Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
[Mark One]
x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended October 31, 2013
OR
o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15 (d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 0-15266
BIO-REFERENCE LABORATORIES, INC.
(Exact name of registrant as specified in its charter)
New Jersey | | 22-2405059 |
(State of incorporation) | | (I.R.S. Employer |
| | Identification No.) |
481 Edward H. Ross Drive, Elmwood Park, New Jersey | | 07407 |
(Address of principal executive offices) | | (Zip Code) |
Registrant’s telephone number, including area code 201-791-2600
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | | Name of each exchange on which registered |
Common Stock, $.01 par value | | NASDAQ Global Select Market |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x.
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes o No x.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o.
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o.
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or in any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer.” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer o | | Accelerated filer x | | Non-Accelerated filer o | | Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x.
The aggregate market value of the voting stock of Bio-Reference Laboratories, Inc. (consisting of Common Stock, $.01 par value) held by non-affiliates of the registrant was approximately $623,072,381 based upon the last sale price for the Common Stock on April 30, 2013, the last trading date of the registrant’s most recently completed second quarter, as reported on the NASDAQ Global Market System.
On January 9, 2014, there were 27,695,213 shares of Common Stock issued and outstanding.
Table of Contents
PART I
Forward Looking Statements
Statements included in this Annual Report on Form 10-K (Annual Report) that are not historical in nature, are intended to be, and are hereby identified as “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements may be identified by words such as “expects,” “anticipates,” “intends,” “plans,” “believes,” “seeks,” “estimates,” “will” or words of similar meaning and include, but are not limited to, statements about the expected future business and financial performance of Bio-Reference Laboratories, Inc. and its subsidiaries. Such statements concern matters that involve known and unknown risks and uncertainties that may cause the Company’s actual results in future periods, performance or achievements, or industry results, to be materially different from any future results, performance or achievements described, implied or suggested herein. Although we believe our expectations are based upon reasonable assumptions, there can be no assurance that our financial goals will be realized.
Factors could cause actual results, performance or achievement to differ materially from those expressed or implied from these forward-looking statements include, but are not limited to, the factors discussed under “Risk Factors” as well as elsewhere herein, which may include:
Loss or suspension of a license or imposition of a fine or penalties under, or future changes in, the law or regulations of CLIA, or those of state laboratory licensing laws;
Failure to comply with HIPAA, which could negatively impact profitability and cash flows;
FDA regulation of Laboratory Developed Tests and clinical laboratories;
Failure to comply with federal and state anti-kickback laws;
Failure to maintain the security of patient-related information;
Failure to comply with the Federal Occupational Safety and Health Administration requirements and Needlestick Safety and Prevention Act;
Failure to comply with federal and state laws and regulations related to submission of claims for our services;
Changes in regulation and policies, including increasing downward pressure on health care reimbursement;
Changing relationships with payers, including the various state and multi-state Blues programs, suppliers and strategic partners; Efforts by third-party payors to reduce utilization and reimbursement for clinical testing services;
Failure to timely or accurately bill for our services;
Failure to integrate newly acquired businesses and the costs related to such integration;
Increased competition, including price competition;
Ability to attract and retain experienced and qualified personnel;
Failure to obtain and retain new clients and business partners, or a reduction in tests ordered or specimens submitted by existing clients;
Adverse litigation results; and
Failure to establish, and perform to, appropriate quality standards to assure that the highest level of quality is observed in the performance of our testing services.
Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date of this filing. We assume no obligation to update the forward-looking statements to reflect actual results or changes in the factors affecting such forward-looking statements.
Item. 1. — Business
Overview
We are a clinical testing laboratory offering testing, information and related services to physician offices, clinics, hospitals, employers and governmental units. We believe that we are the fourth largest full-service laboratory in the United States and the largest independent regional laboratory in the Northeastern market. We offer a comprehensive list of laboratory testing services utilized by healthcare providers in the detection, diagnosis, evaluation, monitoring and treatment of diseases. We primarily focus on esoteric testing, molecular diagnostics, anatomical pathology, genetics, women’s health and correctional health care.
We currently process approximately 7.8 million laboratory test requisitions each year. A requisition form accompanies a patient specimen, indicating the tests to be performed and the party to be invoiced for the tests. We have a network of approximately 116 patient service centers located in the Northeast (primarily in New York metropolitan super-regional area) for collection of patient specimens. We currently conduct business in most New York State counties, as well as in most of New Jersey and Maryland as well as some parts of Pennsylvania, Delaware and Connecticut. We primarily offer laboratory services to physician offices in these areas with an infrastructure that includes a comprehensive logistical department, extensive phlebotomy services and phlebotomy draw stations scattered around our geographic area. In October 2012, we launched Laboratorio Buena Salud, the first national testing laboratory dedicated to serving Spanish-speaking populations in the United States. All business will be conducted in Spanish, including patient and physician interactions.
In addition to our clinical testing operations, we operate a clinical knowledge management service through our PSIMedica business unit. This system uses customer data from laboratory results, pharmaceutical data, claims data and other data sources to provide administrative and clinical decision support systems that enable our customers to provide quality and efficient healthcare to their populations.
We also operate a web-based connectivity portal solution for laboratories and physicians through our CareEvolve subsidiary. We use this portal ourselves to provide laboratory ordering and results as well as connectivity to our physician customers. We also market and license this connectivity solution to other laboratories throughout the country.
We are a New Jersey corporation. We are the successor to Med-Mobile, Inc., a New Jersey corporation that was organized in 1981. Our executive offices are located at 481 Edward H. Ross Drive, Elmwood Park, NJ 07407, and our telephone number is 201-791-2600. In this Form 10-K, we may at times refer to ourselves and our subsidiaries as “we,” “us” or “the Company.”
The Clinical Laboratory Testing Market in the United States
We believe that the U.S. market for clinical laboratory testing generated approximately $68 billion in annual revenue in 2013. Nearly all laboratory tests are performed by one of three types of laboratories: hospital laboratories, physician office laboratories or independent clinical laboratories. We believe approximately 60% of the clinical laboratory tests done in the United States are currently performed in a hospital laboratory, approximately 35% performed by an independent clinical laboratory and the balance in a physician office or other laboratory.
Commencing with the advent of managed care cost containment in the 1990s, the industry has been impacted by the rapid growth of managed care arrangements, increasingly stringent government regulation and escalating numbers of investigations into fraud and abuse. Among other things, these factors have led to revenue and profit declines for many smaller and mid-sized clinical laboratories, and industry consolidations. As a result, fewer but larger commercial clinical laboratories have emerged with greater economies of scale, more effective compliance with government billing regulations and other laws and a better approach to pricing their services. These changes have resulted in improved profitability for these larger commercial laboratories. In addition, new and emerging technologies continue to provide greater testing opportunities for clinical laboratories.
2
Table of Contents
We believe that the clinical laboratory testing industry will continue to experience growth in testing volume due to the following factors:
the aging of the population of the United States;
patient awareness of the value of laboratory tests;
a decrease in the cost of tests;
the development of sophisticated and specialized tests for early detection of disease and disease management;
the diagnosis and monitoring of infectious diseases, such as AIDS and Hepatitis C;
increased recognition of early detection and prevention as a means of reducing healthcare costs;
the emergence of employer-sponsored wellness programs; and
additional research and development in genomics.
In addition, new and emerging technologies continue to provide greater testing opportunities for clinical laboratories. As the result of health insurance coverage to uninsured Americans under the Patient Protection and Affordable Care Act as amended by the Health Care and Education Reconciliation Act, each enacted in March 2010 (the “Health Care Reform Law”) the demand for our services may increase.
Business Strengths and Focus
We operate as a national oncology laboratory through our GenPath business unit. Our expertise in cancer pathology and diagnostics as well as molecular diagnostics has enabled GenPath to grow as a national provider.
Our innovative technology platform for sexually transmitted infections has enabled us to expand from a regional service offering to a national offering with specimens coming from throughout the contiguous 48 states in the area of Women’s Health, through our GenPath business unit.
GeneDx, our wholly owned subsidiary, is our genetics laboratory and is typically recognized as the leading national laboratory for testing of rare and ultra-rare genetic diseases.
We have one of the largest regional marketing staffs of any laboratory in the country, with approximately 336 managers and sales and service representatives working for us. We have groups dedicated to the Metropolitan regional market, the Oncology market, the Women’s Health market, the Genetic testing market and the Correctional Health market. We have also recently created a new marketing group that will cross over into the genetics and Women’s Health groups to market to physicians who offer pre-natal testing.
We believe that our large marketing staff and strong infrastructure within our designated area can be leveraged to bring new technologies to physicians and healthcare providers. Over the past year, our volume of testing in the area of molecular diagnostics has increased by approximately 35%.
We believe that laboratory data has great value in managing the healthcare of a population, but can only be properly utilized when combined with medical claims and pharmacy data. Our medical information unit, PSIMedica, as well as our connectivity solution, CareEvolve, seek to combine laboratory data with these other data elements in order to provide actionable analytics designed to help to improve the quality and efficiency of healthcare.
Strategy
We seek to continue our strong growth not only through our marketing organization, new technologies and superior service, but also by providing value-added analytics in conjunction with laboratory results. Our mission is to be recognized by our clients as the best provider of clinical laboratory testing, information and related services. The principal components of our strategy to achieve our mission are as follows:
capitalize on our position within the clinical market;
lead in providing medical information;
provide the highest quality service; and
pursue strategic growth opportunities, both through development of new testing services and through acquisitions.
Our Testing Services
Our laboratory testing business consists of routine testing and esoteric testing. Routine testing generates approximately 40% and esoteric testing generates approximately 60% of our net revenues.
Routine Testing
Routine tests measure various health parameters, such as the functions of the heart, kidney, liver, thyroid and other organs. Below is an abbreviated list of some commonly ordered routine tests:
Blood cell counts
Cholesterol levels
Pregnancy
Substance abuse
Urinalysis
We perform these tests at our main processing facility in Elmwood Park, New Jersey. We operate 24 hours a day, 365 days a year. We perform and report most routine tests within 24 hours. Tests results are delivered via driver or electronically.
Esoteric Tests
We also perform esoteric tests that require sophisticated equipment and materials, highly skilled personnel and professional attention. These tests are ordered less frequently than routine tests. They are also generally priced higher than routine tests. Esoteric tests are typically related to the following medical fields:
Endocrinology (the study of glands and their hormone secretions)
Genetics (the study of chromosomes, genes and their protein products)
Immunology (the study of the immune system)
Microbiology (the study of microscopic forms of life)
HIV-related tests
Molecular diagnostics (the study of genetic content for disease information)
Oncology (the study of abnormal cell growth)
Serology (the study of body fluids)
Toxicology (the study of chemicals and drugs and their effects on the body)
3
Table of Contents
We perform cancer cytogenetic testing at our leased facilities in at our main processing facility in Elmwood Park, Smithtown, NY, Clarksburg, MD and Milford, MA and genetic testing at our GeneDx leased facility in Gaithersburg, MD, as well as at our Elmwood Park facility. We perform cytology testing in Frederick, MD, Milford, MA, Columbus, OH, Houston, TX, Melbourne, FL, and Campbell, CA, and at our Elmwood Park facility.
PSIMedica—Medical Information
Our PSIMedica business unit is based on a clinical knowledge management, or CKM, system that uses data derived from various disparate sources to provide both administrative and clinical analysis of a population. The source data consists of enrollment (demographic) data, claims data, pharmacy data, laboratory data and any other data that may be available. The system uses sophisticated algorithms to cleanse and configure the data to facilitate comprehensive and meaningful analysis. The data is maintained on multiple levels enabling review of data from the global level to the granular transactional detail. The system includes a base set of queries that provide basic functionality and also provides on-line real-time ad hoc query capability enabling the user to customize analysis to the needs of the user’s organization. In addition to the basic queries provided by the system, PSIMedica Quality Indicators, or PQI, provide comprehensive, disease-state-oriented queries that disclose the quality and efficiency of the care and service. These indicators have been designed to provide the user with standards and outcome predictors on a medical standards basis. We are using PSIMedica to market value-added clinical laboratory services to bulk purchasers of clinical laboratory solutions, as well as marketing our PSIMedica programs to businesses such as health plans, integrated delivery networks, disease management companies, insurers, clinical trial companies and other healthcare providers that benefit from the ability of the system to combine both clinical and administrative analysis.
CareEvolve—Connectivity Solutions
Through our CareEvolve subsidiary, we offer a physician-based connectivity solution. This system provides a complex, sophisticated portal for ordering laboratory services and delivering laboratory results, along with ancillary connectivity services. The system is designed to be physician-centric and to provide a highly flexible, scalable, comprehensive desktop solution for physicians to manage their day-to-day practice needs, as well as to handle their clinical laboratory ordering and reporting. This product has been designed to work as a platform with plug and play capability that can easily be used by other laboratories that also need a web-based solution for their physician customers.
Payors and Clients
We provide laboratory services to a range of healthcare providers. A “payor” is the party who pays for the tests while the “client” is the party that refers the tests to us. An organization that has a contract with us, such as a clinic or governmental agency, may be both a payor and a client. Some states, such as New York and New Jersey, prohibit us from billing physician clients. During fiscal year 2013, no single client accounted for more than 10% of our net revenues.
The following table reflects our estimate of the breakdown of net revenue by type of payor for the fiscal years ended October 31, 2011, 2012, and 2013.
| | Fiscal Year Ended October 31, | |
| | 2013 | | 2012 | | 2011 | |
| | | | | | | |
Direct Patient Billing | | 2 | % | 2 | % | 2 | % |
Commercial Insurance | | 64 | % | 62 | % | 61 | % |
Professional Billing | | 16 | % | 17 | % | 17 | % |
Medicare | | 17 | % | 18 | % | 19 | % |
Medicaid | | 1 | % | 1 | % | 1 | % |
| | 100 | % | 100 | % | 100 | % |
Physicians who order clinical tests for their patients represent one of the primary sources of our testing volume. Fees invoiced to patients and third parties are based on our fee schedule, which may be subject to limitations imposed by third-party payors. Fees invoiced to federal health care programs such as Medicare and Medicaid, are based on fee schedules set by applicable governmental authorities, such as the Center for Medicare and Medicaid Services (“CMS”).
We provide laboratory services to governmental agencies and large employer groups. We believe that we are the largest regional laboratory providing laboratory testing services to correctional facilities in the United States. All of these clients are charged on a contractual basis.
Billing
Billing for laboratory services is extremely complicated. We must bill various payors, such as patients, the Medicare program and state Medicaid programs, insurance companies and employer groups, all of which have different billing requirements. Compliance with applicable laws and regulations as well as internal compliance procedures adds complexity to this process.
Our bad debt expense is not the result of credit-related issues, as is the case in most industries. Our bad debt expense is due primarily to missing or incorrect demographic and billing information on our requisitions. We depend on the healthcare provider to supply us with this information. We perform the tests and report the test results as requested on the requisition regardless of whether the demographic and billing information is correct or even missing altogether. We then attempt to obtain missing and to correct incorrect information. This adds to the complexity, slows the invoicing process and generally increases the aging of our accounts receivable. In addition, we perform all tests requested by the healthcare provider even though all of the tests ordered by the healthcare provider may not be reimbursed by the payor; it is our obligation to provide all tests requested by the healthcare provider. When all issues are not resolved in a timely manner, the item is written-off to bad debt expense through the allowance for doubtful accounts. Other items such as pricing differences and payor disputes also complicate billing. Adjustments to receivables as a result of these types of matters are accounted for as revenue adjustments and are not written-off to bad debt expense.
Sales and Marketing
We employ full and part-time sales and marketing representatives. With approximately 336 managers and sales and service representatives working for us, we have groups dedicated to the New York metropolitan regional market, the oncology market, the women’s health market, the genetic testing market and the correctional health market. We are currently building a new marketing group that will cross over into the genetics and women’s health groups to market to physicians who offer pre-natal testing.
All of our sales and marketing personnel operate in a dual capacity, as both marketing and client support representatives. This ensures that all of our salespersons are intimately involved with the client. We believe that this is extremely helpful in client retention, since it provides a strong connection between our physician clients and us.
Client Service Coordinators
We utilize the services of full and part-time client service coordinators at our Elmwood Park, Clarksburg and Gaithersburg facilities, all of whom are trained in medical and laboratory terminology. This staff is used as an interface with physicians and nurses and supplements the
4
Table of Contents
client support provided by our sales force. They also report highly abnormal and life threatening results to the ordering physician via telephone in order to assist speedy medical resolution of patient problems.
Logistical Support
We employ full and part-time couriers. Our couriers pick up patient specimens from and deliver printed reports to physician offices, nursing homes, clinics and correctional facilities.
Acquisitions
On December 21, 2012, the Company entered into an agreement with Meridian Clinical Laboratory Corporation, a Florida corporation having its place of business in Miami, Florida (“Meridian”), pursuant to which the Company purchased all issued and outstanding common stock of Meridian for approximately $1,848 of which $250 is deferred for one year.
On December 31, 2012, Bio-Reference Laboratories, Inc. (the “Company”) entered into an agreement with Florida Clinical Laboratory, Inc., a Florida corporation having its place of business in Melbourne, Florida (“FCL”), pursuant to which the Company purchased all issued and outstanding shares of capital stock of FCL for approximately $7,016, of which $1,000 is deferred for eighteen months assuming certain conditions are met.
On August 7, 2013 the Company purchased substantially all of the operating assets and certain of the operating liabilities of Hunter Laboratories, Inc., (“Hunter”) a California corporation having its principal place of business in Campbell, California. The gross purchase price was $15,215 plus payroll adjustment of $111 totaling $15,326. Of that amount $3,000 was deferred to cover anticipated pre closing liabilities.
On August 20, 2013 the Company through its subsidiary GeneDx, Inc. purchased the entire membership interest in Edge BioServ, LLC, (“Edge Bio”) a Delaware limited liability company having its place of business in Gaithersburg, Maryland. The gross purchase price was approximately $2,502. Of that $375 was deferred to cover anticipated pre closing liabilities.
Competition
We compete with three types of providers in a highly fragmented and competitive industry: hospital laboratories, physician-office laboratories and other independent clinical laboratories. Our major competitors in the New York metropolitan area are two of the largest national laboratories, Quest Diagnostics (DGX) and Laboratory Corporation of America (LH). Although we are much smaller than these national laboratories, we believe that we compete successfully with them in our region due to our innovative testing services and our level of service. We believe our responses to medical consultation are faster and more personalized than those of the national laboratories. Our client service staff deals only with basic technical questions and those that have medical or scientific significance are referred directly to our senior scientists and medical staff.
Quality Assurance
In order to provide accurate and precise clinical information to the physician, it is essential that we maintain a well structured and vigorous quality assurance program. We hold the required Federal and state licenses necessary for the operation of a clinical laboratory at our facilities in New Jersey, New York, Maryland, Massachusetts, Texas and Ohio. We submit to vigorous proficiency tests (or surveys) for all tests that we perform. We are also subject to unannounced inspections from the various state and federal licensing agencies.
Our laboratories are accredited by the College of American Pathologists, or CAP. This accreditation includes on-site inspections and participation in the CAP (or equivalent) proficiency testing program. CAP is an independent organization of board certified pathologists approved by the Center for Medicare and Medicaid Services (CMS), to inspect clinical laboratories in order to determine compliance with the standards required by the Clinical Laboratory Improvement Amendments of 1988, or CLIA.
We have a Quality Assurance Committee, headed by a Quality Assurance Coordinator and composed of supervisors from all of our departments. The Committee meets each day to assess and evaluate our laboratory quality. Based on the information received from the Committee, recommendations are made to correct conditions that have led to errors. Management, department supervisors and members of the Committee continually monitor laboratory quality. Depending on the test, two or three levels of quality control materials are run in each analytical assay to enhance precision and accuracy. Patient population statistics are evaluated each day. Testing of highly abnormal samples is repeated to maximize accuracy.
We believe that all of these procedures are necessary, not only in maintaining Federal and state licensing, but also in assuring a quality product. We believe that our high standards of quality are an important factor in client retention.
Regulation of Our Clinical Laboratory Operations
The clinical laboratory industry is highly regulated and subjected to significant and changing Federal and state laws and regulations. These laws and regulations affect key aspects of our business, including licensure and operations, billing and payment for laboratory services, sales and marketing interactions with ordering physicians, security and confidentiality of health information, and environmental and occupational safety. Oversight by government officials includes regular inspections and audits. Failure to comply with applicable requirements, which are sometimes vague or indefinite, may result in substantial fines, criminal penalties, or other enforcement actions, such as suspension or revocation of a clinical laboratory’s license. Changes in regulations often increase the cost of testing or processing claims. Also, these laws may be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that could require us to make changes in our operations, including in our pricing, billing and/or marketing practices in a manner that could adversely affect operations. We seek to and believe that we do conduct our business in compliance with all applicable laws and regulations. Set forth below are highlights of the key regulatory areas applicable to our business.
Reimbursement for Laboratory Services
We typically bill third party payors such as Medicare, Medicaid, Governmental programs and private insurers for our services. Billing for clinical testing services is very complicated, and our payors often have different coverage, billing and reimbursement requirements, and change those requirements on an ongoing basis. Also, submissions of our claims are particularly complex because we provide both anatomic pathology services and clinical laboratory tests, which generally are paid using different reimbursement principals. The clinical laboratory tests are often paid under a clinical laboratory fee schedule, and the anatomic pathology services are often paid under a physician fee schedule. If ordering physician requisitions contain incorrect or incomplete information, we may also be unable to collect reimbursement from payors. The increased use of electronic ordering reduces, but does not eliminate, the incidence of missing or incorrect information.
In addition, both government and private sector payors have engaged in ongoing efforts in recent years to contain or reduce health care costs, including reimbursement for clinical laboratory services. The combination of complex billing requirements and ongoing pressure with respect to reimbursement levels, presents substantial challenges to the clinical laboratory business. Through the Health Care Reform Law substantial changes are being made to the current system for paying for healthcare in the United States, including programs to extend medical benefits to millions of individuals who currently lack insurance coverage, coupled with measures to cut Medicare spending for most health care
5
Table of Contents
services, including clinical laboratories. The changes contemplated by the Health Care Reform Law are subject to rule-making and implementation timelines that extend for several years, and this uncertainty limits our ability to forecast changes that may occur in the future.
The U.S. Congress has considered, at least yearly in conjunction with budgetary legislation, changes to one or both of the Medicare fee schedules under which we receive reimbursement, which include the physician fee schedule for anatomical pathology services, and the clinical laboratory fee schedule for our clinical laboratory services. For example, currently there is no copayment or coinsurance required for clinical laboratory services, although there is for our physician services. However, Congress has periodically considered imposing a 20 percent coinsurance on laboratory services. If enacted, this would require us to attempt to collect this amount from patients, although in many cases the costs of collection would exceed the amount actually received.
For most of the tests performed for Medicare or Medicaid beneficiaries, laboratories are required to bill Medicare or Medicaid directly, and to accept Medicare or Medicaid reimbursement as payment in full. Part B of the Medicare program contains fee schedule payment methodologies for clinical laboratory services, and the Medicare approach and reimbursement levels often serve as a benchmark for commercial payors. Payment under Medicare is generally the lesser of billed charges, the local fee for a geographic area, and a national limitation amount that is set at a percent of the median of all local fee schedule amounts for each laboratory test code. Each year, subject to federal legislation, fees may be updated for inflation based on the percentage change in the Consumer Price Index, or CPI. From 2004 through 2008 the clinical laboratory fee schedule remained frozen, with no CPI increases. Then, for the first time in five years, as of January 1, 2009 laboratories received a 4.5% across the board increase in reimbursements. For 2010, the clinical laboratory fee schedule was decreased by 1.9 percent. For 2011, under the Health Care Reform Law, it was decreased by 1.75 percent, the first of a series of such annual reductions effective from 2011 to 2015, and in 2011, certain “productivity adjustments were instituted that have functioned to decrease rate increases under the CPI update. For 2012, the clinical laboratory fee schedule was increased by .65%, and in February 2012, Congress passed legislation that reduced payment rates under the clinical laboratory fee schedule by 2%, effective January 1, 2013 and an additional 2% reduction in Medicare rates is scheduled to take effect on March 1, 2013 under a “sequestration” mandate, unless Congress acts to prevent this reduction.
Under the CMS framework, the national limitation amount for clinical laboratory services had been reduced in a number of instances over the past several years to a present level equal to 74% of the national median of laboratory charges, and a number of proposals for legislation or regulation are under discussion which could have the effect of substantially reducing reimbursements to clinical laboratories through reduction of the present allowable percentage or through other means. We are unable to predict the outcome of these discussions. Depending upon the nature of congressional and/or regulatory action, if any, which is taken and the content of legislation, if any, which is adopted, we could experience a significant decrease in revenues from CMS, which could have a material adverse effect on us.
Also, CMS and other payors have expressed some concern regarding “billed charges” reporting by large clinical laboratories, in light of the common practice, among major clinical laboratories, of providing discounted pricing to certain clients that order testing services on a “bulk” basis, such as certain physicians, hospitals, and other institutions, resulting in economies of scale and relatively low administrative costs, as compared with the higher fees charged to individual patients and third party payors, including Medicare, who generally require separate bills or claims for each patient encounter and which involve relatively high administrative costs). If this issue were decided in a manner that required the downward adjustment of billed charges reporting, it could adversely affect the Company.
Clinical Laboratory Improvement Amendments of 1988 (“CLIA”)
CLIA extends Federal licensing requirements to all clinical laboratories (regardless of the location, size or type of laboratory), including those operated by physicians in their offices, based on the complexity of the tests they perform. CLIA also establishes a stringent proficiency testing program for laboratories and includes substantial sanctions, such as suspension, revocation or limitation of a laboratory’s CLIA certificate (which is necessary to conduct business), cancellation or suspension of the laboratory’s approval to receive Medicare and Medicaid reimbursement, and significant fines and/or criminal penalties.
CLIA, and its implementing regulations, includes quality standards (establishing Federal quality standards for all clinical laboratories); application and user fee requirements; and enforcement procedures. The quality standard regulations establish varying levels of regulatory scrutiny depending upon the complexity of testing performed. Under these regulations, a laboratory that performs only one or more of routine “waived” tests may apply for a waiver from most requirements of CLIA. We believe that most tests performed by physician office laboratories will fall into either the “waived” or the “moderately complex” category. The latter category applies to simple or automated tests and generally permits existing personnel in physicians’ offices to continue to perform testing under the implementation of systems that insure the integrity and accurate reporting of results, establishment of quality control systems, proficiency testing by approved agencies, and biannual inspection.
Under CLIA, the company remains subject to state and local laboratory regulations. CLIA provides that a state may adopt laboratory regulations that are more stringent than those under federal law, and some states require additional personnel qualifications, quality control, record maintenance and other requirements.
Our laboratory completed its first CLIA inspection under CLIA guidelines and received its certificate of compliance effective February 7, 1996. It has been reinspected since on a bi-annual basis and found to be in compliance. We believe the Company is in compliance with all applicable federal and state laboratory requirements.
Compliance Program
The Office of Inspector General has published a Model Compliance Program for the clinical laboratory industry. This is a voluntary program for laboratories to demonstrate to the Federal government that they are responsible providers. In addition, certain states, such as New York, require that health care providers, such as clinical laboratories, that engage in substantial business under the state Medicaid program have a compliance program that general adheres to the standards set forth in the Model Compliance Program. Also, under the Health Care Reform Law, the U.S. Department of Health and Human Services, or HHS, will require suppliers, such as the Company, to adopt, as a condition of Medicare participation, compliance programs that meet a core set of requirements. This mandate has not yet been implemented with respect to clinical laboratories, and HHS has not yet provided a time frame for implementation. We have implemented a comprehensive voluntary compliance program adhering to the standards set forth in the Model Compliance Program.
Health Insurance Portability and Accountability Act of 1996, as amended (“HIPAA”)
Both as a health care provider of clinical laboratory services, and in connection with the services we furnish to health plans and others as a business associate though medical information services, we are required to comply with federal and state laws that protect the privacy and security of certain healthcare and personal information. These include HIPAA, which establishes comprehensive standards with respect to the privacy and security of medical information, including requirements for safeguarding electronic protected health information, and comprehensive standards regarding uses and disclosures of protected health information. The HIPAA standards create a complex regulatory framework, including penalties for non-compliance, requirements to respond to patient requests to review and amend their medical records, certain limitations regarding the use of patient information, and notification obligations in the event of certain breaches of patient information. In addition to HIPAA, we are required to abide by various state laws protecting healthcare information, that impose standards that are stricter than those of HIPAA, such as state laws governing sensitive health information regarding HIV status and genetic testing.
HIPAA provides for significant fines as well as substantial criminal penalties for violations of the Act. The federal Health Information Technology for Economic and Clinical Health, or HITCH, Act, strengthened and expanded HIPAA, including to require certain breach notification obligations, to extend a number of HIPAA requirements directly to business associates, to heighten penalties and enforcement
6
Table of Contents
provisions (including requiring HHS to conduct periodic audits to confirm compliance), and to extend enforcement authority over HIPAA to state attorney generals.
In addition, the HITECH Act established a program of Medicare and Medicaid incentive payments available to certain health care providers including, among others, physicians and dentists, if they meaningfully use certified electronic health record technology (“EHR”). Also, eligible providers that fail to adopt certified EHR systems may be subject to Medicare reimbursement reductions beginning in 2015. Qualification for the incentive payments requires the use of EHRs that are certified as having certain capabilities for meaningful use pursuant to standards adopted by the Department of Health and Human Services. Initial (“stage one”) standards addressed criteria for periods beginning in 2011. CMS has also issued a final rule with “stage two” criteria, for periods beginning in 2014, which are more demanding, and new, incrementally more rigorous criteria are expected to be issued for stage three compliance, however final standards have not yet been issued and so these criteria are not yet certain. Certain of our businesses involve the marketing and distribution of certified EHR products, and these products must maintain compliance with these evolving governmental criteria.
In addition, HIPAA requires health care providers, such as clinical laboratories, and other covered entities, to use certain transaction and code set rules for specified electronic transactions, such as transactions involving claims submissions. The Company believes it is in compliance in all material respects with the current rules. With respect to these rules, commencing July 1, 2012, CMS required that all HIPAA-covered entities, such as the Company, conduct electronic claim submissions and related electronic transactions under a new HIPAA transaction standard, called Version 5010. CMS has required this upgrade in connection with another new requirement applicable to the industry, the implementation of new diagnostic code sets to be used in claims submission. The new diagnostic code sets are called the ICD-10-CM, and are to be implemented on October 1, 2014. The Company has been aware of these changes for some time, and believes it is prepared to timely adopt the new standards. However, it is expected that these changes, in particular the adoption of new diagnostic codes — which must be provided to us accurately by referring physicians in order for us to receive payment from payors, such as Medicare — will result in a degree of disruption and confusion, which may adversely affect Company operations, including reimbursement rates.
Laboratory Developed Tests (LDTs)
The federal Food and Drug Administration, or FDA, has regulatory responsibility over, among other areas, instruments, test kits reagents and other medical devices used by clinical laboratories to perform diagnostic testing. High complexity and CLIA-certified laboratories, such as ours, frequently develop internal testing procedures to provide diagnostic results to customers. These tests are referred to as laboratory developed tests, or LDTs. LTD’s are subject to CMS oversight through its enforcement of CLIA. The FDA has also claimed regulatory authority over all LDTs, but indicates that it has exercised enforcement discretion with regard to most LDTs offered by high complexity CLIA-certified laboratories, and has not subjected these tests to the panoply of FDA rules and regulations governing medical devices. However, the FDA has stated that it has been considering changes in the way it believes that laboratories ought to be allowed to offer these LDTs, and during 2010 publicly announced that it would be exercising regulatory authority over LDTS, using a risk-based approach that will direct more resources to tests with the highest risk of injury. The FDA has not announced a framework or timetable for implementing its announced approach and in its 2013 Work Plan, the U.S. Department of Health & Human Services Office of Inspector General announced that it would examine the oversight of the clinical effectiveness of LDTs given the current approaches by CMS and FDA with respect to LDTs. Depending upon the manner in which the FDA’s new regulatory framework is implemented, there may be an adverse affect on Company operations.
Fraud and Abuse Regulations
Since we supply services that are reimbursed by federal health care programs such as Medicare and Medicaid, our activities are also subject to regulation by CMS and enforcement by the Office of Inspector General, or OIG, within the HHS. A provision of the U.S. Social Security Act known as the “Anti-Kickback Law” prohibits providers and others from directly or indirectly soliciting, receiving, offering or paying any remuneration with the intent of generating referrals or orders for services or items covered by a federal health care program. Many states have similar laws. Courts have interpreted this law very broadly, including holding that a violation has occurred if even one purpose of the remuneration is to generate referrals, even if there are other lawful purposes. There are statutory and regulatory exceptions (known as safe harbors) that outline arrangements that are deemed lawful. However, the fact that an arrangement does not fall within a safe harbor does not necessarily render the conduct illegal under the Anti-Kickback Law. In sum, even legitimate business arrangements between the Company and referral sources, such as physicians, could lead to scrutiny by government enforcement agencies, and require extensive company resources to respond to government investigations. Violations of the Anti-Kickback Law may be punished by civil and criminal penalties and/or exclusion from participation in federal health care programs, including Medicare and Medicaid. The Health Care Reform Law strengthened provisions of the Anti-Kickback Law.
