
TCR Bispecific Molecule TCER® IMA402 Targeting PRAME - Phase 1 Dose Escalation Clinical Data Update November 18, 2024 Data cut-off Nov 6, 2024 Exhibit 99.3
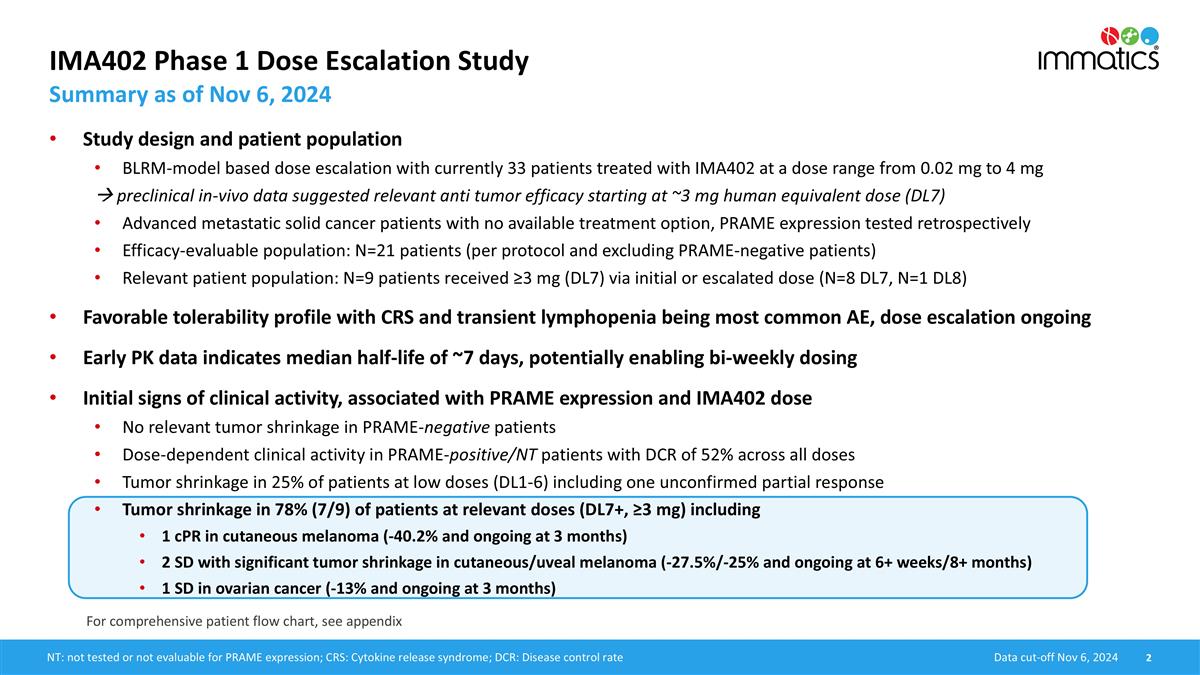
IMA402 Phase 1 Dose Escalation Study Summary as of Nov 6, 2024 Data cut-off Nov 6, 2024 NT: not tested or not evaluable for PRAME expression; CRS: Cytokine release syndrome; DCR: Disease control rate For comprehensive patient flow chart, see appendix Study design and patient population BLRM-model based dose escalation with currently 33 patients treated with IMA402 at a dose range from 0.02 mg to 4 mg à preclinical in-vivo data suggested relevant anti tumor efficacy starting at ~3 mg human equivalent dose (DL7) Advanced metastatic solid cancer patients with no available treatment option, PRAME expression tested retrospectively Efficacy-evaluable population: N=21 patients (per protocol and excluding PRAME-negative patients) Relevant patient population: N=9 patients received ≥3 mg (DL7) via initial or escalated dose (N=8 DL7, N=1 DL8) Favorable tolerability profile with CRS and transient lymphopenia being most common AE, dose escalation ongoing Early PK data indicates median half-life of ~7 days, potentially