UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
(Mark One)
| x | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended June 30, 2007
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 (NO FEE REQUIRED) |
For the transition period from to
Commission File Number 0-27558

CYTYC CORPORATION
(Exact name of registrant as specified in its charter)
| | |
| DELAWARE | | 02-0407755 |
(State or other jurisdiction of incorporation or organization) | | (I.R.S. Employer Identification No.) |
250 Campus Drive, Marlborough, MA 01752
(Address of principal executive offices, including Zip Code)
(508) 263-2900
(Registrant’s telephone number, including area code)
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer x Accelerated filer ¨ Non-accelerated filer ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
Indicate the number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date: The number of shares of the issuer’s Common Stock, $0.01 par value per share, outstanding as of July 27, 2007 was 116,676,670.
Total Number of Pages: 45
Exhibit index located on page 45
CYTYC CORPORATION
INDEX TO FORM 10-Q
2
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Cytyc Corporation
250 Campus Drive
Marlborough, Massachusetts
We have reviewed the accompanying condensed consolidated balance sheet of Cytyc Corporation and subsidiaries (the “Company”) as of June 30, 2007, and the related condensed consolidated statements of income (loss) for the three-month and six-month periods ended June 30, 2007 and 2006, and of cash flows for the six-month periods ended June 30, 2007 and 2006. These interim financial statements are the responsibility of the Company’s management.
We conducted our reviews in accordance with the standards of the Public Company Accounting Oversight Board (United States). A review of interim financial information consists principally of applying analytical procedures and making inquiries of persons responsible for financial and accounting matters. It is substantially less in scope than an audit conducted in accordance with standards of the Public Company Accounting Oversight Board (United States), the objective of which is the expression of an opinion regarding the financial statements taken as a whole. Accordingly, we do not express such an opinion.
Based on our reviews, we are not aware of any material modifications that should be made to such condensed consolidated interim financial statements for them to be in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 13 to the condensed consolidated financial statements, the Company adopted FASB Interpretation No. 48,Accounting for Uncertainty in Income Taxes,on January 1, 2007.
We have previously audited, in accordance with standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheet of the Company as of December 31, 2006, and the related consolidated statements of income, stockholders’ equity, and cash flows for the year then ended (not presented herein); and in our report dated March 1, 2007 (May 18, 2007 as to the effects of the restatement discussed in Note 15) we expressed an unqualified opinion and included explanatory paragraphs relating to the restatement of the Company’s consolidated financial statements and the adoption of Statement of Financial Standards No. 123(R),Share-Based Payment on January 1, 2006. In our opinion, the information set forth in the accompanying condensed consolidated balance sheet as of December 31, 2006 is fairly stated, in all material respects, in relation to the consolidated balance sheet from which it has been derived.
/s/ Deloitte & Touche LLP
Boston, Massachusetts
August 8, 2007
3
Part I FINANCIAL INFORMATION
Item 1. Condensed Consolidated Financial Statements
CYTYC CORPORATION
CONDENSED CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share amounts)
(unaudited)
| | | | | | | | |
| | | June 30,
2007 | | | December 31,
2006 | |
| ASSETS | | | | | | | | |
Current assets: | | | | | | | | |
Cash and cash equivalents | | $ | 31,268 | | | $ | 140,680 | |
Investment securities | | | 2,055 | | | | 157,030 | |
Accounts receivable, net of allowance of $1,787 and $1,951 at June 30, 2007 and December 31, 2006, respectively | | | 113,742 | | | | 94,943 | |
Inventories, net | | | 32,653 | | | | 29,503 | |
Deferred tax assets, net | | | 21,869 | | | | 9,065 | |
Prepaid expenses and other current assets | | | 18,092 | | | | 5,932 | |
| | | | | | | | |
Total current assets | | | 219,679 | | | | 437,153 | |
| | | | | | | | |
Property and equipment, net | | | 161,001 | | | | 149,007 | |
| | | | | | | | |
Intangible assets: | | | | | | | | |
Patents and developed technology, net of accumulated amortization of $32,682 and $23,914 at June 30, 2007 and December 31, 2006, respectively | | | 316,959 | | | | 182,477 | |
Goodwill | | | 595,100 | | | | 386,533 | |
| | | | | | | | |
Total intangible assets | | | 912,059 | | | | 569,010 | |
| | | | | | | | |
Other assets, net | | | 10,079 | | | | 9,544 | |
| | | | | | | | |
Total assets | | $ | 1,302,818 | | | $ | 1,164,714 | |
| | | | | | | | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | | | | | | |
Current liabilities: | | | | | | | | |
Accounts payable | | $ | 13,766 | | | $ | 12,514 | |
Accrued expenses | | | 63,336 | | | | 56,688 | |
Deferred revenue | | | 5,523 | | | | 4,935 | |
Line-of-credit | | | 55,244 | | | | — | |
| | | | | | | | |
Total current liabilities | | | 137,869 | | | | 74,137 | |
| | | | | | | | |
Deferred tax liabilities, net | | | 92,570 | | | | 64,145 | |
| | |
Convertible debt | | | 250,000 | | | | 250,000 | |
| | |
Other non-current liabilities | | | 31,040 | | | | 17,459 | |
| | |
Commitments and contingencies (Note 12) | | | | | | | | |
| | |
Stockholders’ equity: | | | | | | | | |
Preferred stock, $0.01 par value—Authorized—5,000,000 shares No shares issued or outstanding | | | — | | | | — | |
Common stock, $0.01 par value—Authorized—400,000,000 shares Issued— 138,733,815 and 136,305,345 shares in 2007 and 2006, respectively Outstanding— 116,581,045 and 114,726,133 shares in 2007 and 2006, respectively | | | 1,387 | | | | 1,363 | |
Additional paid-in capital | | | 739,952 | | | | 673,168 | |
Treasury stock, at cost: 22,152,770 and 21,579,212 shares in 2007 and 2006, respectively | | | (332,607 | ) | | | (316,153 | ) |
Accumulated other comprehensive income | | | 3,130 | | | | 2,956 | |
Retained earnings | | | 379,477 | | | | 397,639 | |
| | | | | | | | |
Total stockholders’ equity | | | 791,339 | | | | 758,973 | |
| | | | | | | | |
Total liabilities and stockholders’ equity | | $ | 1,302,818 | | | $ | 1,164,714 | |
| | | | | | | | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
4
CYTYC CORPORATION
CONDENSED CONSOLIDATED STATEMENTS OF INCOME (LOSS)
(in thousands, except per share data)
(unaudited)
| | | | | | | | | | | | | | | | |
| | | Three Months Ended
June 30, | | | Six Months Ended
June 30, | |
| | | 2007 | | | 2006 | | | 2007 | | | 2006 | |
Net sales | | $ | 188,837 | | | $ | 150,397 | | | $ | 357,721 | | | $ | 290,937 | |
Cost of sales | | | 48,283 | | | | 33,063 | | | | 90,379 | | | | 62,852 | |
| | | | | | | | | | | | | | | | |
Gross profit | | | 140,554 | | | | 117,334 | | | | 267,342 | | | | 228,085 | |
| | | | | | | | | | | | | | | | |
Operating expenses: | | | | | | | | | | | | | | | | |
Research and development | | | 11,371 | | | | 10,681 | | | | 21,063 | | | | 20,992 | |
Sales and marketing | | | 48,183 | | | | 41,989 | | | | 89,352 | | | | 82,122 | |
General and administrative | | | 27,042 | | | | 14,809 | | | | 46,490 | | | | 28,834 | |
In-process research and development | | | — | | | | — | | | | 89,500 | | | | — | |
| | | | | | | | | | | | | | | | |
Total operating expenses | | | 86,596 | | | | 67,479 | | | | 246,405 | | | | 131,948 | |
| | | | | | | | | | | | | | | | |
Income from operations | | | 53,958 | | | | 49,855 | | | | 20,937 | | | | 96,137 | |
Other (expense) income, net: | | | | | | | | | | | | | | | | |
Interest income | | | 713 | | | | 1,826 | | | | 3,342 | | | | 3,656 | |
Interest expense | | | (3,765 | ) | | | (1,792 | ) | | | (6,159 | ) | | | (3,584 | ) |
Other | | | (11 | ) | | | — | | | | 761 | | | | (77 | ) |
| | | | | | | | | | | | | | | | |
Total other (expense) income, net | | | (3,063 | ) | | | 34 | | | | (2,056 | ) | | | (5 | ) |
| | | | | | | | | | | | | | | | |
Income before provision for income taxes | | | 50,895 | | | | 49,889 | | | | 18,881 | | | | 96,132 | |
Provision for income taxes | | | 17,355 | | | | 18,209 | | | | 36,550 | | | | 35,088 | |
| | | | | | | | | | | | | | | | |
Net income (loss) | | $ | 33,540 | | | $ | 31,680 | | | $ | (17,669 | ) | | $ | 61,044 | |
| | | | | | | | | | | | | | | | |
Net income (loss) per common share and potential common share: | | | | | | | | | | | | | | | | |
Basic | | $ | 0.29 | | | $ | 0.28 | | | $ | (0.15 | ) | | $ | 0.53 | |
| | | | | | | | | | | | | | | | |
Diluted | | $ | 0.27 | | | $ | 0.27 | | | $ | (0.15 | ) | | $ | 0.51 | |
| | | | | | | | | | | | | | | | |
Weighted average common and potential common shares outstanding: | | | | | | | | | | | | | | | | |
Basic | | | 115,656 | | | | 114,356 | | | | 115,197 | | | | 114,917 | |
Diluted | | | 127,398 | | | | 123,582 | | | | 115,197 | | | | 125,014 | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
5
CYTYC CORPORATION
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
(unaudited)
| | | | | | | | |
| | | Six Months Ended
June 30, | |
| | | 2007 | | | 2006 | |
Cash flows from operating activities: | | | | | | | | |
Net (loss) income | | $ | (17,669 | ) | | $ | 61,044 | |
| | |
Adjustments to reconcile net (loss) income to net cash provided by operating activities: | | | | | | | | |
| | |
Revenue relating to license issued in exchange for preferred stock | | | — | | | | (1,933 | ) |
Stock-based compensation expense | | | 9,190 | | | | 11,763 | |
Compensation expense related to issuance of stock to directors and executives | | | 220 | | | | 395 | |
Depreciation and amortization of property and equipment | | | 17,119 | | | | 10,820 | |
Amortization of intangible assets | | | 8,768 | | | | 4,904 | |
Amortization of deferred financing costs | | | 930 | | | | 772 | |
Acquired in-process research and development | | | 89,500 | | | | — | |
Gain on equity investments | | | (496 | ) | | | — | |
Loss on disposal of fixed assets | | | 947 | | | | 91 | |
(Benefit) provision for doubtful accounts | | | (43 | ) | | | 425 | |
Tax benefit from exercise of stock options and employee stock purchase plan | | | 4,770 | | | | 1,682 | |
Deferred tax expense | | | 8,599 | | | | 564 | |
Changes in assets and liabilities, excluding effects of acquisitions: | | | | | | | | |
Accounts receivable | | | (9,878 | ) | | | (2,630 | ) |
Inventories | | | (2,070 | ) | | | (2,374 | ) |
Prepaid expenses and other current assets | | | (12,120 | ) | | | 1,021 | |
Accounts payable | | | (1,144 | ) | | | 1,149 | |
Accrued expenses | | | (12,056 | ) | | | (16,747 | ) |
Deferred revenue | | | 506 | | | | 1,661 | |
| | | | | | | | |
Net cash provided by operating activities | | | 85,073 | | | | 72,607 | |
| | | | | | | | |
Cash flows from investing activities: | | | | | | | | |
Acquisition of Adeza Biomedical Corporation, net of cash acquired | | | (427,697 | ) | | | — | |
Acquisition of Adiana, Inc., net of cash acquired | | | (58,385 | ) | | | — | |
Acquisition of Proxima Therapeutics, Inc. | | | (3,613 | ) | | | (21,074 | ) |
Increase in other assets | | | (291 | ) | | | (981 | ) |
Increase in equipment under customer usage agreements | | | (12,400 | ) | | | (13,458 | ) |
Purchases of property and equipment | | | (9,479 | ) | | | (8,624 | ) |
Increase in patents and developed technology | | | (250 | ) | | | (473 | ) |
Purchases of investment securities | | | (130,544 | ) | | | (59,567 | ) |
Proceeds from sales and maturities of investment securities | | | 357,568 | | | | 66,973 | |
| | | | | | | | |
Net cash used in investing activities | | | (285,091 | ) | | | (37,204 | ) |
| | | | | | | | |
| Cash flows from financing activities: | | | | | | | | |
Net proceeds from line-of-credit | | | 55,015 | | | | (609 | ) |
Purchase of treasury shares | | | (16,454 | ) | | | (78,577 | ) |
Proceeds from issuance of shares under employee stock purchase plan | | | 2,253 | | | | 2,029 | |
Proceeds from exercise of stock options | | | 41,793 | | | | 16,787 | |
Excess tax benefit from exercise of stock options and employee stock purchase plan | | | 8,582 | | | | 1,386 | |
| | | | | | | | |
Net cash provided by (used in) financing activities | | | 91,189 | | | | (58,984 | ) |
| | | | | | | | |
Effect of exchange rate changes on cash | | | (583 | ) | | | 145 | |
| | | | | | | | |
Net decrease in cash and cash equivalents | | | (109,412 | ) | | | (23,436 | ) |
Cash and cash equivalents, beginning of period | | | 140,680 | | | | 123,468 | |
| | | | | | | | |
Cash and cash equivalents, end of period | | $ | 31,268 | | | $ | 100,032 | |
| | | | | | | | |
The accompanying notes are an integral part of these condensed consolidated financial statements.
6
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited)
The accompanying condensed consolidated financial statements of Cytyc Corporation and subsidiaries (the “Company” or “Cytyc”) have been prepared pursuant to the rules and regulations of the Securities and Exchange Commission (the “SEC”). Certain information and footnote disclosures normally included in financial statements prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) have been condensed or omitted pursuant to such rules and regulations. These financial statements should be read in conjunction with the financial statements and notes included in Amendment No. 2 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006.
The notes and accompanying condensed consolidated financial statements are unaudited. The information furnished reflects all adjustments, which, in the opinion of management, are necessary for a fair presentation of results for the interim periods. Such adjustments consisted only of normal recurring items. The interim periods are not necessarily indicative of the results expected for the full year or any future period.
The preparation of these condensed consolidated financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of net sales and expenses during the reporting period. Actual results could differ from those estimates.
| (2) | Plan of Merger with Hologic, Inc. |
On May 20, 2007, the Company entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Hologic, Inc., a Delaware corporation (“Hologic”), and Nor’easter Corp. (“MergerSub”), a Delaware corporation and wholly-owned subsidiary of Hologic. The Merger Agreement provides that, upon the terms and subject to the conditions set forth in the Merger Agreement, the Company will merge with and into MergerSub, with MergerSub continuing as the surviving corporation under the name “Cytyc Corporation” and as a wholly-owned subsidiary of Hologic.
Subject to the terms and conditions of the Merger Agreement, which has been unanimously approved by the boards of directors of both companies, at the effective time and as a result of the merger, each share of the Company’s common stock, par value $0.01 per share, issued and outstanding immediately prior to the effective time of the merger will be cancelled and converted into the right to receive a combination of (i) 0.52 of a share of common stock of Hologic, par value $0.01 per share, and (ii) $16.50 in cash without interest. The combined company will not issue any fractional shares of Hologic common stock in the merger. Instead, the Company’s stockholders will receive cash in lieu of any fractional shares of Hologic common stock that they would have otherwise received in the merger. Based on Hologic’s closing stock price (as reported on the NASDAQ Global Select Market) of $57.61 per share on May 18, 2007 (the last trading day prior to the public announcement of the transaction), the transaction represents a value of $46.46 per share of the Company’s common stock, or a total consideration of approximately $6.2 billion. Under the Merger Agreement, the Company’s stockholders will receive an aggregate of an estimated 69 million shares of Hologic common stock and $2.2 billion in cash, assuming the conversion of the Company’s outstanding convertible notes.
The market prices of Hologic common stock and the Company’s common stock likely will fluctuate between the date of the Merger Agreement and the completion of the merger. No assurance can be given concerning the market prices of Hologic common stock or the Company’s common stock before the completion of the merger or the market price of Hologic common stock after the completion of the merger. The merger consideration is fixed in the Merger Agreement and will not be adjusted for changes in the market value of the common stock of Hologic or the Company.
The Merger Agreement contains customary representations, warranties and covenants of the Company and Hologic, including, among others, covenants (i) to conduct their respective businesses in the ordinary course consistent with past practice during the interim period between the date of the execution of the Merger Agreement and the consummation of the merger, (ii) not to engage in certain kinds of transactions during such period, (iii) to use commercially reasonable efforts to convene and hold a meeting of their respective stockholders to consider and vote upon the approval of the transaction and (iv) that, subject to certain exceptions, the boards of directors of Hologic and the Company will each recommend that their respective stockholders approve the transaction. Each party has also agreed not to (a) solicit proposals relating to alternative business combination transactions or (b) subject to certain exceptions, enter into discussions or an agreement concerning or provide confidential information in connection with any proposals for alternative business combination transactions.
Consummation of the merger is subject to customary conditions, including (i) approval of the transaction by the common stockholders of both Hologic and the Company in accordance with Delaware law and the requirements of the NASDAQ Stock Market, (ii) absence of any applicable law prohibiting the merger, (iii) expiration or termination of the Hart-Scott-Rodino Act waiting
7
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
period and certain other regulatory approvals, (iv) subject to certain exceptions, the accuracy of the representations and warranties of each party, (v) performance in all material respects of each party of its obligations under the Merger Agreement and (vi) the delivery of customary opinions from counsel to Hologic and counsel to the Company to the effect that the receipt of stock merger consideration by the Company’s stockholders will be a tax-free reorganization for federal income tax purposes, subject to the exceptions provided therein.
Under the Merger Agreement, upon completion of the merger, John W. Cumming, Chief Executive Officer of Hologic, will become Chief Executive Officer of the combined company, and Patrick J. Sullivan, Chairman, Chief Executive Officer and President of the Company, will become Chairman of the board of directors of the combined company. The combined company’s board of directors will be comprised of 11 members, with six nominated by Hologic and five nominated by the Company.
The Merger Agreement contains certain termination rights for both Hologic and the Company and further provides that, upon termination of the Merger Agreement under specified circumstances, the Company may be required to pay Hologic a termination fee of $50 million or $150 million or Hologic may be required to pay the Company a termination fee of $33 million or $100 million, in each case depending on the termination event.