The federal “Stark Law” or “self-referral” prohibition, subject to certain exceptions, prohibits payment under Medicare or Medicaid for certain designated health services, including, among others, clinical laboratory services, where the referring physician has a financial relationships with the entity that furnishes the clinical laboratory service. The applicable exceptions permitting federal reimbursement generally require written agreements and fair market value payments that do not vary based upon the volume or value of referrals. Many states have similar self-referral laws that regulate the financial relationships between referring physicians and clinical laboratories, which extend to all referrals, not only referrals for services reimbursed by Medicare or Medicaid. Another federal law, known as the “Anti-Markup Rule,” and similar state laws, address the practice of an independent clinical laboratory performing and then billing to the ordering physician a component of a diagnostic test, such as diagnostic pathology services, where the ordering physician bills the test to Medicare. In this circumstance, penalties may apply to the physician if Medicare or other payor is billed at a rate that exceeds the laboratory’s charges to the physician, and the laboratory could be at risk under false claims laws, described below, for causing the submission of a false claim, if it advised the physician to submit claims to payors in violation of these provisions.
The federal False Claims Act, or FCA, is violated by any entity that “presents or causes to be presented” knowingly false claims for payment to the federal government and many states have similar laws that apply to governmental and private payors. In addition, the Health Care Reform Law amended the FCA to create a cause of action against any person who knowingly makes a false statement material to an obligation to pay money to the government, or knowingly conceals or improperly decreases an obligation to pay or transmit money or property to the government. For the purposes of these recent amendments, an “obligation” includes an overpayment, which is defined broadly to include “any funds that a person receives or retains under Medicare and Medicaid to which the person, after applicable reconciliation, is not entitled . . . .”
The FCA is commonly used to sue those who submit allegedly false Medicare or Medicaid claims, as well as those who induce or assist others to submit a false claim. Courts and government officials have found that “false claims” can result not only from noncompliance with the express requirements of applicable governmental reimbursement programs, such as Medicare and Medicaid, but also from noncompliance with other laws, such as provisions of the Food, Drug and Cosmetic Act, or laws that require quality care in service delivery. In addition, the Health Care Reform Law amended the FCA to specify that a claim to federal health care programs that includes items or services resulting from a violation of the Anti-Kickback Law constitutes a false claim under the FCA. The qui tam or whistleblower provisions of the FCA allow private individuals to bring actions on behalf of the government alleging that the government was defrauded, with tremendous potential financial gain to private citizens in the event they prevail. When a private party brings a whistleblower action under the FCA, the defendant is not made aware of the lawsuit until the government starts its own investigation or makes a decision on whether it will intervene. Many states have enacted similar laws that also apply to claims submitted to commercial insurance companies. The bringing of any FCA action could require us to devote resources to investigate and defend the action. Violations of the FCA could result in enormous economic liability. The law provides that all damages are trebled, and each false claim submitted is subject to a penalty of up to $11,000.
Historically, the clinical laboratory industry has been the focus of major governmental enforcement initiatives, and within the past few years federal and state governments continue to strengthen their enforcement efforts, such as through new laws that increase funding, powers and remedies to pursue suspected cases of fraud and abuse. We believe we operate lawfully within these statutes; however, we cannot predict if some of our practices may be interpreted as violating these statutes and regulations.
7
Table of Contents
Waste Management, Health and Safety
We are subject to federal and state laws and regulations regarding the protection of the environment, the health and safety of employees, and the handling, transportation and disposal of medical specimens, and infectious and hazardous wastes. For example, federal regulations require licensure of interstate transporters of medical waste. In New Jersey, we are subject to the Comprehensive Medical Waste Management Act, or CMWMA, which requires us to register as a generator of special medical waste. All of our medical waste is disposed of by a licensed interstate hauler. The hauler provides a manifest of the disposition of the waste products as well as a certificate of incineration which is retained by us. These records are audited by the State of New Jersey on a yearly basis. We are also subject to the Federal Hazardous Materials Transportation Law, 49 U.S.C. 5101 et seq., and the Hazardous Materials Regulations, or HMR, 49 CFR parts 171-180. In addition, the federal Occupational Safety and Health Administration, or OSHA, has established extensive requirements relating specifically to workplace programs to protect workers from exposure to blood-borne pathogens, such as HIV and the hepatitis B virus, including work practice controls, protective clothing and equipment, training, vaccinations and other measures designed to minimize hazardous exposures.
Intellectual Property Rights
The Company relies on a combination of patents, trademarks, copyrights, trade secrets and nondisclosure and non-competition agreements to establish and protect its proprietary technology. The Company believes, however, that no single patent, technology, trademark, intellectual property asset or license is material to its business as a whole.
Insurance
We maintain professional liability insurance. We believe that our present insurance coverage is sufficient to cover currently estimated exposures, but we cannot assure that we will not incur liabilities in excess of the policy limits. In addition, although we believe that we will be able to continue to obtain adequate insurance coverage, we cannot assure that we will be able to do so at acceptable cost.
Employees
At October 31, 2013, we had 3,338 full-time and 1,089 part-time employees serving in executive positions, as technicians and technologists (including physicians, pathologists and PhDs), in marketing, in logistics and in bookkeeping, clerical and administrative positions. None of our employees are represented by a labor union. We regard relations with our employees as satisfactory.
Available Information
Our Internet website address is www.bioreference.com. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to section 13(a) or 15(d) of the Exchange Act are available free of charge through our website as soon as reasonably practicable after we electronically file with or furnish them to the Securities and Exchange Commission, or SEC, and are available in print to any stockholder who requests a copy. Additionally, the charters of the standing committees of our board of directors are available on our website under “Board Committee Charters”. Information on our website shall not be deemed incorporated into, or to be a part of, this Annual Report on Form 10-K.
The public may also read and copy any materials that we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. Additionally, the SEC maintains a website that contains reports, proxy statements, information statements and other information regarding issuers, including us, that file electronically with the SEC at www.sec.gov.
Item 1A. Risk Factors
You should carefully consider each of the following risk factors and all other information set forth in this report. Any of the following risks could materially and adversely affect our business, financial condition or results of operations. They are not, however, the only risks we face. Additional risks and uncertainties not presently known to us or that we currently believe not to be material may also adversely affect our business, financial condition or results of operations. This report also includes forward-looking statements that involve risks or uncertainties. Our results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks we face described below and elsewhere. See “Forward Looking Statements”.
Complying with numerous regulations pertaining to our business is an expensive and time-consuming process, and any failure to comply could result in substantial penalties.
The clinical laboratory testing industry is highly regulated and there can be no assurance that the regulatory environment in which we operate will not change significantly and adversely in the future. Areas of the regulatory environment that may affect our ability to conduct business include, without limitation:
federal and state laws applicable to billing and claims payment;
federal and state laboratory anti-mark-up laws;
federal and state anti-kickback laws;
federal and state false claims laws;
federal self-referral and financial inducement prohibition laws, commonly known as the Stark Law, and the state equivalents;
federal and state laws governing laboratory licensing and testing, including CLIA;
federal and state laws governing the development, use and distribution of diagnostic medical tests known as laboratory developed tests or “LDTs”;
HIPAA, along with the revisions to HIPPA as a result of the HITECH Act, and analogous state laws;
federal, state and foreign regulation of privacy, security, electronic transactions and identity theft;
federal, state and local laws governing the handling, transportation and disposal of medical and hazardous waste;
Occupational Safety and Health Administration rules and regulations;
changes to laws, regulations and rules as a result of the Health Care Reform Law; and
changes to other federal, state and local laws, regulations and rules, including tax laws.
We have adopted policies and procedures designed to comply with these laws. In the ordinary course of business, we conduct internal reviews of our compliance with these laws. The growth of our business and sales organization may increase the potential for violating these laws or our internal policies and procedures. The risk of our being found in violation of these or other laws and regulations is further increased by the fact that many of them are extremely complex and in many instances, there are no significant regulatory or judicial interpretations of these laws and regulations. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could
8
Table of Contents
cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. Any determination that we have violated these laws or regulations, or the public announcement that we are being investigated for possible violations of these laws or regulations, could harm our operating results and financial condition. If our operations are found to be in violation of any of these laws and regulations, we may be subject to any applicable penalty associated with the violation, including civil and criminal penalties, damages and fines, we could be required to refund payments received by us, and we could be required to curtail or cease our operations. In addition, a significant change in any of these laws or regulations may require us to change our business model in order to maintain compliance with these laws or regulations, which could harm our operating results and financial condition.
Our business could be harmed from the loss or suspension of a license or imposition of a fine or penalties under, or future changes in, or changing interpretations of, CLIA or state laboratory licensing laws to which we are subject.
The clinical laboratory testing industry is subject to extensive federal and state regulation, and many of these statutes and regulations have not been interpreted by the courts. The Clinical Laboratory Improvement Amendments of 1988, or CLIA, are federal regulatory standards that apply to virtually all clinical laboratories (regardless of the location, size or type of laboratory), including those operated by physicians in their offices, by requiring that they be certified by the federal government or by a federally approved accreditation agency. CLIA does not preempt state law, which in some cases may be more stringent than federal law and require additional personnel qualifications, quality control, record maintenance and proficiency testing. The sanction for failure to comply with CLIA and state requirements may be suspension, revocation or limitation of a laboratory’s CLIA certificate, which is necessary to conduct business, as well as significant fines and/or criminal penalties. Several states have similar laws and we may be subject to similar penalties.
We cannot assure you that applicable statutes and regulations will not be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that would adversely affect our business. Potential sanctions for violation of these statutes and regulations include significant fines and the suspension or loss of various licenses, certificates and authorizations, which could have a material adverse effect on our business. In addition, compliance with future legislation could impose additional requirements on us, which may be costly.
Failure to comply with HIPAA, including regarding the use of new “standard transactions,” may negatively impact our profitability and cash flows.
Pursuant to HIPAA, we must comply with comprehensive privacy and security standards with respect to the use and disclosure of protected health information, as well as standards for electronic transactions, including specified transaction and code set rules. Under recent HITECH amendments to HIPAA, the law was expanded, including to require certain data breach notification, direct patient access to laboratory records, the extension of certain HIPAA privacy and security standards directly to business associates, and to heighten penalties for noncompliance, and enforcement efforts. While the Company maintains policies and procedures to comply with HIPAA, HHS has not yet issued final regulations to implement all HITECH requirements, and while the Company believes compliance will increase Company costs, it is difficult to predict precisely the costs involved.
In addition, the HIPAA transaction standards are complex, and subject to differences in interpretation by payors. For instance, some payors may interpret the standards to require us to provide certain types of information, including demographic information not usually provided to us by physicians. As a result of inconsistent application of transaction standards by payors or the our inability to obtain certain billing information not usually provided to us by physicians, we could face increased costs and complexity, a temporary disruption in receipts and ongoing reductions in reimbursements and net revenues. In addition, new requirements for additional standard transactions, such as claims attachments, Version 5010 of the HIPAA Transaction Standards and the ICD-10-CM Code Set, could prove technically difficult, time-consuming or expensive to implement. We are working closely with our payors to establish acceptable protocols for claim submission and with our trade association and an industry coalition to present issues and problems as they arise to the appropriate regulators and standards setting organizations.
FDA regulation of laboratory-developed tests (“LDTs”) may result in significant change, and our business could be adversely impacted if we fail to adapt.
High complexity, CLIA-certified laboratories, such as ours, frequently develop testing procedures internally to provide diagnostic results to customers, which are offered as laboratory-developed tests. The FDA claims to have regulatory authority over these LDTs and has stated that it intends to issue guidance to the industry regarding its regulatory approach. In such discussions, the FDA has indicated that it would use a risk-based approach to regulation and would direct more resources to tests with wider distribution and with the highest risk of injury, but that it will be sensitive to the need to not adversely impact patient care or innovation. To date, the FDA has not announced a framework or timetable for implementing a new regulatory approach, and in its 2013 Work Plan, the U.S. Department of Health & Human Services Office of Inspector General announced that it would examine the oversight of the clinical effectiveness of LDTs given the current approaches by CMS and FDA with respect to LDTs. To date, the FDA has not issued any of these planned guidance documents. We cannot predict the ultimate timing or form of any such guidance or regulation and the potential impact on our existing tests, our tests in development [or the materials used to perform our tests].If adopted, such a regulatory approach by the FDA may lead to an increased regulatory burden, including additional costs and delays in introducing new tests. While the ultimate impact of the FDA’s approach is unknown, it may be extensive and may result in significant change. Our failure to adapt to these changes could have a material adverse effect on our business.
A failure to comply with any of federal or state laws applicable to our business, particularly laws related to the elimination of healthcare fraud, may adversely impact our business.
Federal officials responsible for administering and enforcing the healthcare laws and regulations have made a priority of eliminating healthcare fraud. For example, the Health Care Reform Law includes significant new fraud and abuse measures, including required disclosures of financial arrangements with physician customers, lower thresholds for violations and increasing potential penalties for such violations. Federal funding available for combating health care fraud and abuse generally has increased. While we seek to conduct our business in compliance with all applicable laws and regulations, many of the laws and regulations applicable to our business, particularly those relating to billing and reimbursement of tests and those relating to relationships with physicians, hospitals and patients, contain language that has not been interpreted by courts. We must rely on our interpretation of these laws and regulations based on the advice of our counsel and regulatory or law enforcement authorities may not agree with our interpretation of these laws and regulations and may seek to enforce legal remedies or penalties against us for violations. From time to time we may need to change our operations, particularly pricing or billing practices, in response to changing interpretations of these laws and regulations or regulatory or judicial determinations with respect to these laws and regulations. These occurrences, regardless of their outcome, could damage our reputation and harm important business relationships that we have with healthcare providers, payors and others. Furthermore, if a regulatory or judicial authority finds that we have not complied with applicable laws and regulations, we would be required to refund amounts that were billed and collected in violation of such laws and regulations. In addition, we may
9
Table of Contents
voluntarily refund amounts that were alleged to have been billed and collected in violation of applicable laws and regulations. In either case, we could suffer civil and criminal damages, fines and penalties, exclusion from participation in governmental healthcare programs and the loss of licenses, certificates and authorizations necessary to operate our business, as well as incur liabilities from third-party claims, all of which could harm our operating results and financial condition. Moreover, regardless of the outcome, if we or physicians or other third parties with whom we do business are investigated by a regulatory or law enforcement authority we could incur substantial costs, including legal fees, and our management may be required to divert a substantial amount of time to an investigation.
To enhance compliance with applicable health care laws, and mitigate potential liability in the event of noncompliance, regulatory authorities, such as the United States Health and Human Services Department Office of Inspector General (“OIG”), have recommended the adoption and implementation of a comprehensive health care compliance program that generally contains the elements of an effective compliance and ethics program described in Section 8B2.1 of the United States Sentencing Commission Guidelines Manual, and for many years the OIG has made available a model compliance program targeted to the clinical laboratory industry. In addition, certain states, such as New York, require that health care providers, such as clinical laboratories, that engage in substantial business under the state Medicaid program have a compliance program that general adheres to the standards set forth in the Model Compliance Program. Also, under the Health Care Reform Law, the U.S. Department of Health and Human Services, or HHS, will require suppliers, such as the Company, to adopt, as a condition of Medicare participation, compliance programs that meet a core set of requirements. This mandate has not yet been implemented with respect to clinical laboratories, and HHS has not yet provided a time frame for implementation. While we have adopted U.S. healthcare compliance and ethics programs that generally incorporate the OIG’s recommendations, and train our applicable employees in such compliance, having such a program can be no assurance that we will avoid any compliance issues.
Failure to maintain the security of patient-related information or compliance with security requirements could damage our reputation with customers, cause us to incur substantial additional costs and become subject to litigation.
Pursuant to HIPAA, and certain similar state laws, we must comply with comprehensive privacy and security standards with respect to the use and disclosure of protected health information. Under the HITECH amendments to HIPAA, HIPAA was expanded to require certain data breach notification, to extend certain HIPAA privacy and security standards directly to business associates, to heighten penalties for noncompliance, and enhance enforcement efforts.
We receive certain personal and financial information about our clients and their patients. In addition, we depend upon the secure transmission of confidential information over public networks. A compromise in our security systems that results in client or patient personal information being obtained by unauthorized persons or our failure to comply with security requirements for financial transactions could adversely affect our reputation with our clients and result in litigation against us or the imposition of penalties, all of which may adversely impact our results of operations, financial condition and liquidity.
Failure to comply with environmental, health and safety laws and regulations, including the federal Occupational Safety and Health Administration Act, the Needlestick Safety and Prevention Act and the Comprehensive Medical Waste Management Act, could result in fines and penalties and loss of licensure, and have a material adverse effect upon our business.
We are subject to licensing and regulation under federal, state and local laws and regulations relating to the protection of the environment and human health and safety, including laws and regulations relating to the handling, transportation and disposal of medical specimens, infectious and hazardous waste and radioactive materials, as well as regulations relating to the safety and health of laboratory employees. The federal Occupational Safety and Health Administration has established extensive requirements relating to workplace safety for health care employers, including clinical laboratories, whose workers may be exposed to blood-borne pathogens such as HIV and the hepatitis B virus. These requirements, among other things, require work practice controls, protective clothing and equipment, training, medical follow-up, vaccinations and other measures designed to minimize exposure to, and transmission of, blood-borne pathogens. In addition, the Needlestick Safety and Prevention Act requires, among other things, that we include in our safety programs the evaluation and use of engineering controls such as safety needles if found to be effective at reducing the risk of needlestick injuries in the workplace.
Waste management is subject to federal and state regulations governing the transportation and disposal of medical waste including bodily fluids. Federal regulations require licensure of interstate transporters of medical waste. In New Jersey, we are subject to the Comprehensive Medical Waste Management Act (“CMWMA”), which requires us to register as a generator of special medical waste. All of our medical waste is disposed of by a licensed interstate hauler. The hauler provides a manifest of the disposition of the waste products as well as a certificate of incineration, which is retained by us. These records are audited by the State of New Jersey on a yearly basis. We are also subject to the Federal Hazardous materials transportation law, 49 U.S.C. 5101 et seq., and the Hazardous Materials Regulations (HMR), 49 CFR parts 171-180. The federal government has classified hazardous medical waste as hazardous materials for the purpose of regulation. These regulations preempt state regulation, which must be “substantively the same,” “the non-federal requirement must conform “in every significant respect to the federal requirement. Editorial and other similar de minimis changes are permitted,” 49 CFR 107.202(d).
Failure to comply with such federal, state and local laws and regulations could subject us to denial of the right to conduct business, fines, criminal penalties and/or other enforcement actions, any of which could have a material adverse effect on our business. In addition, compliance with future legislation could impose additional requirements us, which may be costly.
Failure to comply with complex federal and state laws and regulations related to submission of claims for clinical laboratory services could result in significant monetary damages and penalties and exclusion from the Medicare and Medicaid programs.
We are subject to extensive federal and state laws and regulations relating to the submission of claims for payment for clinical laboratory services, including those that relate to coverage of our services under Medicare, Medicaid and other governmental health care programs, the amounts that may be billed for our services and to whom claims for services may be submitted. These rules may also affect the Company in light of the practice management products that we market, to the extent that these products are considered to affect the manner in which our customers’ submit their own claims for services. Submission of our claims is particularly complex because we provide both anatomic pathology services and clinical laboratory tests, which generally are paid using different reimbursement principles. The clinical laboratory tests are often paid under a clinical laboratory fee schedule, and the anatomic pathology services are often paid under a physician fee schedule.
Our failure to comply with applicable laws and regulations could result in our inability to receive payment for our services or result in attempts by third-party payors, such as Medicare and Medicaid, to recover payments from us that have already been made. Submission of claims in violation of certain statutory or regulatory requirements can result in penalties, including substantial civil money penalties for each item or service billed to Medicare in violation of the legal requirement, and exclusion from participation in Medicare and Medicaid. Government
10
Table of Contents
authorities may also assert that violations of laws and regulations related to submission or causing the submission of claims violate the federal False Claims Act (“FCA”) or other laws related to fraud and abuse, including submission of claims for services that were not medically necessary. Violations of the FCA could result in enormous economic liability. The FCA provides that all damages are trebled, and each false claim submitted is subject to a penalty of up to $11,000. For example, we could be subject to FCA liability if it was determined that the services we provided were not medically necessary and not reimbursable, particularly if it were asserted that we contributed to the physician’s referrals of unnecessary services to us. It is also possible that the government could attempt to hold us liable under fraud and abuse laws for improper claims submitted by an entity for services that we performed if we were found to have knowingly participated in the arrangement that resulted in submission of the improper claims.
Changes in regulation and policies, including increasing downward pressure on health care reimbursement, may adversely affect reimbursement for diagnostic services and could have a material adverse impact on our business.
Reimbursement levels for health care services are subject to continuous and often unexpected changes in policies, and we face a variety of efforts by government payors to reduce utilization and reimbursement for diagnostic testing services. Changes in governmental reimbursement may result from statutory and regulatory changes, retroactive rate adjustments, administrative rulings, competitive bidding initiatives, and other policy changes.
The U.S. Congress has considered, at least yearly in conjunction with budgetary legislation, changes to one or both of the Medicare fee schedules under which we receive reimbursement, which include the physician fee schedule for anatomical pathology services, and the clinical laboratory fee schedule for our clinical laboratory services. For example, currently there is no copayment or coinsurance required for clinical laboratory services, although there is for our physician services. However, Congress has periodically considered imposing a 20 percent coinsurance on laboratory services. If enacted, this would require us to attempt to collect this amount from patients, although in many cases the costs of collection would exceed the amount actually received.
Our reimbursement for our pathology services is paid primarily under the physician fee schedule of Medicare and Medicaid and is therefore governed by a complex formula, referred to as the Sustainable Growth Rate, or SGR. As the use of this formula could result in a significant reduction in reimbursement for all physician services, Congress usually acts each year to prevent the full amount of such reductions from taking effect. In 2011, Congress acted to prevent reductions in for 2012, and on January 1, 2013, Congress acted to prevent significant reductions for 2013. The SGR has currently been postponed until March 2014 and Congress continues to work on both a short term and a long term fix to this annual problem. If Congress fails to take such action in the future, implementation of this formula could adversely affect our business.
The Center for Medicare and Medicaid Services (CMS) pays laboratories on the basis of a fee schedule that is reviewed and re-calculated on an annual basis. CMS may change the fee schedule upward or downward on billing codes that we submit for reimbursement on a regular basis. Our revenue and business may be adversely affected if the reimbursement rates associated with such codes are reduced. Even when reimbursement rates are not reduced, policy changes add to our costs by increasing the complexity and volume of administrative requirements. Medicaid reimbursement, which varies by state, is also subject to administrative and billing requirements and budget pressures. Recently, state budget pressures have caused states to consider several policy changes that may impact our financial condition and results of operations, such as delaying payments, reducing reimbursement, restricting coverage eligibility and service coverage, and imposing taxes on our services.
Healthcare policy changes, including recently enacted legislation reforming the U.S. healthcare system, may have a material adverse effect on our financial condition and results of operations.
The Health Care Reform Law makes changes that are expected to significantly impact clinical laboratories, among others. Beginning in 2013, each medical device manufacturer will pay a sales tax in an amount equal to 2.3% of the price for which such manufacturer sells its medical devices that are listed with the FDA. Although the FDA has contended that LDTs are medical devices, none of our products are currently listed with the FDA. We cannot assure you that the tax will not be extended to services such as ours in the future. The Health Care Reform Law also mandates a reduction in payments for clinical laboratory services paid under the Medicare Clinical Laboratory Fee Schedule, or CLFS, of 1.75% through 2015 and a productivity adjustment to the CLFS.
Other significant measures contained in the Health Care Reform Law include, for example, coordination and promotion of research on comparative clinical effectiveness of different technologies and procedures, initiatives to revise Medicare payment methodologies, such as bundling of payments across the continuum of care by providers and physicians, and initiatives to promote quality indicators in payment methodologies. The Health Care Reform Law also includes significant new fraud and abuse measures, including required disclosures of financial arrangements with physician customers, lower thresholds for violations and increasing potential penalties for such violations. In addition, the Health Care Reform Law establishes an Independent Payment Advisory Board, or IPAB, to reduce the per capita rate of growth in Medicare spending. The IPAB has broad discretion to propose policies to reduce expenditures, which may have a negative impact on payment rates for services. The IPAB proposals may impact payments for clinical laboratory services beginning in 2016. We are monitoring the impact of the Health Care Reform Law in order to enable us to determine the trends and changes that may be necessitated by the legislation that may potentially impact on our business over time.
In addition to the Health Care Reform Law, various healthcare reform proposals have also emerged from federal and state governments. For example, in February 2012, Congress passed the “Middle Class Tax Relief and Job Creation Act of 2012” which in part reduced the potential future cost-based increases to the Medicare Clinical Laboratory Fee Schedule by 2%. Overall the expected total fee cut to the CLFS for 2013 is 2.95% not including a further reduction of 2% anticipated from implementation of the automatic expense reductions (sequester) under the Budget Control Act of 2011 which went into effect for dates of service on or after April 1, 2013. Reductions made by the Congressional sequester are applied to total claims payments made. While these reductions do not result in a rebasing of the negotiated or established Medicare or Medicaid reimbursement rates, rebasing could occur as a result of future legislation. In 2014, the total fee cut to the CLFS will be 2.55% plus another 2% that will again take effect from the sequester on April 1, 2014.
We cannot be certain that these or future changes will not affect payment rates in the future. We also cannot predict whether future healthcare initiatives will be implemented at the federal or state level, or the effect any future legislation or regulation will have on us. The taxes imposed by the new federal legislation, cost reduction measures and the expansion in government’s role in the U.S. healthcare industry may result in decreased profits to us, lower reimbursements by payors for our products or reduced medical procedure volumes, all of which may adversely affect our business, financial condition and results of operations.
11
Table of Contents
Change in the billing and/or reimbursement procedures by the federal government could affect our ability to be paid as we have in the past for services rendered.
CMS has changed or discussed making changes to certain types of reimbursement which could affect our rate of reimbursement. Certain cases are comprised of both a technical component (TC) and a professional component (PC). In certain specified areas of testing, primarily in the area of anatomic pathology, CMS has determined that some providers have over-utilized these testing procedures and CMS has introduced changes in reimbursement policies to discourage over-utilization. While the Company does not currently over-utilize services for self-gain and does not perform any significant amounts revenue for the areas of testing currently being changed by CMS, we are always subject to review by CMS and cannot be certain that CMS won’t interpret our practices differently than we do. In addition, CMS may extend this logic and approach to other areas of testing that might affect work performed by us.
CMS has announced planned changes in the area of Molecular Diagnostics’ reimbursement, primarily designed to improve transparency in billing. Molecular Diagnostics is a rapidly changing and evolving area of clinical testing. Whereas other areas of clinical testing are well vetted and established with specific codes for reimbursement, Molecular Diagnostics has moved at a faster pace than CMS can proceed. Clinical laboratories accordingly use a process called cross-walking to get reimbursed by CMS. Cross-walking requires that the clinical laboratory identify the individual processes used to process the patient’s specimen and identify diagnostic results that are already reimbursed in established tests. CMS seeks to specifically identify the testing routine being done and reimburse providers universally for the test actually being performed. CMS has not established all of the molecular diagnostic tests that will be included in this revised schedule for reimbursement and it has not determined how much will be reimbursed to providers for these tests. We expect CMS to implement fair and reasonable reimbursement for such tests, but until such pricing decisions are disclosed we cannot be certain what CMS will finally implement.
Effective July 1, 2012, CMS eliminated an exemption that had been in place since 1999, which allowed commercial laboratories to bill for certain diagnostic tests performed on in-patient and certain outreach recipients by commercial laboratories. From 1999 through July 1, 2012, commercial laboratories were allowed to bill CMS for such tests despite the fact that the recipient was a hospital patient as long as the hospital had been submitting such tests for diagnosis to commercial laboratories prior to 1999. Upon termination of the exemption, we were required to find out from the hospital submitting the test whether the recipient’s bill for diagnostic testing will be reimbursed by the hospital or should be billed to CMS. We have systems in place to manage this change, but these systems are dependent upon our getting proper information from the hospital clients.
The Federal Government is faced with significant economic decisions in the coming years. Some solutions being offered in the government could substantially change the way laboratory testing is reimbursed by government entities. We cannot be certain what or how any such government changes may affect our business.”
Healthcare plans have taken steps to control the utilization and reimbursement of healthcare services, including clinical test services.
We also face efforts by non-governmental third-party payors, including healthcare plans, to reduce utilization and reimbursement for clinical testing services.
The healthcare industry has experienced a trend of consolidation among healthcare insurance plans, resulting in fewer but larger insurance plans with significant bargaining power to negotiate fee arrangements with healthcare providers, including clinical testing providers. These healthcare plans, and independent physician associations, may demand that clinical testing providers accept discounted fee structures or assume all or a portion of the financial risk associated with providing testing services to their members through capped payment arrangements. In addition, some healthcare plans have been willing to limit the PPO or POS laboratory network to only a single national laboratory to obtain improved fee-for-service pricing. There are also an increasing number of patients enrolling in consumer driven products and high deductible plans that involve greater patient cost-sharing.
The increased consolidation among healthcare plans also has increased the potential adverse impact of ceasing to be a contracted provider with any such insurer. The Health Care Reform Law includes provisions, such as the creation of healthcare exchanges, which may encourage healthcare insurance plans to increase exclusive contracting.
We expect continuing efforts to reduce reimbursements, to impose more stringent cost controls and to reduce utilization of clinical test services. These efforts, including future changes in third-party payor rules, practices and policies, or ceasing to be a contracted provider to a healthcare plan, may have a material adverse effect on our business.
Increased competition, including price competition, could have a material adverse impact on our net revenues and profitability.
Our industry is characterized by intense competition. Our major competitors in the New York metropolitan super-region, Quest Diagnostics and Laboratory Corporation of America, are large national laboratories that possess greater name recognition, larger customer bases, significantly greater financial resources and employ substantially more personnel than we do. Many of our competitors have long established relationships with their customers and third-party payors. We cannot assure you that we will be able to compete successfully with such entities in the future.
The clinical laboratory business is intensely competitive both in terms of price and service. Pricing of laboratory testing services is often one of the most significant factors used by health care providers and third-party payors in selecting a laboratory. As a result of the clinical laboratory industry undergoing significant consolidation, larger clinical laboratory providers are able to increase cost efficiencies afforded by large-scale automated testing. This consolidation results in greater price competition. We may be unable to increase cost efficiencies sufficiently, if at all, and as a result, our net earnings and cash flows could be negatively impacted by such price competition. Additionally, we may also face changes in fee schedules, competitive bidding for laboratory services or other actions or pressures reducing payment schedules as a result of increased or additional competition.
Additional competition, including price competition, could have a material adverse impact on our net revenues and profitability.
A failure to obtain and retain new clients and business partners, a loss of existing clients or material contracts, or a reduction in tests ordered or specimens submitted by existing clients, could impact our ability to successfully grow our business.
To offset efforts by payors to reduce the cost and utilization of clinical laboratory services, we need to obtain and retain new clients and business partners. In addition, a reduction in tests ordered or specimens submitted by existing clients, without offsetting growth in our client
12
Table of Contents
base, could impact our ability to successfully grow our business and could have a material adverse impact on our net revenues and profitability. We compete primarily on the basis of the quality of testing, reporting and information systems, reputation in the medical community, the pricing of services and ability to employ qualified personnel. Our failure to successfully compete on any of these factors could result in the loss of clients and a reduction in our ability to expand our customer base.
Failure to timely or accurately bill for our services could have a material adverse effect on our business
Billing for clinical testing services is extremely complicated and is subject to extensive and non-uniform rules and administrative requirements. Depending on the billing arrangement and applicable law, we bill various payors, such as patients, insurance companies, Medicare, Medicaid, physicians, hospitals and employer groups. Changes in laws and regulations could increase the complexity and cost of our billing process. Additionally, auditing for compliance with applicable laws and regulations as well as internal compliance policies and procedures adds further cost and complexity to the billing process. Further, our billing systems require significant technology investment and, as a result of marketplace demands, we need to continually invest in our billing systems.
