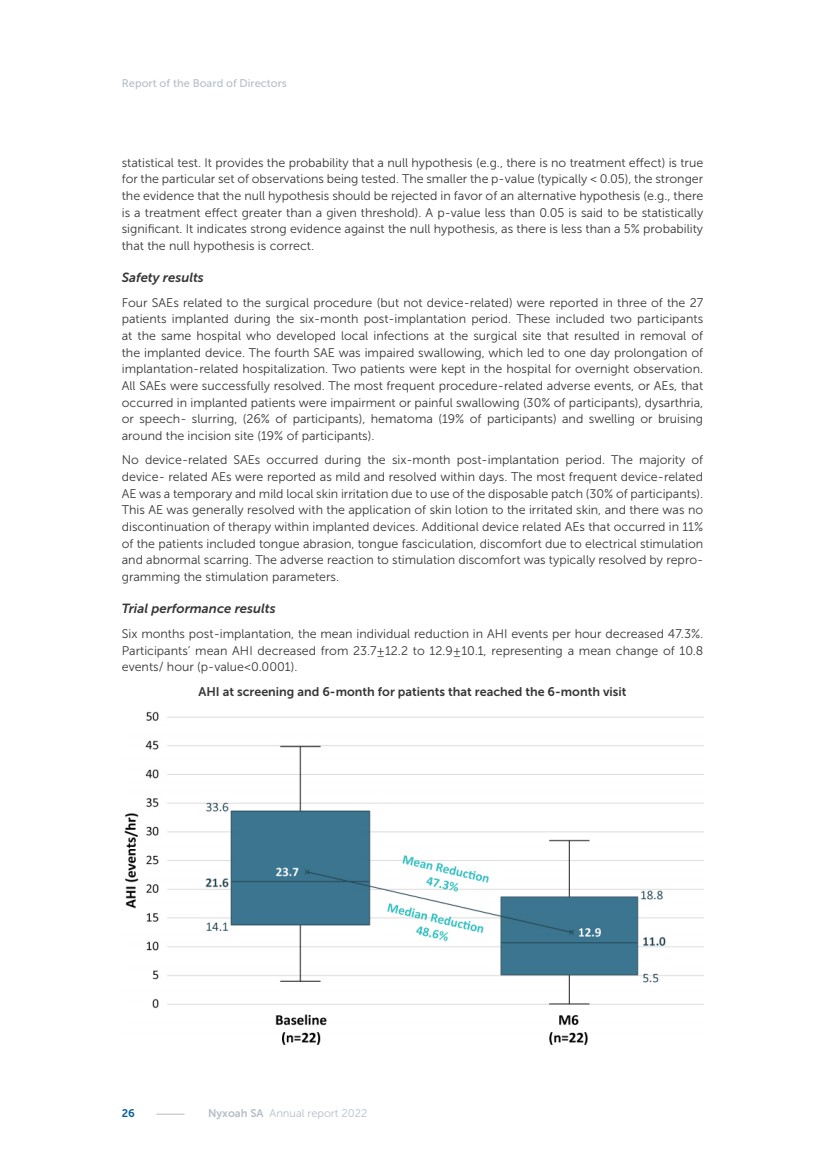
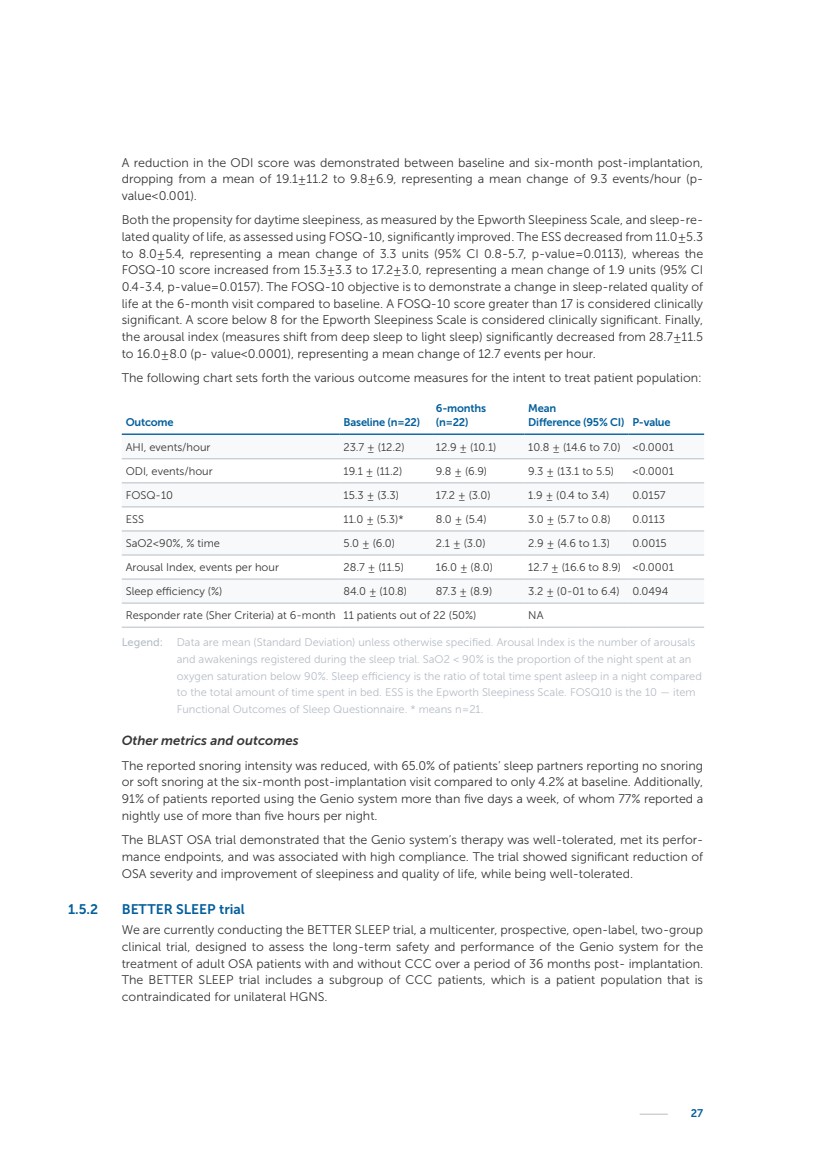
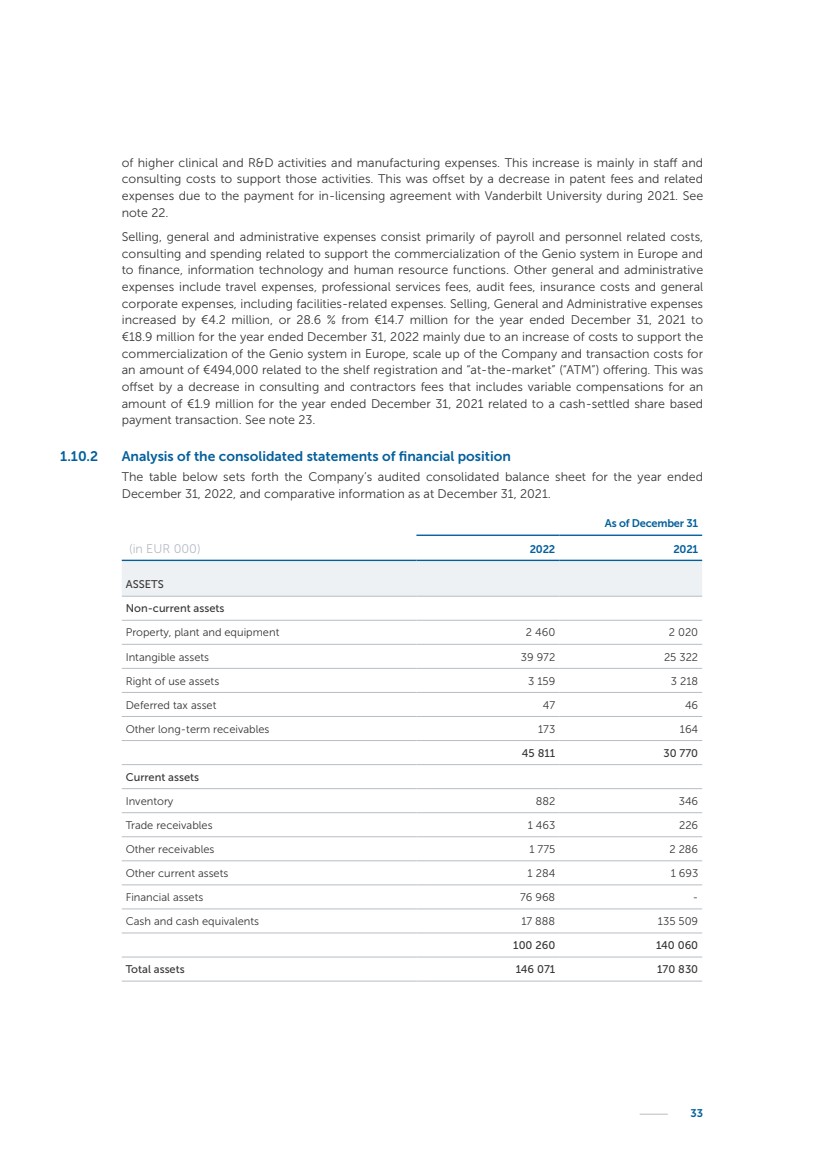
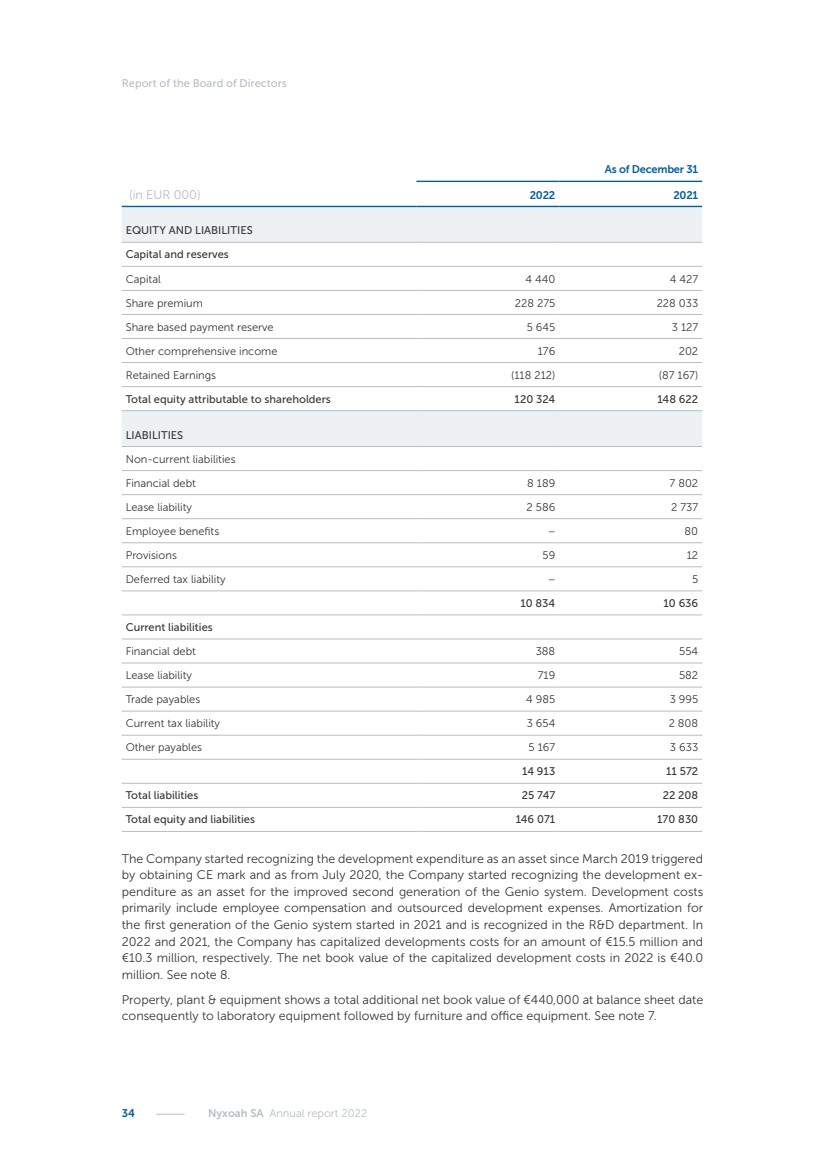
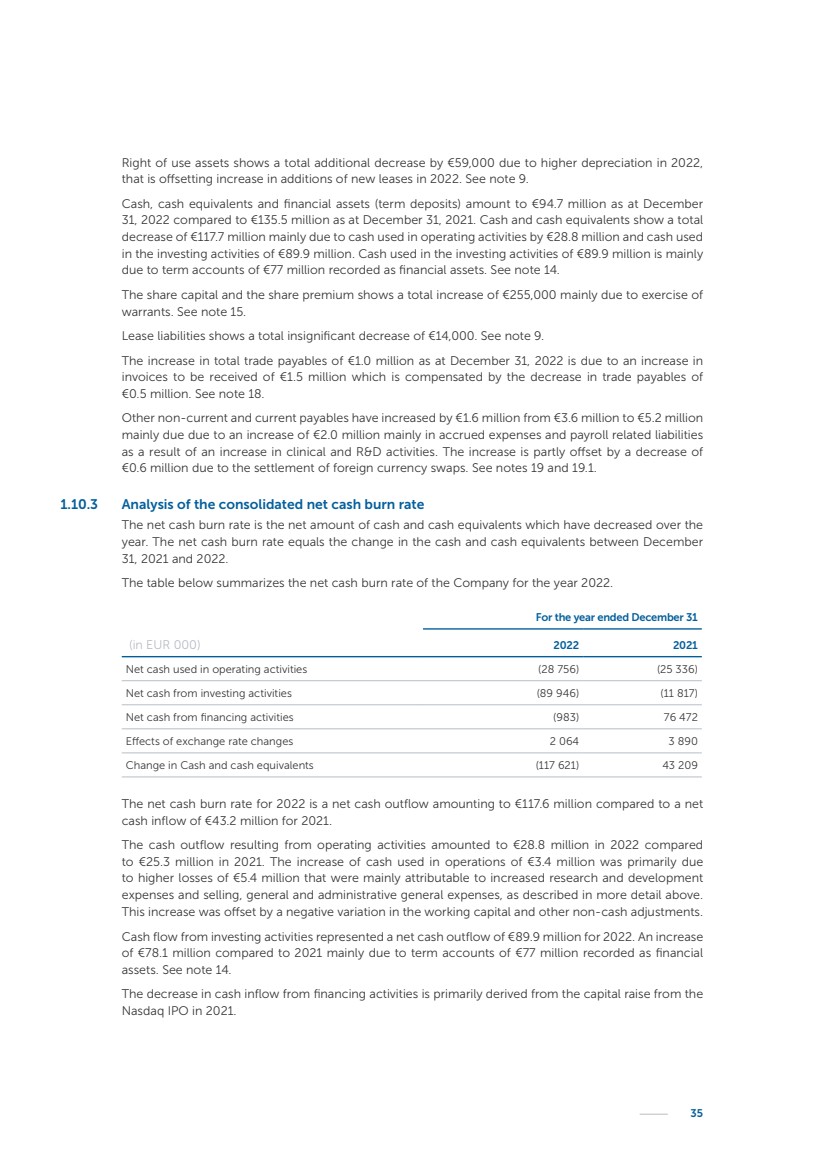
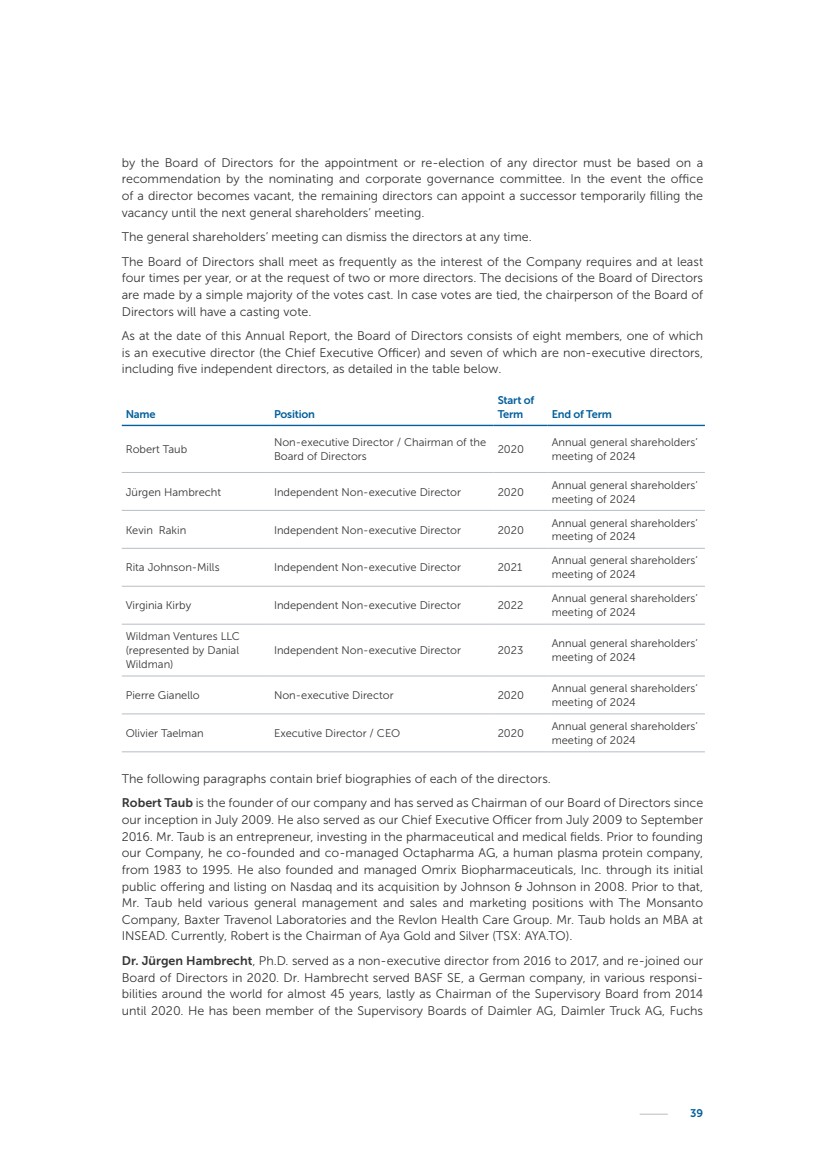
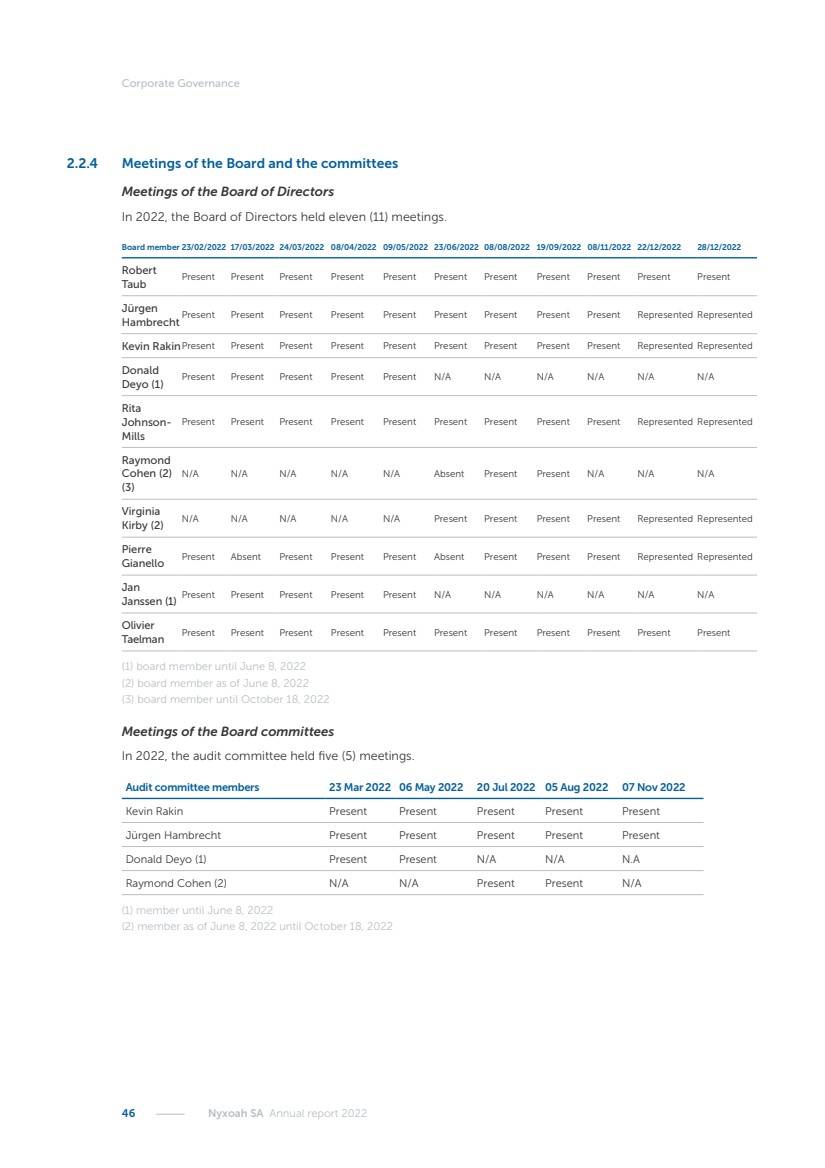
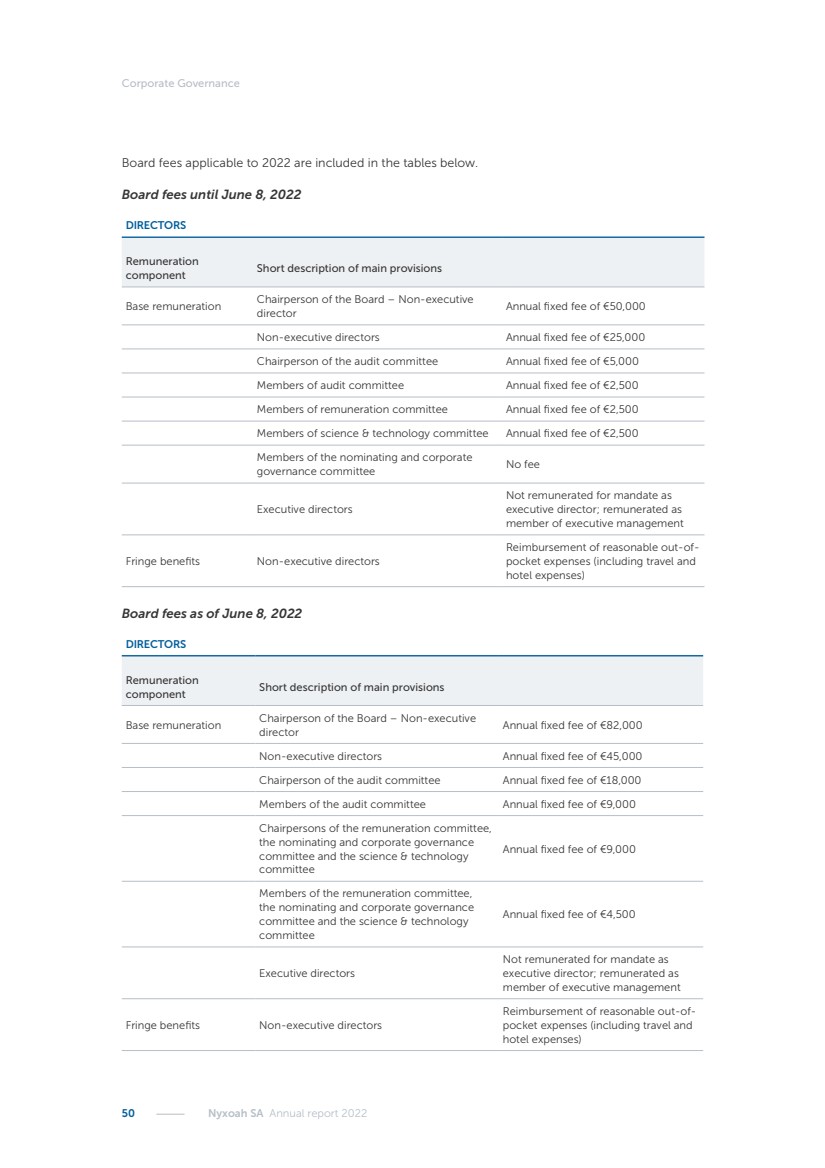
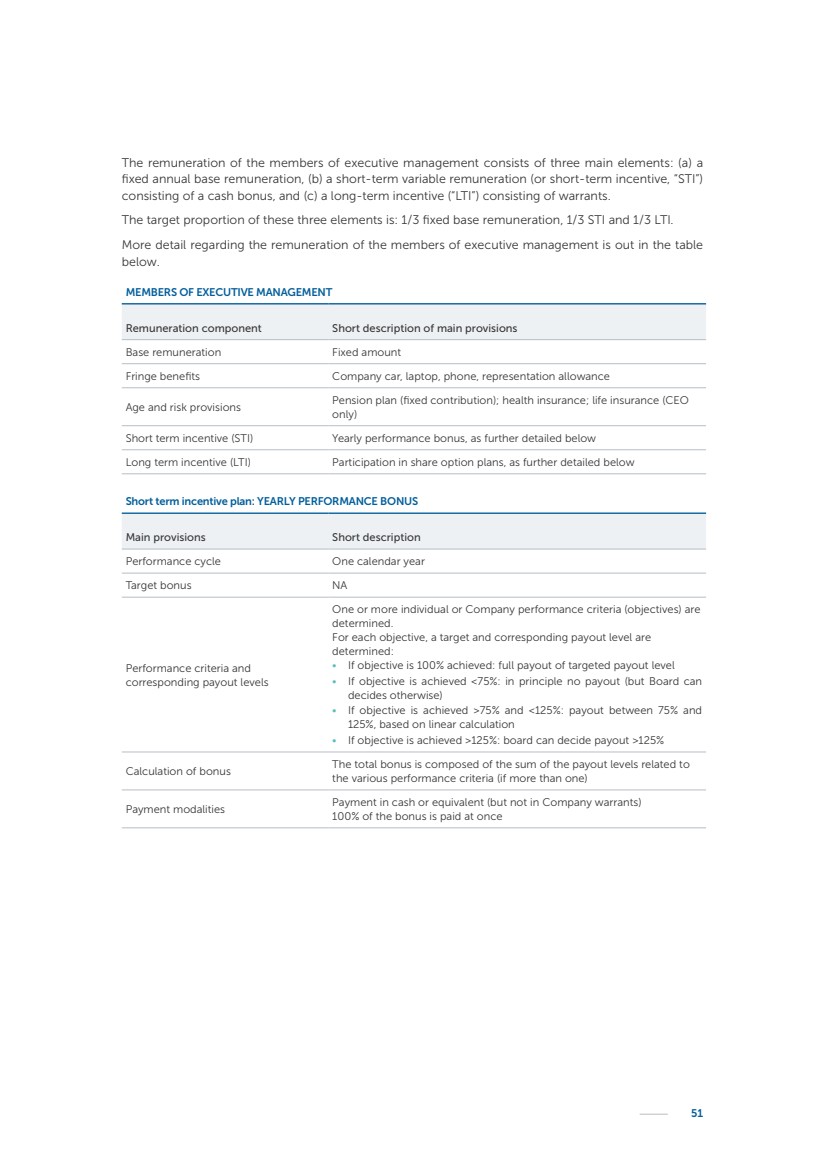
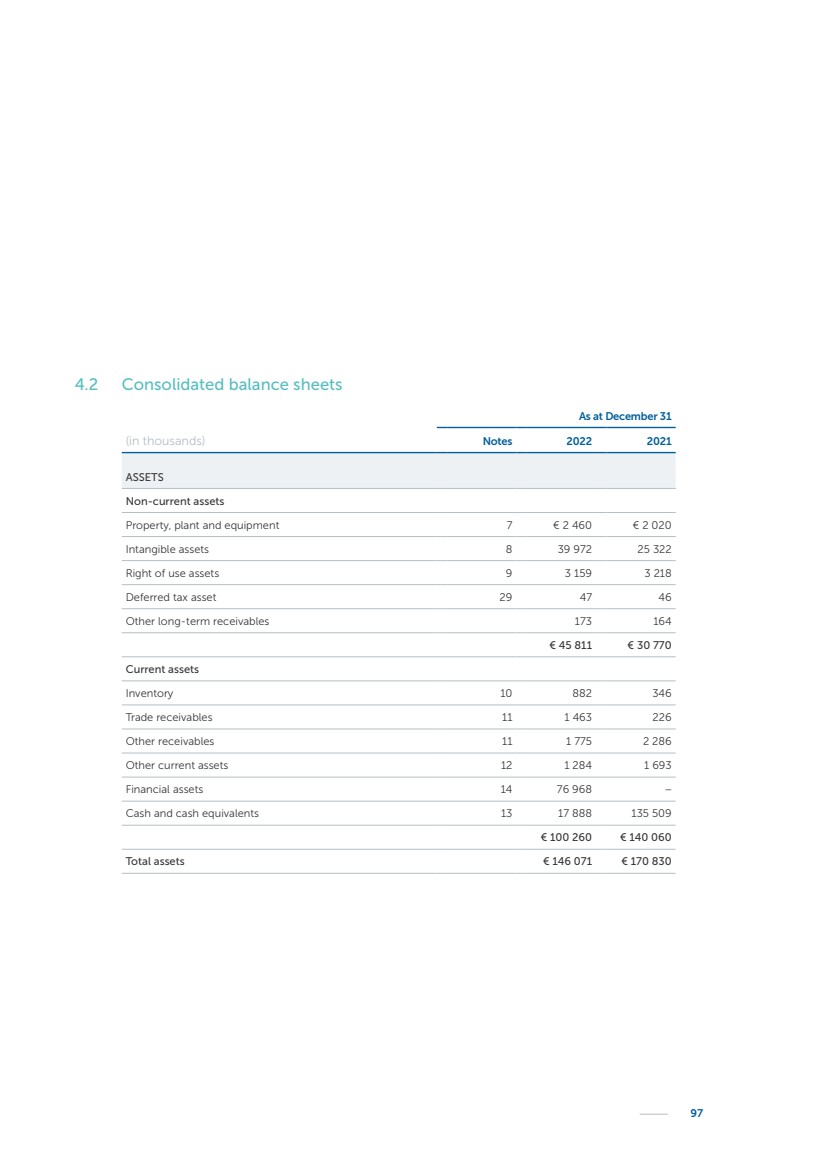
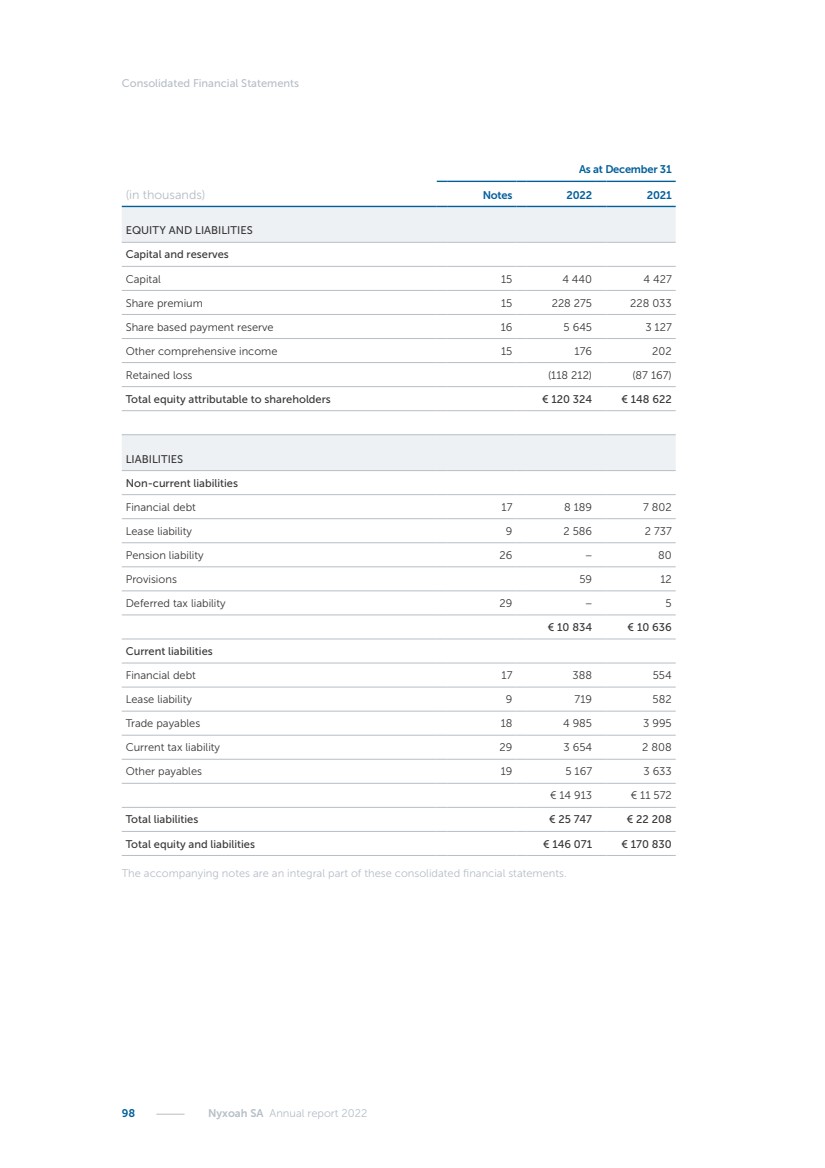
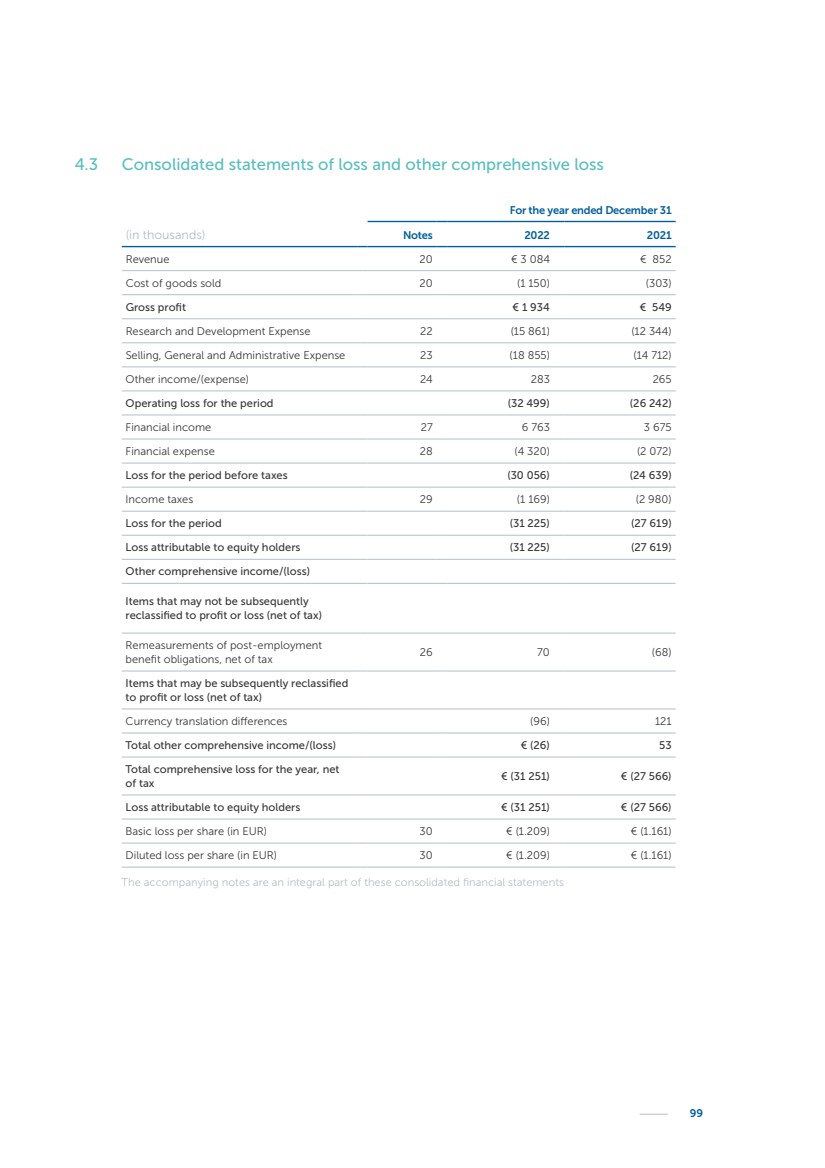
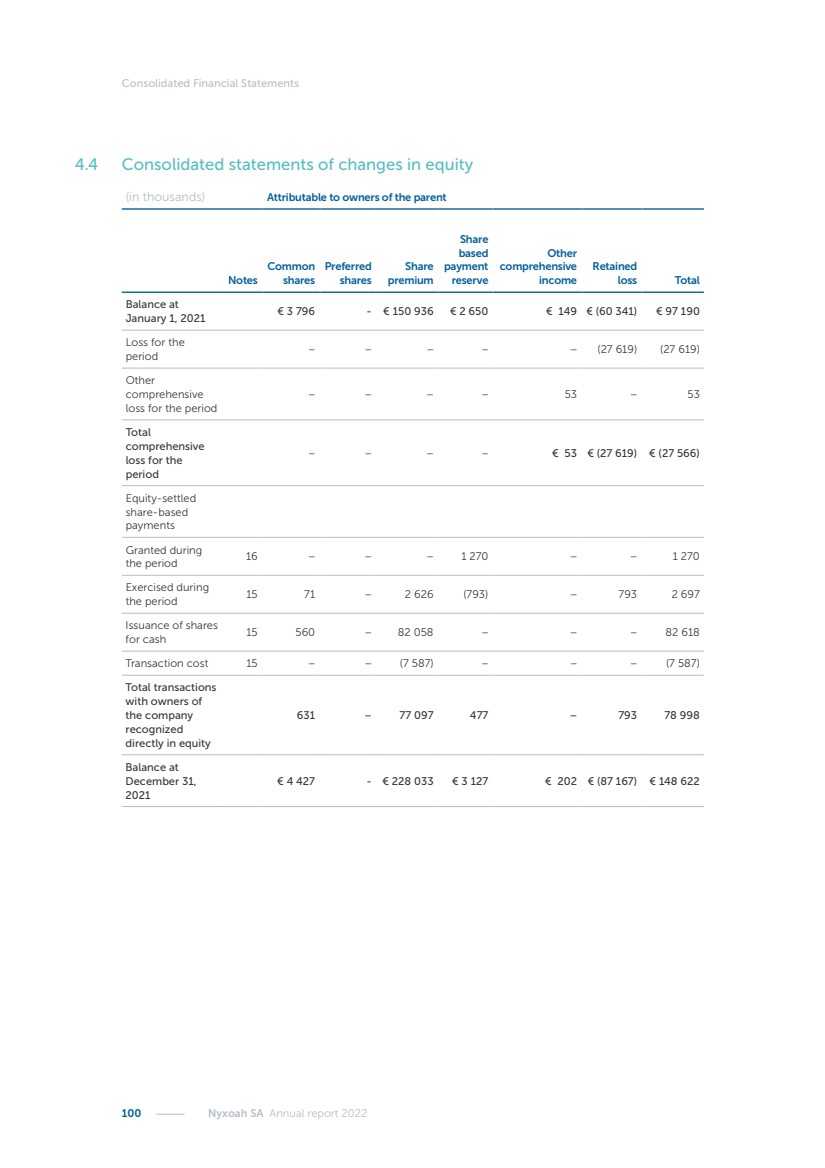
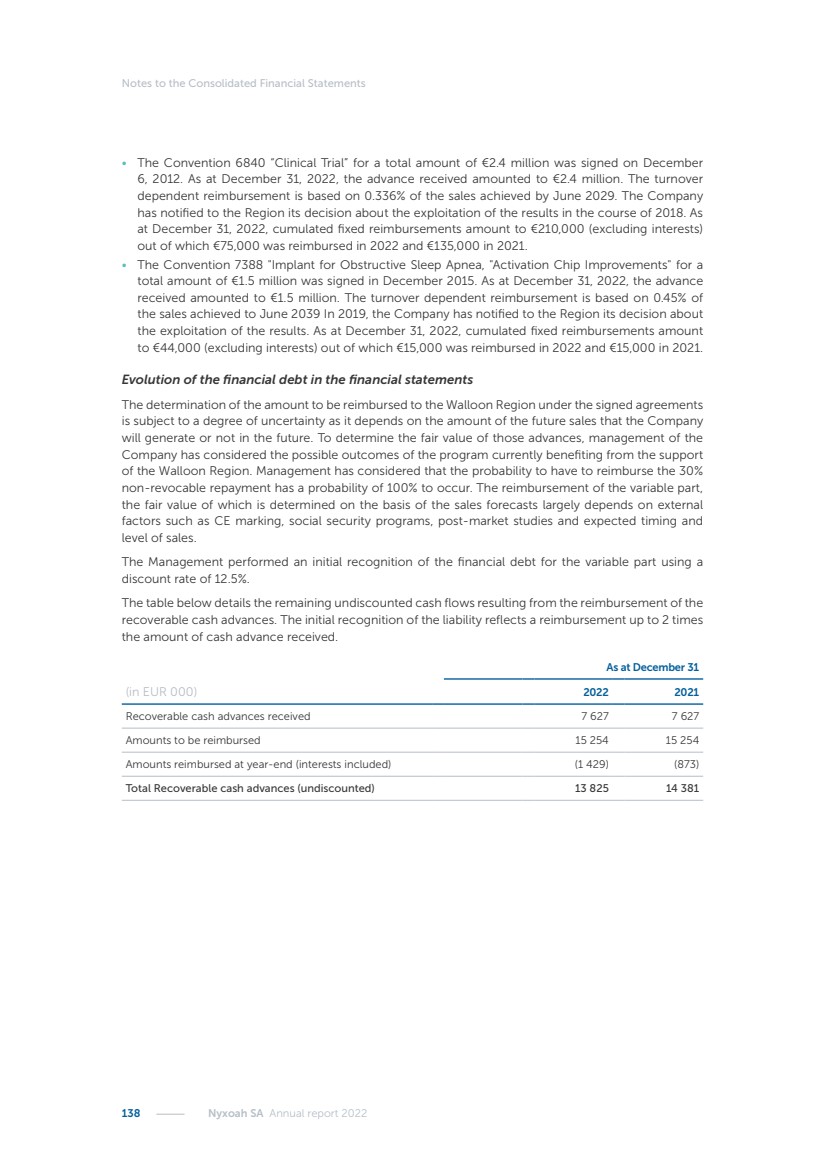
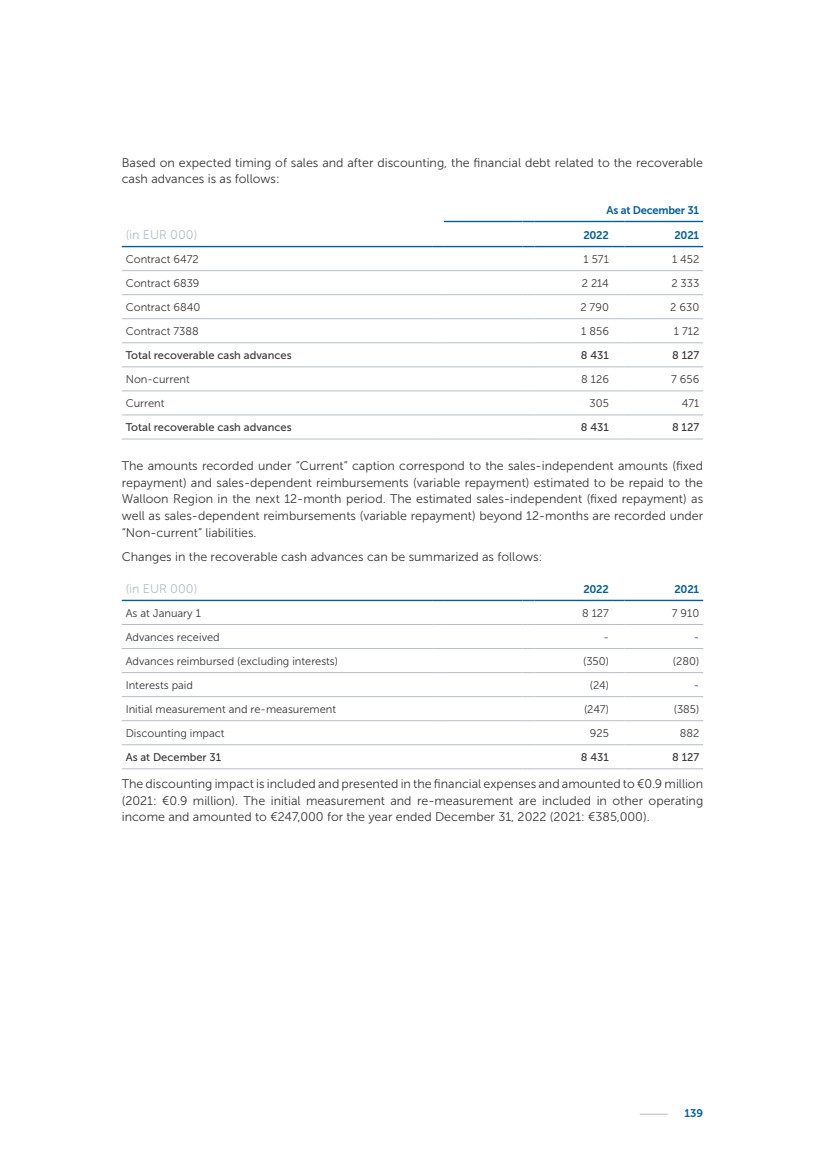
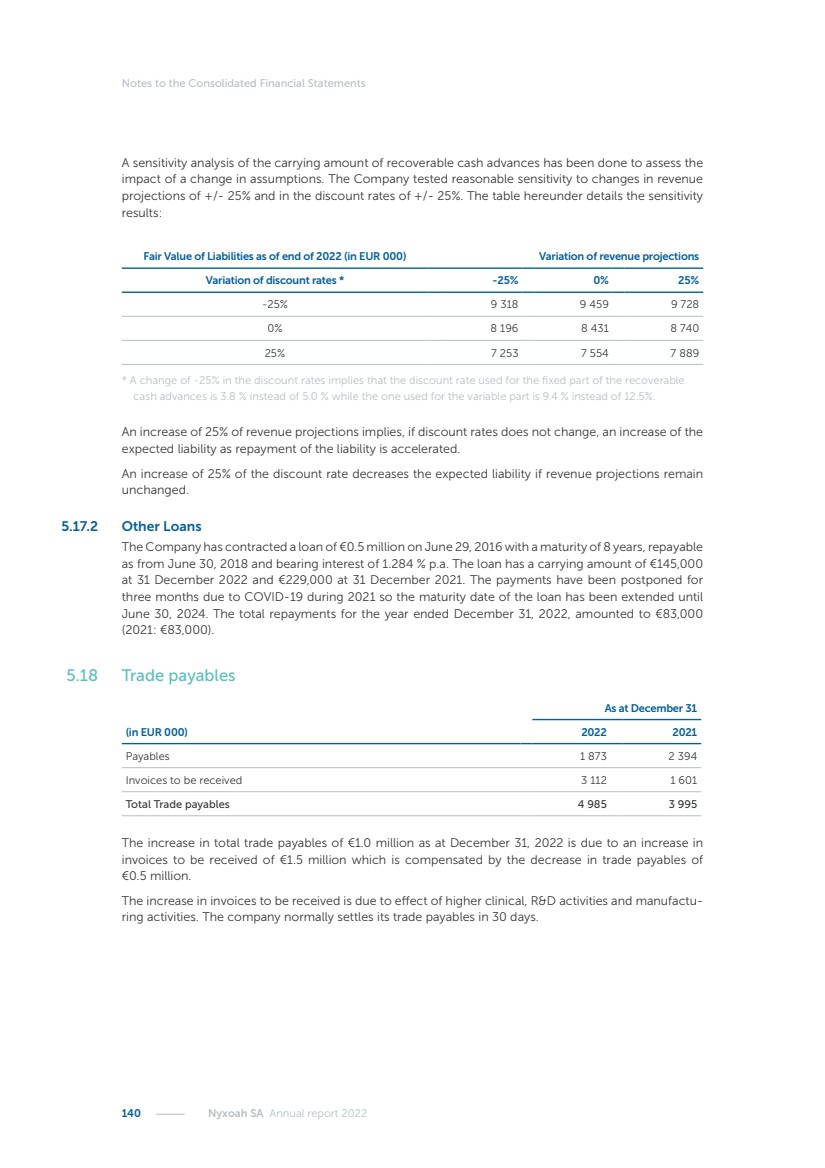
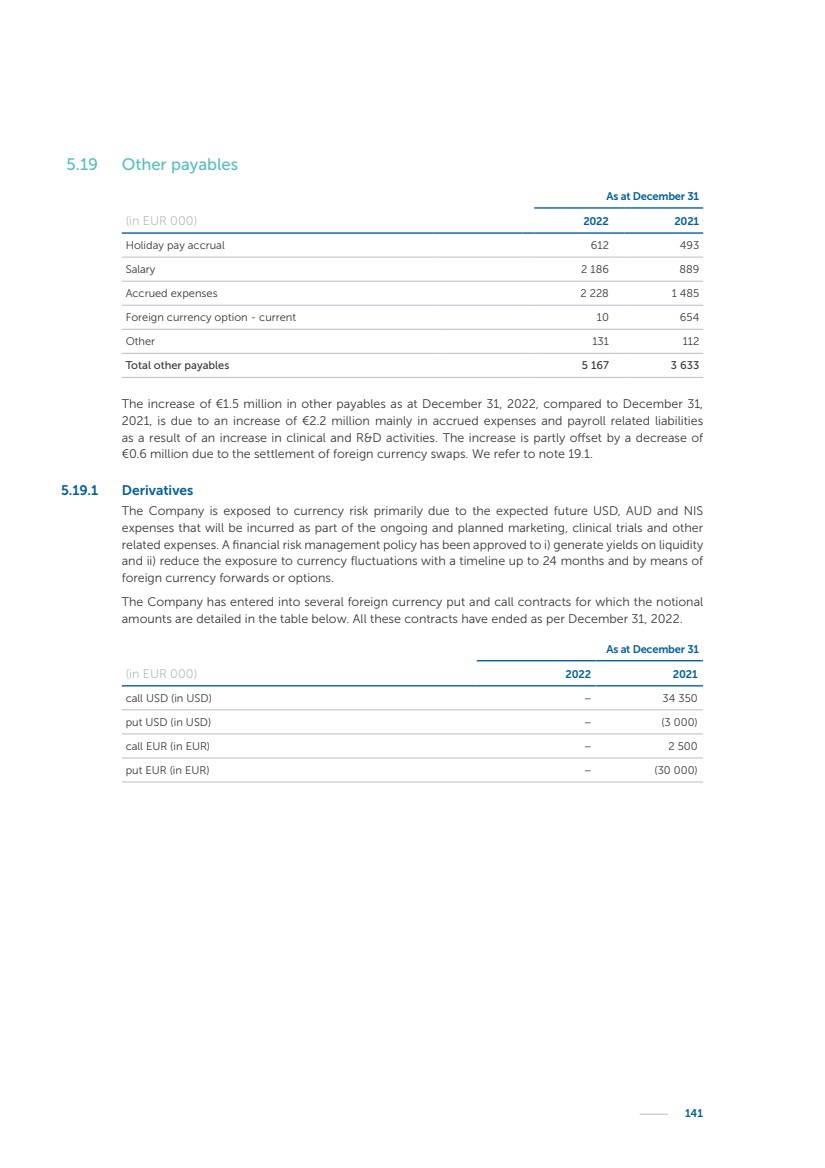
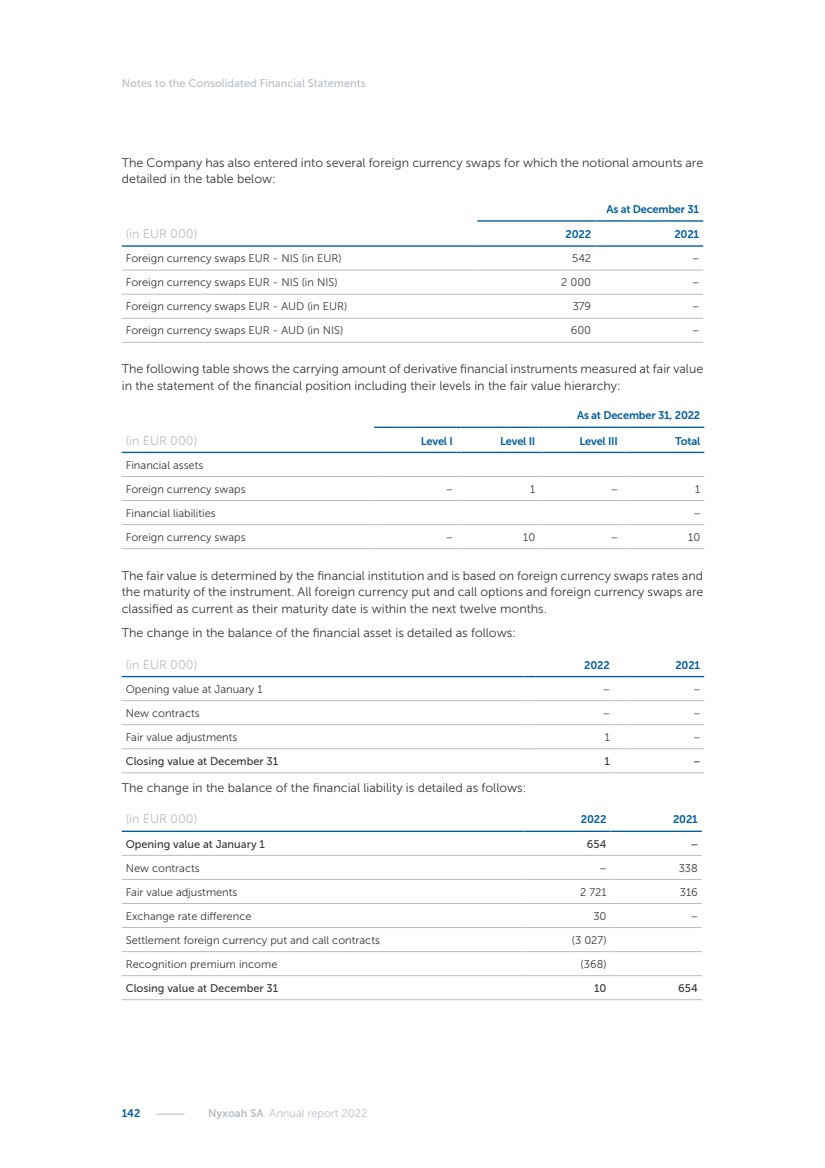
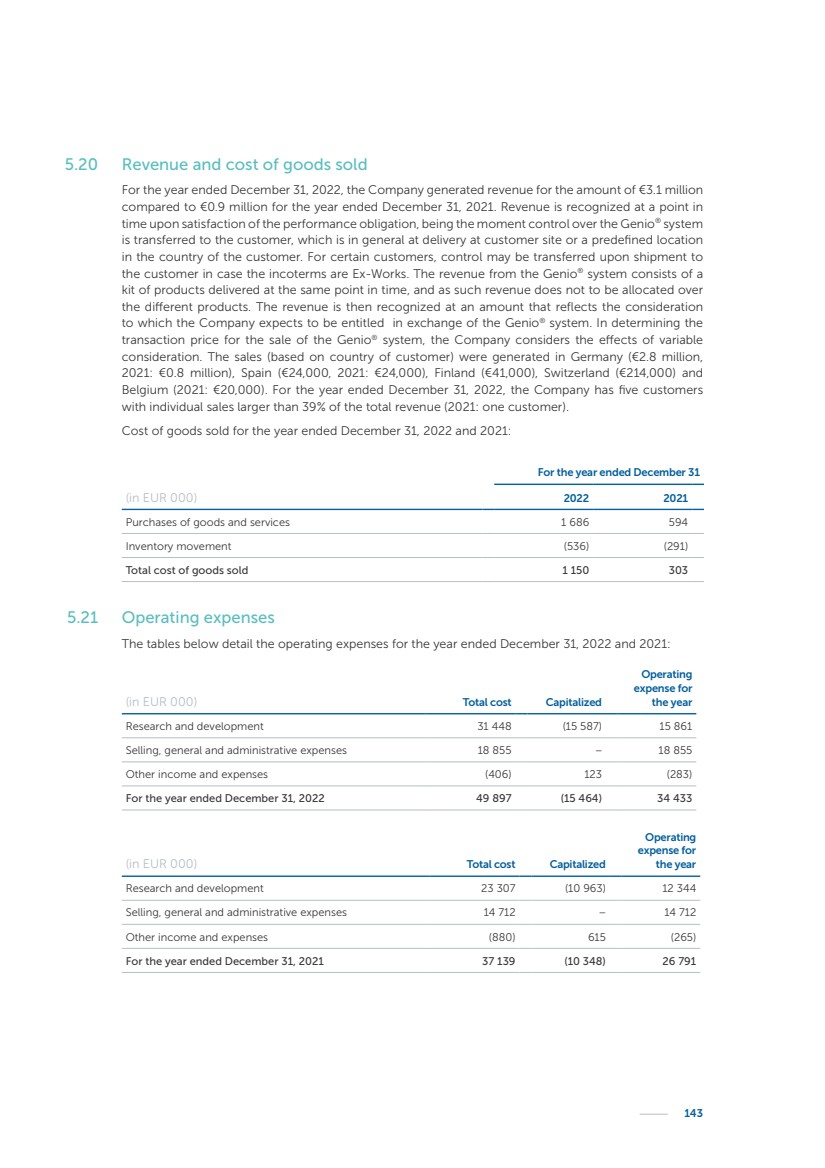