The accompanying condensed consolidated financial statements have been prepared assuming the Company continues on a stand-alone basis and do not reflect any adjustments or disclosures that may be required upon consummation of the merger. Refer to the Registration Statement on Form S-4 (File No. 333- 144238), as may be amended from time to time, filed by Hologic with the SEC, for a more complete description of the merger and related agreements. The merger is expected to close in late September or early October of 2007.
| | (a) | Acquisition of Adeza Biomedical Corporation |
On February 11, 2007, the Company entered into an Agreement and Plan of Merger (“Plan of Merger”) with Augusta Medical Corporation, a Delaware corporation and a newly-formed wholly-owned subsidiary of Cytyc (the “Purchaser”), and Adeza Biomedical Corporation, a Delaware corporation (“Adeza”). Pursuant to the Plan of Merger, the Purchaser commenced a cash tender offer to purchase all outstanding shares of Adeza’s common stock (the “Shares”) in exchange for $24.00 per share in cash (the “Offer Price”). On March 19, 2007, the Purchaser acquired a majority of Adeza’s outstanding Shares through the tender offer. On April 2, 2007, pursuant to the terms of the Plan of Merger, Cytyc acquired Adeza through the merger (the “Merger”) of Purchaser with and into Adeza. The Merger was consummated without a meeting of the stockholders of Adeza in accordance with the Delaware General Corporation Law. In connection with the Merger, Adeza’s name was changed from “Adeza Biomedical Corporation” to “Cytyc Prenatal Products Corp.” As a result of the Merger, all remaining outstanding Shares were converted into the right to receive $24.00 per share in cash, without interest, other than Shares held by Cytyc or any of its subsidiaries or Shares held by Adeza stockholders that perfect their rights to appraisal in accordance with the Delaware General Corporation Law. The purchase price was paid out of the Company’s existing cash and the Company’s existing credit facility.
The aggregate purchase price for Adeza was $457.3 million, of which $452.3 million represented cash payable to Adeza shareholders and $5.0 million represented acquisition-related fees and expenses. As of June 30, 2007, the Company paid $427.7 million of the purchase price (net of $26.3 million of cash acquired) and has $3.3 million accrued related to the Adeza acquisition. The acquisition was accounted for as a purchase in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 141, Business Combinations, and accordingly, the results of operations of Adeza were included in the consolidated statements of income (loss) from the date of acquisition of March 19, 2007. The purchase price was supported by estimates of future sales and earnings of Adeza, as well as the value of sales force and other projected synergies.
8
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
Purchase Price Allocation
The following table summarizes the preliminary fair values of the assets acquired and liabilities assumed at the date of acquisition (“Original Amount”), for an aggregate purchase price of $457.3 million, including acquisition costs:
| | | | | | | | | | | | |
| | | Original
Amount | | | Adjustments | | | Amount as
of June 30,
2007 | |
| | | (in thousands) | |
Cash and cash equivalents | | $ | 26,346 | | | $ | — | | | $ | 26,346 | |
Investment securities | | | 72,019 | | | | — | | | | 72,019 | |
Other current assets | | | 10,865 | | | | — | | | | 10,865 | |
Property and equipment | | | 431 | | | | — | | | | 431 | |
Patents and developed technology | | | 142,800 | | | | 200 | | | | 143,000 | |
Goodwill | | | 208,451 | | | | 83 | | | | 208,534 | |
Other assets | | | 72 | | | | — | | | | 72 | |
Current liabilities | | | (19,676 | ) | | | 429 | | | | (19,247 | ) |
Non-current liabilities | | | (1,251 | ) | | | — | | | | (1,251 | ) |
Deferred tax liabilities, net | | | (26,881 | ) | | | (80 | ) | | | (26,961 | ) |
In-process research and development | | | 43,500 | | | | — | | | | 43,500 | |
| | | | | | | | | | | | |
| | $ | 456,676 | | | $ | 632 | | | $ | 457,308 | |
| | | | | | | | | | | | |
As part of the purchase price allocation, all tangible and intangible assets and liabilities were identified and valued. Of the total purchase price, the Company allocated $62.3 million to acquired net tangible assets. The net deferred tax liabilities of $27.0 million are primarily comprised of deferred tax liabilities of $57.2 million relating to patents and developed technology, offset by $30.2 million of deferred tax assets relating to acquired net operating losses primarily related to net operating loss carry forwards and tax credits. The Company determined the fair value of Adeza’s tangible assets and liabilities based on a review of Adeza’s historical and then current financial statements and an understanding of the ongoing nature of the assets and liabilities.
The Company also allocated $143.0 million to the acquired patents and developed technology. The acquired patents expire at various dates through 2018. Based on the average life of the patent portfolio, the remaining economic life of the developed technology is expected to be approximately 12-15 years. The Company is amortizing the patents and developed technology over this period using the cash flow method, under which amortization is calculated and recognized based upon the Company’s estimated net cash flows over the life of the intangible asset, reflecting the pattern in which the economic benefits of the intangible asset are consumed in accordance with SFAS No. 142,Goodwill and Other Intangible Assets. The Company believes the patents provide sufficient coverage for differentiated products to sustain some competitive advantage in the marketplace over the average remaining life of the patents.
The Company also allocated $43.5 million of the purchase price to in-process research and development projects. This allocation represents the estimated fair value based on risk-adjusted cash flows related to the incomplete research and development activities. The incomplete research and development activities primarily were associated with Adeza’s development of Gestiva™, a therapeutic drug, which is pending final approval from the Food and Drug Administration (“FDA”), as well as an induction of labor diagnostic product. The acquired in-process research and development was charged to expense as of the date of the acquisition and is included in the Company’s statement of loss for the six months ended June 30, 2007.
The Company valued the intangible assets acquired, including the portfolio of patents and technologies, and the in-process research and development projects, based on present value calculations of income using risk-adjusted cash flows for each product, an analysis of project accomplishments and remaining outstanding items, an assessment of overall contributions, as well as project risks. The value assigned to the in-process research and development projects was determined by estimating the costs to develop the acquired technology into commercially viable products, estimating the net cash flows resulting from the projects, and discounting the net cash flows to their present value. The Company expects to continue its efforts on the in-process research and development projects through the end of 2008.
The projections used to value the acquired intangible assets and the in-process research and development projects were based on estimates of relevant market sizes and growth factors, expected trends in technology, and the nature and expected timing of new product introductions by the Company and its competitors, as well as estimates of cost of sales, operating expenses, and income taxes resulting from the acquired products and the in-process research and development projects. In addition, the projections reflect the Company’s expectation that improvements to the current products will continue to be made over the life cycle of the product lines. As a result, the completed technology that existed as of the acquisition date was assumed to represent a declining percentage of the products’ technological composition over time.
9
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
The rate utilized to discount the net cash flows to their present value was based on estimated weighted-average cost of capital calculations. A discount rate of 14% was used to value the acquired intangible assets and the in-process research and development projects. This discount rate was higher than the Company’s weighted-average cost of capital due to the earlier-stage life cycles of the acquired products, the inherent uncertainties surrounding the outcome of the projects and commercialization and development of the acquired intangible assets, the useful life of the acquired technology, the profitability levels of such technology, and the uncertainty of technological advances that were unknown at that time.
The adjustments recorded during the three months ended June 30, 2007 relate primarily to the finalization of the valuation of the acquired intangible assets, as well as adjustments to goodwill resulting from changes in the original severance accrual recorded. The allocation of the purchase price is substantially complete, with the remaining allocation to be completed primarily related to the resolution of tax matters, which the Company expects to record as adjustments to goodwill and deferred taxes.
Goodwill
The excess of the purchase price over the fair value of tangible and identifiable intangible net assets, as well as the in-process research and development projects, was allocated to goodwill, which is non-deductible for tax purposes and totaled $208.5 million. In accordance with SFAS No. 142, this goodwill will not be systematically amortized. Instead, the Company will perform an annual assessment for impairment by applying a fair-value-based test.
| | (b) | Acquisition of Adiana Inc. |
On March 16, 2007, the Company acquired Adiana, Inc. (“Adiana”), a privately-held company located in Redwood City, California, in a non-taxable transaction. As a result of the acquisition of Adiana, all of its fully-diluted equity immediately prior to the acquisition was automatically converted into the right to receive an initial cash payment of $60 million, plus milestone payments. The milestone payments include (i) payment of up to $25 million tied to the timing of certain FDA milestone achievements of the Adiana permanent contraception product and (ii) potential contingent payments tied to future revenue performance milestones. The contingent payments are based on incremental sales growth of the Adiana permanent contraception product during the four-year period following FDA approval of this product, are subject to an aggregate cap of $130 million, and will be recorded as additional goodwill when paid, if at all. No payments can be earned after December 31, 2012. According to the terms of the related merger agreement, total payments, including the initial cash payment, the potential FDA milestone payment and the four-year contingent payments, will not exceed $215 million.
The initial purchase price payment was paid out of the Company’s existing cash. Pursuant to the merger agreement, $3.0 million of the purchase price was placed in escrow to satisfy potential claims. The purchase price was supported by estimates of future sales and earnings of Adiana, as well as other projected synergies.
The aggregate purchase price for Adiana was $60.5 million, of which $59.4 million represented cash payable to Adiana shareholders and $1.1 million represented acquisition-related fees and expenses. As of June 30, 2007, the Company paid $58.4 million of the purchase price (net of $1.9 million of cash acquired) and has $0.2 million accrued related to the Adiana acquisition. The acquisition was accounted for as a purchase in accordance with SFAS No. 141, and accordingly, the results of operations of Adiana were included in the consolidated statement of loss from the date of acquisition.
Purchase Price Allocation
The following table summarizes the preliminary fair values of the assets acquired and liabilities assumed at the date of acquisition for an aggregate purchase price of $60.5 million, including acquisition costs:
10
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
| | | | |
| | | Amount (in thousands) | |
Cash and cash equivalents | | $ | 1,931 | |
Other current assets | | | 210 | |
Property and equipment | | | 151 | |
Other assets | | | 18 | |
Deferred tax assets, net | | | 19,973 | |
Current liabilities | | | (4,412 | ) |
Non-current liabilities | | | (3,401 | ) |
In-process research and development | | | 46,000 | |
| | | | |
| | $ | 60,470 | |
| | | | |
As part of the purchase price allocation, all tangible assets and liabilities were identified and valued. Of the total purchase price, the Company allocated $14.5 million to acquired net tangible assets. The net deferred tax assets of $20.0 million primarily relate to acquired net operating losses and tax credits. The Company determined the fair value of Adiana’s tangible assets and liabilities based on a review of Adiana’s historical and then current financial statements and an understanding of the ongoing nature of the assets and liabilities.
The Company also allocated $46.0 million of the purchase price to an in-process research and development project. This allocation represents the estimated fair value based on risk-adjusted cash flows related to the incomplete research and development activities. The incomplete research and development activities are associated with the Adiana Complete Transcervical Sterilization (“TCS”) System®, a form of permanent female contraception intended as an alternative to tubal ligation, and primarily relate to activities to be performed in order to obtain FDA approval. The acquired in-process research and development was charged to expense as of the date of the acquisition and included in the Company’s statement of loss for the six months ended June 30, 2007.
The Company valued the in-process research and development project based on present value calculations of income using risk-adjusted cash flows, an analysis of project accomplishments and remaining outstanding items, an assessment of overall contributions, as well as project risks. The value assigned to the in-process research and development project was determined by estimating the costs to develop the acquired technology into a commercially viable product, estimating the net cash flows resulting from the project, and discounting the net cash flows to their present value. The Company expects to complete its efforts on the in-process research and development project in 2008.
The projections used to value the in-process research and development project were based on estimates of relevant market sizes and growth factors, expected trends in technology, and the nature and expected timing of new product introductions by the Company and its competitors, as well as estimates of cost of sales, operating expenses, and income taxes resulting from the in-process research and development project.
The rate utilized to discount the net cash flows to their present value was based on estimated weighted-average cost of capital calculations. A discount rate of 26% was used to value the in-process research and development project. The discount rate was higher than the Company’s weighted-average cost of capital due to the early-stage life cycle of the in-process research and development project, the inherent uncertainties surrounding the commercialization and development of TCS, the useful life of the future product, the profitability levels of such technology, and the uncertainty of technological advances that were unknown at that time.
The excess of the fair value of tangible net assets over the purchase price of $3.2 million was allocated to expected contingent earn-out payments related to future milestones in accordance with SFAS No. 142 and recorded in current liabilities.
The initial allocation of the purchase price is substantially complete, with the remaining allocation to be completed primarily related to the resolution of tax matters, which the Company expects to record as adjustments to goodwill and deferred taxes.
The following unaudited pro forma financial information for the three and six months ended June 30, 2007 presents the combined results of operations of Cytyc, Adeza and Adiana as if the acquisitions had occurred as of January 1, 2007. The following unaudited pro forma financial information for the three and six months ended June 30, 2006 presents the combined results of operations of Cytyc, Adeza and Adiana as if the acquisitions had occurred as of January 1, 2006.
11
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
Pro forma results for the three and six months ended June 30, 2007 and 2006 are as follows:
| | | | | | | | | | | | |
| | | Three Months Ended | | Six Months Ended |
| | | June 30, | | June 30, |
| | | 2007 | | 2006 | | 2007 | | 2006 |
| | | (in thousands, except per share data) |
Net sales | | $ | 188,837 | | $ | 163,426 | | $ | 368,582 | | $ | 314,759 |
Net income | | $ | 33,540 | | $ | 26,604 | | $ | 58,857 | | $ | 50,297 |
| | | | |
Basic | | $ | 0.29 | | $ | 0.23 | | $ | 0.51 | | $ | 0.44 |
| | | | | | | | | | | | |
Diluted | | $ | 0.27 | | $ | 0.22 | | $ | 0.49 | | $ | 0.42 |
| | | | | | | | | | | | |
All periods presented include pro forma adjustments to reflect interest costs related to the borrowings on the line-of-credit facility (see Note 12). The pro forma results for the six months ended June 30, 2007 include $10.5 million of transaction fees and expenses incurred by Adeza and Adiana prior to the acquisitions related to the merger, but exclude $89.5 million of in-process research and development costs. The pro forma adjustments are based upon available information and certain assumptions that management believes are reasonable. The unaudited pro forma financial information is not intended to represent or be indicative of the consolidated results of operations or financial condition of the Company that would have been reported had the acquisition been completed as of the dates presented.
| (4) | Amortization of Intangible Assets |
Amortization expense related to identifiable intangible assets, which consists of the Company’s acquired patents and developed technology, was approximately $5.2 million and $2.5 million for the three months ended June 30, 2007 and 2006, respectively, and approximately $8.8 million and $4.9 million for the six months ended June 30, 2007 and 2006, respectively (see Note 11 for amortization expense by operating segment). Prior to 2007, the Company allocated amortization of all of its intangible assets to research and development within its condensed consolidated statements of income as the developed technology has benefited multiple line items (i.e., cost of sales and research and development) and any allocation that would have been made to cost of sales was not deemed to be material. During 2007, the Company acquired a significant amount of developed technology ($143.0 million) as part of its acquisition of Adeza and is including the amortization of such developed technology within cost of sales based upon its intended use. For the amortization of its other developed technology, beginning in 2007, the Company is allocating the majority to cost of sales based upon its determination that such developed technology is primarily being used to generate sales of its products. A portion of the amortization expense is also being allocated to research and development based upon the Company’s determination that such developed technology is primarily being utilized to support the Company’s research and development efforts (e.g., FirstCyte Breast Test).
Amortization expense related to identifiable intangible assets, which is an estimate for each future year and subject to change, is as follows:
| | | |
| | | Amount |
| | | (in thousands) |
Remaining six months ending December 31, 2007 | | $ | 11,500 |
Year ending December 31, 2008 | | | 25,988 |
Year ending December 31, 2009 | | | 29,448 |
Year ending December 31, 2010 | | | 29,272 |
Year ending December 31, 2011 | | | 28,016 |
Thereafter | | | 192,735 |
| | | |
Total | | $ | 316,959 |
| | | |
Investment securities at June 30, 2007 and December 31, 2006 consist of municipal bonds, all of which are classified as available-for-sale. The fair value of available-for-sale securities was determined based on quoted market prices at the reporting date for those securities. Available-for-sale securities are shown in the consolidated financial statements at fair market value with unrealized gains or losses recorded as a component of other comprehensive income (loss).
12
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
At June 30, 2007 and December 31, 2006, the cost basis, aggregate fair value, and gross unrealized holding gains by major security type were as follows:
| | | | | | | | | |
| | | Amortized Cost | | Gross Unrealized Holding Gains | | Fair Value |
| | | (in thousands) |
June 30, 2007 | | | | | | | | | |
Municipal bonds (average maturity of 0.2 months) | | $ | 2,055 | | $ | — | | $ | 2,055 |
| | | | | | | | | |
| December 31, 2006 | | | | | | | | | |
Municipal bonds (average maturity of 0.7 months) | | $ | 157,030 | | $ | — | | $ | 157,030 |
| | | | | | | | | |
A significant portion of the Company’s investment portfolio was converted into cash and cash equivalents during the first quarter of 2007 to fund the Company’s acquisitions of Adeza and Adiana.
| (6) | Other Balance Sheet Information |
Components of selected captions in the condensed consolidated balance sheets at June 30, 2007 and December 31, 2006 consisted of:
| | | | | | |
| | | June 30, 2007 | | December 31, 2006 |
| | | (in thousands) |
| Inventories, net | | | | | | |
Raw material | | $ | 11,396 | | $ | 10,305 |
Work-in-process | | | 2,267 | | | 2,275 |
Finished goods | | | 18,990 | | | 16,923 |
| | | | | | |
| | $ | 32,653 | | $ | 29,503 |
| | | | | | |
| Property and Equipment, net | | | | | | |
Equipment | | $ | 47,657 | | $ | 45,271 |
Equipment under customer usage agreements | | | 103,115 | | | 92,136 |
Computer equipment and software | | | 39,388 | | | 34,662 |
Furniture and fixtures | | | 6,292 | | | 5,186 |
Building | | | 20,960 | | | 20,401 |
Leasehold improvement | | | 18,781 | | | 14,350 |
Land | | | 6,268 | | | 3,224 |
Construction-in-process | | | 12,787 | | | 12,178 |
| | | | | | |
| | | 255,248 | | | 227,408 |
Less — accumulated depreciation and amortization | | | 94,247 | | | 78,401 |
| | | | | | |
| | $ | 161,001 | | $ | 149,007 |
| | | | | | |
| Accrued Expenses | | | | | | |
Accrued compensation | | $ | 31,143 | | $ | 27,343 |
Accrued acquisitions (see Note 3) | | | 7,029 | | | 3,613 |
Accrued transaction costs associated with Hologic merger (see Note 2) | | | 5,854 | | | — |
Accrued sales and marketing | | | 3,230 | | | 5,041 |
Accrued taxes | | | 4,776 | | | 9,174 |
Other accruals | | | 11,304 | | | 11,517 |
| | | | | | |
| | $ | 63,336 | | $ | 56,688 |
| | | | | | |
13
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
The Company records a liability for product warranty obligations at the time of sale based upon historical warranty experience. The term of the warranty is generally twelve months. Product warranty obligations are included in accrued expenses. Changes in the product warranty obligations for the six months ended June 30, 2007 and 2006 were as follows:
| | | | | | | | |
| | | Six Months Ended | |
| | | June 30, | |
| | | 2007 | | | 2006 | |
| | | (in thousands) | |
Balance, beginning of year | | $ | 381 | | | $ | 426 | |
New warranties | | | 401 | | | | 241 | |
Payments | | | (257 | ) | | | (184 | ) |
Adjustments | | | 39 | | | | (173 | ) |
| | | | | | | | |
Balance, June 30 | | $ | 564 | | | $ | 310 | |
| | | | | | | | |
| (7) | Net Income (Loss) Per Common Share |
Basic net income (loss) per share is computed by dividing net income by the weighted average number of common shares outstanding. Diluted net income per share is computed by dividing net income by the weighted average number of common shares and potential common shares from outstanding stock options, restricted stock units, and convertible debt. As a result of the Company’s net loss during the six months ended June 30, 2007, all potential common shares from outstanding stock options, restricted stock units and convertible debt, which totaled 11.0 million weighted average shares, were anti-dilutive and were excluded from the diluted net loss per share calculation. Potential common shares for outstanding stock options and restricted stock units are calculated using the treasury stock method and represent incremental shares issuable upon exercise of the Company’s outstanding stock options and vesting of the Company’s restricted stock units. The treasury stock method is affected by the amount of stock-based compensation attributable to future services and therefore not yet recognized. Restricted stock units with vesting subject to future performance milestones are not included in the calculation of diluted earnings per share until the performance milestones are achieved or if deemed to have been achieved for accounting purposes pursuant to the provisions of SFAS No. 128,Earnings Per Share. At June 30, 2007, there were 160,700 restricted stock units outstanding with vesting subject to future performance milestones, none of which were achieved or deemed to have been achieved. The 200,024 restricted stock units outstanding with vesting that is not contingent upon future performance milestones were included in the calculation of diluted earnings per share as of June 30, 2007.