enabling bi-weekly dosing Initial signs of clinical activity, associated with PRAME expression and IMA402 dose No relevant tumor shrinkage in PRAME-negative patients Dose-dependent clinical activity in PRAME-positive/NT patients with DCR of 52% across all doses Tumor shrinkage in 25% of patients at low doses (DL1-6) including one unconfirmed partial response Tumor shrinkage in 78% (7/9) of patients at relevant doses (DL7+, ≥3 mg) including 1 cPR in cutaneous melanoma (-40.2% and ongoing at 3 months) 2 SD with significant tumor shrinkage in cutaneous/uveal melanoma (-27.5%/-25% and ongoing at 6+ weeks/8+ months) 1 SD in ovarian cancer (-13% and ongoing at 3 months)
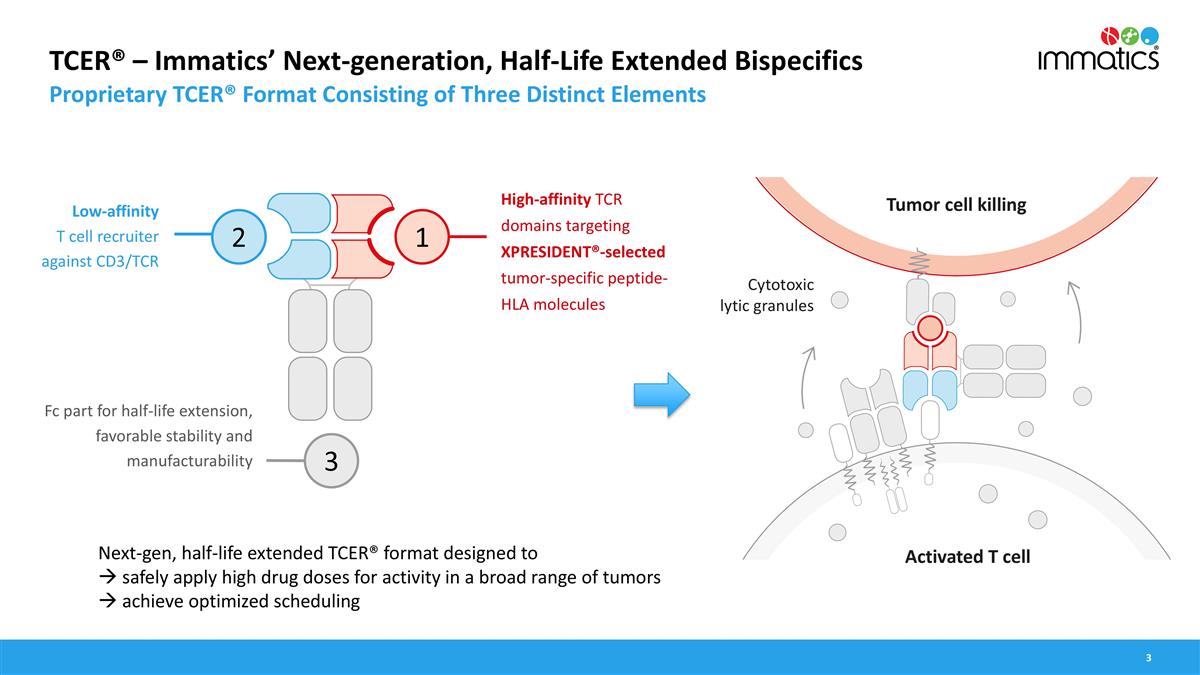
TCER® – Immatics’ Next-generation, Half-Life Extended Bispecifics Proprietary TCER® Format Consisting of Three Distinct Elements High-affinity TCR domains targeting XPRESIDENT®-selected tumor-specific peptide-HLA molecules Low-affinity T cell recruiter against CD3/TCR Fc part for half-life extension, favorable stability and manufacturability Next-gen, half-life extended TCER® format designed to à safely apply high drug doses for activity in a broad range of tumors à achieve optimized scheduling 2 1 3 Cytotoxic lytic granules Tumor cell killing Activated T cell