Missing or incorrect information on requisitions adds complexity to and slows the billing process, creates backlogs of unbilled requisitions, and generally increases the aging of accounts receivable and bad debt expense. We believe that much of our bad debt expense in recent years is attributable to the lack of, or inaccurate, billing information. Failure to timely or correctly bill may lead to our not being reimbursed for our services or an increase in the aging of our accounts receivable, which could adversely affect our results of operations and cash flows. Failure to comply with applicable laws relating to billing government healthcare programs could lead to various penalties, including: (1) exclusion from participation in CMS and other government programs; (2) asset forfeitures; (3) civil and criminal fines and penalties; and (4) the loss of various licenses, certificates and authorizations necessary to operate our business, any of which could have a material adverse effect on our results of operations or cash flows.
There have been times when our accounts receivable have increased at a greater rate than revenue growth and, therefore, have adversely affected our cash flows from operations. We have taken steps to implement systems and processing changes intended to improve billing procedures and related collection results. We believe that we have made progress by reorganizing our accounts receivable and billing functions and that our allowance for doubtful accounts is adequate. However, we cannot assure that our ongoing assessment of accounts receivable will not result in the need for additional provisions. Such additional provisions, if implemented, could have a material adverse effect on our operating results.
Our failure or the failure of third-party payors or physicians to comply with ICD-10-CM Code Set, and our failure to comply with other emerging electronic transaction standards could adversely impact our business.
We are within the assessment and inventory phase to adopt the ICD-10-CM Code Set issued by HHS on January 16, 2009. Compliance with the ICD-10-CM Code Set is currently required to be in place by October 1, 2014. The Company will continue its assessment of information systems, applications and processes for compliance with these requirements. Clinical laboratories are typically required to submit health care claims with diagnosis codes to third party payors. The diagnosis codes must be obtained from the ordering physician. Our failure or the failure of third party payors or physicians to transition within the required timeframe could have an adverse impact on reimbursement, days sales outstanding and cash collections.
Also, the failure of our IT systems to keep pace with technological advances may significantly reduce our revenues or increase our expenses. Public and private initiatives to create healthcare information technology (“HCIT”) standards and to mandate standardized clinical coding systems for the electronic exchange of clinical information, including test orders and test results, could require costly modifications to our existing HCIT systems. While we do not expect HCIT standards to be adopted or implemented without adequate time to comply, if we fail to adopt or delay in implementing HCIT standards, we could lose customers and business opportunities.
Failure in our information technology systems could significantly increase testing turn-around time or billing processes and otherwise disrupt our operations.
Our laboratory operations depend, in part, on the continued performance of our information technology systems. Our information technology systems are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptions. In addition, we are in the process of integrating the information technology systems of our recently acquired subsidiaries, and we may experience system failures or interruptions as a result of this process. Sustained system failures or interruption of our systems in one or more of our laboratory operations could disrupt our ability to process laboratory requisitions, perform testing, provide test results in a timely manner and/or bill the appropriate party. Breaches with respect to protected health information could result in violations of HIPAA and analogous state laws, and risk the imposition of significant fines and penalties. Failure of our information technology systems could adversely affect our business, profitability and financial condition.
Adverse results in material litigation matters could have a material adverse effect upon our business.
We may become subject in the ordinary course of business to material legal action related to, among other things, professional liability and employee-related matters, as well as inquiries from governmental agencies and Medicare or Medicaid carriers requesting comment on allegations of billing irregularities that are brought to their attention through billing audits or third parties. Legal actions could result in substantial monetary damages as well as damage to our reputation with clients, which could have a material adverse effect upon our business.
We may be unable to obtain, maintain or enforce our intellectual property rights and may be subject to intellectual property litigation that could adversely impact our business.
We may be unable to obtain or maintain adequate patent or other proprietary rights for our products and services or to successfully enforce our proprietary rights. In addition, we may be subject to intellectual property litigation and we may be found to infringe on the proprietary rights of others, which could force us to do one or more of the following:
cease developing, performing or selling products or services that incorporate the challenged intellectual property;
obtain and pay for licenses from the holder of the infringed intellectual property right;
redesign or reengineer our tests;
change our business processes; or
13
Table of Contents
pay substantial damages, court costs and attorneys’ fees, including potentially increased damages for any infringement held to be willful.
The Company believes that no single patent, technology, trademark, intellectual property asset or license is material to its business as a whole.
If patent regulations or standards are modified, such changes could have a negative impact on our business.
From time to time, the U.S. Supreme Court, other federal courts, the U.S. Congress or the U.S. Patent and Trademark Office, USPTO, may change the standards of patentability and validity and any such changes could have a negative impact on our business. In addition, competitors may develop their own versions of our test in countries where we did not apply for patents or where our patents have not issued and compete with us in those countries, including encouraging the use of their test by physicians or patients in other countries.
There have been several cases involving “gene patents” and diagnostic claims that have been considered by the U.S. Supreme Court. A suit brought by multiple plaintiffs, including the American Civil Liberties Union, or ACLU, against Myriad Genetics, or Myriad, and the USPTO involves certain of Myriad’s U.S. patents related to the breast cancer susceptibility genes BRCA1 and BRCA2. The Federal Circuit issued a written decision on July 29, 2011 that reversed the U.S. District Court for the Southern District of New York holding instead that the breast cancer genes are patentable subject matter. Subsequently, on March 20, 2012, the Supreme Court issued a decision in Mayo Collaborative v. Prometheus Laboratories, or Prometheus, a case involving patent claims directed to optimizing the amount of drug administered to a specific patient. According to that decision, Prometheus’ claims failed to add enough inventive content to the underlying correlations to allow the processes they describe to qualify as patent-eligible processes that apply natural laws. The Supreme Court subsequently granted certiorari in the Myriad case, vacated the judgment, and remanded the case back to the Federal Circuit for further consideration in light of their decision in the Prometheus case. The Federal Circuit issued a decision on August 16, 2012, reaffirming its earlier decision.
On July 3, 2012, the USPTO issued a memorandum to patent examiners providing guidelines for examining process claims for patent eligibility in view of the Supreme Court decision in Prometheus. The guidance indicates that claims directed to a law of nature, a natural phenomenon, or an abstract idea that do not meet the eligibility requirements should be rejected as non-statutory subject matter. We cannot assure you that our patent portfolio will not be negatively impacted by the decision described above, rulings in other cases or changes in guidance or procedures issued by the USPTO.
The Supreme Court granted ACLU’s petition for a writ of certiorari and issued a decision on June 13, 2013. In the ruling, the Supreme Court held that claims to “isolated” DNA molecules and the information they encode are not patent eligible, whereas DNA, not a “product of nature,” is patent eligible. On July 12, 2013, Senator Patrick Leahy wrote to the National Institutes of Health, or NIH, urging that the NIH take the extremely unusual step of exercising its “march in” rights to force Myriad to license certain patents to others on “reasonable” terms due to the public health and cost considerations.
Congress directed the USPTO to study effective ways to provide independent, confirming genetic diagnostic test activity where gene patents and exclusive licensing for primary genetic diagnostic tests exist. This study will examine the impact that independent second opinion testing has on providing medical care to patients; the effect that providing independent second opinion genetic diagnostic testing would have on the existing patent and license holders of an exclusive genetic test; the impact of current practices on testing results and performance; and the role of insurance coverage on the provision of genetic diagnostic tests. The USPTO was directed to report the findings of the study to Congress and provide recommendations for establishing the availability of independent confirming genetic diagnostic test activity by June 16, 2012. In August 2012, the Department of Commerce advised the House and Senate Judiciary Committee leadership that given the complexity and significant policy implications, that further review, discussion and analysis are required before a final report can be submitted to Congress. To that end, the USPTO held an additional public hearing in late fall 2012, plans to review the comments received during the last year, and then plans to finalize its recommendations to Congress. It is unclear whether the results of this study will be acted upon by the USPTO or result in Congressional efforts to change the law or process in a manner that could negatively impact our patent portfolio or our future research and development efforts.
In addition, the Leahy-Smith America Invents Act, or the America Invents Act, which was signed into law in 2011, includes a number of significant changes to U.S. patent law. These include changes to transition from a “first-to-invent” system to a “first-to-file” system, changes to the way issued patents are challenged and changes to the way patent applications are disputed during the examination process. These changes may favor larger and more established companies that have more resources to devote to patent application filing and prosecution. The USPTO has developed new regulations and procedures to govern the full implementation of the America Invents Act, and many of the substantive changes to patent law associated with the Act, and in particular the first to file provisions, which became effective in March 2013. Substantive changes to patent law associated with the Act may affect our ability to obtain, enforce or defend our patents. Accordingly, it is not clear what, if any, impact the America Invents Act will ultimately have on the cost of prosecuting our patent applications, our ability to obtain patents based on our discoveries and our ability to enforce or defend our issued patents, all of which could have a material adverse effect on our business.
We are facing, and may in the future face, intellectual property infringement claims that could be time-consuming and costly to defend, and could result in our loss of significant rights and the assessment of treble damages.
In October 2013, a lawsuit was filed against us by Myriad Genetics alleging we are infringing upon their intellectual property by offering OncoGeneDx, our comprehensive series of inherited cancers testing, including testing for BRCA1/2. We may from time to time receive additional notices of claims of infringement and misappropriation or misuse of other parties’ proprietary rights. Some of these additional claims may also lead to litigation. We cannot assure you that we will prevail in such actions, or that other actions alleging misappropriation or misuse by us of third-party trade secrets, infringement by us of third-party patents and trademarks or the validity of our patents, will not be asserted or prosecuted against us.
We may also initiate claims to defend our intellectual property or to seek relief on allegations that we use, sell, or offer to sell technology that incorporates third party intellectual property. Intellectual property litigation, regardless of outcome, is expensive and time-consuming, could divert management’s attention from our business and have a material negative effect on our business, operating results or financial condition. If there is a successful claim of infringement against us, we may be required to pay substantial damages (including treble damages if we were to be found to have willfully infringed a third party’s patent) to the party claiming infringement, develop non-infringing technology, stop selling our tests or using technology that contains the allegedly infringing intellectual property or enter into royalty or license agreements that may not be available on acceptable or commercially practical terms, if at all. Our failure to develop non-infringing technologies or license the proprietary rights on a timely basis could harm our business.
14
Table of Contents
It is possible that a third party or patent office might take the position that one or more patents or patent applications constitute prior art in the field of genomic-based diagnostics. In such a case, we might be required to pay royalties, damages and costs to firms who own the rights to these patents, or we might be restricted from using any of the inventions claimed in those patents.
Failure to establish, and perform to, appropriate quality standards to assure that the highest level of quality is observed in the performance of our testing services and in the design, manufacture and marketing of our products could adversely affect the results of our operations and adversely impact our reputation.
The provision of clinical testing services, including anatomic pathology services, and related services, and the design, manufacture and marketing of diagnostic products involve certain inherent risks. The services that we provide and the products that we design, manufacture and market are intended to provide information for healthcare providers in providing patient care. Therefore, users of our services and products may have a greater sensitivity to errors than the users of services or products that are intended for other purposes.
Manufacturing or design defects, unanticipated use of our products, or inadequate disclosure of risks relating to the use of the products can lead to injury or other adverse events. These events could lead to recalls or safety alerts relating to our products (either voluntary or required by governmental authorities) and could result, in certain cases, in the removal of a product from the market. Any recall could result in significant costs as well as negative publicity that could reduce demand for our products. Personal injuries relating to the use of our products can also result in product liability claims being brought against us. In some circumstances, such adverse events could also cause delays in new product approvals.
Similarly, negligence in performing our services can lead to injury or other adverse events. We may be sued under physician liability or other liability law for acts or omissions by our pathologists, laboratory personnel and hospital employees who are under the supervision of our hospital-based pathologists. We are subject to the attendant risk of substantial damages awards and risk to our reputation.
Discontinuation or recalls of existing testing products, failure to develop, or acquire, licenses for new or improved testing technologies; or our clients using new technologies to perform their own tests could adversely affect our business.
From time to time, manufacturers discontinue or recall reagents, test kits or instruments used by us to perform laboratory testing. Such discontinuations or recalls could adversely affect our costs, testing volume and revenue.
The clinical laboratory industry is subject to changing technology and new product introductions. Our success in maintaining a leadership position in genomic and other advanced testing technologies will depend, in part, on our ability to develop, acquire or license new and improved technologies on favorable terms and to obtain appropriate coverage and reimbursement for these technologies. We may not be able to negotiate acceptable licensing arrangements and it cannot be certain that such arrangements will yield commercially successful diagnostic tests. If we are unable to license these testing methods at competitive rates, our research and development costs may increase as a result. In addition, if we are unable to license new or improved technologies to expand our esoteric testing operations, our testing methods may become outdated when compared with our competition and testing volume and revenue may be materially and adversely affected.
In addition, advances in technology may lead to the development of more cost-effective technologies such as point-of-care testing equipment that can be operated by physicians or other healthcare providers in their offices or by patients themselves without requiring the services of freestanding clinical laboratories. Development of such technology and its use by our clients could reduce the demand for our laboratory testing services and negatively impact our revenues.
Currently, most clinical laboratory testing is categorized as “high” or “moderate” complexity, and thereby is subject to extensive and costly regulation under CLIA. The cost of compliance with CLIA makes it impractical for most physicians to operate clinical laboratories in their offices, and other laws limit the ability of physicians to have ownership in a laboratory and to refer tests to such a laboratory. Manufacturers of laboratory equipment and test kits could seek to increase their sales by marketing point-of-care laboratory equipment to physicians and by selling test kits approved for home or physician office use to both physicians and patients. Diagnostic tests approved for home use are automatically deemed to be “waived” tests under CLIA and may be performed in physician office laboratories as well as by patients in their homes with minimal regulatory oversight. Other tests meeting certain FDA criteria also may be classified as “waived” for CLIA purposes. The FDA has regulatory responsibility over instruments, test kits, reagents and other devices used by clinical laboratories and has taken responsibility from the Centers for Disease Control for classifying the complexity of tests for CLIA purposes. Increased approval of “waived” test kits could lead to increased testing by physicians in their offices or by patients at home, which could affect our market for laboratory testing services and negatively impact our revenues.
Clinicians or patients using our services may sue us, and our insurance may not sufficiently cover all claims brought against us, which will increase our expenses.
The development, marketing, sale and performance of healthcare services expose us to the risk of litigation, including professional negligence. Damages assessed in connection with, and the costs of defending, any legal action could be substantial. We may be faced with litigation claims that exceed our insurance coverage or are not covered under any of our insurance policies. In addition, litigation could have a material adverse effect on our business if it impacts our existing and potential customer relationships, creates adverse public relations, diverts management resources from the operation of the business, or hampers our ability to otherwise conduct our business.
A failure to integrate newly acquired businesses and the costs related to such integration could have a material adverse impact on our net revenues and profitability.
The successful integration of any business that we may acquire entails numerous risks, including, among others:
issues related to revenue recognition and/or cash collections;
loss of key customers or employees;
difficulty in consolidating redundant facilities and infrastructure and in standardizing information and other systems;
failure to maintain quality of services that we and any such acquired companies have historically provided;
coordination of geographically separated facilities and workforces; and
diversion of management’s attention from our day-to-day business.
15
Table of Contents
We cannot assure you that current or future acquisitions, if any, or any related integration efforts will be successful, or that our business will not be adversely affected by any future acquisitions. Even if we are able to successfully integrate the operations of companies or businesses that we may acquire in the future, we may not be able to realize the benefits that we expect to result from such integration, including projected cost savings.
Our operations may be adversely impacted by the effects of extreme weather conditions, natural disasters such as hurricanes and earthquakes, health pandemics, hostilities or acts of terrorism and other criminal activities.
Our operations were adversely impacted by the effects of Hurricane Sandy, and may be adversely impacted by other extreme weather conditions, natural disasters, health pandemics, hostilities or acts of terrorism or other criminal activities from time to time. Such events may result in a temporary decline in the number of patients who seek clinical testing services or in our employees’ ability to perform their job duties. In addition, such events may temporarily interrupt our ability to transport specimens, to receive materials from our suppliers or otherwise to provide our services. The occurrence of any such event and/or a disruption to our operations as a result may adversely impact our results of operations.
An inability to attract and retain experienced and qualified personnel could adversely affect our business.
The loss of key management personnel or the inability to attract and retain experienced and qualified employees at our clinical laboratories and research centers could adversely affect our business. Our success is dependent in part on the efforts of key members of our management team, including Marc D. Grodman, M.D., our founder, president and chief executive officer. Success in maintaining our leadership position in genomic and other advanced testing technologies will depend in part on our ability to attract and retain skilled research professionals. In addition, the success of our clinical laboratories also depends on employing and retaining qualified and experienced laboratory professionals, including specialists, who perform clinical laboratory testing services. In the future, if competition for the services of these professionals increases, we may not be able to continue to attract and retain individuals in its markets. Our net revenues and earnings could be adversely affected if a significant number of professionals terminate their relationship with us or become unable or unwilling to continue their employment.
Our outstanding debt may impair our financial and operating flexibility.
As of October 31, 2013, we had approximately $30,302 million of debt outstanding. Our debt agreements contain various restrictive covenants. These restrictions could limit our ability to use operating cash flow in other areas of our business because we must use a portion of these funds to make principal and interest payments on our debt.
We or our subsidiaries may incur additional indebtedness in the future. Our ability to make principal and interest payments will depend on our ability to generate cash in the future. If we incur additional debt, a greater portion of our cash flows may be needed to satisfy our debt service obligations and if we do not generate sufficient cash to meet our debt service requirements, we may need to seek additional financing. In that case, it may be more difficult, or we may be unable, to obtain financing on terms that are acceptable to us. As a result, we would be more vulnerable to general adverse economic, industry and capital markets conditions as well as the other risks associated with indebtedness
Possible volatility in our stock price could negatively affect us and our stockholders.
The trading price of our common stock on the NASDAQ Global Select Market has fluctuated significantly in the past. During the period from November 1, 2010 through October 31, 2013, the trading price of our common stock fluctuated from a high of $33.46 per share to a low of $12.03 per share. In the past, we have experienced a drop in stock price following an announcement of disappointing earnings or earnings guidance. Any such announcement in the future could lead to a similar drop in stock price. The price of our common stock could also be subject to wide fluctuations in the future as a result of a number of other factors, including the following:
changes in expectations as to future financial performance or buy/sell recommendations of securities analysts;
our, or a competitor’s, announcement of new products or services, or significant acquisitions, strategic partnerships, joint ventures or capital commitments;
the operating and stock price performance of other comparable companies; and adverse publicity.
In addition, the U.S. securities markets have experienced significant price and volume fluctuations. These fluctuations often have been unrelated to the operating performance of companies in these markets. Broad market and industry factors may lead to volatility in the price of our common stock, regardless of our operating performance. Moreover, our stock has limited trading volume, and this illiquidity may increase the volatility of our stock price.
In the past, following periods of volatility in the market price of an individual company’s securities, securities class action litigation often has been instituted against that company. The institution of similar litigation against us could result in substantial costs and a diversion of management’s attention and resources, which could negatively affect our business, results of operations or financial condition.
Certain provisions of our charter, by-laws and New Jersey law may delay or prevent a change of control of our company.
Our certificate of incorporation, as amended, requires the approval of 80% of our outstanding shares for any merger or consolidation unless the business combination has been approved or authorized by our board of directors. As a New Jersey corporation with a class of securities registered with the SEC, we are governed by certain provisions of the New Jersey Business Corporation Act that also restrict business combinations with shareholders owning 10% or more of our outstanding shares (or other “interested stockholders” as the term is defined by the New Jersey Shareholders’ Protection Act) for a period of five years after such interested shareholder achieves such status unless the business combination is approved by our board of directors prior to the shareholder becoming an interested shareholder. The New Jersey Shareholders’ Protection Act also restricts business combinations with an interested shareholder after the five-year period unless the transaction receives the approval of two-thirds of the shares outstanding, exclusive of the shares held by the interested shareholder or the transaction satisfies certain fair price requirements. In addition, with certain limited exceptions, federal regulations prohibit a person or company or a group of persons deemed to be “acting in concert” from, directly or indirectly, acquiring more than 10% (5% if the acquirer is a bank holding company) of any class of our voting stock or obtaining the ability to control in any manner the election of a majority of our directors or otherwise direct the management or policies of our company without prior notice or application to and the approval of the Federal Reserve.
16
Table of Contents
A significant deterioration in the economy could negatively impact testing volumes, cash collections and the availability of credit.
Our operations are dependent upon ongoing demand for diagnostic testing services by patients, physicians, hospitals and others. A significant downturn in the economy could negatively impact the demand for diagnostic testing as well as the ability of patients and other payors to pay for services ordered. In addition, uncertainty in the credit markets could reduce the availability of credit and impact our ability to meet our financing needs in the future.
Item 1B. - Unresolved Staff Comments
None.
Item 2. -Properties.
We operate through a regional network of laboratories. The table below summarizes certain information as to our principal facilities as of October 31, 2012.
Location | | Purpose | | Type of
Occupancy |
Clarksburg, MD | | Pathology Laboratory | | Leased |
Elmwood Park, NJ | | Main Laboratory | | Leased |
Elmwood Park, NJ | | Corporate Headquarters | | Leased |
Gaithersburg, MD | | Genetics Laboratory | | Leased |
Houston, TX | | Pathology Laboratory | | Leased |
Milford, MA | | Oncology Laboratory | | Leased |
Poughkeepsie, NY | | Pathology Laboratory | | Leased |
Campbell, CA | | Main Laboratory | | Leased |
Miami, FL | | Main Laboratory | | Leased |
Melbourne, FL | | Main Laboratory | | Leased |
We perform cancer cytogenetic testing at our leased facilities in at our main processing facility in Elmwood Park, Smithtown, NY, Clarksburg, MD and Milford, MA and genetic testing at our GeneDx leased facility in Gaithersburg, MD, as well as at our Elmwood Park facility. We perform cytology testing in Frederick, MD, Milford, MA, Columbus, OH, Houston, TX and at our Elmwood Park facility. We believe that each of these facilities as presently equipped has the production capacity for its currently foreseeable level of operations. We also lease additional space for patient service centers throughout the New York metropolitan area to collect specimens from physician-referred patients for testing at our processing facilities.
Item 3. - Legal Proceedings
In the normal course of business, we have been named, from time to time, as a defendant in various legal actions, which may include lawsuits alleging negligence or other similar legal claims, which could involve claims for substantial compensatory and/or punitive damages or claims for indeterminate amounts of damages, and could have an adverse impact on our client base and reputation. We may also be involved, from time to time, in other reviews, investigations and proceedings by governmental agencies regarding our business. In addition, as a health care provider and in connection with health care billing-related products, the Company may also be named from time to time in suits brought under the qui tam provisions of the False Claims Act and comparable state laws in which allegations may be made that the Company has submitted or cause to be submitted false claims in connection with claims for payment from federal or state health care programs. In addition, the Company may, from time to time, receive subpoenas from state agencies and from the Office of the Inspector General of the U.S. Department of Health and Human Services seeking documents relating to the Company’s billing-related activities. These types of legal proceedings could result in adverse judgments, including substantial monetary settlements, significant fines and penalties, as well as injunctions or other relief.
BioReference Laboratories, Inc. v. Horizon Healthcare Services, Inc. d/b/a Horizon Blue Cross Blue Shield of New Jersey
On December 18, 2013, the Company filed an action in the Superior Court of New Jersey against Horizon Blue Cross Blue Shield of New Jersey (“Horizon”), captioned BioReference Laboratories, Inc. v. Horizon Healthcare Services, Inc. d/b/a Horizon Blue Cross Blue Shield of New Jersey, Docket No. BER L-009748-13 (N.J. Super. Ct. Bergen Cnty.). The Company asserts claims for breach of contract, breach of the implied covenant of good faith and fair dealing, and fraud against Horizon relating to the Ancillary Services Provider Agreement entered into by the parties in 2003 and amended in 2007. More specifically, the central claims in the lawsuit arise from the Company’s performance of laboratory services since at least 2008 for members of Horizon’s NJ DIRECT plan, who receive benefits under a program that Horizon has bid, promoted, and represented to be a PPO product for New Jersey state, county, and municipal workers and teachers. The lawsuit alleges that, despite these representations, Horizon has been improperly treating NJ DIRECT as a Managed Care program in its dealings with the Company, thereby costing the Company more than $20,000,000 in unreimbursed services and depriving state beneficiaries of valuable rights and benefits to which they are entitled. In addition to compensatory damages, the Company seeks to recover punitive damages from Horizon due to Horizon’s intentional and malicious misconduct. The Company also seeks declaratory and injunctive relief. Horizon’s response to the Company’s complaint is currently due on or before January 22, 2014. The lawsuit has been placed on a pretrial discovery schedule. The Company intends to vigorously prosecute its claims against Horizon.
Univ. Utah Research Foundation et al. v. GeneDx, Inc., Case No. 2:13-CV-00954-RJS (D. Utah)
On October 16, 2013, University of Utah Research Foundation, the University of Pennsylvania, the Hospital for Sick Children, Endorecherche, Inc., and Myriad Genetics, Inc., filed a civil complaint against GeneDx, , in the United States District Court for the District of Utah, Central Division in Salt Lake City, Utah. The complaint alleges that certain genetic tests offered by GeneDx infringe, willfully infringe, contribute to the infringement of, and/or induce others to infringe a number of issued U.S. patents. The plaintiffs seek, among other relief, injunctive relief, unspecified compensatory damages, destruction of infringing products, accounting for unjust enrichments, attorneys’ fees, and costs.
On December 9, 2013, GeneDx, timely served and filed an answer to the plaintiffs’ complaint denying the plaintiffs’ claims and asserted affirmative defenses and counterclaims for invalidity of the asserted patents. GeneDx, Inc. is defending by alleging non-infringement and invalidity of the patents-in-suit, and is seeking attorneys’ fees and costs on the counterclaims. On December 23, 2013, the plaintiffs filed a reply to the counterclaims denying them and the requested relief. The case is currently in the very early stages. No schedule has been set and discovery
17
Table of Contents
has not begun. Plaintiffs are also in litigation with industry competitors and are attempting to consolidate the actions in the District of Utah through the United States Judicial Panel on Multidistrict Litigation (MDL No. 2510). We intend to vigorously defend ourselves in this matter.
Item 4. — Mine Safety Disclosure
Not Applicable.
18
Table of Contents
PART II
Item 5. - Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our Common Stock is listed for trading on The NASDAQ Global Select Market under the symbol “BRLI.”
The following table sets forth the range of high and low closing prices on the NASDAQ Stock Market for our Common Stock for the periods indicated.
Fiscal Year | | Prices ($) | |
2012 | | High | | Low | |
First Quarter (11/1/2011-1/31/2012) | | 19.86 | | 12.03 | |
Second Quarter (2/1/2012-4/30/2012) | | 24.18 | | 18.93 | |
Third Quarter (5/1/2012-7/31/2012) | | 28.12 | | 18.38 | |
Fourth Quarter (8/1/2012-10/31/2012) | | 32.42 | | 25.10 | |
2013 | | High | | Low | |
First Quarter (11/1/2012-1/31/2013) | | 31.05 | | 24.68 | |
Second Quarter (2/1/2013-4/30/2013) | | 28.22 | | 23.58 | |
Third Quarter (5/1/2013-7/31/2013) | | 31.90 | | 25.25 | |
Fourth Quarter (8/1/2013-10/31/2013) | | 33.46 | | 25.78 | |
On January 3, 2014 the last sale price for the Common Stock on NASDAQ was $24.76 per share.
Stockholders
At January 3, 2014, the number of record owners of the Common Stock was 270. Such number of record owners was determined from our shareholder records and does not include beneficial owners whose shares are held in nominee accounts with brokers, dealers, banks and clearing agencies.
Dividends
We have not paid any dividends on our Common Stock since our inception and, do not contemplate or anticipate paying any dividends in the foreseeable future. Furthermore, our loan agreement with PNC Bank prohibits us from paying any cash dividends or making any cash distributions with respect to shares of our Common Stock.
Recent Sales of Unregistered Securities
In 2010, we entered into an employment agreement with Wendy Chung. Upon execution of the employment agreement, 11,432 shares of Common Stock were issued to Ms. Chung and another 34,293 shares were held in escrow, to be released subject to certain terms and conditions set forth in the employment agreement. Certain terms and conditions of the employment agreement were met as of December 1, 2011 and on February 3, 2012, another 11,431 shares of Common Stock were delivered to Ms. Chung. The terms and conditions of Ms. Chung’s employment agreement were met again on December 1, 2012 and, on December 14, 2012, another 11,431 shares of Common Stock were delivered to her. On December 1 2013 Ms Chung yet again met the terms of her employment agreement and the final 11,431 shares of our common stock were delivered to her.
Performance Graph
We have presented below the cumulative total return to our stockholders during the period from November 1, 2008, through October 31, 2013 in comparison to the cumulative return on the S&P 500 Index and a customized peer group of eight companies during that same period.
19
Table of Contents
Peer Group |
Covance Inc |
Enzo Biochem Inc. |
Genomic Health Inc |
Laboratory Corporation of America Holdings |
Myriad Genetics Inc |
Neogenomics Inc |
Quest Diagnostics Inc |
Response Genetics Inc |
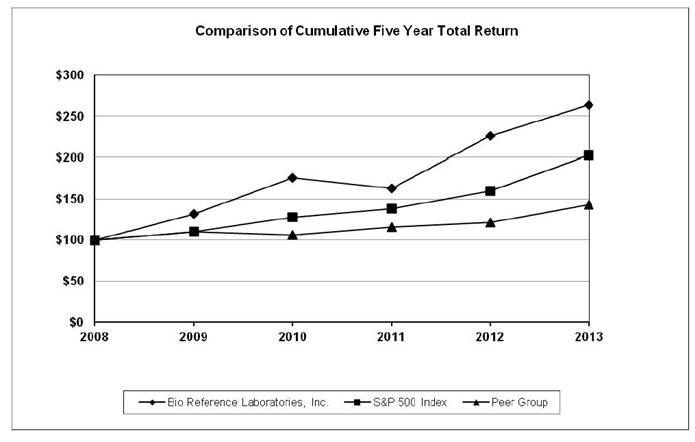
The results assume that $100 (with reinvestment of all dividends) was invested in our common stock, in the peer group, and in the index on October 31, 2008 and its relative performance tracked through October 31, 2013. The comparisons are based on historical data and are not indicative of, nor intended to forecast, the future performance of our common stock. The performance graph set forth above shall not be deemed incorporated by reference into any filing by us under the Securities Act of 1933 or the Securities Exchange Act of 1934 except to the extent that we specifically incorporate such information by reference therein.
20
Table of Contents
Item 6. - Selected Financial Data
The following is a summary of our historical consolidated financial data for the periods ended and at the dates indicated below. You are encouraged to read this information together with our audited consolidated financial statements and the related footnotes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this Annual Report.
The historical consolidated financial data for the years ended October 31, 2013, 2012, and 2011, 2010 and 2009 has been derived from our audited consolidated financial statements. The historical consolidated financial data for the years ended October 31, 2009 and 2010, has been derived from our audited consolidated financial statements, which are not included in this Annual Report.
We believe that the comparability of our financial results between the periods presented in the table below is significantly impacted by factors which are more fully described in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the Consolidated Financial Statements and the notes thereto included elsewhere in this Annual Report.
All comparisons to prior periods are adjusted in accordance with the Accounting Standards Update 2011-7 under Topic 954 of FASB codification. The appended table reflects the adjustments for the prior period.
| | Fiscal Years Ended October 31, | |
| | 2013 | | 2012 | | 2011 | | 2010 | | 2009 | |
| | [In Thousands Except Per Share Data] | |
Operating Data: | | | | | | | | | | | |
Net Revenues | | $ | 715,354 | | $ | 614,255 | | $ | 522,081 | | $ | 424,559 | | $ | 336,895 | |
Cost of Services | | 392,815 | | 337,644 | | 287,853 | | 232,252 | | 183,524 | |
Gross Profit | | 322,539 | | 276,611 | | 234,228 | | 192,307 | | 153,371 | |
General and Administrative Expenses | | 240,566 | | 200,480 | | 174,454 | | 143,929 | | 115,049 | |
Income From Operations | | 81,973 | | 76,131 | | 59,774 | | 48,378 | | 38,322 | |
Other Expenses [Income] - Net | | 876 | | 1,615 | | -5,072 | | 1,415 | | -267 | |
Provision for Income Tax Expense | | 35,272 | | 32,360 | | 28,487 | | 20,582 | | 16,739 | |
Net Income | | $ | 45,825 | | $ | 42,156 | | $ | 36,359 | | $ | 26,381 | | $ | 21,850 | |
Net Income Per Share - Basic | | $ | 1.65 | | $ | 1.52 | | $ | 1.30 | | $ | 0.95 | | $ | 0.79 | |
Net Income Per Share - Diluted | | $ | 1.65 | | $ | 1.51 | | $ | 1.29 | | $ | 0.94 | | $ | 0.78 | |
Other Data: | | | | | | | | | | | |
Net Cash - Operating Activities | | $ | 17,662 | | $ | 53,098 | | $ | 30,946 | | $ | 14,305 | | $ | 24,366 | |
Net Cash - Investing Activities | | $ | (44,113 | ) | $ | (21,390 | ) | $ | (15,542 | ) | $ | (18,411 | ) | $ | (10,807 | ) |
Net Cash - Financing Activities | | $ | 19,260 | | $ | (29,056 | ) | $ | (11,170 | ) | $ | 5,790 | | $ | (9,260 | ) |
| | As of October 31, | |
| | 2013 | | 2012 | | 2011 | | 2010 | | 2009 | |
Balance Sheet Data: | | | | | | | | | | | |
Total Assets | | $ | 421,528 | | $ | 312,347 | | $ | 283,259 | | $ | 244,131 | | $ | 197,390 | |
Total Long-Term Liabilities | | $ | 14,382 | | $ | 13,626 | | $ | 10,978 | | $ | 8,405 | | $ | 8,378 | |
Total Liabilities | | $ | 149,934 | | $ | 85,100 | | $ | 93,492 | | $ | 91,743 | | $ | 72,867 | |
Working Capital | | $ | 161,116 | | $ | 151,625 | | $ | 124,266 | | $ | 89,459 | | $ | 75,984 | |
Shareholder’s Equity | | $ | 271,594 | | $ | 227,247 | | $ | 189,767 | | $ | 152,388 | | $ | 124,523 | |
Item 7. - Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You are encouraged to read the following discussion and analysis of our financial condition and results of operations together with our audited consolidated financial statements and related footnotes included at the end of this Annual Report. This discussion and analysis contains forward-looking statements that involve risks and uncertainties. See “Risk Factors” included elsewhere in this Annual Report for a discussion of some of the important factors that could cause actual results to differ materially from those described or implied by the forward-looking statements contained in the following discussion and analysis. See “Special Note Regarding Forward-Looking Statements” included elsewhere in this Annual Report.