The following table provides a reconciliation of the net income (loss) and weighted average common shares used in calculating basic and diluted net income (loss) per share for the three and six months ended June 30, 2007 and 2006:
| | | | | | | | | | | | | |
| | | Three Months Ended
June 30, | | Six Months Ended
June 30, |
| | | 2007 | | 2006 | | 2007 | | | 2006 |
| | | (in thousands, except per share data) |
Numerator: | | | | | | | | | | | | | |
Net income (loss), as reported, for basic earnings per share | | $ | 33,540 | | $ | 31,680 | | $ | (17,669 | ) | | $ | 61,044 |
Interest expense on convertible debt, net of tax | | | 1,181 | | | 1,138 | | | — | | | | 2,276 |
| | | | | | | | | | | | | |
Net income (loss), as adjusted, for diluted earnings per share | | $ | 34,721 | | $ | 32,818 | | $ | (17,669 | ) | | $ | 63,320 |
| | | | | | | | | | | | | |
Denominator: | | | | | | | | | | | | | |
Basic weighted average common shares outstanding | | | 115,656 | | | 114,356 | | | 115,197 | | | | 114,917 |
Dilutive effect of assumed exercise of stock options and restricted stock units | | | 3,316 | | | 800 | | | — | | | | 1,671 |
Dilutive effect of assumed conversion of convertible debt | | | 8,426 | | | 8,426 | | | — | | | | 8,426 |
| | | | | | | | | | | | | |
Weighted average common shares outstanding assuming dilution | | | 127,398 | | | 123,582 | | | 115,197 | | | | 125,014 |
| | | | | | | | | | | | | |
Basic net income (loss) per common share | | $ | 0.29 | | $ | 0.28 | | $ | (0.15 | ) | | $ | 0.53 |
| | | | | | | | | | | | | |
Diluted net income (loss) per common and potential common share | | $ | 0.27 | | $ | 0.27 | | $ | (0.15 | ) | | $ | 0.51 |
| | | | | | | | | | | | | |
14
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
Diluted weighted average common shares outstanding for the three months ended June 30, 2007 and 2006 excluded 23,499 and 4,544,854 potential common shares, respectively, from stock options outstanding and diluted weighted average common shares outstanding for the six months ended June 30, 2007 excluded interest expense on convertible debt, as well as the effect on weighted average diluted common stock outstanding of the assumed exercise of stock options and restricted stock units and the assumed conversion of convertible debt, as such amounts would have been anti-dilutive. Diluted weighted average common stock outstanding for the six months ended June 30, 2006 excluded 2,545,387 potential common shares from stock options outstanding, because the exercise prices of such stock options were higher than the average closing price of the Company’s common stock as quoted on The NASDAQ Global Select Market during the period and, accordingly, their effect would be anti-dilutive.
| (8) | Stock-Based Compensation and Stock Incentive Plans |
| | (a) | Stock-Based Compensation |
Stock-based compensation to employees, including grants of employee stock options, is recognized in the financial statements based on their fair values in accordance with SFAS No. 123R,Share-Based Payment. In order to determine the fair value of stock options and employee stock purchase plan shares, the Company is using the Black-Scholes option pricing model and is applying the multiple-option valuation approach to the stock option valuation. The Company is recognizing stock-based compensation expense on a straight-line basis over the requisite service period of the awards for options and restricted stock units granted following the adoption of SFAS No. 123R. For unvested stock options outstanding as of January 1, 2006, the Company will continue to recognize stock-based compensation expense using the accelerated amortization method prescribed in the Financial Accounting Standards Board (“FASB”) Interpretation (“FIN”) No. 28,Accounting for Stock Appreciation Rights and Other Variable Stock Option or Award Plans.
Estimates of the fair value of equity awards will be affected by the future market price of the Company’s common stock, as well as the actual results of certain assumptions used to value the equity awards. These assumptions include, but are not limited to, the related income tax impact, the expected volatility of the common stock, and the expected term of options granted.
As noted above, the fair value of stock options and employee stock purchase plan shares is determined by using the Black-Scholes option pricing model and applying the multiple-option valuation approach to the stock option valuation. The options have graded-vesting on an annual basis over an average vesting period of four years. In applying the multiple-option approach, each option “tranche” is separately valued based upon when the tranche vests. The Company estimates the expected option term by calculating the average period of time before the employees exercise their options and adds this to the vesting period of each tranche. Historically, this period of time has averaged one year from the date the options vest. The expected term of employee stock purchase plan shares is the average of the remaining purchase periods under each offering period. For equity awards granted prior to the adoption of SFAS No. 123R, the volatility of the common stock was estimated using historical volatility. For equity awards granted since January 1, 2006, the volatility of the common stock is estimated using a combination of historical and implied volatility, as discussed in Staff Accounting Bulletin (“SAB”) No. 107,Considering the interaction between SFAS No. 123R and Certain Securities and Exchange Commission Rules and Regulations. By using this combination, the Company is taking into consideration the historical realized volatility, as well as factoring in estimates of future volatility that the Company believes will differ from historical volatility as a result of the Company’s product diversification over the last three years, the market performance of the common stock, the volume of activity of the underlying shares, the availability of actively traded common stock options, and overall market conditions.
The risk-free interest rate used in the Black-Scholes option pricing model is determined by looking at historical U.S. Treasury zero-coupon bond issues with remaining terms equal to the expected terms of the equity awards. In addition, an expected dividend yield of zero is used in the option valuation model, because the Company does not expect to pay any cash dividends in the foreseeable future. Lastly, in accordance with SFAS No. 123R, the Company is required to estimate forfeitures at the time of grant and revise those estimates in subsequent periods if actual forfeitures differ from those estimates. In order to determine an estimated pre-vesting forfeiture rate for equity awards, the Company used historical forfeiture data. This estimated forfeiture rate has been applied to all unvested options outstanding as of January 1, 2006 and to all options and restricted stock units granted since January 1, 2006. Therefore, stock-based compensation expense is recorded for only those options and restricted stock units that are expected to vest.
In February 2007, the Company issued 160,700 performance-based restricted stock units (“Performance Shares”) that vest over a three-year period based upon specific future performance milestones. A grantee may also earn, over the course of three years, a total number of shares ranging from 0% to 175% of the number of shares equal to his or her initial grant of Performance Shares, which range is based on the actual achievement of the performance milestones. The market price of the Company’s common stock on the date of grant of the Performance Shares was $30.44. Each period the Company will assess its estimate of the probability that such performance milestones will be met and, if necessary, will adjust the related stock-based compensation expense. The Company is recording stock-based compensation expense for these equity awards over the three-year vesting period for those Performance Shares that are expected to vest, assuming all future performance milestones will be met.
15
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
In May 2007, the Company issued 200,024 restricted stock units that vest over a four-year period and are not subject to any performance milestones. The market price of the Company’s common stock on the date of grant was $35.12. The Company is recording stock-based compensation expense for these equity awards over the four-year vesting period for the restricted stock units that are expected to vest.
Total stock-based compensation recognized in the Company’s condensed consolidated statements of income (loss) for the three and six months ended June 30, 2007 and 2006 was as follows:
| | | | | | | | | | | | |
| | | Three Months
Ended June 30, | | Six Months Ended
June 30, |
| | | 2007 | | 2006 | | 2007 | | 2006 |
| | | (in thousands) |
Cost of sales | | $ | 290 | | $ | 344 | | $ | 551 | | $ | 705 |
Research and development | | | 677 | | | 803 | | | 1,286 | | | 1,647 |
Sales and marketing | | | 2,129 | | | 2,525 | | | 4,044 | | | 5,176 |
General and administrative | | | 1,742 | | | 2,066 | | | 3,309 | | | 4,235 |
| | | | | | | | | | | | |
Total stock-based compensation expense | | $ | 4,838 | | $ | 5,738 | | $ | 9,190 | | $ | 11,763 |
| | | | | | | | | | | | |
The underlying assumptions used in the Black-Scholes model were as follows for options granted during the three and six months ended June 30, 2007 and 2006:
| | | | | | | | | | | | |
| | | Three Months Ended June 30, | | | Six Months Ended June 30, | |
| | | 2007 | | | 2006 | | | 2007 | | | 2006 | |
Risk-free interest rate | | 4.8 | % | | 5.0 | % | | 4.7 | % | | 4.6 | % |
Expected dividend yield | | — | | | — | | | — | | | — | |
Expected lives (in years) | | 3.5 | | | 3.5 | | | 3.5 | | | 3.5 | |
Expected volatility | | 30 | % | | 32 | % | | 29 | % | | 32 | % |
As of June 30, 2007, total unrecognized stock-based compensation expense relating to unvested employee stock awards, adjusted for estimated forfeitures, was $44.1 million. This amount is expected to be recognized over a weighted-average period of 2.8 years. If actual forfeitures differ from current estimates, total unrecognized stock-based compensation expense will be adjusted for future changes in estimated forfeitures.
| | (b) | Employee and Director Incentive Plans |
The Cytyc Corporation 2004 Omnibus Stock Plan (the “2004 Omnibus Plan”), which is the Company’s primary plan for grants of equity, provides for the issuance of up to 12,250,000 shares of the Company’s common stock, no more than 8,200,000 of which shares may be issued as awards other than stock options or stock appreciation rights. The 2004 Omnibus Plan provides for grants of various incentives, including stock options and other stock-based awards. At June 30, 2007, 3,060,228 shares were available for future grant under the 2004 Omnibus Plan.
The following table summarizes options outstanding, by stock plan, as of June 30, 2007:
| | |
2004 Omnibus Plan | | 8,294,205 |
1995 Stock Plan | | 5,806,721 |
1998 Stock Plan | | 5,307 |
1995 Non-Employee Director Stock Option Plan | | 180,000 |
2001 Non-Employee Director Stock Option Plan | | 898,000 |
| | |
| | 15,184,233 |
| | |
16
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
The following schedule summarizes the activity under the Company’s stock option plans during the six months ended June 30, 2007:
| | | | | | | | | | | | | | | |
| | | Number of Shares | | | Range of Exercise Prices | | | Weighted Average Exercise Price per share |
Outstanding, December 31, 2006 | | 15,755,150 | | | $ | 0.44 | | — | | $ | 30.11 | | | $ | 20.82 |
Granted | | 2,314,125 | | | | 28.14 | | — | | | 43.00 | | | | 32.74 |
Exercised | | (2,321,614 | ) | | | 2.33 | | — | | | 30.11 | | | | 18.00 |
Canceled | | (563,428 | ) | | | 9.86 | | — | | | 35.12 | | | | 24.46 |
| | | | | | | | | | |
Outstanding, June 30, 2007 | | 15,184,233 | | | $ | 0.44 | | — | | $ | 43.00 | | | $ | 22.94 |
| | | | | | | | | | |
Exercisable, June 30, 2007 | | 7,794,694 | | | $ | 0.44 | | — | | $ | 35.08 | | | $ | 19.46 |
| | | | | | | | | | |
Exercisable, December 31, 2006 | | 8,482,964 | | | $ | 0.44 | | — | | $ | 28.45 | | | $ | 18.12 |
| | | | | | | | | | |
The weighted average fair value per share of options granted during the three months ended June 30, 2007 and 2006 was $10.13 and $7.91, respectively and for the six months ended June 30, 2007 and 2006 was $9.25 and $8.41, respectively.
The total intrinsic value of options exercised during the three months ended June 30, 2007 and 2006 was $29.7 million and $1.1 million, respectively, and for the six months ended June 30, 2007 and 2006 was $43.8 million and $10.2 million, respectively. The intrinsic value is calculated as the difference between the market value of the Company’s common stock on date of exercise and the exercise price per share.
The following table summarizes information about stock options outstanding at June 30, 2007:
| | | | | | | | | | | | |
| | | Options Outstanding | | Options Exercisable |
Range of Exercise Prices | | Number of Shares | | Weighted Average Remaining Contractual Life (in years) | | Weighted Average Exercise Price per Share | | Number of Shares | | Weighted Average Exercise Price per share |
$ 0.44 – $ 12.65 | | 2,751,637 | | 2.59 | | $ | 11.19 | | 2,067,233 | | $ | 10.72 |
12.71 – 20.75 | | 1,864,995 | | 4.46 | | | 17.99 | | 1,655,064 | | | 18.15 |
21.07 – 23.48 | | 1,864,049 | | 4.76 | | | 22.35 | | 1,200,273 | | | 22.17 |
23.59 – 24.31 | | 1,668,461 | | 5.49 | | | 24.13 | | 752,981 | | | 24.12 |
24.32 – 25.71 | | 1,662,454 | | 5.72 | | | 24.67 | | 1,272,433 | | | 24.58 |
25.74 – 28.17 | | 1,533,906 | | 6.94 | | | 27.24 | | 353,048 | | | 26.46 |
28.18 – 28.37 | | 155,675 | | 9.19 | | | 28.29 | | 12,733 | | | 28.30 |
28.39 – 28.53 | | 2,087,733 | | 4.88 | | | 28.39 | | 455,715 | | | 28.39 |
28.68 – 35.12 | | 1,472,198 | | 7.87 | | | 34.33 | | 25,214 | | | 31.83 |
35.13 – 43.00 | | 123,125 | | 9.91 | | | 40.28 | | — | | | — |
| | | | | | | | | | | | | |
$0.44 – $43.00 | | 15,184,233 | | 5.14 | | $ | 22.94 | | 7,794,694 | | $ | 19.46 |
| | | | | | | | | | | | | |
The aggregate intrinsic value of dilutive options outstanding and options exercisable as of June 30, 2007 was $306.3 million and $184.3 million, respectively. The intrinsic value is calculated as the difference between the market value of the Company’s common stock as of June 30, 2007 and the exercise price per share. The market value as of June 30, 2007 was $43.11 per share as quoted on The NASDAQ Global Select Market.
The weighted average remaining contractual life for options exercisable as of June 30, 2007 was 4.2 years.
17
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
| (9) | Comprehensive Income (Loss) |
Comprehensive income (loss) for the three and six months ended June 30, 2007 and 2006 was as follows:
| | | | | | | | | | | | | |
| | | Three Months Ended
June 30, | | Six Months Ended
June 30, |
| | | 2007 | | 2006 | | 2007 | | | 2006 |
| | | (in thousands) |
Net income (loss) | | $ | 33,540 | | $ | 31,680 | | $ | (17,669 | ) | | $ | 61,044 |
Other comprehensive income, net of tax: | | | | | | | | | | | | | |
Unrealized gain on investment securities | | | — | | | 19 | | | — | | | | 38 |
Foreign currency translation adjustments | | | 60 | | | 488 | | | 174 | | | | 596 |
| | | | | | | | | | | | | |
Comprehensive income (loss) | | $ | 33,600 | | $ | 32,187 | | $ | (17,495 | ) | | $ | 61,678 |
| | | | | | | | | | | | | |
| (10) | Stock Repurchase Program |
Under the current stock repurchase program, the Company is authorized to repurchase up to $200 million of its common stock through open market purchases or private transactions that will be made from time to time as market conditions allow. The stock repurchase program is expected to be in effect until November 15, 2009. Shares repurchased under this program will be held in the Company’s treasury. The stock repurchase program may be suspended or discontinued at any time without prior notice. During the three and six months ended June 30, 2007, the Company repurchased zero and 573,558 shares, respectively, with an aggregate cost of zero and $16.5 million, respectively. During the three and six months ended June 30, 2006, the Company repurchased 2,161,790 and 2,926,308 shares, respectively, with an aggregate cost of $56.3 million and $78.6 million, respectively.
SFAS No. 131,Disclosures About Segments of an Enterprise and Related Information, requires certain financial and supplementary information to be disclosed for each reportable operating segment of an enterprise, as defined. During the third quarter of 2006, the Company established the domestic diagnostic products and domestic surgical products divisions, each managed by a divisional president. Effective for the first quarter of 2007, as a result of the oversight of each segment by a divisional president, including review and ownership of discrete financial information, operating expenses are captured in the segment to which they relate, except for certain corporate expenses that benefit multiple operating segments and stock-based compensation expense (such expenses are included in “Corporate” below). Segment figures for 2006 have been restated to reflect the changes described above.
Financial information for the three segments is included below for all periods presented and each segment is described as follows:
Domestic Diagnostic Products— This segment develops and markets the ThinPrep® System in the United States primarily for use in diagnostic cytology testing applications focused on women’s health. The ThinPrep System is widely used for cervical cancer screening. The ThinPrep System consists of any one or more of the following: the ThinPrep 2000 Processor, ThinPrep 3000 Processor, ThinPrep Imaging System, and related reagents, filters, and other supplies, such as the ThinPrep Pap Test and the Company’s proprietary ThinPrep PreservCyt Solution. This segment also develops and markets the FullTerm® Fetal Fibronectin Test, which offers clinical and cost benefits for the assessment of the risk of pre-term birth.
Domestic Surgical Products— This segment develops and markets the NovaSure® System, an innovative endometrial ablation device to treat menorrhagia, or excessive menstrual bleeding, the MammoSite® Radiation Therapy System, a device for the treatment of early-stage breast cancer, and the GliaSite® Radiation Therapy System for the treatment of malignant brain tumors, and markets these products in the United States. This segment also develops the Adiana TCS System, which is a form of permanent female contraception intended as an alternative to tubal ligation and for which the Company is in the process of seeking a pre-market approval from the FDA.
International— This segment markets the Company’s diagnostic and surgical products outside of the United States through the Company’s subsidiaries, branch office and distributors. Products sold by the Company’s international segment are manufactured at domestic and international manufacturing locations.
18
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
The Company operates manufacturing facilities in the United States and Costa Rica and has several offices, primarily for sales and distribution, throughout the world. Property and equipment is primarily located within the domestic diagnostic products segment in the United States.
