UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
| | | |
| þ | | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
For the quarterly period ended December 31, 2005
OR
| | | |
| o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
For the transition period from to .
Commission File No. 1-15803
AVANIR PHARMACEUTICALS
(Exact name of registrant as specified in its charter)
| | | |
| California | | 33-0314804 |
| (State or other jurisdiction of | | (I.R.S. Employer Identification No.) |
| incorporation or organization) | | |
| | | |
| 11388 Sorrento Valley Road, San Diego, California | | 92121 |
| (Address of principal executive offices) | | (Zip Code) |
(858) 622-5200
(Registrant’s telephone number, including area code)
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities and Exchange Act of 1934 during the preceding 12 months (or for such shorter periods that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YESþ NOo
Indicate by check mark whether the registrant is an accelerated filer (as defined in Rule 12b-2 of the Exchange Act). YESþ NOo
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YESo NOþ
As of February 8, 2006, the registrant had 31,348,705 shares of common stock issued and outstanding.
PART I. FINANCIAL INFORMATION
Item 1. FINANCIAL STATEMENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Shareholders
Avanir Pharmaceuticals
We have reviewed the accompanying condensed consolidated balance sheet ofAvanir Pharmaceuticals and subsidiaries (the “Company”) as of December 31, 2005 and the related condensed consolidated statements of operations and cash flows for the three-month periods ended December 31, 2005 and 2004. These interim financial statements are the responsibility of the Company’s management.
We conducted our reviews in accordance with the standards of the Public Company Accounting Oversight Board (United States). A review of interim financial information consists principally of applying analytical procedures and making inquiries of persons responsible for financial and accounting matters. It is substantially less in scope than an audit conducted in accordance with the standards of the Public Company Accounting Oversight Board (United States), the objective of which is the expression of an opinion regarding the financial statements taken as a whole. Accordingly, we do not express such an opinion.
Based on our reviews, we are not aware of any material modifications that should be made to such condensed consolidated interim financial statements for them to be in conformity with accounting principles generally accepted in the United States of America.
We have previously audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheet ofAvanir Pharmaceuticals and subsidiaries as of September 30, 2005, and the related consolidated statements of operations, shareholders’ equity, and cash flows for the year then ended (not presented herein); and in our report dated December 14, 2005, we expressed an unqualified opinion on those consolidated financial statements. In our opinion, the information set forth in the accompanying condensed consolidated balance sheet as of September 30, 2005 is fairly stated, in all material respects, in relation to the consolidated balance sheet from which it has been derived.
DELOITTE & TOUCHE LLP
San Diego, California
February 9, 2006
3
AVANIR Pharmaceuticals
CONDENSED CONSOLIDATED BALANCE SHEETS (UNAUDITED)
| | | | | | | | | |
| | | December 31, | | | September 30, | |
| | | 2005 | | | 2005 | |
ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 30,801,894 | | | $ | 8,620,143 | |
| Short-term investments in securities | | | 17,476,908 | | | | 14,215,005 | |
| Receivables, net | | | 6,598,575 | | | | 1,169,654 | |
| Inventory | | | 136,487 | | | | 27,115 | |
| Prepaid expenses | | | 3,050,191 | | | | 2,370,801 | |
| | | | | | | |
| Total current assets | | | 58,064,055 | | | | 26,402,718 | |
| | | | | | | | | |
| Investments in securities | | | 2,206,450 | | | | 3,845,566 | |
| Restricted investments in securities | | | 856,872 | | | | 856,872 | |
| Property and equipment, net | | | 6,147,438 | | | | 6,004,527 | |
| Intangible assets, net | | | 3,591,003 | | | | 3,665,086 | |
| Long-term inventory | | | 602,619 | | | | 347,424 | |
| Other assets | | | 274,828 | | | | 279,797 | |
| | | | | | | |
| TOTAL ASSETS | | $ | 71,743,265 | | | $ | 41,401,990 | |
| | | | | | | |
| | | | | | | | | |
LIABILITIES AND SHAREHOLDERS’ EQUITY | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 3,083,727 | | | $ | 6,751,781 | |
| Accrued expenses and other liabilities | | | 7,079,723 | | | | 4,094,295 | |
| Accrued compensation and payroll taxes | | | 1,188,347 | | | | 1,272,231 | |
| Current portion of deferred revenue | | | 2,002,922 | | | | 1,970,989 | |
| Current portion of notes payable | | | 325,408 | | | | 317,667 | |
| Current portion of capital lease obligations | | | 26,983 | | | | 26,305 | |
| | | | | | | |
| Total current liabilities | | | 13,707,110 | | | | 14,433,268 | |
| Deferred revenue, net of current portion | | | 16,601,691 | | | | 17,187,221 | |
| Notes payable, net of current portion | | | 552,972 | | | | 637,285 | |
| Capital lease obligations, net of current portion | | | 2,330 | | | | 9,337 | |
| | | | | | | |
| Total liabilities | | | 30,864,103 | | | | 32,267,111 | |
| | | | | | | |
| | | | | | | | | |
COMMITMENTS AND CONTINGENCIES(Note 13) | | | | | | | | |
| | | | | | | | | |
| SHAREHOLDERS’ EQUITY: | | | | | | | | |
| Preferred stock — no par value, 10,000,000 shares authorized, no shares issued and outstanding as of December 31, 2005 and September 30, 2005 | | | — | | | | — | |
| Common stock — no par value, Class A, 200,000,000 shares authorized, 30,569,493 and 27,341,732 shares issued and outstanding as of December 31, 2005 and September 30, 2005, respectively (Note 2) | | | 201,739,213 | | | | 167,738,303 | |
| Unearned compensation | | | — | | | | (3,477,144 | ) |
| Accumulated deficit | | | (160,686,900 | ) | | | (155,012,466 | ) |
| Accumulated other comprehensive loss | | | (173,151 | ) | | | (113,814 | ) |
| | | | | | | |
| Total shareholders’ equity | | | 40,879,162 | | | | 9,134,879 | |
| | | | | | | |
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | | $ | 71,743,265 | | | $ | 41,401,990 | |
| | | | | | | |
See notes to consolidated financial statements.