All amounts are presented in thousands, except share and per share amounts and per patient data.
21
Table of Contents
Overview
We are a national clinical diagnostic laboratory located in northeastern New Jersey. We are a national laboratory in certain focused areas of laboratory testing and a full service laboratory in the New York super-region. We have developed a national reputation for our expertise in certain focused areas of clinical testing. GenPath, the name by which we are known for our cancer and oncology services, is recognized for the superior hematopathology services it provides throughout the country. Our Women’s Health initiative, through which we provide dedicated services for obstetrics and gynecology practices, including a unique, technically advanced multiplex process for identifying sexually transmitted infections, is also offered as GenPath. Our regional footprint lays within the New York City metropolitan area and the surrounding areas of New Jersey and southern New York State as well eastern Pennsylvania and some areas of western Connecticut; we also provide services further into New York State, Pennsylvania, Delaware and Maryland. As a regional provider, we are a full-service laboratory that primarily services physician office practices; our drivers pick up samples and deliver reports and supplies, we provide sophisticated technical support, phlebotomy services or patient service centers where appropriate, and electronic communication services in many cases. Physicians outside of our regional footprint send samples to our laboratory in order to take advantage of the expertise that we are able to provide in blood-based cancer pathology and associated diagnostics or to take advantage of the superior service, support and technologically advanced testing we offer in our Women’s Health initiative. These accounts frequently send routine testing to us for processing along with specialized testing in order to simplify their diagnostic ordering and review procedures and to take advantage of our outstanding capability, service and support. Our correctional healthcare services are used throughout the country at prisons and jails. The focused markets we serve on a national basis outside of our regional footprint do not require many of the logistical and other ancillary support services required within the region. Even within our regional footprint, we provide the same services that we provide on a national basis as well as some regional focused diagnostic services, such as histology and pathology support services, substance abuse testing, fertility testing, hemostasis testing, women’s health testing, and molecular diagnostics that are unavailable from many of the smaller regional competitors; testing in some of these areas may be provided outside of physician offices. In October 2012, we launched Laboratorio Buena Salud, the first national testing laboratory dedicated to serving Spanish-speaking populations in the United States. All business will be conducted in Spanish, including patient and physician interactions.
Over the last few years, there have been fundamental changes in the laboratory services industry. In the 1990s, the industry was negatively impacted by the growth of managed care, increased government regulation, and investigations into fraud and abuse. These factors led to revenue and profit declines and industry consolidations, especially among commercial laboratories. There are currently only three US publicly traded full service laboratories operating primarily in the U.S. While that means that the two national mega-laboratories and Bio-Reference Laboratories are the only remaining publicly traded full service commercial laboratories, there are numerous hospital outreach programs and smaller reference laboratories that compete for the commercial clinical laboratory business scattered throughout the country. Clinical laboratories have had to improve efficiency, leverage economies of scale, comply with government regulations and other laws and develop more profitable approaches to pricing. Moreover, there has been a proliferation of technology advancements in clinical diagnostics over the last decade that has created significant opportunities for new testing and growth.
As a full service clinical laboratory, we are constantly looking for new technologies and new methodologies that will help us to grow. Since the turn of the century, our size alone has made us attractive to companies that are driving the advances in technology. We represent a significant opportunity for these companies to market their products with a nationally recognized specialty provider in our focused areas of specialty or in one of the major population centers of the world—the New York Metropolitan area. We have had several successful strategic relationships with such technology opportunities. In addition to new technology opportunities, we have an extremely seasoned and talented management staff that has been able to identify emerging laboratory markets that are under-served or under-utilized. We have recently developed programs for cardiology, histology and women’s health to go along with our existing hemostasis, hematopathology and correctional healthcare initiatives which have already been established and in which we have been increasing our market share for the past several years. We are currently preparing to launch a comprehensive pre-natal program to leverage our presence in the women’s health environment and we will continue to vigilantly seek focused diagnostic marketing opportunities where we can provide information, technology, service or support that expand and grow our clinical laboratory.
On December 21, 2012, the Company entered into an agreement with Meridian Clinical Laboratory Corporation, a Florida corporation having its place of business in Miami, Florida (“Meridian”), pursuant to which the Company purchased all issued and outstanding common stock of Meridian for approximately $1,848 of which $250 is deferred for one year.
On December 31, 2012, Bio-Reference Laboratories, Inc. (the “Company”) entered into an agreement with Florida Clinical Laboratory, Inc., a Florida corporation having its place of business in Melbourne, Florida (“FCL”), pursuant to which the Company purchased all issued and outstanding shares of capital stock of FCL for approximately $7,016, of which $1,000 is deferred for eighteen months assuming certain conditions are met.
On August 7, 2013 the Company purchased substantially all of the operating assets and certain of the operating liabilities of Hunter Laboratories, Inc., (“Hunter”) a California corporation having its principal place of business in Campbell, California. The gross purchase price was $15,215 plus payroll adjustment of $111 totaling $15,326. Of that amount $3,000 was deferred to cover anticipated pre closing liabilities.
On August 20, 2013 the Company through its subsidiary GeneDx, Inc. purchased the entire membership interest in Edge BioServ, LLC, (“Edge Bio”) a Delaware limited liability company having its place of business in Gaithersburg, Maryland. The gross purchase price was approximately $2,502. Of that $375 was deferred to cover anticipated pre closing liabilities.
While we recognize that we are a clinical laboratory that processes samples, we also understand that we are an information company that needs to effectively communicate the results of our efforts back to healthcare providers. Laboratory results play a major role in the implementation of physician healthcare. Laboratory results are used to diagnose, monitor and classify health concerns. In many cases, laboratory results represent the confirming data in diagnosing complicated health issues. Since laboratory results play such an important role in routine physician care, we have developed informatics solutions that leverage our role in healthcare. We built a web-based solution to quickly, accurately, conveniently and competitively collect ordering information and deliver results. That solution is called CareEvolve. CareEvolve has been essential to our own operations. We license the technology to other laboratories throughout the country that they utilize to more effectively compete against the national laboratories. These other laboratories licensing our technology are typically not our competitors since they are outside our regional footprint.
We have also created our PSIMedica business unit that has developed a Clinical Knowledge Management (CKM) System that takes data from enrollment, claims, pharmacy, laboratory results and any other available electronic source to provide both administrative and clinical analysis of a population. The system uses proprietary algorithms to cleanse and configure the data and transfer the resulting information into a healthcare data repository. Using advanced cube technology methodologies, the data can be analyzed from a myriad of views and from highly granular transactional detail to global trended overview. Events such as the Katrina disaster in Louisiana and general pressures from the government have made development of an electronic medical record system and Pay-for Performance reimbursement priority goals in the healthcare industry. A large portion of an individual’s medical record consists of laboratory data and a key performance indicator in any Pay-for-Performance initiative is laboratory result data. Our CKM system is a mature, full functioning solution that will allow us to play a role in these important national initiatives.
To date, neither our PSIMedica business unit nor CareEvolve has produced significant revenues relative to the primary laboratory operations.
22
Table of Contents
Results of Operations
All comparisons to prior periods are adjusted in accordance with the Accounting Standards Update 2011-7 under Topic 954 of FASB codification.
Fiscal Year 2013 Compared to Fiscal Year 2012
NET REVENUES:
Net revenues for the year ended October 31, 2013 were $715,354 as compared to $614,255 for the year ended October 31, 2012; this represents a 16% increase in net revenues. This increase is due to a 10% increase in patients serviced and a 6% increase in net revenue per patient. Our laboratory operations had net revenues of $709,592 in fiscal 2013 and $609,763 in fiscal 2012.
The number of patients serviced during the year ended October 31, 2013 was 8,549, which was 10% greater when compared to the prior fiscal year. Net revenue per patient for the year ended October 31, 2013 was $83.00 compared to net revenue per patient for the year ended October 31, 2012 of $78.16, an increase of 6% as a result of increases in esoteric testing.
Despite continued strong volume growth, the Company believes there is an ongoing recalibration of reimbursement for the industry, which has resulted in substantial downward pressure from many payers regarding reimbursement in FY13. Over the past year, the Company has had to negotiate contract modifications to reimbursement rates, conditions of payment and / or eligibility with dozens of health plans representing a substantial numbers of lives nationwide; most of these changes became effective toward the end of FY13 and especially in Q4FY13.
Our revenues and patient counts could be adversely affected by a number of factors, including, but not limited, to an extended economic downturn in general or healthcare economic conditions, an unexpected reduction in reimbursement rates, increased market penetration by our competitors or a substantial adverse change in federal regulatory requirements governing our industry as well as a failure to continue the sizeable annual percentage increase in base business from significantly higher levels after 19 years of sustained growth.
COST OF SERVICES:
Cost of services for the year ended October 31, 2013 was $392,815 as compared to $337,644 for the year ended October 31, 2012, an increase of 16% as compared to a 16% increase in net revenues. This is basically in line with the increase in our net revenues.
GROSS PROFIT:
Gross profit on net revenues increased to $322,539 for the year ended October 31, 2013 from $276,611 for the year ended October 31, 2012; an increase of $45,928 (17%), primarily attributable to the increase in net revenues. Gross profit margins remained consistent at 45% from fiscal 2012 to fiscal 2013.
GENERAL AND ADMINISTRATIVE EXPENSES:
General and administrative expenses for the year ended October 31, 2013 were $240,566 as compared to $200,480 for the year ended October 31, 2012, an increase of $40,086 or 20%. This increase is slightly more than the increase in net revenues due to additional bad debt expenses the Company recorded as the result of the ongoing reimbursement changes in the marketplace. We expect this trend to continue in the near future.
INTEREST EXPENSE:
Interest expense increased from $1,455 during the year ended October 31, 2012 to $1,606 during the year ended October 31, 2013; an increase of $151 or 10%. This increase is due to an increase in utilization of the PNC Bank line of credit. Management believes that this trend will continue in the near term.
NET INCOME:
We realized net income of $45,825 for the twelve month period ended October 31, 2013 as compared to $42,156 for the twelve month period ended October 31, 2012, an increase of 9%.
Pre-tax income for the period ended October 31, 2013 was $81,097, as compared to $74,516 for the period ended October 31, 2012, an increase of $6,581 (9%) and was caused primarily by an increase in net revenues. The provision for income taxes increased from $32,360 for the period ended October 31, 2012, to $35,272 (9%) for the current twelve month period.
During this fiscal year the Company received a refund of $1,062 for its New York State clinical laboratory inspection fee that was included in other income.
Our diluted net income per share went from $1.51 in fiscal 2012 to $1.65 in fiscal 2013.
Fiscal Year 2012 Compared to Fiscal Year 2011
NET REVENUES:
Net revenues for the year ended October 31, 2012 were $614,255 as compared to $522,081 for the year ended October 31, 2011; this represents an 18% increase in net revenues. This increase is due to a 16% increase in patients serviced and a 2% increase in net revenue per patient. Our laboratory operations had net revenues of $609,763 in fiscal 2012 and $517,721 in fiscal 2011.
The number of patients serviced during the year ended October 31, 2012 was 7,801, which was 16% greater when compared to the prior fiscal year. Net revenue per patient for the year ended October 31, 2012 was $78.16 compared to net revenue per patient for the year ended October 31, 2011 of $76.82, an increase of 2% as a result of increases in esoteric testing.
During the fiscal year ended October 31, 2012, we increased our sales force by approximately 12%, mostly in the specialty testing services that we market nationally. We believe that this increase in sales personnel accounted for a majority of the 16% increase in our patient volume. This allowed us to expand or increase our presence in a number of markets and we expect this trend to continue.
While there is always uncertainty as to the sustainability of such growth in the future, we believe that our historical performance of 20% compound annual growth rate for the past 18 years, the current demand for our services and our continued corporate focus on strategic
23
Table of Contents
growth, together with our expertise in the industry, should enable us to sustain continued strong growth in the near term. Going beyond that, however, our revenues and patient counts could be adversely affected by a number of factors including, but not limited to an extended downturn in general or healthcare economic conditions, an unexpected reduction in reimbursement rates, increased market penetration by our competitors, or a substantial adverse change in federal regulatory requirements governing our industry as well as a failure to continue the sizeable annual percentage increase in base business from significantly higher levels after 17 years of sustained growth.
Our net revenues for the fourth quarter of fiscal 2012 and cumulative results for the fiscal 2012 as well as operating results during the first quarter of fiscal 2013 have been affected by the adverse weather conditions associated with the hurricane Sandy. Based on actual revenues and expenses from the period immediately preceding the storm as well as the analysis of the period following the storm, management has calculated the damage from, the hurricane Sandy resulted in a loss of net revenues of approximately $5,000 for the fourth quarter of fiscal 2012 and approximately $3,000 for the first quarter of fiscal 2013.
COST OF SERVICES:
Cost of services for the year ended October 31, 2012 was $337,644 as compared to $287,853 for the year ended October 31, 2011, an increase of 17% as compared to an 18% increase in net revenues. The Company’s medical supplies expense increased by 34% due to the higher cost of specialty testing supplies. Our medical equipment repair costs increased by 26% year over year due to higher equipment utilization rate. We expect these trends to continue.
GROSS PROFIT:
Gross profit on net revenues increased to $276,611 for the year ended October 31, 2012 from $234,229 for the year ended October 31, 2011; an increase of $42,382 (18%), primarily attributable to the increase in net revenues. Gross profit margins remained consistent at a rate of 45% from fiscal 2011 to fiscal 2012.
GENERAL AND ADMINISTRATIVE EXPENSES:
General and administrative expenses for the year ended October 31, 2012 were $200,480 as compared to $174,454 for the year ended October 31, 2011, an increase of $26,026 or 15%. This is basically in line with the increase in net revenues. We expect this trend to continue in the near future.
INTEREST EXPENSE:
Interest expense decreased from $1,747 during the year ended October 31, 2011 to $1,455 during the year ended October 31, 2012; a decrease of $292 or 17%. This decrease is due to a decrease in utilization of the PNC Bank line of credit. Management believes that this trend will continue in the near term due to the decrease in utilization of this credit facility.
NET INCOME:
We realized net income of $42,156 for the twelve month period ended October 31, 2012 as compared to $36,359 for the twelve month period ended October 31, 2011, an increase of 16%.
Pre-tax income for the period ended October 31, 2012 was $74,516, as compared to $64,846 for the period ended October 31, 2011, an increase of $9,670 (15%) and was caused primarily by an increase in net revenues. The provision for income taxes increased from $28,487 for the period ended October 31, 2011, to $32,360 (14%) for the current twelve month period.
Our diluted net income per share went from $1.29 in fiscal 2011 to $1.51 in fiscal 2012.
Our operating results for the fourth quarter of fiscal 2012 and cumulative results for the fiscal 2012 as well as operating results during the first quarter of fiscal 2013 have been affected by hurricane Sandy. Based on actual revenues and expenses from the period immediately preceding the storm as well as the analysis of the period following the storm, Management has calculated the damage from the storm resulted in a loss of earnings approximately $0.06 per share for the fourth quarter of fiscal 2012 and approximately $0.03 per share for the first quarter of fiscal 2013.
Liquidity and Capital Resources
Our working capital at October 31, 2013 was approximately $161,116 as compared to approximately $151,625 at October 31, 2012, an increase of $9,491 (6%). Our cash position decreased by approximately $7,191 during the current twelve month period. We increased our short term borrowing by approximately $26,139 and decreased our long term debt by approximately $435. We had current liabilities of approximately $133,762 at October 31, 2013. We generated approximately $17,662 in cash from operations, a decrease of approximately $35,436 as compared to the year ended October 31, 2012.
The decrease is primarily due to slower cash collections. These slower collections are, in the opinion of management, attributable first in part to the expired “grandfather” provision, a rule that allowed us to bill Medicare directly for in hospital laboratory work that was performed on Medicare patients even though the patient was in the hospital at the time the service was rendered. This exemption expired at the end of 2012 and starting in 2013 we were required to bill the hospital directly for such laboratory work instead of billing Medicare for it. As a consequence of this change collection cycle has increased and in some cases prices may have decreased. The second reason for slower collection, in our opinion, involves changes in the molecular coding around the country by Medicare and in some cases Medicaid, who have simply stopped paying at all for these molecular tests. These reimbursement levels are set my CMS. With regard to payments by Medicare, there still remain many of these tests whose reimbursement has not been determined by the carrier. Another reason for slower collections is the change in the Blue Cross Blue Shield (“BCBS”) reimbursement practices whereby instead of billing BCBS for all of the laboratory services preformed nationwide on one bill we are now required to bill each local BCBS only for the services preformed based on the location where a patient’s sample was drawn. This new practice, also effective in 2013, significantly increased the complexity of our BCBS billing and collection processes. In many cases billings were delayed for some time until prices were loaded in the various systems around the country. Lastly, slower collections occurred due to a dispute with Horizon Blue Cross Blue Shield of New Jersey (“Horizon BCBSNJ”) concerning Horizon BCBSNJ’s obligation to pay Bio-Reference with respect to certain of its insurance plans. Please see “Legal Proceedings” above. .
Accounts receivable, net of allowance for doubtful accounts, totaled approximately $206,261 at October 31, 2013, an increase of approximately $53,014 from October 31, 2012, or 35%. This increase was primarily attributable to increased revenue and slowdown in the collection cycle. Cash collected over the twelve month period ended October 31, 2013 increased 11% over the prior twelve month period.
24
Table of Contents
Net service revenues on the statements of operations are as follows:
| | ($) | |
| | Year Ended | |
| | October 31, | |
| | 2013 | | 2012 | | 2011 | |
Gross Service Revenues | | 3,524,108 | | 3,052,431 | | 2,482,349 | |
| | | | | | | |
Contractual Adjustments and Discounts: | | | | | | | |
Medicare/Medicaid Portion | | 354,638 | | 320,697 | | 293,874 | |
All Other Third Party Payors* | | 2,393,872 | | 2,070,073 | | 1,629,833 | |
Total Contractual Adjustments and Discounts | | 2,748,510 | | 2,390,770 | | 1,923,707 | |
Service Revenues Net of Contractual Adjustments and Discounts | | 775,598 | | 661,661 | | 558,642 | |
Patient Service Revenue Provision for Bad Debts** | | 60,244 | | 47,406 | | 36,561 | |
Net Revenues | | 715,354 | | 614,255 | | 522,081 | |
| | | | | | | |
Percent of Contractual Allowances, Discounts and Patient Service Provision for Bad Debts to Gross Revenue. | | 79.7 | % | 79.9 | % | 79.0 | % |
* All Other Third Party and Direct Payors consists of almost eight hundred distinct payors, including commercial health insurers and administrators as well as professionally billed accounts such as physicians, hospitals, clinics and other direct billed accounts.
** Represents the amount of Bad Debt Expense that is now required to be presented as a deduction from patient service revenue (net of contractual allowances and discounts) pursuant to ASU No. 2011-7.
Credit risk with respect to accounts receivable is generally diversified due to the large number of patients comprising our client base. We have significant receivable balances with government payors and various insurance carriers. Generally, we do not require collateral or other security to support customer receivables. However, we continually monitor and evaluate our client acceptance and collection procedures to minimize potential credit risks associated with our accounts receivable and to establish an allowance for uncollectible accounts. As a consequence, we believe that our accounts receivable credit risk exposure beyond such allowance is not material to the financial statements.
A number of proposals for legislation continue to be under discussion which could substantially reduce Medicare and Medicaid (CMS) reimbursements to clinical laboratories. Depending upon the nature of regulatory action, and the content of legislation, we could experience a significant decrease in revenues from Medicare and Medicaid (CMS), which could have a material adverse effect on us. We are unable to predict, however, the extent to which such actions will be taken.
25
Table of Contents
LABORATORY GROSS RECEIVABLES BY PAYOR GROUP
| | | | | | | | | | ($) | | | | | | | | | | | |
| | | | | | | | | | FY 2013 | | | | | | | | | | | |
| | 30 Days | | % | | 60 Days | | % | | 90 Days | | % | | >90 Days | | % | | Total | | % | |
Self Pay | | 12,723 | | 18 | % | 12,934 | | 18 | % | 11,244 | | 15 | % | 35,367 | | 49 | % | 72,269 | | 100 | % |
Medicare | | 35,783 | | 45 | % | 12,801 | | 16 | % | 4,732 | | 6 | % | 25,841 | | 33 | % | 79,158 | | 100 | % |
Medicaid | | 5,625 | | 20 | % | 4,377 | | 15 | % | 4,321 | | 15 | % | 14,145 | | 50 | % | 28,469 | | 100 | % |
Pro Bill | | 16,103 | | 51 | % | 6,162 | | 20 | % | 3,085 | | 10 | % | 6,140 | | 19 | % | 31,491 | | 100 | % |
Commercial Insurance | | 196,097 | | 46 | % | 59,571 | | 14 | % | 33,559 | | 8 | % | 137,204 | | 32 | % | 426,432 | | 100 | % |
Total | | 266,332 | | 42 | % | 95,847 | | 15 | % | 56,942 | | 9 | % | 218,698 | | 34 | % | 637,819 | | 100 | % |
| | ($)
FY 2012 | |
| | 30 DAYS | | % | | 60 DAYS | | % | | 90 DAYS | | % | | >90 DAYS | | % | | TOTAL | | % | |
Self Pay | | 9,620 | | 18 | % | 10,633 | | 20 | % | 9,474 | | 18 | % | 24,202 | | 44 | % | 53,929 | | 100 | % |
Medicare | | 25,898 | | 56 | % | 6,550 | | 14 | % | 2,992 | | 6 | % | 11,186 | | 24 | % | 46,626 | | 100 | % |
Medicaid | | 4,881 | | 23 | % | 3,804 | | 18 | % | 3,829 | | 18 | % | 8,714 | | 41 | % | 21,228 | | 100 | % |
Pro Bill | | 17,697 | | 64 | % | 4,706 | | 17 | % | 1,771 | | 6 | % | 3,657 | | 13 | % | 27,832 | | 100 | % |
Commercial Insurance | | 154,294 | | 47 | % | 53,097 | | 17 | % | 24,752 | | 8 | % | 88,747 | | 28 | % | 320,889 | | 100 | % |
Total | | 212,390 | | 45 | % | 78,790 | | 17 | % | 42,818 | | 9 | % | 136,507 | | 29 | % | 470,505 | | 100 | % |
Billing for laboratory services is complicated and we must bill various payors, such as the individual, the insurance company, the government (federal or state), the private company or the health clinic. Other factors that may complicate billing include:
Differences between fee schedules and reimbursement rates;
Incomplete or inaccurate billing information as provided by the physician;
Disparity in coverage and information requirements;
Disputes with payors; and
Internal and external compliance policies and procedures.
Significant costs are incurred as a result of our participation in government programs since billing and reimbursement for laboratory tests are subject to complex regulations. We perform the requested tests and report the results whether the information is correct or not or even missing. This adds to the complexity and slows the collection process and increases the aging of our accounts receivable (“A/R”). When patient invoices are not collected in a timely manner the item is written off to the allowance.
Days Sales Outstanding (“DSO”) for fiscal years 2012 and 2013 were 86 and 100, respectively, an increase of approximately 16%, computed under the new method taking into account the change in presentation for patient service revenue provision for bad debts. Depending on the period in question, our actual collections represent between 98% and 102% of our net collectable revenues after giving effect to our DSO lag.
Overall, the components of A/R as shown above for the two most recently completed fiscal years under review have not varied much year over year. The percent of A/R over 90 days has increased to 34% as of October 31, 2013 as compared to 29% as of October 31, 2012, an increase of 5%.
See Note 5 and Note 6 to our consolidated financial statements for information regarding outstanding loans.
See Note 18 to our consolidated financial statements describing our merger and acquisition activities.
The weighted average interest rate on short-term borrowings outstanding as of October 31, 2013 was 3.50% and as of October 31, 2012 was approximately 3.25%.
We intend to expand our laboratory operations through aggressive marketing an while also attempting to diversify into related medical fields through acquisitions. These acquisitions may involve cash, notes, Common Stock, and/or combinations thereof.
Subsequent to our year end, on December 19, 2013 the Company approved a new stock repurchase program authorizing buyback of up to 2,000,000 shares of Common Stock in the over the counter market at prevailing market prices through October 31, 2015.
Contractual Obligations
The following table summarizes our significant contractual obligations as of October 31, 2013:
| | Total | | FY 2014 | | FY 2015 | | FY 2016 | | FY 2017 | | FY 2018
and thereafter | |
Long-Term Debt | | 4,163 | | 486 | | 518 | | 551 | | 585 | | 2,023 | |
Capital Leases | | 16,846 | | 5,622 | | 4,630 | | 3,727 | | 2,192 | | 675 | |
Operating Leases | | 14,847 | | 9,015 | | 2,556 | | 1,509 | | 558 | | 1,209 | |
Purchase Obligations | | 45,556 | | 12,505 | | 11,938 | | 10,854 | | 6,559 | | 3,700 | |
Long-Term Liabilities under Employment and Consultant Contracts | | 16,860 | | 5,169 | | 3,873 | | 3,657 | | 2,918 | | 1,243 | |
26
Table of Contents
No one supplier who is counterparty to any particular supply agreement is contracted to provide more than one percent of our Cost of Services in any future period. Such contracts are made in the ordinary course of business. No directors, officers, promoters, voting trustees or individuals known to be Bio-Reference Laboratories, Inc (“BRLI”) security holders are counterparties to these agreements. Management does not believe that BRLI is substantially dependent upon these supply agreements, as the goods may be obtained from different suppliers or wholesalers, if needed. None of these agreements are leases or call for the acquisition or sale of property, plant and equipment.
Our cash balances at October 31, 2013 totaled approximately $17,952 as compared to approximately $25,143 at October 31, 2012. We believe that our cash position, the anticipated cash generated from future operations, and the availability of our credit line with PNC Bank, will meet our anticipated cash needs in fiscal 2014.
Off-Balance Sheet Arrangements
As of October 31, 2013, we did not have any off-balance sheet items.
Impact of Inflation
To date, inflation has not had a material effect on our operations.
Critical Accounting Policies
The preparation of financial statements in conformity with U.S. generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reported periods.
Accounting for Intangible and Other Long-Lived Assets
We evaluate the possible impairment of our long-lived assets, including intangible assets on annual basis or earlier if events or changes in circumstances occur that indicate that the carrying value of the asset(s) may not be recoverable. The Company assessed qualitative factors to determine whether events and circumstances lead to the conclusion that it is necessary to perform the two-step goodwill impairment test have occurred and determined that no such events had occurred. Under ASU No. 2011-08, entities are not required to calculate the fair value of a reporting unit unless they conclude that it is more likely than not that the unit’s carrying value is greater than its fair value based on an assessment of events and circumstances. The “more likely than not” threshold is when there is a likelihood of more than 50% that a reporting unit’s carrying value is greater than its fair value. No impairment loss was recognized in the years ended October 31, 2013, 2012 and 2011.
Accounting for Revenue
Service revenues are principally generated from laboratory testing services including chemical diagnostic tests such as blood analysis, urine analysis and genetic testing among others. Service revenues are recognized at the time the testing services are performed and are reported at their estimated net realizable amounts.
Service revenues before provision for bad debts are determined utilizing gross service revenues net of contractual adjustments and discounts. Even though it is the responsibility of the patient to pay for laboratory service bills, most individuals in the United States have an agreement with a third party payor such as Medicare, Medicaid or a commercial insurance provider to pay all or a portion of their healthcare expenses. The majority of services provided by Bio-Reference Laboratories, Inc. (“BRLI”) are to patients covered under a third party payor contract. In certain cases, the individual has no insurance or does not provide insurance information and in other cases tests are performed under contract to a professional organization (such as physicians, hospitals, and clinics) which reimburse BRLI directly. In the remainder of the cases, BRLI is provided the third party billing information and seeks payment from the third party under the terms and conditions of the third party payor for health service providers like BRLI. Each of these third party payors may differ not only with regard to rates, but also with regard to terms and conditions of payment and providing coverage (reimbursement) for specific tests. Estimated revenues are established based on a series of highly complex procedures and judgments that require industry specific healthcare experience and an understanding of payor methods and trends. We review our calculations on a monthly basis in order to make certain that we are properly allowing for the uncollectable portion of our gross billings due to the contractual adjustments and discounts and that our estimates remain sensitive to variances and changes within our payor groups. The contractual allowance calculation is made on the basis of historical allowance rates for the various specific payor groups on a monthly basis with a greater weight being given to the most recent trends This process is adjusted based on recent changes in underlying contract provisions and shifts in the testing being performed. This calculation is routinely analyzed by BRLI on the basis of actual allowances issued by payors and the actual payments made to determine what adjustments, if any, are needed. The table below shows the adjustments made to gross service revenues to arrive at net revenues, the amount reported on our statement of operations.
| | ($) | |
| | Year Ended | |
| | October 31, | |
| | 2013 | | 2012 | | 2011 | |
Gross Service Revenues | | 3,524,108 | | 3,052,431 | | 2,482,349 | |
| | | | | | | |
Contractual Adjustments and Discounts: | | | | | | | |
Medicare/Medicaid Portion | | 354,638 | | 320,697 | | 293,874 | |
All Other Third Party Payors* | | 2,393,872 | | 2,070,073 | | 1,629,833 | |
Total Contractual Adjustments and Discounts | | 2,748,510 | | 2,390,770 | | 1,923,707 | |
Service Revenues Net of Contractual Adjustments and Discounts | | 775,598 | | 661,661 | | 558,642 | |
Patient Service Revenue Provision for Bad Debts** | | 60,244 | | 47,406 | | 36,561 | |
Net Revenues | | 715,354 | | 614,255 | | 522,081 | |
| | | | | | | |
Percent of Contractual Allowances, Discounts and Patient Service Provision for Bad Debts to Gross Revenue. | | 79.7 | % | 79.9 | % | 79.0 | % |
27
Table of Contents
* All Other Third Party and Direct Payors consists of almost eight hundred distinct payors, including commercial health insurers and administrators as well as professionally billed accounts such as physicians, hospitals, clinics and other direct billed accounts.
** Represents the amount of Bad Debt Expense that is now required to be presented as a deduction from patient service revenue (net of contractual allowances and discounts) pursuant to ASU No. 2011-7.
When new business is received by BRLI, service revenues net of contractual adjustments and discounts are calculated by reducing gross service revenues by the estimated contractual allowance. The Patient Service Revenue Provision for Bad Debts represents the amount of bad debt expense expected to occur on patient service revenue based upon our experience. The remaining bad debt expense is presented as part of operating expenses. The bad debt expense presented as part of operating expense represents the bad debt expense related to receivables from service revenues determined after taking into account our ability to collect on such revenue. BRLI recognized the amounts in subsequent periods for actual allowances/discounts to gross service revenue; bad debt may have been adjusted over the same periods of time to maintain an accurate balance between net revenues and actual revenues. Management has reviewed the allowances/discounts recognized in subsequent periods and believes the amounts to be immaterial. A number of proposals for legislation or regulation continue to be under discussion which could have the effect of substantially reducing Medicare reimbursements for clinical laboratories or introducing cost sharing to beneficiaries. Depending upon the nature of regulatory action, if any, which is taken and the content of legislation, if any, which is adopted, the Company could experience a significant decrease in revenues from Medicare and Medicaid, which could have a material adverse effect on the Company. The Company is unable to predict, however, the extent to which such actions will be taken.