The Company’s operating results, amortization of intangible assets, and intangible assets by segment, are as follows (in thousands):
| | | | | | | | | | | | | | | | |
Three Months Ended June 30, 2007 | | Domestic Diagnostic Products | | Domestic Surgical Products | | International | | Corporate | | | Consolidated |
Net sales | | $ | 100,336 | | $ | 65,873 | | $ | 22,628 | | $ | — | | | $ | 188,837 |
Amortization of intangible assets | | | 1,917 | | | 2,750 | | | 525 | | | — | | | | 5,192 |
Income from operations | | | 51,883 | | | 26,028 | | | 5,283 | | | (29,236 | ) | | | 53,958 |
| | | | | |
Six Months Ended June 30, 2007 | | | | | | | | | | | |
Net sales | | $ | 189,512 | | $ | 125,137 | | $ | 43,072 | | $ | — | | | $ | 357,721 |
In-process research and development | | | 43,500 | | | 46,000 | | | — | | | — | | | | 89,500 |
Amortization of intangible assets | | | 2,478 | | | 5,376 | | | 914 | | | — | | | | 8,768 |
Income from operations | | | 56,273 | | | 4,469 | | | 10,380 | | | (50,185 | ) | | | 20,937 |
| | | | | |
As of June 30, 2007 | | | | | | | | | | | |
Intangible assets: | | | | | | | | | | | | | | | | |
Patents and developed technology, net | | $ | 139,517 | | $ | 141,261 | | $ | 36,181 | | $ | — | | | $ | 316,959 |
Goodwill | | | 275,287 | | | 261,225 | | | 58,588 | | | — | | | | 595,100 |
| | | | | |
Three Months Ended June 30, 2006 | | | | | | | | | | | |
Net sales | | $ | 84,087 | | $ | 50,024 | | $ | 16,286 | | $ | — | | | $ | 150,397 |
Amortization of intangible assets | | | 361 | | | 1,912 | | | 233 | | | — | | | | 2,506 |
Income from operations | | | 47,292 | | | 18,684 | | | 2,365 | | | (18,486 | ) | | | 49,855 |
| | | | | |
Six Months Ended June 30, 2006 | | | | | | | | | | | |
Net sales | | $ | 164,783 | | $ | 94,728 | | $ | 31,426 | | $ | — | | | $ | 290,937 |
Amortization of intangible assets | | | 723 | | | 3,737 | | | 444 | | | — | | | | 4,904 |
Income from operations | | | 93,943 | | | 33,578 | | | 4,932 | | | (36,316 | ) | | | 96,137 |
During the first quarter of 2007, the Company acquired $143.0 million of patents and developed technology and $208.5 million of goodwill, as part of its acquisition of Adeza (see Note 3(a)), of which $129.9 million and $189.4 million is included in the domestic diagnostic products segment, respectively, and $13.1 million and $19.1 million, respectively, is included in the international segment as of June 30, 2007. There were no other material changes within the Company’s other operating segments during the three and six months ended June 30, 2007.
SFAS No. 131 also requires that certain enterprise-wide disclosures be made related to products and services, geographic areas and significant customers. During the three and six months ended June 30, 2007 and 2006, the Company derived its sales from the following geographies (as a percentage of net sales):
| | | | | | | | | | | | |
| | | Three Months Ended June 30, | | | Six Months Ended
June 30, | |
| | | 2007 | | | 2006 | | | 2007 | | | 2006 | |
United States | | 88 | % | | 89 | % | | 88 | % | | 89 | % |
International | | 12 | % | | 11 | % | | 12 | % | | 11 | % |
| | | | | | | | | | | | |
| | 100 | % | | 100 | % | | 100 | % | | 100 | % |
| | | | | | | | | | | | |
During the three and six months ended June 30, 2007 and 2006, no customer represented 10% or more of consolidated net sales.
19
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
| (12) | Long-Term Debt, Contractual Obligations, Commitments and Contingencies |
Long-Term Debt and Contractual Obligations:
Credit Agreement— On June 30, 2006, the Company entered into a five-year Credit Agreement (the “Credit Agreement”) with a syndicate of lenders. The Credit Agreement provided for a $150 million senior unsecured revolving credit facility. On October 6, 2006, the Company and its related lenders amended the Credit Agreement in order to, among other items, increase the committed amount of the revolving credit facility to $345 million and to include one additional lender into the syndicate of lenders. The Company may request an increase in available borrowings under the Credit Agreement by an additional amount of up to $155 million (for a maximum amount of $500 million) upon satisfaction of certain conditions. These increased borrowings may be provided either by one or more existing lenders upon the Company obtaining the agreement of such lender(s) to increase commitments or by new lenders being added to the credit facility. The loan proceeds are available to be used by the Company and its subsidiaries to finance working capital needs and for general corporate purposes, including certain permitted business acquisitions. On May 14, 2007, the Company and its related lenders amended the Credit Agreement in order to: (1) extend the delivery deadline for both the audited financial statements for the fiscal year ended December 31, 2006 and the unaudited financial statements for the fiscal quarter ended March 31, 2007 and (2) amend the negative covenant concerning Indebtedness (as such term is defined in the Credit Agreement) to increase the maximum size of the receivables financing facility permitted thereunder from $50 million to $100 million.
Amounts under the Credit Agreement may be borrowed, repaid and re-borrowed by the Company from time to time until the maturity of the Credit Agreement on June 30, 2011. Voluntary prepayments and commitment reductions requested by the Company under the Credit Agreement are permitted at any time without penalty (other than customary breakage costs relating to the prepayment of any drawn loans) upon proper notice and subject to a minimum dollar requirement. Borrowings under the Credit Agreement bear interest at a floating rate, which will be, at the Company’s option, either LIBOR plus an applicable margin (which is subject to adjustment based on financial ratios), or a base rate.
The Credit Agreement requires the Company to comply with maximum leverage and minimum fixed charge coverage ratios. The Credit Agreement contains affirmative and negative covenants, including limitations on additional debt, liens, certain investments, and acquisitions outside of the healthcare business. The Credit Agreement also includes events of default customary for facilities of this type. Upon the occurrence of an event of default, all outstanding loans may be accelerated and/or the lenders’ commitments terminated.
As of June 30, 2007, the Company had $55.2 million outstanding under this Credit Agreement at an interest rate of 5.8%, of which $15.2 million was repaid in July 2007.
Convertible Notes— On March 22, 2004, the Company completed the sale (the “Offering”) of $250 million aggregate principal amount of its 2.25% convertible notes due 2024. The convertible notes were sold to qualified institutional buyers pursuant to an exemption from the registration requirements of the Securities Act of 1933, as amended. The notes bear interest at a rate of 2.25% per year on the principal amount, payable semi-annually in arrears in cash on March 15 and September 15 of each year, beginning September 15, 2004. The holders of the notes may convert the notes into shares of the Company’s common stock at a conversion price of $29.67 per share, subject to adjustment, prior to the close of business on March 15, 2024, subject to prior redemption or repurchase of the notes, under any of the following circumstances: (1) during any calendar quarter commencing after June 30, 2004 if the closing sale price of the Company’s common stock exceeds 120% of the conversion price for at least 20 trading days in the 30 consecutive trading days ending on the last trading day of the preceding calendar quarter (if the specified threshold is met, the notes will thereafter be convertible at any time at the option of the holder prior to the close of business on March 15, 2024); (2) during the five business day period after any five consecutive trading day period in which the trading price per $1,000 principal amount of notes for each day of such period was less than 98% of the product of the closing sale price of the Company’s common stock and the number of shares issuable upon conversion of $1,000 principal amount of the notes; (3) if the notes have been called for redemption; or (4) upon the occurrence of specified corporate events.
Holders may require the Company to repurchase the notes on March 15 of 2009, 2014 and 2019 at a repurchase price equal to 100% of their principal amount, plus accrued and unpaid interest, including contingent interest and liquidated damages, if any, to, but excluding, the repurchase date. The Company may redeem any of the notes beginning March 20, 2009, by giving holders at least 30 days’ notice.
On July 19, 2007, the Company announced that it is delivering a notice to the holders of the convertible notes in connection with Section 15.01(a)(i) of the indenture between the Company and U.S. Bank Trust National Association, as trustee, under which the convertible notes were issued (the “Indenture”). The notice advised that effective July 1, 2007, holders are entitled to convert the convertible notes into shares of the Company’s common stock due to the closing sale price of the Company’s common stock exceeding 120% of the convertible notes conversion price for at least 20 trading days out of the last 30 trading days in the quarter ended June 30, 2007. The conversion price of the convertible notes is $29.67 under the Indenture.
20
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
As of the filing of this Form 10-Q, none of the convertible notes have been converted into the Company’s common stock.
Commitments:
Earn-out Payments— As part of its acquisition of Adiana, the Company may be required to make contingent earn-out payments tied to future performance milestones (see Note 3(b)).
Finance Lease Obligations—On April 23, 2007, the Company signed a non-cancelable lease agreement for a building with approximately 164,000 square feet located in Alajuela, Costa Rica, to be used as a manufacturing and office facility to replace its current facility, the lease for which expires on December 31, 2008. The Company is responsible for a significant portion of the construction costs and therefore was deemed, for accounting purposes, to be the owner of the building during the construction period, in accordance with Emerging Issues Task Force (“EITF”) No. 97-10,The Effect of Lessee Involvement in Asset Construction.During the three months ended June 30, 2007, the Company recorded the $3.0 million fair market value of the land and the $2.7 million fair market value of the portion of the building constructed. The Company has recorded such fair market value within property and equipment on its consolidated balance sheet, with an offsetting increase to non-current liabilities. The Company will record the remainder of the building’s fair market value (estimated to have a total fair market value of $12.1 million), as well as the related leasehold improvements, as construction occurs. The term of the lease is for a period of approximately ten years with the option to extend for two consecutive five-year terms. The lease term will commence on or around February 2008 and the Company is expected to transfer most of its Costa Rica operations to this facility during the first half of calendar year 2008.
Future minimum lease payments, including principal and interest, under this lease were as follows at June 30, 2007:
| | | |
| | | Amount |
| | | (in thousands) |
Remaining six months ending December 31, 2007 | | $ | — |
Year ending December 31, 2008 | | | 1,316 |
Year ending December 31, 2009 | | | 1,481 |
Year ending December 31, 2010 | | | 1,533 |
Year ending December 31, 2011 | | | 1,587 |
Thereafter | | | 10,921 |
| | | |
Total minimum payments | | | 16,838 |
Less-amount representing interest | | | 8,334 |
| | | |
Total | | $ | 8,504 |
| | | |
On July 11, 2006, the Company signed a non-cancelable lease agreement for a building with approximately 146,000 square feet located in Marlborough, Massachusetts, to be principally used as an additional manufacturing facility. In 2011, the Company will have an option to lease an additional 30,000 square feet. As part of the lease agreement, the lessor agreed to allow the Company to make significant renovations to the facility to prepare the facility for the Company’s manufacturing needs. The Company is responsible for a significant amount of the construction costs and therefore was deemed under Generally Accepted Accounting Principles to be the owner of the building during the construction period.During the year ended December 31, 2006, the Company recorded the fair market value of the facility of $13.2 million within property and equipment on its consolidated balance sheet, with an offsetting increase to current and non-current liabilities. The Company began occupying a portion of the facility effective June 1, 2007. The term of the lease is for a period of approximately 12 years commencing on November 14, 2006.
21
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
Future minimum lease payments, including principal and interest, under this lease were as follows at June 30, 2007:
| | | |
| | | Amount |
| | | (in thousands) |
Remaining six months ending December 31, 2007 | | $ | 430 |
Year ending December 31, 2008 | | | 924 |
Year ending December 31, 2009 | | | 939 |
Year ending December 31, 2010 | | | 982 |
Year ending December 31, 2011 | | | 982 |
Thereafter | | | 7,913 |
| | | |
Total minimum payments | | | 12,170 |
Less-amount representing interest | | | 5,596 |
| | | |
Total | | $ | 6,574 |
| | | |
Restructuring— During the fourth quarter of 2006, Company management approved a restructuring plan designed to reduce future operating expenses by consolidating its Mountain View, California operations into its existing operations in Costa Rica and Massachusetts. In connection with this plan, the Company incurred $2.9 million of restructuring costs during the fourth quarter of 2006 related to severance expenses and $0.3 million related to retention costs. During the three and six months ended June 30, 2007, the Company incurred additional restructuring costs of $0.5 million and $1.7 million, respectively, related to retention costs for employees. The Company does not expect to incur significant additional severance or retention costs related to this restructuring. Changes in the restructuring accrual for the six months ended June 30, 2007 were as follows:
| | | | |
| | | Six Months Ended June 30, 2007 | |
| | | (in thousands) | |
Balance, beginning of year | | $ | 3,227 | |
Additions | | | 1,685 | |
Payments | | | (2,556 | ) |
| | | | |
Balance, June 30 | | $ | 2,356 | |
| | | | |
During the three months ended June 30, 2007, the Company entered into an arrangement in which the Company is sub-leasing all of its Mountain View facility to a third party for a term of approximately five years, a period of time equivalent to the remainder of the Company’s lease of this facility. The sub-lease commenced on July 1, 2007. The Company will record the payments it will receive under the sub-lease as other income within its consolidated statement of income.
Contingencies:
On June 16, 2003, Cytyc filed a suit for Declaratory Judgment in United States District Court for the District of Massachusetts asking the court to determine and declare that certain of TriPath Imaging, Inc.’s (“TriPath”) patents are invalid and not infringed by the Company’s ThinPrep Imaging System. On June 17, 2003, TriPath announced that it had filed a lawsuit against the Company in the United States District Court for the Middle District of North Carolina alleging patent infringement, false advertising, defamation, intentional interference, unfair competition, and unfair and deceptive trade practices. The non-patent claims have been dismissed and the patent cases have since been consolidated into a single action. A hearing occurred on August 2, 2006 in the United States District Court for the District of Massachusetts to hear oral arguments on summary judgment motions. The Court has scheduled a trial start date of October 29, 2007. The Company continues to believe that the claims against it are without merit and intends to vigorously defend this suit. Given the current status of the litigation, the Company is unable to reasonably estimate the ultimate outcome of this case.
The Company is also involved in ordinary, routine litigation incidental to its business. Although the outcomes of these other lawsuits and claims are uncertain, management does not believe that, individually or in the aggregate, these other lawsuits and claims will have a material adverse effect on the Company’s business, financial condition, results of operations or liquidity.
22
CYTYC CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (Unaudited) — Continued
The Company’s effective tax rate for the three and six months ended June 30, 2007 was 34.1% and 193.6%, respectively. Exclusive of the effects of the non-deductible in-process research and development charges incurred in connection with the Company’s acquisitions of Adeza and Adiana in March 2007, the Company’s effective tax rate for the six months ended June 30, 2007 would have been 33.7%. This rate includes the impact of the recognition of a $1.6 million tax benefit related to a tax examination that closed during the first quarter of 2007, as well as the reversal during the second quarter of 2007 of $1.4 million of deferred tax liabilities related to the Company permanently reinvesting the undistributed earnings realized by its Costa Rica subsidiary in accordance with the Company’s election during the period to maintain cash generated from profits at its Costa Rica subsidiary outside the United States in accordance with Accounting Principles Board (“APB”) Opinion No. 23,Accounting for Income Taxes—Special Areas. The effective rate for the three and six months ended June 30, 2007 also reflects $4.4 million of non-deductible costs associated with the Company’s pending merger with Hologic incurred during the second quarter of 2007. The effective tax rate was 36.5% for both the three and six months ended June 30, 2006.
On January 1, 2007, the Company adopted FIN No. 48,Accounting for Uncertainty in Income Taxes—an interpretation of FASB Statement No. 109, which clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements in accordance with FASB Statement No. 109,Accounting for Income Taxes. FIN No. 48 prescribes a recognition threshold and measurement criteria for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. FIN No. 48 also provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition and defines the criteria that must be met for the benefits of a tax position to be recognized. As a result of its adoption of FIN No. 48, the Company has recorded the cumulative effect of the change in accounting principle of $0.5 million as a decrease to opening retained earnings.
The Company had gross unrecognized tax benefits of approximately $12.8 million as of January 1, 2007. Of this amount, $6.8 million (net of federal benefit on state issues) represents the amount of unrecognized tax benefits as of January 1, 2007 that, if recognized, would result in a reduction of the Company’s effective tax rate. At March 31, 2007, the Company had $11.3 million of unrecognized tax benefits, $5.3 million of which, if recognized, would result in the reduction of the Company’s effective tax rate. The decrease during the three months ended March 31, 2007 in unrecognized tax benefits is primarily the result of the recognition of approximately $1.6 million of tax benefits related to an examination that closed during the first quarter of 2007. There were no significant changes during the second quarter of 2007.
The Company’s policy is to recognize accrued interest and penalties related to unrecognized tax benefits and income tax liabilities, when applicable, as part of income tax expense in its consolidated statements of income (loss). As of January 1, 2007, accrued interest was approximately $0.9 million, net of federal benefit. As of January 1, 2007, no penalties have been accrued.
The Company and its subsidiaries are subject to United Sates federal income tax, as well as income tax of multiple state income and foreign jurisdictions. The last years examined by the Internal Revenue Service and the Massachusetts Department of Revenue were 2004 and 2003, respectively, and all years up through and including those years are closed by examination.
| (14) | Recent Accounting Pronouncements |
In September 2006, the FASB issued SFAS No. 157,Fair Value Measurements.Among other requirements, SFAS No. 157 defines fair value and establishes a framework for measuring fair value and also expands disclosure requirements regarding fair value measurements. SFAS No. 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007 and interim periods within those years. The Company is evaluating the impact of adopting SFAS No. 157 on its consolidated financial statements.
In February 2007, the FASB issued SFAS No. 159,The Fair Value Option for Financial Assets and Financial Liabilities—Including an amendment of FASB Statement No.115, which permits entities to measure various financial instruments and certain other items at fair value at specified election dates. The election must be made at initial recognition of the financial instrument, and any unrealized gains or losses must be reported at each reporting date. SFAS No. 159 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. The Company is evaluating the potential impact that adopting SFAS No. 159 will have on its consolidated financial statements.
23
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Thefollowing discussion should be read in conjunction with our consolidated financial statements and the related notes appearing in Amendment No. 2 to our Annual Report on Form 10-K (“2006 Form 10-K/A”) for the year ended December 31, 2006. Our discussion contains forward-looking statements that involve risks and uncertainties, such as our plans, objectives, expectations and intentions. Actual results and events could differ materially from those anticipated in these forward-looking statements as a result of a number of factors, including those referred to or set forth below under Part II. Item 1A. “Risk Factors”.
Overview
Cytyc Corporation is a diversified diagnostic and medical device company that designs, develops, manufactures, and markets innovative and clinically effective diagnostic and surgical products. Our products cover a range of cancer and women’s health applications, including cervical cancer screening, pre-term birth risk assessment, treatment of excessive menstrual bleeding, radiation treatment of early-stage breast cancer, and radiation treatment of patients with malignant brain tumors. We operate our business in three reportable segments: domestic diagnostic products, domestic surgical products and international. Our domestic diagnostic products segment develops and markets the ThinPrep® System in the United States primarily for use in cytology testing applications, such as cervical cancer screening, and the FullTerm® Fetal Fibronectin Test, which offers clinical and cost benefits for the assessment of the risk of pre-term birth. Our domestic surgical products segment develops and markets the NovaSure® System, an innovative endometrial ablation device to treat menorrhagia, or excessive menstrual bleeding, the MammoSite® Radiation Therapy System, a device for the treatment of early-stage breast cancer that positions radiation sources directly into the post-lumpectomy site to optimize radiation treatment delivery while minimizing damage to healthy tissue, and the GliaSite® Radiation Therapy System for the treatment of malignant brain tumors. Our international segment markets our diagnostic and surgical products outside of the United States.