4
AVANIR Pharmaceuticals
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (UNAUDITED)
| | | | | | | | | |
| | | Three months ended | |
| | | December 31, | |
| | | 2005 | | | 2004 | |
REVENUES: | | | | | | | | |
| Licenses | | $ | 5,000,000 | | | $ | 200,000 | |
| Research and development services | | | 2,478,018 | | | | — | |
| Royalties and sale of royalty rights | | | 582,045 | | | | 510,314 | |
| Government research grants | | | 84,825 | | | | 160,651 | |
| Product sales | | | — | | | | 17,400 | |
| | | | | | | |
| Total revenues | | | 8,144,888 | | | | 888,365 | |
| | | | | | | |
| | | | | | | | | |
OPERATING EXPENSES: | | | | | | | | |
| Research and development | | | 9,363,402 | | | | 5,054,241 | |
| Selling, general and administrative | | | 4,768,743 | | | | 2,951,003 | |
| Cost of product sales | | | — | | | | 3,102 | |
| | | | | | | |
| Total operating expenses | | | 14,132,145 | | | | 8,008,346 | |
| | | | | | | |
| | | | | | | | | |
LOSS FROM OPERATIONS | | | (5,987,257 | ) | | | (7,119,981 | ) |
| Interest income | | | 328,166 | | | | 121,832 | |
| Interest expense | | | (23,438 | ) | | | (21,621 | ) |
| Other income (expense), net | | | 10,512 | | | | (66,921 | ) |
| | | | | | | |
| | | | | | | | | |
LOSS BEFORE INCOME TAXES | | | (5,672,017 | ) | | | (7,086,691 | ) |
| Provision for income taxes | | | (2,417 | ) | | | (1,898 | ) |
| | | | | | | |
NET LOSS | | $ | (5,674,434 | ) | | $ | (7,088,589 | ) |
| | | | | | | |
| | | | | | | | | |
NET LOSS PER SHARE: | | | | | | | | |
BASIC AND DILUTED (Note 2) | | $ | (0.20 | ) | | $ | (0.30 | ) |
| | | | | | | |
| | | | | | | | | |
WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING: | | | | | | | | |
BASIC AND DILUTED (Note 2) | | | 28,579,357 | | | | 23,962,989 | |
| | | | | | | |
See notes to consolidated financial statements.
5
AVANIR Pharmaceuticals
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS (UNAUDITED)
| | | | | | | | | |
| | | Three Months Ended December 31, | |
| | | 2005 | | | 2004 | |
OPERATING ACTIVITIES: | | | | | | | | |
| Net loss | | $ | (5,674,434 | ) | | $ | (7,088,589 | ) |
| Adjustments to reconcile net loss to net cash used for operating activities: | | | | | | | | |
| Depreciation and amortization | | | 480,127 | | | | 470,289 | |
| Share-based compensation expense | | | 462,013 | | | | 2,701 | |
| Other-than-temporary impairment of investment | | | — | | | | 78,012 | |
| Loss on disposal of assets | | | 1,225 | | | | 785 | |
| Intangible assets abandoned | | | 34,728 | | | | 170,328 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Receivables | | | (5,428,921 | ) | | | (51,815 | ) |
| Inventory | | | (364,567 | ) | | | 3,102 | |
| Prepaid expenses and other assets | | | (679,390 | ) | | | 344,077 | |
| Accounts payable | | | (3,581,490 | ) | | | (229,828 | ) |
| Accrued expenses and other liabilities | | | 2,887,539 | | | | 592,289 | |
| Accrued compensation and payroll taxes | | | (83,884 | ) | | | (42,406 | ) |
| Deferred revenue | | | (553,597 | ) | | | (608,329 | ) |
| | | | | | | |
| Net cash used for operating activities | | | (12,500,651 | ) | | | (6,359,384 | ) |
| | | | | | | |
| | | | | | | | | |
INVESTING ACTIVITIES: | | | | | | | | |
| Investments in securities | | | (13,532,124 | ) | | | (4,259,665 | ) |
| Proceeds from sales and maturities of investments in securities | | | 11,850,000 | | | | 4,900,000 | |
| Patent costs | | | (25,314 | ) | | | (203,937 | ) |
| Purchases of property and equipment | | | (543,300 | ) | | | (57,652 | ) |
| | | | | | | |
| Net cash (used for) provided by investing activities | | | (2,250,738 | ) | | | 378,746 | |
| | | | | | | |
| | | | | | | | | |
FINANCING ACTIVITIES: | | | | | | | | |
| Proceeds from issuance of common stock, net | | | 37,016,041 | | | | 7,055,590 | |
| Proceeds from issuance of notes payable | | | — | | | | 136,438 | |
| Payments on notes and capital lease obligations | | | (82,901 | ) | | | (90,079 | ) |
| | | | | | | |
| Net cash provided by financing activities | | | 36,933,140 | | | | 7,101,949 | |
| | | | | | | |
| | | | | | | | | |
| Net increase in cash and cash equivalents | | | 22,181,751 | | | | 1,121,311 | |
| Cash and cash equivalents at beginning of period | | | 8,620,143 | | | | 13,494,083 | |
| | | | | | | |
| Cash and cash equivalents at end of period | | $ | 30,801,894 | | | $ | 14,615,394 | |
| | | | | | | |
| | | | | | | | | |
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | | | | | | | | |
| Interest paid | | $ | 23,438 | | | $ | 21,621 | |
| Income taxes paid | | $ | 2,417 | | | $ | 1,898 | |
| | | | | | | | | |
NONCASH INVESTING ACTIVITIES: | | | | | | | | |
| Purchases of property and equipment which are included in accounts payable and accrued expenses | | $ | 253,538 | | | $ | 5,253 | |
| Elimination of unearned compensation against common stock | | $ | 3,477,144 | | | $ | — | |
See notes to consolidated financial statements.