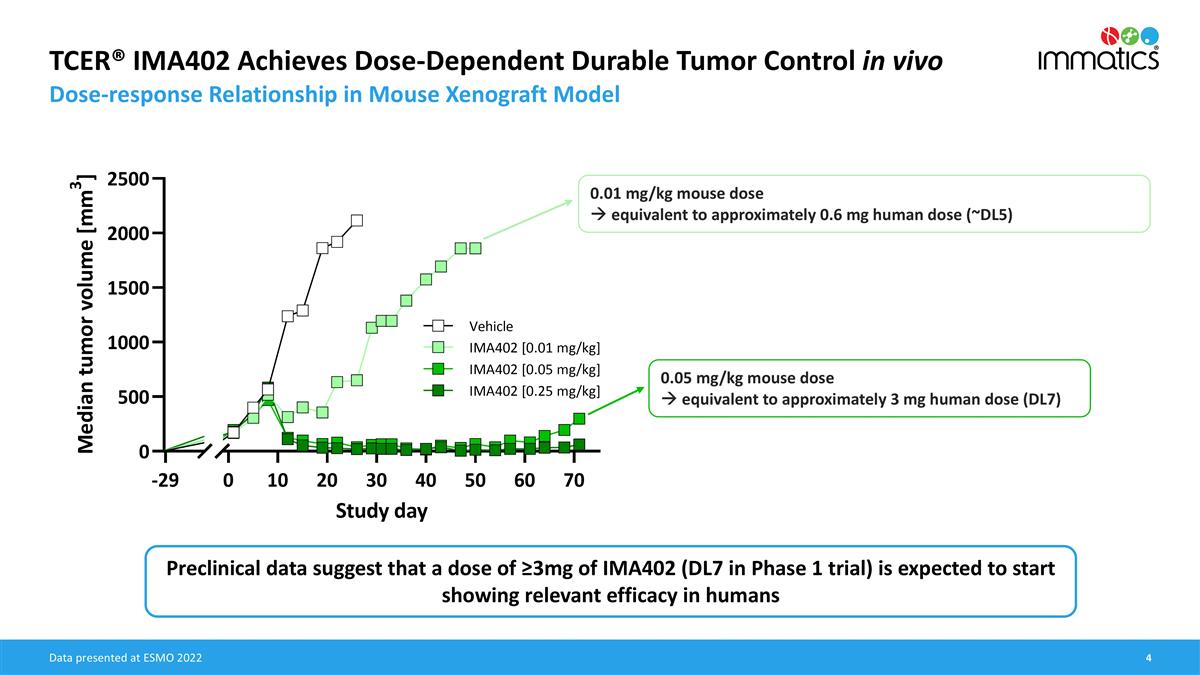
TCER® IMA402 Achieves Dose-Dependent Durable Tumor Control in vivo Dose-response Relationship in Mouse Xenograft Model 0.01 mg/kg mouse dose à equivalent to approximately 0.6 mg human dose (~DL5) 0.05 mg/kg mouse dose à equivalent to approximately 3 mg human dose (DL7) Data presented at ESMO 2022 Preclinical data suggest that a dose of ≥3mg of IMA402 (DL7 in Phase 1 trial) is expected to start showing relevant efficacy in humans
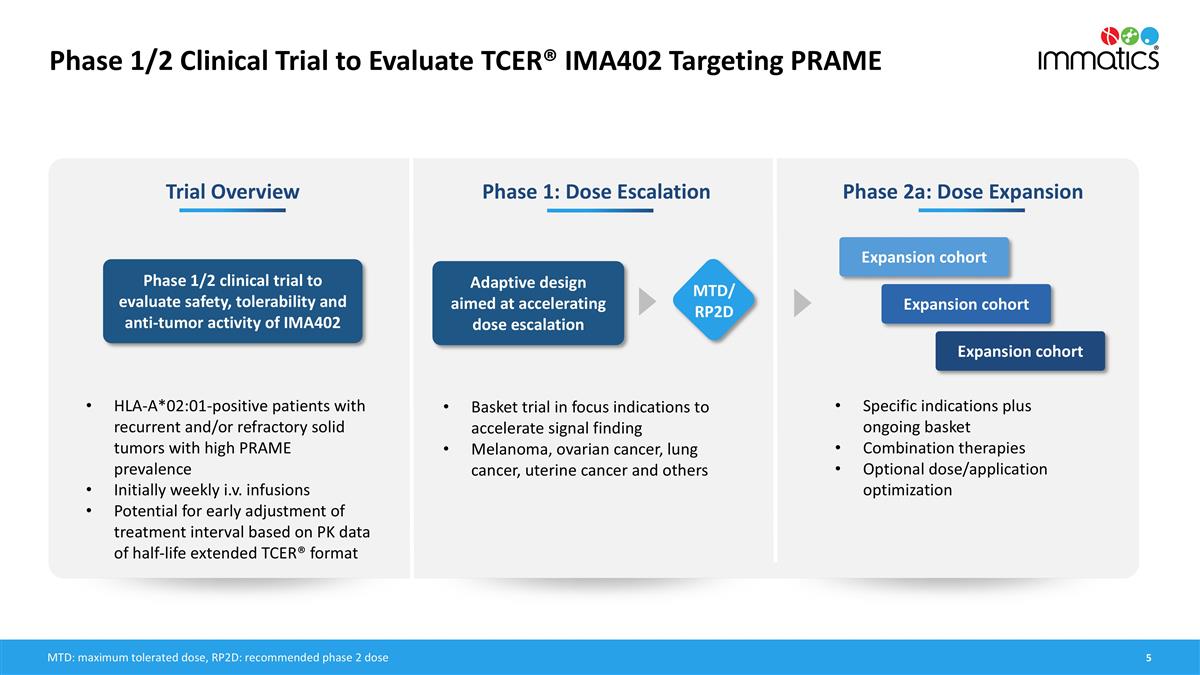
Phase 1/2 Clinical Trial to Evaluate TCER® IMA402 Targeting PRAME Phase 1: Dose Escalation Phase 2a: Dose Expansion Adaptive design aimed at accelerating dose escalation Specific indications plus ongoing basket Combination therapies Optional dose/application optimization Expansion cohort Expansion cohort Expansion cohort Trial Overview Phase 1/2 clinical trial to evaluate safety, tolerability and anti-tumor activity of IMA402 HLA-A*02:01-positive patients with recurrent and/or refractory solid tumors with high PRAME prevalence Initially weekly i.v. infusions Potential for early adjustment of treatment interval based