On May 20, 2007, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Hologic, Inc., a Delaware corporation (“Hologic”), and Nor’easter Corp. (“MergerSub”), a Delaware corporation and wholly-owned subsidiary of Hologic. The Merger Agreement provides that, upon the terms and subject to the conditions set forth in the Merger Agreement, we will merge with and into MergerSub, with MergerSub continuing as the surviving corporation under the name “Cytyc Corporation” and as a wholly-owned subsidiary of Hologic. Subject to the terms and conditions of the Merger Agreement, which has been unanimously approved by the boards of directors of both companies, at the effective time and as a result of the merger, each share of common stock of Cytyc, par value $0.01 per share, issued and outstanding immediately prior to the effective time of the merger will be cancelled and converted into the right to receive a combination of (i) 0.52 of a share of common stock, par value $0.01 per share, of Hologic, and (ii) $16.50 in cash without interest. The combined company will not issue any fractional shares of Hologic common stock in the merger. Instead, our stockholders will receive cash in lieu of any fractional shares of Hologic common stock that they would have otherwise received in the merger. Based on Hologic’s closing stock price (as reported on the NASDAQ Global Select Market) of $57.61 per share on May 18, 2007 (the last trading day prior to the public announcement of the transaction), the transaction represents a value of $46.46 per share of our common stock, or a total consideration of approximately $6.2 billion. Under the Merger Agreement, our stockholders will receive an aggregate of an estimated 69 million shares of Hologic common stock and $2.2 billion in cash, assuming the conversion of our outstanding convertible notes.
The market prices of Hologic common stock and our common stock likely will fluctuate between the date of the Merger Agreement and the completion of the merger. No assurance can be given concerning the market prices of Hologic common stock or our common stock before the completion of the merger or the market price of Hologic common stock after the completion of the merger. The merger consideration is fixed in the Merger Agreement and will not be adjusted for changes in the market value of our or Hologic’s common stock.
The Merger Agreement contains customary representations, warranties and covenants of us and Hologic, including, among others, covenants (i) to conduct their respective businesses in the ordinary course consistent with past practice during the interim period between the date of the execution of the Merger Agreement and the consummation of the merger, (ii) not to engage in certain kinds of transactions during such period, (iii) to use commercially reasonable efforts to convene and hold a meeting of their respective stockholders to consider and vote upon the approval of the transaction and (iv) that, subject to certain exceptions, the boards of directors of Hologic and Cytyc will each recommend that their respective stockholders approve the transaction. Each party has also agreed not to (a) solicit proposals relating to alternative business combination transactions or (b) subject to certain exceptions, enter into discussions or an agreement concerning or provide confidential information in connection with any proposals for alternative business combination transactions.
Consummation of the merger is subject to customary conditions, including (i) approval of the transaction by the common stockholders of both Hologic and Cytyc in accordance with Delaware law and the requirements of the NASDAQ Stock Market, (ii) absence of any applicable law prohibiting the merger, (iii) expiration or termination of the Hart-Scott-Rodino Act waiting period and certain other regulatory approvals, (iv) subject to certain exceptions, the accuracy of the representations and warranties of each party, (v) performance in all material respects of each party of its obligations under the Merger Agreement and (vi) the delivery of customary opinions from counsel to Hologic and counsel to us to the effect that the receipt of stock merger consideration by our stockholders will be a tax-free reorganization for federal income tax purposes, subject to the exceptions provided therein.
24
Under the Merger Agreement, upon completion of the merger, John W. Cumming, Chief Executive Officer of Hologic, will become Chief Executive Officer of the combined company, and Patrick J. Sullivan, Chairman, Chief Executive Officer and President of Cytyc, will become Chairman of the board of directors of the combined company. The combined company’s board of directors will be comprised of 11 members, with six nominated by Hologic and five nominated by us.
The Merger Agreement contains certain termination rights for both Hologic and us and further provides that, upon termination of the Merger Agreement under specified circumstances, we may be required to pay Hologic a termination fee of $50 million or $150 million or Hologic may be required to pay us a termination fee of $33 million or $100 million, in each case depending on the termination event.
The accompanying condensed consolidated financial statements have been prepared assuming we continue on a stand-alone basis and do not reflect any adjustments or disclosures that may be required upon consummation of the merger. Refer to the Registration Statement on Form S-4 (File No. 333- 144238), as may be amended from time to time, filed by Hologic with the Securities Exchange Commission (“SEC”), for a more complete description of the merger and related agreements. The merger is expected to close in late September or early October of 2007.
Critical Accounting Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. A “critical accounting estimate” is one which is both important to the portrayal of our financial condition and results and requires management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. We continuously evaluate our critical accounting estimates. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Valuation of Long-Lived Assets, Intangibles and Goodwill. Tangible and intangible assets acquired in a business combination, including acquired in-process research and development, are recorded under the purchase method of accounting at their estimated fair values at the date of acquisition. The fair values of acquired assets are determined by management using relevant information and assumptions and assisted, in certain situations, by independent appraisers. Fair value of acquired intangible assets is generally calculated as the present value of estimated future cash flows using a risk-adjusted discount rate, which requires significant management judgment with respect to revenue and expense growth rates, analyses of project accomplishments, assessment of overall contributions, project risks and the selection and use of an appropriate discount rate. Amortization of all our intangible assets with defined lives, including those acquired individually, is calculated either using the straight-line or cash flow method. The cash flow method requires management’s estimate of net cash flows over the life of the intangible asset, reflecting the pattern in which the economic benefits of the intangible asset are expected to be consumed, and is subject to change.
Prior to 2007, we allocated amortization of all of our intangible assets to research and development within our condensed consolidated statements of income as the developed technology has benefited multiple line items (i.e., cost of sales and research and development) and any allocation that would have been made to cost of sales was not deemed to be material. During 2007, we acquired a significant amount of developed technology ($143.0 million) as part of our acquisition of Adeza Biomedical Corporation (“Adeza”) and are including the amortization of such developed technology within cost of sales based upon its intended use. For the amortization of our other developed technology, beginning in 2007, we are allocating the majority to cost of sales based upon our determination that such developed technology is primarily being used to generate sales of our products. A portion of the amortization expense also will be allocated to research and development based upon our determination that such developed technology is primarily being utilized to support our research and development efforts (e.g., FirstCyte Breast Test).
We assess the impairment of long-lived assets, identifiable intangible assets and goodwill whenever events or changes in circumstances indicate that the carrying value may not be recoverable and at least annually in the case of goodwill. If it is determined that the carrying value of long-lived assets, intangible assets or goodwill might not be recoverable based upon the existence of one or more indicators of impairment, we would measure any impairment based on a projected discounted cash flow method if the undiscounted cash flows did not exceed the carrying value of such assets. No significant impairment charges have been recorded to date. We are required to perform an impairment review for goodwill on an annual basis, or earlier if indicators of potential impairment exist. Based on our impairment review during 2006, the carrying amount of goodwill did not exceed its fair value and, accordingly, no impairment loss exists. We are in the process of updating our impairment review as of July 31, 2007, but do not expect any impairment loss. At June 30, 2007, we had $912.1 million of net intangible assets, of which $595.1 million represented goodwill. An impairment of our intangible assets could result in a material, non-cash expense in our consolidated statements of income (loss).
25
Income Taxes and Deferred Taxes. We file income tax returns in eleven countries as well as many states and other localities. We must estimate our income tax expense after considering, among other factors, differing tax rates between jurisdictions, allocation factors, tax credits, non-deductible items and changes in enacted tax rates. A 1% change in our 2007 effective income tax rate would have the effect of changing net income (loss) for the three and six months ended June 30, 2007 by approximately $0.5 million and $1.1 million, respectively, or less than $.01 and zero per common share, respectively. The carrying value of our deferred tax assets assumes that we will be able to generate sufficient future taxable income in certain tax jurisdictions, based on estimates and assumptions, to fully recover the net carrying value of the assets. If these estimates and related assumptions change in the future, we may be required to record a valuation allowance against our deferred tax assets resulting in additional income tax expense in our consolidated statement of income.
Effective January 1, 2007, we adopted Financial Accounting Standards Board (“FASB”) Interpretation (“FIN”), No. 48,Accounting for Uncertainty in Income Taxes-an interpretation of FASB Statement No.109.FIN No. 48 requires significant judgment as to what constitutes a tax position as well as assessing the outcome of each tax position. Changes in facts or judgments surrounding recognition or measurement will result in a tax benefit or charge to our consolidated statements of income (loss).
Legal Proceedings. We are involved in various legal actions, the outcomes of which are not within our complete control and may not be known for prolonged periods of time. In some actions, the claimants seek damages, as well as other relief, which, if granted, would require significant expenditures. We record a liability in our consolidated financial statements for these actions when a loss is known or considered probable and the amount can be reasonably estimated. We review these estimates each accounting period as additional information is known and adjust the loss provision when appropriate. If the loss is not probable or cannot be reasonably estimated, a liability is not recorded in the consolidated financial statements. Our significant legal proceedings are discussed in Note 12 to our condensed consolidated financial statements and in Part II, Item 1. “Legal Proceedings” of this Form 10-Q, as well as in our 2006 Form 10-K/A.
Stock-based Compensation. Stock-based compensation to employees, including grants of employee stock options, is recognized in the financial statements based on their fair values in accordance with Statement of Financial Accounting Standards (“SFAS”) No. 123R,Share-Based Payment. In order to determine the fair value of our stock options and employee stock purchase plan shares, we are using the Black-Scholes option pricing model and are applying the multiple-option valuation approach to the stock option valuation. We are recognizing stock-based compensation expense on a straight-line basis over the requisite service period of the awards for options and restricted stock units granted following the adoption of SFAS No. 123R. For unvested stock awards outstanding as of January 1, 2006, we will continue to recognize stock-based compensation expense using the accelerated amortization method prescribed in FIN No. 28,Accounting for Stock Appreciation Rights and Other Variable Stock Option or Award Plans.
Our estimates of the fair value of equity awards will be affected by the future market price of our common stock, as well as the actual results of certain assumptions used to value the equity awards. These assumptions include, but are not limited to, the related income tax impact, the expected volatility of the common stock, and the expected term of options granted.
As noted above, we determine the fair value of stock options and employee stock purchase plan shares by using the Black-Scholes option pricing model and applying the multiple-option valuation approach to the stock option valuation. The options have graded-vesting on an annual basis over an average vesting period of four years. In applying the multiple-option approach, we separately value each option “tranche” based on when the tranche vests. We estimate the expected option term by calculating the average period of time before the employees exercise their options and add this to the vesting period of each tranche. Historically, this period of time has averaged one year from the date the options vest. The expected term of employee stock purchase plan shares is the average of the remaining purchase periods under each offering period. For equity awards granted prior to the adoption of SFAS No. 123R, we estimated the volatility of the common stock using historical volatility. For equity awards granted since January 1, 2006, we are estimating the volatility of the common stock using a combination of historical and implied volatility, as discussed in Staff Accounting Bulletin No. 107,Considering the Information Between SFAS No. 123R and Certain Securities and Exchange Commissions Rules and Regulations. By using this combination, we are taking into consideration the historical realized volatility, as well as factoring in estimates of future volatility that we believe will differ from historical as a result of our product diversification over the last three years, the market performance of our common stock, the volume of activity of the underlying shares, the availability of actively traded options of our common stock, and overall market conditions.
We determine the risk-free interest rate used in the Black-Scholes option pricing model by looking at historical U.S. Treasury zero-coupon bond issues with remaining terms equal to the expected terms of the equity awards. In addition, we use an expected dividend yield of zero in the option valuation model, because we do not expect to pay any cash dividends in the foreseeable future. Lastly, in accordance with SFAS No. 123R, we are required to estimate forfeitures at the time of grant and revise those estimates in subsequent periods if actual forfeitures differ from those estimates. In order to determine an estimated pre-vesting forfeiture rate for equity awards, we used historical forfeiture data. This estimated forfeiture rate has been applied to all unvested options outstanding as of January 1, 2006 and to all options and restricted stock units granted since January 1, 2006. Therefore, stock-based compensation expense is recorded for only those options and restricted stock units that are expected to vest.
26
In February 2007, we issued performance-based restricted stock units (“Performance Shares”) that vest over a three-year period based upon specific future performance milestones. A grantee may also earn, over the course of three years, a total number of shares ranging from 0% to 175% of the number of shares equal to his or her initial grant of Performance Shares, which range is based on the actual achievement of the performance milestones. Each period we will assess its estimate of the probability that such performance milestones will be met and, if necessary, will adjust the related stock-based compensation expense. We are recording stock-based compensation expense for these equity awards over the three-year vesting period for those Performance Shares that are expected to vest, assuming all future performance milestones will be met.
The statements of income (loss) classification of our stock-based compensation is consistent with the classification of our cash compensation expense related to the salaries of the employee equity award holders. Total stock-based compensation recognized in our condensed consolidated statements of income (loss) for the three and six months ended June 30, 2007 and 2006 was as follows:
| | | | | | | | | | | | |
| | | Three Months Ended
June 30, | | Six Months Ended
June 30, |
| | | 2007 | | 2006 | | 2007 | | 2006 |
| | | (in thousands) |
Cost of sales | | $ | 290 | | $ | 344 | | $ | 551 | | $ | 705 |
Research and development | | | 677 | | | 803 | | | 1,286 | | | 1,647 |
Sales and marketing | | | 2,129 | | | 2,525 | | | 4,044 | | | 5,176 |
General and administrative | | | 1,742 | | | 2,066 | | | 3,309 | | | 4,235 |
| | | | | | | | | | | | |
Total stock-based compensation expense | | $ | 4,838 | | $ | 5,738 | | $ | 9,190 | | $ | 11,763 |
| | | | | | | | | | | | |
As of June 30, 2007, total unrecognized stock-based compensation expense relating to unvested employee stock awards, adjusted for estimated forfeitures, was $44.1 million. This amount is expected to be recognized over a weighted-average period of 2.8 years. If actual forfeitures differ from current estimates, total unrecognized stock-based compensation expense will be adjusted for future changes in estimated forfeitures.
The above list is not intended to be a comprehensive list of all of our accounting estimates. In many cases, the accounting treatment of a particular transaction is specifically dictated by accounting principles generally accepted in the United States (“GAAP”), with little need for management’s judgment in their application. There are also areas in which management’s judgment in selecting any available alternative would not produce a materially different result. See our audited consolidated financial statements and notes thereto included in our 2006 Form 10-K/A which contain accounting policies and other disclosures required by GAAP.
Results of Operations
Net Sales
| | | | | | | | | | | | | | | | | | |
| | | Three Months Ended June 30, | | | Six Months Ended June 30, | |
| | | 2007 | | 2006 | | %
Change | | | 2007 | | 2006 | | %
Change | |
| | | ($ in millions) | |
Domestic Diagnostic Products (1) | | $ | 100.3 | | $ | 84.1 | | 19 | % | | $ | 189.5 | | $ | 164.8 | | 15 | % |
Domestic Surgical Products | | | 65.9 | | | 50.0 | | 32 | % | | | 125.1 | | | 94.7 | | 32 | % |
International (2) | | | 22.6 | | | 16.3 | | 39 | % | | | 43.1 | | | 31.4 | | 37 | % |
| | | | | | | | | | | | | | | | | | |
Total Company | | $ | 188.8 | | $ | 150.4 | | 26 | % | | $ | 357.7 | | $ | 290.9 | | 23 | % |
| | | | | | | | | | | | | | | | | | |
| (1) | Our net sales for the three and six months ended June 30, 2007 include the results of Adeza from the date of acquisition (March 19, 2007, the date on which Cytyc owned the majority of Adeza’s outstanding shares). |
| (2) | The international segment includes international sales of our diagnostic products of $19.8 million and $14.9 million during the three months ended June 30, 2007 and 2006, respectively, and $37.9 million and $28.9 million during the six months ended June 30, 2007 and 2006, respectively. This segment also includes international sales of our surgical products of $2.8 million and $1.4 million during the three months ended June 30, 2007 and 2006, respectively, and $5.2 million and $2.5 million during the six months ended June 30, 2007 and 2006, respectively. |
27
Net sales for each segment as a percentage of total consolidated net sales is as follows (dollars in millions) for the six months ended June 30, 2007 and 2006:
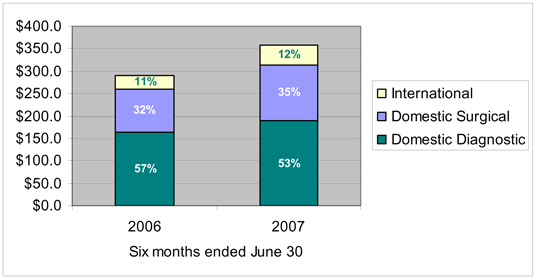
Our domestic surgical net sales increased 32% during the three and six months ended June 30, 2007, as compared to the same periods of 2006, primarily reflecting growth in unit sales and increased average sales price of single-use disposable devices of both the NovaSure System and MammoSite Radiation Therapy System. Domestic net sales of our NovaSure System represented 86% and 85% of the domestic surgical products segment’s net sales during the three months ended June 30, 2007 and 2006, respectively, and represented 86% and 84% of the domestic surgical products segment’s net sales during the six months ended June 30, 2007 and 2006, respectively. Net sales of the MammoSite Radiation Therapy System represented 13% of the domestic surgical products segment’s net sales during the three months ended June 30, 2007 and 2006, respectively, and 14% of the domestic surgical products segment’s net sales during the six months ended June 30, 2007 and 2006, respectively. During the three and six months ended June 30, 2007, no customer represented more than 10% of total domestic surgical net sales.
Our domestic diagnostic net sales increased 19% and 15% during the three and six months ended June 30, 2007, respectively, as compared to the same periods of 2006, due primarily to the continued increase in adoption and utilization of the ThinPrep Imaging System across our customer base. In addition, net sales during the three and six months ended June 30, 2007 include $14.0 million and $17.6 million of net sales of the FullTerm Fetal Fibronectin Test, acquired as part of our purchase of Adeza, from the date of acquisition. During the three months ended June 30, 2007, two customers represented 12% and 14%, respectively, of total domestic diagnostic net sales. During the six months ended June 30, 2007, two customers represented 13% and 14%, respectively, of total domestic diagnostic net sales.
Net sales in our international segment, which is comprised of net sales to customers outside the United States of both diagnostic and surgical products, increased 39% and 37% during the three and six months ended June 30, 2007, respectively, as compared to the same periods of 2006, primarily reflecting higher unit sales of the ThinPrep Pap Test and the NovaSure single-use disposable devices. During the three and six months ended June 30, 2007, no customer represented more than 10% of total international net sales.
During the three and six months ended June 30, 2007 and 2006, no customer represented 10% or more of our consolidated net sales.
Gross Margin
Our gross margin was 74% and 78% during the three months ended June 30, 2007 and 2006, respectively, and was 75% and 78% during the six months ended June 30, 2007 and 2006, respectively. Our gross margin decreased during the three and six months ended June 30, 2007, primarily as a result of: (1) slightly lower gross margins in our domestic diagnostic products segment as a result of increased placements of the ThinPrep Imaging System which generally carry lower gross margins than domestic sales of the ThinPrep Pap Test; (2) our inclusion within cost of sales of $4.8 million and $8.0 million of non-cash amortization expense related to our intangible assets during the three and six months ended June 30, 2007, respectively and (3) growth in net sales at our international segment, which generally carries lower margins than sales in the United States, although there was an increase in gross margins within the international segment during the three and six months ended June 30, 2007 as compared to the same periods of 2006, reflecting product mix.