6
AVANIR Pharmaceuticals
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
Avanir Pharmaceuticals (“Avanir,” “we,” or the “Company”) prepared the accompanying unaudited condensed consolidated financial statements in accordance with the rules and regulations of the Securities and Exchange Commission (“SEC”) including the instructions to Form 10-Q and Rule 10-01 of Regulation S-X. Such rules and regulations allow us to condense and omit certain information and footnote disclosures normally included in audited financial statements prepared in accordance with accounting principles generally accepted in the United States. We believe these condensed consolidated financial statements reflect all adjustments (consisting only of normal, recurring adjustments) that are necessary for a fair presentation of our financial position and results of operations for the periods presented. Results of operations for the interim periods presented are not necessarily indicative of results to be expected for the year. The information included in this Form 10-Q should be read in conjunction with the financial statements and notes thereto included in our fiscal 2005 Form 10-K.
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts and the disclosure of contingent amounts in the financial statements and accompanying notes. Actual results could differ from those estimates. Certain prior year amounts have been reclassified to conform to the current year presentation. These reclassifications had no impact on net earnings as previously reported.
The unaudited condensed consolidated financial statements include the accounts ofAvanir Pharmaceuticals and its wholly-owned subsidiaries, Xenerex Biosciences and Avanir Holding Company. Intercompany accounts and transactions have been eliminated.
On January 5, 2006, our Board of Directors approved a one-for-four reverse stock split of our common stock. The reverse stock split was previously approved by our shareholders at our annual shareholder meeting held on March 17, 2005 and was implemented on January 17, 2006. Our common stock began trading on a split-adjusted basis on January 18, 2006, under the new ticker symbol of “AVN.R”. All share and per share information herein (including shares outstanding, earnings per share and warrant and stock option exercise prices) reflect this reverse stock split.
| 3. | | SIGNIFICANT ACCOUNTING POLICIES |
Change in Accounting Method for Share-Based Compensation
In December 2004, the Financial Accounting Standards Board (“FASB”) revised Statement of Accounting Standards No. 123 (“SFAS 123(R)”), “Share-Based Payment.” On April 14, 2005, the U.S. Securities and Exchange Commission adopted a new rule amending the effective dates for SFAS 123(R). In accordance with the new rule, the accounting provisions of SFAS 123(R) became effective for us beginning in the quarter ended December 31, 2005.
We adopted the provisions of SFAS 123(R) on October 1, 2005, the first day of our fiscal 2006. Accordingly, compensation costs for all share-based awards to employees are measured based on the grant date fair value of those awards and recognized over the period during which the employee is required to perform service in exchange for the award (generally over the vesting period of the award). We have no awards with market or performance conditions. Excess tax benefits, as defined by SFAS 123(R), will be recognized as an addition to common stock. Effective October 1, 2005 and for all periods
7
subsequent to that date, SFAS 123(R) supersedes our previous accounting under Accounting Principles Board Opinion No. 25, “Accounting for Stock Issued to Employees”(“APB 25”). In March 2005, the Securities and Exchange Commission issued Staff Accounting Bulletin No. 107 (“SAB 107”) relating to SFAS 123(R). The Company has applied the provisions of SAB 107 in its adoption of SFAS 123(R).
On November 10, 2005, the FASB issued FASB Staff Position No. FAS 123(R)-3, “Transition Election Related to Accounting for Tax Effects of Share-Based Payment Awards” (“FAS 123(R)-3”. The alternative transition method includes simplified methods to establish the beginning balance of the additional paid-in capital pool (“APIC pool”) related to the tax effects of employee share-based compensation, and to determine the subsequent impact on the APIC pool and consolidated statements of cash flows of the tax effects of employee share-based compensation awards that are outstanding upon adoption of SFAS 123(R). An entity may make a one-time election to adopt the transition method described in this guidance. An entity may take up to one year from the later of its initial adoption of SFAS 123(R) or the effective date of this guidance, which was November 11, 2005. We are in the process of determining whether to adopt the alternative transition method provided in FAS 123(R)-3 for calculating the tax effects of share-based compensation pursuant to SFAS 123(R).
We adopted SFAS 123(R) using the modified prospective transition method, which provides for certain changes to the method for valuing share-based compensation. The valuation provisions of SFAS 123(R) apply to new awards and to awards that are outstanding at the effective date and subsequently modified or cancelled. Estimated compensation expense for awards outstanding at the effective date will be recognized over the remaining service period using the compensation cost calculated for pro forma disclosure purposes under FASB Statement No. 123, “Accounting for Stock-Based Compensation” (“SFAS 123”). Our consolidated financial statements as of and for the quarter ended December 31, 2005 reflect the impact of SFAS 123(R). In accordance with the modified prospective transition method, our consolidated financial statements for prior periods were not restated to reflect, and do not include, the impact of SFAS 123(R).