on PK data of half-life extended TCER® format MTD/ RP2D Basket trial in focus indications to accelerate signal finding Melanoma, ovarian cancer, lung cancer, uterine cancer and others MTD: maximum tolerated dose, RP2D: recommended phase 2 dose
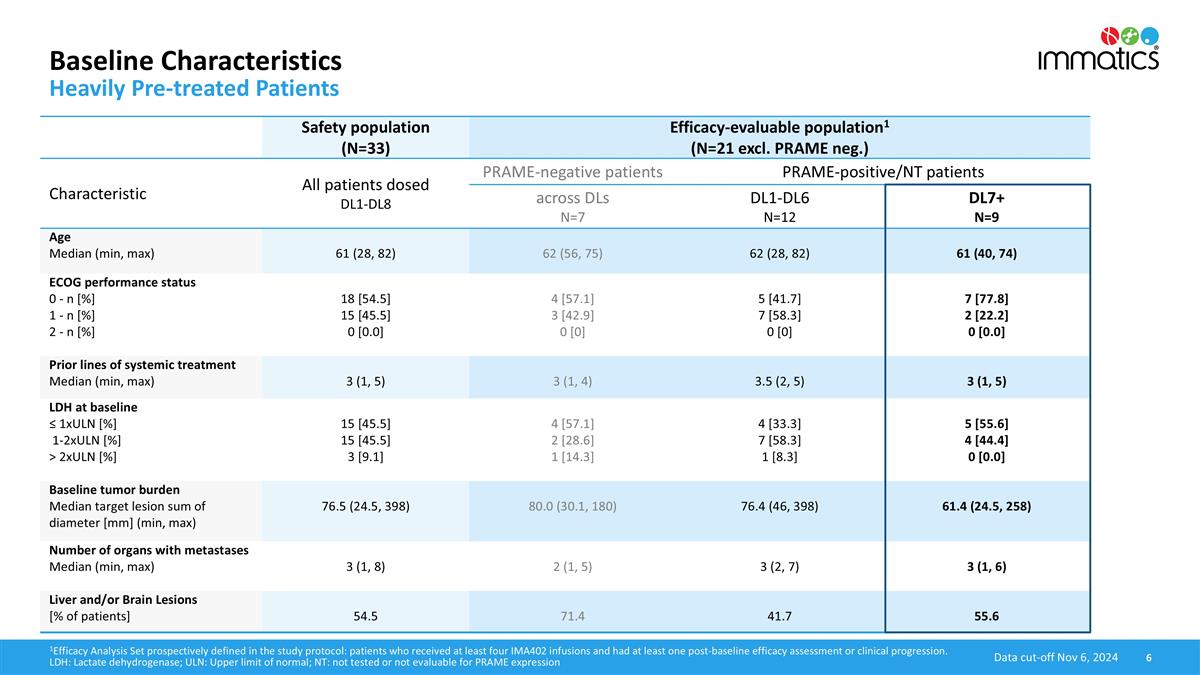
Baseline Characteristics Heavily Pre-treated Patients 1Efficacy Analysis Set prospectively defined in the study protocol: patients who received at least four IMA402 infusions and had at least one post-baseline efficacy assessment or clinical progression. LDH: Lactate dehydrogenase; ULN: Upper limit of normal; NT: not tested or not evaluable for PRAME expression Data cut-off Nov 6, 2024 Safety population (N=33) Efficacy-evaluable population1 (N=21 excl. PRAME neg.) Characteristic All patients dosed DL1-DL8 PRAME-negative patients PRAME-positive/NT patients across DLs N=7 DL1-DL6 N=12 DL7+ N=9 Age Median (min, max) 61 (28, 82) 62 (56, 75) 62 (28, 82) 61 (40, 74) ECOG performance status 0 - n [%] 1 - n [%] 2 - n [%] 18 [54.5] 15 [45.5] 0 [0.0] 4 [57.1] 3 [42.9] 0 [0] 5 [41.7] 7 [58.3] 0 [0] 7 [77.8] 2 [22.2] 0 [0.0] Prior lines of systemic treatment Median (min, max) 3 (1, 5) 3 (1, 4) 3.5 (2, 5) 3 (1, 5) LDH at baseline ≤ 1xULN [%] 1-2xULN [%] > 2xULN [%] 15 [45.5] 15 [45.5] 3 [9.1] 4 [57.1] 2 [28.6] 1 [14.3] 4 [33.3] 7 [58.3] 1 [8.3] 5 [55.6] 4 [44.4] 0 [0.0] Baseline tumor burden Median target lesion sum of diameter [mm] (min, max) 76.5 (24.5, 398) 80.0 (30.1, 180) 76.4 (46, 398) 61.4 (24.5, 258) Number of organs with metastases Median (min, max) 3 (1, 8) 2 (1, 5) 3 (2, 7) 3 (1, 6) Liver and/or Brain Lesions [% of patients] 54.5 71.4 41.7 55.6
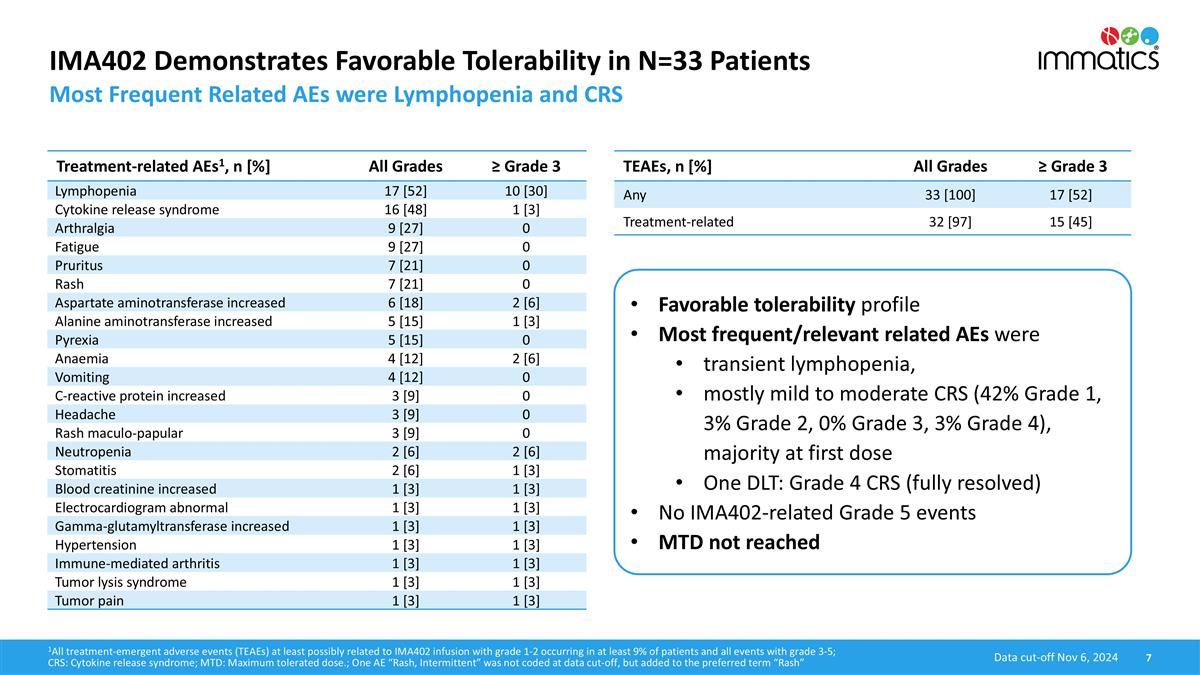
IMA402 Demonstrates Favorable Tolerability in N=33 Patients Most Frequent Related AEs were Lymphopenia and CRS TEAEs, n [%] All Grades ≥ Grade 3 Any 33 [100] 17 [52] Treatment-related 32 [97] 15 [45] Treatment-related AEs1, n [%] All Grades ≥ Grade 3 Lymphopenia 17 [52] 10 [30] Cytokine release syndrome 16 [48] 1 [3] Arthralgia 9 [27] 0 Fatigue 9 [27] 0 Pruritus 7 [21] 0 Rash 7 [21] 0 Aspartate aminotransferase increased 6 [18] 2 [6] Alanine aminotransferase increased 5 [15] 1 [3] Pyrexia 5 [15] 0 Anaemia 4 [12] 2 [6] Vomiting 4 [12] 0 C-reactive protein increased 3 [9] 0 Headache 3 [9] 0 Rash maculo-papular 3 [9] 0 Neutropenia 2 [6] 2 [6] Stomatitis 2 [6] 1 [3] Blood creatinine increased 1 [3] 1 [3] Electrocardiogram abnormal 