A*02:01 and PRAME-positive uveal melanoma patients annually in the US2 Favorable tolerability profile mostly mild to moderate CRS, infrequent ICANS (6% Gr1, 4% Gr2, 4% Gr3), no treatment related deaths Promising anti-tumor activity (cORR, mDOR, PFS) Leukapharesis as source for cell product, no surgery required Short manufacturing time of 7 days plus 7 days of QC release testing Low dose IL-2 post IMA203 infusion with better tolerability profile than high dose IL-2 CPI: Checkpoint inhibitor; 1 Based on annual mortality of ~7,700 cutaneous melanoma patients in the US, HLA-A*02:01 prevalence of 41% in the US and PRAME prevalence of 95% (TCGA RNAseq data combined with proprietary MS-guided RNA expression threshold); 2 Based on annual mortality of ~800 uveal melanoma patients in the US, HLA-A*02:01 prevalence of 41% in the US and PRAME prevalence of 89% (IMADetect® qPCR testing of screening biopsies from clinical trial patients (n=33)) ≥95% of cutaneous melanoma patients are PRAME-positive Data cut-off Aug 23, 2024 IMA203 in Melanoma Targeted to Enter Randomized Phase 3 Trial in 2L+ Melanoma in 2024 IMA203
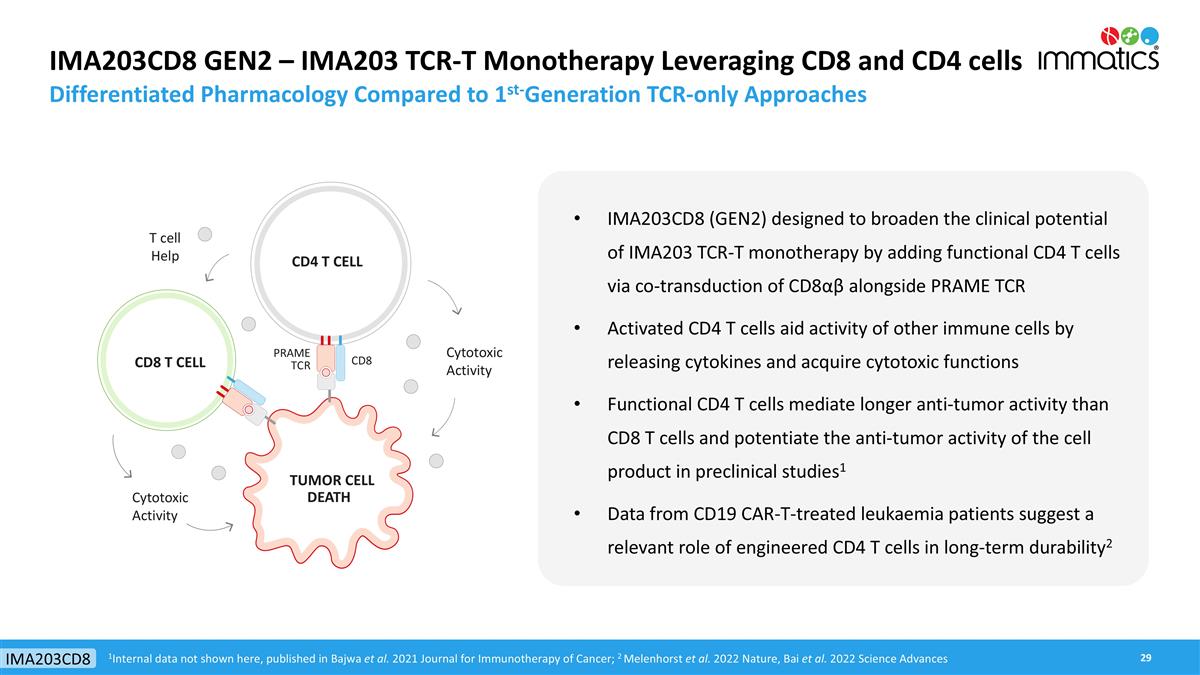
IMA203CD8 GEN2 – IMA203 TCR-T Monotherapy Leveraging CD8 and CD4 cells Differentiated Pharmacology Compared to 1st-Generation TCR-only Approaches IMA203CD8 (GEN2) designed to broaden the clinical potential of IMA203 TCR-T monotherapy by adding functional CD4 T cells via co-transduction of CD8αβ alongside PRAME TCR Activated CD4 T cells aid activity of other immune cells by releasing cytokines and acquire cytotoxic functions Functional CD4 T cells mediate longer anti-tumor activity than CD8 T cells and potentiate the anti-tumor activity of the cell product in preclinical studies1 Data from CD19 CAR-T-treated leukaemia patients suggest a relevant role of engineered CD4 T cells in long-term durability2 TUMOR CELL DEATH CD4 T CELL Cytotoxic Activity CD8 T CELL T cell Help Cytotoxic Activity 1Internal data not shown here, published in Bajwa et al. 2021 Journal for Immunotherapy of Cancer; 2 Melenhorst et al. 2022 Nature, Bai et al. 2022 Science Advances CD8 PRAME TCR IMA203CD8
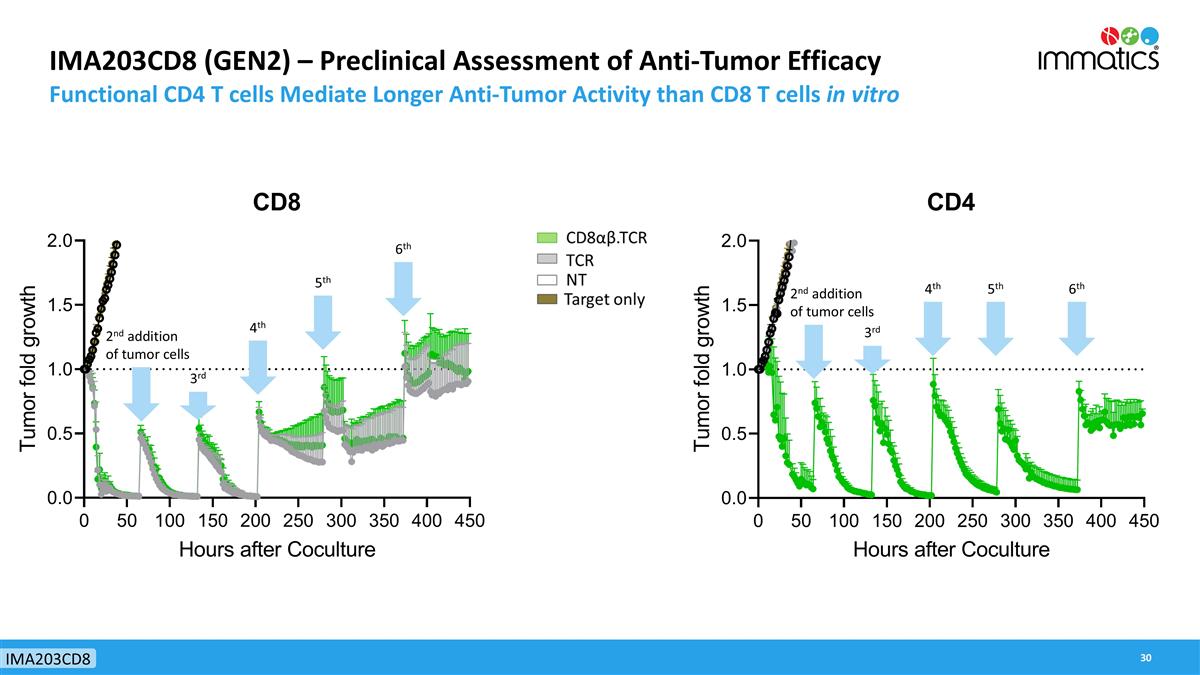
IMA203CD8 (GEN2) – Preclinical Assessment of Anti-Tumor Efficacy Functional CD4 T cells Mediate Longer Anti-Tumor Activity than CD8 T cells in vitro 2nd addition of tumor cells 3rd 4th 5th 6th 2nd addition of tumor cells 3rd 4th 5th 6th IMA203CD8
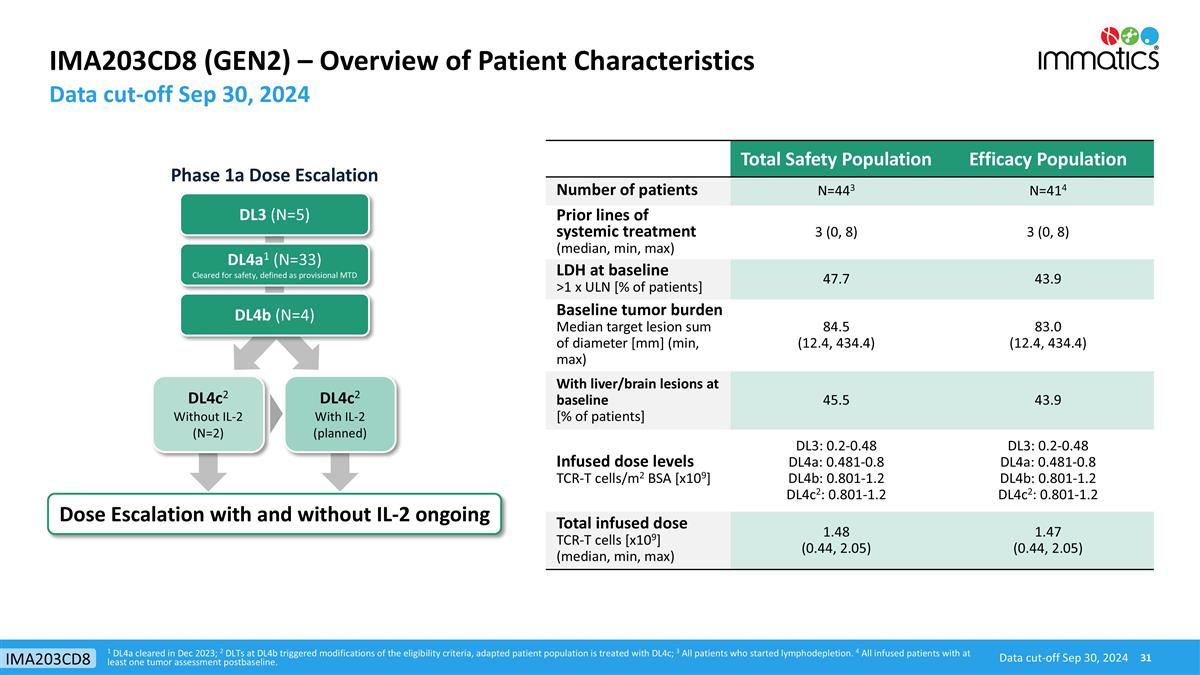
IMA203CD8 (GEN2) – Overview of Patient Characteristics 1 DL4a cleared in Dec 2023; 2 DLTs at DL4b triggered modifications of the eligibility criteria, adapted patient population is treated with DL4c; 3 All patients who started lymphodepletion. 4 All infused patients with at least one tumor assessment postbaseline. Data cut-off Sep 30, 2024 Data cut-off Sep 30, 2024 IMA203CD8 DL3 (N=5) DL4a1 (N=33) Cleared for safety, defined as provisional MTD Dose Escalation with and without IL-2 ongoing DL4b (N=4) DL4c2 Without IL-2 (N=2) DL4c2 With IL-2 (planned) Phase 1a Dose Escalation Total Safety Population Efficacy Population Number of patients N=443 N=414 Prior lines of systemic treatment (median, min, max) 3 (0, 8) 3 (0, 8) LDH at baseline >1 x ULN [% of patients] 47.7 43.9 Baseline tumor burden Median target lesion sum of diameter [mm] (min, max) 84.5 (12.4, 434.4) 83.0 (12.4, 434.4) With liver/brain lesions at baseline [% of patients] 45.5 43.9 Infused dose levels TCR-T cells/m2 BSA [x109] DL3: 0.2-0.48 DL4a: 0.481-0.8 DL4b: 0.801-1.2 DL4c2: 0.801-1.2 DL3: 0.2-0.48 DL4a: 0.481-0.8 DL4b: 0.801-1.2 DL4c2: 0.801-1.2 Total infused dose TCR-T cells [x109] (median, min, max) 1.48 (0.44, 2.05) 1.47 (0.44, 2.05)
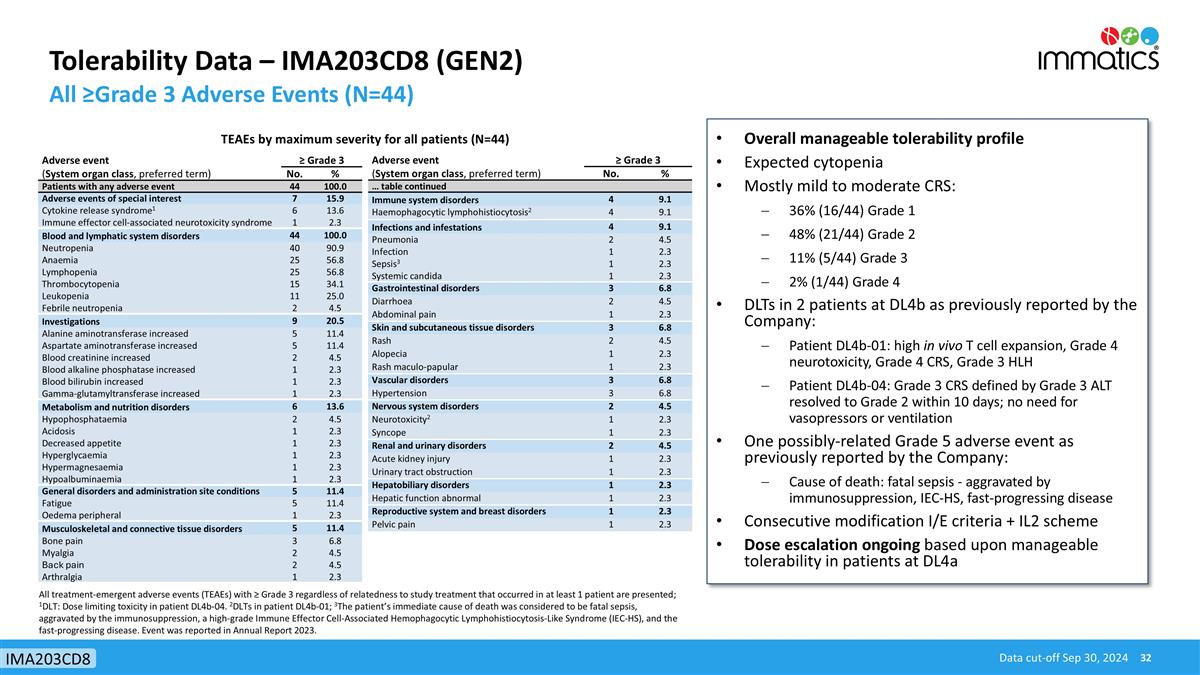
Tolerability Data – IMA203CD8 (GEN2) All ≥Grade 3 Adverse Events (N=44) Data cut-off Sep 30, 2024 IMA203CD8 Adverse event ≥ Grade 3 (System organ class, preferred term) No. % Patients with any adverse event 44 100.0 Adverse events of special interest 7 15.9 Cytokine release syndrome1 6 13.6 Immune effector cell-associated neurotoxicity syndrome 1 2.3 Blood and lymphatic system disorders 44 100.0 Neutropenia 40 90.9 Anaemia 25 56.8 Lymphopenia 25 56.8 Thrombocytopenia 15 34.1 Leukopenia 11 25.0 Febrile neutropenia 2 4.5 Investigations 9 20.5 Alanine aminotransferase increased 5 11.4 Aspartate aminotransferase increased 5 11.4 Blood creatinine increased 2 4.5 Blood alkaline phosphatase increased 1 2.3 Blood bilirubin increased 1 2.3 Gamma-glutamyltransferase increased 1 2.3 Metabolism and nutrition disorders 6 13.6 Hypophosphataemia 2 4.5 Acidosis 1 2.3 Decreased appetite 1 2.3 Hyperglycaemia 1 2.3 Hypermagnesaemia 1 2.3 Hypoalbuminaemia 1 2.3 General disorders and administration site conditions 5 11.4 Fatigue 5 11.4 Oedema peripheral 1 2.3 Musculoskeletal and connective tissue disorders 5 11.4 Bone pain 3 6.8 Myalgia 2 4.5 Back pain 2 4.5 Arthralgia 1 2.3 TEAEs by maximum severity for all patients (N=44) Adverse event ≥ Grade 3 (System organ class, preferred term) No. % … table continued Immune system disorders 4 9.1 Haemophagocytic lymphohistiocytosis2 4 9.1 Infections and infestations 4 9.1 Pneumonia 2 4.5 Infection 1 2.3 Sepsis3 1 2.3 Systemic candida 1 2.3 Gastrointestinal disorders 3 6.8 Diarrhoea 2 4.5 Abdominal pain 1 2.3 Skin and subcutaneous tissue disorders 3 6.8 Rash 2 4.5 Alopecia 1 2.3 Rash maculo-papular 1 2.3 Vascular disorders 3 6.8 Hypertension 3 6.8 Nervous system disorders 2 4.5 Neurotoxicity2 1 2.3 Syncope 1 2.3 Renal and urinary disorders 2 4.5 Acute kidney injury 1 2.3 Urinary tract obstruction 1 2.3 Hepatobiliary disorders 1 2.3 Hepatic function abnormal 1 2.3 Reproductive system and breast disorders 1 2.3 Pelvic pain 1 2.3 All treatment-emergent adverse events (TEAEs) with ≥ Grade 3 regardless of relatedness to study treatment that occurred in at least 1 patient are presented; 1DLT: Dose limiting toxicity in patient DL4b-04. 2DLTs in patient DL4b-01; 3The patient’s immediate cause of death was considered to be fatal sepsis, aggravated by the immunosuppression, a high-grade Immune Effector Cell-Associated Hemophagocytic Lymphohistiocytosis-Like Syndrome (IEC-HS), and the fast-progressing disease. Event was reported in Annual Report 2023. Overall manageable tolerability profile Expected cytopenia Mostly mild to moderate CRS: 36% (16/44) Grade 1 48% (21/44) Grade 2 11% (5/44) Grade 3 2% (1/44) Grade 4 DLTs in 2 patients at DL4b as previously reported by the Company: Patient DL4b-01: high in vivo T cell expansion, Grade 4 neurotoxicity, Grade 4 CRS, Grade 3 HLH Patient DL4b-04: Grade 3 CRS defined by Grade 3 ALT resolved to Grade 2 within 10 days; no need for vasopressors or ventilation One possibly-related Grade 5 adverse event as previously reported by the Company: Cause of death: fatal sepsis - aggravated by immunosuppression, IEC-HS, fast-progressing disease Consecutive modification I/E criteria + IL2 scheme Dose escalation ongoing based upon manageable tolerability in patients at DL4a