Share-based compensation expense recognized during the period is based on the value of the portion of share-based payment awards that is ultimately expected to vest during the period. Share-based compensation expense recognized in our consolidated statement of operations for the first quarter of fiscal 2006 included compensation expense for share-based payment awards granted prior to, but not yet vested as of, September 30, 2005 based on the grant date fair value estimated in accordance with the pro forma provisions of SFAS 123 and share-based payment awards granted subsequent to September 30, 2005 based on the grant date fair value estimated in accordance with SFAS 123(R). For share awards granted in fiscal 2006, expenses are amortized under the straight-line attribution method. For share awards granted prior to fiscal 2006, expenses are amortized under the straight-line single option method prescribed by SFAS 123. As share-based compensation expense recognized in the consolidated statement of operations for the first quarter of fiscal 2006 is based on awards ultimately expected to vest, it has been reduced for estimated forfeitures. SFAS 123(R) requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Pre-vesting forfeitures were estimated to be approximately 8% for both officers and directors and 13% for employees in the first quarter of fiscal 2006 based on our historical experience. In our pro forma information required under SFAS 123 for the periods prior to fiscal 2006, we accounted for forfeitures as they occurred.
8
Total estimated share-based compensation expense, related to all of our share-based awards, recognized under SFAS 123(R) for the quarter ended December 31, 2005 was comprised of the following:
| | | | | |
| | | Quarter Ended | |
| | | December 31, 2005 | |
| Research and development | | $ | 76,347 | |
| Selling, general and administrative | | | 385,666 | |
| | | | |
| Share-based compensation expense before taxes | | | 462,013 | |
| Related income tax benefits | | | — | |
| Share-based compensation expense | | $ | 462,013 | |
| | | | |
| Net share-based compensation expense per basic and diluted common share | | $ | (0.02 | ) |
| | | | |
Share-based compensation expense recognized under SFAS 123(R) for the quarter ended December 31, 2005 included $247,000 from stock options and $215,000 from restricted stock awards. Since we have a net operating loss carryforward as of December 31, 2005, no excess tax benefits for the tax deductions related to share-based awards were recognized in the consolidated statement of operations. Additionally, no incremental tax benefits were recognized from stock options exercised in the quarter ended December 31, 2005 which would have resulted in a reclassification to reduce net cash provided by operating activities with an offsetting increase in net cash provided by financing activities. Share-based compensation expense was not recognized during the quarter ended December 31, 2004. As of December 31, 2005, $6.0 million of total unrecognized compensation costs related to non-vested awards is expected to be recognized over a weighted average period of 2.7 years.
As of December 31, 2005, we had five equity incentive plans (the “Plans”): the 2005 Equity Incentive Plan, the 2003 Equity Incentive Plan, the 2000 Stock Option Plan, the 1998 Stock Option Plan and the 1994 Stock Option Plan. All of the Plans were approved by the shareholders, except for the 2003 Equity Incentive Plan, which was approved solely by the Board of Directors. Under the Plans we had an aggregate of 2,712,468 shares of our common stock reserved for issuance as of December 31, 2005. Of those shares, 1,765,345 were subject to outstanding options and other awards and 947,123 shares were available for future grants of share-based awards. The share amounts have been adjusted for a one-for-four reverse stock split (see Note 2, “Reverse Stock Split”). Options are subject to terms and conditions established by the Compensation Committee of our Board of Directors. At the present time, we intend to issue new common shares upon the exercise of stock options. None of the share-based awards are classified as a liability as of December 31, 2005.
Stock Options. Options awards are generally granted with an exercise price equal to the fair market value of our common stock at the grant date and have 10-year contractual terms. Options awards typically vest in accordance with one of the following schedules:
| | a. | | 25% of the option shares vest and become exercisable on the first anniversary of the grant date and the remaining 75% of the option shares vest and become exercisable quarterly in equal installments thereafter over three years. |
| |
| | b. | | One-third of the option shares vest and become exercisable on the first anniversary of the grant date and the remaining two-thirds of the option shares vest and become exercisable daily or quarterly in equal installments thereafter over two years. |
| |
| | c. | | Fully vest and become exercisable at the date of grant. |
Certain option awards provide for accelerated vesting if there is a change in control (as defined in the Plans).
9
The fair value of each option award is estimated on the date of grant using a Black-Scholes-Merton option pricing model (“Black-Scholes model”) that uses the assumptions noted in the following table. Expected volatilities are based on historical volatility of our common stock and other factors. The expected term of options granted is based on analyses of historical employee termination rates and option exercises. The risk-free interest rates are based on the U.S. Treasury yield for a period consistent with the expected term of the option in effect at the time of the grant. Assumptions used in the Black-Scholes model for the quarter ended December 31, 2005 were as follows:
| | | | | |
| Expected volatility | | | 79.1% - 80.4 | % |
| Weighted-average volatility | | | 79.1 | % |
| Average expected term in years | | | 4.5 | |
| Risk-fee interest rate (zero coupon U.S. Treasury Note) | | | 4.2 | % |
| Expected dividend yield | | | 0 | % |
A summary of option activity under the Plans as of December 31, 2005 and changes during the quarter then ended is presented below. The option share amounts and exercise prices have been adjusted for a one-for-four reverse stock split (see Note 2, “Reverse Stock Split”).
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Weighted | | | | |
| | | | | | | Weighted | | | average | | | | |
| | | | | | | average | | | remaining | | | Aggregate | |
| | | | | | | exercise | | | contractual | | | intrinsic | |
| | | Number of | | | price per | | | term | | | value | |
| | | shares | | | share | | | (in years) | | | (in millions) | |
| Outstanding at September 30, 2005 | | | 1,600,032 | | | $ | 8.76 | | | | | | | | | |
| Granted | | | 221,813 | | | $ | 11.71 | | | | | | | | | |
| Exercised | | | (58,000 | ) | | $ | 3.29 | | | | | | | | | |
| Forfeited or expired | | | (11,000 | ) | | $ | 12.08 | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| Outstanding at December 31, 2005 | | | 1,752,845 | | | $ | 9.27 | | | | 6.20 | | | $ | 8.1 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Exercisable at December 31, 2005 | | | 1,245,735 | | | $ | 8.26 | | | | 4.85 | | | $ | 7.1 | |
The weighted average grant-date fair values of options granted during the quarters ended December 31, 2005 and 2004 were $7.44 per share and $10.70 per share, respectively. The total intrinsic value of options exercised during the quarters ended December 31, 2005 and 2004 was $604,000 and $100,000, respectively.