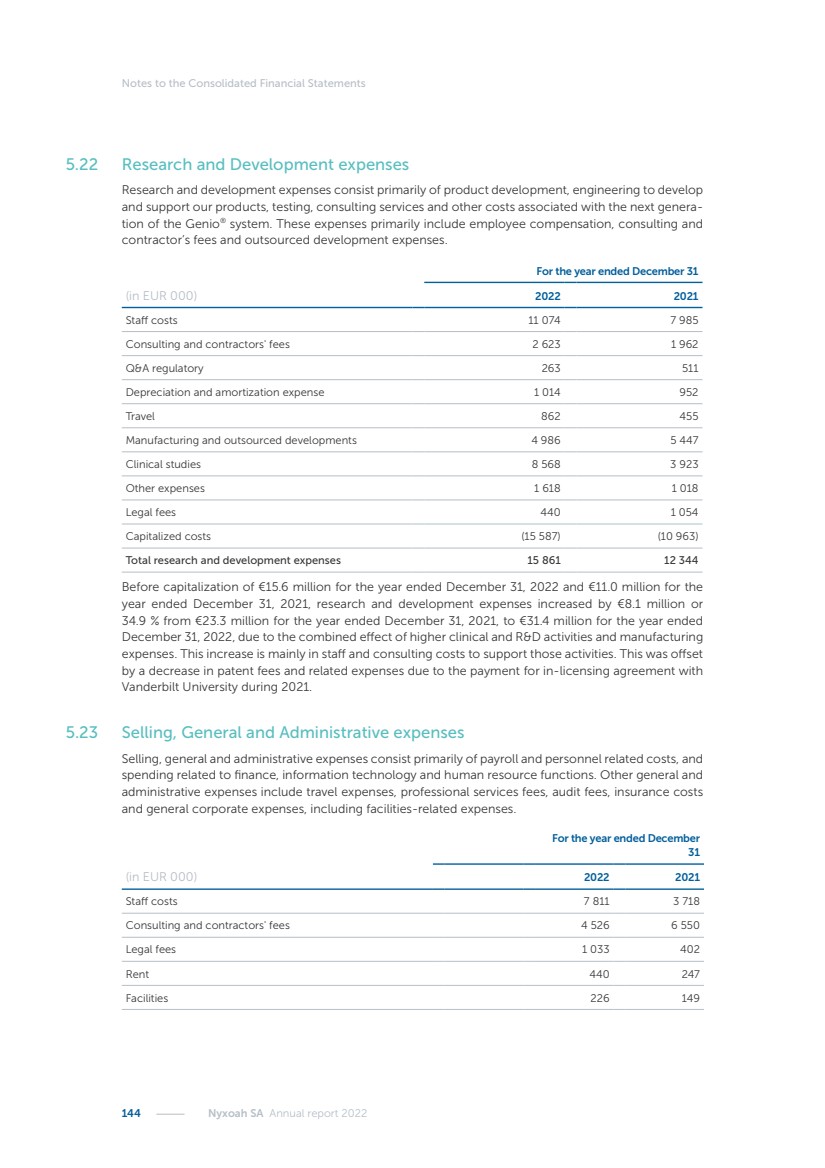
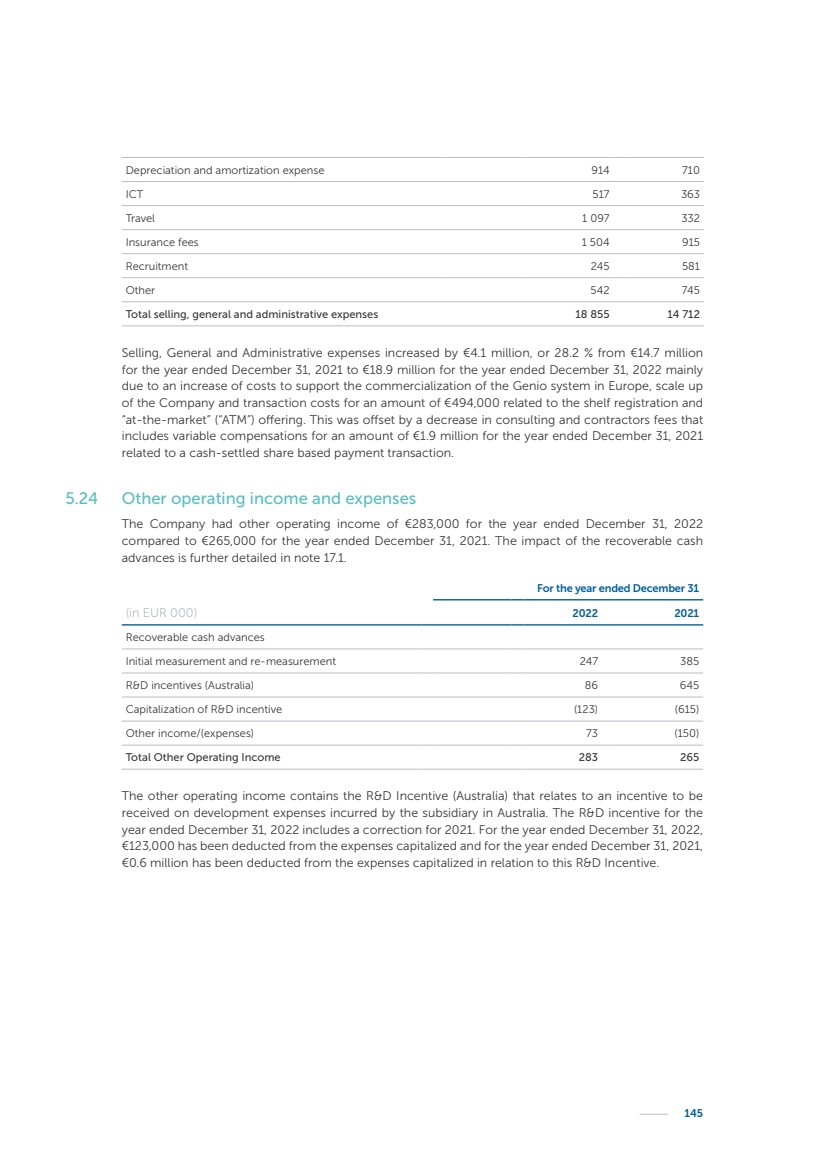
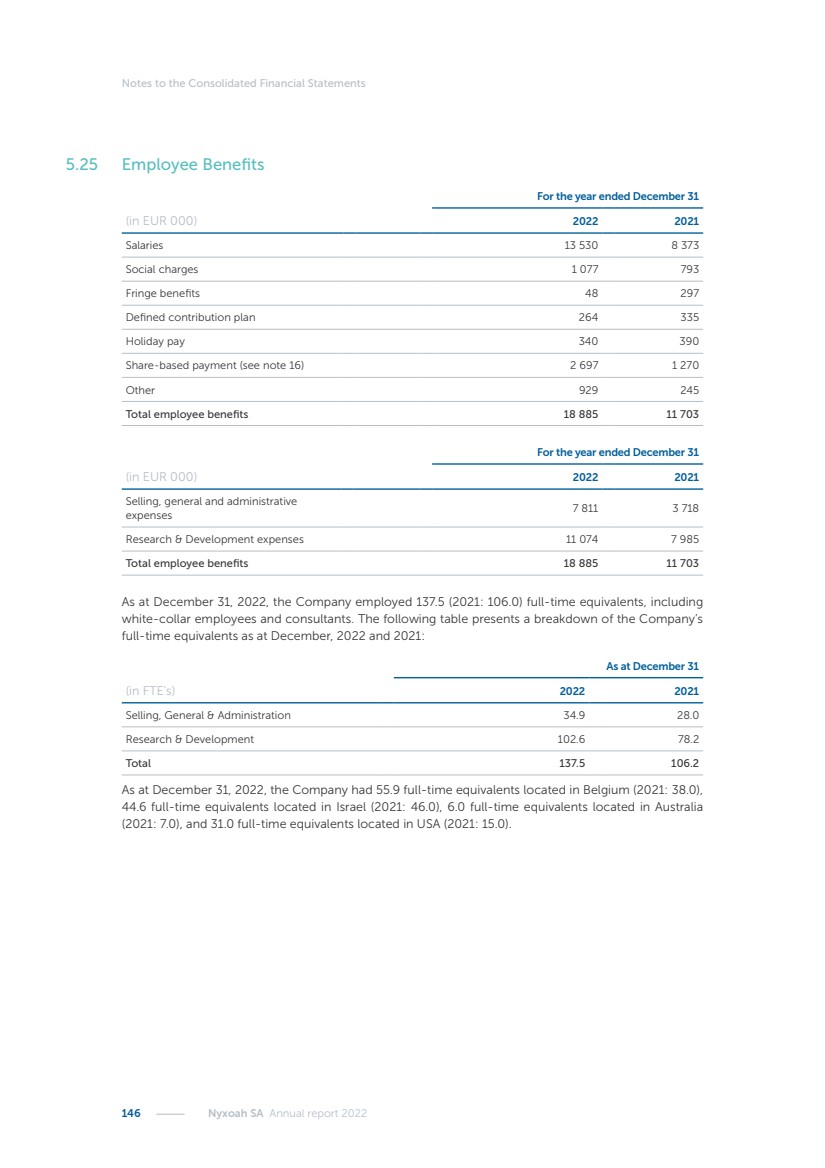
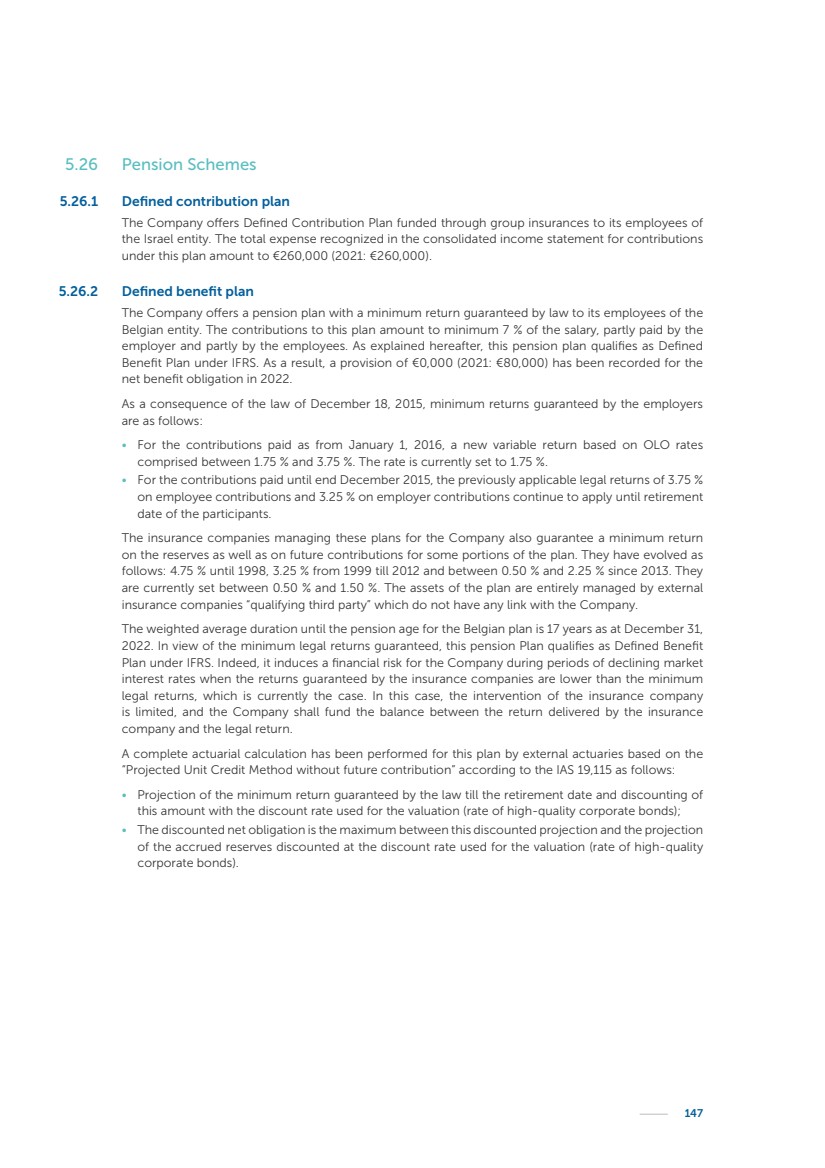
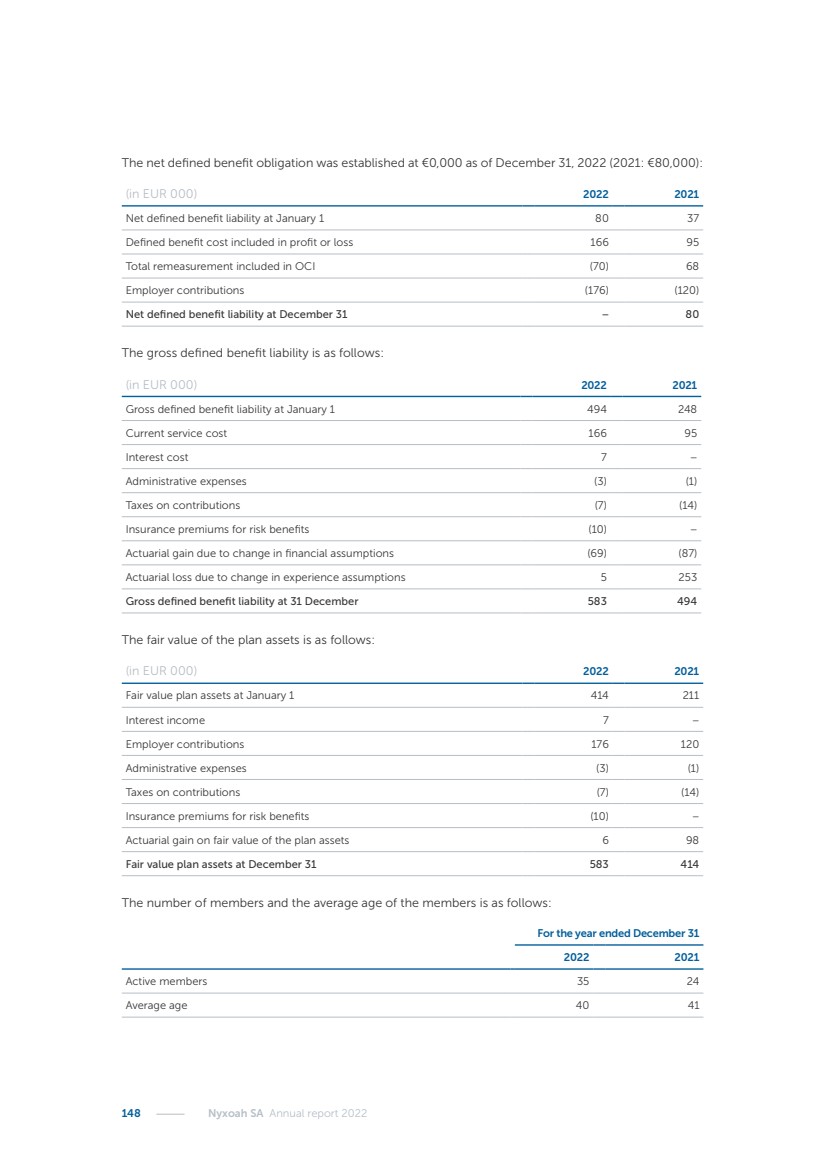
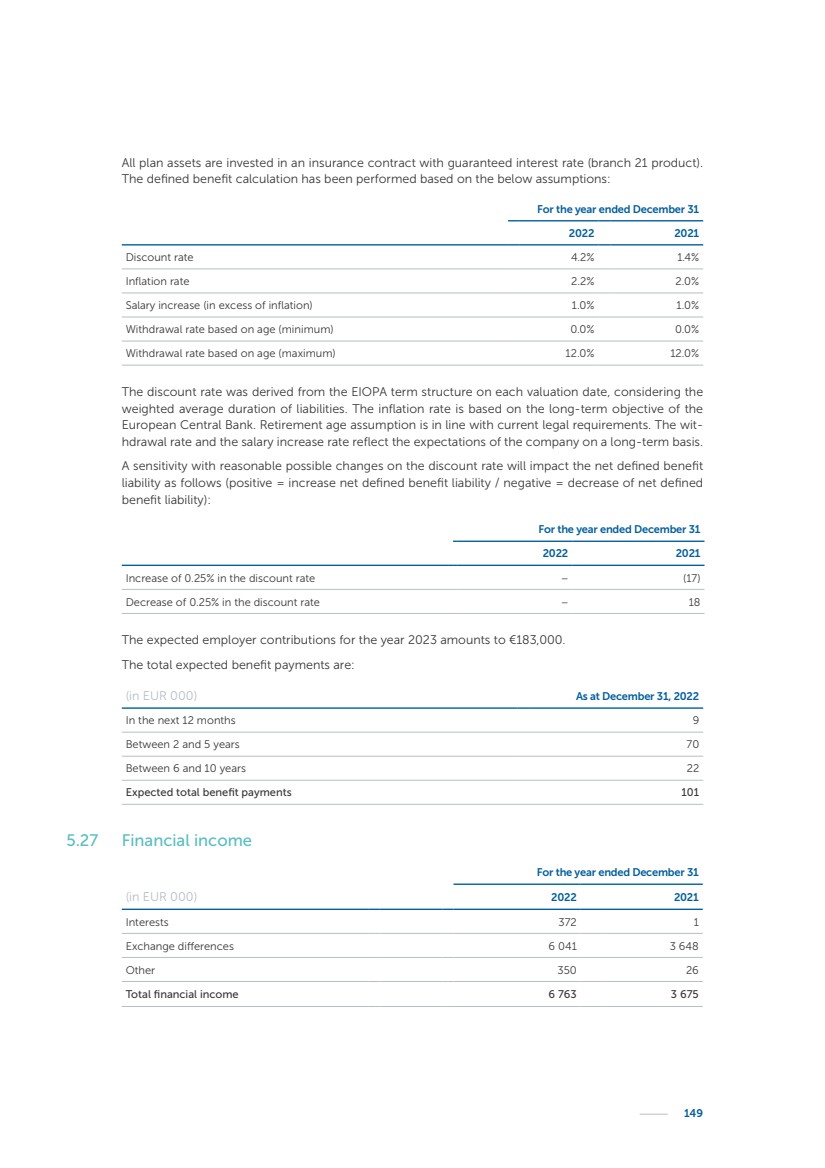
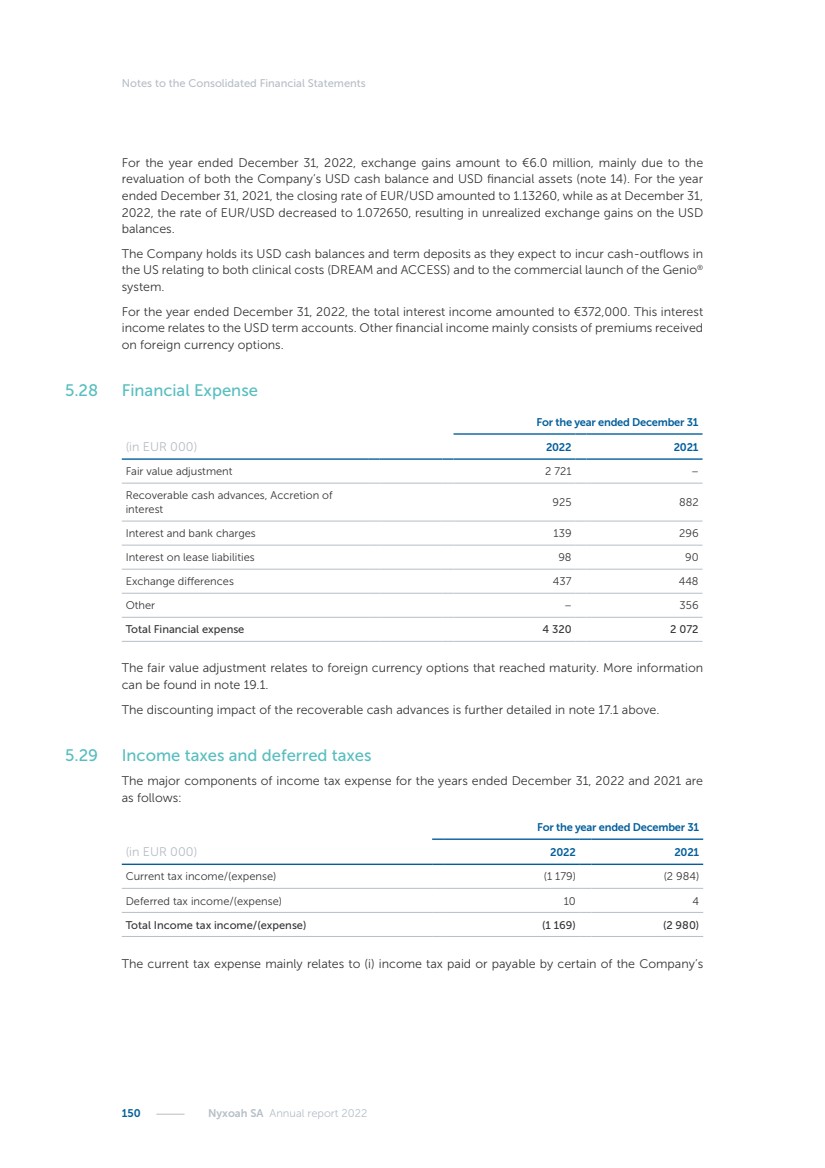
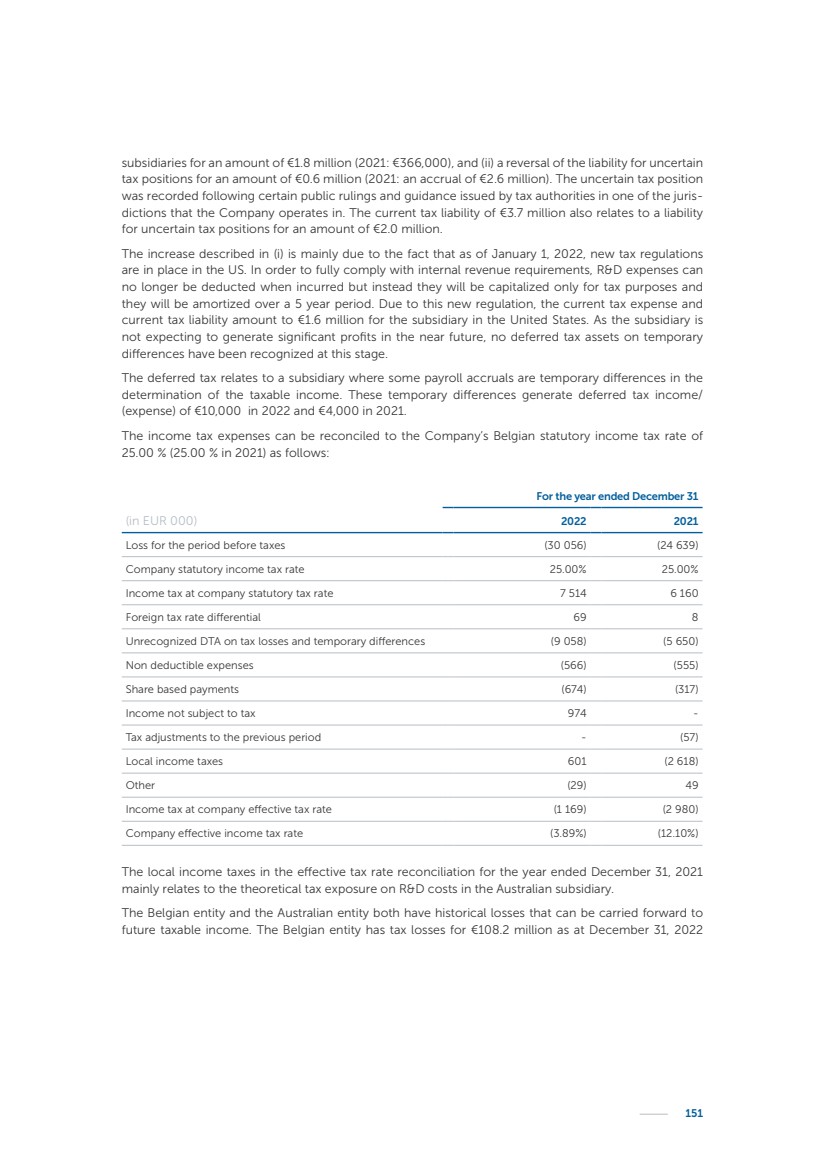
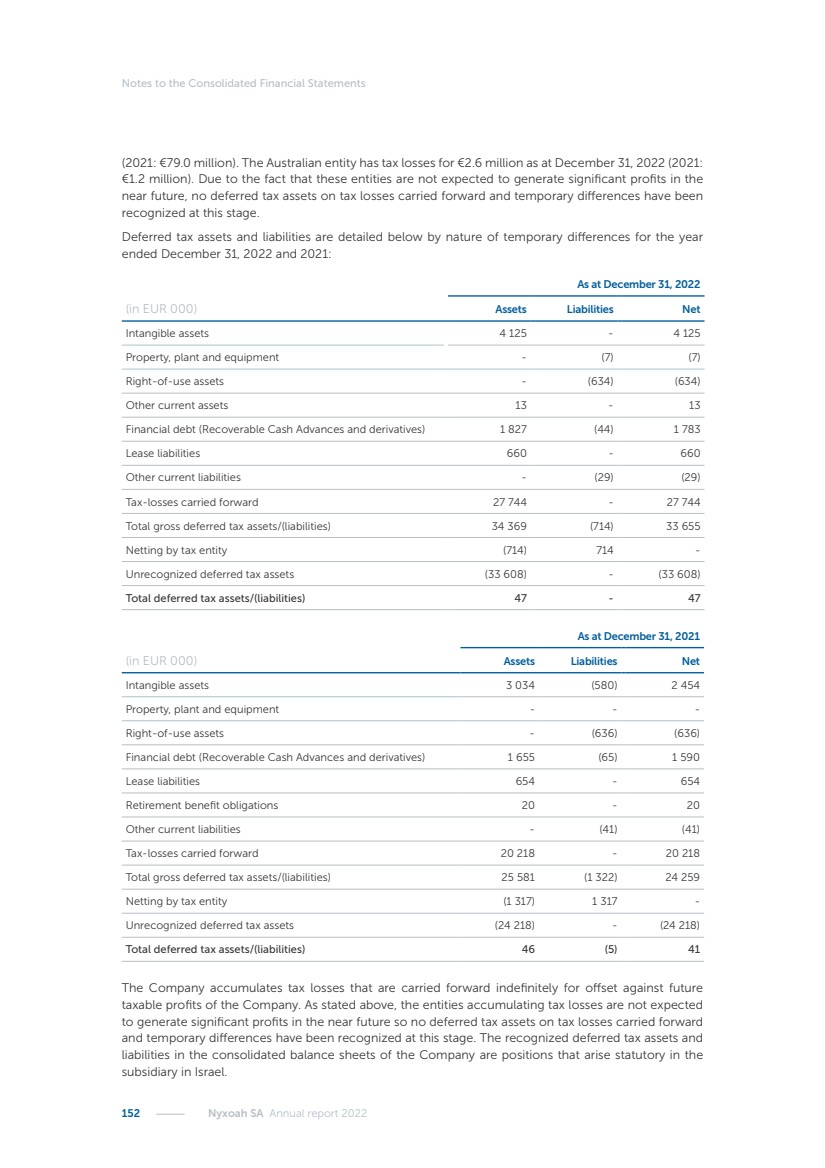
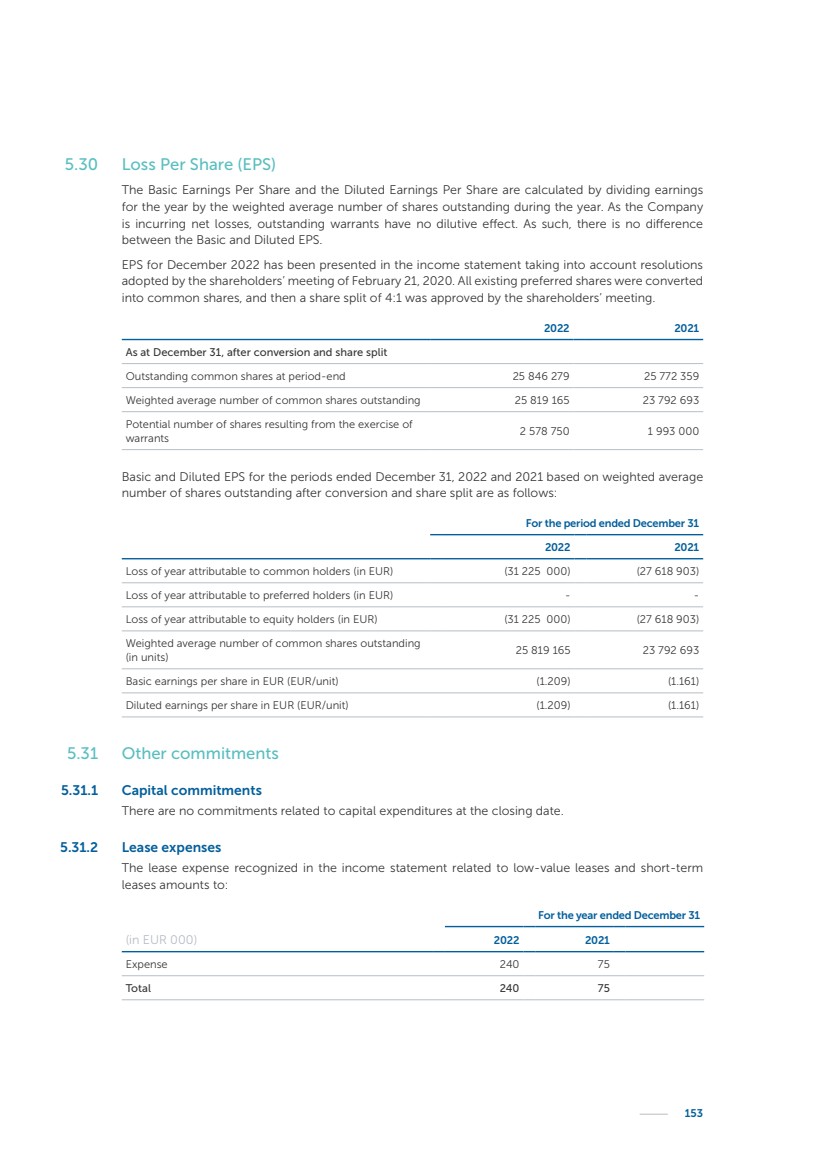
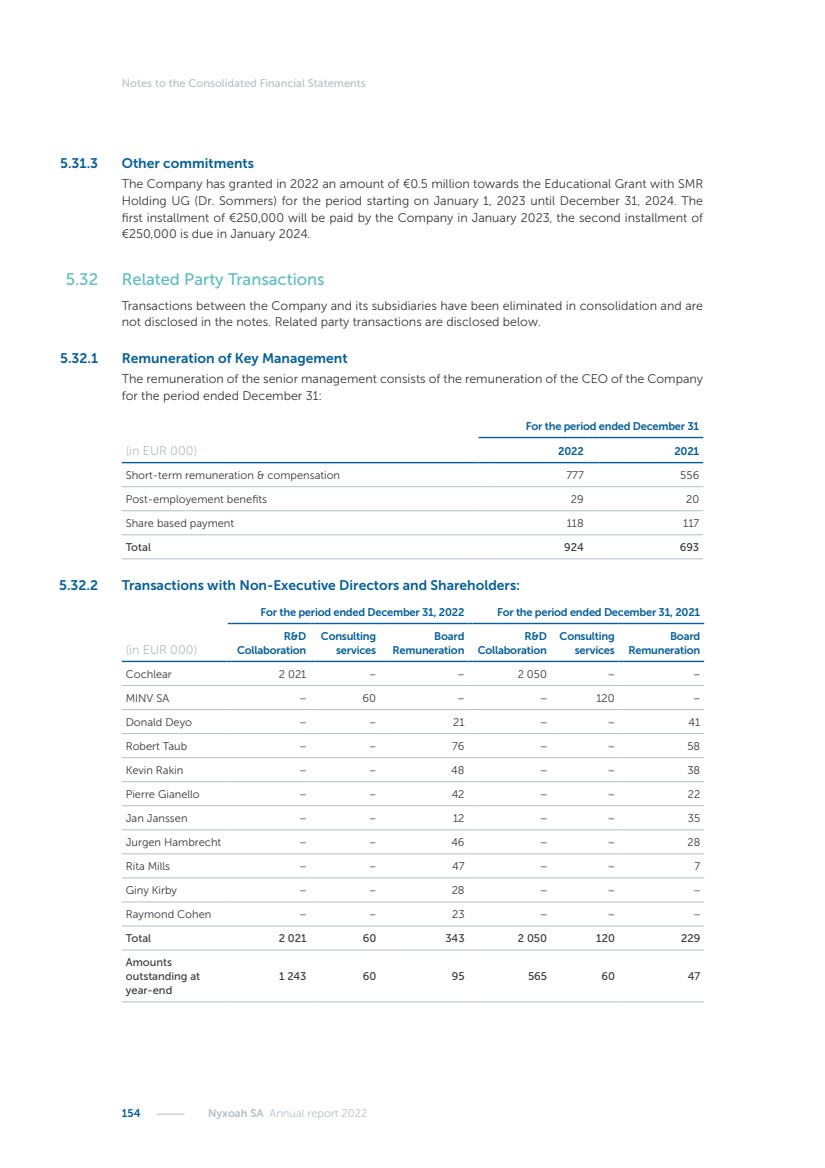
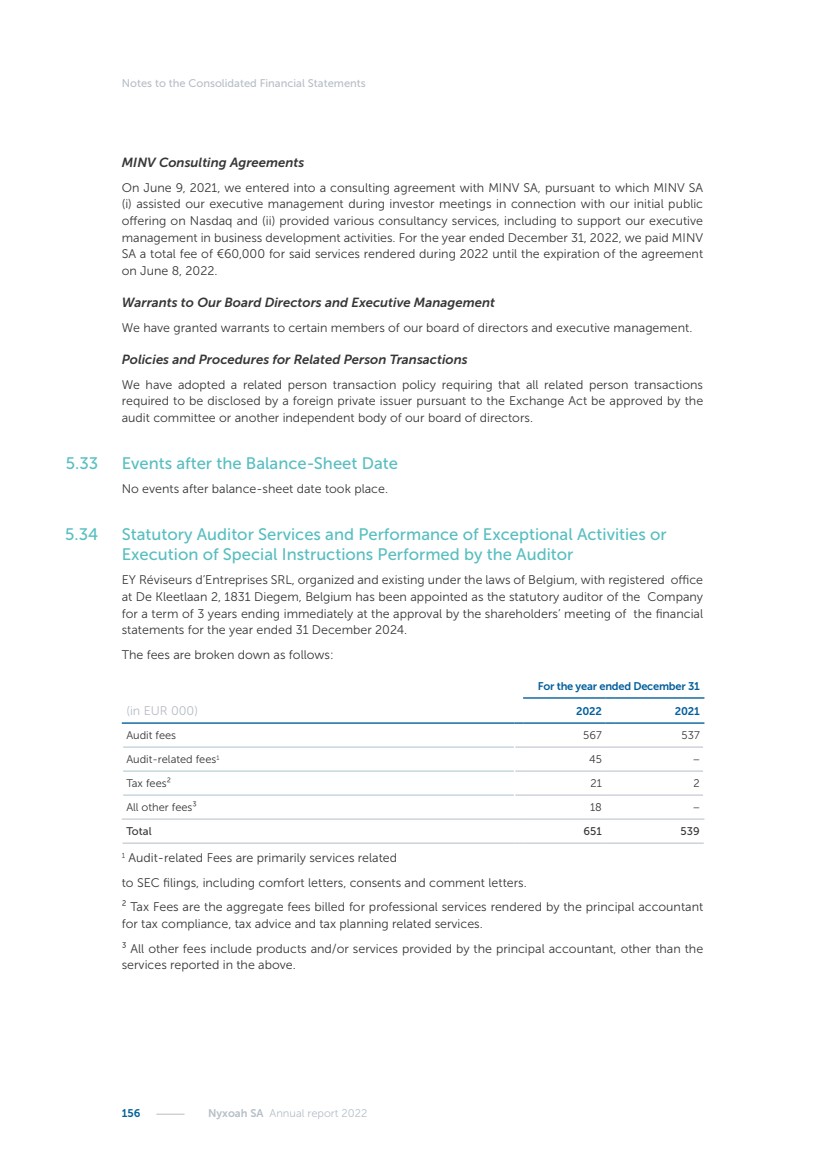
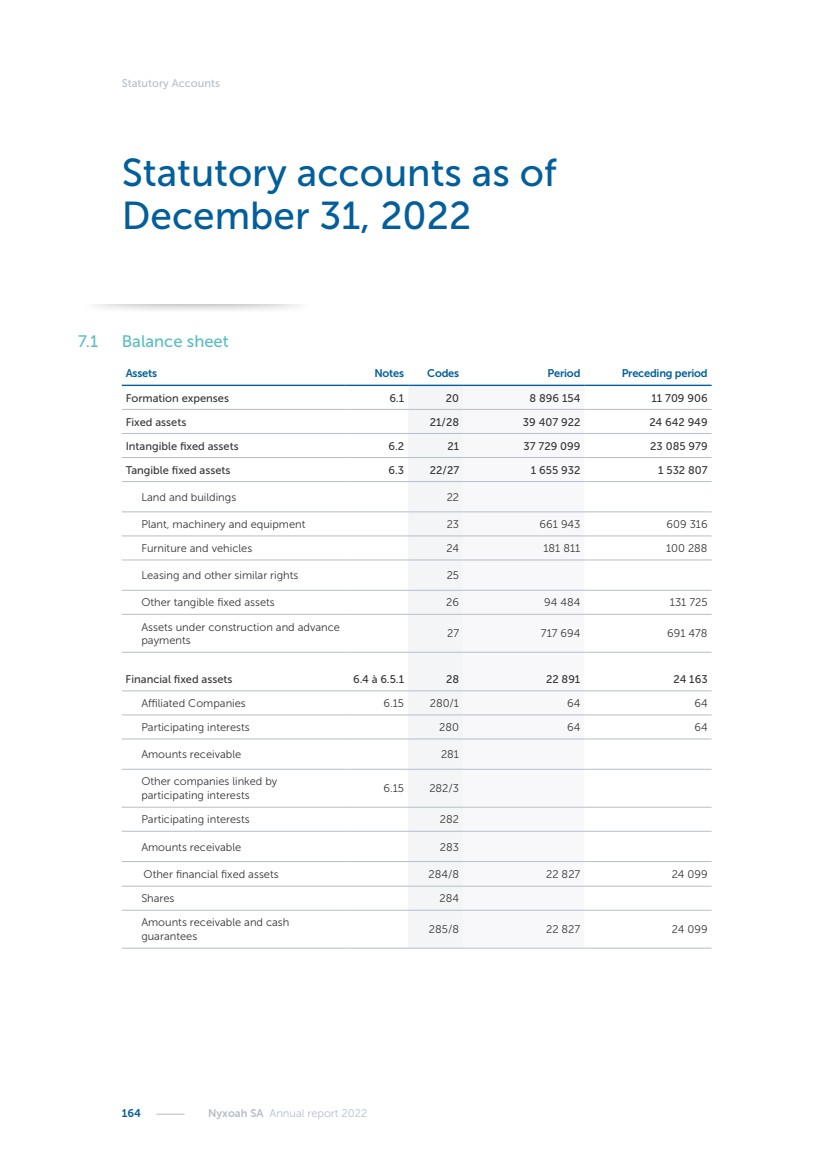
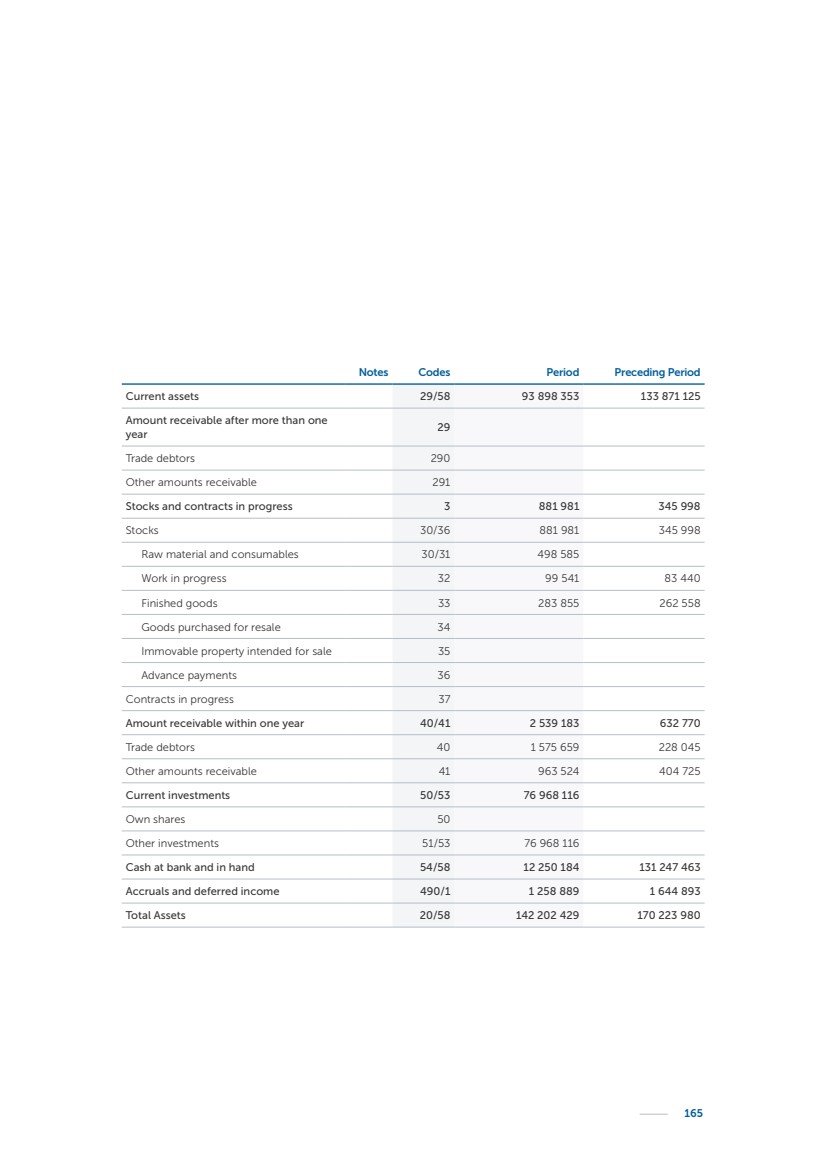
| 16 Nyxoah SA Annual report 2022 replace depleted batteries and enables software, firmware or external hardware updates and upgrades to be implemented without the need for