1 [3] 1 [3] Gamma-glutamyltransferase increased 1 [3] 1 [3] Hypertension 1 [3] 1 [3] Immune-mediated arthritis 1 [3] 1 [3] Tumor lysis syndrome 1 [3] 1 [3] Tumor pain 1 [3] 1 [3] Favorable tolerability profile Most frequent/relevant related AEs were transient lymphopenia, mostly mild to moderate CRS (42% Grade 1, 3% Grade 2, 0% Grade 3, 3% Grade 4), majority at first dose One DLT: Grade 4 CRS (fully resolved) No IMA402-related Grade 5 events MTD not reached 1All treatment-emergent adverse events (TEAEs) at least possibly related to IMA402 infusion with grade 1-2 occurring in at least 9% of patients and all events with grade 3-5; CRS: Cytokine release syndrome; MTD: Maximum tolerated dose.; One AE “Rash, Intermittent” was not coded at data cut-off, but added to the preferred term “Rash” Data cut-off Nov 6, 2024
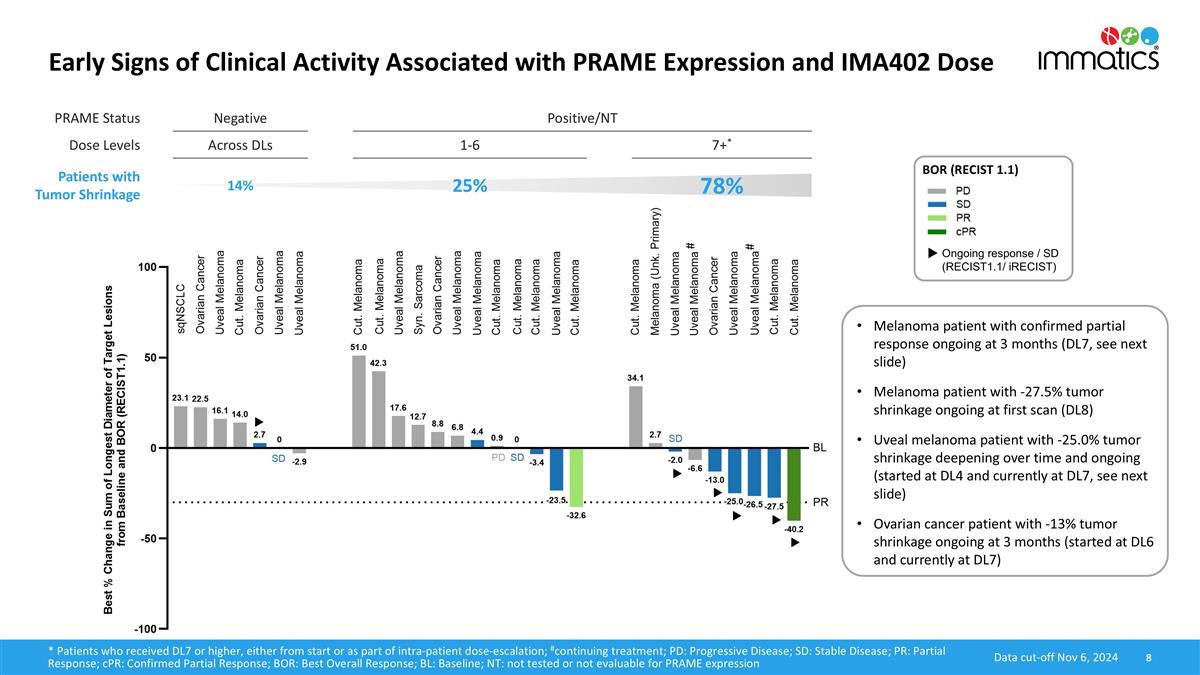
PRAME Status Negative Positive/NT Dose Levels Across DLs 1-6 7+* Patients with Tumor Shrinkage 14% 25% 78% Early Signs of Clinical Activity Associated with PRAME Expression and IMA402 Dose 8 BOR (RECIST 1.1) Ongoing response / SD (RECIST1.1/ iRECIST) Data cut-off Nov 6, 2024 * Patients who received DL7 or higher, either from start or as part of