Accounting for Contractual Credits and Doubtful Accounts
It is typically the responsibility of the patient to pay for laboratory service bills. Most individuals in the United States have an agreement with a third party payor such as Medicare, Medicaid or commercial insurance to pay all or a portion of their healthcare expenses; this represents the major portion of payment for all services provided by BRLI. In certain cases, the individual has no insurance or does not provide insurance information; in the remainder of the cases, BRLI is provided the third party billing information, usually by the referring physician, and seeks payment from the third party under the terms and conditions of the third party payor for health service providers like BRLI. Each of these third party payors may differ not only with regard to rates, but also with regard to terms and conditions of payment and coverage of specific tests. BRLI routinely reviews the reimbursement policies and subsequent payments and collection rates from these different types of payors. Contractual adjustments and discounts are recorded as reductions to gross service revenues and are collectively referred to as the contractual allowance. BRLI has not been required to record an adjustment in a subsequent period related to revenue recorded in a prior period which was material in nature. Aging of accounts receivable is monitored by billing personnel and follow-up activities including collection efforts are conducted as necessary. BRLI writes off receivables against the allowance for doubtful accounts when they are deemed uncollectible. For client billing, accounts are written off when all reasonable collection efforts prove to be unsuccessful. Patient accounts, where the patient is directly responsible for all or a remainder portion of the account after partial payment or denial by a third party payor, are written off after the normal dunning cycle has occurred, although these may be subsequently transferred to a third party collection agency after being written off. Third party payor accounts are written off when they exceed the payer’s timely filing limits. Accounts Receivable on the balance sheet is net of the following amounts for contractual credits and doubtful accounts:
| | ($) | |
| | October 31, | | October 31, | |
| | 2013 | | 2012 | |
| | | | | |
Contractual Credits/Discounts | | 342,297 | | 267,921 | |
Doubtful Accounts | | 89,261 | | 51,274 | |
Total Allowance | | 431,558 | | 319,195 | |
Accounting for Employee Benefit Plans
See Note 21 to our consolidated financial statements for a discussion on Employee Benefit Plans.
New Authoritative Pronouncements
See Note 22 to our consolidated financial statement that discusses new authoritative pronouncements.
Item 7A. - Quantitative and Qualitative Disclosures about Market Risk
We do not invest in or trade instruments which are sensitive to market risk. We also do not have any material foreign operations or foreign sales so we have no exposure to foreign currency exchange rate risk.
We do have exposure to both rising and falling interest rates. At October 31, 2013, advances of approximately $26,139under our Loan Agreement with PNC Bank were subject to interest charges at the bank’s then prime rate of 3.50 %.
We estimate that our monthly cash interest expense at October 31, 2013 was approximately $143and that a one percentage point increase or decrease in short-term rates would increase or decrease our monthly interest expense by approximately $22. However we expect to utilize the credit line in the future and we thus expect exposure to short term interest rates changes that will depend on the utilization rate at the time.
See Note 5 and Note 6 to the Consolidated Financial Statements contained herein for information on our loans.
Item 8. - Financial Statements and Supplementary Data
Financial Statements are annexed hereto.
28
Table of Contents
Item 9. - Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None
Item 9A. - Controls and Procedures
(a) Evaluation of Disclosure Controls and Procedures
An evaluation was performed under the supervision and with the participation of our management, including our principal executive officer and our principal financial officer as to the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report. Any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. Based on that evaluation, the principal executive officer and the principal financial officer of the Company have concluded that, as of the end of the period covered by this report, our disclosure controls and procedures are effective at a reasonable assurance level.
(b) Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles in the United States of America. Internal control over financial reporting includes those policies and procedures that:
(i) pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
(ii) provide reasonable assurance that the transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with the authorization of management and/or our Board of Directors; and
(iii) provide reasonable assurance regarding the prevention or timely detection of any unauthorized acquisition, use or disposition of our assets that could have a material effect on our financial statements.
Due to its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate due to changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework published in 1992. Based on its evaluation, our management concluded that our internal control over financial reporting was effective as of the end of the period covered by this Annual Report on Form 10-K.
MSPC, Certified Public Accountants and Advisors, A Professional Corporation, an independent registered public accounting firm, has audited the Consolidated Financial Statements included in this Annual Report on Form l0-K and, as part of their audit, has issued its attestation report, included herein, on the effectiveness of our internal control over financial reporting. See “Report of Independent Registered Public Accounting Firm” included in this filling.
(c) Changes in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting that occurred during the fourth quarter of fiscal 2013 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. - Other Information
None.
29
Table of Contents
PART III
Item 10. - Directors, Executive Officers and Corporate Governance
Executive Officers and Directors
The following table sets forth certain information with respect to each of our directors and executive officers.
Name | | Age | | Position |
| | | | |
Marc D. Grodman, M.D | | 62 | | Chairman of the Board, President, Chief Executive Officer and Director |
| | | | |
Howard Dubinett | | 62 | | Executive Vice President, Chief Operating Officer and Director |
| | | | |
Sam Singer | | 70 | | Senior Vice President, Chief Financial Officer, Chief Accounting Officer and Director |
| | | | |
Joseph Benincasa(a)(c)(e) | | 64 | | Director |
| | | | |
Harry Elias(a)(c)(e) | | 83 | | Director |
| | | | |
Gary Lederman, Esq. (b)(c)(e) | | 79 | | Director |
| | | | |
John Roglieri, M.D. (a)(d)(e) | | 74 | | Director |
(a) Member of the Audit Committee
(b) Chairman of the Audit Committee
(c) Member of the Compensation Committee
(d) Chairman of the Compensation Committee
(e) Member of Nominating Committee
Marc D. Grodman, M.D. founded the Company in December 1981 and has been our Chairman of the Board, President, Chief Executive Officer and a director since our formation. Dr. Grodman is an Assistant Professor of Clinical Medicine at Columbia University’s College of Physicians and Surgeons and Assistant Attending Physician at Presbyterian Hospital, New York City. Since January 2005, Dr. Grodman has been a member of the board of directors, served as Chairman and currently serves as Vice Chairman of the American Clinical Laboratory Association, an industry organization comprised of the largest and most significant commercial clinical laboratories in the United States. From 1980 to 1983, Dr. Grodman attended the Kennedy School of Government at Harvard University and was a Primary Care Clinical Fellow at Massachusetts General Hospital. From 1982 to 1984, he was a medical consultant to the Metal Trades Department of the AFL-CIO. Dr. Grodman received a B.A. degree from the University of Pennsylvania in 1973 and an M.D. degree from Columbia University’s College of Physicians and Surgeons in 1977. Except for his part-time duties as Assistant Professor of Clinical Medicine and Assistant Attending Physician at Columbia University and Presbyterian Hospital, Dr. Grodman devotes all of his working time to our business. We believe that Dr. Grodman is qualified to serve on our board of directors because of his extensive medical expertise, his experience on the faculty at Columbia University College of Physicians and Surgeons, his leadership role in our industry and his knowledge of trends in the healthcare industry.
Howard Dubinett has been our Executive Vice-President and Chief Operating Officer of the Company since our formation in 1981. He became a director in April 1986. Mr. Dubinett attended Rutgers University. We believe that Mr. Dubinett is qualified to serve on our board of directors because of his extensive knowledge of and experience in our business and his knowledge of healthcare regulation.
Sam Singer has been our Chief Financial Officer since October 1987, a director since November 1989, and a Senior Vice President since 2007. Mr. Singer was the Controller for Sycomm Systems Corporation, a data processing and management consulting company, from 1981 to 1987, prior to joining us. Mr. Singer also serves on the boards of several not-for-profit institutions. He received a B.A. degree from Strayer University and an M.B.A. from Rutgers University. We believe that Mr. Singer is qualified to serve on our board of directors because of his extensive experience in financial matters, including financial reporting, and his experience with our business gained through his tenure as our Chief Financial Officer.
Joseph Benincasa joined our board of directors in June 2005. Mr. Benincasa currently serves as the executive director of The Actors’ Fund of America, a position he has held since 1989. The Actors’ Fund is the leading national, non-profit human services organization providing comprehensive social and health care services, employment, training and housing support to the entertainment profession. For six years, from 2000 to 2006, Mr. Benincasa served as a director of St. Peter’s University Medical Center, a major hospital in northern New Jersey. He also sits on the board of directors of Broadway Cares/Equity Fights AIDS; the National Theatre Workshop of the Handicapped; Career Transition for Dancers; the Times Square Alliance; the New York Society of Association Executives and the Somerset Patriots, a minor league baseball team. Mr. Benincasa holds a B.A. degree from St. Joseph’s University, an M. Ed. Degree from Rutgers University and also attended the Fordham University Graduate School of Business. We believe that Mr. Benincasa is qualified to serve on our board of directors because of his familiarity with healthcare issues gained through his board service at St. Peter’s University Medical Center and his extensive experience with administrative matters.
Harry Elias became a member of the board of directors in March 2004. Mr. Elias commenced his employment in sales and marketing with JVC Company of America (“JVC”), a distributor of audio and video products, in 1967, subsequently being appointed as JVC’s Senior Vice President of Sales and Marketing in 1983 and as Executive Vice President of Sales and Marketing in 1990. In 1995, Mr. Elias was named as JVC’s Chief Operating Officer, a position he occupied until April 2003 when he resigned his positions upon his appointment as JVC’s “Honorable Chairman.” In January 2005, after retiring from JVC, Mr. Elias was appointed Chairman of the Board of and commenced to serve as a consultant to AKAI USA, the sole distributor in the United States of electronic products produced by AKAI, a Chinese manufacturer. Mr. Elias retired from AKAI in 2007 and currently is self-employed as a business consultant. We believe that Mr. Elias is qualified to serve on our board of directors because of the experience and skills he gained in running a large business operation.
Gary Lederman, Esq. became a member of our board of directors in May 1997. He received his B.A. degree from Brooklyn College in 1954 and his J.D. degree from NYU Law School in 1957. He was manager of Locals 370, 491 and 662 of the U.F.C.W. International Union from 1961 to 1985.. During the 1970s, Mr. Lederman also served as a member of the New York Attorney General’s Consumer Fraud Advisory Committee. He is retired from the unions and has been a lecturer at Queensboro Community College in the field of insurance. He served on an institutional review board for RTL, a pharmaceutical drug testing laboratory until his retirement in February 2007. We believe that Mr. Lederman
30
Table of Contents
is qualified to serve on our board of directors as a result of his legal expertise, his union manager experience and responsibilities and his experience with RTL, including his involvement with health and welfare funds and his familiarity with consumer regulation and the activities of pharmaceutical companies.
John Roglieri, M.D. became a member of our board of directors in September 1995. He is an Assistant Professor of Clinical Medicine at Columbia University’s College of Physicians and Surgeons and an Assistant Attending Physician at Presbyterian Hospital, New York City. Dr. Roglieri received a B.S. degree in Chemical Engineering and a B.A. degree in Applied Sciences from Lehigh University in 1960, an M.D. degree from Harvard Medical School in 1966, and a Masters degree from Columbia University’s School of Business in 1978. From 1969 until 1971, he was a Senior Assistant Surgeon in the U.S. Public Health Service in Washington, D.C. From 1971 until 1973 he was a Clinical and Research Fellow at Massachusetts General Hospital. From 1973 until 1975, he was director of the Robert Wood Johnson Clinical Scholars program at Columbia University. In 1975 he was appointed Vice-President, Ambulatory Services at Presbyterian Hospital, a position which he held until 1980. Since 1980, he has maintained a private practice of internal medicine at Columbia-Presbyterian Medical Center. From 1988 until 1992, he was also director of the Employee Health Service at Presbyterian Hospital. From 1992 through 1999, Dr. Roglieri was the corporate medical director of NYLCare, a managed care subsidiary of New York Life Insurance Company. Dr. Roglieri was chief medical officer of Physician WebLink, a national physician practice management company, from 1999 to 2000. Since 2001, he has been a medical director for New York Life in Manhattan. He is a member of advisory boards to several pharmaceutical companies, a member of the Editorial Advisory Board of the journals Managed Care and Seminars in Medical Practice. We believe that Dr. Roglieri is qualified to serve on our board of directors due to his extensive medical background, his role as director of the Employee Health service at Presbyterian Hospital, his role as corporate medical director of a managed care organization and the skill and expertise gained through his many other activities.
There are no family relationships between or among any directors or executive officers of Bio-Reference Laboratories.
Director Independence
Our board of directors has determined that each of Messrs. Benincasa, Elias and Lederman and Dr. Roglieri are independent within the applicable rules of the SEC and the NASDAQ Stock Market, and that each of them is also an independent director under Rule 10A-3 of the Exchange Act for the purpose of audit committee membership.
Audit Committee
We have a separately-designated standing Audit Committee established in accordance with Section 3(a)(58)(A) of the Exchange Act and the Nasadq Listing Rules. The Audit Committee is comprised of Gary Lederman (Chairman), Joseph Benincasa, Harry Elias and John Roglieri. Our Board has determined that Mr. Lederman is an audit committee financial expert within the meaning of the applicable rules of the SEC and the NASDAQ Stock Market.
Code of Ethics
Our Code of Ethics is applicable to our senior management, as well as our key financial and accounting personnel. It has been designed to deter wrongdoing and to promote:
honest and ethical conduct including the ethical handling of actual or apparent conflicts of interest between personal and professional relationships;
full, fair, accurate, timely and understandable disclosure in our public communications and reports filed with the SEC;
compliance with applicable governmental laws, rules and regulations;
prompt internal reporting of violations of the Code to an appropriate person or persons identified in the Code; and
accountability to ensure adherence to the Code.
The Code requires each covered person to deal ethically and honestly with the company and to avoid business, financial or other direct or indirect interests or relationships that conflict with those of the company or divide the covered person’s loyalty to the company. Each covered person is required to sign an attestation of compliance with the Code at the end of each fiscal year.
The Code of Ethics is available on our internet Web site (www.bioreference.com) and will be provided in print without charge to any stockholder who submits a request in writing to Bio-Reference Laboratories, Inc. Investor Relations, 481 Edward H. Ross Drive, Elmwood Park, New Jersey 07407. Any amendment to and waivers from the Code of Ethics with respect to the Company’s Chief Executive Officer or Chief Financial Officer will be posted on the Company’s Web site.
Section 16(a) Beneficial Ownership Reporting Compliance
Based solely on a review of Forms 3 and 4 and any amendments thereto furnished to us pursuant to Rule 16a-3(e) under the Exchange Act, or representations that no Forms 5 were required, we believe that with respect to fiscal 2013, our officers, directors and beneficial owners of more than 10% of our equity timely complied with all applicable Section 16(a) filing requirements.
31
Table of Contents
Item 11. — Executive Compensation
The table below summarizes the total compensation paid or accrued by us with respect to the fiscal years ended October 31, 2011, 2012 and 2013 to our named executive officers (“NEOs”). Our NEOs for fiscal 2013 are Marc D. Goodman, our President and Chief Executive Officer; Howard Dubinett, our Executive Vice President and Chief Operating Officer; and Sam Singer, our Senior Vice President and Chief Financial Officer. This table does not include any amount for our group life, health, hospitalization or medical reimbursement plans, if any, as such benefits do not discriminate in scope, terms or operation, in favor of any or our officers, senior management members or directors, and are generally available to all salaried employees.
SUMMARY COMPENSATION TABLE
Name and
Principal
Position | | Fiscal
Year | | Salary($) | | Bonus
($)(1) | | Stock
Awards($) | | Option
Awards($) | | Non-Equity
Incentive Plan
Compensation
($)(2) | | Change in
Pension
Value and
Nonqualified
Deferred
Compensation
Earnings ($) | | All Other
Compensation
($)(3) | | Total($) | |
| | | | | | | | | | | | | | | | | | | |
Marc D. Grodman M.D., | | 2013 | | 1,136,700 | | 70,000 | | 0 | | 0 | | 0 | | 0 | | 233,832 | | 1,444,532 | |
President and Chief | | 2012 | | 1,092,933 | | 70,000 | | 0 | | 0 | | 131,152 | | 0 | | 245,359 | | 1,539,444 | |
Executive Officer | | 2011 | | 1,059,044 | | 1,321,241 | | 0 | | 0 | | 63,543 | | 0 | | 229,638 | | 2,673,466 | |
| | | | | | | | | | | | | | | | | | | |
Howard Dubinett, | | 2013 | | 449,200 | | 0 | | 0 | | 0 | | 0 | | 0 | | 45,685 | | 494,885 | |
Executive Vice President and | | 2012 | | 431,911 | | 100,000 | | 0 | | 0 | | 51,829 | | 0 | | 42,474 | | 626,214 | |
Chief Operating Officer | | 2011 | | 418,518 | | 0 | | 0 | | 0 | | 25,111 | | 0 | | 45,044 | | 488,673 | |
| | | | | | | | | | | | | | | | | | | |
Sam Singer, | | 2013 | | 449,200 | | 0 | | 0 | | 0 | | 0 | | 0 | | 47,145 | | 496,345 | |
Senior Vice President and | | 2012 | | 431,911 | | 300,000 | | 0 | | 0 | | 51,829 | | 0 | | 48,998 | | 832,738 | |
Chief Financial Officer | | 2011 | | 418,518 | | 110,000 | | 0 | | 0 | | 25,111 | | 0 | | 45,087 | | 598,716 | |
(1) The amounts shown in this column for fiscal 2013 represent (i) with respect to Dr. Grodman, a cash bonus of $70,000 paid in connection with his payment of the premium costs for an insurance policy owned by the Company insuring the life of Dr. Grodman pursuant to an Endorsement Split-Dollar Insurance Agreement among the Company, Dr. Grodman and an Insurance Trust established by Dr. Goodman (“Premium Payments”). The amounts shown in this column for fiscal 2012 represent (i) with respect to Dr. Grodman, a cash bonus of $70,000 paid in connection with his Premium Payments; and (ii) with respect to Mr. Singer and Mr. Dubinett, a one-time cash bonus for their entering into new employment contracts during the year. The amounts shown in this column for fiscal 2011 represent (i) with respect to Dr. Grodman, cash bonuses of $1,202,241 and $119,000 paid in connection with his payment of Premium Payments; and (ii) with respect to Mr. Singer, a one-time cash bonus for his activity in the collection of $6.7 million sales tax refund from the State of New Jersey.
(2) The amounts shown in this column represent amounts earned under the Senior Management Incentive Bonus Plan adopted by the Compensation Committee for the applicable fiscal year. No payments were made to NEOs pursuant to the 2013 Senior Management Incentive Bonus Plan.
(3) The amounts in the “All Other Compensation” column for fiscal 2013 are detailed below.
Name | | Personal Use of
Company Leased
Automobile ($)(a) | | Personal Use of
Company Airplane
($) (a) | | Life Insurance
Premium ($)(b) | | 401(k) Plan
Contribution ($) | | Total ($) | |
Marc D. Grodman | | 27,247 | | 5,585 | | 200,000 | | 1,000 | | 233,832 | |
Howard Dubinett | | 19,685 | | 0 | | 25,000 | | 1,000 | | 45,685 | |
Sam Singer | | 21,145 | | 0 | | 25,000 | | 1,000 | | 47,145 | |
(a) Represents our aggregate incremental costs for personal use of a Company leased automobile or the Company’s aircraft, as applicable.
(b) See “Split Dollar Life Insurance” below.
32
Table of Contents
GRANTS OF PLAN-BASED AWARDS
This table provides information regarding awards that could be granted to our NEOs under the 2013 Senior Management Incentive Bonus Plan.
| | Estimated Future Payouts Under Non-Equity Incentive Plan
Awards (1) | |
Name | | Threshold ($) | | Maximum ($) | |
Marc D. Grodman M.D. | | 45,468 | | 170,505 | |
| | | | | |
Howard Dubinett | | 17,968 | | 67,380 | |
| | | | | |
Sam Singer | | 17,968 | | 67,380 | |
(1) The amounts represent the range of annual cash incentive awards the NEO was potentially entitled to receive based on the achievement of the performance goals for fiscal 2013 under the 2013 Senior Management Incentive Bonus Plan. These cash incentive awards were granted with no specified target level, as defined under SEC Regulation S-K, rule 402(d).
Employment Agreements with NEOs
Dr. Grodman
On December 31, 2010, the Company executed an employment agreement with Dr. Grodman (the “Grodman Contract”), employing him as President and Chief Executive Officer through October 31, 2017. The Grodman Contract is automatically renewable for one additional two year period subject to the right of either party to elect not to renew at least four months prior thereto. The Grodman Contract provides Dr. Grodman with a minimum annual base compensation of $1,059,044, subject to annual percentage increases based on the Consumer Price Index as well as to increases at the discretion of the Compensation Committee. Dr. Grodman’s minimum annual base compensation for fiscal 2012 as determined by the Compensation Committee was $1,092,933 and for fiscal 2013 is $1,136,700. Under the Grodman Contract we agreed to lease and insure an automobile for his benefit and agreed to provide him with access for personal use our airplane, which use will be taxable to him. The Grodman Contract also provides Dr. Grodman with participation rights in any fringe benefit and bonus plans available to the Company’s employees to the extent determined by the Compensation Committee. The Grodman Contract provides that in the event of Dr. Grodman’s total disability we may continue to employ him and compensate him at his then current base compensation for the month the disability occurs and a period of 36 months thereafter followed by an unpaid 3 month period, following which his employment will terminate unless we grant an additional leave of absence. If Dr. Grodman incurs a partial disability then his base compensation will be equitably adjusted based on the time he is able to devote to the Company. In the event of Dr. Grodman’s termination due to his death, the Company will pay his estate a death benefit equal to 24 times his monthly base compensation in effect at the date of his death, paid over 24 months. Under the Grodman Contract we may terminate Dr. Grodman’s employment for “Cause” and Dr. Grodman has the right to terminate his employment for “Good Reason”. If he terminates for Good Reason he will be entitled to continuation of his base compensation and employee benefits through the end of the period he otherwise would have been employed under the Grodman Contract.
“Cause” is defined in the Grodman Contact to mean: any act or acts of dishonesty by Dr. Grodman constituting criminal acts resulting or intending to result directly or indirectly in his gain or personal enrichment at our expense; his commission of a crime involving fraud, embezzlement or theft; or his material breach of the Grodman Contract. “Good Reason” is defined in the Grodman Contract to mean: a material diminution of Dr. Grodman’s base compensation; a material diminution in his authority, duties or responsibilities; a material diminution of the authority, duties or responsibilities of any supervisor he reports to; a material diminution in the budget over which he retains authority; a material change in the geographic location at which he provides services; or any other action or inaction that constitutes a material breach by us of the Grodman Contract.
In the event of a Change in Control of the Company, Dr. Grodman can elect to terminate his employment by providing written notice within 30 days following the Change in Control, with a termination date effective at the earlier of 45 days after the Change in Control or the next to last day of the calendar year in which the Change in Control occurs. In that event, he will be entitled to be paid a lump sum severance payment equal to 2.99 times the average of the annual compensation paid to him by the Company for the five calendar years preceding the earlier of the calendar year in which the Change of Control occurred or the calendar year of the date of termination, reduced by the amount of any other payment or the value of any other benefit received or to be received by him in connection with the termination of his employment or contingent on a Change in Control that are not deductible by us pursuant to Section 280G of the Internal Revenue Code of 1986, as amended (the “Tax Code”). “Change in Control” is defined in the Grodman Contact to mean a “change in effective control” or a “change in the ownership of a substantial portion of a corporation’s assets” as such terms are defined under Section 409A of the Tax Code, and means either the acquisition within 12 months by a person or group of ownership of 30% or more of the total voting power of our stock; the replacement of a majority of the member’s of our Board of Directors during any 12 month period by directors not endorsed prior to their appointment or election by a majority of the Board of Directors; or the acquisition by a person or group within 12 months of our assets with a total gross fair market value equal to more than 40% of the total gross fair market value of our assets prior to such acquisition.
Dr. Grodman is also subject to certain non-competition restrictions preventing him from competing with the Company after termination of his employment. Such restrictions will run for one year from the date of his termination, other than following a termination by him for Good Reason in which case they will continue until the greater of one year from the date of termination or ½ of the period remaining from the date of termination through October 31, 2017.
Pursuant to the Grodman Contract, the Company agreed to transfer to an Insurance Trust (the “1999 Trust”) established by Dr. Grodman, an insurance policy (“Policy A”) owned by the Company insuring the life of Dr. Grodman pursuant to an Endorsement Split-Dollar Insurance Agreement (“Split-Dollar Agreement No. 1”) among the Company, Dr. Grodman and the 1999 Trust, by paying a $1,202,411 bonus (the “Initial Bonus”) to Dr. Grodman, which is equal to the amount of the premiums paid by the Company on Policy A through the date of the Grodman Contract. Split-Dollar Agreement No. 1 required the Company to pay the annual premiums on Policy A and provided that in the event of Dr. Grodman’s death while serving as a full time Company employee, the Company would receive that amount out of the policy death proceeds equal to its “interest in the policy” (i.e. the greater of the premiums it had paid on the policy or the policy cash value at the date of death) and the balance of the death proceeds would be paid to Dr. Grodman’s designated beneficiaries. Pursuant to the Grodman Contract, Split-Dollar Agreement No. 1 was terminated and in a “book entry” transaction, the Initial Bonus was “paid” to Dr. Grodman who in turn “transferred” the Initial Bonus amount to the 1999 Trust which in turn “repaid” the Initial Bonus amount back to the Company. The Company then, in accordance with Split-Dollar Agreement No. 1, transferred ownership of Policy A to the 1999 Trust. To facilitate these transactions, the parties
33
Table of Contents
agreed that the actual monetary funds did not need to change hands but agreed to treat the transactions appropriately for tax and accounting purposes. The Company also agreed to pay bonuses to Dr. Grodman of $119,000 in 2011, $70,000 in 2012 and $70,000 in 2013 unless his employment was terminated for “Cause” prior to a payment. These three bonuses were equal in amount to the remaining premiums payable on Policy A. The Company will expense the Initial Bonus ratably over the term of the New Contract. If Dr. Grodman’s employment is terminated for “Cause”, he is obligated to pay back the unexpended portion of the Initial Bonus back to the Company.
The Company also agreed to obtain a second insurance policy, a second-to-die policy (“Policy B”) insuring the lives of Dr. Grodman and his wife. Policy B will be owned by the Company pursuant to a second Endorsement Split-Dollar Insurance Agreement (“Split-Dollar Agreement No. 2”) among the Company, Dr. Grodman and an Insurance Trust established by Dr. Grodman. Policy B provides for seven years of annual premiums of approximately $200,000 each, to be paid by the Company unless Dr. Grodman’s employment is terminated for “Cause.” At Dr. Grodman’s death, if his wife survives him, or in the event his employment is terminated for “Cause”, Dr. Grodman’s estate or Dr. Grodman, as the case may be, will cause the premiums paid by the Company under Policy B up to said date, to be paid back to the Company and the Company will transfer ownership of Policy B to Dr. Grodman’s estate, or to Dr. Grodman, as the case may be. If Dr. Grodman survives his wife, and assuming his employment has not been terminated for “Cause,” at his death, the Company will be paid the greater of the premiums it paid on Policy B or the Policy B cash value out of the death proceeds and Dr. Grodman’s estate will be paid the balance of the death proceeds, provided, however, that if Dr. Grodman survives his wife and assuming his employment has not been terminated for “Cause,” at his wife’s death, Dr Grodman or his designee shall have the option, exercisable within 90 days of her death, to purchase Policy B from the Company for the greater of the premiums paid or the cash value at the date of her death.
Mr. Singer
On June 14, 2012, effective February 1, 2012, the Company executed a new employment agreement with Mr. Singer (the “Singer Contract”), employing him as Senior Vice President, Chief Financial Officer and Chief Accounting Officer through January 31, 2015. The Singer Contract replaced Mr. Singer’s employment agreement that expired January 31, 2012. Mr. Singer’s minimum annual base compensation under the Singer Contract is $431,911 subject to increases based on increases in the Consumer Price Index as well as to increases at the discretion of the Compensation Committee. The Singer Contract provides for the leasing of an automobile for his use and participation in fringe benefit, bonus, pension, profit sharing, and similar plans maintained for the Company’s employees. In consideration for his entering into the Singer Contract, the company paid Mr. Singer a sign-on bonus in the amount of $300,000 subject to a pro rata claw-back provision in the event Mr. Singer is terminated for “cause” or if he resigns prior to the earlier of a “change of control” or January 31, 2015. In the event of Mr. Singer’s total disability we may continue to employ him and compensate him at his then current base compensation for the month the disability occurs and a period of 12 months thereafter followed by an unpaid 3 month period, following which his employment will terminate unless we grant an additional leave of absence. If Mr. Singer incurs a partial disability then his base compensation will be equitably adjusted based on the time he is able to devote to the Company. In the event of Mr. Singer’s termination due to his death, we will continue to pay his beneficiary his base salary for 12 months. Under the Singer Contract we may terminate Mr. Singer’s employment for “Cause” and Mr. Singer has the right to terminate for “Good Reason”. If he terminates for Good Reason he will be entitled, subject to his execution of a release, to continuation of his base compensation and employee benefits through the end of the employment period he otherwise would have been employed under his employment agreement. In the event of termination due to a Change in Control of the Company, Mr. Singer will be entitled to the same severance payment described above for Dr. Grodman. Mr. Singer’s agreement does not contain non-competition restrictions.
“Cause” is defined in Mr. Singer’s employment agreement to mean: an act or acts of dishonesty by Mr. Singer constituting criminal acts resulting or intended to result directly or indirectly in his gain or personal enrichment at our expense; his commission of a crime involving fraud, embezzlement or theft against us; or his engaging in competition with us. Good Reason and Change in Control under Mr. Singer’s employment agreement has the same meanings as provided in the Grodman Contract.
Mr. Dubinett
On June 14, 2012, effective February 1, 2012 the Company executed an employment agreement with Mr. Dubinett’s (the “Dubinett Contract”), employing him as Executive Vice President and Chief Operating Officer through January 31, 2015. The Dubinett Contract replaced Mr. Dubinett’s employment agreement with us that expired on October 31, 2011. The Dubinett Contract is substantially identical with the Singer Contract except that Mr. Dubinett’s sign-on bonus was $100,000.
Potential Payments Upon Termination or Change in Control as of October 31, 2013
The following table sets out the estimated payments that would have been paid to each of our NEOs upon termination of employment due to Death, for Good Reason or following a Change in Control in accordance with their employment agreements as described above as in effect, and in each case assuming such termination had occurred, as of October 31, 2013. We have calculated these estimated payments to meet SEC disclosure requirements. The estimated payments are not necessarily indicative of the actual amounts any of our NEOs would receive in such circumstances. The table excludes compensation amounts accrued through October 31, 2013 that would be paid in the normal course of continued employment, such as accrued but unpaid base compensation, and vested account balances under our retirement plans that are generally available to all of our salaried employees.
| | Base Compensation
Continuation/ Lump
Sum ($) (a) | | Benefits Continuation
($) (b) | | Total ($) | |
Marc D. Grodman M.D. | | | | | | | |
Good Reason | | 5,683,500 | | 12,217 | | 5,695,717 | |
Death | | 2,273,400 | | 0 | | 2,273,400 | |
Change in Control | | 3,398,733 | | 0 | | 3,398,733 | |
Howard Dubinett | | | | | | | |
Good Reason | | 1,010,700 | | 5,169 | | 1,015,869 | |
Death | | 449,200 | | 0 | | 449,200 | |
Change in Control | | 1,343,108 | | 0 | | 1,343,108 | |
Sam Singer | | | | | | | |
Good Reason | | 1,010,700 | | 5,169 | | 1,015,869 | |
Death | | 449,200 | | 0 | | 449,200 | |
Change in Control | | 1,343,108 | | 0 | | 1,343,108 | |
34
Table of Contents
(a) For a Good Reason termination this payment reflects a continuation of base compensation through the end of period the NEO otherwise would have been employed under his employment agreement. For death this payment reflects a continuation of base compensation for 24 months in the case of Dr. Grodman and 12 months in the case of Messrs. Dubinett and Singer. For Change in Control this payment reflects an amount equal to 2.99 times the NEOs five-year average compensation, without reduction.
(b) For a Good Reason termination this payment reflects our estimated costs for a continuation of the NEOs benefits under our medical plan through the end of period the NEO otherwise would have been employed under his employment agreement.