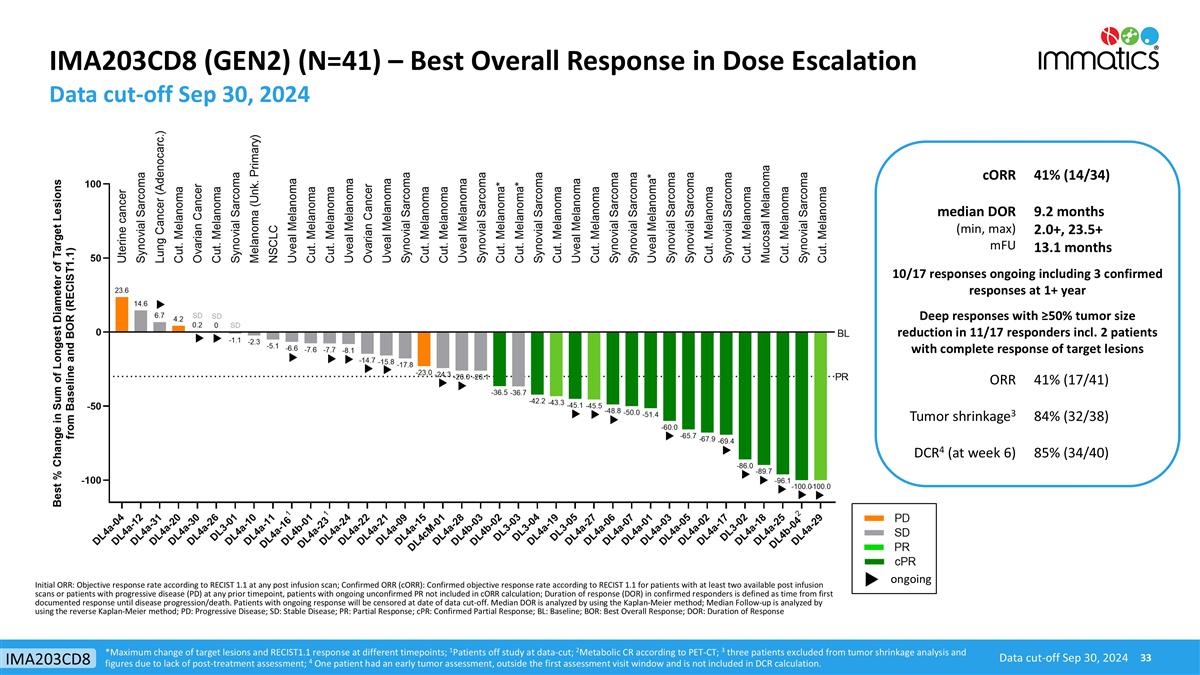
IMA203CD8 (GEN2) (N=41) – Best Overall Response in Dose Escalation Data cut-off Sep 30, 2024 Data cut-off Sep 30, 2024 IMA203CD8 cORR 41% (14/34) median DOR (min, max) mFU 9.2 months 2.0+, 23.5+ 13.1 months 10/17 responses ongoing including 3 confirmed responses at 1+ year Deep responses with ≥50% tumor size reduction in 11/17 responders incl. 2 patients with complete response of target lesions ORR 41% (17/41) Tumor shrinkage3 84% (32/38) DCR4 (at week 6) 85% (34/40) ongoing *Maximum change of target lesions and RECIST1.1 response at different timepoints; 1Patients off study at data-cut; 2Metabolic CR according to PET-CT; 3 three patients excluded from tumor shrinkage analysis and figures due to lack of post-treatment assessment; 4 One patient had an early tumor assessment, outside the first assessment visit window and is not included in DCR calculation. Initial ORR: Objective response rate according to RECIST 1.1 at any post infusion scan; Confirmed ORR (cORR): Confirmed objective response rate according to RECIST 1.1 for patients with at least two available post infusion scans or patients with progressive disease (PD) at any prior timepoint, patients with ongoing unconfirmed PR not included in cORR calculation; Duration of response (DOR) in confirmed responders is defined as time from first documented response until disease progression/death. Patients with ongoing response will be censored at date of data cut-off. Median DOR is analyzed by using the Kaplan-Meier method; Median Follow-up is analyzed by using the reverse Kaplan-Meier method; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response; BL: Baseline; BOR: Best Overall Response; DOR: Duration of Response
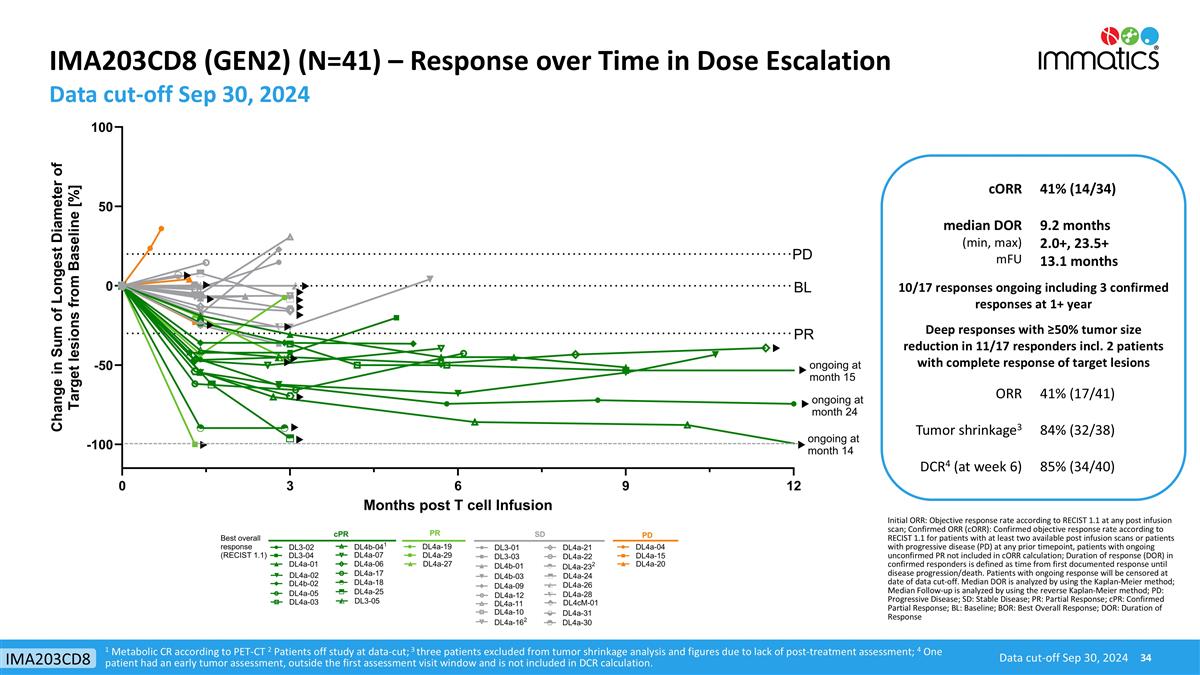
IMA203CD8 (GEN2) (N=41) – Response over Time in Dose Escalation Data cut-off Sep 30, 2024 Data cut-off Sep 30, 2024 IMA203CD8 1 Metabolic CR according to PET-CT 2 Patients off study at data-cut; 3 three patients excluded from tumor shrinkage analysis and figures due to lack of post-treatment assessment; 4 One patient had an early tumor assessment, outside the first assessment visit window and is not included in DCR calculation. cORR 41% (14/34) median DOR (min, max) mFU 9.2 months 2.0+, 23.5+ 13.1 months 10/17 responses ongoing including 3 confirmed responses at 1+ year Deep responses with ≥50% tumor size reduction in 11/17 responders incl. 2 patients with complete response of target lesions ORR 41% (17/41) Tumor shrinkage3 84% (32/38) DCR4 (at week 6) 85% (34/40) Initial ORR: Objective response rate according to RECIST 1.1 at any post infusion scan; Confirmed ORR (cORR): Confirmed objective response rate according to RECIST 1.1 for patients with at least two available post infusion scans or patients with progressive disease (PD) at any prior timepoint, patients with ongoing unconfirmed PR not included in cORR calculation; Duration of response (DOR) in confirmed responders is defined as time from first documented response until disease progression/death. Patients with ongoing response will be censored at date of data cut-off. Median DOR is analyzed by using the Kaplan-Meier method; Median Follow-up is analyzed by using the reverse Kaplan-Meier method; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response; BL: Baseline; BOR: Best Overall Response; DOR: Duration of Response
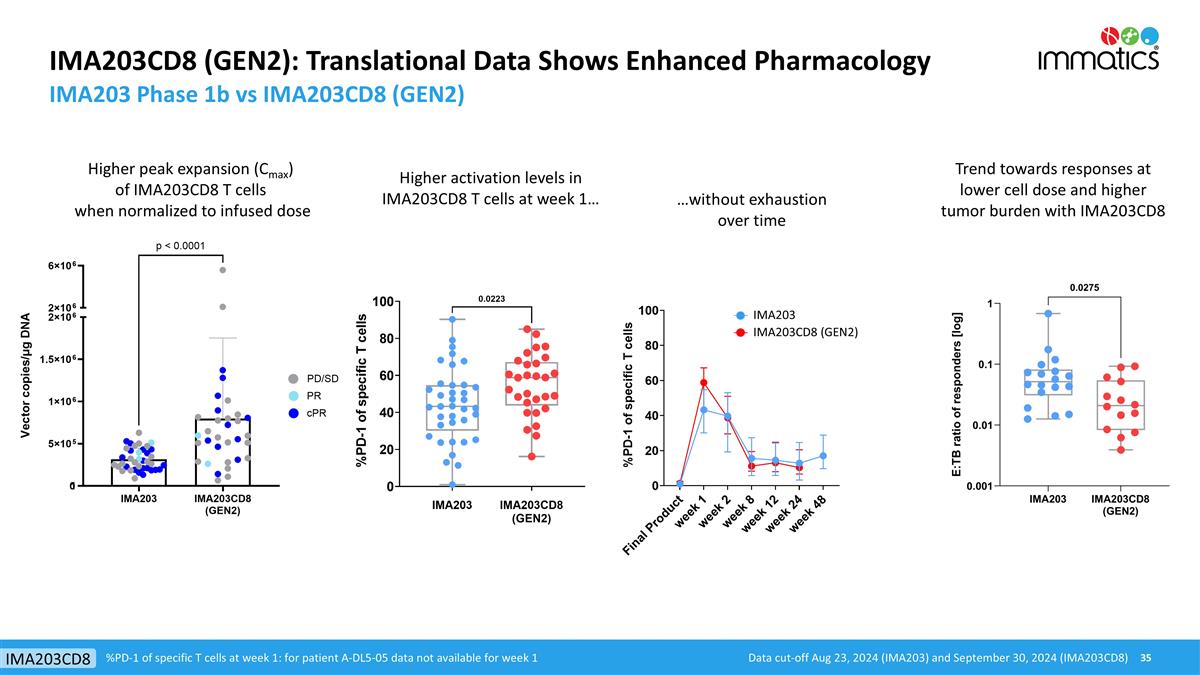
IMA203CD8 (GEN2): Translational Data Shows Enhanced Pharmacology IMA203 Phase 1b vs IMA203CD8 (GEN2) Trend towards responses at lower cell dose and higher tumor burden with IMA203CD8 Higher peak expansion (Cmax) of IMA203CD8 T cells when normalized to infused dose Higher activation levels in IMA203CD8 T cells at week 1… …without exhaustion over time %PD-1 of specific T cells at week 1: for patient A-DL5-05 data not available for week 1 Data cut-off Aug 23, 2024 (IMA203) and September 30, 2024 (IMA203CD8) IMA203CD8 IMA203 IMA203CD8 (GEN2)
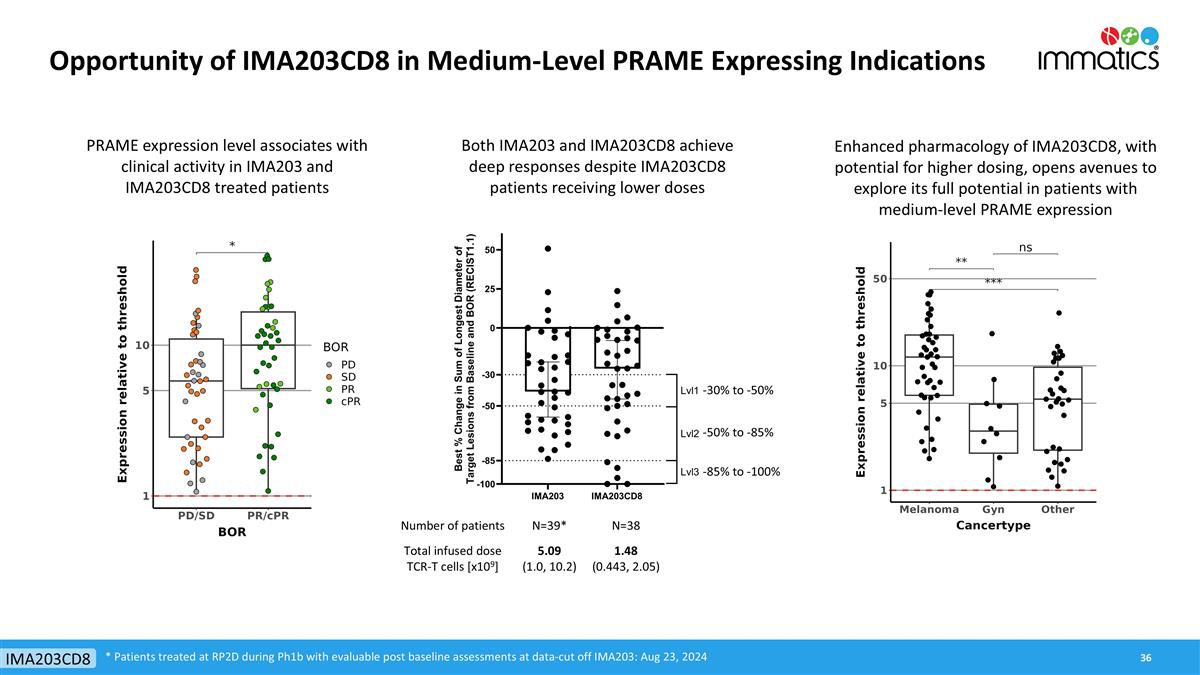
Opportunity of IMA203CD8 in Medium-Level PRAME Expressing Indications * Patients treated at RP2D during Ph1b with evaluable post baseline assessments at data-cut off IMA203: Aug 23, 2024 IMA203CD8 PRAME expression level associates with clinical activity in IMA203 and IMA203CD8 treated patients Enhanced pharmacology of IMA203CD8, with potential for higher dosing, opens avenues to explore its full potential in patients with medium-level PRAME expression Both IMA203 and IMA203CD8 achieve deep responses despite IMA203CD8 patients receiving lower doses Number of patients N=39* N=38 Total infused dose TCR-T cells [x109] 5.09 (1.0, 10.2) 1.48 (0.443, 2.05) -30% to -50% -50% to -85% -85% to -100%
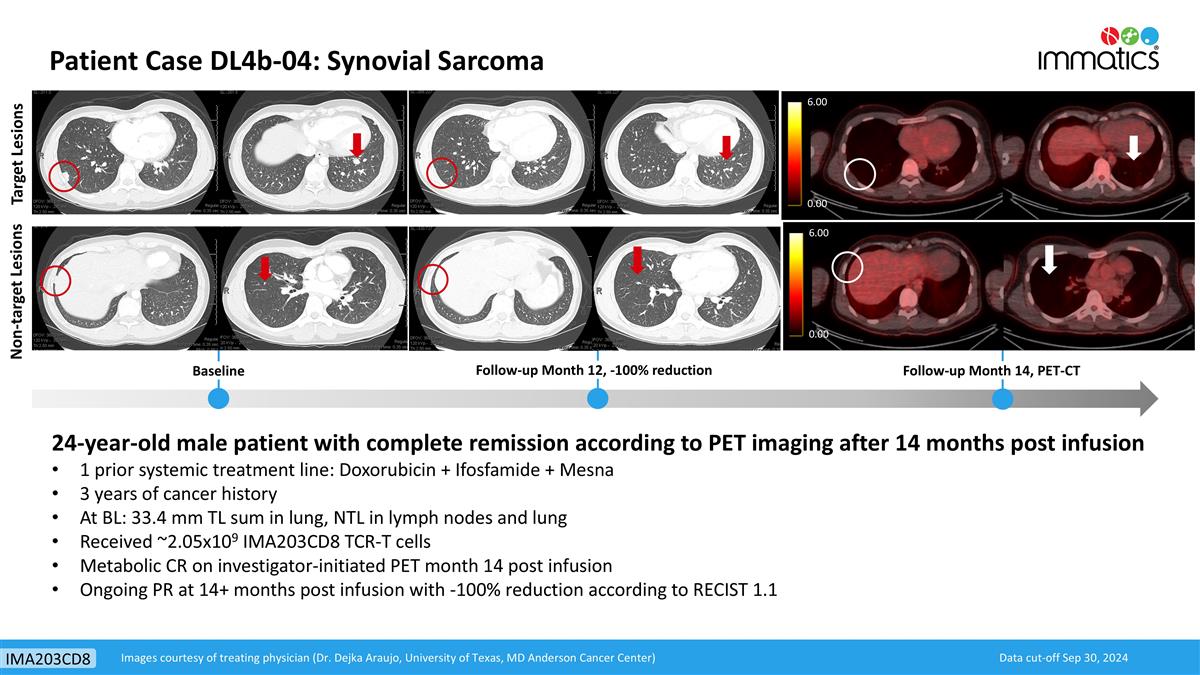
Non-target Lesions Target Lesions Baseline Follow-up Month 12, -100% reduction 24-year-old male patient with complete remission according to PET imaging after 14 months post infusion 1 prior systemic treatment line: Doxorubicin + Ifosfamide + Mesna 3 years of cancer history At BL: 33.4 mm TL sum in lung, NTL in lymph nodes and lung Received ~2.05x109 IMA203CD8 TCR-T cells Metabolic CR on investigator-initiated PET month 14 post infusion Ongoing PR at 14+ months post infusion with -100% reduction according to RECIST 1.1 Follow-up Month 14, PET-CT 6.00 0.00 6.00 0.00 Data cut-off Sep 30, 2024 Images courtesy of treating physician (Dr. Dejka Araujo, University of Texas, MD Anderson Cancer Center) Patient Case DL4b-04: Synovial Sarcoma IMA203CD8
ACTengine® IMA203CD8 (GEN2) TCR-T Monotherapy Targeting PRAME Summary of IMA203CD8 Clinical Data and Planned Next Steps Data cut-off Sep 30, 2024 IMA203CD8 Manageable tolerability with most frequent ≥Grade 3 AEs being expected cytopenia DLTs in 2 patients at DL4b triggered dosing adjustment to DL4a Manageable tolerability in patients at DL4a combined with modifications of the eligibility criteria and IL-2 scheme allows further exploration of higher doses Deep and durable objective responses already observed at low doses (median: 1.48 x109 T cells) 41% (14/34) cORR and tumor shrinkage in 84% (32/38) of patients including two patients with complete response of target lesions 9.2 months median DOR with 3 confirmed responses ongoing at 1+ year Opportunity of IMA203CD8 in medium-level PRAME expressing indications Association of PRAME expression with clinical activity in IMA203 and IMA203CD8 treated patients Deep responses with IMA203CD8, even though applied dose still lower than IMA203 Dose escalation with and without post-infusion low-dose IL-2 is ongoing to investigate the full clinical potential of IMA203CD8 in hard-to-treat solid tumors such as ovarian cancer, endometrial cancers and triple-negative breast cancer