28
Operating Expenses
Total operating expenses increased to $86.6 million and $246.4 million for the three and six months ended June 30, 2007, respectively, an increase of 28% and 87%, respectively, as compared to $67.5 million and $131.9 million for the same periods of 2006, primarily as a result of the in-process research and development charges totaling $89.5 million associated with our March 2007 acquisitions of Adeza and Adiana Inc. (“Adiana”), incremental operating expense from these two subsidiaries, and fees of approximately $6.2 million related to our pending merger with Hologic. The following is a summary of operating expenses for the three and six months ended June 30, 2007 and 2006:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Three Months Ended June 30, | | | Six Months Ended June 30, | |
| | | 2007 | | | 2006 | | | 2007 | | | 2006 | |
| | | $ | | % of
Sales | | | $ | | % of
Sales | | | $ | | % of
Sales | | | $ | | % of
Sales | |
| | | ($ in millions) | |
Research and development | | $ | 11.4 | | 6 | % | | $ | 10.7 | | 7 | % | | $ | 21.1 | | 6 | % | | $ | 21.0 | | 7 | % |
Sales and marketing | | | 48.2 | | 26 | % | | | 42.0 | | 28 | % | | | 89.3 | | 25 | % | | | 82.1 | | 28 | % |
General and administrative | | | 27.0 | | 14 | % | | | 14.8 | | 10 | % | | | 46.5 | | 13 | % | | | 28.8 | | 10 | % |
In-process research and development | | | — | | — | % | | | ��� | | — | % | | | 89.5 | | 25 | % | | | — | | — | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total operating expense | | $ | 86.6 | | 46 | % | | $ | 67.5 | | 45 | % | | $ | 246.4 | | 69 | % | | $ | 131.9 | | 45 | % |
| | | | | | | | | | | | | | | | | | | | | | | | |
Research and Development
Our core research and development strategy is to (1) enhance our existing product lines, such as the ThinPrep Imaging System, the NovaSure System, and the MammoSite Radiation Therapy System, through operational enhancements and cost reductions, and (2) develop additional innovative medical diagnostic and surgical devices and therapeutic applications for cancer and women’s health, such as the Adiana Complete Transcervical Sterilization (“TCS”) System®, GestivaTM, CellientTM Automated Cell Block System and the Helica Thermal Coagulator System products. Our research and development costs increased to $11.4 million and $21.1 million for the three and six months ended June 30, 2007, respectively, an increase of 6% and less than 1%, respectively, as compared to the same periods of 2006, primarily reflecting: (1) $2.4 million and $2.9 million of incremental expenses during the three and six months ended June 30, 2007, respectively, from the inclusion of the research and development activities of Adeza and Adiana in our consolidated results from the dates of acquisition, including continued development efforts surrounding the Gestiva product and the FDA approval process for the TCS System; and (2) increased engineering costs to continue to improve the ThinPrep System, NovaSure System and the MammoSite Radiation Therapy System and to develop related technologies to grow our product lines. These costs were slightly offset by a $2.1 million and $4.2 million reduction of non-cash amortization as a result of recording this amortization within cost of sales during the three and six months ended June 30, 2007, respectively, where all such non-cash amortization had been included within research and development expenses in prior years.
Sales and Marketing
Sales and marketing costs increased to $48.2 million and $89.3 million for the three and six months ended June 30, 2007, respectively, an increase of 15% and 9%, respectively, as compared to the same period of 2006, due primarily to (1) $4.5 million and $5.4 million of incremental expenses during the three and six months ended June 30, 2007, respectively, from the inclusion of the sales and marketing activities of Adeza in our consolidated results from the date of acquisition, including costs associated with the integration of the Adeza sales force and (2) incremental sales and marketing costs, primarily related to headcount to support the growth in sales and commissions on higher net sales in our domestic surgical products business. These higher sales and marketing costs were partially offset by a $0.4 million and $1.1 million reduction in non-cash stock-based compensation expense during the three and six months ended June 30, 2007, respectively, as compared to the same periods in 2006.
General and Administrative
General and administrative costs increased to $27.0 million and $46.5 million for the three and six months ended June 30, 2007, respectively, an increase of 83% and 61%, respectively, as compared to the same periods of 2006, primarily due to: (1) $6.2 million of costs incurred during the three months ended June 30, 2007 related to our pending merger with Hologic, (2) $1.2 million and $1.6 million of incremental expenses during the three and six months ended June 30, 2007, respectively, from the inclusion of the general
29
and administrative activities of Adeza in our consolidated results from the date of acquisition, (3) increased personnel and facility costs to support the growth of our business; and (4) increased efforts to enhance our management information systems. These increased costs were partially offset by $0.3 million and $0.9 million reduction in non-cash stock-based compensation expense during the three and six months ended June 30, 2007, respectively, as compared to the same periods in 2006.
Other (Expense) Income, net
We recorded interest expense of $3.8 million and $1.8 million for the three months ended June 30, 2007 and 2006, respectively, and $6.2 million and $3.6 million for the six months ended June 30, 2007 and 2006, respectively, related primarily to: (1) $1.8 million and $3.6 million of interest expense during both the three and six months ended June 30, 2007 and 2006, respectively, from our 2.25% convertible notes due 2024, which were issued on March 22, 2004, including amortization of $0.4 million and $0.8 million of deferred financing costs for both the three and six months ended June 30, 2007 and 2006, respectively, associated with the issuance of these notes; and (2) $1.9 million and $2.5 million of interest expense during the three and six months ended June 30, 2007, related to our borrowing on our line-of-credit facility during the first quarter of 2007, to facilitate the purchases of Adiana and Adeza. Interest income decreased to $0.6 million and $3.3 million for the three and six months ended June 30, 2007, respectively, as compared to $1.8 million and $3.7 million for the same periods of 2006, respectively, as the average total balance of our cash, cash equivalents and investment securities was lower during the three and six months ended June 30, 2007 as compared to the same periods of 2006 due to the acquisitions of Adeza and Adiana.
Income Taxes
Our effective tax rate for the three and six months ended June 30, 2007 was 34.1% and 193.6%, respectively. Exclusive of the effects of the non-deductible in-process research and development charges incurred in connection with our acquisitions of Adeza and Adiana in March 2007, our effective tax rate for the six months ended June 30, 2007 would have been 33.7%, including the impact of the recognition of a $1.6 million tax benefit related to a tax examination that closed during the first quarter of 2007, as well as the reversal during the second quarter of 2007 of $1.4 million of deferred tax liabilities related to our permanently reinvesting the undistributed earnings realized by our Costa Rica subsidiary. The effective tax rate for the three and six months ended June 30, 2007 also reflected $4.4 million of non-deductible costs associated with our pending merger with Hologic incurred during the second quarter of 2007. The effective tax rate was 36.5% for both the three and six months ended March 31, 2006. We believe we have adequate liabilities for uncertain tax positions. If we ultimately determine that these liabilities are greater or less than the amounts paid, we will adjust the liabilities for uncertain tax positions and recognize a tax benefit or charge in the consolidated statement of income (loss) during the period in which we make the determination or, if the adjustments relate to unrecognized tax benefits for deferred tax assets recorded in connection with an acquisition, we will adjust the carrying value of goodwill. We estimate our effective tax rate for 2007 will be in the range of 55% to 56% and, exclusive of the effects of the in-process research and development charges, we estimate our effective tax rate will be in the range of 35% to 36%.
Liquidity and Capital Resources
At June 30, 2007, we had total cash, cash equivalents and investment securities of $33.3 million. Cash provided by operations was $85.1 million for the six months ended June 30, 2007, an increase of 17% compared to $72.6 million during the same period of 2006, primarily as a result of increased net sales as compared to the same period in the prior year and our $11.4 million payment to DEKA during the first quarter of 2006 relating to the arbitration panel decision. Our net accounts receivable increased 20% to $113.7 million at June 30, 2007 as compared to $94.9 million at December 31, 2006, reflecting continued growth in net sales, the timing of cash collections, as well as the addition of receivables obtained in the acquisition of Adeza in March 2007. Our Days Sales Outstanding (“DSO”) was 54 days and 52 days at June 30, 2007 and December 31, 2006, respectively. We have had no significant issues of collectibility. The slight increase in DSO is largely the result of our acquisition of Adeza, which has generally had a higher DSO than our historical average. The term “Days Sales Outstanding”, which we calculate by dividing gross trade accounts receivable at the end of the quarter by our average consolidated daily net sales for the quarter, refers to the estimated number of days’ worth of sales that are outstanding and unpaid at any given time. Our inventories increased 11% to $32.7 million at June 30, 2007 as compared to $29.5 million at December 31, 2006, primarily reflecting an increase in inventory to support the growth in net sales in our domestic diagnostic and surgical products businesses, as well as an increase as a result of our acquisition of Adeza during the first quarter of 2007.
Our investing activities used cash of $285.1 million during the six months ended June 30, 2007, primarily related to the acquisitions of Adeza and Adiana in March 2007. The total purchase prices for Adeza and Adiana were $457.3 million and $60.5 million (excluding future contingent payments), respectively, including acquisition-related costs (see Note 3 to our condensed consolidated financial statements). During the six months ended June 30 2007, we paid $427.7 million and $58.4 million, respectively (net of cash acquired), related to these acquisitions using a combination of cash on hand and borrowings on our line-of-credit. Also during the six months ended June 30, 2007: (1) we paid $3.6 million to the former shareholders of Proxima Therapeutics, Inc. (“Proxima”), a company we acquired in 2005, for contingent earn-out payments relating to incremental sales growth in our breast-related products during 2006 (there will be no further contingent earn-out payments associated with the Proxima acquisition); (2) we
30
invested $12.4 million in equipment under customer usage agreements, of which $10.8 million represented ThinPrep Imaging System units; and (3) we made $9.5 million of capital expenditures related primarily to our investment in manufacturing processes to support the growth of our business. During the six months ended June 30, 2006, our investing activities used cash of $37.2 million, primarily related to the payment of $21.1 million to the former Proxima shareholders for the contingent earn-out payments relating to incremental sales growth in our breast-related products during 2005 pursuant to the terms of the related merger agreement. In addition, during the six months ended June 30, 2006, we invested $13.5 million in equipment under customer usage agreements, of which $11.5 million represented ThinPrep Imaging System units, and we made $8.6 million of capital expenditures.
Our financing activities during the six months ended June 30, 2007 provided cash of $91.2 million, primarily reflecting our net borrowings of $55.0 million on our line-of-credit facility to facilitate our purchases of Adeza and Adiana, as well as $41.8 million and $2.3 million of proceeds from the exercise of stock options and employee purchases of our common stock under the employee stock purchase plan, respectively, along with related tax benefits of $8.6 million. Partially offsetting these cash inflows was the repurchase of $16.5 million of our common stock during the six months ended June 30, 2007 as part of our stock repurchase program. Our financing activities during the six months ended June 30, 2006 used cash of $59.0 million, primarily reflecting our repurchase of $78.6 million of our common stock as part of our repurchase program, partially offset by proceeds and tax benefits of $16.8 million and $2.0 million from the exercise of stock options and employee purchases of our common stock under the employee stock purchase plan, respectively.
Long-Term Debt and Contractual Obligations. In addition to the contractual cash obligations described in our 2006 Form 10-K/A, changes to such obligations during the six months ended June 30, 2007 are as described below:
Credit Agreement— We have a five-year Credit Agreement (the “Credit Agreement”) with a syndicate of lenders, which provides for a $345 million senior unsecured revolving credit facility. We may request an increase in available borrowings under the Credit Agreement by an additional amount of up to $155 million (for a maximum amount of $500 million) upon satisfaction of certain conditions. The loan proceeds are available to be used by us and our subsidiaries to finance working capital needs and for general corporate purposes, including future business acquisitions, as defined. Four of our wholly-owned subsidiaries are guarantors under the Credit Agreement. On May 14, 2007, we and our related lenders amended the Credit Agreement in order to: (1) extend the delivery deadline for both the audited financial statements for the fiscal year ended December 31, 2006 and the unaudited financial statements for the fiscal quarter ended March 31, 2007 and (2) amend the negative covenant concerning Indebtedness (as such term is defined in the Credit Agreement) to increase the maximum size of the receivables financing facility permitted thereunder from $50 million to $100 million.
The Credit Agreement requires us to comply with maximum leverage and minimum fixed charge coverage ratios. The Credit Agreement contains affirmative and negative covenants, including limitations on additional debt, liens, certain investments, and acquisitions outside of the healthcare business. The Credit Agreement also includes events of default customary for facilities of this type. Upon the occurrence of an event of default, all outstanding loans may be accelerated and/or the lenders’ commitments terminated.
As of June 30, 2007, we had $55.2 million outstanding under this Credit Agreement at an interest rate of 5.8%, of which $15.2 million was repaid in July 2007.
Adiana Earn-out Payments— As part of our acquisition of Adiana, we may be required to make contingent earn-out payments tied to future performance milestones (see Note 3(b)).
Our future contractual cash obligations as of June 30, 2007 are as follows (in thousands):
| | | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period |
Contractual Obligations | | Total | | 2007 | | 2008 | | 2009 | | 2010 | | 2011 | | Thereafter |
Operating leases | | $ | 76,078 | | $ | 4,530 | | $ | 8,662 | | $ | 7,575 | | $ | 7,165 | | $ | 7,144 | | $ | 41,002 |
Finance lease obligation (1) | | | 29,008 | | | 430 | | | 2,240 | | | 2,420 | | | 2,515 | | | 2,569 | | | 18,834 |
Inventory purchase commitments | | | 18,000 | | | 2,000 | | | 4,000 | | | 3,000 | | | 3,000 | | | 3,000 | | | 3,000 |
Private equity investments (2) | | | 2,950 | | | 2,950 | | | — | | | — | | | — | | | — | | | — |
Line-of-credit facility (3) | | | 55,244 | | | — | | | — | | | — | | | — | | | 55,244 | | | — |
Convertible debt: (4) | | | | | | | | | | | | | | | | | | | | | |
Principal | | | 250,000 | | | — | | | — | | | 250,000 | | | — | | | — | | | — |
Interest | | | 11,250 | | | 2,812 | | | 5,625 | | | 2,813 | | | — | | | — | | | — |
| | | | | | | | | | | | | | | | | | | | | |
Total contractual cash obligations (5) | | $ | 442,530 | | $ | 12,722 | | $ | 20,527 | | $ | 265,808 | | $ | 12,680 | | $ | 67,957 | | $ | 62,836 |
| | | | | | | | | | | | | | | | | | | | | |
| (1) | Represents: a) the lease of a facility located in Marlborough, Massachusetts to be used principally as a manufacturing facility. The lease |
31
| | was entered into on July 11, 2006. Total minimum payments due under the lease as of June 30, 2007 were $12.2 million; and b) the lease of a facility located in Alajuela, Costa Rica to be used as a manufacturing and office facility. The lease was entered into on April 23, 2007. Total minimum payments due under the lease as of June 30, 2007 were $16.8 million. |
| (2) | Primarily relates to the remainder of our $5 million private equity investment. |
| (3) | The line-of-credit borrowings bear a variable interest rate as such interest payments are not included in this table. We have included this amount in current liabilities in our condensed consolidated balance sheet as we expect to repay this amount within one year. |
| (4) | Relates to our $250 million aggregate principal amount of 2.25% convertible senior notes described above. The amounts in the table above include interest and principal payable assuming redemption at the earliest redemption date of March 5, 2009. In July 2007, these notes became convertible into common stock at the option of the note holders (see Note 12). |
| (5) | We have not included $5.8 million of liabilities relating to uncertain tax positions within this schedule due to uncertainty of the payment date, if any. In addition, we have not included any contingent earn-out payments relating to our acquisition of Adiana (see Note 3(b)) as payment of such earn-outs, if any, are tied to future performance milestones. |
We expect that our cash and cash equivalents, investment securities, cash flows from operating activities and funds available under our Credit Agreement will be sufficient to meet our projected operating cash needs for the next twelve months, including capital expenditures, lease and purchase commitments and tax payments. However, from time to time, we review our capital structure and financing arrangements. As a result of these reviews, we may periodically elect to pursue alternatives to our current structure, including the refinancing of our existing debt securities, the issuance of additional debt securities, the expansion of the existing credit facility, and the emplacement of an additional credit facility. In addition, if we make future acquisitions, we may be required to seek additional capital.
Recent Accounting Pronouncements
In September 2006, the FASB issued SFAS No. 157,Fair Value Measurements. Among other requirements, SFAS No. 157 defines fair value and establishes a framework for measuring fair value and also expands disclosure requirements regarding fair value measurements. SFAS No. 157 is effective for financial statements issued for fiscal years beginning after November 15, 2007 and interim periods within those years. We are evaluating the potential impact that adopting SFAS No. 157 will have on our consolidated financial statements.
In February 2007, the FASB issued SFAS No.159,The Fair Value Option for Financial Assets and Financial Liabilities — Including an amendment of FASB Statement No.115, which permits entities to measure various financial instruments and certain other items at fair value at specified election dates. The election must be made at initial recognition of the financial instrument, and any unrealized gains or losses must be reported at each reporting date. SFAS No.159 is effective for financial statements issued for fiscal years beginning after November 15, 2007, and interim periods within those fiscal years. We are evaluating the potential impact that adopting SFAS No.159 will have on our consolidated financial statements.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Derivative Financial Instruments, Other Financial Instruments, and Derivative Commodity Instruments.At June 30, 2007, we were not a party to any derivative financial instruments, other financial instruments for which the fair value disclosure would be required under SFAS No. 107,Disclosures about Fair Value of Financial Instruments, or derivative commodity instruments. At June 30, 2007, all of our investments were in municipal bonds that are carried at fair value on our books. Accordingly, we have no quantitative information concerning the market risk of participating in such investments.
Primary Market Risk Exposures.Our primary market risk exposures are in the areas of interest rate risk and foreign currency exchange rate risk. Our investment portfolio of cash equivalents and investment securities is subject to interest rate fluctuations, but we believe this risk is immaterial due to the short-term nature of these investments. Our business outside the United States is conducted primarily in local currencies, except in Costa Rica, where the majority of business is conducted in the U.S. dollar. We have no foreign exchange contracts, option contracts, or other foreign hedging arrangements. We estimate that any market risk associated with our international operations is unlikely to have a material adverse effect on our business, financial condition or results of operations.
Interest Rate Risk and Management.As of June 30, 2007, our debt portfolio included both a fixed rate instrument ($250 million) and a floating rate instrument (approximately $55.2 million). The weighted average interest rate during the three and six months ended June 30, 2007 of the fixed instrument portfolio was 2.25% and the weighted average interest rate during the three and six months ended June 30, 2007 for the variable instrument portfolio was approximately 5.8%. While our fixed rate instrument guarantees that the impact on our consolidated statements of income (loss) and cash flows will be predictable, changes in interest rates can cause the value of our fixed rate debt to change. However, such a value change has no impact on either our consolidated statements of income or cash flows unless we determine that we wish to retire a fixed rate debt obligation on the open market.
32
Alternatively, our future consolidated statements of income (loss) and future cash flows can fluctuate with our floating rate borrowings. However, the impact would be partially mitigated by the floating rate interest earned on excess cash. If there were a hypothetical 10% change in interest rates, the net impact to our consolidated statements of income (loss) and cash flows would not be material. The potential change in cash flows and earnings is calculated based on the change in the net interest expense over both one and two quarters, due to an immediate 10% change in rates.
Item 4. Controls and Procedures
(a)Evaluation of Disclosure Controls and Procedures.As of the end of the period covered by this report, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934). Based on this evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures are effective in timely notification to them of information we are required to disclose in our periodic SEC filings and in ensuring that this information is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and regulations.