surgical intervention thereby limiting potential infection risk due to an additional procedure. We continue to develop a substantial body of clinical evidence on the Genio system. In 2019, we completed our BiLAteral hypoglossal nerve STimulation for treatment of Obstructive Sleep Apnea, or BLAST OSA, trial, a prospective, open label, non-randomized, single arm treatment trial involving 27 implanted participants. Twenty-two patients completed the protocol, and the trial met all primary, secondary and exploratory endpoints. In the six-month data, the mean individual reduction in the Apnea-Hypopnea Index, or AHI, events per hour was 47.3%. Participants’ AHI decreased from 23.7±12.2 to 12.9±10.1, representing a mean change of 10.8 events per hour. The results of the trial were published in the European Respiratory Journal in October 2019 and were the basis for receiving CE-Mark on the Genio system. We are seeking to expand indications of the Genio system by obtaining clinical evidence through our ongoing multicenter, prospective, open-label BilatEral Hypoglossal Nerve StimulaTion for TreatmEnt of ObstRuctive SLEEP Apnoea With and Without Complete Concentric Collapse clinical trial in Australia and New Zealand, or the BETTER SLEEP trial, to evaluate the effectiveness of the Genio system for patients suffering from CCC. We believe that positive results from this trial may eliminate the need for Genio system patients to be selected based on a DISE procedure prior to implantation of the Genio system, thereby leading