A summary of the status of our non-vested stock options as of December 31, 2005 and changes during the quarter then ended are presented below. The option share amounts and exercise prices were adjusted for a one-for-four reverse stock split (see Note 2, “Reverse Stock Split).
| | | | | | | | | |
| | | | | | | Weighted | |
| | | | | | | average | |
| | | | | | | grant-date | |
| | | Number of | | | fair value per | |
| | | shares | | | share | |
| Nonvested at September 30, 2005 | | | 342,137 | | | $ | 8.37 | |
| Granted | | | 221,813 | | | $ | 7.44 | |
| Vested | | | (56,839 | ) | | $ | 8.22 | |
| | | | | | | | |
| Nonvested at December 31, 2005 | | | 507,111 | | | $ | 7.87 | |
| | | | | | | | |
Share-based compensation expense recognized under SFAS 123(R) from stock options was $247,000 for the quarter ended December 31, 2005. As of December 31, 2005, there was $2.6 million of total
10
unrecognized compensation cost, related to non-vested stock options, which is expected to be recognized over a weighted-average period of 2.7 years. The total fair values of shares vested during the quarters ended December 31, 2005 and 2004 were $343,000 and $282,000 respectively.
Restricted stock awards. Restricted stock awards are grants that entitle the holder to acquire shares of restricted common stock at a fixed price, which is typically nominal. The shares of restricted stock cannot be sold, pledged, or otherwise disposed of until the award vests and any unvested shares may be reacquired by us for the original purchase price following the awardee’s termination of service. This repurchase option lapses as the awards vest, which is typically on the second or third anniversary of the grant date or on a graded vesting schedule over three years of employment. The value of such stock is based on a five-day average market price including the two days prior, the day of and the two days after the date of grant. A summary of our outstanding restricted stock awards as of December 31, 2005 and changes during the quarter period then ended are presented below. The share amounts and exercise prices have been adjusted for a one-for-four reverse stock split (see Note 2, “Reverse Stock Split”).
| | | | | | | | | | | | | |
| | | | | | | | | | | Weighted | |
| | | | | | | | | | | average | |
| | | | | | | Weighted | | | remaining | |
| | | Number of | | | average grant | | | contractual life | |
| | | shares | | | date fair value | | | (in years) | |
| Unvested, September 30, 2005 | | | 250,000 | | | $ | 3.56 | | | | 2.9 | |
| Granted | | | 12,500 | | | $ | 3.27 | | | | | |
| | | | | | | | | | | | |
| Unvested, December 31, 2005 | | | 262,500 | | | $ | 3.54 | | | | 2.7 | |
| | | | | | | | | | | | |
Share-based compensation expense recognized under SFAS 123(R) from restricted stock awards was $215,000 for the quarter ended December 31, 2005. The grant-date fair value of a restricted stock award granted during the quarter ended December 31, 2005 was $164,000. No restricted stock awards were granted in the quarter ended December 31, 2004. As of December 31, 2005, the total unrecognized compensation cost related to unvested shares was $3.4 million, which is expected to be recognized over a weighted-average period of 2.7 years. The total fair value of shares vested during the quarters ended December 31, 2005 and 2004 was $299,000 and $0, respectively.
For the quarter ended December 31, 2005, we received a total of $191,000 in cash from options exercised under all share-based payment arrangements.
Pro Forma Information under SFAS 123 for Periods Prior to Fiscal 2006. Through fiscal 2005, we accounted for share-based awards to employees using the intrinsic value method in accordance with APB 25 and related interpretations and provided the required pro forma disclosures of SFAS 123. Under the intrinsic value method, no share-based compensation expense had been recognized in our consolidated statement of operations for share-based awards to employees, because the exercise price of our stock options granted to employees equaled the fair market value of the underlying stock at the date of grant.
11
The following table summarizes the pro forma effect on our net loss and per share data if we had applied the fair value recognition provisions of SFAS 123 to share-based employee compensation for the quarter period ended December 31, 2004.
| | | | | |
| | | Quarter Ended | |
| | | December 31, 2004 | |
| Net loss, as reported | | $ | (7,088,589 | ) |
| Add: Share-based employee compensation expense | | | — | |
| Deduct: Total share-based employee compensation expense determined under fair value based method for all awards | | | (281,691 | ) |
| | | | |
| Pro forma net loss | | $ | (7,370,280 | ) |
| | | | |
| Net loss per share (adjusted for a one-for-four reverse stock split. See Note 2, “Reverse Stock Split”): | | | | |
| Basic and diluted — as reported | | $ | (0.30 | ) |
| Basic and diluted — pro forma | | $ | (0.31 | ) |
For employee stock options granted during the quarter ended December 31, 2004, we determined pro forma compensation expense under the provisions of SFAS 123 using the Black-Scholes model and the following assumptions: (1) an expected volatility of 130%, (2) an expected term of 3.4 years, (3) a risk-free interest rate of 3.2% and (4) an expected dividend yield of 0%. The weighted average fair value of options granted during the quarter ended December 31, 2004 was $10.70 per share.
We account for stock options granted to non-employees in accordance with Emerging Issues Task Force No. 96-18, “Accounting for Equity Instruments That Are Issued to Other Than Employees for Acquiring, or in Conjunction with Selling, Goods or Services,” (“EITF 96-18”). Under EITF 96-18, we determine the fair value of the stock options granted as either the fair value of the consideration received or the fair value of the equity instruments issued, whichever is more reliably measurable.