Split-Dollar Life Insurance
We have established split-dollar life insurance programs for each of our NEOs under which we are entitled to receive the net cash surrender value of the policies. We have entered into Endorsement Split-Dollar Life Insurance Agreements with each of the NEOs pursuant to which we have agreed to continue to pay the annual premiums on the policies during the period of the NEO’s full-time employment by the Company ($200,000 under Dr. Grodman’s policy and $25,000 each under Messrs. Dubinett’s and Singer’s policies). In the case of Dr. Grodman, the insurance policy is a second-to-die policy on the lives of Dr. Grodman and his wife. In the event of an NEO’s death while serving as a full-time employee of the Company, we will be entitled to receive that amount of the death proceeds equal to our interest in the policy (the aggregate amount of premiums paid by the Company with respect to the policy less the amount of any loans, if any, from the Insurer to the Company against the cash value or policy proceeds, and less the aggregate amount of any premiums paid by the NEO to the Company in reimbursement of premiums paid by the Company) and the balance of the death proceeds will be paid to the NEO’s designated beneficiaries. The premiums paid by the Company on such policies are approximately $250,000 at October 31, 2013. As of such date the aggregate net cash surrender value of the three policies was approximately $1,165,000 and is recorded on the books of the Company at such value.
Stock Options
See Note 11 of Notes to the Consolidated Financial Statements for information on the company’s stock option plans.
Option Grants to Our NEOs in Last Fiscal Year
No options to purchase shares of our Common Stock were granted to any of our NEOs in fiscal 2013.
Option Exercises and Stock Vested
At October 31, 2013, there were no outstanding options held by our NEOs or any of our directors. During fiscal 2013 no options were exercised by any member of the Board of Directors.
Director Compensation
During fiscal 2013, each director who was not a Company employee was compensated for his services as a director with a quarterly fee of $16,250. In addition, Gary Lederman as chairman of the Audit Committee, was compensated with an additional quarterly fee of $4,500. For his service as chairman of both the Nominating Committee and the Compensation Committee, John Roglieri M.D., was compensated with additional quarterly fees of $3,000 and $2,000, respectively. No director’s fees were paid to our employee directors.
The following table sets forth the compensation paid to our directors in fiscal 2013.
Fiscal 2013
Director Name: | | Fees Earned or paid in
Cash ($) | | Chairman Fees ($) | | Total ($) | |
Joseph Benincasa | | 91,250 | | — | | 91,250 | |
Harry Elias | | 91,250 | | — | | 91,250 | |
Gary Lederman (a) | | 91,250 | | 17,250 | (a) | 108,500 | |
John Roglieri M.D (b) | | 91,250 | | 18,750 | (b) | 110,000 | |
(a) Chairman of the Audit Committee
(b) Chairman of the Nominating Committee and the Compensation Committee
Compensation Discussion and Analysis
Executive Compensation Philosophy
The objective of our compensation program for our NEOs is to reward them for their leadership and efficiency in their areas of responsibility and for their overall contribution to the Company’s performance. Our NEOs for fiscal 2012 are Dr. Grodman, our President and Chief Executive officer, Mr. Dubinett, our Executive Vice President Chief Operating officer who is responsible for healthcare regulatory compliance and insurance matters, and Mr. Singer, our Senior Vice President and Chief Financial Officer who is responsible for all financial matters.
We seek to maintain a uniform approach in how we compensate our NEOs. Accordingly, as Dr. Grodman already owns a substantial equity interest in the Company and we believe that further equity compensation would not provide him with an effective incentive, we do not currently provide equity compensation to any of our NEOs. Our compensation program for our NEOs therefore focuses primarily on the following cash based incentives:
(i) Annual base compensation consisting of a set annual cash amount that is subject to annual increase based upon a review of the NEO’s and the Company’s performance and increases in the Consumer Price Index; and
(ii) Participation in the annual Senior Management Incentive Bonus Plan (“Annual Bonus Plan”), which provides cash incentives based on the level of achievement of specific performance objectives. We annually establish targets under the Annual Bonus Plan that are designed
35
Table of Contents
to assist in the Company’s profitability by encouraging a “team effort” that rewards participants based on the level of achievement of Company financial targets set by the Compensation Committee with no reward if minimum targets are not achieved. Annual Bonus Plan targets were achieved with respect to fiscal 2011, fiscal 2012 so that bonuses were earned and paid to our NEOs under the Annual Bonus Plan for fiscal 2011 (the “2011 Bonus Plan”), the Annual Bonus Plan for fiscal 2012 (the “2012 Bonus Plan”). Annual Bonus Plan targets were not achieved with respect to fiscal 2013, so no bonuses were paid to our NEOs under the Annual Bonus Plan for fiscal 2013 (the “2013 Bonus Plan”). See “Senior Management Incentive Bonus Plan” herein.
Advisory Vote on Executive Compensation
The Company provides its stockholders with the opportunity to cast an annual vote on executive compensation. At the 2013 Annual Meeting of Stockholders held in July 2013, 64.9% of the votes cast on the advisory vote on executive compensation proposal were in favor of our NEO compensation as described in the proxy statement for the 2013 Annual Meeting of Stockholders. The Compensation Committee reviewed these final vote results and took them into account when considering its compensation decisions for fiscal 2014. The Compensation Committee determined that given the leadership role of the NEOs in the Company’s continued steady performance the Company’s executive compensation program remains appropriate and no changes were necessary. However, the Compensation Committee continues to review our executive compensation program consistent with the compensation goals set forth herein and will continue to consider the outcome of the stockholder votes on the annual executive compensation proposal when making future decisions regarding our executive officers.
Process for Determining Executive Compensation
Our Compensation Committee reviews and approves the annual base compensation and other compensation of our NEOs. Our Compensation Committee also establishes and reviews the achievement of performance goals and other matters relating to the Annual Bonus Plans. There were no changes to our executive compensation structure in fiscal 2013.
In connection with its review of NEO compensation in fiscal 2012, the Compensation Committee considered an executive compensation study furnished by Compensation Resources, Inc., an independent executive compensation consulting firm (“CRI”) engaged by the Compensation Committee. After taking into account the compensation paid to similar executive officers of a peer group of eleven publicly owned clinical testing laboratories (including the two major national laboratories, Quest Diagnostics, Inc. and Laboratory Corp. of America Holdings), CRI concluded that Dr. Grodman’s total direct compensation was then above the total direct compensation provided to the chief executive officers in the peer group, and that the total direct compensation provided to Messrs. Singer and Dubinett were below the total direct compensation provided to similar executive officers in the peer group. Notwithstanding the report, the Compensation Committee believes that Dr. Grodman’s compensation level is reasonable as the current levels were determined based on a CRI report for fiscal 2011 which showed that Dr. Grodman’s total direct compensation was 20% below the peer group.
For 2012, CRI selected the following eleven peer group of publicly owned clinical testing laboratories to be used as a benchmark, most of which generally have revenue in a range of ½ to 2 times that of the Company, but was expanded to include companies with revenues of up to $8 billion:
Alliance Healthcare Services, Inc.
Bioclinica Inc.
Enzo Biochem Inc.
eResearch Technology
Laboratory CP of Amer Hldgs
Medtox Scientific Inc.
Neogenomics Inc.
Psychemedics Corp.
Quest Diagnostics Inc.
Radnet Inc.
Response Genetics Inc.
Two companies that were part of the 2011 peer group, Genoptix Inc. and Orchid Cellmark Inc., were excluded for fiscal 2012 as they no longer met the peer group criteria and were replaced by Alliance Healthcare Services, Inc., Enzo Biochem Inc., eResearch Technology, and Radnet Inc.
Base Compensation
In accordance with the Grodman Contract, the Compensation Committee determined that Dr. Grodman’s base compensation be increased to $1,136,700 for fiscal 2013, which reflects a 4% increase based on increases in the Consumer Price Index.
Since fiscal 2008, the base compensation and the increase in base compensation in each year for Mr. Dubinett and for Mr. Singer have been identical. This is because the Compensation Committee continues to believe that Mr. Dubinett and Mr. Singer perform their duties equally well and to distinguish between them in compensation could cause the Company to lose the services of one of them. The increases in their base compensation in each of the past three fiscal years have been as follows, which reflect annual increases for each fiscal year based on increases in the Consumer Price Index.
Increases in Base Compensation for Each of
Mr. Dubinett and Mr. Singer Over the Prior Three Fiscal Years
Period | | Amount ($) | | Percentage Increase | |
| | | | | |
Fiscal 2011 | | 18,022 | | 4 | % |
Fiscal 2012 | | 13,393 | | 3 | % |
Fiscal 2013 | | 17,289 | | 4 | % |
| | | | | | |
Benefits
Our policy is to provide health benefits as well as access to our 401(k) Plan to which we contribute a maximum of $1,000 per employee each year, to all of our employees including our NEOs.
36
Table of Contents
All senior officers of the Company, including our NEOs, are entitled to an automobile leased by the Company and access to the Company’s airplane for personal use to the extent the airplane is not in use for business purposes. The costs for insurance and maintenance of such automobiles are paid by the Company. All amounts reflecting the personal use of such perquisites are reported as income to them subject to tax in accordance with the Tax Code. The amounts reflecting the Company’s incremental costs for the NEO’s personal use of the Company leased automobile and the Company’s airplane are reflected in Footnote (3) to the “Summary Compensation Table” below.
Change in Control Benefits
The employment agreements with our NEOs provide for substantial severance payments to them in the event of a change in control of the Company. This provision provides an additional level of financial security for our NEOs as they may be asked to evaluate a transaction purportedly expected to maximize shareholder value while resulting in the elimination of their jobs. The severance payment provision (2.99 times the annual average of the preceding five years of compensation) is designed to minimize the distraction caused by concerns over personal financial security in the context of a proposed change in control.
Annual Bonus Plans for fiscal years 2013 and 2012
The Compensation Committee adopts an Annual Bonus Plans for each year which it believes incentivizes senior management to push to achieve operating results that the Compensation Committee believes will inure to the benefit of stockholders as well as management. Each Annual Bonus Plan provides goals which the Compensation Committee believes could only be achieved through extraordinary team efforts by senior management and that are designed to incentivize senior management to operate the Company in the most efficient manner possible. In developing the Annual Bonus Plan for each year, the Compensation Committee takes into consideration the economy in general and the goals of the Company that it wished to reward, namely to improve Company margins within attainable goals for management.
The Compensation Committee has at all times sought (and continues to seek) to provide a mechanism to reward outstanding efforts that enhance shareholder value. The following is a description of the Annual Bonus Plan for fiscal 2012 (the “2012 Bonus Plan”) and fiscal 2013 (the “2013 Bonus Plan”). The Compensation Committee has adopted an Annual Bonus Plan for fiscal 2014 that is substantially similar to the 2013 Bonus Plan and is attached hereto as Exhibit 10.5. Any bonuses required to be paid under the provision of any Annual Bonus Plan is required to be paid to each participant on the pro-rata formula established upon the adoption of the plan and not at the discretion of the Compensation Committee.
2013 Bonus Plan
The 2013 Bonus Plan was based on two separate financial formula calculations. The first formula provided for bonuses (up to a maximum of 10% of the participant’s annual gross wages for 2013, less any bonus, auto or airplane usage expense charge-back or other unearned revenue (“2013 Wages”)) based on the level of the Company’s achievement of total operating income (TOI) as a percentage of our net revenues for fiscal 2013 as follows:
If TOI is greater than or equal
to: | | and less than: | | Bonus equal to the following
percentage of the participant’s
2013 wages: | |
12.25 | % | 12.75 | % | 4 | % |
12.75 | % | 13.25 | % | 6 | % |
13.25 | % | 13.75 | % | 8 | % |
13.75 | % | N/A | | 10 | % |
The second formula provided for bonuses (up to a maximum of 15% of the participant’s 2013 Wages ) based on the percentage increase on a year over year basis in the Company’s operating income before interest and taxes (“OIBIT”) from fiscal 2012 to fiscal 2013, determined by subtracting the Company’s fiscal 2012 OIBIT from the Company’s fiscal 2013 OIBIT and dividing the difference by the Company’s OIBIT for fiscal 2012 to determine the percentage of change (“2013 PC”) , as follows:
If 2013 PC is greater than: | | and less than: | | Bonus equal to the following
percentage of the participant’s
2013 Wages: | |
25.00 | % | 30.00 | % | 6 | % |
30.00 | % | 35.00 | % | 9 | % |
35.00 | % | 40.00 | % | 12 | % |
40.00 | % | N/A | | 15 | % |
Actual results under the 2013 Bonus Plan were as follows:
| | | | Bonus equal to the following percentage of the
participant’s 2013 Wages: | |
TOI as a percentage of our net revenues for fiscal 2013 | | 11.46 | % | 0 | % |
2013 PC | | 7.67 | % | 0 | % |
Total Bonus Percentage: | | — | | 0 | % |
2012 Bonus Plan
The 2012 Bonus Plan was based on two separate financial formula calculations. The first formula provided for bonuses (up to a maximum of 10% of the participant’s annual gross wages for 2012, less any bonus, auto or airplane usage expense charge-back or other unearned revenue (“2012 Wages”)) based on the level of the Company’s achievement of total operating income (TOI) as a percentage of our net revenues for fiscal 2012 as follows:
37
Table of Contents
If TOI is greater than or equal
to: | | and less than: | | Bonus equal to the following
percentage of the participant’s
2012 wages: | |
11.00 | % | 11.50 | % | 4 | % |
11.50 | % | 12.00 | % | 6 | % |
12.00 | % | 12.50 | % | 8 | % |
12.50 | % | N/A | | 10 | % |
The second formula provided for bonuses (up to a maximum of 15% of the participant’s 2012 Wages ) based on the percentage increase on a year over year basis in the Company’s operating income before interest and taxes (“OIBIT”) from fiscal 2011 to fiscal 2012, determined by subtracting the Company’s fiscal 2011 OIBIT from the Company’s fiscal 2012 OIBIT and dividing the difference by the Company’s OIBIT for fiscal 2011 to determine the percentage of change (“2012 PC”) , as follows:
If 2012 PC is greater than: | | and less than: | | Bonus equal to the following
percentage of the participant’s
2012 Wages: | |
25.00 | % | 30.00 | % | 6 | % |
30.00 | % | 35.00 | % | 9 | % |
35.00 | % | 40.00 | % | 12 | % |
40.00 | % | N/A | | 15 | % |
Actual results under the 2012 Bonus Plan were as follows:
| | | | Bonus equal to the following percentage of the
participant’s 2012 Wages: | |
TOI as a percentage of our net revenues for fiscal 2012 | | 11.51 | % | 6 | % |
2010 PC | | 27.36 | % | 6 | % |
Total Bonus Percentage: | | — | | 12 | % |
See Item 11 — the “Summary Compensation Table”, column (g) “Non-Equity Incentive Plan Compensation” as to the bonuses paid under the Senior Management Incentive Compensation Plan with respect to fiscal 2013 and 2012.
Tax Compliance Policy
Section 162(m) of the Code generally disallows a tax deduction to public corporations for compensation in excess of $1,000,000 paid for any fiscal year to a corporation’s chief executive officer and to the three other most highly compensated executive officers in office as of the end of the fiscal year, other than the chief financial officer. The statute exempts qualifying performance-based compensation from the deduction limit if certain requirements are met. However, shareholder interests may at times be best served by not restricting the Compensation Committee’s discretion and flexibility in developing compensation programs, even though the programs may result in non-deductible compensation expenses. Accordingly, the Compensation Committee may from time to time approve elements of compensation for certain officers that are not fully deductible.
Compensation Committee Interlocks and Insider Participation
During fiscal 2013, the members of the Company’s Compensation Committee were:
John Roglieri M.D. — Chairman
Joseph Benincasa
Harry Elias
Gary Lederman
No member of the Compensation Committee was an officer or employee of the Company in fiscal 2013 or was formerly an officer of the Company.
Compensation Committee Report
The members of the Company’s Compensation Committee hereby state:
We have reviewed and discussed the Compensation Discussion and Analysis contained in this Annual Report on Form 10-K for the year ended October 31, 2013 with the Company’s Management, and
Based on such review and discussions, we have recommended to the Company’s Board of Directors that the Compensation Discussion and Analysis be included in the Company’s Annual Report on Form 10-K for the year ended October 31, 2013.
| | COMPENSATION COMMITTEE |
| |
| By: | John Roglieri M.D., Chairman |
| | Joseph Benincasa |
| | Harry Elias |
| | Gary Lederman |
38
Table of Contents
Item 12. - Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth information as of January, 2014 with respect to the ownership of Common Stock by (i) each person known to us to be the beneficial owner of more than 5% of our outstanding Common Stock, (ii) each of our directors, (iii) each of our executive officers, and (iv) all directors and executive officers as a group.
Name and Address of Beneficial
Owner* | | Shares of Common Stock
Beneficially Owned(1) | | Percentage
Ownership | |
| | | | | |
Marc D. Grodman(2) | | 2,741,800 | | 9.90 | % |
Howard Dubinett(3) | | 365,138 | | 1.32 | % |
Sam Singer(4) | | 14,132 | | | ** |
Joseph Benincasa | | 0 | | 0 | % |
Harry Elias | | 0 | | 0 | % |
Gary Lederman(5) | | 30,400 | | | ** |
John Roglieri(6) | | 10,000 | | | ** |
Executive Officers and Directors as a group (seven persons) (2)(3)(4)(5)(6) | | 3,161,470 | | 11.42 | % |
| | | | | |
Black Rock, Inc(7)
40 East 52nd Street
New York, NY 10022 | | 1,859,554 | | 6.71 | % |
| | | | | |
Jennison Associates LLC(9)
466 Lexington Avenue
New York, NY 10017 | | 2,583,152 | | 9.3 | % |
| | | | | |
Riverbridge Partners LLC(11)
801 Nicollet Mall, Suite 600
Minneapolis, MN 55402 | | 1,767,970 | | 6.38 | % |
| | | | | |
The Vanguard Group (12)
100 Vanguard Blvd.
Malvem, PA 19355 | | 1,748,989 | | 6.31 | % |
| | | | | |
Manulife Asset Management Limited(13)
200 Bloor Street East
Toronto, ON, Canada M4W 1E5 | | 2,146,944 | | 7.75 | % |
|
* | The address of all of the Company’s directors and executive officers is c/o the Company, 481 Edward H. Ross Drive, Elmwood Park, New Jersey 07407. |
| |
** | Less than one (1%) percent. |
| |
(1) | Except as otherwise noted, each holder named in the table has sole voting and investment power with respect to all shares of Common Stock shown as beneficially owned. |
| |
(2) | Includes 2,296,966 shares owned directly. 32,210 of these shares are pledged as security in a brokerage margin account. Also includes 145,834 shares owned directly by Dr. Grodman’s wife, Pam Grodman, and 200,000 shares held in trust for the benefit of Pam Grodman, and 99,000 shares owned by their children. Dr. Grodman disclaims beneficial ownership of these 444,834 shares. |
| |
(3) | Includes 365,138 shares owned directly. All of these shares are pledged as security in a brokerage margin account. |
| |
(4) | Includes 1,000 shares owned directly and 13,132 shares owned by trusts for the benefit of Mr. Singer and his family members, of which Nancy Kelly-Singer, Mr. Singer's wife, and Mr. Singer are co-trustees. |
| |
(5) | Includes 30,400 shares owned directly. |
| |
(6) | Includes 10,000 shares owned directly. |
| |
(7) | Black Rock, Inc. (“Black Rock”) is the beneficial owner of these 1,859,554 shares. In its Schedule 13G filing dated February 4, 2013 filed with the Securities and Exchange Commission, Black Rock stated that to the best of its knowledge, these 1,859,554 shares were acquired in the ordinary course of business; were not acquired for the purpose of and do not have the effect of changing or influencing the control of the Company; and were not acquired in connection with or as a participant in any transaction having such purpose or effect. |
| |
(9) | Jennison Associates LLC (“Jennison”) is the beneficial owner of these 2,583,152 shares. In its Schedule 13G filing dated February 12, 2013 (as of December 31, 2012) filed with the Securities and Exchange Commission, Jennison stated that to the best of its knowledge, these 2,583,152 shares were acquired in the ordinary course of business; were not acquired for the purpose of and do not have the effect of changing or influencing the control of the Company; and were not acquired in connection with or as a participant in any transaction having such purpose or effect. |
| |
(11) | Riverbridge Partners LLC (“Riverbridge”) is the beneficial owner of these 1,767,970 shares. In its Schedule 13G filing dated February 4, 2013 (as of December 31, 2012) filed with the Securities and Exchange Commission, Riverbridge stated that to the best of its knowledge, these 1,767,970 shares were acquired in the ordinary course of business; were not acquired for the purpose of and do not have the effect of changing or influencing the control of the Company; and were not acquired in connection with or as a participant in any transaction having such purpose or effect. |
| |
(12) | The Vanguard Group (“Vanguard”) is the beneficial owner of these 1,748,989 shares. In its Schedule 13G filing dated February 7, 2013 filed with the Securities and Exchange Commission, Vanguard stated that to the best of its knowledge, these 1,748,989 shares were acquired |
39
Table of Contents
| in the ordinary course of business; were not acquired for the purpose of and do not have the effect of changing or influencing the control of the Company; and were not acquired in connection with or as a participant in any transaction having such purpose or effect. |
| |
(13) | Manulife Asset Management Limited (“MAML”) is the beneficial owner of these 2,146,944 shares. In Its Schedule 13G filing dated February 13, 2013 filed with the Securities and Exchange Commission, MAML stated that to the best of its knowledge, these 2,146,944 shares were acquired in the ordinary course of business; were not acquired for the purpose of and do not have the effect of changing or influencing the control of the company; and were not acquired in connection with or as a participant in any transaction having such purpose or effect. |
Equity Compensation Plan Information
The following table provides information as of October 31, 2013 regarding shares of Common Stock that may be issued pursuant to the Company’s equity compensation plans:
| | | | | | (c) | |
| | | | | | Number of Shares Remaining | |
| | (a) | | (b) | | Available for Future Issuances Under | |
| | Number of Shares Issuable | | Weighted-Average Exercise | | Equity Compensation Plans | |
| | upon Exercise of Outstanding | | Price per Share of Outstanding | | (Excluding Shares Reflected in | |
| | Options | | Options | | Column (a)) | |
| | | | | | | |
Equity Compensation Plans Approved by Stockholders | | 255,000 | (1) | $ | 9.97 | | — | (2) |
| | | | | | | | |
(1) Reflects shares issuable upon exercise of outstanding ISOs granted pursuant to the Company’s 2000 and 2003 Employee Stock Option Plans.
(2) No additional options may be granted under the Company’s stock options plans.
Item 13. — Certain Relationships and Related Transactions
No material transactions occurred between the Company and related parties during fiscal 2013.
It is the Company’s policy that transactions involving related persons (excluding executive officer compensation which is determined by the Compensation Committee) are to be presented to and assessed by the independent members of the board of directors. Related persons include the Company’s directors and executive officers, immediate family members of the directors and executive officers, and certain large security holders and their family members. If the determination is made that a related person has or may have a material direct or indirect interest in any Company transaction and that the amount involved equals or exceeds $120,000, the Company’s independent directors will review, approve and ratify the transaction, if appropriate, and the transaction will be disclosed if required under SEC rules. If the related party at issue is a director of the Company or a family member of a director, then that director will not participate in the relevant discussion and review.
Information considered in evaluating such transactions include the nature of the related person’s interest in the transaction, the material terms of the transaction, the importance of the transaction to the Company and the related person, whether the transaction would impair the judgment of a director or an executive officer to act in the best interests of the Company, and any other matters that management or the independent directors deem appropriate. Corporate policy requires all directors and employees, including all executives, to disclose their interests (including indirect interests through family members) with individuals or entities doing business with the Company, to management and/or the Board of Directors, and to remove themselves from all decisions related to that organization. No such transactions with related parties occurred in fiscal year 2011 through 2013.
Item 14. - Principal Accountant Fees and Services
The firm of MSPC, Certified Public Accountants and Advisors, A Professional Corporation (“MSPC”) audited our accounts and the accounts of our subsidiaries for the fiscal years ended October 31, 2013 and 2012. MSPC and its predecessor firm have been our auditors since 1988. The table set forth below lists the fees billed to the company by MSPC for audit services rendered in connection with the audits of our consolidated financial statements for the years ended October 31, 2013 and 2012, and fees billed for other services rendered by MSPC during these periods.
| | 2013 | | 2012 | |
| | (in thousands) ($) | |
(1) Audit Fees | | 288 | | 376 | |
(2) Audit-Related Fees | | 90 | | 40 | |
(3) Tax Fees | | 49 | | 15 | |
(4) All Other Fees | | 0 | | 0 | |
Total | | 427 | | 431 | |
(1) Audit Fees
MSPC billed us approximately $288 for professional services rendered in connection with the audit of our annual financial statements for the fiscal year ended October 31, 2013 and the review of the financial statements included in our quarterly reports on Form 10-Q for such fiscal year compared to approximately $376 in billings for such services for the fiscal year ended October 31, 2012. In addition, MSPC billed us approximately $9 in fiscal 2013 for its audit of our 401(k) Plan for calendar year 2012 as compared to approximately $29 of such fees in fiscal 2012 with respect to calendar year 2011.
40
Table of Contents
(2) Audit-Related Fees
MSPC billed us approximately $90 during fiscal 2013 and approximately $40 during fiscal 2012 for Sarbanes-Oxley (“SOX”) related audit fees.
(3) Tax Fees
MSPC billed us approximately $49 for tax services for fiscal 2012 and approximately $15 for tax services for fiscal 2012.
(4) All Other Fees
No fees were billed to us by MSPC with respect to fiscal 2013 or fiscal 2012 other than for services described in Item 14 (1), (2) and (3) herein.
(5) Pre-Approval Policies and Procedures
The engagement of MSPC to render the above audit and tax services was approved by our audit committee prior to the engagement.
41
Table of Contents
PART IV
Item 15. — Exhibits and Financial Statement Schedules
(a) 1. Financial Statements
The following financial statements of the Company are included in Part II, Item 8, “Financial Statements and Supplementary Data:”
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets - October 31, 2013 and 2012
Consolidated Statements of Operations for the Years ended October 31, 2013, 2012 and 2011
Consolidated Statements of Shareholders’ Equity for the Years ended October 31, 2013, 2012, and 2011
Consolidated Statements of Cash Flows for the Years ended October 31, 2013, 2012 and 2011
Notes to Consolidated Financial Statements
2. Financial Statements Schedule
The following is included in Item 8, “Financial Statements and Supplementary Data:”
Schedule II — Valuation and Qualifying Accounts for the Years ended October 31, 2013, 2012 and 2011
(b) Exhibits
Exhibit No. | | Item |
| | |
3.1* | | Amended and Restated Certificate of Incorporation dated November 15, 1989 (Incorporated by reference to exhibit filed with the Company’s annual report on form 10-K for the year ended October 31, 2011. (SEC File No. 0-15266). |
| | |
3.1.1* | | Amendment to Certificate of Incorporation dated August 23, 1993 (Incorporated by reference to exhibit filed with the Company’s annual report on form 10-K for the year ended October 31, 2011. (SEC File No. 0-15266). |
| | |
3.1.2* | | Amendment to Certificate of Incorporation dated August 23, 1993 (Incorporated by reference to exhibit filed with the Company’s annual report on form 10-K for the year ended October 31, 2011. (SEC File No. 0-15266). |
| | |
3.1.3* | | Amendment to Certificate of Incorporation dated March 27, 1998 (Incorporated by reference to exhibit filed with the Company’s annual report on form 10-K for the year ended October 31, 2011. (SEC File No. 0-15266). |
| | |
3.1.4* | | Amendment to Certificate of Incorporation dated March 31, 1998 (Incorporated by reference to exhibit filed with the Company’s annual report on form 10-K for the year ended October 31, 2011. (SEC File No. 0-15266). |
| | |
3.1.5* | | Amendment to Certificate of Incorporation dated September 26, 2003 (Incorporated by reference to exhibit filed with the Company’s annual report on form 10-K for the year ended October 31, 2011. (SEC File No. 0-15266). |
| | |
3.2.2* | | By-laws, as amended. (Incorporated by reference to exhibit filed with the Company’s annual report on form 10-K for the year ended October 31, 2011. (SEC File No. 0-15266). |
| | |
4.1* | | Form of Common Stock Certificate, $.01 par value (Incorporated by reference to exhibit filed with the Company’s annual report on form 10-K for the year ended October 31, 2004 (SEC File No. 0-15266)). |
| | |
10.1* | | Lease Agreement for Elmwood Park, New Jersey Premises, expiring in February, 2004 (Incorporated by reference to exhibit filed with the Company’s annual report on Form 10-K for the year ended October 31, 1999 (SEC File No. 0-15266)). |
| | |
10.1.1* | | Fifth Amendment dated as of July 16, 2004 to Lease for Elmwood Park, New Jersey Premises (Incorporated by reference to exhibit filed with the Company’s annual report on Form 10-K for the year ended October 31, 2004 (SEC File No. 0-15266)). |
| | |
10.1.2* | | Sixth Amendment dated as of October 27, 2004 to Lease for Elmwood Park, New Jersey Premises (Incorporated by reference into exhibit filed with the Company’s annual report on Form 10-K for the year ended October 31, 2004 (SEC File No. 0-15266)). |
| | |
10.2* | | Employment Agreement between the Company and Marc Grodman expiring on October 31, 2017. (Incorporated by reference to exhibit filed with the Company’s current report on Form 8-K for December 31, 2010 (SEC File No. 0-15266)). |
| | |
10.3* | | Employment Agreement between the Company and Sam Singer expiring on January 31, 2015 (Incorporated by reference to exhibit filed with the Company’s current report on Form 8-K for June 15, 2012 (SEC File No. 0-15266)). |
| | |
10.3.1* | | Employment Agreement between the Company and Howard Dubinett expiring on January 31, 2015 (Incorporated by reference to exhibit filed with the Company’s current report on Form 8-K for June 15, 2012 (SEC File No. 0-15266)). |
| | |
10.4* | | The Company’s 2000 Employee Incentive Stock Option Plan. (Incorporated by reference to exhibit filed with the Company’s annual report on Form 10-K for the year ended October 31, 2000 (SEC File No. 0-15266)). |
| | |
10.4.1* | | The Company’s 2003 Employee Incentive Stock Option Plan. (Incorporated by reference to exhibit filed with the Company’s Registration Statement on Form S-8 (File No. 333-111578)). |
| | |
10.5 | | The Company’s 2014 Senior Management Incentive Bonus Plan. |
| | |
10.6* | | Amended and Restated Loan and Security Agreement as of September 30, 2004 between the Company and PNC Bank, National Association. (Incorporated by reference to exhibit filed with the Company’s annual report on Form 10-K for the year ended October 31, 2004 (SEC File No. 0-15266)). |
| | |
10.6.1* | | Fourth Amendment as of October 31, 2006 to Loan and Security Agreement as of September 30, 2004 between the Company and PNC Bank, National Association (Incorporated by reference to exhibit filed with the Company’s annual report on Form 10-K for the year ended October 31, 2006 (SEC File No. 0-15266)). |
42
Table of Contents
10.6.2* | | Fifth Amendment as of October 31, 2007 to Loan and Security Agreement as of September 30, 2004 between the Company and PNC Bank, National Association (Incorporated by reference to exhibit filed with the Company’s annual report on Form 10-K for the year ended October 31, 2007 (SEC File No. 0-15266)). |
| | |
10.6.3* | | Sixth Amendment as of May 12, 2008 to Loan and Security Agreement as of September 30, 2004 between the Company and PNC Bank, National Association (Incorporated by reference to exhibit filed with the Company’s current report on Form 8-K (for December 31, 2010) (SEC File No. 0-15266)). |
| | |
10.6.4* | | Seventh Amendment as of October 22, 2010 to Loan and Security Agreement between the Company and PNC Bank, National Association. (Incorporated by reference to exhibit filed with the Company’s annual report on form 10-K for the year ended October 31, 2011. (SEC File No. 0-15266). |
| | |
10.6.5* | | Eighth Amendment as of November 2, 2011 to Loan and Security Agreement as of October 31, 2010 between the Company and PNC Bank, National Association. (Incorporated by reference to exhibit filed with the Company’s annual report on form 10-K for the year ended October 31, 2011. (SEC File No. 0-15266). |
| | |
21 | | Subsidiaries of the Company |
| | |
23.1 | | Consent of Independent Registered Public Accounting Firm |
| | |
31.1 | | Certification of Chief Executive Officer |
| | |
31.2 | | Certification of Chief Financial Officer |
| | |
32.1 | | Certification pursuant to 18 U.S.C. Section 1350 of Chief Executive Officer |
| | |
32.2 | | Certification pursuant to 18 U.S.C. Section 1350 of Chief Financial Officer |
| | |
101 | | Interactive Data File |
The exhibits designated above with an asterisk (*) have previously been filed with the Commission and, pursuant to 17 C.F.R. Secs. 201.24 and 240.12b-32, are incorporated by reference to the documents as indicated.