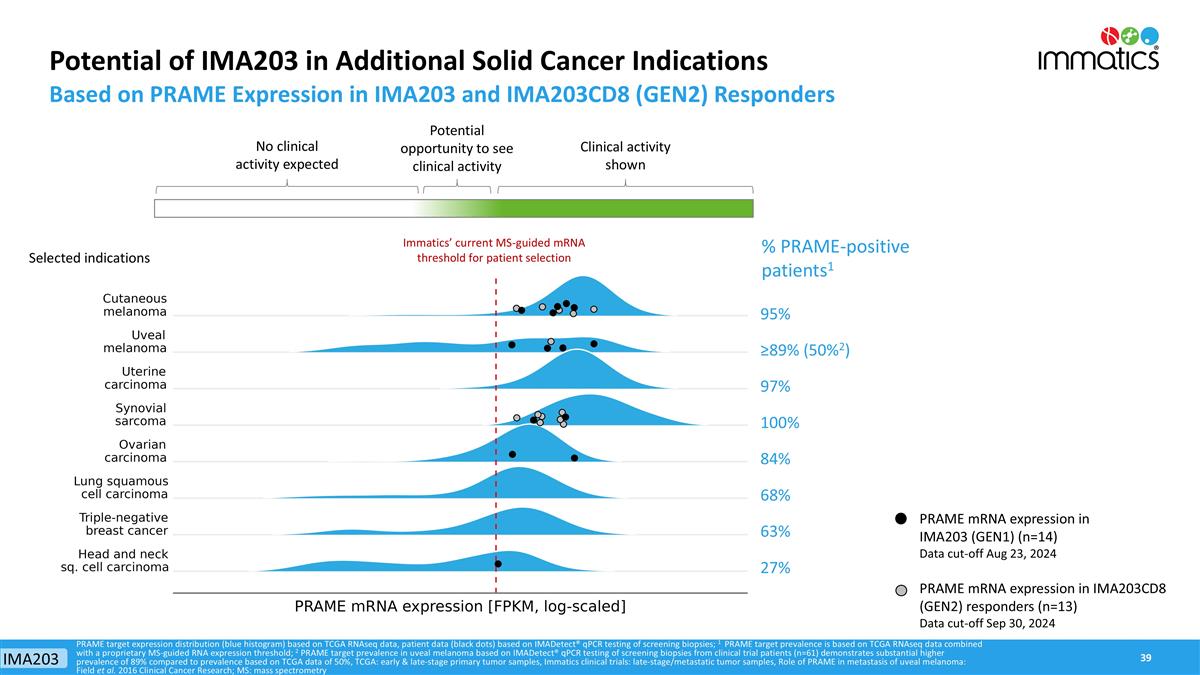
PRAME mRNA expression in IMA203 (GEN1) (n=14) Data cut-off Aug 23, 2024 PRAME mRNA expression in IMA203CD8 (GEN2) responders (n=13) Data cut-off Sep 30, 2024 Potential of IMA203 in Additional Solid Cancer Indications Based on PRAME Expression in IMA203 and IMA203CD8 (GEN2) Responders % PRAME-positive patients1 PRAME target expression distribution (blue histogram) based on TCGA RNAseq data, patient data (black dots) based on IMADetect® qPCR testing of screening biopsies; 1 PRAME target prevalence is based on TCGA RNAseq data combined with a proprietary MS-guided RNA expression threshold; 2 PRAME target prevalence in uveal melanoma based on IMADetect® qPCR testing of screening biopsies from clinical trial patients (n=61) demonstrates substantial higher prevalence of 89% compared to prevalence based on TCGA data of 50%, TCGA: early & late-stage primary tumor samples, Immatics clinical trials: late-stage/metastatic tumor samples, Role of PRAME in metastasis of uveal melanoma: Field et al. 2016 Clinical Cancer Research; MS: mass spectrometry 95% ≥89% (50%2) 97% 100% 84% 68% 63% 27% Immatics’ current MS-guided mRNA threshold for patient selection Selected indications Clinical activity shown No clinical activity expected Potential opportunity to see clinical activity IMA203

TCER® – TCR Bispecifics
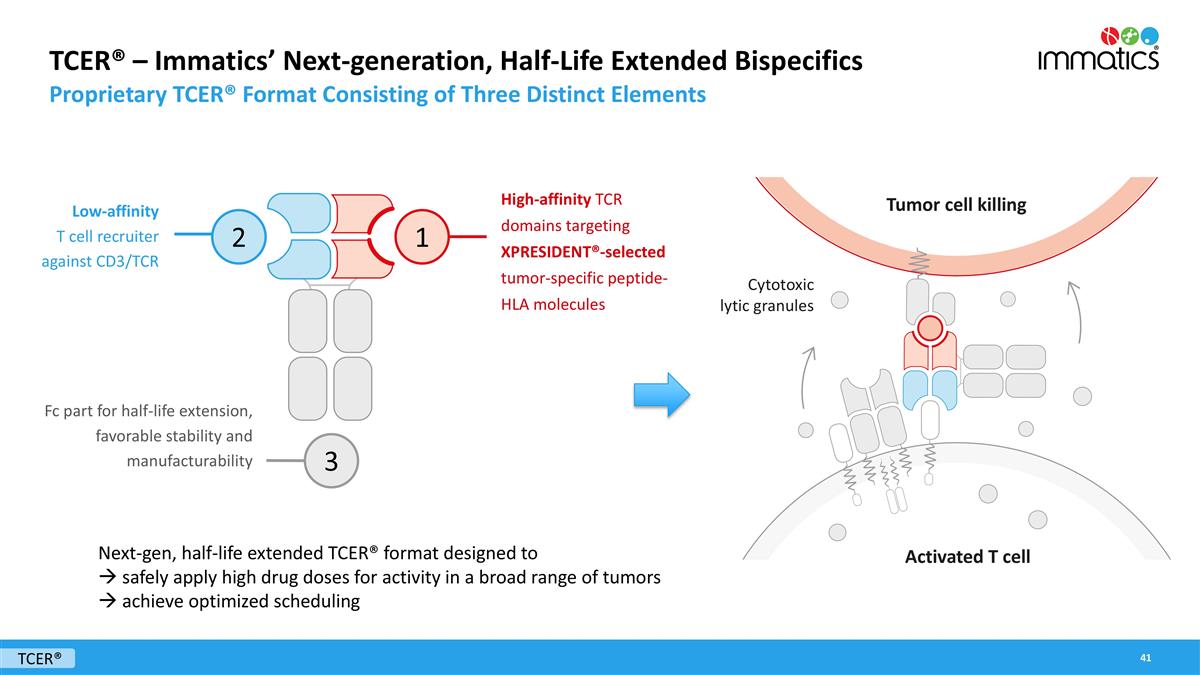
TCER® – Immatics’ Next-generation, Half-Life Extended Bispecifics Proprietary TCER® Format Consisting of Three Distinct Elements High-affinity TCR domains targeting XPRESIDENT®-selected tumor-specific peptide-HLA molecules Low-affinity T cell recruiter against CD3/TCR Fc part for half-life extension, favorable stability and manufacturability Next-gen, half-life extended TCER® format designed to à safely apply high drug doses for activity in a broad range of tumors à achieve optimized scheduling 2 1 3 Cytotoxic lytic granules Tumor cell killing Activated T cell TCER®
TCER® – Immatics’ Next-generation, Half-Life Extended Bispecifics pHLA targeting TCR High-affinity (single digit nM) TCR targeting XPRESIDENT®-selected tumor-specific peptide-HLA molecules Broad therapeutic window through XPRESIDENT®-guided affinity maturation (>1000x)1 Complete tumor eradication in mouse xenograft models at low doses T cell recruiting antibody Low-affinity (triple digit nM) T cell recruiter against both TCR & CD3 Optimized biodistribution aiming for enrichment at tumor site and prevention of CRS2 Superior anti-tumor activity in mouse models as compared to widely used CD3 recruiters Next-generation TCER® format Off-the-shelf biologic with antibody-like manufacturability3 and low cost of goods Superior anti-tumor activity4 compared to six alternative bispecific formats Half-life of several days expected in humans Our TCER® format is designed to maximize efficacy while minimizing toxicities in patients 1 As compared to natural TCR; 2 Based on literature data for other low-affinity recruiters (e.g. Harber et al., 2021, Nature; Trinklein et al., 2019, mAbs); 3 Production in mammalian cells (CHO cells); 4 Based on preclinical testing TCER® 1 2 3
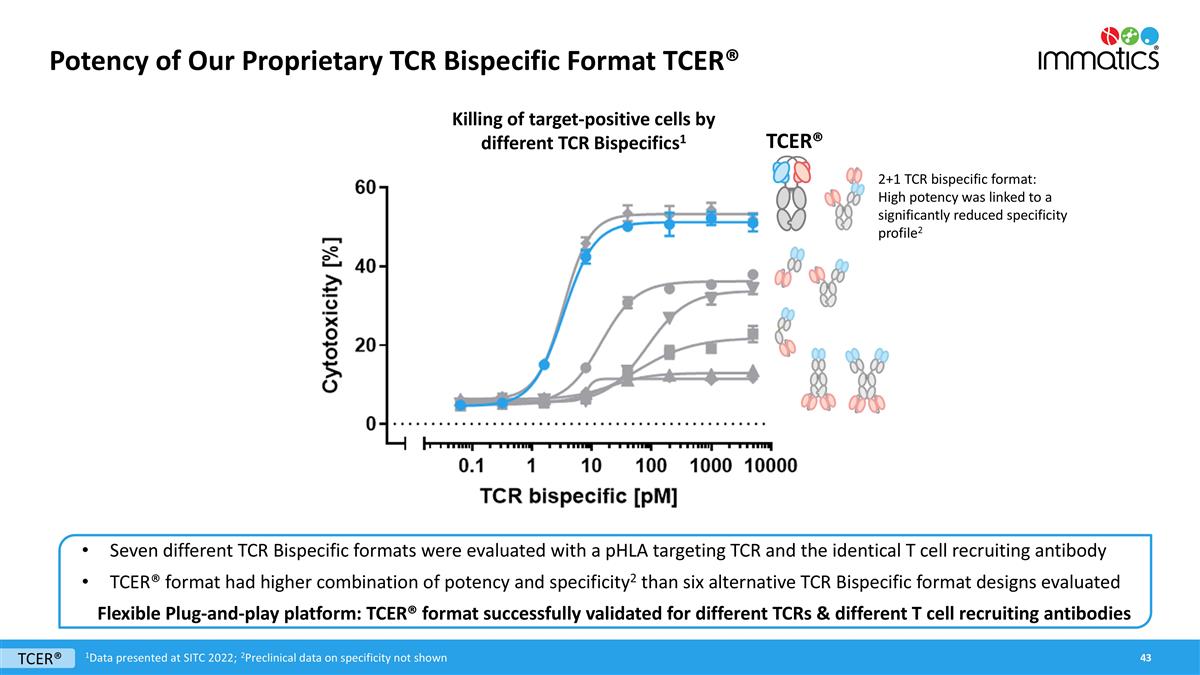
Potency of Our Proprietary TCR Bispecific Format TCER® Seven different TCR Bispecific formats were evaluated with a pHLA targeting TCR and the identical T cell recruiting antibody TCER® format had higher combination of potency and specificity2 than six alternative TCR Bispecific format designs evaluated Flexible Plug-and-play platform: TCER® format successfully validated for different TCRs & different T cell recruiting antibodies TCER® 2+1 TCR bispecific format: High potency was linked to a significantly reduced specificity profile2 Killing of target-positive cells by different TCR Bispecifics1 1Data presented at SITC 2022; 2Preclinical data on specificity not shown TCER®
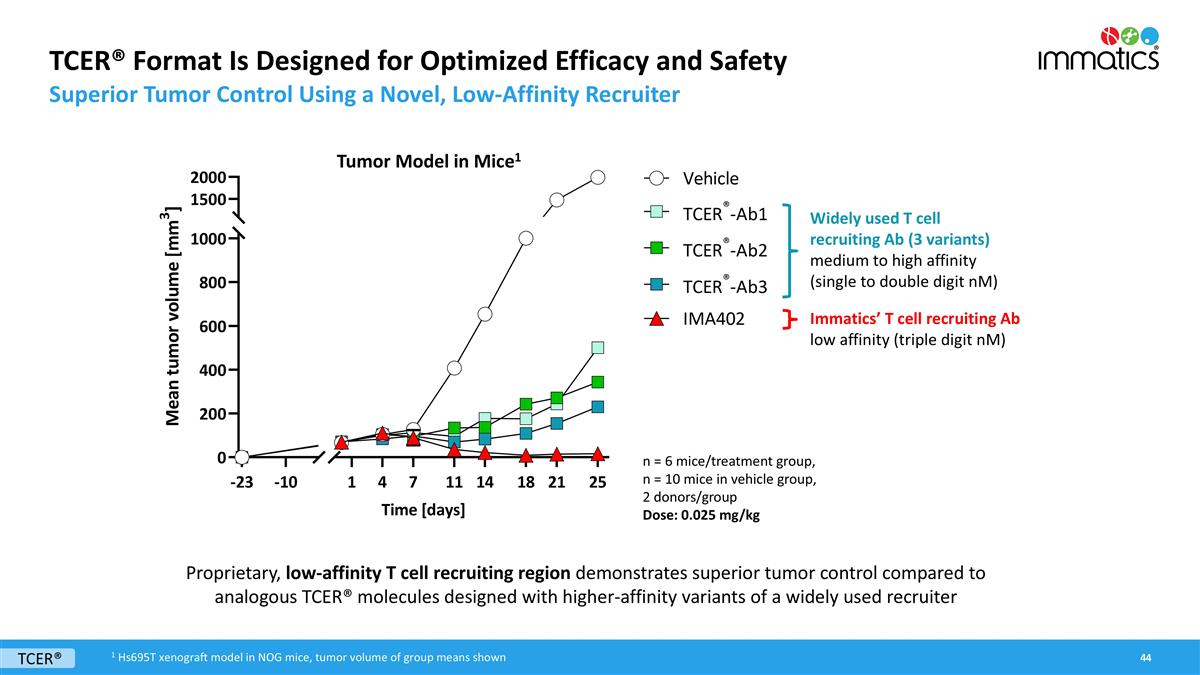
TCER® Format Is Designed for Optimized Efficacy and Safety Superior Tumor Control Using a Novel, Low-Affinity Recruiter Widely used T cell recruiting Ab (3 variants) medium to high affinity (single to double digit nM) n = 6 mice/treatment group, n = 10 mice in vehicle group, 2 donors/group Dose: 0.025 mg/kg Proprietary, low-affinity T cell recruiting region demonstrates superior tumor control compared to analogous TCER® molecules designed with higher-affinity variants of a widely used recruiter Immatics’ T cell recruiting Ab low affinity (triple digit nM) TCER® Tumor Model in Mice1 1 Hs695T xenograft model in NOG mice, tumor volume of group means shown
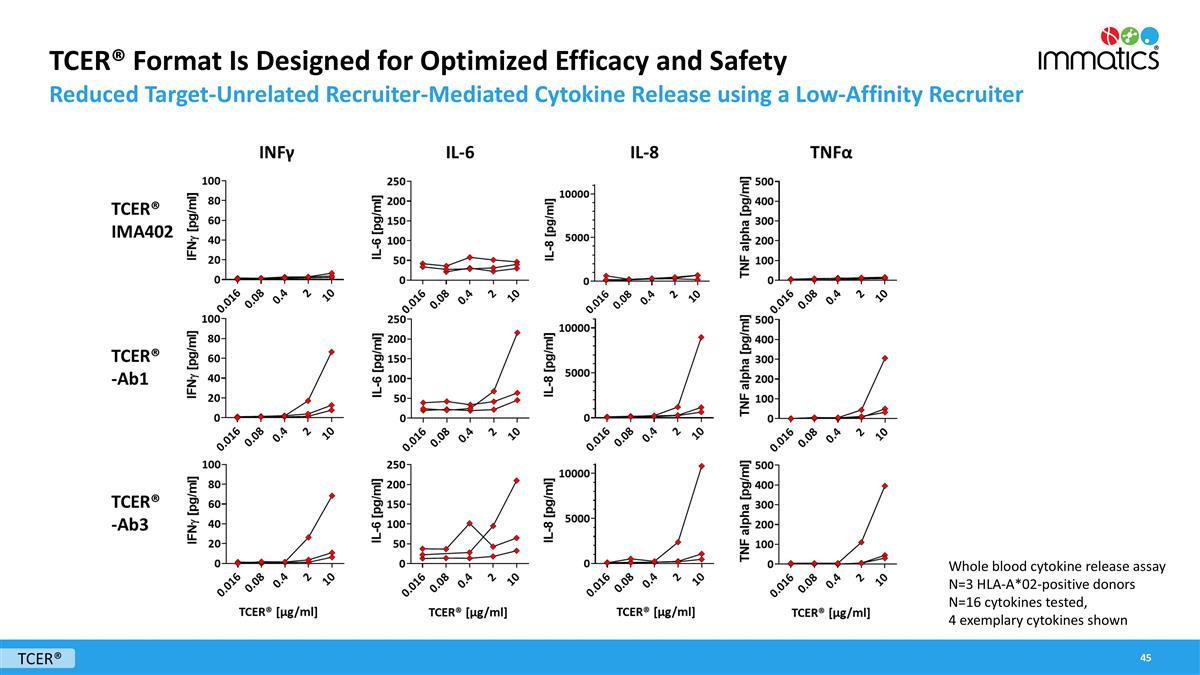
TCER® Format Is Designed for Optimized Efficacy and Safety Reduced Target-Unrelated Recruiter-Mediated Cytokine Release using a Low-Affinity Recruiter TCER® Whole blood cytokine release assay N=3 HLA-A*02-positive donors N=16 cytokines tested, 4 exemplary cytokines shown
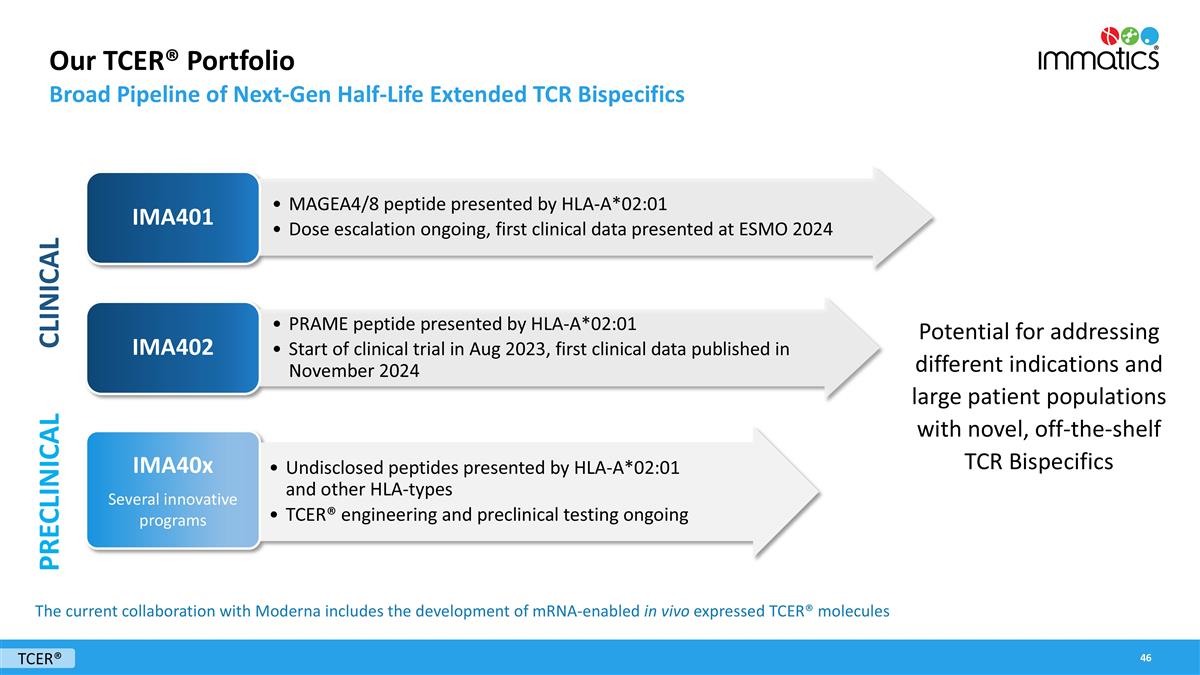
Our TCER® Portfolio Broad Pipeline of Next-Gen Half-Life Extended TCR Bispecifics TCER® PRAME peptide presented by HLA-A*02:01 Start of clinical trial in Aug 2023, first clinical data published in November 2024 IMA402 Potential for addressing different indications and large patient populations with novel, off-the-shelf TCR Bispecifics MAGEA4/8 peptide presented by HLA-A*02:01 Dose escalation ongoing, first clinical data presented at ESMO 2024 IMA401 Undisclosed peptides presented by HLA-A*02:01 and other HLA-types TCER® engineering and preclinical testing ongoing IMA40x Several innovative programs CLINICAL PRECLINICAL The current collaboration with Moderna includes the development of mRNA-enabled in vivo expressed TCER® molecules
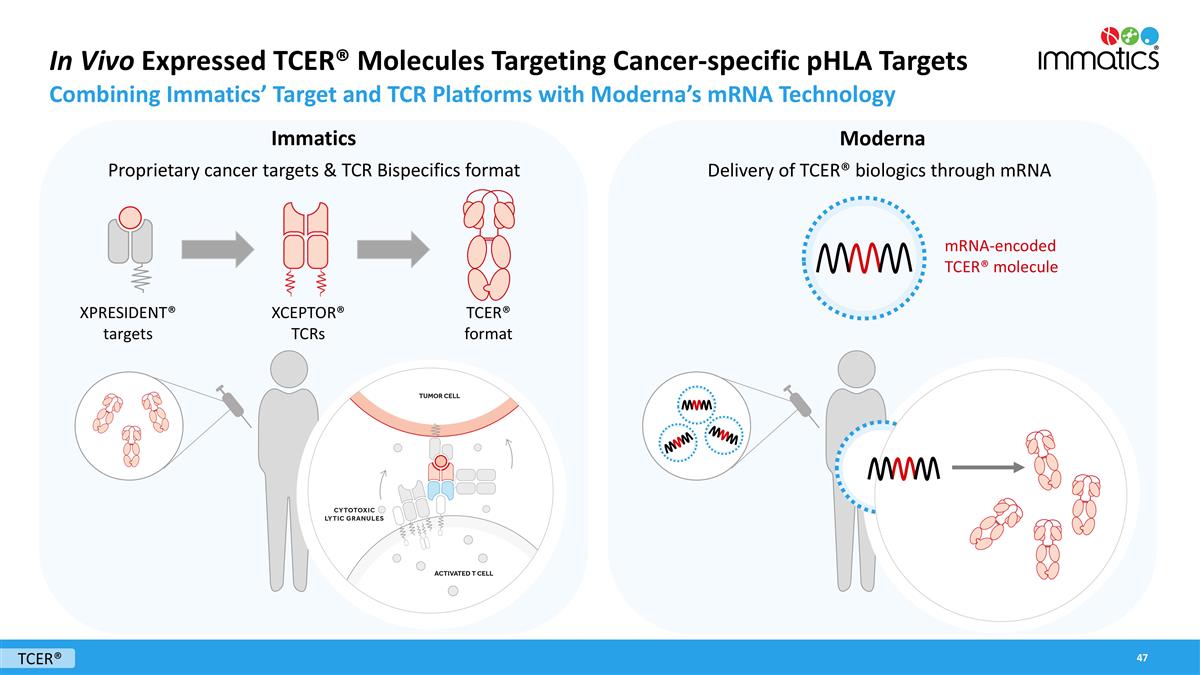
In Vivo Expressed TCER® Molecules Targeting Cancer-specific pHLA Targets Combining Immatics’ Target and TCR Platforms with Moderna’s mRNA Technology Immatics Moderna Delivery of TCER® biologics through mRNA Proprietary cancer targets & TCR Bispecifics format mRNA-encoded TCER® molecule XPRESIDENT® targets XCEPTOR® TCRs TCER® format TCER®

TCER® IMA401 Targeting MAGEA4/8
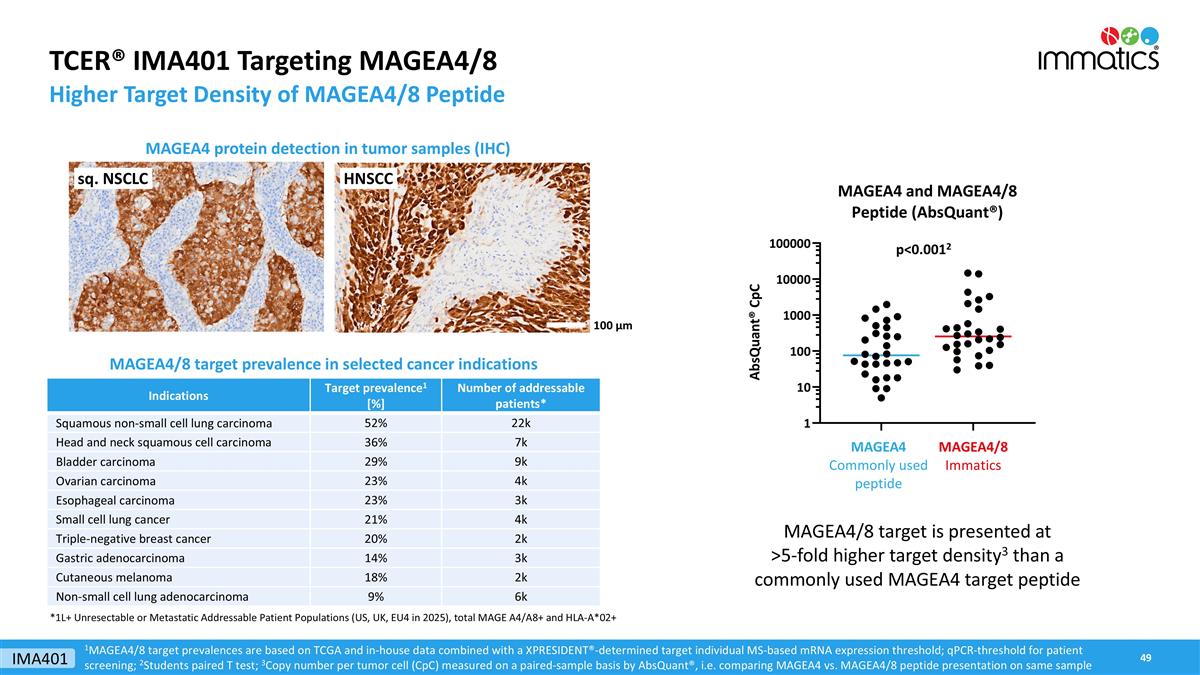