Revenue Recognition
We recognize revenue in accordance with SEC Staff Accounting Bulletin Topic 13, “Revenue Recognition” and Emerging Issues Task Force No. 00-21 (“EITF 00-21”), “Accounting for Revenue Arrangements with Multiple Deliverables.” Revenue is recognized when four basic criteria of revenue recognition are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services rendered; (3) the fee is fixed or determinable; and (4) collectibility is reasonably assured.
Contract Revenue from Collaborations. Collaborative arrangements typically consist of non-refundable and/or exclusive technology access or data transfer fees, reimbursements for costs of specific research and development, and various milestone and future product royalty payments. If the delivered technology does not have standalone value or if we do not have objective or reliable evidence of the fair value of the undelivered component, the amount of revenue allocable to the delivered technology is deferred. Nonrefundable, up-front license or technology access fees with standalone value that are not dependent on any future performance by us under the arrangements are recognized as revenue upon the earlier of when payments are received or collection is assured, but are deferred if we have continuing performance obligations. If we have continuing involvement through research and development collaborations or other contractual obligations, such up-front fees are deferred and recognized over the collaboration period or the period for which we continue to have a performance obligation, unless all of the following criteria exist: (1) the collaboration or contractual obligations have standalone value to the customer, (2) there is objective and reliable evidence of the fair value of the undelivered item(s), and (3) there is no general right to return the delivered item(s).
12
Payments related to substantive, performance-based milestones are recognized as revenue upon the achievement of the milestones as specified in the underlying agreements, which represent the culmination of the earnings process. Revenue from research services is recognized during the period in which the services are performed and is based upon the number of FTE personnel working on specific project at the agreed-upon rate. Reimbursements from collaborative partners for agreed upon direct costs including research stock and outsourced pre-clinical studies are recognized as revenues in the period the reimbursable expenses are incurred. Payments received in advance are recorded as deferred revenue until the research is performed, costs are incurred, or a milestone is reached.
Royalty Revenues.We recognize royalty revenues from licensed products when earned in accordance with the terms of the license agreements. Net sales figures used for calculating royalties include deductions for costs of unsaleable returns, managed care chargebacks, cash discounts, freight and warehousing, and miscellaneous write-offs, which may vary over the course of the license agreement.
Revenues from Sale of Royalty Rights.In agreements where we have sold our rights to future royalties under license agreements and we maintain continuing involvement in earning such royalties, we defer revenues and recognize them over the life of the license agreement. For example, in the sale of an undivided interest of our abreva® license agreement to Drug Royalty USA, revenue recognition is being determined under the “units-of-revenue method”. Under this method, the amount of deferred revenue to be recognized as revenue in each period is calculated by multiplying the following: (1) the ratio of the royalty payments due to Drug Royalty USA for the period to the total remaining royalties that we expect GlaxoSmithKline will pay Drug Royalty USA over the term of the agreement by (2) the unamortized deferred revenue amount.
Revenue Arrangements with Multiple Deliverables.We have revenue arrangements whereby we are obligated to deliver to the customer multiple products and/or services (multiple deliverables). Such arrangements may include antibody generation services agreements and other forms of research collaborations. In accordance with EITF 00-21, we analyze our multiple element arrangements to determine whether the elements can be separated and accounted for individually as separate units of accounting. The evaluation is performed at the inception of the arrangement. The delivered item(s) is (are) considered a separate unit of accounting if all of the following criteria are met: (1) the delivered item(s) has (have) value to the customer on a standalone basis; (2) there is objective and reliable evidence of the fair value of the undelivered item(s); and (3) if the arrangement includes a general right of return relative to the delivered item, delivery, or performance of the undelivered item(s) is (are) considered probable and substantially in our control.
Government Research Grant Revenue.We recognize revenues from federal research grants during the period in which the related expenditures are incurred.
Product Sales — Active Pharmaceutical Ingredient Docosanol.Revenue from product sales, which consist of sales of our active pharmaceutical ingredient docosanol, is recorded when the above criteria are met, generally when title and risk of loss have passed to the buyer upon delivery. We sell the active pharmaceutical ingredient docosanol to various licensees and ship only on written order for the materials. Total shipments generally occur fewer than five times a year. Our contracts for sales of the active pharmaceutical ingredient docosanol include buyer acceptance provisions that give our buyers the right of replacement if the delivered materials do not meet specified criteria by giving notice within 30 days after receipt of such defective materials. We have the option to refund or replace any such defective materials however, we have historically demonstrated that the materials shipped from the same pre-inspected lot have consistently met the specified criteria and no buyer has rejected any of our shipments from the same pre-inspected lot to date. Therefore, we recognize revenue from sales of the active pharmaceutical ingredient docosanol without providing for such contingency upon shipment.
13
Recognition of Expenses in Outsourced Contracts
Pursuant to management’s assessment of the services that have been performed on clinical trials and other contracts, we recognize expenses as the services are provided. Such management assessments include, but are not limited to: (1) an evaluation by the project manager of the work that has been completed during the period, (2) measurement of progress prepared internally and/or provided by the third-party service provider, (3) analyses of data that justify the progress, and (4) management’s judgment.
Research and Development Expenses
Research and development expenses consist of expenses incurred in performing research and development activities including salaries and benefits, facilities and other overhead expenses, clinical trials, contract services and other outside expenses. Research and development expenses are charged to operations as they are incurred. Up-front payments to collaborators made in exchange for the avoidance of potential future milestone and royalty payments on licensed technology are also charged to research and development expense when the drug is still in the development stage, has not been approved by the FDA for commercialization and concurrently has no alternative uses.