43
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
BIO-REFERENCE LABORATORIES, INC. | |
| | |
By | /S/ Marc D. Grodman | |
Marc D. Grodman | |
Chairman of the Board, President, | |
Chief Executive Officer and Director | |
Dated: January 10, 2014
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
/S/ Marc D. Grodman | |
Marc D. Grodman | |
Chairman of the Board, President, | |
Chief Executive Officer and Director | |
January 10, 2014 | |
| |
/S/ Howard Dubinett | |
Howard Dubinett | |
Executive Vice President, | |
Chief Operating Officer and Director | |
January 10, 2014 | |
| |
/S/ Sam Singer | |
Sam Singer | |
Sr. Vice President, Chief Financial Officer, | |
Chief Accounting Officer and Director | |
January 10, 2014 | |
| |
/S/ Joseph Benincasa | |
Joseph Benincasa | |
Director | |
January 10, 2014 | |
| |
/S/ Harry Elias | |
Harry Elias | |
Director | |
January 10, 2014 | |
| |
/S/ Gary Lederman | |
Gary Lederman | |
Director | |
January 10, 2014 | |
| |
/S/ John Roglieri | |
John Roglieri | |
Director | |
January 10, 2014 | |
44
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders
Bio-Reference Laboratories, Inc.
We have audited the accompanying consolidated balance sheets of Bio-Reference Laboratories, Inc. and its subsidiaries (the “Company”) as of October 31, 2013 and 2012, and the related consolidated statements of operations, shareholders’ equity, and cash flows for each of the years in the three-year period ended October 31, 2013. We also have audited the Company’s internal control over financial reporting as of October 31, 2013, based on criteria established in Internal Control - Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). The Company’s management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on these consolidated financial statements and an opinion on the Company’s internal control over financial reporting based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the consolidated financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall consolidated financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (a) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (b) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (c) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respect, the financial position of Bio-Reference Laboratories, Inc. and its subsidiaries as of October 31, 2013 and 2012, and the results of their operations and their cash flows for each of the years in the three-year period ended October 31, 2013 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, Bio-Reference Laboratories Inc. and its subsidiaries maintained, in all material respects, effective internal control over financial reporting as of October 31, 2013, based on criteria established in Internal Control - Integrated Framework (1992) issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”).
MSPC | |
Certified Public Accountants and Advisors, | |
A Professional Corporation | |
| |
Cranford, New Jersey | |
January 10, 2014 | |
46
Table of Contents
BIO-REFERENCE LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
[Dollars In Thousands, Except Share Data]
| | October 31, | | October 31, | |
| | 2013 | | 2012 | |
| | | | | |
CURRENT ASSETS: | | | | | |
Cash and Cash Equivalents | | $ | 17,952 | | $ | 25,143 | |
Accounts Receivable - Net | | 206,261 | | 153,247 | |
Inventory | | 19,095 | | 14,902 | |
Other Current Assets | | 9,416 | | 5,373 | |
Deferred Tax Assets | | 42,154 | | 24,912 | |
TOTAL CURRENT ASSETS | | 294,878 | | 223,577 | |
| | | | | |
PROPERTY AND EQUIPMENT - AT COST | | 133,599 | | 102,701 | |
LESS: Accumulated Depreciation | | (67,950 | ) | (52,261 | ) |
PROPERTY AND EQUIPMENT - NET | | 65,649 | | 50,440 | |
| | | | | |
OTHER ASSETS: | | | | | |
Investments | | 5,237 | | 4,977 | |
Deposits | | 1,017 | | 956 | |
Goodwill - Net | | 35,185 | | 23,408 | |
Intangible Assets - Net | | 16,320 | | 6,323 | |
Other Assets | | 1,165 | | 866 | |
Deferred Tax Assets | | 2,077 | | 2,278 | |
TOTAL OTHER ASSETS | | 61,001 | | 38,808 | |
| | | | | |
TOTAL ASSETS | | $ | 421,528 | | $ | 312,825 | |
The Accompanying Notes are an Integral Part of These Consolidated Financial Statements.
47
Table of Contents
BIO-REFERENCE LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
[Dollars In Thousands, Except Share Data]
| | October 31, | | October 31, | |
| | 2013 | | 2012 | |
| | | | | |
CURRENT LIABILITIES: | | | | | |
Accounts Payable | | $ | 61,614 | | $ | 41,288 | |
Accrued Salaries and Commissions Payable | | 19,601 | | 16,490 | |
Accrued Taxes and Expenses | | 18,292 | | 9,753 | |
Other Short Term Acquisition Payable | | 2,438 | | — | |
Revolving Note Payable - Bank | | 26,139 | | — | |
Current Maturities of Long-Term Debt | | 493 | | 464 | |
Capital Lease Obligations - Short-Term Portion | | 5,185 | | 3,957 | |
TOTAL CURRENT LIABILITIES | | 133,762 | | 71,952 | |
| | | | | |
LONG-TERM LIABILITIES: | | | | | |
Capital Lease Obligations - Long-Term Portion | | 10,712 | | 9,463 | |
Long Term Debt - Net of Current Portion | | 3,670 | | 4,163 | |
Other Long Term Acquisition Payable | | 1,789 | | — | |
TOTAL LONG-TERM LIABILITIES | | 16,171 | | 13,626 | |
| | | | | |
SHAREHOLDERS’ EQUITY: | | | | | |
Preferred Stock $.10 Par Value; | | | | | |
Authorized 1,666,667 shares, including 3,000 shares of Series A Junior Preferred Stock | | | | | |
None Issued | | — | | — | |
Common Stock, $.01 Par Value; | | | | | |
Authorized 35,000,000 shares: | | | | | |
Issued and Outstanding 27,683,213 and 27,707,382 at October 31, 2013 and at October 31, 2012, respectively | | 277 | | 277 | |
| | | | | |
Additional Paid-In Capital | | 39,430 | | 40,907 | |
| | | | | |
Retained Earnings | | 231,888 | | 186,063 | |
| | | | | |
TOTAL SHAREHOLDERS’ EQUITY | | 271,595 | | 227,247 | |
TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | | $ | 421,528 | | $ | 312,825 | |
The Accompanying Notes are an Integral Part of These Consolidated Financial Statements.
48
Table of Contents
BIO-REFERENCE LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
[Dollars In Thousands, Except Share Data]
| | Years Ended | |
| | October 31, | |
| | 2013 | | 2012 | | 2011 | |
| | | | | | | |
NET REVENUES: | | $ | 715,354 | | $ | 614,255 | | $ | 522,081 | |
| | | | | | | |
COST OF SERVICES: | | | | | | | |
Depreciation and Amortization | | 15,598 | | 13,101 | | 11,036 | |
Employee Related Expenses | | 173,137 | | 146,292 | | 126,354 | |
Reagents and Lab Supplies | | 135,486 | | 121,446 | | 100,569 | |
Other Cost of Services | | 68,594 | | 56,805 | | 49,894 | |
| | | | | | | |
TOTAL COST OF SERVICES | | 392,815 | | 337,644 | | 287,853 | |
| | | | | | | |
GROSS PROFIT ON REVENUES | | 322,539 | | 276,611 | | 234,228 | |
| | | | | | | |
General and Administrative Expenses: | | | | | | | |
| | | | | | | |
Depreciation and Amortization | | 4,141 | | 3,562 | | 3,969 | |
Other General and Administrative Expenses | | 177,508 | | 154,928 | | 131,967 | |
Bad Debt Expense | | 58,917 | | 41,990 | | 38,518 | |
| | | | | | | |
TOTAL GENERAL AND ADMIN. EXPENSES | | 240,566 | | 200,480 | | 174,454 | |
| | | | | | | |
OPERATING INCOME | | 81,973 | | 76,131 | | 59,774 | |
| | | | | | | |
OTHER (INCOME) EXPENSES: | | | | | | | |
| | | | | | | |
Interest Expense | | 1,606 | | 1,455 | | 1,747 | |
Other (Income) Expense | | (612 | ) | 323 | | (6,656 | ) |
Interest Income | | (118 | ) | (163 | ) | (163 | ) |
| | | | | | | |
TOTAL OTHER EXPENSES - NET | | 876 | | 1,615 | | (5,072 | ) |
| | | | | | | |
INCOME BEFORE INCOME TAXES | | 81,097 | | 74,516 | | 64,846 | |
| | | | | | | |
Provision for Income Taxes | | 35,272 | | 32,360 | | 28,487 | |
| | | | | | | |
NET INCOME | | 45,825 | | 42,156 | | 36,359 | |
| | | | | | | |
NET INCOME PER SHARE - BASIC: | | $ | 1.65 | | $ | 1.52 | | $ | 1.30 | |
WEIGHTED AVERAGE NUMBER OF SHARES - BASIC: | | 27,690,677 | | 27,742,257 | | 27,971,100 | |
| | | | | | | |
NET INCOME PER SHARE - DILUTED: | | $ | 1.65 | | $ | 1.51 | | $ | 1.29 | |
WEIGHTED AVERAGE NUMBER OF SHARES - DILUTED: | | 27,851,720 | | 27,920,920 | | 28,207,358 | |
The Accompanying Notes are an Integral Part of These Consolidated Financial Statements.
49
Table of Contents
BIO-REFERENCE LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
[Dollars In Thousands Except Number of Shares]
| | Series A | | | | | | Additional | | | | | | | |
| | Preferred Stock | | Common Stock | | Paid-in | | Retained | | Deferred | | Total Shareholders’ | |
| | Shares | | Amount | | Shares | | Amount | | Capital | | Earnings | | Compensation | | Equity | |
| | | | | | | | | | | | | | | | | |
Balance on October 31, 2010 | | | | | | 27,847,204 | | $ | 278 | | $ | 44,562 | | $ | 107,548 | | — | | $ | 152,388 | |
| | | | | | | | | | | | | | | | | |
Exercise of Options - Employees | | | | | | 79,600 | | 1 | | 497 | | | | | | 498 | |
Stock Based Compensation | | | | | | | | | | 40 | | | | | | 40 | |
Stock Issued for Acquisition | | | | | | 23,096 | | — | | 482 | | | | | | 482 | |
Net Income | | | | | | | | | | | | 36,359 | | | | 36,359 | |
| | | | | | | | | | | | | | | | | |
Balance on October 31, 2011 | | | | | | 27,949,900 | | $ | 279 | | $ | 45,581 | | $ | 143,907 | | — | | $ | 189,767 | |
| | | | | | | | | | | | | | | | | |
Exercise of Options - Employees | | | | | | 31,500 | | 1.00 | | 226 | | | | | | 227 | |
Stock Based Compensation | | | | | | 11,432 | | — | | 290 | | | | | | 290 | |
Net Income | | | | | | | | | | | | 42,156 | | | | 42,156 | |
Common Stock Repurchased and Canceled | | | | | | (285,450 | ) | (3 | ) | (5,190 | ) | | | | | (5,193 | ) |
| | | | | | | | | | | | | | | | | |
Balance on October 31, 2012 | | | | | | 27,707,382 | | $ | 277 | | $ | 40,907 | | $ | 186,063 | | | | $ | 227,247 | |
| | | | | | | | | | | | | | | | | |
Exercise of Options - Employees | | | | | | 46,000 | | | | | 263 | | | | | | 263 | |
Stock Based Compensation | | | | | | 11,431 | | | | | 290 | | | | | | 290 | |
Net Income | | | | | | | | | | | | | 45,825 | | | | 45,825 | |
Common Stock Repurchased and Canceled | | | | | | (81,600 | ) | | | | (2,030 | ) | | | | | (2,030 | ) |
| | | | | | | | | | | | | | | | | |
Balance on October 31, 2013 | | | | | | 27,683,213 | | $ | 277 | | $ | 39,430 | | $ | 231,888 | | | | $ | 271,595 | |
The Accompanying Notes are an Integral Part of These Consolidated Financial Statements.
50
Table of Contents
BIO-REFERENCE LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
[Dollars In Thousands]
| | Years Ended October 31, | |
| | 2013 | | 2012 | | 2011 | |
OPERATING ACTIVITIES: | | | | | | | |
Net Income | | $ | 45,825 | | $ | 42,156 | | $ | 36,359 | |
Adjustments to Reconcile Net Income to | | | | | | | |
Cash Provided by Operating Activities: | | | | | | | |
Depreciation and Amortization | | 19,739 | | 16,663 | | 15,005 | |
Deferred Income Taxes (Benefit) | | (17,041 | ) | (3,638 | ) | (5,911 | ) |
Stock - Based Compensation Expense | | 290 | | 290 | | 40 | |
Loss on Disposal of Property and Equipment | | 1,408 | | 537 | | 1,680 | |
Undistributed Equity Method (Income) Loss | | 450 | | 323 | | — | |
Change in Assets and Liabilities: | | | | | | | |
(Increase) Decrease in: | | | | | | | |
Accounts Receivable | | (91,002 | ) | (11,240 | ) | (29,254 | ) |
Provision for Doubtful Accounts | | 37,988 | | 6,053 | | 10,316 | |
Inventory | | (4,193 | ) | (5,211 | ) | (3,498 | ) |
Other Current Assets | | (4,043 | ) | (916 | ) | (1,637 | ) |
Other Assets | | (299 | ) | (141 | ) | 798 | |
Deposits | | (61 | ) | (74 | ) | 507 | |
Increase (Decrease) in: | | | | | | | |
Accounts Payable and Accrued Liabilities | | 28,601 | | 8,296 | | 6,541 | |
| | | | | | | |
NET CASH - OPERATING ACTIVITIES | | 17,662 | | 53,098 | | 30,946 | |
| | | | | | | |
INVESTING ACTIVITIES: | | | | | | | |
Business Acquisitions Related Costs | | (19,013 | ) | (5,675 | ) | (1,425 | ) |
Acquisition of Equipment and Leasehold Improvements | | (25,100 | ) | (15,715 | ) | (14,117 | ) |
| | | | | | | |
NET CASH - INVESTING ACTIVITIES | | (44,113 | ) | (21,390 | ) | (15,542 | ) |
| | | | | | | |
FINANCING ACTIVITIES: | | | | | | | |
Payments of Long-Term Debt | | (464 | ) | (1,270 | ) | (1,178 | ) |
Payments of Capital Lease Obligations | | (4,648 | ) | (3,710 | ) | (2,968 | ) |
Increase (Decrease) in Revolving Note Payable | | 26,139 | | (18,632 | ) | (7,522 | ) |
Proceeds from Exercise of Options | | 263 | | 227 | | 498 | |
Common Stock Repurchased | | (2,030 | ) | (5,193 | ) | — | |
| | | | | | | |
NET CASH - FINANCING ACTIVITIES | | 19,260 | | (28,578 | ) | (11,170 | ) |
| | | | | | | |
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | | (7,191 | ) | 3,130 | | 4,234 | |
| | | | | | | |
CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIODS | | $ | 25,143 | | $ | 22,013 | | $ | 17,779 | |
| | | | | | | |
CASH AND CASH EQUIVALENTS AT END OF PERIODS | | $ | 17,952 | | $ | 25,143 | | $ | 22,013 | |
| | | | | | | |
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | | | | | | | |
Cash paid during the period for: | | | | | | | |
Interest | | $ | 1,503 | | $ | 1,547 | | $ | 1,754 | |
Income Taxes | | $ | 44,312 | | $ | 36,697 | | $ | 32,773 | |
The Accompanying Notes are an Integral Part of These Consolidated Financial Statements
51
Table of Contents
BIO-REFERENCE LABORATORIES, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
[Dollars In Thousands]
Supplemental Schedule of Non-Cash Investing and Financing Activities:
During fiscal 2013, 2012 and 2011, the Company wrote-off approximately $4,464, $2,508 and $5,944 of property which was fully depreciated.
During fiscal 2011, the Company disposed of certain equipment with an initial cost of $4,558. During the same period the Company financed the purchase of new equipment through a term note of $5,408.
During fiscal 2013, 2012, and 2011, the Company incurred capital lease obligations totaling approximately $7,125, $7,777, and $5,444 in connection with the acquisition of property and equipment.
The Accompanying Notes are an Integral Part of These Consolidated Financial Statements
52
Table of Contents
BIO-REFERENCE LABORATORIES, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
[Dollars In Thousands Except Share Data or Unless Otherwise Indicated]
[1] Organization and Business
Bio-Reference Laboratories, Inc. [“Bio-Reference”, “BRLI”, or the “Company”] was incorporated on December 24, 1981. Bio-Reference is principally engaged in providing laboratory testing services, primarily to customers in the greater New York metropolitan area as well as to customers in a number of other states. Bio-Reference offers a comprehensive list of chemical diagnostic tests including blood and urine analysis, blood chemistry, hematology services, serology, radio-immuno analysis, toxicology (including drug screening), pap smears, tissue pathology (biopsies) and other tissue analysis. We perform cancer cytogenetic testing at our leased facilities in at our main processing facility in Elmwood Park, Smithtown, NY, Clarksburg, MD and Milford, MA and genetic testing at our GeneDx leased facility in Gaithersburg, MD, as well as at our Elmwood Park facility. We perform cytology testing in Frederick, MD, Milford, MA, Columbus, OH, Houston, TX and at our Elmwood Park facility. Bio-Reference markets its laboratory testing services directly to physicians, geneticists, hospitals, clinics, correctional and other health facilities.
The Company’s laboratory testing business currently represents its one reportable business segment. The laboratory testing business accounts for over 98% of consolidated assets, net revenues and net income in each of the three years ended October 31, 2013. All other operating segments include the Company’s non-clinical laboratory testing businesses and consist of our clinical knowledge management service through our PSIMedica business unit and a web-based connectivity portal solution for laboratories and physicians through its Care Evolve subsidiary.
[2] Summary of Significant Accounting Policies
Principles of Consolidation - The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All significant intercompany accounts and transactions have been eliminated.
Cash and Cash Equivalents - Cash equivalents are comprised of certain highly liquid investments with a maturity of three months or less when purchased. The Company had $17,952 and $25,143 in cash and cash equivalents at October 31, 2013 and 2012, respectively.
Inventory - Inventory is stated at the lower of cost [determined on a first-in, first-out basis] or market. Inventory consists of purchased laboratory supplies, which is used in our various testing laboratories.
Property and Equipment - Property and equipment are carried at cost. Depreciation is computed by the straight-line method over the estimated useful lives of the respective assets, which generally range from 2 to 15 years. Leasehold improvements are amortized over the life of the lease or improvement, which is typically five years.
The statements of operations reflect depreciation expense related to property and equipment of $18,745, $16,082 and $13,684 for the years ended October 31, 2013, 2012 and 2011, respectively.
On sale or retirement, the asset cost and related accumulated depreciation or amortization are removed from the accounts, and any related gain or loss is reflected in general and administrative expenses. Repairs and maintenance are charged to expense when incurred.
Goodwill - Effective November 1, 2011, the Company adopted revised Financial Accounting Standards Board (“FASB”) rules promulgated under Accounting Standards Update (“ASU”) No. 2011-08 issued on September 15, 2011, Intangibles—Goodwill and Other (Topic 350) Testing Goodwill for Impairment. Under these simplified goodwill impairment testing rules the Company assessed qualitative factors to determine whether events and circumstances lead to the conclusion that it is necessary to perform the two-step goodwill impairment test have occurred and determined that no such events had occurred. Under ASU No. 2011-08, entities are not required to calculate the fair value of a reporting unit unless they conclude that it is more likely than not that the unit’s carrying value is greater than its fair value based on an assessment of events and circumstances. The “more likely than not” threshold is when there is a likelihood of more than 50% that a reporting unit’s carrying value is greater than its fair value. No impairment loss was recognized in the years ended October 31, 2013, 2012 and 2011.
The balance sheet reflects prior Goodwill accumulated amortization of $2,401 as of October 31, 2013 and 2012, respectively.
Other Intangible Assets - Intangible assets are amortized using the straight-line method. The estimated useful life of costs capitalized is evaluated for each specific project when completed, at which time such costs begin to be amortized. The statements of operations reflect amortization expense related to intangible assets of $994, $581, and $1,322 for the years ended October 31, 2013, 2012 and 2011, respectively. The balance sheet reflects accumulated amortization of $8,846, and $7,852 as of October 31, 2013, and 2012, respectively. During the 2013 and 2012 fiscal years, the Company did not write off any intangible assets.
New Accounting pronouncements
Certain prior year amounts have been reclassified to conform to the current year presentation. The Company adopted Accounting Standard Update (“ASU”) No. 2011-7: Health Care Entities (Topic 954) — Presentation and Disclosure of Patient Service Revenue, Provision for Bad Debts, and the Allowance for Doubtful Accounts for Certain Health Care Entities commencing with the current fiscal year, the first year such standard is required for the Company. The adoption of this update did not have a material impact on the Company’s financial statements.
Although this update does not have a material impact on the Company’s financial statements as a whole, it requires that we adjust our presentation of our statement of operations along with prior periods presented in this report to maintain comparability. As the result of this change in presentation, our “Net Revenues”, “Gross Profit on Revenues” and our “General and Administrative Expenses” would change while our “Operating Income”, “Net Income” and “Earnings per Share” will remain the same. The presentation is adjusted for a portion of our “Bad Debt Expense” that is now reported in our Net Revenues as required under ASU No. 2011-7.
Accounting for Revenue
Service revenues are principally generated from laboratory testing services including chemical diagnostic tests such as blood analysis, urine analysis and genetic testing among others. Service revenues are recognized at the time the testing services are performed and are reported at their estimated net realizable amounts.
53
Table of Contents
Service revenues before provision for bad debts are determined utilizing gross service revenues net of contractual adjustments and discounts. Even though it is the responsibility of the patient to pay for laboratory service bills, most individuals in the United States have an agreement with a third party payor such as Medicare, Medicaid or a commercial insurance provider to pay all or a portion of their healthcare expenses. The majority of services provided by Bio-Reference Laboratories, Inc. (“BRLI”) are to patients covered under a third party payor contract. In certain cases, the individual has no insurance or does not provide insurance information and in other cases tests are performed under contract to a professional organization (such as physicians, hospitals, and clinics) which reimburses BRLI directly. In the remainder of the cases, BRLI is provided the third party billing information and seeks payment from the third party under the terms and conditions of the third party payor for health service providers like BRLI. Each of these third party payors may differ not only with regard to rates, but also with regard to terms and conditions of payment and providing coverage (reimbursement) for specific tests. Estimated revenues are established based on a series of highly complex procedures and judgments that require industry specific healthcare experience and an understanding of payor methods and trends. We review our calculations on a monthly basis in order to make certain that we are properly allowing for the uncollectable portion of our gross billings due to the contractual adjustments and discounts and that our estimates remain sensitive to variances and changes within our payor groups. The contractual allowance calculation is made on the basis of historical allowance rates for the various specific payor groups on a monthly basis with a greater weight being given to the most recent trends This process is adjusted based on recent changes in underlying contract provisions and shifts in the testing being performed. This calculation is routinely analyzed by BRLI on the basis of actual allowances issued by payors and the actual payments made to determine what adjustments, if any, are needed. The table below shows the adjustments made to gross service revenues to arrive at net revenues, the amount reported on our statement of operations.
| | ($) | |
| | Year Ended | |
| | October 31, | |
| | 2013 | | 2012 | | 2011 | |
Gross Service Revenues | | 3,524,108 | | 3,052,431 | | 2,482,349 | |
| | | | | | | |
Contractual Adjustments and Discounts: | | | | | | | |
Medicare/Medicaid Portion | | 354,638 | | 320,697 | | 293,874 | |
All Other Third Party Payors* | | 2,393,872 | | 2,070,073 | | 1,629,833 | |
Total Contractual Adjustments and Discounts | | 2,748,510 | | 2,390,770 | | 1,923,707 | |
Service Revenues Net of Contractual Adjustments and Discounts | | 775,598 | | 661,661 | | 558,642 | |
Patient Service Revenue Provision for Bad Debts** | | 60,244 | | 47,406 | | 36,561 | |
Net Revenues | | 715,354 | | 614,255 | | 522,081 | |
| | | | | | | |
Percent of Contractual Allowances, Discounts and Patient Service Provision for Bad Debts to Gross Revenue. | | 79.7 | % | 79.9 | % | 79.0 | % |
* All Other Third Party and Direct Payors consists of almost eight hundred distinct payors, including commercial health insurers and administrators as well as professionally billed accounts such as physicians, hospitals, clinics and other direct billed accounts.
** Represents the amount of Bad Debt Expense that is now required to be presented as a deduction from patient service revenue (net of contractual allowances and discounts) pursuant to ASU No. 2011-7.
When new business is received by BRLI, service revenues net of contractual adjustments and discounts are calculated by reducing gross service revenues by the estimated contractual allowance. The Patient Service Revenue Provision for Bad Debts represents the amount of bad debt expense expected to occur on patient service revenue based upon our experience. The remaining bad debt expense is presented as part of operating expenses. The bad debt expense presented as part of operating expense represents the bad debt expense related to receivables from service revenues determined after taking into account our ability to collect on such revenue. BRLI recognized the amounts in subsequent periods for actual allowances/discounts to gross service revenue; bad debt may have been adjusted over the same periods of time to maintain an accurate balance between net revenues and actual revenues. Management has reviewed the allowances/discounts recognized in subsequent periods and believes the amounts to be immaterial. A number of proposals for legislation or regulation continue to be under discussion which could have the effect of substantially reducing Medicare reimbursements for clinical laboratories or introducing cost sharing to beneficiaries. Depending upon the nature of regulatory action, if any, which is taken and the content of legislation, if any, which is adopted, the Company could experience a significant decrease in revenues from Medicare and Medicaid, which could have a material adverse effect on the Company. The Company is unable to predict, however, the extent to which such actions will be taken.
Accounting for Contractual Credits and Doubtful Accounts
It is typically the responsibility of the patient to pay for laboratory service bills. Most individuals in the United States have an agreement with a third party payor such as Medicare, Medicaid or commercial insurance to pay all or a portion of their healthcare expenses; this represents the major portion of payment for all services provided by BRLI. In certain cases, the individual has no insurance or does not provide insurance information; in the remainder of the cases, BRLI is provided the third party billing information, usually by the referring physician, and seeks payment from the third party under the terms and conditions of the third party payor for health service providers like BRLI. Each of these third party payors may differ not only with regard to rates, but also with regard to terms and conditions of payment and coverage of specific tests. BRLI routinely reviews the reimbursement policies and subsequent payments and collection rates from these different types of payors. Contractual adjustments and discounts are recorded as reductions to gross service revenues and are collectively referred to as the contractual allowance. BRLI has not been required to record an adjustment in a subsequent period related to revenue recorded in a prior period which was material in nature. Aging of accounts receivable is monitored by billing personnel and follow-up activities including collection efforts are conducted as necessary. BRLI writes off receivables against the allowance for doubtful accounts when they are deemed uncollectible. For client billing, accounts are written off when all reasonable collection efforts prove to be unsuccessful. Patient accounts, where the patient is directly responsible for all or a remainder portion of the account after partial payment or denial by a third party payor, are written off after the normal
54
Table of Contents
dunning cycle has occurred, although these may be subsequently transferred to a third party collection agency after being written off. Third party payor accounts are written off when they exceed the payer’s timely filing limits. Accounts Receivable on the balance sheet is net of the following amounts for contractual credits and doubtful accounts:
| | ($) | |
| | October 31, | | October 31, | |
| | 2013 | | 2012 | |
| | | | | |
Contractual Credits/Discounts | | 342,297 | | 267,921 | |
Doubtful Accounts | | 89,261 | | 51,274 | |
Total Allowance | | 431,558 | | 319,195 | |
Current Income Taxes — The Company recognizes interest and penalties on settlement of tax liabilities in its income from operations. For the fiscal years 2011 through 2013, no material amounts for interest and penalties have been recorded.
Deferred Income Taxes - Deferred income tax assets and liabilities are computed annually for differences between the financial statement and tax bases of assets and liabilities that will result in taxable or deductible amounts in the future based on enacted tax laws and rates applicable to the periods in which the differences are expected to affect taxable income.
The Company adopted GAAP guidance with respect to uncertain tax positions when it became effective. Under these rules the Company may recognize the tax benefit from an uncertain tax position only if it meets the more-likely-than-not criteria (over 50% likelihood) of being realized on an examination by taxing authorities. For the years ended October 31, 2011 through October 31, 2013 the Company had no material uncertain tax positions to report.
Earnings Per Share - Basic earnings per share [“EPS”] reflects the amount of income attributable to each share of common stock based on average common shares outstanding during the period. Diluted EPS reflects Basic EPS while giving effect to all potential dilutive common shares that were outstanding during the period, such as common shares that could result from the exercise or conversion of securities into common stock. The computation of Diluted EPS is calculated by using the treasury stock method, which assumes that any proceeds obtained from the exercise of such dilutive securities would be used to purchase common stock at the average market price of the common stock during the period. This reduces the gross number of dilutive shares by the number of shares purchasable from the proceeds of the securities assumed to be exercised. Securities whose conversion would have an anti-dilutive effect on EPS are not assumed converted. Securities that could potentially dilute earnings in the future are disclosed in Note 10.
Use of Estimates - The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Recoverability and Impairment of Intangible Assets and Other Long-Lived Assets — The Company evaluates the possible impairment of its long-lived assets under the provisions of FASB codification 350-30-35 and 360-10-35. The Company reviews the recoverability of its long-lived assets on an annual basis. Evaluation of possible impairment is based on the Company’s ability to recover the asset from the expected future pretax cash flows (undiscounted and without interest charges) of the related operations. If the expected undiscounted pretax cash flows are less than the carrying amount of such asset, an impairment loss is recognized for the difference between the estimated fair value and carrying amount of the asset. No impairment loss was recognized in the fiscal years ended October 31, 2013, 2012 and 2011.
Advertising Costs -Advertising costs are expensed when incurred. Advertising costs amounted to approximately $3,200, $2,366 and $2,026 for the years ended October 31, 2013, 2012 and 2011, respectively.
Other Income — During the year the Company recorded a loss of $450on its investment in IncellDx. The loss represents the Company’s share of IncellDX undistributed net loss under the equity method of accounting. During the year ended October 31, 2013 the Company received a refund of $1,062 for its New York State clinical laboratory inspection fee that was included in other income.
Subsequent Events — The management considered subsequent events through the date the financial statements are issued as defined in FASB Codification 855-10-50.
[3] Property and Equipment - Property and equipment - at cost is summarized as follows:
| | October 31 | |
| | 2013 | | 2012 | |
| | | | | |
Medical Equipment | | $ | 65,762 | | $ | 50,338 | |
Leasehold Improvements | | 27,974 | | 19,768 | |
Furniture, Fixtures and Office & Computer Equipment | | 21,621 | | 16,503 | |
Automobiles and Aircraft | | 18,242 | | 16,092 | |
| | | | | |
Sub Totals | | 133,599 | | 102,701 | |
Less Accumulated Depreciation | | 67,950 | | 52,261 | |
| | | | | |
Totals — Net of Accumulated Depreciation | | $ | 65,649 | | $ | 50,440 | |
55
Table of Contents
[4] Intangible Assets
Intangible assets are summarized as follows:
October 31, 2013
| | Weighted-Average | | | | Accumulated | | Net of
Accumulated | |
Intangible Asset | | Amortization Period | | Cost | | Amortization | | Amortization | |
| | | | | | | | | |
Customer Lists | | 20 | | $ | 8,739 | | $ | 2,879 | | $ | 5,860 | |
Covenants Not-to-Compete | | 3 | | 11,131 | | 4,560 | | 6,571 | |
Patents and Licenses | | 17 | | 5,297 | | 1,408 | | 3,889 | |
| | | | | | | | | |
Totals | | | | $ | 25,166 | | $ | 8,847 | | $ | 16,320 | |
October 31, 2012
| | Weighted-Average | | | | Accumulated | | Net of
Accumulated | |
Intangible Asset | | Amortization Period | | Cost | | Amortization | | Amortization | |
| | | | | | | | | |
Customer Lists | | 20 | | $ | 4,573 | | $ | 2,537 | | $ | 2,036 | |
Covenants Not-to-Compete | | 5 | | 4,305 | | 4,257 | | 48 | |
Patents and Licenses | | 17 | | 5,297 | | 1,058 | | 4,239 | |
| | | | | | | | | |
Totals | | | | $ | 14,175 | | $ | 7,852 | | $ | 6,323 | |
The estimated amortization expense related to intangible assets for each of the five succeeding fiscal years and thereafter as of October 31, 2013 is as follows:
October 31, | | | |
2014 | | $ | 1,917 | |
2015 | | 1,852 | |
2016 | | 1,540 | |
2017 | | 1,063 | |
2018 | | 946 | |
Thereafter | | 9,002 | |
| | | |
Total | | $ | 16,320 | |
[5] Revolving Note Payable - Bank
In October 2011, the Company entered into an amended revolving note payable loan agreement with PNC Bank, N.A. The maximum amount of the credit line available to the Company pursuant to the loan agreement is the lesser of (i) $45,000 or (ii) 50% of the Company’s qualified accounts receivable [as defined in the agreement]. The amendment to the Loan and Security Agreement provides for an interest rate on advances to be subject, at the election of the Company, to either the bank’s base rate or the Eurodollar rate of interest plus, in certain instances, an additional interest percentage. The additional interest percentage charge on bank’s base rate borrowings and on Eurodollar rate borrowings ranges from 1% to 4% and is determined based upon certain financial ratios achieved by the Company. At October 31, 2013, the Company had elected to have all of the total advances outstanding to be subject to the bank’s base rate of interest of 3.50%. The credit line is collateralized by substantially all of the Company’s assets. The line of credit is available through October 2016 and may be extended for annual periods by mutual consent, thereafter. The terms of this agreement contain, among other provisions, requirements for maintaining defined levels of capital expenditures, fixed charge coverage, and the prohibition of the payment by the Company of cash dividends. As of October 31, 2013 and October 31, 2012, the Company utilized $26,139 and $0 of the credit line, respectively.