TCER® IMA401 Targeting MAGEA4/8 Higher Target Density of MAGEA4/8 Peptide MAGEA4 protein detection in tumor samples (IHC) 1MAGEA4/8 target prevalences are based on TCGA and in-house data combined with a XPRESIDENT®-determined target individual MS-based mRNA expression threshold; qPCR-threshold for patient screening; 2Students paired T test; 3Copy number per tumor cell (CpC) measured on a paired-sample basis by AbsQuant®, i.e. comparing MAGEA4 vs. MAGEA4/8 peptide presentation on same sample p<0.0012 MAGEA4/8 target is presented at >5-fold higher target density3 than a commonly used MAGEA4 target peptide HNSCC sq. NSCLC 100 µm MAGEA4/8 target prevalence in selected cancer indications Indications Target prevalence1 [%] Number of addressable patients* Squamous non-small cell lung carcinoma 52% 22k Head and neck squamous cell carcinoma 36% 7k Bladder carcinoma 29% 9k Ovarian carcinoma 23% 4k Esophageal carcinoma 23% 3k Small cell lung cancer 21% 4k Triple-negative breast cancer 20% 2k Gastric adenocarcinoma 14% 3k Cutaneous melanoma 18% 2k Non-small cell lung adenocarcinoma 9% 6k *1L+ Unresectable or Metastatic Addressable Patient Populations (US, UK, EU4 in 2025), total MAGE A4/A8+ and HLA-A*02+ IMA401
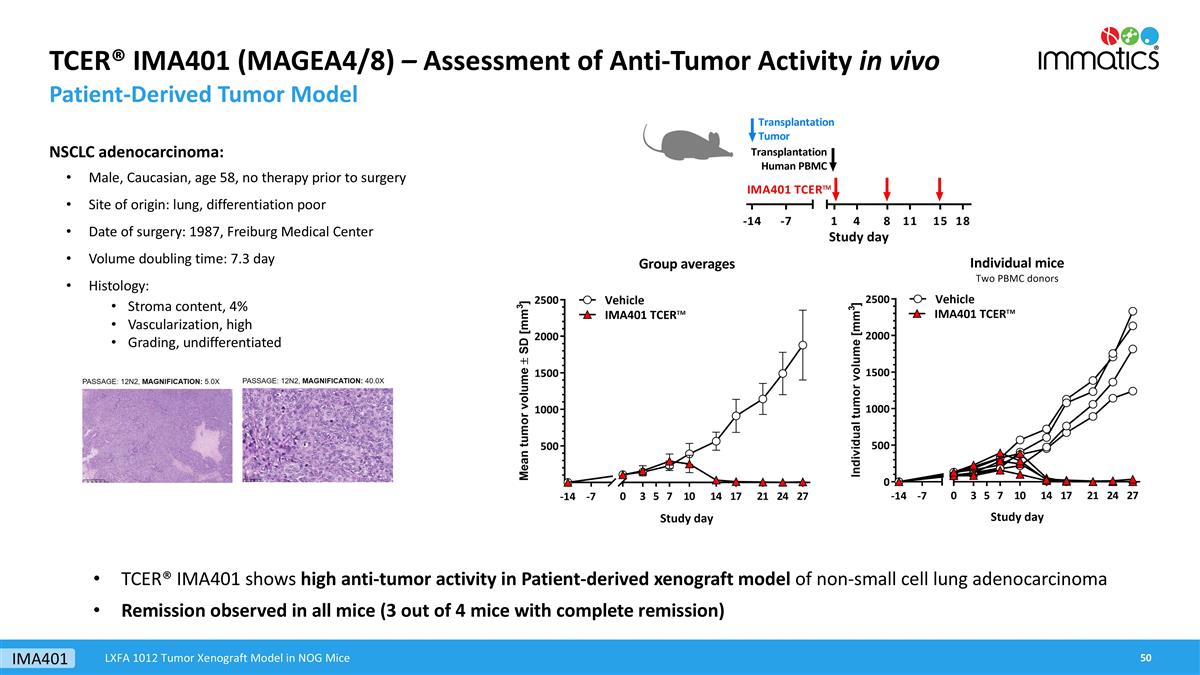
TCER® IMA401 (MAGEA4/8) – Assessment of Anti-Tumor Activity in vivo Patient-Derived Tumor Model NSCLC adenocarcinoma: Male, Caucasian, age 58, no therapy prior to surgery Site of origin: lung, differentiation poor Date of surgery: 1987, Freiburg Medical Center Volume doubling time: 7.3 day Histology: Stroma content, 4% Vascularization, high Grading, undifferentiated TCER® IMA401 shows high anti-tumor activity in Patient-derived xenograft model of non-small cell lung adenocarcinoma Remission observed in all mice (3 out of 4 mice with complete remission) LXFA 1012 Tumor Xenograft Model in NOG Mice IMA401
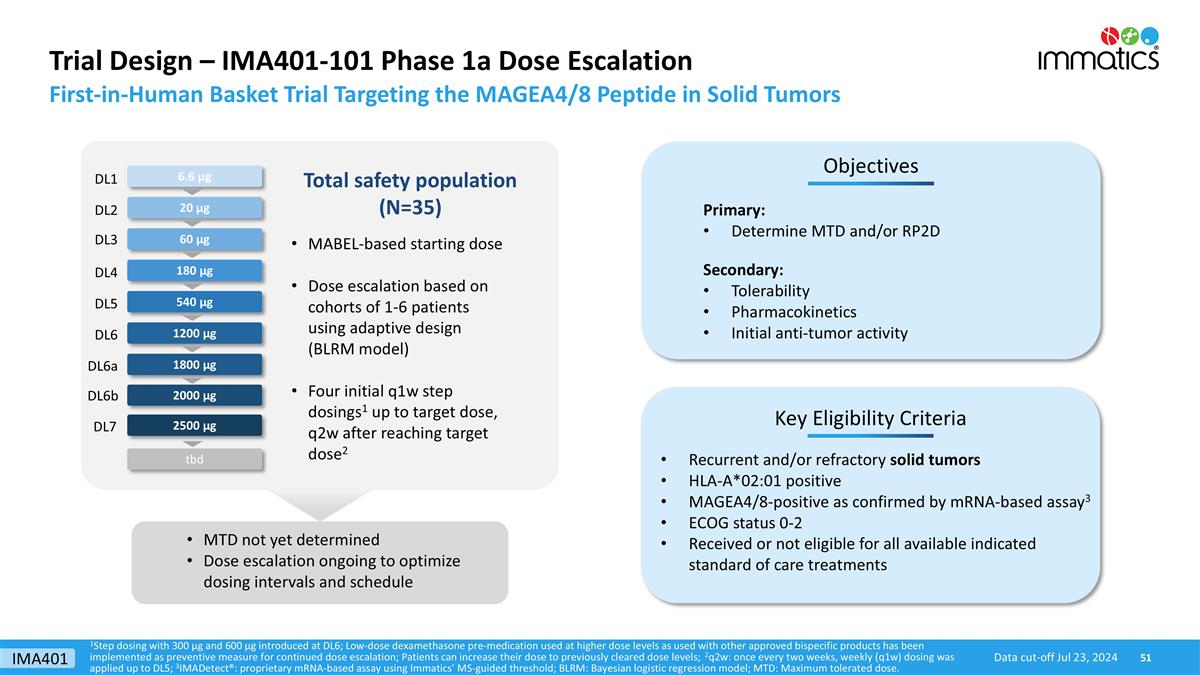
Trial Design – IMA401-101 Phase 1a Dose Escalation 180 µg 540 µg 1800 µg 2500 µg Key Eligibility Criteria Objectives Primary: Determine MTD and/or RP2D Secondary: Tolerability Pharmacokinetics Initial anti-tumor activity Recurrent and/or refractory solid tumors HLA-A*02:01 positive MAGEA4/8-positive as confirmed by mRNA-based assay3 ECOG status 0-2 Received or not eligible for all available indicated standard of care treatments 60 µg 1200 µg Total safety population (N=35) 20 µg 6.6 µg MTD not yet determined Dose escalation ongoing to optimize dosing intervals and schedule MABEL-based starting dose Dose escalation based on cohorts of 1-6 patients using adaptive design (BLRM model) Four initial q1w step dosings1 up to target dose, q2w after reaching target dose2 First-in-Human Basket Trial Targeting the MAGEA4/8 Peptide in Solid Tumors 1Step dosing with 300 µg and 600 µg introduced at DL6; Low-dose dexamethasone pre-medication used at higher dose levels as used with other approved bispecific products has been implemented as preventive measure for continued dose escalation; Patients can increase their dose to previously cleared dose levels; 2q2w: once every two weeks, weekly (q1w) dosing was applied up to DL5; 3IMADetect®: proprietary mRNA-based assay using Immatics’ MS-guided threshold; BLRM: Bayesian logistic regression model; MTD: Maximum tolerated dose. DL1 DL2 DL3 DL4 DL5 DL6a DL7 DL6 tbd 2000 µg DL6b Data cut-off Jul 23, 2024 IMA401
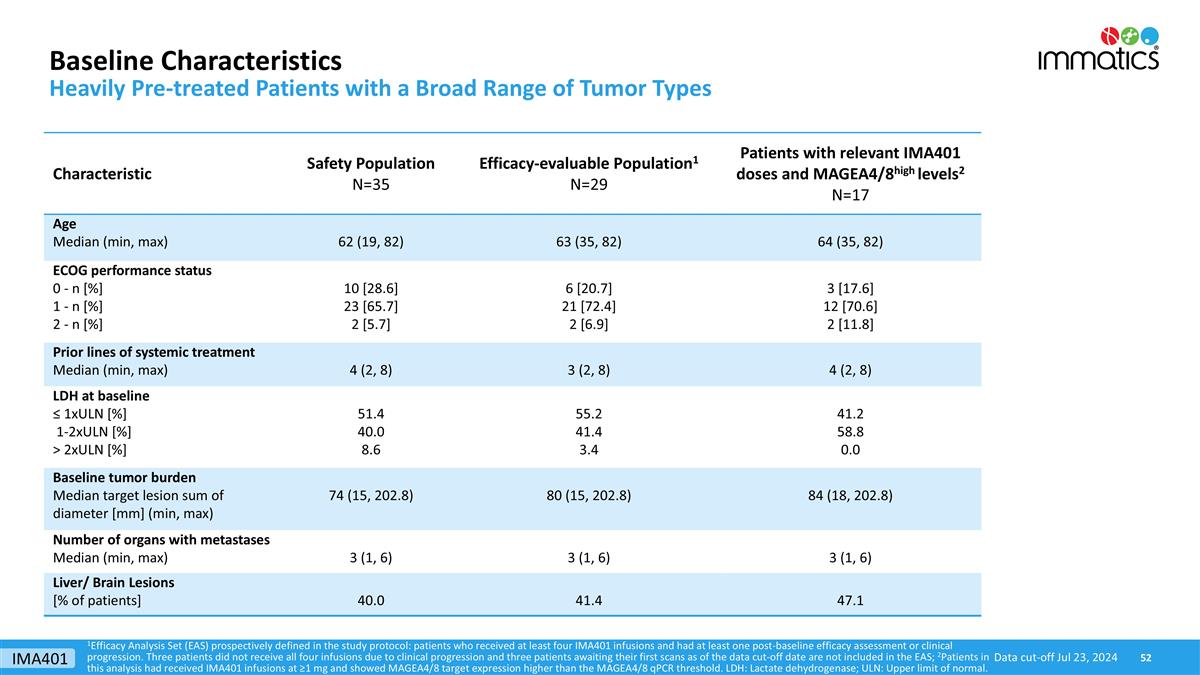
Baseline Characteristics Heavily Pre-treated Patients with a Broad Range of Tumor Types 1Efficacy Analysis Set (EAS) prospectively defined in the study protocol: patients who received at least four IMA401 infusions and had at least one post-baseline efficacy assessment or clinical progression. Three patients did not receive all four infusions due to clinical progression and three patients awaiting their first scans as of the data cut-off date are not included in the EAS; 2Patients in this analysis had received IMA401 infusions at ≥1 mg and showed MAGEA4/8 target expression higher than the MAGEA4/8 qPCR threshold. LDH: Lactate dehydrogenase; ULN: Upper limit of normal. Data cut-off Jul 23, 2024 Characteristic Safety Population N=35 Efficacy-evaluable Population1 N=29 Patients with relevant IMA401 doses and MAGEA4/8high levels2 N=17 Age Median (min, max) 62 (19, 82) 63 (35, 82) 64 (35, 82) ECOG performance status 0 - n [%] 1 - n [%] 2 - n [%] 10 [28.6] 23 [65.7] 2 [5.7] 6 [20.7] 21 [72.4] 2 [6.9] 3 [17.6] 12 [70.6] 2 [11.8] Prior lines of systemic treatment Median (min, max) 4 (2, 8) 3 (2, 8) 4 (2, 8) LDH at baseline ≤ 1xULN [%] 1-2xULN [%] > 2xULN [%] 51.4 40.0 8.6 55.2 41.4 3.4 41.2 58.8 0.0 Baseline tumor burden Median target lesion sum of diameter [mm] (min, max) 74 (15, 202.8) 80 (15, 202.8) 84 (18, 202.8) Number of organs with metastases Median (min, max) 3 (1, 6) 3 (1, 6) 3 (1, 6) Liver/ Brain Lesions [% of patients] 40.0 41.4 47.1 IMA401
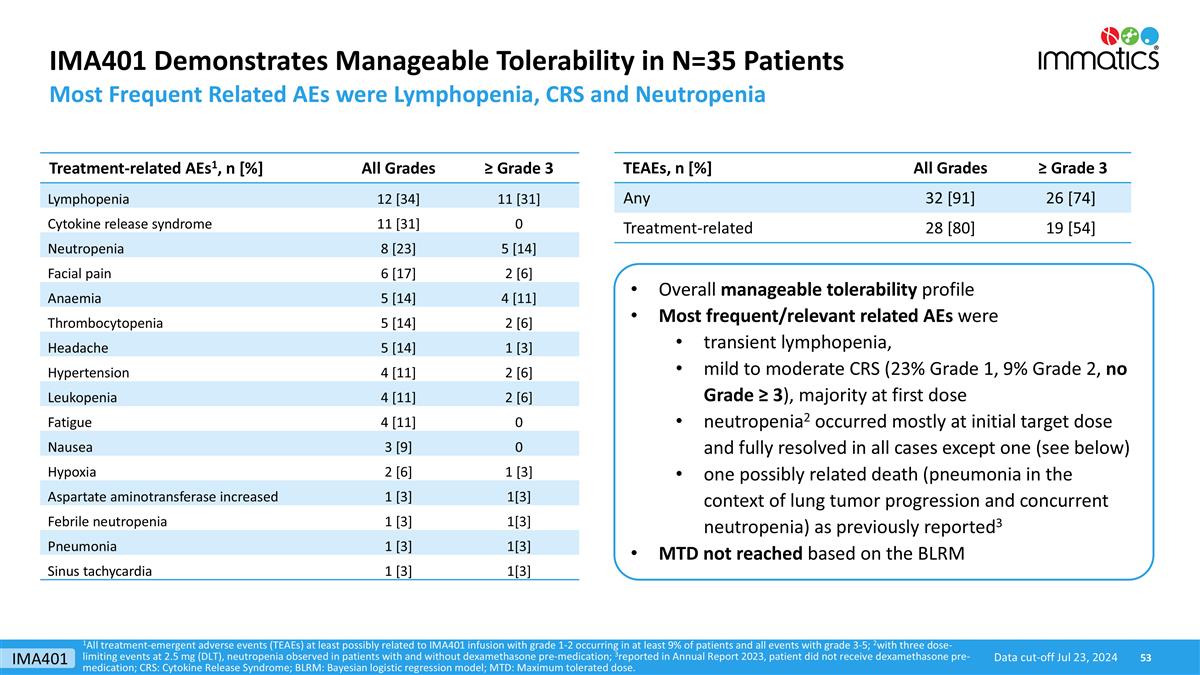
IMA401 Demonstrates Manageable Tolerability in N=35 Patients Most Frequent Related AEs were Lymphopenia, CRS and Neutropenia TEAEs, n [%] All Grades ≥ Grade 3 Any 32 [91] 26 [74] Treatment-related 28 [80] 19 [54] Treatment-related AEs1, n [%] All Grades ≥ Grade 3 Lymphopenia 12 [34] 11 [31] Cytokine release syndrome 11 [31] 0 Neutropenia 8 [23] 5 [14] Facial pain 6 [17] 2 [6] Anaemia 5 [14] 4 [11] Thrombocytopenia 5 [14] 2 [6] Headache 5 [14] 1 [3] Hypertension 4 [11] 2 [6] Leukopenia 4 [11] 2 [6] Fatigue 4 [11] 0 Nausea 3 [9] 0 Hypoxia 2 [6] 1 [3] Aspartate aminotransferase increased 1 [3] 1[3] Febrile neutropenia 1 [3] 1[3] Pneumonia 1 [3] 1[3] Sinus tachycardia 1 [3] 1[3] Overall manageable tolerability profile Most frequent/relevant related AEs were transient lymphopenia, mild to moderate CRS (23% Grade 1, 9% Grade 2, no Grade ≥ 3), majority at first dose neutropenia2 occurred mostly at initial target dose and fully resolved in all cases except one (see below) one possibly related death (pneumonia in the context of lung tumor progression and concurrent neutropenia) as previously reported3 MTD not reached based on the BLRM 1All treatment-emergent adverse events (TEAEs) at least possibly related to IMA401 infusion with grade 1-2 occurring in at least 9% of patients and all events with grade 3-5; 2with three dose-limiting events at 2.5 mg (DLT), neutropenia observed in patients with and without dexamethasone pre-medication; 3reported in Annual Report 2023, patient did not receive dexamethasone pre-medication; CRS: Cytokine Release Syndrome; BLRM: Bayesian logistic regression model; MTD: Maximum tolerated dose. Data cut-off Jul 23, 2024 IMA401
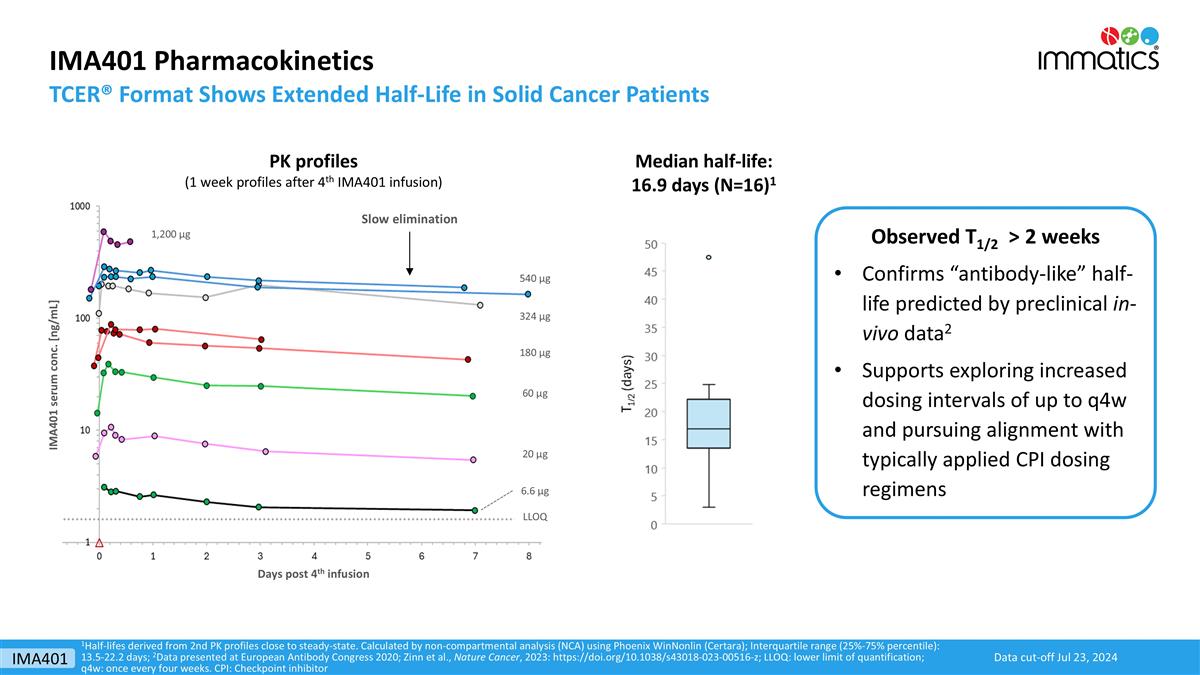
IMA401 Pharmacokinetics TCER® Format Shows Extended Half-Life in Solid Cancer Patients 1Half-lifes derived from 2nd PK profiles close to steady-state. Calculated by non-compartmental analysis (NCA) using Phoenix WinNonlin (Certara); Interquartile range (25%-75% percentile): 13.5-22.2 days; 2Data presented at European Antibody Congress 2020; Zinn et al., Nature Cancer, 2023: https://doi.org/10.1038/s43018-023-00516-z; LLOQ: lower limit of quantification; q4w: once every four weeks. CPI: Checkpoint inhibitor Median half-life: 16.9 days (N=16)1 Slow elimination Days post 4th infusion IMA401 serum conc. [ng/mL] 6.6 µg 20 µg 60 µg 180 µg LLOQ 324 µg 540 µg 1,200 µg PK profiles (1 week profiles after 4th IMA401 infusion) Observed T1/2 > 2 weeks Confirms “antibody-like” half-life predicted by preclinical in-vivo data2 Supports exploring increased dosing intervals of up to q4w and pursuing alignment with typically applied CPI dosing regimens Data cut-off Jul 23, 2024 IMA401