We assess our obligations to make milestone payments that may become due under licensed or acquired technology to determine whether the payments should be expensed or capitalized. We charge milestone payments to research and development expense when:
| | • | | The technology is in the early stage of development and has no alternative uses; |
| |
| | • | | There is substantial uncertainty of the technology or product being successful; |
| |
| | • | | There will be difficulty in completing the remaining development; and |
| |
| | • | | There is substantial cost to complete the work. |
Acquired contractual rights. Payments to acquire contractual rights to a licensed technology or drug candidate are expensed as incurred when there is uncertainty in receiving future economic benefits from the acquired contractual rights. We consider the future economic benefits from the acquired contractual rights to a drug candidate to be uncertain until such drug candidate is approved by the FDA or when other significant risk factors are abated.
Capitalization and Valuation of Long-Lived and Intangible Assets
Intangible assets with finite useful lives consist of capitalized legal costs incurred in connection with patents, patent applications pending and license agreements. We amortize costs of approved patents, patent applications pending and license agreements over their estimated useful lives, or terms of the agreements, whichever are shorter. For patents pending, we amortize the costs over the shorter of a period of twenty years from the date of filing the application or, if licensed, the term of the license agreement. We re-assess the useful lives of patents when they are issued, or whenever events or changes in circumstances indicate the useful lives may have changed. For patent and patent applications pending and trademarks that we abandon, we charge the remaining unamortized accumulated costs to expense.
Intangible assets and long-lived assets are evaluated for impairment whenever events or changes in circumstances indicate that their carrying value may not be recoverable. If the review indicates that intangible assets or long-lived assets are not recoverable (i.e. the carrying amount is less than the future projected undiscounted cash flows), their carrying amount would be reduced to fair value. Factors we consider important that could trigger an impairment review include the following:
14
| | • | | A significant underperformance relative to expected historical or projected future operating results; |
| |
| | • | | A significant change in the manner of our use of the acquired asset or the strategy for our overall business; and/or |
| |
| | • | | A significant negative industry or economic trend. |
When we determine that the carrying value of intangible assets or long-lived assets are not recoverable based upon the existence of one or more of the above indicators of impairment, we may be required to record impairment charges for these assets.
Stock Offering Costs
Expenses incurred in connection with common stock issuances are recorded as an offset to additional paid-in capital on the condensed consolidated balance sheets. These expenses consist of underwriters’ discounts and commissions and third-party related offering expenses.
4. INVESTMENTS
Investments in securities as of December 31, 2005 and September 30, 2005 consisted of the following:
| | | | | | | | | | | | | | | | | |
| | | | | | | Gross | | | Gross | | | | |
| | | Amortized | | | Unrealized | | | Unrealized | | | Fair | |
| | | Cost | | | Gains (1) | | | Losses (1) | | | Value | |
| As of December 31, 2005: | | | | | | | | | | | | | | | | |
| Certificates of deposit | | $ | 856,872 | | | $ | — | | | $ | — | | | $ | 856,872 | |
| Government debt securities | | | 19,856,509 | | | | — | | | | (173,151 | ) | | | 19,683,358 | |
| | | | | | | | | | | | | |
| Total | | $ | 20,713,381 | | | $ | — | | | $ | (173,151 | ) | | $ | 20,540,230 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Classified and reported as: | | | | | | | | | | | | | | | | |
| Short term investments: | | | | | | | | | | | | | | | | |
| Classified as available-for-sale | | | | | | | | | | | | | | $ | 17,476,908 | |
| | | | | | | | | | | | | | | | |
| Long term investments: | | | | | | | | | | | | | | | | |
| Classified as available-for-sale | | | | | | | | | | | | | | | 2,206,450 | |
| Restricted investments in securities (2) | | | | | | | | | | | | | | | 856,872 | |
| | | | | | | | | | | | | | | | |
| Long-term investments | | | | | | | | | | | | | | | 3,063,322 | |
| | | | | | | | | | | | | | | | |
| Total | | | | | | | | | | | | | | $ | 20,540,230 | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | Gross | | | Gross | | | | |
| | | Amortized | | | Unrealized | | | Unrealized | | | Fair | |
| | | Cost | | | Gains (3) | | | Losses (3) | | | Value | |
| As of September 30, 2005: | | | | | | | | | | | | | | | | |
| Certificates of deposit | | $ | 956,872 | | | $ | 48 | | | $ | — | | | $ | 956,920 | |
| Government debt securities | | | 18,074,385 | | | | — | | | | (113,862 | ) | | | 17,960,523 | |
| | | | | | | | | | | | | |
| Total | | $ | 19,031,257 | | | $ | 48 | | | $ | (113,862 | ) | | $ | 18,917,443 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Classified and reported as: | | | | | | | | | | | | | | | | |
| Short term investments: | | | | | | | | | | | | | | | | |
| Classified as available-for-sale | | | | | | | | | | | | | | $ | 14,215,005 | |
| | | | | | | | | | | | | | | | |
| Long term investments: | | | | | | | | | | | | | | | | |
| Classified as available-for-sale | | | | | | | | | | | | | | | 3,845,566 | |
| Restricted investments in securities (2) | | | | | | | | | | | | | | | 856,872 | |