to a potential indication expansion in Europe. In June 2021, we announced initial top-line results from the six-month data for the BETTER SLEEP trial. Based on this data, in October 2021, the EU Notified Body granted CE-Marked indication to include OSA patients with CCC for the Genio system in Europe, which should eliminate the need for a DISE procedure. Additionally, in September 2021, we received breakthrough device designation in the United States for the Genio system from the Food and Drug Administration, or FDA, for the treatment of OSA with CCC, based on the initial clinical evidence from the BETTER SLEEP trial. We plan to continue to obtain authorization in additional target markets and are currently conducting our Dual-sided Hypoglossal neRvE stimulAtion for the treatMent of Obstructive Sleep Apnea clinical trial, or DREAM trial, a multicenter, prospective, open-label, pivotal Investigational Device Exemption, or IDE, trial designed to support marketing authorization in the United States. We anticipate 12 month data for the DREAM trial will be available in early 2024. Assuming a positive outcome from the DREAM trial, we expect to apply for marketing authorization in the United States with the aim of being commercially available in the United States in the second half of 2024. In July 2022, we announced that the FDA approved an IDE to enable us to initiate a clinical trial, called ACCCESS, to evaluate the use of the Genio system for the treatment of adult patients with mode-rate-to-severe OSA with CCC that have failed, did not tolerate, or refused PAP. In the ACCCESS trial, we plan to implant up to 106 subjects with co-primary efficacy endpoints of AHI responder rate, per the Sher criteria, and ODI responder rate, both assessed at twelve months post-implant. The first enrolled subjects have been implanted. We are initially targeting markets in Europe where we have identified a country- specific reimburse-ment pathway or execution strategy. We began our commercial launch in Germany in July 2020. After obtaining reimbursement approval in Germany through the existing HGNS special innovation funding program, or NUB, we generated our first revenue in the second half of 2020. In 2021, we success-fully obtained reimbursement in Germany under a dedicated DRG code for HGNS and also recently obtained reimbursement under an OSA-specific DRG code in Switzerland from the Federal Statistic Office, or BFS. The reimbursement coverage in both Germany and Switzerland includes the cost of the Genio system, implant procedure, hospital stay and follow-up care. In 2021, we began marketing products in Switzerland and also secured first revenue in Spain and we began commercialization in Finland in 2022. Based on market access activities conducted by us over the past several years, we have developed tailored reimbursement strategies using assessments of the local requirements of target countries. In countries where there is existing reimbursement coverage in place, we plan to piggyback Report of the Board of Directors |