(b)Changes in Internal Control.During the period covered by this report, there have been no significant changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
33
PART II — OTHER INFORMATION
Item 1. Legal Proceedings
On June 16, 2003, we filed a suit for Declaratory Judgment in United States District Court for the District of Massachusetts asking the court to determine and declare that certain of TriPath Imaging, Inc.’s (“TriPath”) patents are invalid and not infringed by our ThinPrep Imaging System. On June 17, 2003, TriPath announced that it had filed a lawsuit against us in the United States District Court for the Middle District of North Carolina alleging patent infringement, false advertising, defamation, intentional interference, unfair competition, and unfair and deceptive trade practices. The non-patent claims have been dismissed and the patent cases have since been consolidated into a single action. A hearing occurred on August 2, 2006 in the United States District Court for the District of Massachusetts to hear oral arguments on summary judgment motions. The Court has scheduled a trial start date of October 29, 2007. We continue to believe that the claims against us are without merit and intend to vigorously defend this suit. Given the current status of the litigation, we are unable to reasonably estimate the ultimate outcome of this case.
We are also involved in ordinary, routine litigation incidental to its business. Although the outcomes of these other lawsuits and claims are uncertain, management does not believe that, individually or in the aggregate, these other lawsuits and claims will have a material adverse effect on our business, financial condition, results of operations or liquidity.
Item 1A. Risk Factors
The forward-looking statements in this Form 10-Q are made under the safe harbor provisions of Section 21E of the Securities Exchange Act of 1934, as amended. Our operating results and financial condition have varied and may in the future vary significantly depending on a number of factors. Statements in this Form 10-Q which are not strictly historical statements, including, without limitation, statements regarding management’s expectations for future growth and plans and objectives for future management and operations, domestic and international marketing and sales plans, key customer relationships, product plans and performance, research and development plans, the successful integration of new technologies or businesses, regulatory uncertainties, potential savings to the healthcare system, management’s assessment of market factors, costs and uncertainties related to current or future litigation, as well as statements regarding our strategy and plans, constitute forward-looking statements that involve risks and uncertainties. In some cases these forward-looking statements can be identified by the use of words such as “may,” “will,” “could,” “should,” “would,” “expect,” “project,” “predict,” “potential” or the negative of these words or comparable words.
The following risk factors amend and restate the risk factors contained in our 2006 Form 10-K/A and our quarterly report on Form 10-Q for the quarter ended March 31, 2007. In addition to the risk factors listed below, the risk factors related to the merger of our company and Hologic, which are included in Hologic’s Registration Statement on Form S-4 (File No. 333-144238), as amended from time to time, could cause actual results to differ materially from those contained in forward-looking statements made in this report and presented elsewhere by management from time to time. Such factors, among others, may have a material adverse effect upon our business, financial condition, and results of operations. We undertake no obligation to update publicly or revise any forward-looking statements, whether as a result of new information, future events or otherwise. Accordingly, you are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date on which they are made.
We depend principally on the sale of a limited number of product lines.
Prior to our acquisitions of Novacept in March 2004, Proxima in March 2005 and Adeza in March 2007, we derived most of our revenues from sales of our ThinPrep processor, filters and other disposable supplies for use in gynecological and non–gynecological testing applications. As a result of the Novacept, Proxima and Adeza acquisitions, we manufacture and market the NovaSure, MammoSite, GliaSite, FullTerm® and TLiIQ® Systems, which together represented 44% and 41% of worldwide consolidated net sales in the three and six months ended June 30, 2007. Nevertheless, we still rely heavily on sales of our ThinPrep products to generate a significant portion of our net sales and earnings. If we are unable to continue to successfully develop and commercialize our current products as well as other new or improved products, our business, sales and profits may be materially impaired.
In addition, in October 2003, an article in the New England Journal of Medicine suggested that the interval between screenings for cervical cancer could be extended from one year to three years for women over 30 who have had consistently negative results from previous screenings. If screening periods are extended as suggested, aggregate demand for testing is likely to decrease and if we are unable to successfully develop additional products, our business may be materially adversely affected. Furthermore, we may be required to obtain FDA approval for any other new or improved products that we are able to develop or acquire, and we may not be able to do so.
34
Our sales are dependent on third-party reimbursement.
Widespread adoption of our ThinPrep, ThinPrep Imaging, NovaSure, MammoSite, GliaSite, FullTerm, The Fetal Fibronectin Test and TLiIQ Systems in the United States and other countries is dependent upon the ability of healthcare providers and laboratories to secure adequate reimbursement from third-party payors such as private insurance plans, managed care organizations, Medicare and Medicaid and foreign governmental healthcare agencies. Although a majority of managed care organizations in the United States have added the ThinPrep Pap Test, NovaSure, FullTerm and TLiIQ to their coverage and the MammoSite and GliaSite procedures are reimbursed by many private healthcare insurance and managed care payors, we cannot guarantee that reimbursement will increase or continue to be available, or that reimbursement levels will be adequate to enable healthcare providers and clinical laboratories in the United States and other countries to use our products instead of conventional methods or existing therapies, such as whole breast irradiation and brachy therapy, or the products of our competitors. Reimbursement and healthcare payment systems in international markets vary significantly by country and include both government–sponsored healthcare and private insurance. There can be no assurance that third–party payors will provide or continue to provide such coverage, that third–party reimbursement will be made available at an adequate level, if at all, for our products under any such overseas reimbursement system or that healthcare providers or clinical laboratories will use our products in lieu of other methods. We also will be required to secure adequate reimbursement for any new products we develop or acquire, and we may not be able to do so successfully.
We are dependent upon a relatively small number of large clinical laboratory customers in the United States for a significant portion of our sales of the ThinPrep System.
We are dependent upon a relatively small number of large clinical laboratory customers in the United States for a significant portion of our sales of the ThinPrep System, and our business may materially suffer if we are unable to increase sales to, or maintain our pricing levels with our existing customers and establish new customers both within and outside the United States. Due in part to a trend toward consolidation of clinical laboratories in recent years and the relative size of the largest United States laboratories, it is likely that a significant portion of ThinPrep System sales will continue to be concentrated among a relatively small number of large clinical laboratories.
Our business faces intense competition from other companies.
Our products face direct competition from a number of publicly–traded and privately–held companies. Some of our competitors are large companies that enjoy significant competitive advantages over us, including:
| | • | | significantly greater name recognition; |
| | • | | established distribution networks; |
| | • | | additional lines of products, and the ability to offer rebates or bundle products to offer discounts or incentives to gain competitive advantage; |
| | • | | greater resources for product development, sales and marketing; and |
| | • | | well–established products for procedures such as the radical mastectomy or traditional radiation therapy. |
The markets we sell in are intensely competitive, subject to rapid change and significantly affected by new product introductions and other market activities of industry participants. Other companies may develop products that are superior to ours or less expensive, or both. Improvements in existing competitive products or the introductions of new competitive products may reduce our ability to compete for sales, particularly if those competitive products demonstrate better safety or effectiveness, clinical results, ease of use or lower costs. The development, FDA approval and commercial marketing of competitive systems for cervical cancer screening, treatment of excessive menstrual bleeding, radiation treatment of early–stage breast cancer, and radiation treatment of patients with malignant brain tumors could have a material adverse effect on our business and financial condition. If we are unable to compete effectively against existing and future competitors and existing and future alternative treatments, our sales could decline and our business could be adversely affected.
The success of our business depends on our ability to protect our intellectual property rights.
We rely on a combination of patents, trade secrets, copyrights, trademarks and confidentiality agreements to protect our products’ proprietary technology, rights and know–how. We also are the exclusive licensee of certain patented technology for use in the field of cytology related to the ThinPrep System and in the treatment of any proliferative disease relating to our GliaSite System.
We own all intellectual property rights with respect to the NovaSure System, the MammoSite System and the GliaSite System. However, the medical device industry in general, and the therapeutic use of devices employing radiofrequency energy, such as the NovaSure System, in particular, have been subject to extensive litigation and administrative proceedings regarding patents and other intellectual property rights.
35
If we fail to protect, defend and maintain the intellectual property rights with respect to any of our products or if we are subject to a successful third–party claim of infringement, the competitive position of these products could be impaired. In addition, infringement, interference and other intellectual property claims and proceedings, with or without merit, are expensive and time-consuming to litigate and could adversely affect our business, financial condition and operating results.
If breast surgeons, radiation oncologists and patients do not continue to adopt the MammoSite System as a preferred treatment for early–stage breast cancer, the intended benefits of our commercializing this product may not be realized and the market price of our common stock could decline.
One of our products acquired as a result of our acquisition of Proxima is the MammoSite System, which is used to treat early–stage breast cancer. At the time of acquisition, the MammoSite System was supported by less than four years of patient follow-up studies from the initial 43–patient study that was designed to gain clearance from the FDA and the one–year follow-up study from a 1,600–patient registry following the FDA clearance. Some of the existing data has been produced in studies that involve relatively small patient groups, and such data may not be reproduced in wider patient populations. Currently, with respect to our MammoSite System, we are competing against breast cancer treatment using external beam radiation, which has longer-term data on patient outcome.
We may have difficulty gaining further acceptance of the MammoSite System among breast surgeons, radiation oncologists and patients for a number of reasons including:
| | • | | the presence of competing products sold by companies with longer operating histories, more recognizable names and more established distribution networks; |
| | • | | the introduction or existence of competing products or technologies that may be more effective, safer or easier to use than the MammoSite System; |
| | • | | the results of long-term clinical studies relating to the effectiveness of the MammoSite System; |
| | • | | the availability of alternative treatments or procedures that provide comparable levels of improvement in breast cancer treatment at a lower cost than the MammoSite System; |
| | • | | breast surgeons, radiation oncologists and patient perceptions of the MammoSite System as compared to other treatments for breast cancer; and |
| | • | | the continued availability of satisfactory reimbursement from healthcare payors for breast cancer treatment procedures. |
We believe that continued recommendation and support for the use of the MammoSite System by influential breast surgeons and radiation oncologists and treatment centers are essential for widespread market acceptance. If the MammoSite System does not continue to receive support from these key constituencies, or if longer-term data does not demonstrate continued support for the clinical efficacy of the MammoSite System, breast surgeons and radiation oncologists may not use, and hospitals and outpatient surgery centers may not purchase, the MammoSite System. If this occurs, the intended benefits of the acquisition, such as increased net sales, may not be realized and the market price of our common stock could decline.
Current levels of growth in the market for endometrial ablation procedures for the treatment of excessive menstrual bleeding may not be indicative of future growth.
Demand for newly introduced technologies or treatments can initially be exaggerated as supply increases to meet preexisting demand. However, once the pre–existing demand is met, growth in the market may abruptly stop or significantly slow. We believe that some of the current growth in the market for new endometrial ablation procedures may be the result of a considerable pre–existing unmet demand for products of this nature. We cannot determine what portion of Novacept’s historical sales is attributable to this pre–existing demand, nor can we predict when, or at what rate, this demand may stop or decline in growth. We cannot assure you that we will be successful in continuing to attract physicians and women to use the NovaSure System, or whether or not evolving trends in the treatment of excessive menstrual bleeding will favor new endometrial ablation procedures as compared to traditional approaches. If the demand for treatments like the NovaSure System were to stop abruptly or begin to decline, our operating results and profitability could be adversely affected.
The success of our ThinPrep System depends upon the continued market acceptance of our ThinPrep System products.
Our success and growth depends on the continued market acceptance of our ThinPrep System and ThinPrep Imaging System, including any follow-on applications of ThinPrep technology. The laboratory cost of using the ThinPrep System and ThinPrep Imaging System for cervical cancer screening, both together and individually, is higher than that of a conventional Pap smear and, we believe, competing liquid–based slide preparation systems. Due in part to increased competitive pressures in the healthcare industry to reduce costs, our ability to continue gaining market acceptance of the ThinPrep System and follow-on products depends on our ability to demonstrate that the higher cost of using the ThinPrep System is offset by (i) a reduction in costs often associated with conventional
36
Pap smears or competing liquid–based slide preparation systems, such as inaccurate diagnoses and the need for repeat Pap smears, as well as (ii) the ability to conduct additional testing, such as testing for the HPV, Chlamydia trachomatis and Neisseria gonorrhea on samples collected in a ThinPrep vial of preservative. In particular, for the ThinPrep Imaging System, we need to continue to work with healthcare providers, insurance companies and other third–party payors, and clinical laboratories to reinforce the known clinical efficacy and cost effectiveness of the ThinPrep Imaging System products.
If we have difficulty in achieving and sustaining market acceptance of the products acquired as a result of Adeza, the intended benefits of our acquisition of such products may not be realized and the market price of our common stock could decline.
Our products acquired as a result of our acquisition of Adeza are the FullTerm and the TLiIQ Systems. The commercial success of these products depends in large part on our ability to achieve and sustain market acceptance. We plan to expand sales of our TLiIQ System in hospitals and clinical laboratories and increase the related sales of the Fetal Fibronectin Test and other consumables used in conjunction with the TLiIQ System by convincing healthcare providers of the benefits of these products through various means, including through published papers, presentations at scientific conferences and additional clinical trials. If existing users of these products determine that these products do not satisfy their requirements, or if our competitors develop a product perceived to better satisfy their requirements, our sales of Fetal Fibronectin Tests and other consumables may decline.
We also acquired a product candidate called Gestiva. Even if the FDA approves Gestiva, physicians may adopt Gestiva only if they determine, based on experience, clinical data, side effect profiles and other factors, that it is preferable to other products or treatments then in use. Acceptance of Gestiva among influential practitioners will be essential for market acceptance of Gestiva. If our products acquired from Adeza are unable to achieve or maintain broad market acceptance, the intended benefits of the Adeza acquisition, such as increased net sales, may not be realized and the market price of our common stock could decline.
Our success depends on our ability to manage growth effectively.
The scope of our operations and facilities, the number of our employees and the geographic area of our operations have grown rapidly. If we are not able to manage our growth effectively, our business and financial condition will materially suffer. Our growth may significantly strain our managerial, operational and financial resources and systems. To manage our growth effectively, we will have to continue to implement and improve additional management and financial systems and controls, and to effectively expand, train and manage our employee base.
We may not be able to successfully integrate future acquisitions.
We continually explore opportunities to acquire related businesses, some of which could be material to us. Our ability to continue to grow may depend upon identifying and successfully acquiring attractive companies, effectively integrating such companies, achieving cost efficiencies and managing these businesses as part of our Company. We may not be able to effectively integrate acquired companies and successfully implement appropriate operational, financial and management systems and controls to achieve the benefits expected to result from these acquisitions. Our efforts to integrate these businesses could be affected by a number of factors beyond our control, such as regulatory developments, general economic conditions, increased competition, the loss of customers resulting from the acquisitions and the assumption of unknown liabilities. In addition, the process of integrating these businesses could cause an interruption of, or loss of momentum in, the activities of our existing business and the loss of key personnel and customers. The diversion of management’s attention and any delays or difficulties encountered in connection with the integration of these businesses could negatively impact our business if any of the above adverse effects were to occur. Further, the benefits that we anticipate from any future acquisitions may not develop.
Future acquisitions may also harm our operating results, dilute our stockholders’ equity and create other financial difficulties for us.
We may, in the future, pursue acquisitions that we believe could provide us with new technologies, products or service offerings, or enable us to obtain other competitive advantages.
Acquisitions by us may involve some or all of the following financial risks:
| | • | | use of significant amounts of cash; |
| | • | | potential dilutive issuances of equity securities; |
| | • | | incurrence of debt or amortization expenses related to certain intangible assets; and |
| | • | | future impairment charges related to diminished fair value of businesses acquired as compared to the price we pay for them. |
37
We may not be successful in overcoming the risks described above or any other problems associated with future acquisitions. Any of these risks and problems could materially harm our business, prospects and financial condition. Additionally, we cannot guarantee that any companies we may acquire will achieve anticipated revenues or operating results.
We are subject to the risk of product liability claims relating to our products.
Regardless of the technology employed, surgical procedures involve risk of complications, and companies that produce medical devices for use in surgical procedures are subject to risk of product liability litigation. We have become a party to legal proceedings involving injuries to patients that occurred when one of our products was used. We vigorously defend against any product liability claims that arise related to any of our products. However, there can be no assurance that any defense against alleged claims will be successful.
If one of our products is found to have caused or contributed to injuries or deaths, we could be held liable for substantial damages. Product liability insurance is in place for all of our products. However, we cannot assure that available insurance will be sufficient to cover damages from any possible claims. In addition, claims could adversely affect the reputation of the related product, which could damage its competitive position in the market. The sale and use of one of our diagnostic products could also lead to the filing of product liability claims if someone were to allege that one of our products contained a design or manufacturing defect that resulted in the failure to detect a disorder for which it was being used to screen or caused injuries to a patient. Any product liability claim brought against us, with or without merit, could result in the increase of our product liability insurance rates or the inability to secure additional coverage in the future. Also, even a meritless or unsuccessful product liability claim could be time consuming and expensive to defend, which could result in a diversion of management’s attention from our business and could adversely affect the perceived safety and efficacy of our products, as well as our financial condition and operating results.
Our quarterly operating results may fluctuate.
Our operating results have fluctuated significantly in the past on a quarterly basis. Our operating results may fluctuate significantly from quarter to quarter in the future and we may experience losses in the future depending on a number of factors, including the extent to which our products continue to gain or maintain market acceptance, the rate and size of expenditures incurred as we expand our domestic and establish our international sales and distribution networks, the timing and level of reimbursement for our products by third–party payors, seasonality and other factors, many of which are outside our control.
We may not be successful in growing our international sales, which could have a material adverse effect on our business and financial condition.
We believe the growth in our international sales is a key element to our future success. We commenced sales in countries outside the United States in 1998 and have grown our international sales to 11% and 12% of consolidated net sales for the year ended December 31, 2006 and the six months ended June 30, 2007. However, we cannot guarantee that we will successfully continue to develop international sales channels or capabilities that will enable us to generate significant revenue from international sales. Even though we have established international sales capabilities and have experienced growth in the international markets, we may not be able to obtain favorable third–party reimbursements and required regulatory approvals in foreign countries. While we believe we have developed a comprehensive international patent filing strategy, we have not secured patents in all foreign countries in which we currently sell or plan to sell our products. In some cases our ability to pursue patents in certain countries has lapsed. In those countries where we do have patents covering our products, we cannot guarantee that such patents may be successfully enforced against infringing products. If we fail to continue to increase international sales, our business and financial condition may suffer materially.
The increase in our international operations exposes us to additional operational challenges that we might not otherwise face.
As we increase our international operations, we will have to confront and manage a number of risks and expenses that we would not face if we conducted our operations solely in the United States. Any of these risks or expenses could cause a material adverse effect on our operating results. These risks and expenses include:
| | • | | difficulties in staffing, managing and operating an international operation as a result of, among other things, distance, language and cultural differences; |
| | • | | less flexible labor laws and regulations; |
| | • | | expenses associated with customizing our products for clients in foreign countries; |
| | • | | foreign currency exchange rate fluctuations; |
| | • | | protectionist laws and business practices that favor local companies; |
| | • | | political and economic instability in some international markets; |
| | • | | multiple, conflicting and changing government laws and regulations (including, among other things, antitrust and tax requirements, international trade regulations and the Foreign Corrupt Practices Act); |
38
| | • | | reduced protection for intellectual property rights in some countries; and |
| | • | | potentially adverse tax consequences. |
Our operating results may be adversely affected by currency fluctuations.