[6] Long-Term Debt - Bank
Effective as of October 31, 2007, we executed a fifth amendment to the Loan Agreement formalizing the repayment terms of the $5 million term loan from PNC Bank used by our wholly-owned GeneDX (formerly, BRLI No. 2 Acquisition Corp.) subsidiary to fund the $5 million acquisition cash payment in connection with its purchase of the operating assets of GeneDx, Inc. The term loan is evidenced by a secured promissory note payable over a six year term in equal monthly principal payments of approximately $69, plus interest at an annual rate of 6.85%. The note was paid off on October 31, 2012.
In December 2010, the Company issued a seven year term note for $5,408 at the rate of interest of 6.12% per annum for the financing of new equipment. The note is payable in eighty-four equal monthly installments commencing on January 29, 2011 of $61 including principal and interest followed by a balloon payment of the principal and interest outstanding on the loan repayment date of December 29, 2017. The balance on this note as of October 31, 2013 is approximately $4,163.
56
Table of Contents
Principal repayment for each of the five succeeding fiscal years and thereafter as of October 31, 2013 is as follows:
Year Ended | | | |
October 31, | | | |
2014 | | 493 | |
2015 | | 518 | |
2016 | | 551 | |
2017 | | 585 | |
2018 | | 2,016 | |
Thereafter | | — | |
Totals | | $ | 4,163 | |
| | | | |
[7] Related Party Transactions [Not in Thousands]
There were no material related party transactions during fiscal 2013 and fiscal 2012.
[8] Income Taxes
The reconciliation of income tax from continuing operations computed at the U.S. federal statutory tax rate to the Company’s effective income tax rate is as follows:
| | October 31 | |
| | 2013 | | 2012 | | 2011 | |
| | | | | | | |
U.S. Federal Statutory Rate | | 35 | % | 35 | % | 35 | % |
State and Local Taxes, Net of U.S. Federal Tax Benefit | | 11.20 | % | 8.87 | % | 9.87 | % |
Permanent differences and Other | | (2.78 | )% | (0.44 | )% | (0.94 | )% |
| | | | | | | |
Actual Rate | | 43.42 | % | 43.43 | % | 43.93 | % |
The provision for income taxes shown in the consolidated statements of operations consists of the following:
| | October 31 | |
| | 2013 | | 2012 | | 2011 | |
| | | | | | | |
Current: | | | | | | | |
Federal | | $ | 38,295 | | $ | 25,794 | | $ | 24,573 | |
State & Local | | 14,018 | | 10,203 | | 9,825 | |
| | | | | | | |
Deferred: | | | | | | | |
Federal | | (10,803 | ) | (2,183 | ) | (4,223 | ) |
State and Local | | (6,238 | ) | (1,454 | ) | (1,688 | ) |
| | | | | | | |
Total Provision for Income Taxes | | $ | 35,272 | | $ | 32,360 | | $ | 28,487 | |
At October 31, 2013 and 2012, the Company had a net deferred tax asset of approximately $44,231 and $27,190, respectively. The deferred taxes primarily relate to timing differences associated with the deductibility of depreciation and amortization, bad debts and certain accrued expenses and deferred costs. For fiscal years ended October 31 2011 through October 31, 2013, the Company had no material net operating loss carry-forwards available to reduce current year taxable income.
| | October 31, | |
| | 2013 | | 2012 | |
| | | | | |
Deferred Tax Asset: | | | | | |
Bad Debt Allowance | | $ | 39,275 | | $ | 22,560 | |
Depreciation and amortization | | 1,815 | | 1,963 | |
Accrued Expenses | | 3,141 | | 2,667 | |
| | | | | |
Deferred Tax Asset - Net | | 44,231 | | 27,190 | |
| | | | | |
Current Deferred Tax Asset - Net | | 42,154 | | 24,912 | |
Long Term Deferred Tax Asset - Net | | 2,077 | | 2,278 | |
| | | | | |
Deferred Tax Asset — Net | | $ | 44,231 | | $ | 27,190 | |
During fiscal year ended October 31, 2013 the Company recorded a net deferred tax benefit of $17,041. This reflects a net benefit of approximately $16,715 in allowance for bad debts, a liability of approximately $281 from depreciation and amortization timing differences, and a benefit of approximately $607 in certain accrued expenses. Although realization is not assured and dependent upon things such as generating sufficient taxable income in future periods, management, through sufficient positive evidence, believes it is more likely than not that all of the deferred tax asset will be realized. The amount of the deferred tax asset considered realizable, however, could be reduced in the near term if estimates of future taxable income or changes in the accrued expenses during the future periods are reduced.
At October 31, 2013, fiscal 2010 through 2013 are subject to examination by US federal and state tax authorities. Through the issuance of these financial statements the outcome of all of these examinations has not been determined.
57
Table of Contents
[9] Capital Transactions
[A] Preferred Stock and Common Stock - The Company is authorized to issue an aggregate of 1,666,667 shares of preferred stock, $.10 par value. None was outstanding as of October 31, 2013 and October 31, 2012.
Holders of the Company’s Common Stock are entitled to one vote per share on matters submitted for shareholder vote. Holders are also entitled to receive dividends ratably, if declared. In the event of dissolution or liquidation, holders are entitled to share ratably in all assets remaining after payment of liabilities.
On November 11, 2011, the Company announced that its board of directors has approved a Stock Repurchase Program authorizing the repurchase of up to 1,000,000 shares of its Common Stock in the over-the-counter market at prevailing market prices over the period ending October 31, 2012. During the year ended October 31, 2012 the Company repurchased 285,450 shares at a cost of $5,193. On December 6, 2012, the Company announced that its board of directors has approved a new Stock Repurchase Program authorizing the buyback of up to 714,550 shares of its Common Stock in the over-the-counter market at prevailing market prices through October 31, 2013. As of October 31, 2013, the Company repurchased 81,600 shares at a cost of $2,030. Subsequent to our year end, on December 19, 2013 the Company announced that its board of directors has approved a new Stock Repurchase Program authorizing the buyback of up to 2,000,000 shares of its Common Stock in the over-the-counter market at prevailing market prices through October 31, 2015.
[B] Equity Transactions for Services — For the fiscal years ended in 2013, 2012 and 2011, the Company issued 11,431, 11,432 and 11,432 shares of the Company’s common stock for employment or consulting services [See Note 11 for common stock options issued for employee and consulting services].
[10] Earnings Per Share
The computation of basic and diluted net earnings per common share is as follows [in thousands, except per share data rounded]:
| | For Years Ended October 31, | |
| | 2013 | | 2012 | | 2011 | |
| | | | | | | |
Income Available to Common Stockholders | | $ | 45,825 | | $ | 42,156 | | $ | 36,359 | |
Weighted Average Common Shares Outstanding | | 27,691 | | 27,742 | | 27,971 | |
| | | | | | | |
Effect of Dilutive Securities: | | | | | | | |
Warrants/Options | | 161 | | 179 | | 236 | |
| | | | | | | |
Weighted Average Diluted Common Shares Outstanding | | 27,852 | | 27,921 | | 28,207 | |
| | | | | | | |
Net Income Per Share - Basic | | $ | 1.65 | | $ | 1.52 | | $ | 1.30 | |
Net Income Per Share - Diluted | | $ | 1.65 | | $ | 1.51 | | $ | 1.29 | |
[11] Stock Options and Warrants
Employee Incentive Stock Options - In June 2003, the Board of Directors adopted, and in July 2003, the stockholders approved, the 2003 Employee Incentive Stock Option Plan [the “2003 Plan”]. The 2003 Plan authorizes the grant of stock options, which may be designated as incentive stock options, to purchase up to a maximum aggregate 1,600,000 shares of Company common stock. The 2003 Plan provides that the exercise price of an option granted there under shall not be less than the fair market value of the Common Stock on the date the option is granted. However, in the event an option is granted under the 2003 Plan to a holder of 10% or more of the Company’s outstanding Common Stock, the exercise price must be at least 110% of such fair market value. Under the 2003 Plan, options must be granted before the June 2, 2013 Termination Date. No option may have a term longer than ten years (limited to five years in the case of an option granted to a 10% or greater stockholder of the Company). The aggregate fair market value of the Company’s Common Stock with respect to which options are exercisable for the first time by a grantee under all of the Company’s Stock Option Plans during any calendar year cannot exceed $100. Options granted under the 2003 Plan are non-transferable and must be exercised by an optionee, if at all, while employed by the Company or a subsidiary or within three months after termination of such optionee’s employment due to retirement, or within one year of such termination if due to disability or death. The Board (or a Stock Option Committee, if designated), may, in its sole discretion, cause the Company to lend money to or guaranty any obligation of an employee for the purpose of enabling such employee to exercise an option granted under the 2003 Plan provided that such loan or obligation cannot exceed fifty percent (50%) of the exercise price of such option. In fiscal year ended October 31, 2013, 2012 and 2011, -0-, -0- and -0- options were granted under the Plan, respectively. A total of 4,000, 23,500 and 40,000 incentive stock options issued under the 2003 Plan were exercised in fiscal years ended in October 31, 2013, 2012 and 2011, respectively. A total of 6,000, 13,000 and -0- options were cancelled in fiscal years ended October 31, 2013, 2012 and 2011.
In August 2000, the Company adopted, and on December 15, 2000, the stockholders approved, the 2000 Employee Incentive Stock Option Plan [“2000 Plan”]. The 2000 Plan provided for the granting of stock options, which may have been designated as incentive stock options, to purchase an aggregate of 1,600,000 shares of the Company’s common stock at a price not less than 100% of the fair market value per share of the common stock at the date of grant. However, in the event an option was granted under the 2000 Plan to a holder of 10% or more of the Company’s outstanding common stock, the exercise price must have been at least 110% of fair market value at the date of grant. Employees of the Company or its subsidiary, as determined, were eligible for the 2000 Plan. The term of the options could not exceed ten years from the date of grant. In fiscal years ended October 31, 2013, 2012 and 2011, no options were granted under the Plan. A total of 44,000, 8,000 and 39,600 incentive stock options issued under the 2000 Plan were exercised in fiscal years ended in October 31, 2013, 2012 and 2011, respectively. A total of 2,000, -0-, and -0- options were cancelled in fiscal years ended October 31, 2013, 2012 and 2011, respectively. Options issued under the 2000 Plan must have been granted before the August 2010 termination date.
The following is a summary of Employee Incentive Stock Option Plan transactions:
58
Table of Contents
| | 2003 Plan | |
| | Shares Under Options
[In Thousands] | | Weighted Average
Exercise Price Per Share | |
Outstanding at October 31, 2010* | | 335 | | 9.66 | |
Granted | | — | | — | |
Expired | | — | | — | |
Exercised | | (40 | ) | 8.67 | |
Outstanding at October 31, 2011* | | 295 | | 9.80 | |
| | | | | |
Granted | | — | | — | |
Expired | | (13 | ) | 9.13 | |
Exercised | | (23 | ) | 8.52 | |
Outstanding at October 31, 2012* | | 259 | | $ | 9.95 | |
| | | | | |
Granted | | — | | — | |
Expired | | (6 | ) | 8.64 | |
Exercised | | (4 | ) | 7.25 | |
Outstanding at October 31, 2013* | | 249 | | 10.02 | |
| | | | | | |
*Eligible for exercise at October 31, 2010 were 310 at a weighted average exercise price per share of $9.66
*Eligible for exercise at October 31, 2011 were 275 at a weighted average exercise price per share of $9.80
*Eligible for exercise at October 31, 2012 were 244 at a weighted average exercise price per share of $9.95
*Eligible for exercise at October 31, 2013 were 239 at a weighted average exercise price per share of $9.97
| | 2000 Plan | |
| | Shares Under
Options
[In Thousands] | | Weighted
Average
Exercise Price
Per Share | |
Outstanding and Eligible for Exercise at October 31, 2010 | | 100 | | 4.64 | |
Granted | | — | | — | |
Expired | | — | | — | |
Exercised | | (40 | ) | 3.82 | |
Outstanding and Eligible for Exercise at October 31, 2011 | | 60 | | 5.19 | |
| | | | | |
Granted | | — | | — | |
Expired | | — | | — | |
Exercised | | (8 | ) | 3.39 | |
Outstanding and Eligible for Exercise at October 31, 2012 | | 52 | | $ | 5.47 | |
Granted | | — | | — | |
Expired | | (2 | ) | 3.60 | |
Exercised | | (44 | ) | 5.25 | |
Outstanding and Eligible for Exercise at October 31, 2013 | | | | | |
| | 6 | | 7.67 | |
| | | | | | |
Summary of outstanding options:
| | Weighted Average | | Exercisable | |
| | Shares | | Remaining | | Exercise | | Shares | | Exercise | |
Exercise Price Range | | Outstanding | | Life | | Price | | Outstanding | | Price | |
$6.11 | | to | | $6.11 | | Per Share | | 6 | | 0.75 | | 6.11 | | 6 | | 6.11 | |
$7.41 | | to | | $7.67 | | Per Share | | 62 | | 1.09 | | 7.48 | | 62 | | 7.48 | |
$9.13 | | to | | $9.13 | | Per Share | | 147 | | 1.00 | | 9.13 | | 147 | | 9.13 | |
$17.50 | | to | | $17.50 | | Per Share | | 40 | | 4.08 | | 17.50 | | 30 | | 17.50 | |
| | | | | | | | 255 | | | | | | 245 | | | |
Compensation cost recognized for the years ended October 31, 2013, October 31, 2012 and October 31, 2011 was $40 for each one of the years, with a related tax benefit of $-0- with respect to these options.
[12] Employment Contracts and Consulting Agreements
The Company has multiple employment contracts with its key executives with expiration dates ranging from October 31, 2015 through October 31, 2018. At October 31, 2013, the approximate aggregate minimum commitment under these employment contracts and agreements, excluding commissions or consumer price index increases, is as follows:
59
Table of Contents
October 31 | | Employees and
Consultants | |
2014 | | $ | 5,169 | |
2015 | | 3,873 | |
2016 | | 3,657 | |
2017 | | 2,918 | |
2018 | | 1,243 | |
Thereafter | | — | |
| | | |
Total | | $ | 16,860 | |
Some of these agreements provide bonuses and commissions based on a percentage of collected revenues ranging from 1% to 10% on accounts referred by or serviced by the employee or consultant.
In addition to the above, in fiscal 2013 the Company has entered into seventy at — will employment and consulting agreements which together with prior at — will agreements provide for annual aggregate minimum commitments of approximately $33,555 which have no termination dates.
[13] Capitalized Lease Obligations
The Company leases various assets under capital leases expiring in fiscal 2018 with interest rates ranging between 2% to 8% as follows:
| | October 31 | |
| | 2013 | | 2012 | |
Medical Equipment | | $ | 15,013 | | $ | 12,078 | |
Automobiles | | 10,581 | | 8,711 | |
| | | | | |
Totals | | 25,594 | | 20,789 | |
Less: Accumulated Depreciation | | 13,125 | | 6,776 | |
| | | | | |
Net | | $ | 12,469 | | $ | 14,013 | |
Depreciation expense on assets under capital leases was approximately $6,494, $3,792, and $1,418 for the years ended October 31, 2013, 2012 and 2011, respectively.
Aggregate future minimum rentals under capital leases are:
October 31, | | | |
2014 | | $ | 5,622 | |
2015 | | 4,630 | |
2016 | | 3,727 | |
2017 | | 2,192 | |
2018 | | 660 | |
Thereafter | | 15 | |
| | | |
Total | | 16,846 | |
| | | |
Less Interest: | | 949 | |
| | | |
Present Value of Minimum Lease Payments | | $ | 15,897 | |
[14] Commitments and Contingencies
The Company leases various office and laboratory facilities and equipment under operating leases expiring from 2014 to 2019. Several of these leases contain renewal options for one to five year periods.
Total expense for property and equipment rental for the years ended October 31, 2013, 2012 and 2011 was $10,568, $8,787 and $8,010, respectively. There were no contingent rental amounts due through October 31, 2013.
Aggregate future minimum rental payments on non cancelable operating leases [exclusive of several month to month leases] are as follows:
October 31, | | Property($) | | Equipment($) | |
| | | | | |
2014 | | 8,671 | | 344 | |
2015 | | 2,333 | | 223 | |
2016 | | 1,422 | | 87 | |
2017 | | 558 | | — | |
2018 | | 558 | | — | |
Thereafter | | 651 | | — | |
| | | | | |
Totals: | | 14,193 | | 654 | |
The Company has entered into several purchase agreements for reagent supplies through October, 2018. Minimum purchase commitments as of October 31, 2013 are as follows:
60
Table of Contents
October 31 | | Reagents ($) | |
2014 | | 12,505 | |
2015 | | 11,938 | |
2016 | | 10,854 | |
2017 | | 6,559 | |
2018 | | 3,700 | |
Thereafter | | — | |
| | | |
Totals: | | 45,556 | |
Reagent supplies expensed under purchase agreements amount to $7,873, $13,338, and $8,379 for the years ended October 31, 2013, 2012 and 2011, respectively.
[15] Litigation
In the normal course of business, the Company is exposed to a number of asserted and unasserted potential claims. In the opinion of management, the resolution of these matters will not have a material adverse effect on the Company’s financial position or results of operations.
[16] Insurance
The Company maintains professional liability insurance of $3,000 in the aggregate, with a per occurrence limit of $1,000. In addition, the Company maintains excess commercial insurance of $5,000 per occurrence and $5,000 in aggregate over the primary limits. In addition, the Company also maintains excess umbrella coverage of $15,000.
[17] Significant Risks and Uncertainties
[A] Concentrations of Credit Risk - Cash - At October 31, 2013 and 2012, the Company had approximately $14,720 and $21,260, respectively, in cash and certificate of deposit balances at financial institutions which were in excess of the federally insured limits.
[B] Concentration of Credit Risk - Accounts Receivable - Credit risk with respect to accounts receivable is generally diversified due to the large number of patients comprising the client base. The Company does have significant receivable balances with government payors and various insurance carriers. Generally, the Company does not require collateral or other security to support customer receivables. However, the Company continually monitors and evaluates its client acceptance and collection procedures to minimize potential credit risks associated with its accounts receivable and establishes an allowance for uncollectible accounts and as a consequence, believes that its accounts receivable credit risk exposure beyond such allowance is not material to the financial statements.
A number of proposals for legislation continue to be under discussion which could substantially reduce Medicare and Medicaid (CMS) reimbursements to clinical laboratories. Depending upon the nature of regulatory action, and the content of legislation, the Company could experience a significant decrease in revenues from Medicare and Medicaid (CMS), which could have a material adverse effect on the Company. The Company is unable to predict, however, the extent to which such actions will be taken.
[18] Acquisitions
On April 27, 2012, the Company entered into an agreement pursuant to which the Company purchased preferred shares of IncellDx, Inc. (“IncellDx”), a Delaware corporation. Information about IncellDx and the agreement may be found in the Current Report on Form 8-K the Company filed on May 1, 2012.
As of October 31, 2013 the Company invested a total $6,000.
On December 21, 2012, the Company entered into an agreement with Meridian Clinical Laboratory Corporation, a Florida corporation having its place of business in Miami, Florida (“Meridian”), pursuant to which the Company purchased all issued and outstanding common stock of Meridian for approximately $1,848 of which $250 is deferred for one year.
On December 31, 2012, Bio-Reference Laboratories, Inc. (the “Company”) entered into an agreement with Florida Clinical Laboratory, Inc., a Florida corporation having its place of business in Melbourne, Florida (“FCL”), pursuant to which the Company purchased all issued and outstanding shares of capital stock of FCL for approximately $7,016, of which $1,000 is deferred for eighteen months assuming certain conditions are met.
The following table sets forth these final purchase allocations.
| | ($) | |
| | FCL | | MCL | | Totals: | |
| | | | | | | |
Accounts Receivable | | 1,008 | | 232 | | 1,240 | |
Autos | | 137 | | 48 | | 185 | |
Medical Equipment | | 225 | | 3 | | 228 | |
Computer Equipment | | 21 | | — | | 21 | |
Leasehold Improvements | | 53 | | — | | 53 | |
Other Non-Current Assets | | 3 | | — | | 3 | |
Non-Compete Agreement | | 747 | | 43 | | 790 | |
Deposits | | — | | 2 | | 2 | |
Customer Relationships in Place | | 3,235 | | 930 | | 4,165 | |
Goodwill | | 1,905 | | 673 | | 2,578 | |
Accounts Payable | | -118 | | -83 | | -201 | |
Long Term Debt (Auto-Loans) | | -200 | | — | | -200 | |
Short Term Acquisition Payable | | -1,000 | | -250 | | -1,250 | |
| | | | | | | |
Totals: | | 6,016 | | 1,598 | | 7,614 | |
61
Table of Contents
On August 7, 2013 the Company purchased substantially all of the operating assets and certain of the operating liabilities of Hunter Laboratories, Inc., (“Hunter”) a California corporation having its principal place of business in Campbell, California. The gross purchase price was $15,215 plus payroll adjustment of $111 totaling $15,326. Of that amount $3,000 was deferred to cover anticipated pre closing liabilities.
On August 20, 2013 the Company through its subsidiary GeneDx, Inc., purchased the entire membership interest in Edge BioServ, LLC, (“Edge Bio”) a Delaware limited liability company having its place of business in Gaithersburg, Maryland. The gross purchase price was approximately $2,502. Of that $375 was deferred to cover anticipated pre closing liabilities.
The following table sets forth these final purchase allocations.
| | $ | |
| | Hunter | | Edge Bio | | Totals: | |
Accounts Receivable | | 1,700 | | — | | 1,700 | |
Prepaid Expenses | | 421 | | 70 | | 491 | |
Inventory | | 369 | | — | | 369 | |
Autos | | 62 | | — | | 62 | |
Furniture and Fixtures | | 265 | | 6 | | 271 | |
Leasehold Improvements | | — | | 714 | | 714 | |
Medical Equipment | | 234 | | 906 | | 1,140 | |
Computer Equipment | | 376 | | 574 | | 950 | |
Non-Compete Agreement | | 1,255 | | 926 | | 2,181 | |
Customer Relationships in Place | | 3,852 | | 3 | | 3,855 | |
Goodwill | | 8,573 | | 182 | | 8,755 | |
Accounts Payable | | (1,388 | ) | — | | (1,388 | ) |
Accrued Payroll Benefits | | (393 | ) | — | | (393 | ) |
Notes Payable | | — | | (578 | ) | (578 | ) |
Capital Leases | | — | | (193 | ) | (193 | ) |
Deferred Acquisition Payable | | (3,000 | ) | (375 | ) | (3,375 | ) |
Deferred Rent Payable | | — | | (108 | ) | (108 | ) |
| | | | | | | |
Totals: | | 12,326 | | 2,127 | | 14,453 | |
[19] Fair Value of Financial Instruments
For certain financial instruments, including cash and cash equivalents, trade receivables, trade payables, and short-term debt, it was estimated that the carrying amount approximated fair value for the majority of these items because of their short maturities. The fair value of the Company’s long-term debt is estimated based on the quoted market prices for similar issues or by discounting expected cash flows at the rates currently offered to the Company for debt of the same remaining maturities.
Due to the non-interest bearing nature and unspecified payment terms, it was not practicable to estimate the fair value of amounts due from related parties [See also Note 7].
[20] Health Insurance Plan
The Company has a limited self-funded health insurance plan for its employees under which the Company pays the initial $150 of covered medical expenses per person each year. The Company has a contract with an insurance carrier for any excess up to a maximum of $2,000 per person and $20,504 in the aggregate. Health insurance premium expense for the years ended October 31, 2013, 2012 and 2011 amounted to approximately $6,797, $5,792 and $4,426, respectively. Uninsured employee medical expenses incurred by the Company amounted to approximately $32,016, $25,994 and $19,408 for the years ended October 31, 2013, 2012 and 2011, respectively. During fiscal years ended October 31, 2013, 2012 and 2011, employee contributions of $4,713, $4,124 and $3,593 offset the above health plan costs.
[21] Employee Benefit Plan
The Company sponsors a 401(k) Profit-Sharing Plan [the “Plan”]. Employees become eligible for participation after attaining the age of eighteen and completing one year of service. Participants may elect to contribute up to ten percent of their compensation, as defined in the Plan, to a maximum allowed by the Internal Revenue Service. The Company may choose to make a matching contribution to the plan for each participant who has elected to make tax-deferred contributions for the plan year, at a percentage determined each year by the Company. The Company elected to make a matching contribution which amounted to $1,358 for 2013, $1,106 for 2012, and $993 for 2011. These amounts were charged to the Statement of Operations. The Employer contribution will be fully vested after the third year of service.
[22] New Authoritative Accounting Pronouncements
The Company adopted Accounting Standard Update (“ASU”) No. 2011-7: Health Care Entities (Topic 954) — Presentation and Disclosure of Patient Service Revenue, Provision for Bad Debts, and the Allowance for Doubtful Accounts for Certain Health Care Entities commencing with the current fiscal year, the first year such standard is required for the Company. The adoption of this update did not have a material impact on the Company’s financial statements.
62
Table of Contents
Although this update does not have a material impact on the Company’s financial statements as a whole, it requires that we adjust our presentation of our statement of operations along with prior periods presented in this report to maintain comparability. As the result of this change in presentation, our “Net Revenues”, “Gross Profit on Revenues” and our “General and Administrative Expenses” would change while our “Operating Income”, “Net Income” and “Earnings per Share” will remain the same. The presentation is adjusted for a portion of our “Bad Debt Expense” that is now reported in our Net Revenues as required under ASU No. 2011-7.
63
Table of Contents
[23] Selected Quarterly Financial Data [Unaudited]
| | | | | | | | | | Audited | |
| | Three Month Ended | | Fiscal Year | |
| | 1/31/2013 | | 4/30/2013 | | 7/31/2013 | | 10/31/2013 | | 2013 | |
| | | | | | | | | | | |
Net Revenues | | 161,256 | | 176,452 | | 185,427 | | 192,219 | | 715,355 | |
Gross Profit | | 70,922 | | 80,676 | | 85,660 | | 85,282 | | 322,539 | |
Net Income | | 8,665 | | 11,338 | | 14,701 | | 11,120 | | 45,825 | |
Net Income Per Common Share: | | | | | | | | | | | |
| | | | | | | | | | | |
Basic | | 0.31 | | 0.41 | | 0.53 | | 0.40 | | 1.65 | |
Diluted | | 0.31 | | 0.41 | | 0.53 | | 0.40 | | 1.65 | |
| | | | | | | | | | | |
Weighted Average Common Shares Outstanding — Basic [in thousands] | | 27,716 | | 27,698 | | 27,672 | | 27,677 | | 27,691 | |
| | | | | | | | | | | |
Weighted Average Common Shares Outstanding - Diluted [in thousands] | | 27,912 | | 27,879 | | 27,842 | | 27,844 | | 27,851 | |
| | | | | | | | | | Audited | |
| | Three Month Ended | | Fiscal Year | |
| | 1/31/2012 | | 4/30/2012 | | 7/31/2012 | | 10/31/2012 | | 2012 | |
| | | | | | | | | | | |
Net Revenues | | $ | 138,793 | | $ | 151,443 | | $ | 160,532 | | $ | 163,487 | | $ | 614,255 | |
Gross Profit | | $ | 60,118 | | $ | 67,534 | | $ | 74,279 | | $ | 74,681 | | $ | 276,611 | |
Net Income | | $ | 7,365 | | $ | 9,306 | | $ | 12,596 | | $ | 12,889 | | $ | 42,156 | |
Net Income Per Common Share: | | | | | | | | | | | |
| | | | | | | | | | | |
Basic | | $ | 0.26 | | $ | 0.34 | | $ | 0.45 | | $ | 0.47 | | $ | 1.52 | |
Diluted | | $ | 0.26 | | $ | 0.33 | | $ | 0.45 | | $ | 0.46 | | $ | 1.51 | |
| | | | | | | | | | | |
Weighted Average Common Shares Outstanding — Basic [in thousands] | | 27,888 | | 27,685 | | 27,695 | | 27,705 | | 27,742 | |
| | | | | | | | | | | |
Weighted Average Common Shares Outstanding - Diluted [in thousands] | | 28,041 | | 27,878 | | 27,888 | | 27,906 | | 27,921 | |
| | | | Audited | |
| | Three Month Ended | | Fiscal Year | |
| | 1/31/2011 | | 4/30/2011 | | 7/31/2011 | | 10/31/2011 | | 2011 | |
| | | | | | | | | | | |
Net Revenues | | $ | 114,129 | | $ | 129,465 | | $ | 137,802 | | $ | 140,684 | | $ | 522,081 | |
Gross Profit | | $ | 49,276 | | $ | 57,447 | | $ | 63,206 | | $ | 64,300 | | $ | 234,229 | |
Net Income | | $ | 7,985 | | $ | 7,817 | | $ | 10,081 | | $ | 10,476 | | $ | 36,359 | |
Net Income Per Common Share: | | | | | | | | | | | |
| | | | | | | | | | | |
Basic | | $ | 0.29 | | $ | 0.28 | | $ | 0.36 | | $ | 0.37 | | $ | 1.30 | |
Diluted | | $ | 0.28 | | $ | 0.28 | | $ | 0.36 | | $ | 0.37 | | $ | 1.29 | |
| | | | | | | | | | | |
Weighted Average Common Shares Outstanding — Basic [in thousands] | | 27,884 | | 27,920 | | 27,941 | | 27,949 | | 27,971 | |
| | | | | | | | | | | |
Weighted Average Common Shares Outstanding - Diluted [in thousands] | | 28,122 | | 28,142 | | 28,147 | | 28,138 | | 28,207 | |
64
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
Bio-Reference Laboratories, Inc.
Elmwood Park, New Jersey
Our report on our audit of the basic consolidated financial statements of Bio-Reference Laboratories, Inc. and its subsidiaries appears earlier in this document. That audit was conducted for the purpose of forming an opinion on the consolidated basic financial statements taken as a whole. The supplemental schedule II is presented for purposes of complying with the Securities and Exchange Commission’s Rules and Regulations under the Securities Exchange Act of 1934 and is not otherwise a required part of the basic consolidated financial statements. Such information has been subjected to the auditing procedures applied in the audit of the basic consolidated financial statements, and in our opinion, is fairly stated in all material respects in relation to the basic consolidated financial statements taken as a whole.
| MSPC |
| Certified Public Accountants and Advisors, |
| A Professional Corporation |
Cranford, New Jersey
January 10, 2014
65
Table of Contents
BIO-REFERENCE LABORATORIES, INC. AND SUBSIDIARIES
SCHEDULE II - VALUATION AND QUALIFYING ACCOUNTS FOR THE YEARS ENDED OCTOBER 31, 2013, 2012 AND 2011
[In Thousands]
| | (b) | | (c) | | (d) | | (e) | |
(a)
Description | | Balance at the
Beginning of a Period | | Charged to Cost and
Expenses | | Deductions Charged to Valuation
Allowance Accounts | | Balance at the End
of a Period | |
Year Ended October 31, 2013 | | | | | | | | | |
Allowance for Doubtful Accounts | | 51,274 | | 119,161 | | (81,174 | ) | 89,261 | |
Contractual Credits/Discounts | | 267,921 | | 2,748,510 | | (2,674,134 | ) | 342,297 | |
| | | | | | | | | |
Total Allowance | | 319,195 | | 2,867,671 | | (2,755,308 | ) | 431,558 | |
| | | | | | | | | |
Year Ended October 31, 2012 | | | | | | | | | |
Allowance for Doubtful Accounts | | 45,220 | | 89,396 | | (83,342 | ) | 51,274 | |
Contractual Credits/Discounts | | 235,922 | | 2,390,770 | | (2,358,771 | ) | 267,921 | |
| | | | | | | | | |
Total Allowance | | 281,142 | | 2,480,166 | | (2,442,113 | ) | 319,195 | |
| | | | | | | | | |
Year Ended October 31, 2011 | | | | | | | | | |
Allowance for Doubtful Accounts | | 34,904 | | 75,079 | | (64,763 | ) | 45,220 | |
Contractual Credits/Discounts | | 186,372 | | 1,926,164 | | (1,876,614 | ) | 235,922 | |
| | | | | | | | | |
Total Allowance | | 221,276 | | 2,001,243 | | (1,941,377 | ) | 281,142 | |
66