intra-patient dose-escalation; #continuing treatment; PD: Progressive Disease; SD: Stable Disease; PR: Partial Response; cPR: Confirmed Partial Response; BOR: Best Overall Response; BL: Baseline; NT: not tested or not evaluable for PRAME expression # # Melanoma patient with confirmed partial response ongoing at 3 months (DL7, see next slide) Melanoma patient with -27.5% tumor shrinkage ongoing at first scan (DL8) Uveal melanoma patient with -25.0% tumor shrinkage deepening over time and ongoing (started at DL4 and currently at DL7, see next slide) Ovarian cancer patient with -13% tumor shrinkage ongoing at 3 months (started at DL6 and currently at DL7)
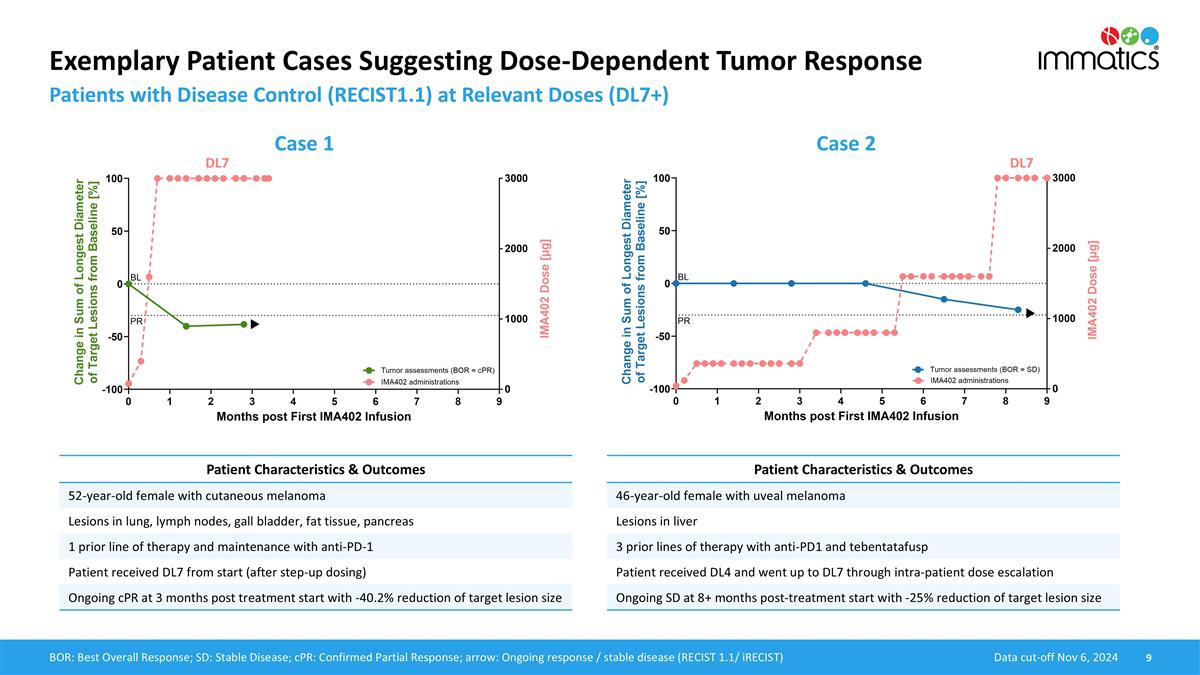
Exemplary Patient Cases Suggesting Dose-Dependent Tumor Response Patients with Disease Control (RECIST1.1) at Relevant Doses (DL7+) Data cut-off Nov 6, 2024 BOR: Best Overall Response; SD: Stable Disease; cPR: Confirmed Partial Response; arrow: Ongoing response / stable disease (RECIST 1.1/ iRECIST) Case 1 Case 2 DL7 DL7 Patient Characteristics & Outcomes 52-year-old female with cutaneous melanoma Lesions in lung, lymph nodes, gall bladder, fat tissue, pancreas 1 prior line of therapy and maintenance with