Our business outside the United States is conducted primarily in local currencies, except at our Costa Rica subsidiary, where the majority of business is conducted in U.S. dollars. Fluctuations in foreign currency exchange rates could affect our cost of goods and operating margins and could result in exchange losses. In addition, currency devaluation can result in a loss to us if we hold deposits of that currency. Hedging foreign currencies can be difficult, especially if the currency is not freely traded. We currently have no foreign exchange contracts, option contracts, or other foreign hedging arrangements. We currently estimate that risks associated with currency fluctuations are unlikely to have a material adverse effect on our operating results. However, our operating results may be adversely affected by currency fluctuations in the future, especially as we expand our international operations.
Our operating results may be adversely affected as a result of restructuring operations and/or reductions in workforce.
In 2006, management approved restructuring plans designed to reduce future operating expenses by consolidating our Mountain View, California operations into our existing operations in Costa Rica and Massachusetts. This restructuring includes the elimination of positions and the incurrence of expenditures related to severance and facility–related costs, all of which have impacted our operating results in 2006 and during the six months ended June 30, 2007. There can be no assurance that our current restructuring, as well as any potential future restructurings, and related transition issues, will not adversely affect our operations or customer perceptions. The uncertainty caused by such restructurings and integration–related events could result in reduced productivity by our remaining employees, which in turn may affect our revenue in the future.
In addition, any decision to limit investment in, dispose of or otherwise exit business activities may result in the recording of special charges, such as technology write-offs, workforce reduction costs, or charges relating to consolidation of excess facilities. Our estimates with respect to the useful life or ultimate recoverability of our carrying basis of assets, including intangible assets, could change as a result of such decisions. Further, our estimates related to the liabilities for excess facilities are affected by changes in real estate market conditions. Additionally, we are required to perform goodwill impairment tests on an annual basis and during the year in certain circumstances. There can be no assurance that future goodwill impairment tests will not result in a charge to earnings.
We are highly dependent on key personnel.
We are highly dependent on the principal members of our management staff. Loss of our key personnel would likely impede achievement of our research and development, operational, or strategic objectives. To be successful, we must retain key employees and attract additional qualified employees.
Our reliance on single source or limited source suppliers could harm our business.
We currently obtain certain key components of our products, including the proprietary filter material and microscope slides used in the ThinPrep Pap Test, radioisotopes, certain balloons and other items used in the design and manufacture of the MammoSite System and the Iotrex liquid isotope used with the GliaSite System, from single or a limited number of sources due to technology, availability, price, quality and other considerations. Additionally, the NovaSure System utilizes several components that may become obsolete or no longer be manufactured. We also rely on a limited number of suppliers for both raw materials and components necessary for the manufacture of FullTerm, The Fetal Fibronectin Test, and TLiIQ System. We have no manufacturing capabilities for Gestiva and we may depend on third parties who are single source suppliers to manufacture Gestiva. We attempt to mitigate these risks by working closely with key suppliers regarding our supply needs and we have qualified backup vendors for several of our key components. Although we believe that alternative sources for these components are available, a supply interruption could harm our ability to manufacture our products until a new source of supply is identified and qualified. If we are unable to obtain sufficient quantities of key components that meet our quality and technical requirements at reasonable prices and in a timely manner, we will not be able to manufacture and sell our products on a timely and cost–competitive basis, which would materially and adversely affect our business and financial condition.
Our lack of redundant manufacturing capabilities could harm our business.
We assemble all of our ThinPrep Processors, ThinPrep Imaging System, TLiIQ System and the single–use disposable devices in the NovaSure System, as well as performing final assembly and testing of our RF Controllers, MammoSite and GliaSite systems. In addition, we contract with third parties to manufacture our MammoSite System and GliaSite System. We have established a certain level of site redundancy on our core products, or have developed alternate sources. While we have made significant efforts to safeguard these core manufacturing processes, the loss or closure of any of these facilities, such as our closure of our Mountain View facility, or third–party manufacturing providers would impede our manufacturing and sales efforts, which would materially and adversely affect our business and financial condition.
39
Because the NovaSure and MammoSite Systems are manufactured in Costa Rica, we will be subject to the political and economic risks of doing business there.
We manufacture the Novasure System and MammoSite System in a facility in Costa Rica and recently signed a non–cancelable, ten–year lease agreement for a building with approximately 164,000 square feet located in Alajuela, Costa Rica, which will be used as an additional manufacturing facility and office building. We cannot guarantee that the political, labor and economic climate in Costa Rica will remain sufficiently stable for uninterrupted manufacturing operations in that country. Our operations relating to the NovaSure System and MammoSite System could be adversely affected by political unrest and we could also be harmed by strikes and other labor disruptions. Any of these events could result in increased costs or in disruptions of supply of the NovaSure and MammoSite disposable devices, or any other product or component we may elect to manufacture in Costa Rica in the future, which could harm our business, financial condition and operating results.
We are subject to numerous FDA laws and regulations, and noncompliance with such laws, as well as changes in such laws or future interpretations of such laws, could cause us to incur significant compliance costs and/or subject us to significant civil and criminal sanctions.
Our products are subject to expansive FDA regulation. Complying with these requirements imposes significant expenses on our operations. If we fail to comply with applicable regulations, we could be subject to a wide range of civil and criminal enforcement sanctions, our promotional practices may be restricted, and our marketed products could be subject to recall or loss of FDA marketing claim approval.
We cannot guarantee that we will obtain or maintain necessary regulatory approvals for our products.
The FDA regulatory approval process can be expensive, lengthy and uncertain for both original and supplemental approvals.
There can be no assurance that we will be able to obtain necessary regulatory clearances or approvals for any proposed future products or modifications of existing products. The failure to obtain clearances or approvals, loss of previously received clearances or approvals, or a failure to comply with existing or future regulatory requirements, would have a material adverse effect on our business, financial condition and operating results.
Any modifications to a device that has received a pre–market approval that affect its safety or effectiveness require a pre–market approval supplement or possibly a separate pre–market approval, either of which is likely to be time-consuming, expensive and uncertain to obtain. The FDA requires the device manufacturer to make and document its determination whether or not a pre–market approval or supplement is required, but the FDA can review the manufacturer’s decision. If the FDA requires us to seek one or more pre–market approval supplements or new pre–market approvals for any modification to a previously approved device, we may be required to cease marketing or to recall the modified device until we obtain approval, and we may be subject to significant criminal and/or civic sanctions, including but not limited to, regulatory fines or penalties.
If we or our contract manufacturers fail to comply with the FDA’s Quality System Regulation, manufacturing operations could be interrupted, and our product sales and operating results could be adversely affected.
Our design and manufacturing processes are required to comply with the FDA’s Quality System Regulation, which covers the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our devices. The FDA enforces its Quality System Regulation through unannounced inspections. If one of our manufacturing facilities fails an inspection, operations and manufacturing could be disrupted. Failure to take adequate and timely corrective action in response to an adverse inspection could force a shutdown of these manufacturing operations or a recall of the product. If we shut down manufacturing operations or recalls our products, we may lose customers and experience a decline in our revenues.
Our products may be subject to recalls even after receiving FDA clearance or approval, and if a recall occurs, the reputation of our products and our operating results could be adversely affected.
The FDA and similar governmental bodies in other countries have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture. A government mandated or voluntary recall by us could occur as a result of component failures, manufacturing errors or design defects, including defects in labeling. Any recall could harm the reputation of our products and adversely affect our financial condition and operating results.
40
Some of our activities may subject us to risks under federal and state laws prohibiting “kickbacks” and false or fraudulent claims.
We are subject to the provisions of a federal law commonly known as the “Medicare/Medicaid anti–kickback law,” and several similar state laws, which prohibit payments intended to induce physicians or others either to refer patients or to acquire or arrange for or recommend the acquisition of healthcare products or services. While the federal law applies only to referrals, products or services for which payment may be made by a federal healthcare program, state laws often apply regardless of whether federal funds may be involved. These laws constrain the sales, marketing and other promotional activities of manufacturers of medical devices, such as us, by limiting the kinds of financial arrangements, including sales programs, with hospitals, physicians, laboratories and other potential purchasers of medical devices. Other federal and state laws generally prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third–party payors that are false or fraudulent, or are for items or services that were not provided as claimed. Anti–kickback and false claims laws prescribe civil and criminal penalties for noncompliance that can be substantial. While we continually strive to comply with these complex requirements, interpretations of the applicability of these laws to marketing practices is ever evolving and even an unsuccessful challenge could cause adverse publicity and be costly to respond to, and thus could have a material adverse effect on our business, operating results and financial condition.
Certain provisions of our certificate of incorporation, bylaws and Delaware law may delay or prevent a change in control of our Company.
Our certificate of incorporation, bylaws and Delaware law contain provisions that may enable our board of directors to resist a change in control of our Company. These provisions include:
| | • | | a staggered board of directors; |
| | • | | limitations on persons authorized to call a special meeting of stockholders; |
| | • | | the authorization of undesignated preferred stock, the terms of which may be established and shares of which may be issued without stockholder approval; |
| | • | | advance notice procedures required for stockholders to nominate candidates for election as directors or to bring matters before an annual meeting of stockholders; and |
| | • | | a stockholder rights agreement or “poison pill.” |
In addition, we are subject to the anti–takeover provisions of Section 203 of the DGCL, which may prohibit large stockholders, in particular those owning 15% or more of our outstanding voting stock, from merging or consolidating with our Company except under certain circumstances.
These anti–takeover defenses could discourage, delay or prevent a transaction involving a change in control of our Company. These provisions could also discourage proxy contests and make it more difficult for stockholders to elect directors of their choosing and cause us to take other corporate actions stockholders desire.
The implementation of SFAS No. 123R has reduced and may continue to reduce our reported earnings, which could result in a decline in our stock price.
As part of our compensation to employees, we issue equity awards, primarily in the form of stock options. Many of the companies within our industry and with whom we compete for skilled employees use stock based compensation as a means to attain these employees. While we have structured the estimated cost of our equity program to be comparable to other companies within our industry and similar to our size, not all companies use equity awards as part of their compensation packages nor do they issue the same level of equity awards. In addition, if we unexpectedly hire additional employees or acquire another company, the impact of the implementation of SFAS No. 123R may be more significant for us than previously forecasted. To the extent investors believe the costs incurred for SFAS No. 123R are higher than those incurred by other companies, our stock price could be negatively impacted.
41
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
| | (b) | Issuer purchases of equity securities: |
We are authorized to repurchase up to $200 million of our common stock through open market purchases or private transactions that will be made from time to time as market conditions allow. The stock repurchase program is authorized to remain in effect until November 15, 2009. Shares repurchased under this program will be held in our treasury. The stock repurchase program may be suspended or discontinued at any time without prior notice. During the three months ended June 30, 2007, we did not repurchase any shares under this program. During the six months ended June 30, 2007, we repurchased a total of 573,558 shares, with an aggregate cost of $16.5 million. As of June 30, 2007, we had 22,152,770 shares held in treasury with an aggregate cost of $332.6 million.
The approximate dollar value of shares that may yet be purchased under the stock repurchase program was $75.0 million as of April 30, 2007, May 31, 2007 and June 30, 2007.
42
Item 6. Exhibits
| | |
| Exhibit No. | | Description |
| 2.1(10) | | Agreement and Plan of Merger, dated as of May 20, 2007, by and among Cytyc Corporation, Hologic, Inc. and Nor’easter Corp. |
| |
| 3.1(1) | | Fourth Restated Certificate of Incorporation of Cytyc Corporation. |
| |
| 3.2(2) | | Second Amended and Restated By-Laws of Cytyc Corporation. |
| |
| 4.1(3) | | Specimen certificate representing the Common Stock. |
| |
| 4.2(4) | | Rights Agreement, dated as of August 27, 1997, between Cytyc Corporation and BankBoston, N.A (the “Rights Agreement”) which includes as Exhibit A the Form of Certificate of Designations, as Exhibit B the Form of Rights Certificate, and as Exhibit C the Summary of Rights to Purchase Preferred Stock. |
| |
| 4.3(5) | | Amendment No. 1 to Rights Agreement, dated as of June 22, 1998, between Cytyc Corporation and BankBoston, N.A., amending the Rights Agreement. |
| |
| 4.4(6) | | Amendment to the Rights Agreement, dated as of January 3, 2003, among Cytyc Corporation, BankBoston, N.A. and EquiServe Trust Company, N.A. |
| |
| 4.5(7) | | Amendment No. 2 to Rights Agreement, dated as of November 6, 2003, between Cytyc Corporation and EquiServe Trust Company, N.A., amending the Rights Agreement. |
| |
| 4.5(11) | | Amendment No. 3 to Rights Agreement, dated as of May 20, 2007, by and between Cytyc Corporation and Computershare Trust Company, N.A. |
| |
| 10.1(8) | | Amendment No. 3, dated May 14, 2007, to the Credit Agreement, dated as of June 30, 2006, among Cytyc Corporation, the lenders and agents party thereto, and SunTrust Bank, as Administrative Agent. |
| |
| 10.2(9) | | Form of Restricted Stock Unit Agreement. |
| |
| 15 * | | Letter on Unaudited Interim Financial Information. |
| |
| 31.1 * | | Certification of Patrick J. Sullivan, Chief Executive Officer and President, pursuant to Rules 13a-14 and 15d-14 under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 31.2 * | | Certification of Timothy M. Adams, Senior Vice President, Chief Financial Officer, pursuant to Rules 13a-14 and 15d-14 under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 32.1 * | | Certification of Patrick J. Sullivan, Chief Executive Officer and President, pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| |
| 32.2 * | | Certification of Timothy M. Adams, Senior Vice President, Chief Financial Officer, pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| (1) | Incorporated herein by reference to Exhibit 3.2 to our Current Report on Form 8-K, filed September 20, 2005. |
| (2) | Incorporated herein by reference to Exhibit 3.1 to our Current Report on Form 8-K, filed September 19, 2005. |
| (3) | Incorporated herein by reference to the exhibits to our Registration Statement on Form S-1 (File No. 333-00300). |
| (4) | Incorporated herein by reference to Exhibit 4.1 to our Current Report on Form 8-K, filed August 29, 1997. |
| (5) | Incorporated herein by reference to Exhibit 4.2 to our Quarterly Report on Form 10-Q, filed August 13, 1998. |
| (6) | Incorporated herein by reference to Exhibit 4.4 to our Annual Report on Form 10-K, filed January 30, 2004. |
| (7) | Incorporated herein by reference to Exhibit 4.4 to our Quarterly Report on Form 10-Q, filed November 12, 2003. |
| (8) | Incorporated herein by reference to Exhibit 10.1 to our Quarterly Report on Form 10-Q, filed May 18, 2007. |
| (9) | Incorporated herein by reference to Exhibit 10.2 to our Quarterly Report on Form 10-Q, filed May 18, 2007. |
| (10) | Incorporated herein by reference to Exhibit 2.1 to our Current Report on Form 8-K, filed May 21, 2007. |
| (11) | Incorporated herein by reference to Exhibit 4.1 to our Current Report on Form 8-K, filed May 22, 2007. |
43
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | |
| | CYTYC CORPORATION |
| | |
| Date: August 8, 2007 | | By: | | /s/ PATRICK J. SULLIVAN |
| | | | Patrick J. Sullivan |
| | | | Chief Executive Officer and President |
| | |
| Date: August 8, 2007 | | By: | | /s/ TIMOTHY M. ADAMS |
| | | | Timothy M. Adams |
| | | | Senior Vice President, Chief Financial Officer and Treasurer |
44
EXHIBIT INDEX
| | |
| Exhibit No. | | Description |
| 2.1(10) | | Agreement and Plan of Merger, dated as of May 20, 2007, by and among Cytyc Corporation, Hologic, Inc. and Nor’easter Corp. |
| |
| 3.1(1) | | Fourth Restated Certificate of Incorporation of Cytyc Corporation. |
| |
| 3.2(2) | | Second Amended and Restated By-Laws of Cytyc Corporation. |
| |
| 4.1(3) | | Specimen certificate representing the Common Stock. |
| |
| 4.2(4) | | Rights Agreement, dated as of August 27, 1997, between Cytyc Corporation and BankBoston, N.A (the “Rights Agreement”) which includes as Exhibit A the Form of Certificate of Designations, as Exhibit B the Form of Rights Certificate, and as Exhibit C the Summary of Rights to Purchase Preferred Stock. |
| |
| 4.3(5) | | Amendment No. 1 to Rights Agreement, dated as of June 22, 1998, between Cytyc Corporation and BankBoston, N.A. |
| |
| 4.4(6) | | Amendment to the Rights Agreement, dated as of January 3, 2003, among Cytyc Corporation, BankBoston, N.A. and EquiServe Trust Company, N.A. |
| |
| 4.5(7) | | Amendment No. 2 to Rights Agreement, dated as of November 6, 2003, between Cytyc Corporation and EquiServe Trust Company, N.A. |
| |
| 4.5(11) | | Amendment No. 3 to Rights Agreement, dated as of May 20, 2007, by and between Cytyc Corporation and Computershare Trust Company, N.A. |
| |
| 10.1(8) | | Amendment No. 3, dated May 14, 2007, to the Credit Agreement, dated as of June 30, 2006, among Cytyc Corporation, the lenders and agents party thereto, and SunTrust Bank, as Administrative Agent. |
| |
| 10.2(9) | | Form of Restricted Stock Unit Agreement. |
| |
| 15 * | | Letter on Unaudited Interim Financial Information. |
| |
| 31.1 * | | Certification of Patrick J. Sullivan, Chief Executive Officer and President, pursuant to Rules 13a-14 and 15d-14 under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 31.2 * | | Certification of Timothy M. Adams, Senior Vice President, Chief Financial Officer, pursuant to Rules 13a-14 and 15d-14 under the Securities Exchange Act of 1934, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
| |
| 32.1 * | | Certification of Patrick J. Sullivan, Chief Executive Officer and President, pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| |
| 32.2 * | | Certification of Timothy M. Adams, Senior Vice President, Chief Financial Officer, pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| (1) | Incorporated herein by reference to Exhibit 3.2 to our Current Report on Form 8-K, filed September 20, 2005. |
| (2) | Incorporated herein by reference to Exhibit 3.1 to our Current Report on Form 8-K, filed September 19, 2005. |
| (3) | Incorporated herein by reference to the exhibits to our Registration Statement on Form S-1 (File No. 333-00300). |
| (4) | Incorporated herein by reference to Exhibit 4.1 to our Current Report on Form 8-K, filed August 29, 1997. |
| (5) | Incorporated herein by reference to Exhibit 4.2 to our Quarterly Report on Form 10-Q, filed August 13, 1998. |
| (6) | Incorporated herein by reference to Exhibit 4.4 to our Annual Report on Form 10-K, filed January 30, 2004. |
| (7) | Incorporated herein by reference to Exhibit 4.4 to our Quarterly Report on Form 10-Q, filed November 12, 2003. |
| (8) | Incorporated herein by reference to Exhibit 10.1 to our Quarterly Report on Form 10-Q, filed May 18, 2007. |
| (9) | Incorporated herein by reference to Exhibit 10.2 to our Quarterly Report on Form 10-Q, filed May 18, 2007. |
| (10) | Incorporated herein by reference to Exhibit 2.1 to our Current Report on Form 8-K, filed May 21, 2007. |
| (11) | Incorporated herein by reference to Exhibit 4.1 to our Current Report on Form 8-K, filed May 22, 2007. |
45