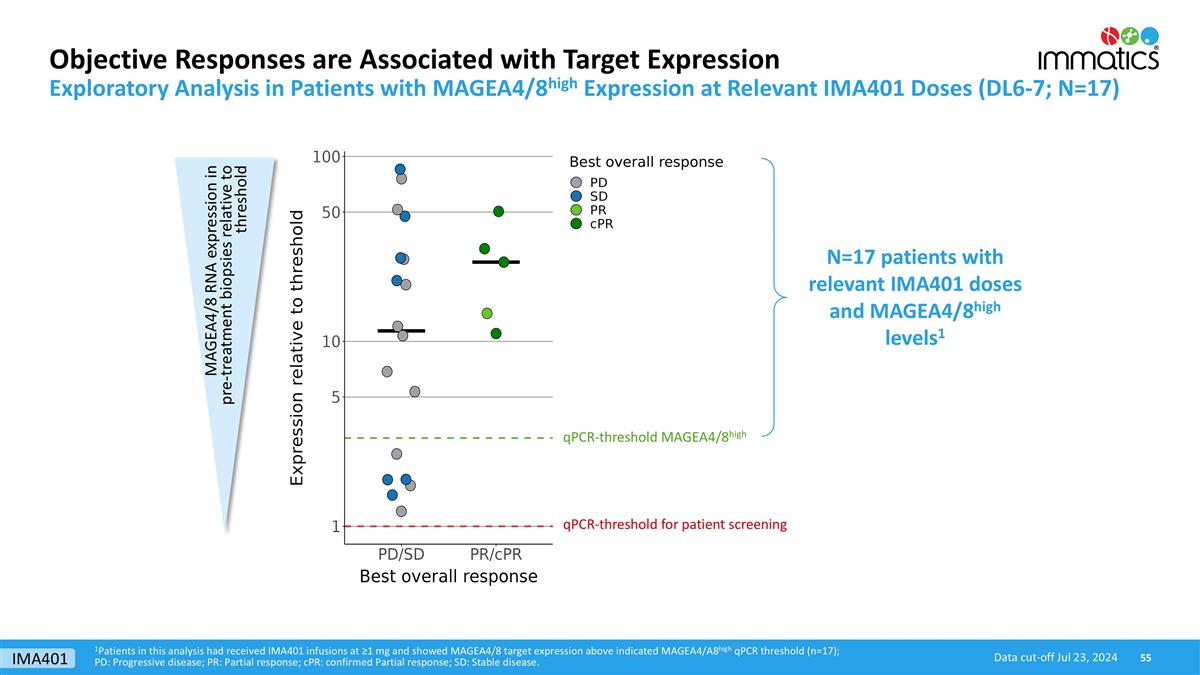
1Patients in this analysis had received IMA401 infusions at ≥1 mg and showed MAGEA4/8 target expression above indicated MAGEA4/A8high qPCR threshold (n=17); PD: Progressive disease; PR: Partial response; cPR: confirmed Partial response; SD: Stable disease. Objective Responses are Associated with Target Expression Data cut-off Jul 23, 2024 Exploratory Analysis in Patients with MAGEA4/8high Expression at Relevant IMA401 Doses (DL6-7; N=17) qPCR-threshold MAGEA4/8high qPCR-threshold for patient screening MAGEA4/8 RNA expression in pre-treatment biopsies relative to threshold N=17 patients with relevant IMA401 doses and MAGEA4/8high levels1 IMA401
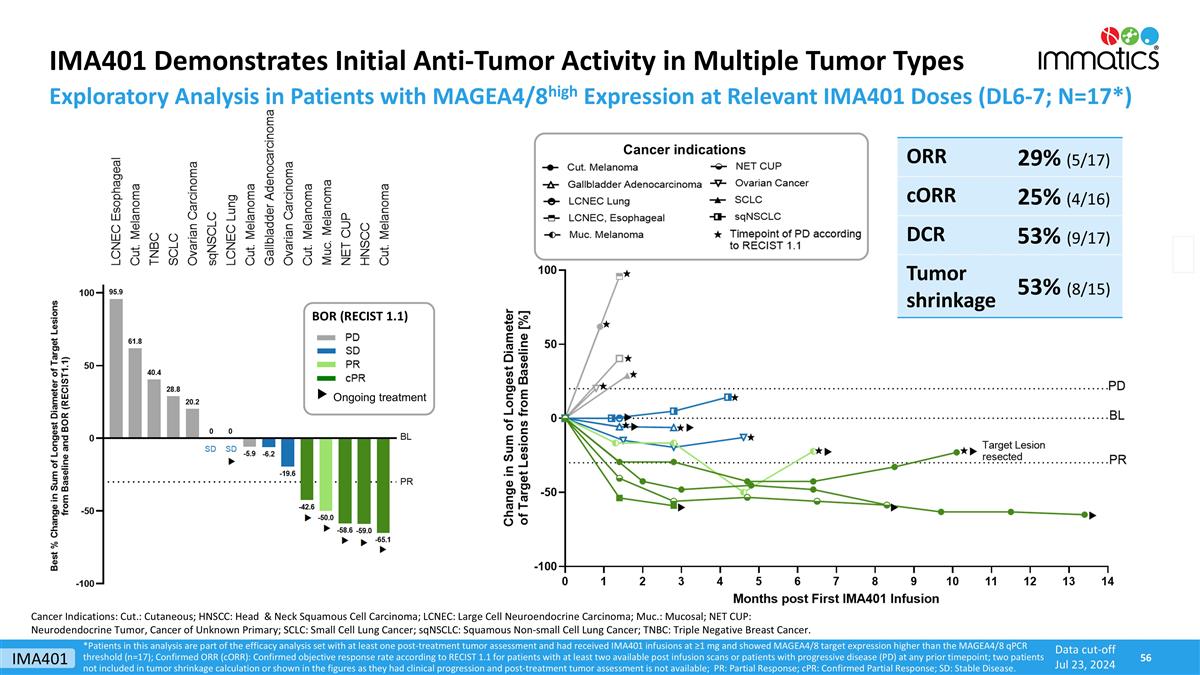
IMA401 Demonstrates Initial Anti-Tumor Activity in Multiple Tumor Types *Patients in this analysis are part of the efficacy analysis set with at least one post-treatment tumor assessment and had received IMA401 infusions at ≥1 mg and showed MAGEA4/8 target expression higher than the MAGEA4/8 qPCR threshold (n=17); Confirmed ORR (cORR): Confirmed objective response rate according to RECIST 1.1 for patients with at least two available post infusion scans or patients with progressive disease (PD) at any prior timepoint; two patients not included in tumor shrinkage calculation or shown in the figures as they had clinical progression and post-treatment tumor assessment is not available; PR: Partial Response; cPR: Confirmed Partial Response; SD: Stable Disease. Data cut-off Jul 23, 2024 Exploratory Analysis in Patients with MAGEA4/8high Expression at Relevant IMA401 Doses (DL6-7; N=17*) ORR 29% (5/17) cORR 25% (4/16) DCR 53% (9/17) Tumor shrinkage 53% (8/15) Cancer indications Cancer Indications: Cut.: Cutaneous; HNSCC: Head & Neck Squamous Cell Carcinoma; LCNEC: Large Cell Neuroendocrine Carcinoma; Muc.: Mucosal; NET CUP: Neurodendocrine Tumor, Cancer of Unknown Primary; SCLC: Small Cell Lung Cancer; sqNSCLC: Squamous Non-small Cell Lung Cancer; TNBC: Triple Negative Breast Cancer. BOR (RECIST 1.1) Ongoing treatment IMA401
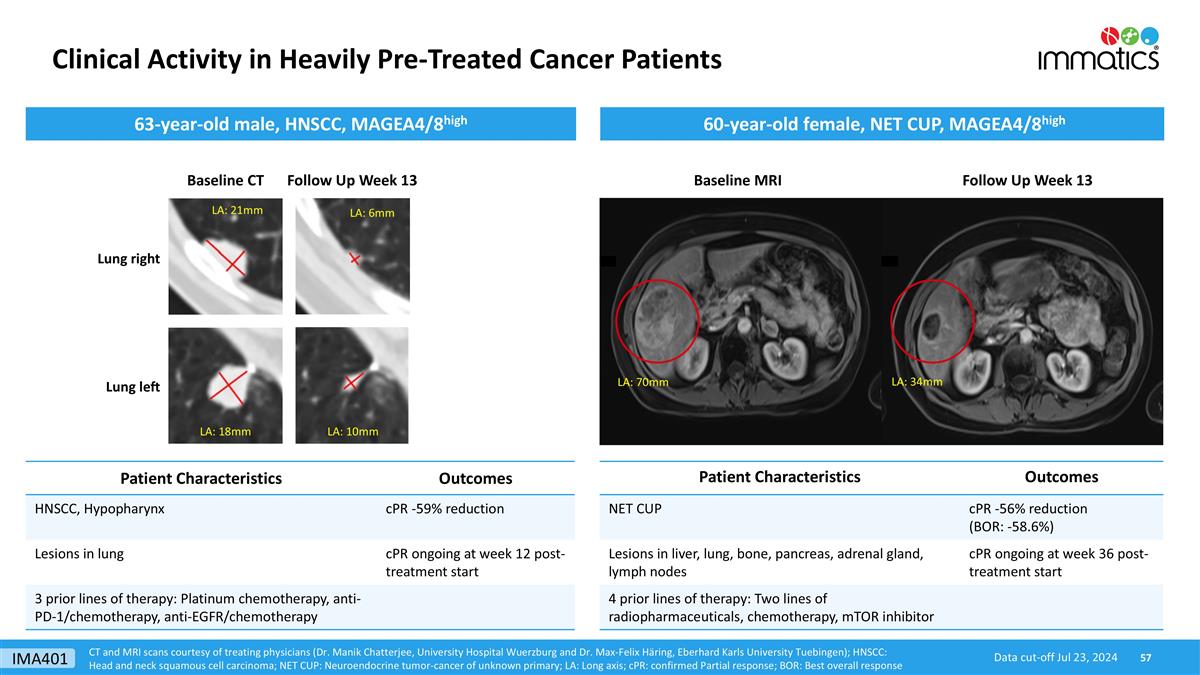
Clinical Activity in Heavily Pre-Treated Cancer Patients CT and MRI scans courtesy of treating physicians (Dr. Manik Chatterjee, University Hospital Wuerzburg and Dr. Max-Felix Häring, Eberhard Karls University Tuebingen); HNSCC: Head and neck squamous cell carcinoma; NET CUP: Neuroendocrine tumor-cancer of unknown primary; LA: Long axis; cPR: confirmed Partial response; BOR: Best overall response Baseline MRI Follow Up Week 13 Patient Characteristics Outcomes NET CUP cPR -56% reduction (BOR: -58.6%) Lesions in liver, lung, bone, pancreas, adrenal gland, lymph nodes cPR ongoing at week 36 post-treatment start 4 prior lines of therapy: Two lines of radiopharmaceuticals, chemotherapy, mTOR inhibitor 60-year-old female, NET CUP, MAGEA4/8high 63-year-old male, HNSCC, MAGEA4/8high Follow Up Week 13 Patient Characteristics Outcomes HNSCC, Hypopharynx cPR -59% reduction Lesions in lung cPR ongoing at week 12 post-treatment start 3 prior lines of therapy: Platinum chemotherapy, anti-PD-1/chemotherapy, anti-EGFR/chemotherapy LA: 18mm LA: 21mm LA: 70mm LA: 34mm Data cut-off Jul 23, 2024 Baseline CT Lung right Lung left LA: 6mm LA: 10mm IMA401
First-in-human Data of IMA401 TCER® Targeting MAGEA4/8 Presentation at ESMO on September 16, 2024 Tolerability: Most common treatment-related AEs are low-grade CRS, transient lymphopenia and neutropenia Pharmacokinetics: Median terminal half-life of 16.9 days supporting potential further flexibility in future dosing schedules incl. combination with CPI and increased dosing intervals up to q4w Initial anti-tumor activity in heavily pre-treated patients Objective responses in HNSCC, neuroendocrine tumor of unknown origin, cutaneous and mucosal melanoma including durable ongoing PRs of up to 13+ months Deep responses (tumor shrinkage of ≥ 50%) in four patients including deepening of responses over time Objective responses are associated with target expression and IMA401 dose: ORR 29%, cORR 25%, and tumor shrinkage in 53% of patients with relevant IMA401 doses and MAGEA4/8high target levels Dose escalation ongoing AE: Adverse Event; CRS: Cytokine Release Syndrome; CPI: checkpoint inhibitors; q4w: once every four weeks; HNSCC: Head and neck squamous cell carcinoma; PR: Partial Response

TCER® IMA402 Targeting PRAME
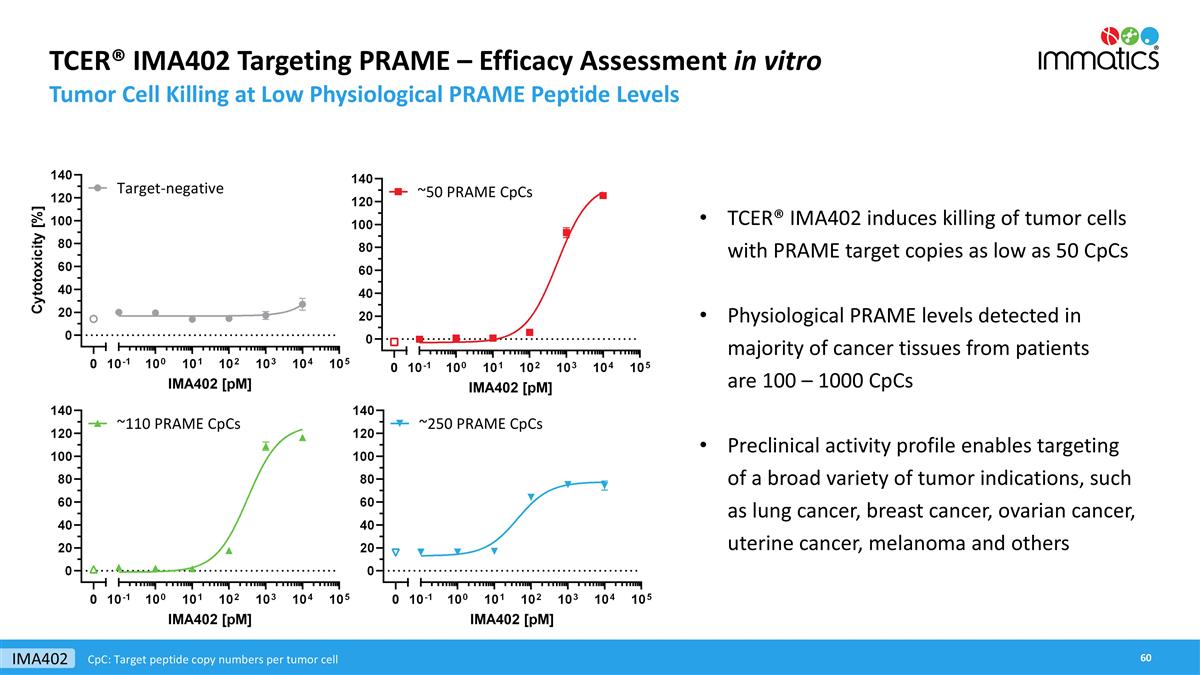
TCER® IMA402 Targeting PRAME – Efficacy Assessment in vitro Tumor Cell Killing at Low Physiological PRAME Peptide Levels TCER® IMA402 induces killing of tumor cells with PRAME target copies as low as 50 CpCs Physiological PRAME levels detected in majority of cancer tissues from patients are 100 – 1000 CpCs Preclinical activity profile enables targeting of a broad variety of tumor indications, such as lung cancer, breast cancer, ovarian cancer, uterine cancer, melanoma and others IMA402 CpC: Target peptide copy numbers per tumor cell
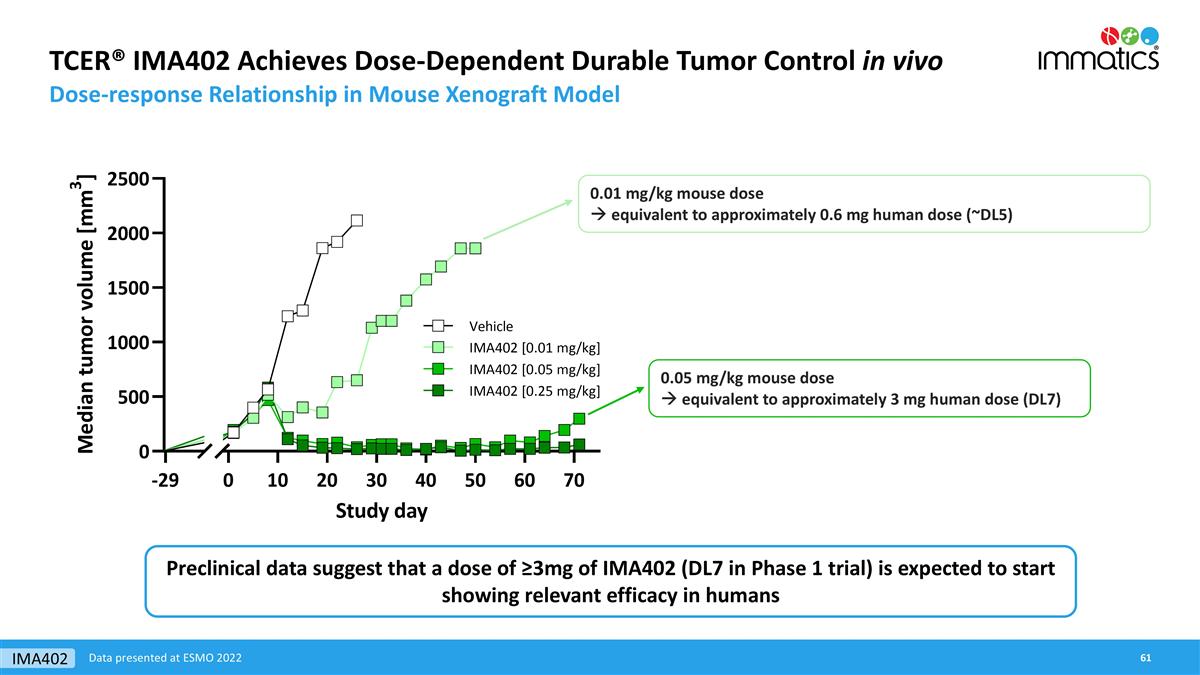
TCER® IMA402 Achieves Dose-Dependent Durable Tumor Control in vivo Dose-response Relationship in Mouse Xenograft Model 0.01 mg/kg mouse dose à equivalent to approximately 0.6 mg human dose (~DL5) 0.05 mg/kg mouse dose à equivalent to approximately 3 mg human dose (DL7) Data presented at ESMO 2022 Preclinical data suggest that a dose of ≥3mg of IMA402 (DL7 in Phase 1 trial) is expected to start showing relevant efficacy in humans IMA402
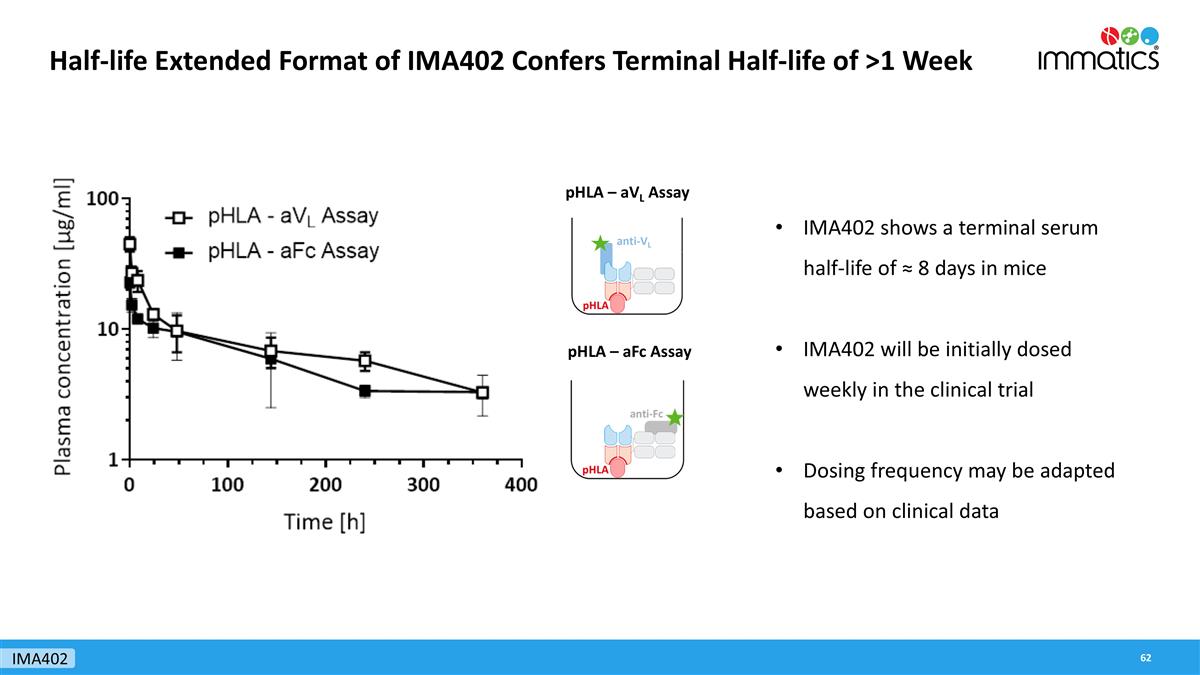
Half-life Extended Format of IMA402 Confers Terminal Half-life of >1 Week pHLA – aVL Assay pHLA – aFc Assay IMA402 shows a terminal serum half-life of ≈ 8 days in mice IMA402 will be initially dosed weekly in the clinical trial Dosing frequency may be adapted based on clinical data IMA402
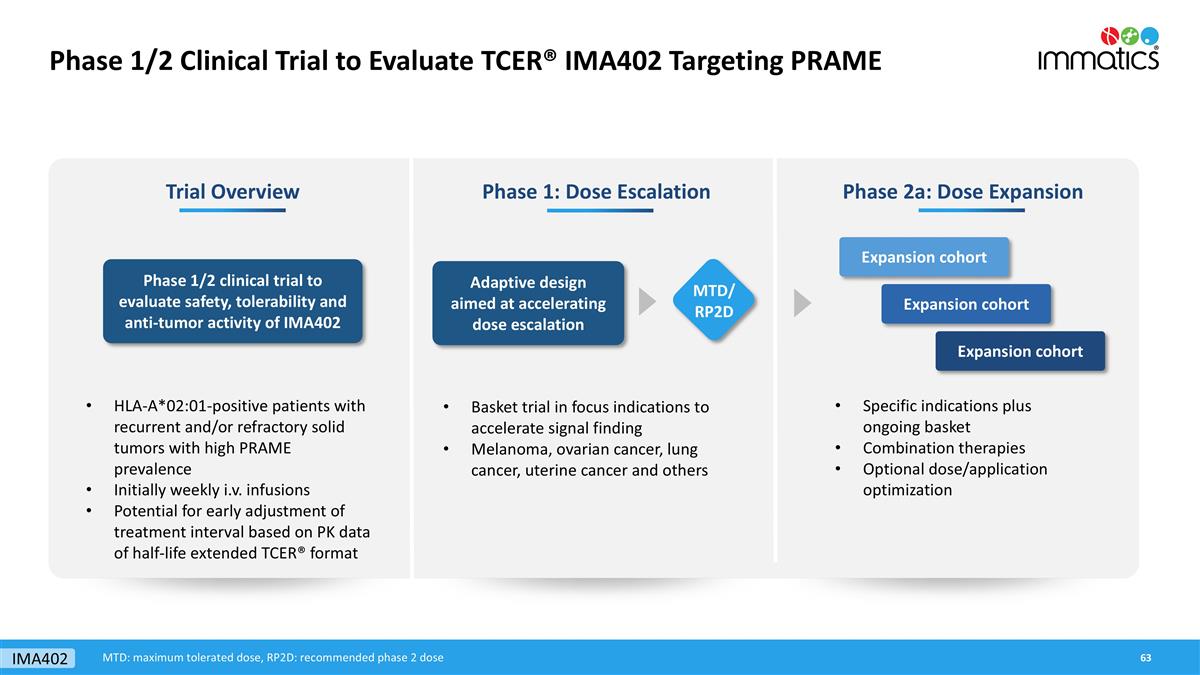
Phase 1/2 Clinical Trial to Evaluate TCER® IMA402 Targeting PRAME Phase 1: Dose Escalation Phase 2a: Dose Expansion Adaptive design aimed at accelerating dose escalation Specific indications plus ongoing basket Combination therapies Optional dose/application optimization Expansion cohort Expansion cohort Expansion cohort Trial Overview Phase 1/2 clinical trial to evaluate safety, tolerability and anti-tumor activity of IMA402 HLA-A*02:01-positive patients with recurrent and/or refractory solid tumors with high PRAME prevalence Initially weekly i.v. infusions Potential for early adjustment of treatment interval based on PK data of half-life extended TCER® format MTD/ RP2D IMA402 Basket trial in focus indications to accelerate signal finding Melanoma, ovarian cancer, lung cancer, uterine cancer and others MTD: maximum tolerated dose, RP2D: recommended phase 2 dose