anti-PD-1 Patient received DL7 from start (after step-up dosing) Ongoing cPR at 3 months post treatment start with -40.2% reduction of target lesion size Patient Characteristics & Outcomes 46-year-old female with uveal melanoma Lesions in liver 3 prior lines of therapy with anti-PD1 and tebentatafusp Patient received DL4 and went up to DL7 through intra-patient dose escalation Ongoing SD at 8+ months post-treatment start with -25% reduction of target lesion size

Appendix
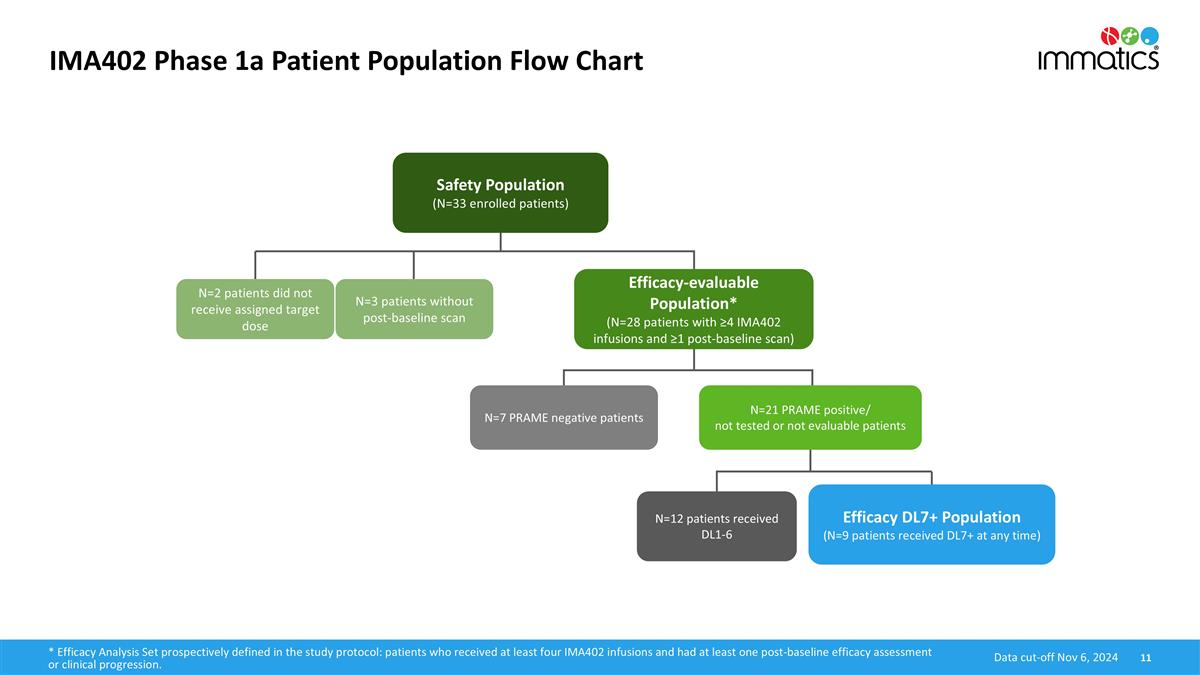
IMA402 Phase 1a Patient Population Flow Chart Safety Population (N=33 enrolled patients) Efficacy-evaluable Population* (N=28 patients with ≥4 IMA402 infusions and ≥1 post-baseline scan) N=21 PRAME positive/ not tested or not evaluable patients N=2 patients did not receive assigned target dose N=3 patients without post-baseline scan N=7 PRAME negative patients N=12 patients received DL1-6 Efficacy DL7+ Population (N=9 patients received DL7+ at any time) * Efficacy Analysis Set prospectively defined in the study protocol: patients who received at least four IMA402 infusions and had at least one post-baseline efficacy assessment or clinical progression. Data cut-off Nov 6, 2024