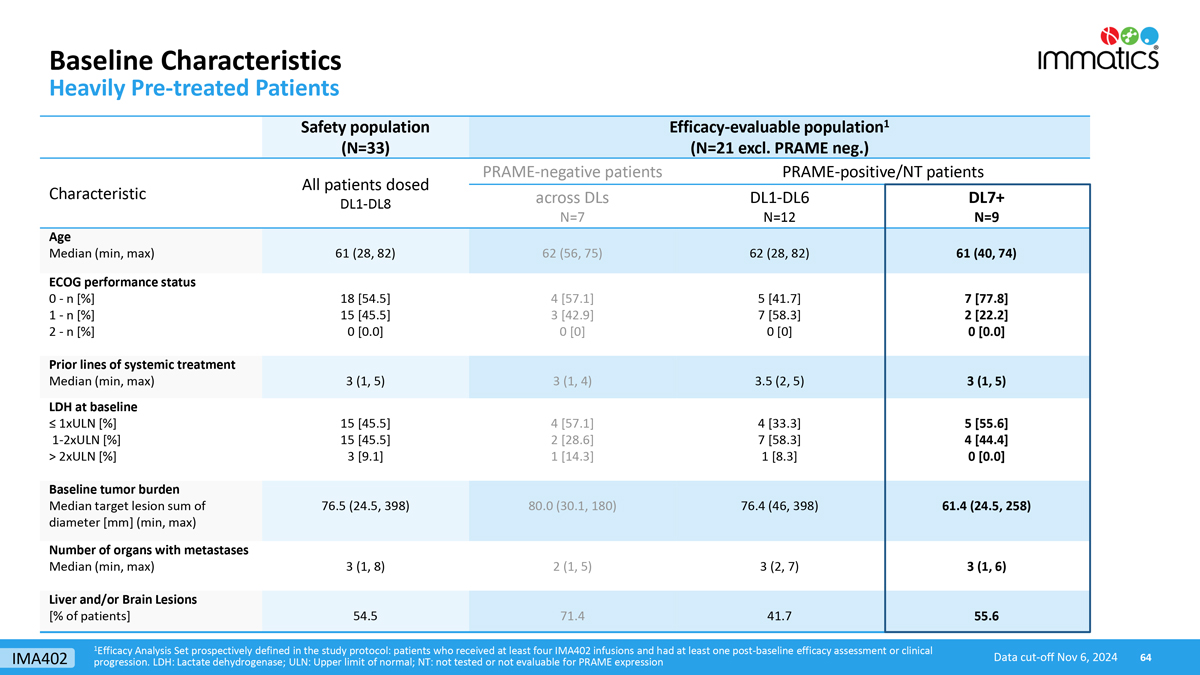
Baseline Characteristics Heavily Pre-treated Patients 1Efficacy Analysis Set prospectively defined in the study protocol: patients who received at least four IMA402 infusions and had at least one post-baseline efficacy assessment or clinical progression. LDH: Lactate dehydrogenase; ULN: Upper limit of normal; NT: not tested or not evaluable for PRAME expression Data cut-off Nov 6, 2024 Safety population (N=33) Efficacy-evaluable population1 (N=21 excl. PRAME neg.) Characteristic All patients dosed DL1-DL8 PRAME-negative patients PRAME-positive/NT patients across DLs N=7 DL1-DL6 N=12 DL7+ N=9 Age Median (min, max) 61 (28, 82) 62 (56, 75) 62 (28, 82) 61 (40, 74) ECOG performance status 0 - n [%] 1 - n [%] 2 - n [%] 18 [54.5] 15 [45.5] 0 [0.0] 4 [57.1] 3 [42.9] 0 [0] 5 [41.7] 7 [58.3] 0 [0] 7 [77.8] 2 [22.2] 0 [0.0] Prior lines of systemic treatment Median (min, max) 3 (1, 5) 3 (1, 4) 3.5 (2, 5) 3 (1, 5) LDH at baseline ≤ 1xULN [%] 1-2xULN [%] > 2xULN [%] 15 [45.5] 15 [45.5] 3 [9.1] 4 [57.1] 2 [28.6] 1 [14.3] 4 [33.3] 7 [58.3] 1 [8.3] 5 [55.6] 4 [44.4] 0 [0.0] Baseline tumor burden Median target lesion sum of diameter [mm] (min, max) 76.5 (24.5, 398) 80.0 (30.1, 180) 76.4 (46, 398) 61.4 (24.5, 258) Number of organs with metastases Median (min, max) 3 (1, 8) 2 (1, 5) 3 (2, 7) 3 (1, 6) Liver and/or Brain Lesions [% of patients] 54.5 71.4 41.7 55.6 IMA402
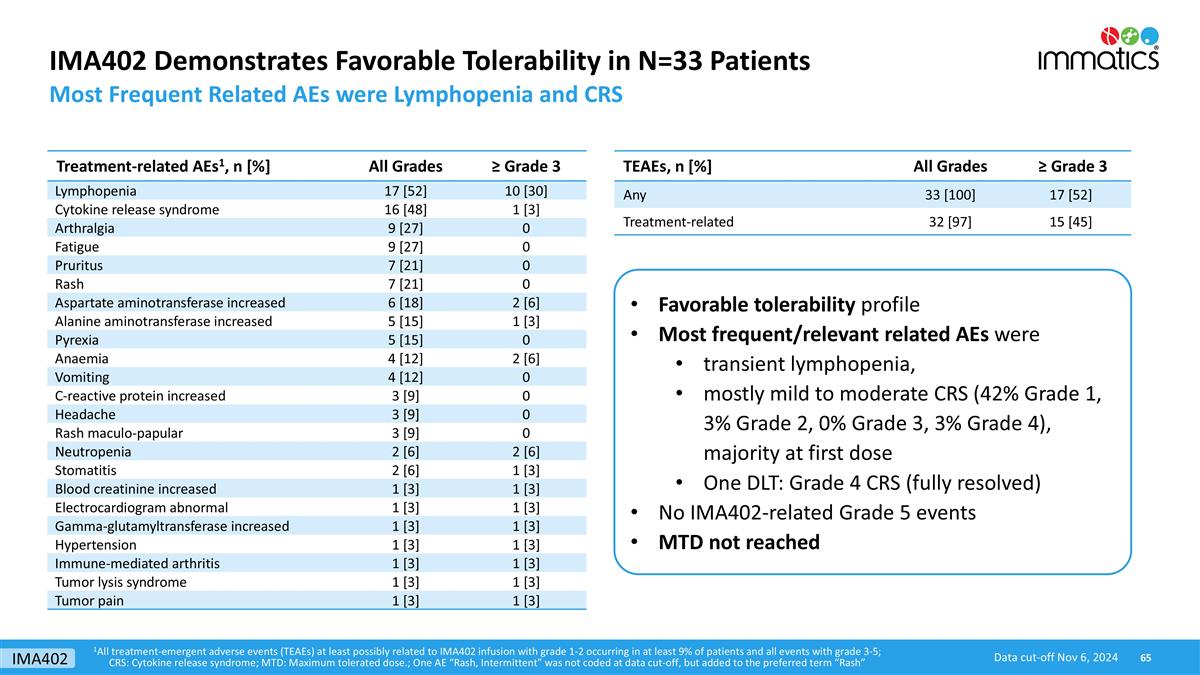
IMA402 Demonstrates Favorable Tolerability in N=33 Patients Most Frequent Related AEs were Lymphopenia and CRS TEAEs, n [%] All Grades ≥ Grade 3 Any 33 [100] 17 [52] Treatment-related 32 [97] 15 [45] Treatment-related AEs1, n [%] All Grades ≥ Grade 3 Lymphopenia 17 [52] 10 [30] Cytokine release syndrome 16 [48] 1 [3] Arthralgia 9 [27] 0 Fatigue 9 [27] 0 Pruritus 7 [21] 0 Rash 7 [21] 0 Aspartate aminotransferase increased 6 [18] 2 [6] Alanine aminotransferase increased 5 [15] 1 [3] Pyrexia 5 [15] 0 Anaemia 4 [12] 2 [6] Vomiting 4 [12] 0 C-reactive protein increased 3 [9] 0 Headache 3 [9] 0 Rash maculo-papular 3 [9] 0 Neutropenia 2 [6] 2 [6] Stomatitis 2 [6] 1 [3] Blood creatinine increased 1 [3] 1 [3] Electrocardiogram abnormal 1 [3] 1 [3] Gamma-glutamyltransferase increased 1 [3] 1 [3] Hypertension 1 [3] 1 [3] Immune-mediated arthritis 1 [3] 1 [3] Tumor lysis syndrome 1 [3] 1 [3] Tumor pain 1 [3] 1 [3] Favorable tolerability profile Most frequent/relevant related AEs were transient lymphopenia, mostly mild to moderate CRS (42% Grade 1, 3% Grade 2, 0% Grade 3, 3% Grade 4), majority at first dose One DLT: Grade 4 CRS (fully resolved) No IMA402-related Grade 5 events MTD not reached 1All treatment-emergent adverse events (TEAEs) at least possibly related to IMA402 infusion with grade 1-2 occurring in at least 9% of patients and all events with grade 3-5; CRS: Cytokine release syndrome; MTD: Maximum tolerated dose.; One AE “Rash, Intermittent” was not coded at data cut-off, but added to the preferred term “Rash” Data cut-off Nov 6, 2024 IMA402
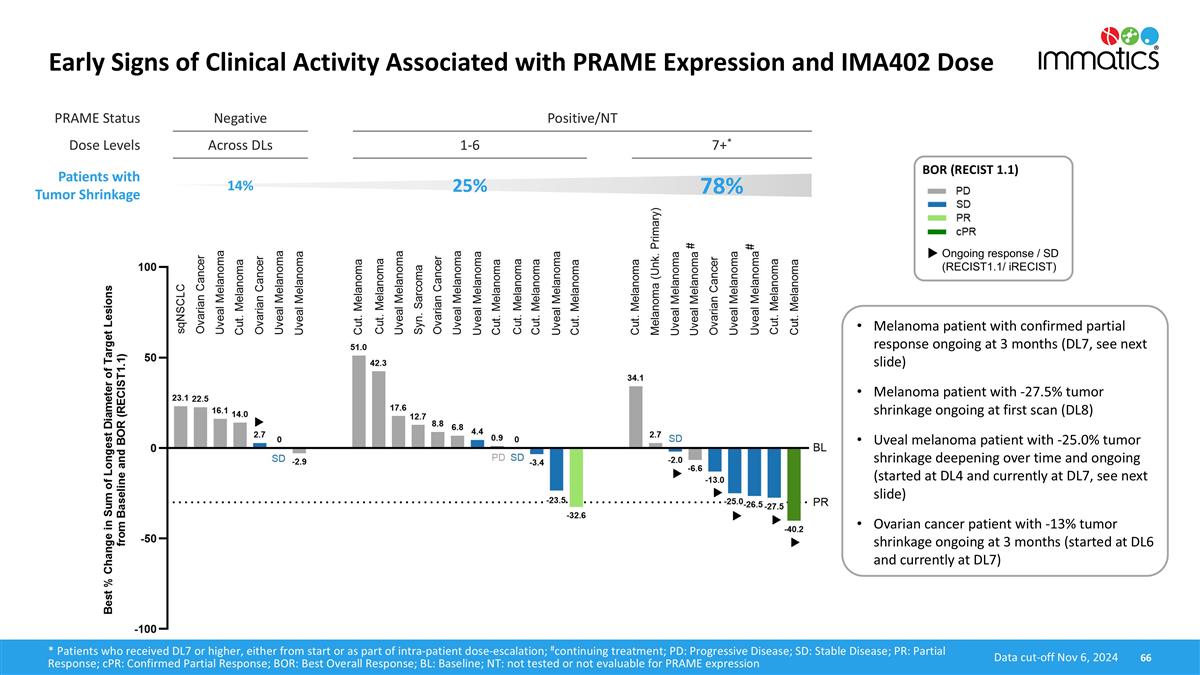
PRAME Status Negative Positive/NT Dose Levels Across DLs 1-6 7+* Patients with Tumor Shrinkage 14% 25% 78% Early Signs of Clinical Activity Associated with PRAME Expression and IMA402 Dose 66 BOR (RECIST 1.1) Ongoing response / SD (RECIST1.1/ iRECIST) Data cut-off Nov 6, 2024 * Patients who received DL7 or higher, either from start or as part of intra-patient dose-escalation; #continuing treatment; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response; BOR: Best Overall Response; BL: Baseline; NT: not tested or not evaluable for PRAME expression # # Melanoma patient with confirmed partial response ongoing at 3 months (DL7, see next slide) Melanoma patient with -27.5% tumor shrinkage ongoing at first scan (DL8) Uveal melanoma patient with -25.0% tumor shrinkage deepening over time and ongoing (started at DL4 and currently at DL7, see next slide) Ovarian cancer patient with -13% tumor shrinkage ongoing at 3 months (started at DL6 and currently at DL7)
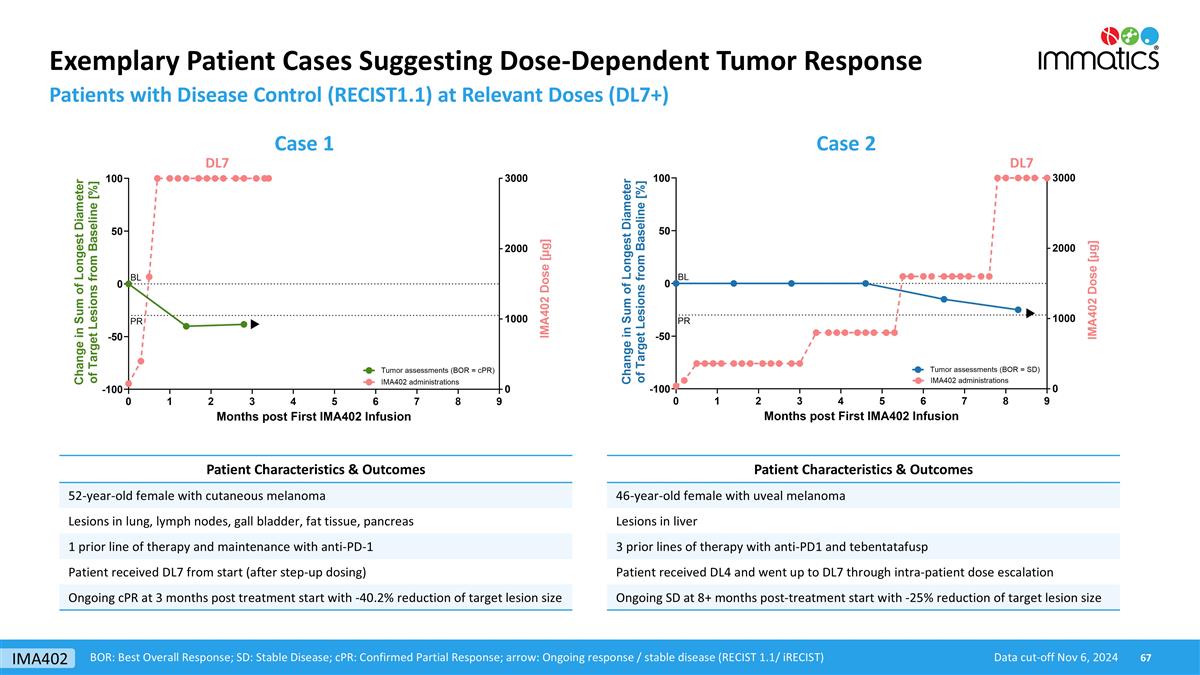
Exemplary Patient Cases Suggesting Dose-Dependent Tumor Response Patients with Disease Control (RECIST1.1) at Relevant Doses (DL7+) Data cut-off Nov 6, 2024 BOR: Best Overall Response; SD: Stable Disease; cPR: Confirmed Partial Response; arrow: Ongoing response / stable disease (RECIST 1.1/ iRECIST) Case 1 Case 2 DL7 DL7 Patient Characteristics & Outcomes 52-year-old female with cutaneous melanoma Lesions in lung, lymph nodes, gall bladder, fat tissue, pancreas 1 prior line of therapy and maintenance with anti-PD-1 Patient received DL7 from start (after step-up dosing) Ongoing cPR at 3 months post treatment start with -40.2% reduction of target lesion size Patient Characteristics & Outcomes 46-year-old female with uveal melanoma Lesions in liver 3 prior lines of therapy with anti-PD1 and tebentatafusp Patient received DL4 and went up to DL7 through intra-patient dose escalation Ongoing SD at 8+ months post-treatment start with -25% reduction of target lesion size IMA402
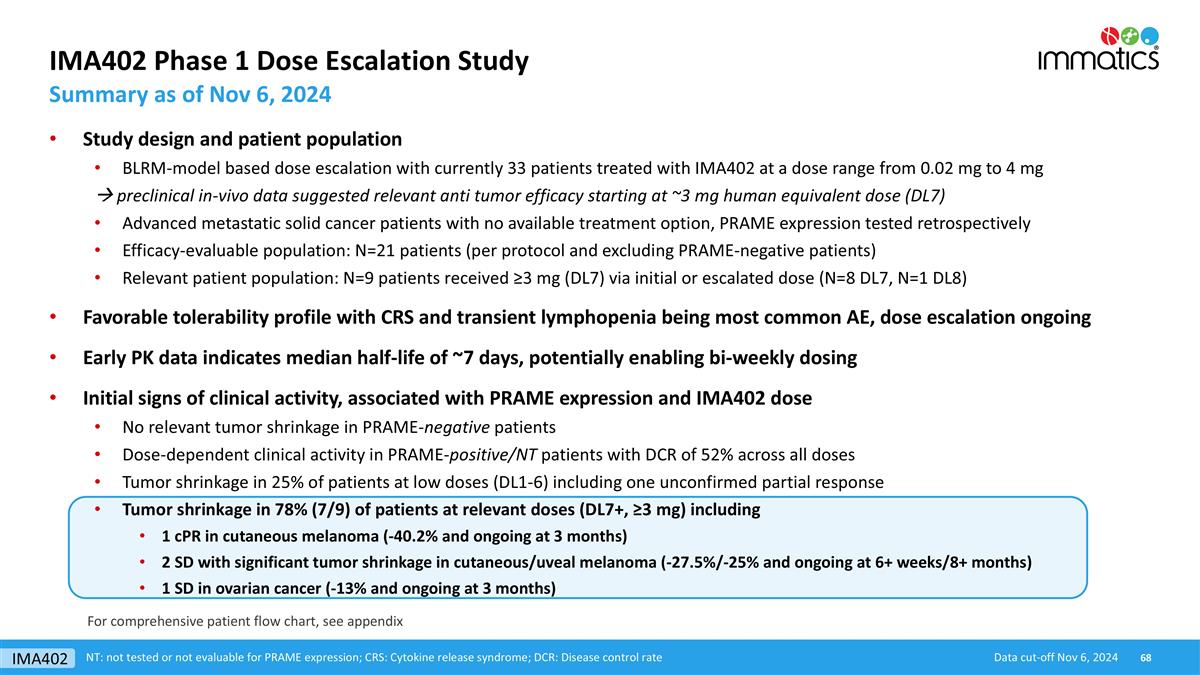
IMA402 Phase 1 Dose Escalation Study Summary as of Nov 6, 2024 Data cut-off Nov 6, 2024 NT: not tested or not evaluable for PRAME expression; CRS: Cytokine release syndrome; DCR: Disease control rate For comprehensive patient flow chart, see appendix Study design and patient population BLRM-model based dose escalation with currently 33 patients treated with IMA402 at a dose range from 0.02 mg to 4 mg à preclinical in-vivo data suggested relevant anti tumor efficacy starting at ~3 mg human equivalent dose (DL7) Advanced metastatic solid cancer patients with no available treatment option, PRAME expression tested retrospectively Efficacy-evaluable population: N=21 patients (per protocol and excluding PRAME-negative patients) Relevant patient population: N=9 patients received ≥3 mg (DL7) via initial or escalated dose (N=8 DL7, N=1 DL8) Favorable tolerability profile with CRS and transient lymphopenia being most common AE, dose escalation ongoing Early PK data indicates median half-life of ~7 days, potentially enabling bi-weekly dosing Initial signs of clinical activity, associated with PRAME expression and IMA402 dose No relevant tumor shrinkage in PRAME-negative patients Dose-dependent clinical activity in PRAME-positive/NT patients with DCR of 52% across all doses Tumor shrinkage in 25% of patients at low doses (DL1-6) including one unconfirmed partial response Tumor shrinkage in 78% (7/9) of patients at relevant doses (DL7+, ≥3 mg) including 1 cPR in cutaneous melanoma (-40.2% and ongoing at 3 months) 2 SD with significant tumor shrinkage in cutaneous/uveal melanoma (-27.5%/-25% and ongoing at 6+ weeks/8+ months) 1 SD in ovarian cancer (-13% and ongoing at 3 months) IMA402

Immatics’ Proprietary Target and TCR Discovery Platforms
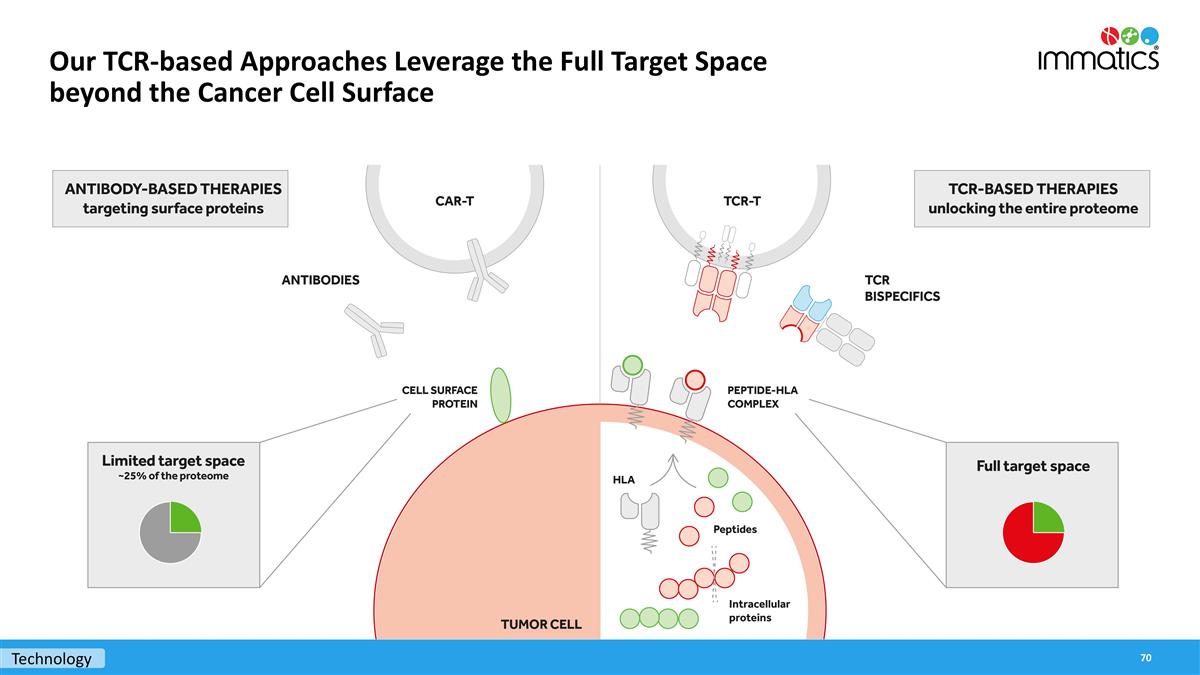
Our TCR-based Approaches Leverage the Full Target Space beyond the Cancer Cell Surface Technology
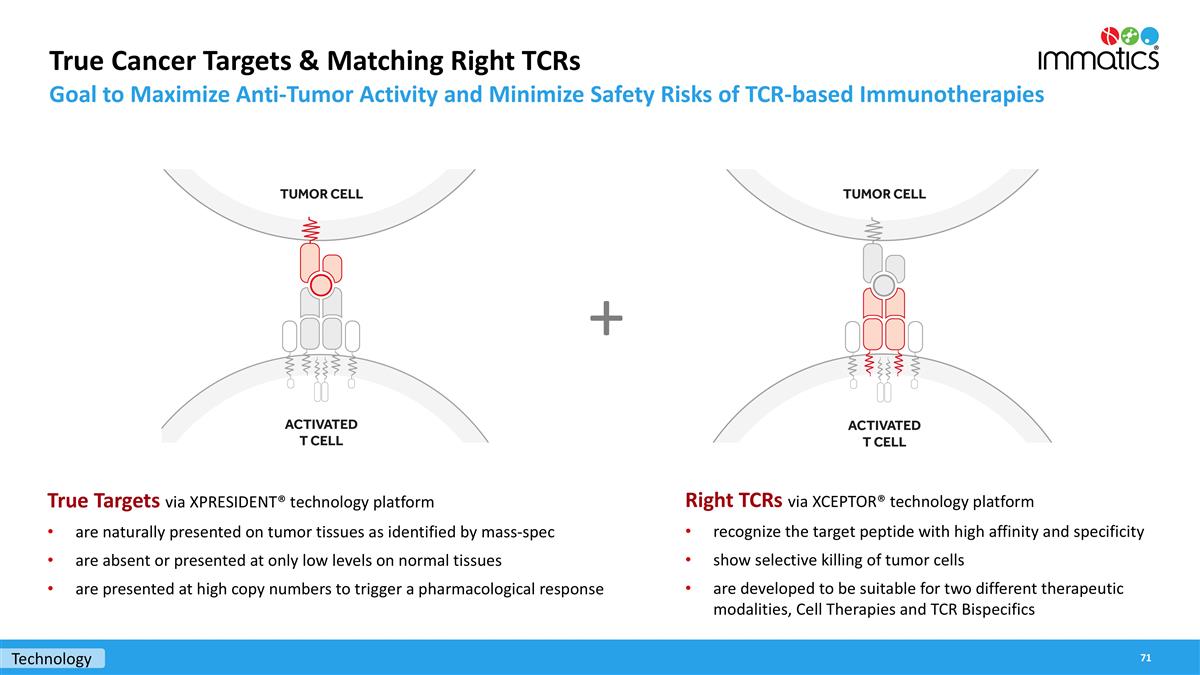
True Cancer Targets & Matching Right TCRs Goal to Maximize Anti-Tumor Activity and Minimize Safety Risks of TCR-based Immunotherapies True Targets via XPRESIDENT® technology platform are naturally presented on tumor tissues as identified by mass-spec are absent or presented at only low levels on normal tissues are presented at high copy numbers to trigger a pharmacological response + Technology Right TCRs via XCEPTOR® technology platform recognize the target peptide with high affinity and specificity show selective killing of tumor cells are developed to be suitable for two different therapeutic modalities, Cell Therapies and TCR Bispecifics
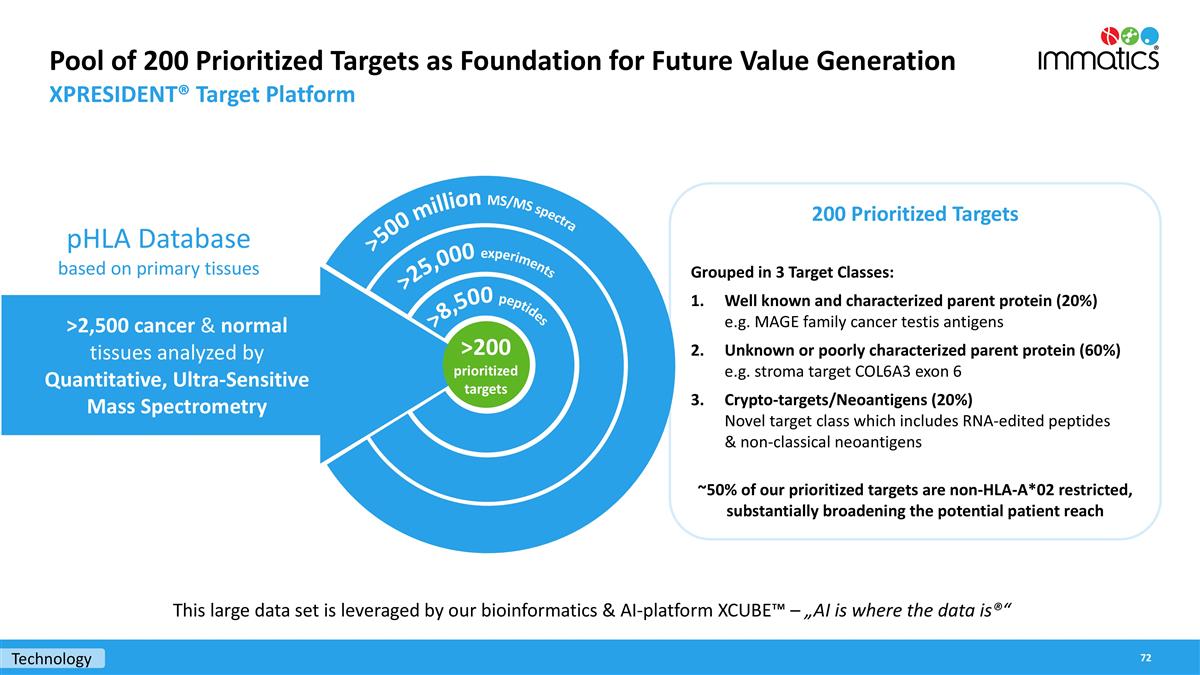
Technology Pool of 200 Prioritized Targets as Foundation for Future Value Generation XPRESIDENT® Target Platform 200 Prioritized Targets Grouped in 3 Target Classes: Well known and characterized parent protein (20%) e.g. MAGE family cancer testis antigens Unknown or poorly characterized parent protein (60%) e.g. stroma target COL6A3 exon 6 Crypto-targets/Neoantigens (20%) Novel target class which includes RNA-edited peptides & non-classical neoantigens ~50% of our prioritized targets are non-HLA-A*02 restricted, substantially broadening the potential patient reach >500 million MS/MS spectra >25,000 experiments >8,500 peptides >2,500 cancer & normal tissues analyzed by Quantitative, Ultra-Sensitive Mass Spectrometry pHLA Database based on primary tissues >200 prioritized targets This large data set is leveraged by our bioinformatics & AI-platform XCUBE™ – „AI is where the data is®“
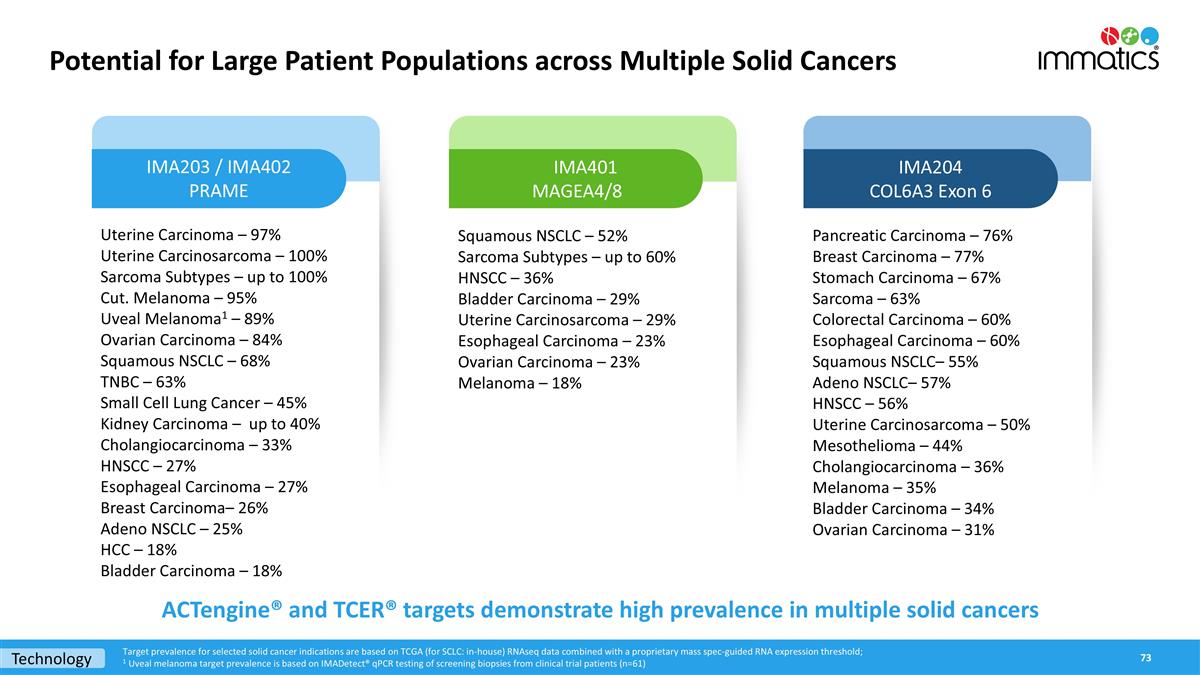
Potential for Large Patient Populations across Multiple Solid Cancers Uterine Carcinoma – 97% Uterine Carcinosarcoma – 100% Sarcoma Subtypes – up to 100% Cut. Melanoma – 95% Uveal Melanoma1 – 89% Ovarian Carcinoma – 84% Squamous NSCLC – 68% TNBC – 63% Small Cell Lung Cancer – 45% Kidney Carcinoma – up to 40% Cholangiocarcinoma – 33% HNSCC – 27% Esophageal Carcinoma – 27% Breast Carcinoma– 26% Adeno NSCLC – 25% HCC – 18% Bladder Carcinoma – 18% Squamous NSCLC – 52% Sarcoma Subtypes – up to 60% HNSCC – 36% Bladder Carcinoma – 29% Uterine Carcinosarcoma – 29% Esophageal Carcinoma – 23% Ovarian Carcinoma – 23% Melanoma – 18% IMA203 / IMA402 PRAME IMA401 MAGEA4/8 IMA204 COL6A3 Exon 6 ACTengine® and TCER® targets demonstrate high prevalence in multiple solid cancers Target prevalence for selected solid cancer indications are based on TCGA (for SCLC: in-house) RNAseq data combined with a proprietary mass spec-guided RNA expression threshold; 1 Uveal melanoma target prevalence is based on IMADetect® qPCR testing of screening biopsies from clinical trial patients (n=61) Pancreatic Carcinoma – 76% Breast Carcinoma – 77% Stomach Carcinoma – 67% Sarcoma – 63% Colorectal Carcinoma – 60% Esophageal Carcinoma – 60% Squamous NSCLC– 55% Adeno NSCLC– 57% HNSCC – 56% Uterine Carcinosarcoma – 50% Mesothelioma – 44% Cholangiocarcinoma – 36% Melanoma – 35% Bladder Carcinoma – 34% Ovarian Carcinoma – 31% Technology
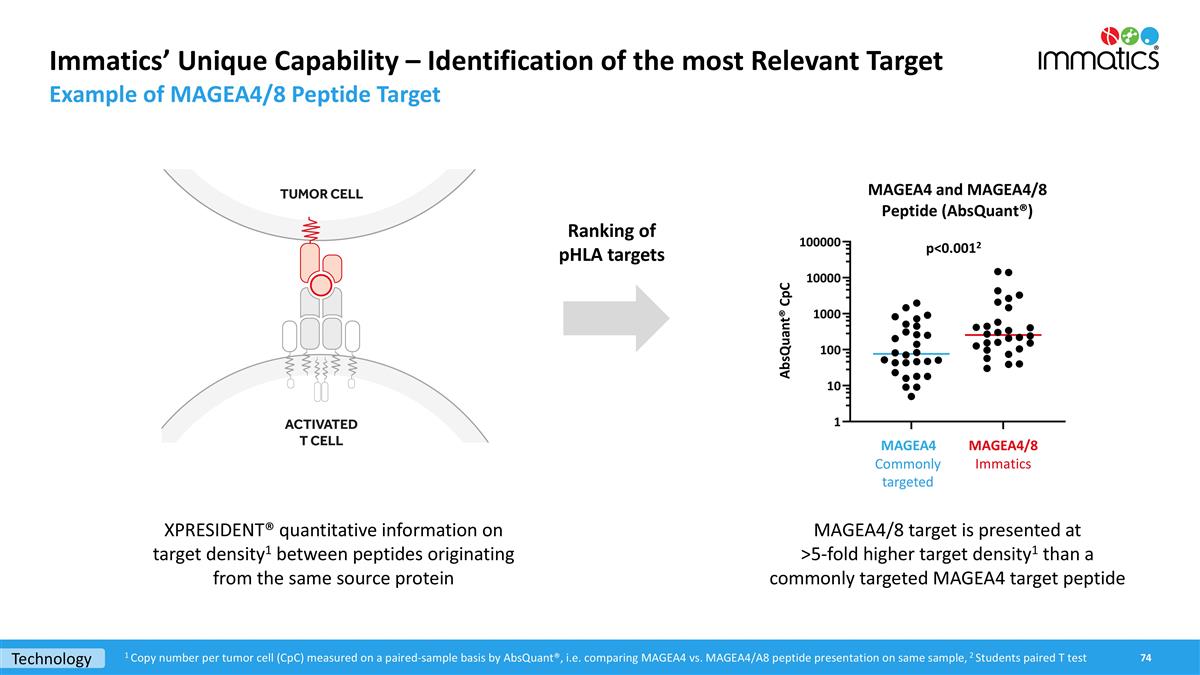
Immatics’ Unique Capability – Identification of the most Relevant Target Example of MAGEA4/8 Peptide Target 1 Copy number per tumor cell (CpC) measured on a paired-sample basis by AbsQuant®, i.e. comparing MAGEA4 vs. MAGEA4/A8 peptide presentation on same sample, 2 Students paired T test p<0.0012 Technology MAGEA4/8 target is presented at >5-fold higher target density1 than a commonly targeted MAGEA4 target peptide XPRESIDENT® quantitative information on target density1 between peptides originating from the same source protein Ranking of pHLA targets Commonly targeted
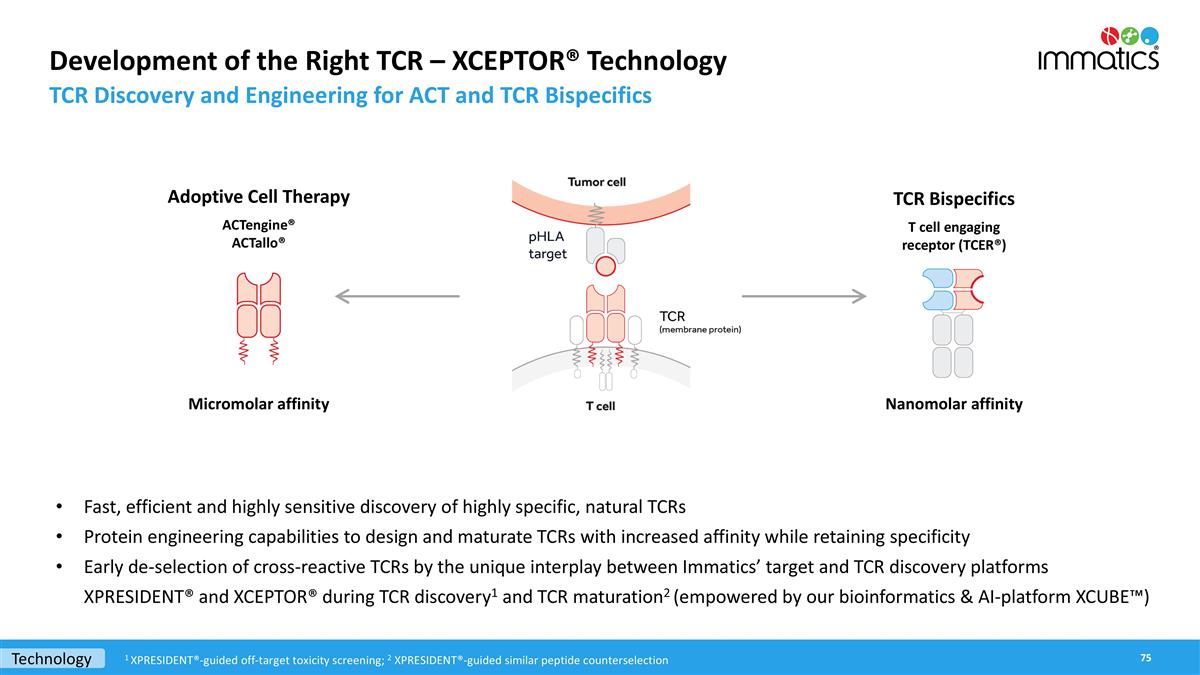
Development of the Right TCR – XCEPTOR® Technology TCR Discovery and Engineering for ACT and TCR Bispecifics TCR Bispecifics T cell engaging receptor (TCER®) Adoptive Cell Therapy ACTengine® ACTallo® Fast, efficient and highly sensitive discovery of highly specific, natural TCRs Protein engineering capabilities to design and maturate TCRs with increased affinity while retaining specificity Early de-selection of cross-reactive TCRs by the unique interplay between Immatics’ target and TCR discovery platforms XPRESIDENT® and XCEPTOR® during TCR discovery1 and TCR maturation2 (empowered by our bioinformatics & AI-platform XCUBE™) Micromolar affinity Nanomolar affinity Technology 1 XPRESIDENT®-guided off-target toxicity screening; 2 XPRESIDENT®-guided similar peptide counterselection
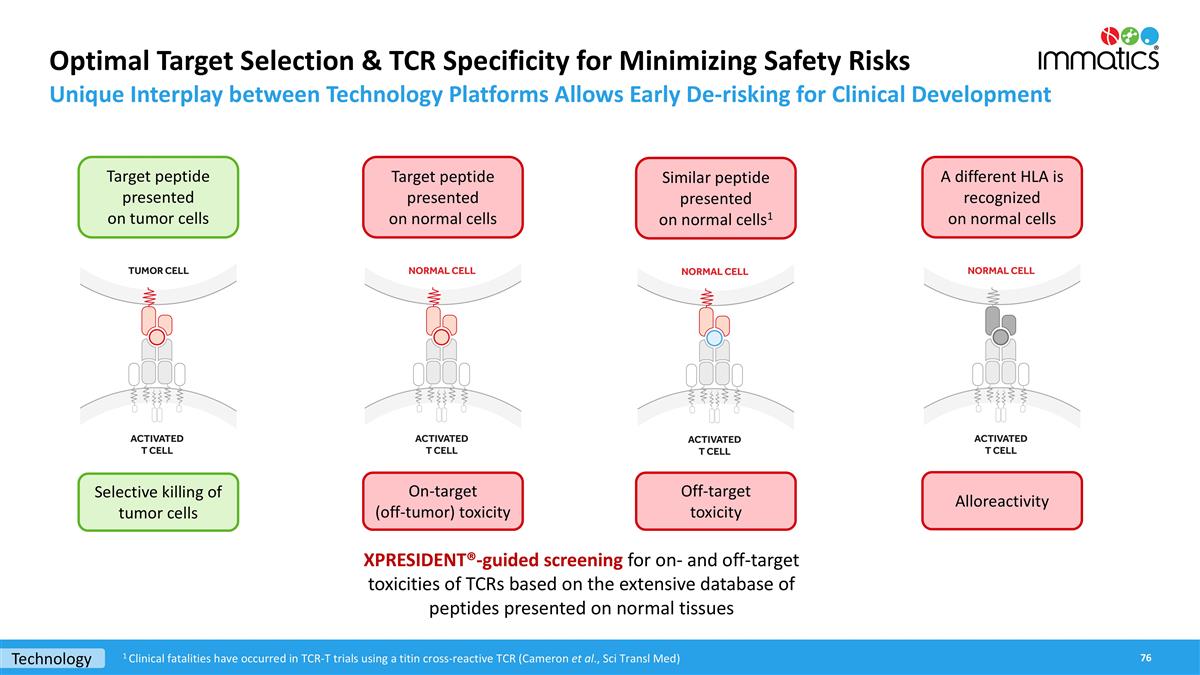
Optimal Target Selection & TCR Specificity for Minimizing Safety Risks Unique Interplay between Technology Platforms Allows Early De-risking for Clinical Development Target peptide presented on tumor cells Selective killing of tumor cells Target peptide presented on normal cells Off-target toxicity On-target (off-tumor) toxicity A different HLA is recognized on normal cells Alloreactivity Similar peptide presented on normal cells1 XPRESIDENT®-guided screening for on- and off-target toxicities of TCRs based on the extensive database of peptides presented on normal tissues Technology 1 Clinical fatalities have occurred in TCR-T trials using a titin cross-reactive TCR (Cameron et al., Sci Transl Med)
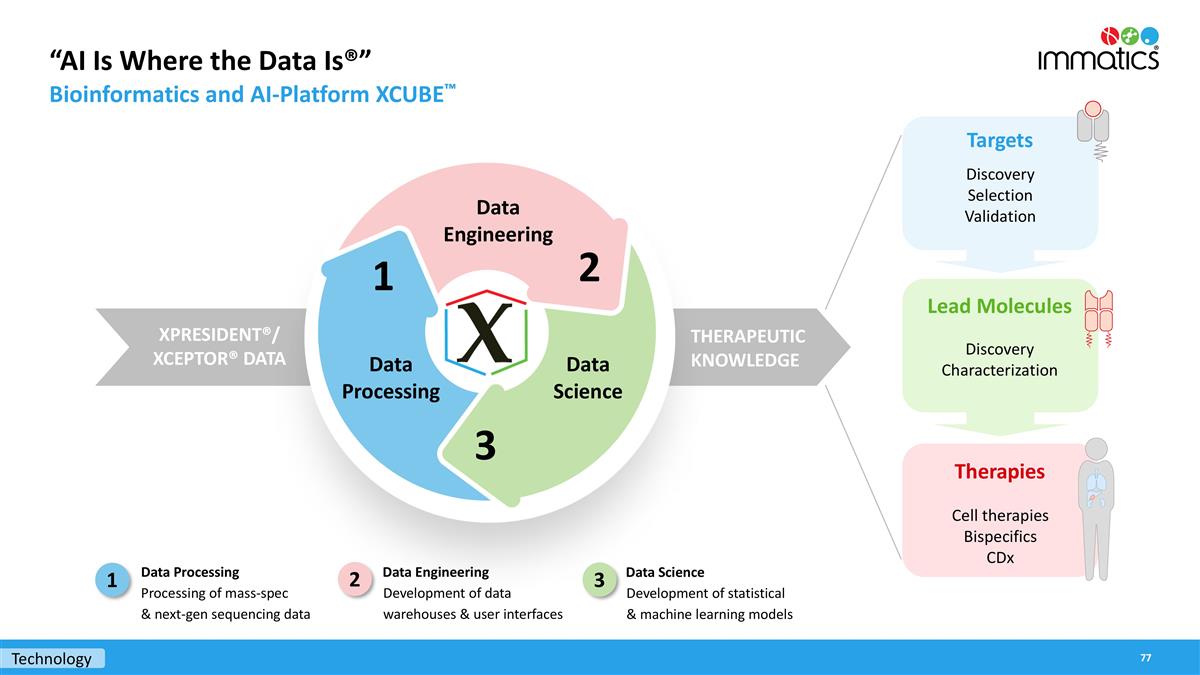
“AI Is Where the Data Is®” Bioinformatics and AI-Platform XCUBE™ Data Engineering Development of data warehouses & user interfaces Data Science Development of statistical & machine learning models Data Processing Processing of mass-spec & next-gen sequencing data 1 THERAPEUTIC KNOWLEDGE XPRESIDENT®/ XCEPTOR® DATA Data Engineering Data Science Data Processing 2 3 1 Cell therapies Bispecifics CDx Therapies Targets Lead Molecules Discovery Characterization Discovery Selection Validation 2 3 Technology
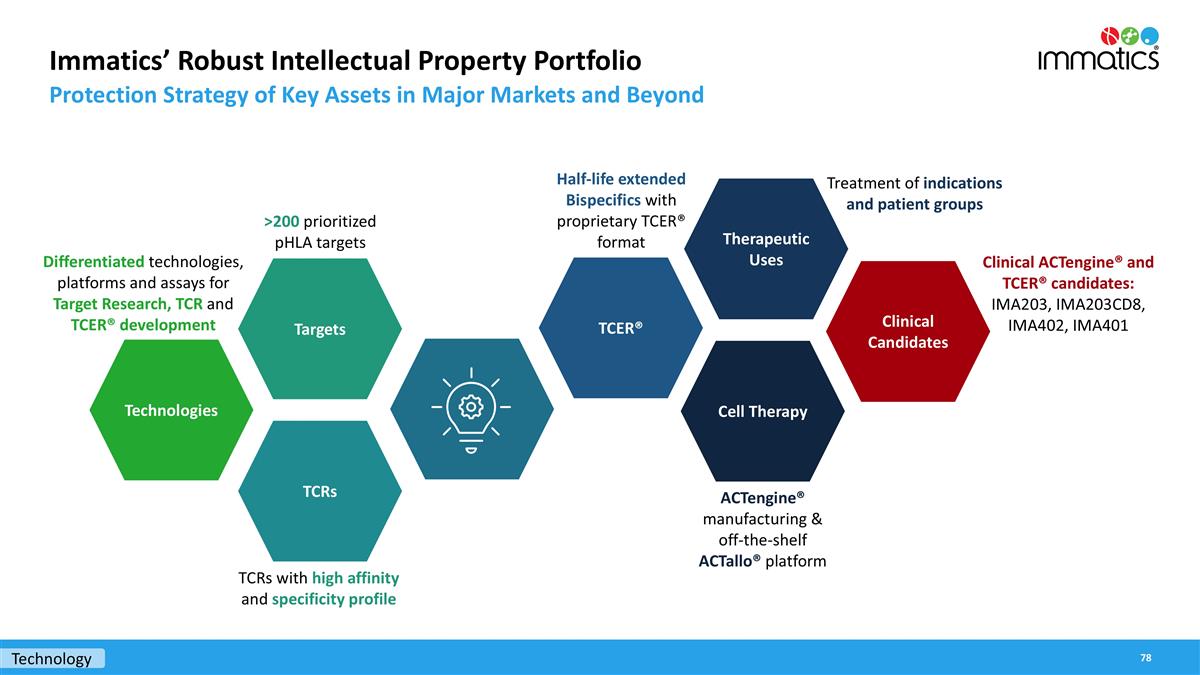
Immatics’ Robust Intellectual Property Portfolio Protection Strategy of Key Assets in Major Markets and Beyond Technologies Targets TCRs TCER® Therapeutic Uses Cell Therapy TCRs with high affinity and specificity profile Differentiated technologies, platforms and assays for Target Research, TCR and TCER® development >200 prioritized pHLA targets Half-life extended Bispecifics with proprietary TCER® format Treatment of indications and patient groups Clinical ACTengine® and TCER® candidates: IMA203, IMA203CD8, IMA402, IMA401 Clinical Candidates ACTengine® manufacturing & off-the-shelf ACTallo® platform Technology

ACTengine® IMA204 – TCR-T Targeting COL6A3 Exon 6
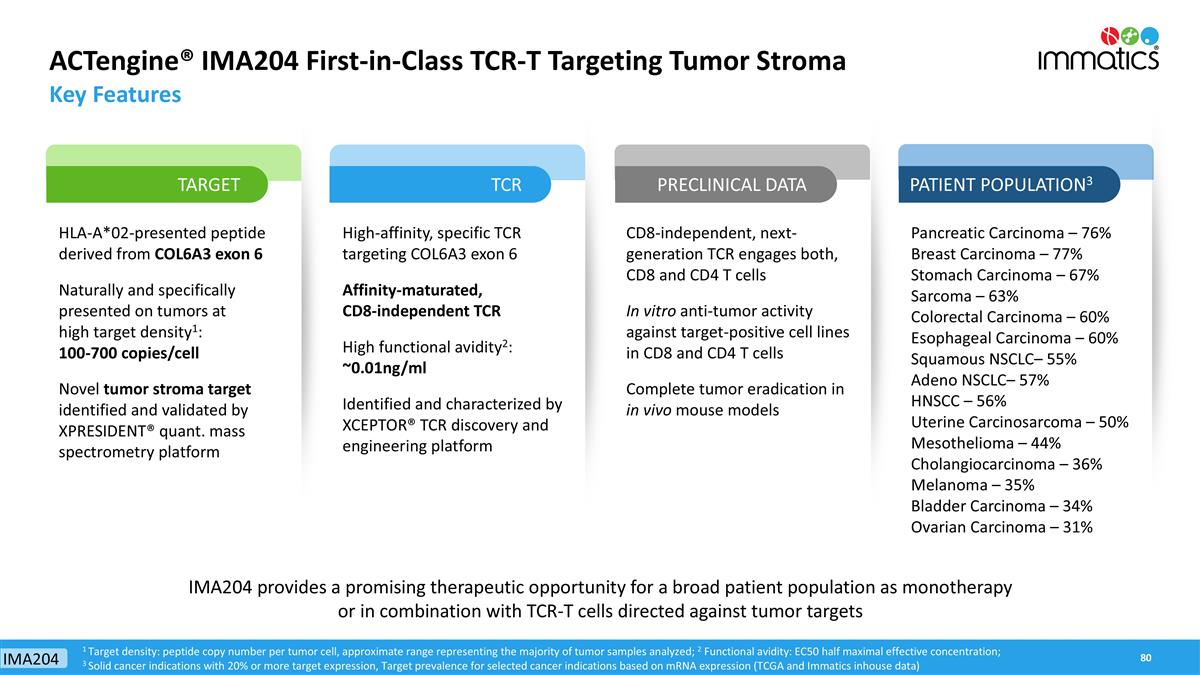
ACTengine® IMA204 First-in-Class TCR-T Targeting Tumor Stroma Key Features HLA-A*02-presented peptide derived from COL6A3 exon 6 Naturally and specifically presented on tumors at high target density1: 100-700 copies/cell Novel tumor stroma target identified and validated by XPRESIDENT® quant. mass spectrometry platform High-affinity, specific TCR targeting COL6A3 exon 6 Affinity-maturated, CD8-independent TCR High functional avidity2: ~0.01ng/ml Identified and characterized by XCEPTOR® TCR discovery and engineering platform CD8-independent, next-generation TCR engages both, CD8 and CD4 T cells In vitro anti-tumor activity against target-positive cell lines in CD8 and CD4 T cells Complete tumor eradication in in vivo mouse models Pancreatic Carcinoma – 76% Breast Carcinoma – 77% Stomach Carcinoma – 67% Sarcoma – 63% Colorectal Carcinoma – 60% Esophageal Carcinoma – 60% Squamous NSCLC– 55% Adeno NSCLC– 57% HNSCC – 56% Uterine Carcinosarcoma – 50% Mesothelioma – 44% Cholangiocarcinoma – 36% Melanoma – 35% Bladder Carcinoma – 34% Ovarian Carcinoma – 31% 1 Target density: peptide copy number per tumor cell, approximate range representing the majority of tumor samples analyzed; 2 Functional avidity: EC50 half maximal effective concentration; 3 Solid cancer indications with 20% or more target expression, Target prevalence for selected cancer indications based on mRNA expression (TCGA and Immatics inhouse data) TARGET TCR PRECLINICAL DATA PATIENT POPULATION3 IMA204 provides a promising therapeutic opportunity for a broad patient population as monotherapy or in combination with TCR-T cells directed against tumor targets IMA204
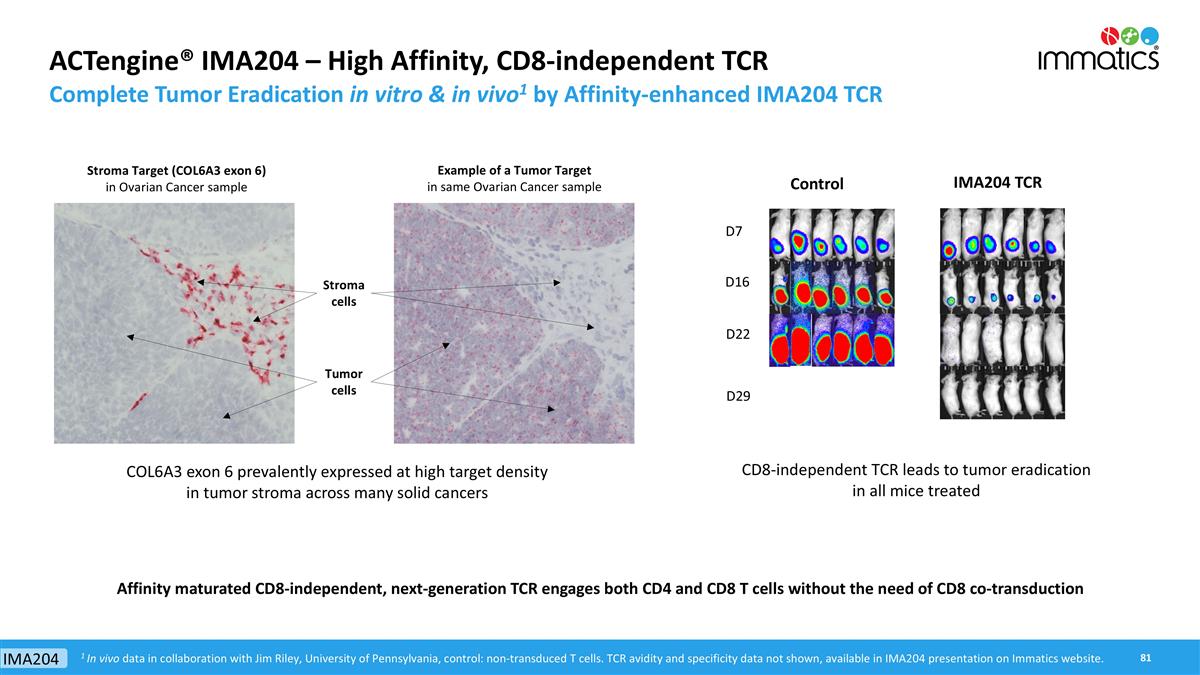
ACTengine® IMA204 – High Affinity, CD8-independent TCR Complete Tumor Eradication in vitro & in vivo1 by Affinity-enhanced IMA204 TCR CD8-independent TCR leads to tumor eradication in all mice treated Control IMA204 TCR D7 D16 D22 D29 Affinity maturated CD8-independent, next-generation TCR engages both CD4 and CD8 T cells without the need of CD8 co-transduction Stroma cells Tumor cells Stroma Target (COL6A3 exon 6) in Ovarian Cancer sample Example of a Tumor Target in same Ovarian Cancer sample 1 In vivo data in collaboration with Jim Riley, University of Pennsylvania, control: non-transduced T cells. TCR avidity and specificity data not shown, available in IMA204 presentation on Immatics website. COL6A3 exon 6 prevalently expressed at high target density in tumor stroma across many solid cancers IMA204

ACTallo® – Our Next-generation Off-the-shelf TCR-T
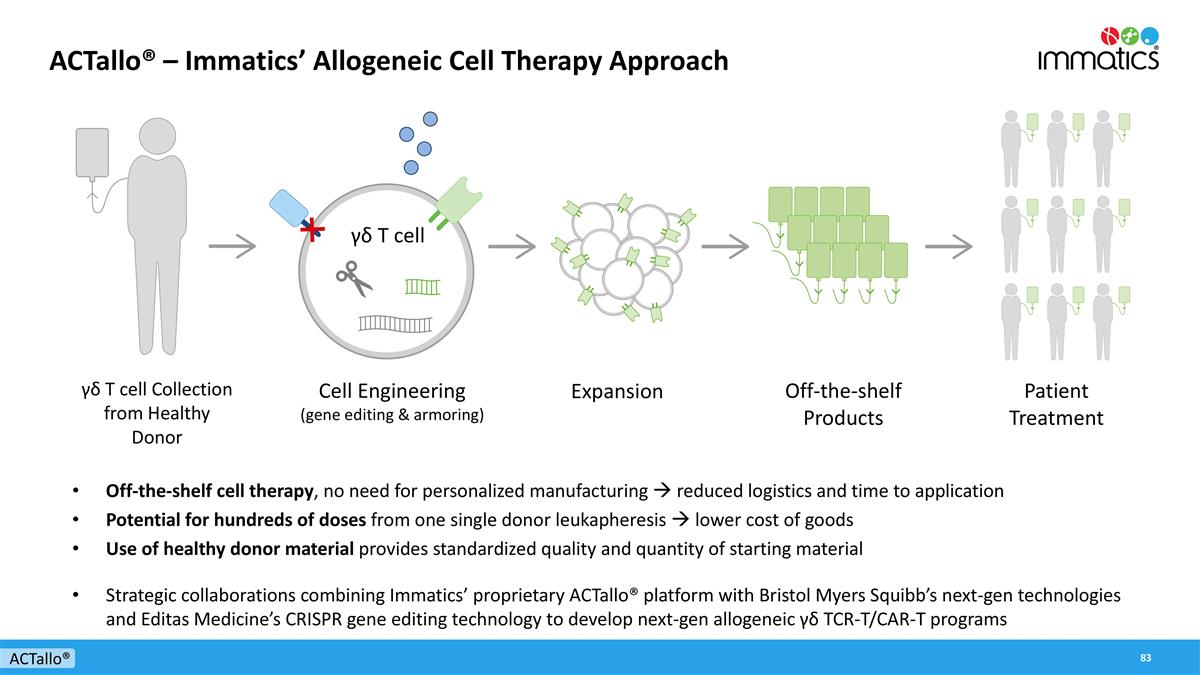
ACTallo® – Immatics’ Allogeneic Cell Therapy Approach Off-the-shelf cell therapy, no need for personalized manufacturing à reduced logistics and time to application Potential for hundreds of doses from one single donor leukapheresis à lower cost of goods Use of healthy donor material provides standardized quality and quantity of starting material Strategic collaborations combining Immatics’ proprietary ACTallo® platform with Bristol Myers Squibb’s next-gen technologies and Editas Medicine’s CRISPR gene editing technology to develop next-gen allogeneic γδ TCR-T/CAR-T programs ACTallo® γδ T cell Cell Engineering (gene editing & armoring) γδ T cell Collection from Healthy Donor Expansion Off-the-shelf Products Patient Treatment
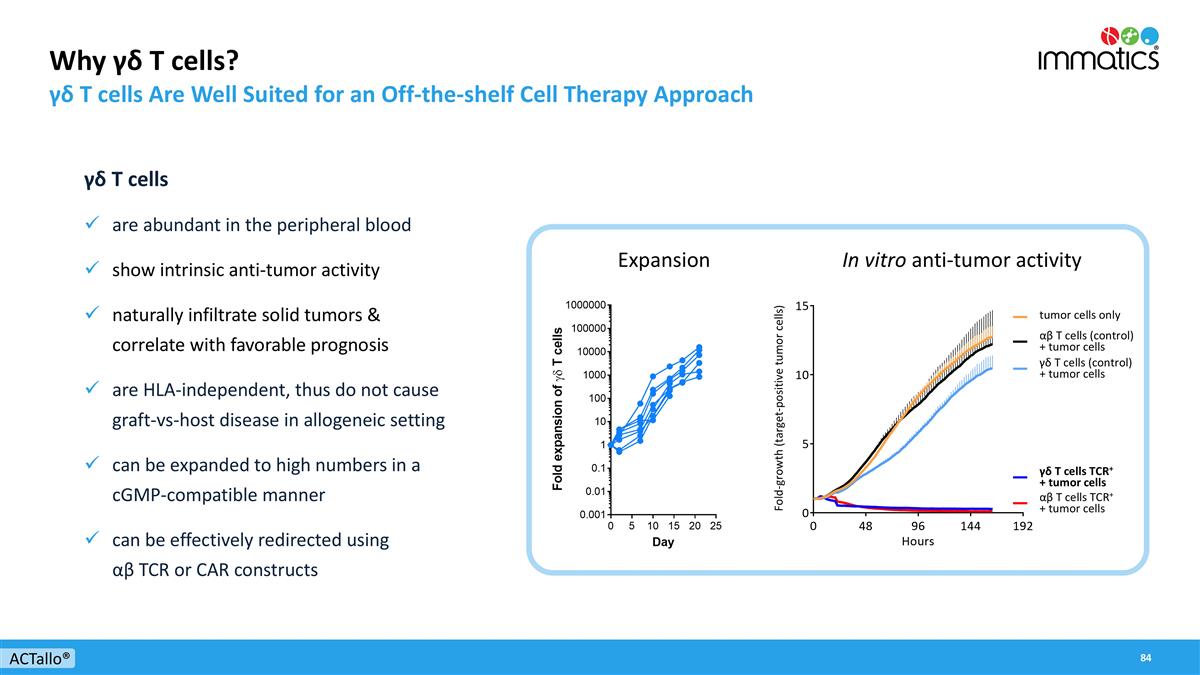
Why γδ T cells? γδ T cells Are Well Suited for an Off-the-shelf Cell Therapy Approach γδ T cells are abundant in the peripheral blood show intrinsic anti-tumor activity naturally infiltrate solid tumors & correlate with favorable prognosis are HLA-independent, thus do not cause graft-vs-host disease in allogeneic setting can be expanded to high numbers in a cGMP-compatible manner can be effectively redirected using αβ TCR or CAR constructs In vitro anti-tumor activity γδ T cells (control) + tumor cells tumor cells only αβ T cells (control) + tumor cells γδ T cells TCR+ + tumor cells αβ T cells TCR+ + tumor cells ACTallo® Expansion Fold-growth (target-positive tumor cells)

Corporate Information

David Leitner Schuldirektor David Leitner Schuldirektor David Leitner Schuldirektor Harpreet Singh Chief Executive Officer Co-Founder >20 yrs biotech experience Arnd Christ Chief Financial Officer >20 yrs biotech experience (InflaRx, Medigene, NovImmune, Probiodrug) Carsten Reinhardt Chief Development Officer >20 yrs pharma & biotech experience (Micromet, Roche, Fresenius) Cedrik Britten Chief Medical Officer >15 yrs pharma & biotech experience (GSK, BioNTech) Rainer Kramer Chief Business Officer >25 yrs pharma & biotech experience (Amgen, MorphoSys, Jerini, Shire, Signature Dx) Steffen Walter Chief Operating Officer Co-Founder Immatics US >15 yrs biotech experience Edward Sturchio General Counsel >15 yrs pharma & biotech experience (Abeona Therapeutics, AAA, Novartis, Merck, Schering) ) Jordan Silverstein Head of Strategy >10 yrs biotech experience (InflaRx, AAA) Toni Weinschenk Chief Innovation Officer Co-Founder >15 yrs biotech experience Experienced Global Leadership Team Across Europe and the US Corporate
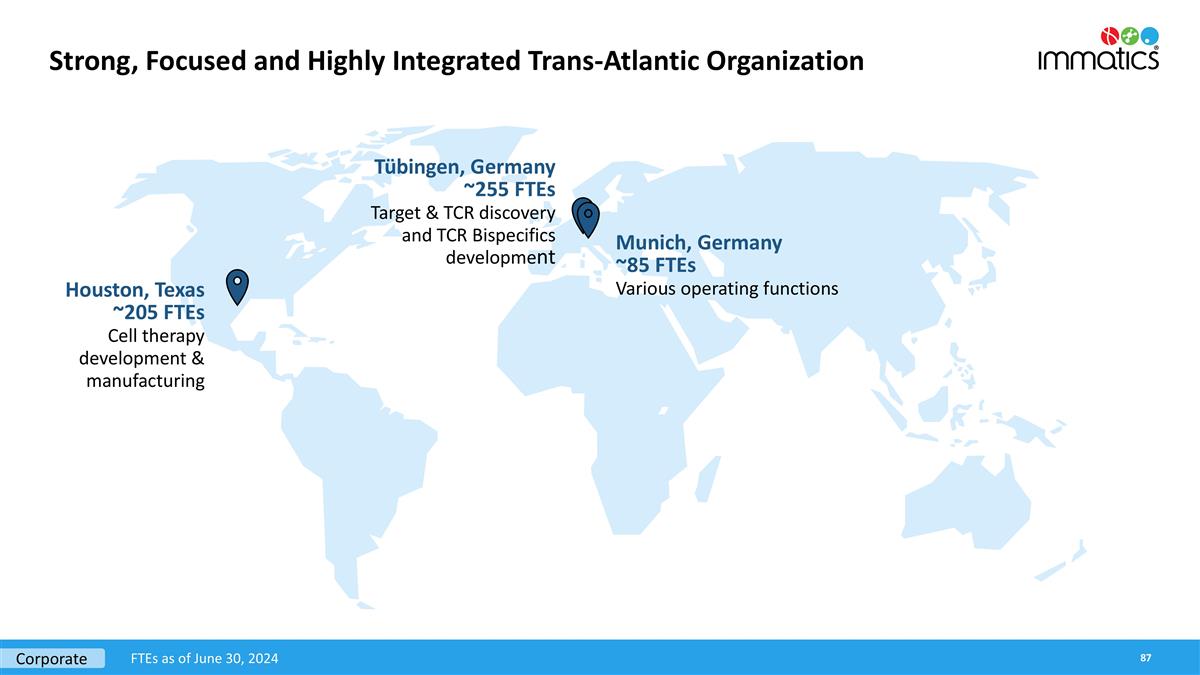
Strong, Focused and Highly Integrated Trans-Atlantic Organization Houston, Texas ~205 FTEs Cell therapy development & manufacturing Munich, Germany ~85 FTEs Various operating functions Tübingen, Germany ~255 FTEs Target & TCR discovery and TCR Bispecifics development Corporate FTEs as of June 30, 2024

Appendix
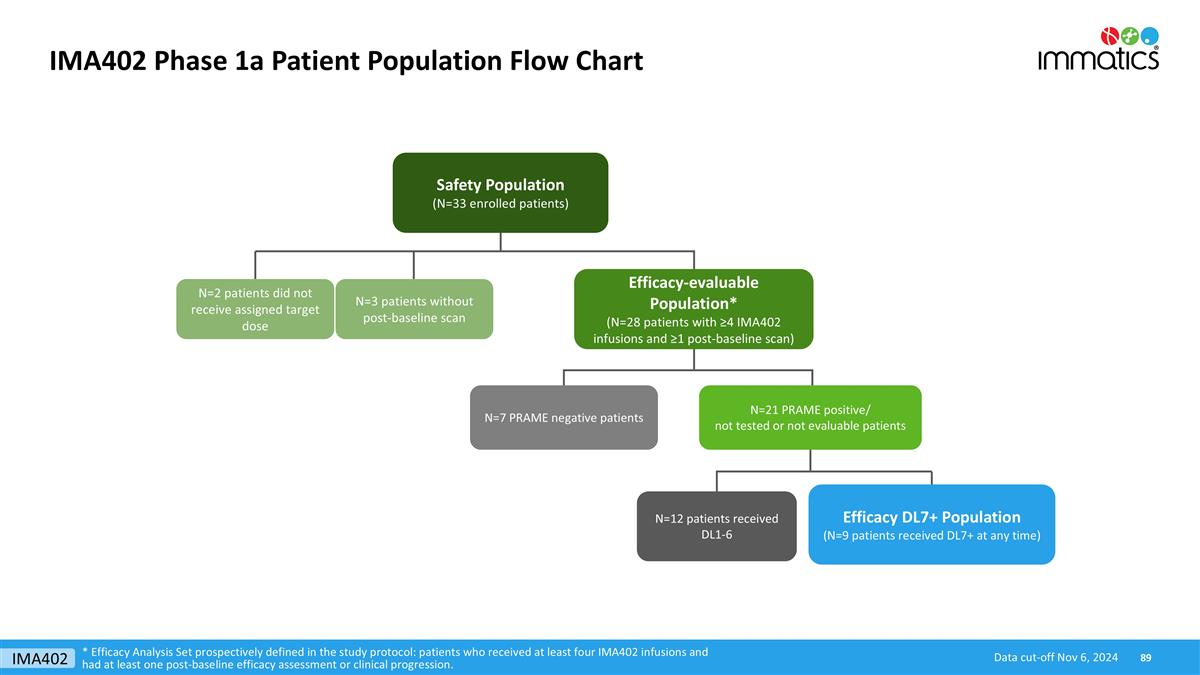
IMA402 Phase 1a Patient Population Flow Chart Safety Population (N=33 enrolled patients) Efficacy-evaluable Population* (N=28 patients with ≥4 IMA402 infusions and ≥1 post-baseline scan) N=21 PRAME positive/ not tested or not evaluable patients N=2 patients did not receive assigned target dose N=3 patients without post-baseline scan N=7 PRAME negative patients N=12 patients received DL1-6 Efficacy DL7+ Population (N=9 patients received DL7+ at any time) * Efficacy Analysis Set prospectively defined in the study protocol: patients who received at least four IMA402 infusions and had at least one post-baseline efficacy assessment or clinical progression. Data cut-off Nov 6, 2024 IMA402
























































