| | | | | | | | | | | | | | | | |
| Long-term investments | | | | | | | | | | | | | | | 4,702,438 | |
| | | | | | | | | | | | | | | | |
| Total | | | | | | | | | | | | | | $ | 18,917,443 | |
| | | | | | | | | | | | | | | | |
| | |
| (1) | | Gross unrealized losses of $173,151 on government debt securities are reported as “accumulated other comprehensive loss” on the consolidated balance sheet as of December 31, 2005. |
| |
| (2) | | Represents amounts pledged to our bank as collateral for letters of credit issued in connection with our leases of office and laboratory space. |
| | |
| |
| (3) | | Gross unrealized gains of $48 and gross unrealized losses of $113,862 on government securities and certificates of deposit represent an accumulated net unrealized loss of $113,814, which is reported as “accumulated other comprehensive loss” on the consolidated balance sheet as of September 30, 2005. |
15
5. RECEIVABLES, NET
Receivables as of December 31 2005 and September 30, 2005 consist of the following:
| | | | | | | | | |
| | | December 31, | | | September 30, | |
| | | 2005 | | | 2005 | |
| Receivables | | $ | 5,181,922 | | | $ | 186,851 | |
| Unbilled receivables | | | 1,444,752 | | | | 1,010,902 | |
| | | | | | | |
| | | | 6,626,674 | | | | 1,197,753 | |
| Allowance for doubtful accounts | | | (28,099 | ) | | | (28,099 | ) |
| | | | | | | |
| Receivables, net | | $ | 6,598,575 | | | $ | 1,169,654 | |
| | | | | | | |
6. INVENTORY
Inventory as of December 31, 2005 and September 30, 2005 is as follows:
| | | | | | | | | |
| | | December 31, | | | September 30, | |
| | | 2005 | | | 2005 | |
| Inventory | | $ | 739,106 | | | $ | 374,539 | |
| Less: current portion | | | 136,487 | | | | 27,115 | |
| | | | | | | |
| Long-term portion | | $ | 602,619 | | | $ | 347,424 | |
| | | | | | | |
7. PROPERTY AND EQUIPMENT
Property and equipment consist of the following:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2005 | | | September 30, 2005 | |
| | | Gross | | | | | | | | | | | Gross | | | | | | | |
| | | Carrying | | | Accumulated | | | | | | | Carrying | | | Accumulated | | | | |
| | | Value | | | Depreciation | | | Net | | | Value | | | Depreciation | | | Net | |
| Research and development equipment | | $ | 3,970,343 | | | $ | (2,721,921 | ) | | $ | 1,248,422 | | | $ | 3,903,735 | | | $ | (2,567,843 | ) | | $ | 1,335,892 | |
| Computer equipment and related software | | | 1,197,575 | | | | (595,478 | ) | | | 602,097 | | | | 1,038,390 | | | | (565,137 | ) | | | 473,253 | |
| Leasehold improvements | | | 5,621,308 | | | | (1,822,656 | ) | | | 3,798,652 | | | | 5,583,177 | | | | (1,641,485 | ) | | | 3,941,692 | |
| Furniture, fixtures and equipment | | | 825,869 | | | | (327,602 | ) | | | 498,267 | | | | 558,911 | | | | (305,221 | ) | | | 253,690 | |
| | | | | | | | | | | | | | | | | | | |
| Total property and equipment | | $ | 11,615,095 | | | $ | (5,467,657 | ) | | $ | 6,147,438 | | | $ | 11,084,213 | | | $ | (5,079,686 | ) | | $ | 6,004,527 | |
| | | | | | | | | | | | | | | | | | | |
Depreciation expense related to property and equipment for the quarters ended December 31, 2005 and 2004 was approximately $410,000 and $418,000, respectively.
16
8. INTANGIBLE ASSETS
At December 31, 2005 and September 30, 2005, the components of amortizable and unamortizable intangibles are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, 2005 | | | September 30, 2005 | |
| | | Gross | | | | | | | | | | | Gross | | | | | | | |
| | | Carrying | | | Accumulated | | | | | | | Carrying | | | Accumulated | | | | |
| | | Value | | | Amortization | | | Net | | | Value | | | Amortization | | | Net | |
| Intangible assets with finite lives: | | | | | | | | | | | | | | | | | | | | | | | | |
| Patent applications pending (1) | | $ | 2,917,181 | | | $ | (363,872 | ) | | $ | 2,553,309 | | | $ | 3,029,127 | | | $ | (336,457 | ) | | $ | 2,692,670 | |
| Patents | | | 1,521,313 | | | | (533,097 | ) | | | 988,216 | | | | 1,429,532 | | | | (506,144 | ) | | | 923,388 | |
| Licenses | | | 42,461 | | | | (18,468 | ) | | | 23,993 | | | | 42,461 | | | | (17,904 | ) | | | 24,557 | |
| | | | | | | | | | | | | | | | | | | |
| Total intangible assets with finite lives | | | 4,480,955 | | | | (915,437 | ) | | | 3,565,518 | | | | 4,501,120 | | | | (860,505 | ) | | | 3,640,615 | |
| Intangible assets with indefinite useful lives: | | | | | | | | | | | | | | | | | | | | | | | | |
| Trademarks | | | 25,485 | | | | — | | | | 25,485 | | | | 24,471 | | | | — | | | | 24,471 | |
| | | | | | | | | | | | | | | | | | | |
| Total intangible assets | | $ | 4,506,440 | | | $ | (915,437 | ) | | $ | 3,591,003 | | | $ | 4,525,591 | | | $ | (860,505 | ) | | $ | 3,665,086 | |
| | | | | | | | | | | | | | | | | | | |
| | |
| (1) | | Patent applications pending include the net effect of $34,728 and $170,328 (net of accumulated amortization of $3,409 and $18,321) in intangible assets abandoned during the quarters ended December 31, 2005 and 2004, respectively. We abandoned certain patents pending related to docosanol 10% cream in selected countries where we determined there are no viable markets. |
Amortization expense related to amortizable intangible assets for the quarters ended December 31, 2005 and 2004 was approximately $65,000 and $47,000, respectively. Based solely on the amortizable intangible assets as of December 31, 2005, the estimated annual amortization expense of intangible assets for the fiscal years ending September 30 is shown in the following table. Actual amortization expense to be reported in future periods could differ from these estimates as a result of acquisitions, divestitures, and other relevant factors.
Amortization Expense
| | | | | |
| Fiscal year ending September 30: | | | | |
| 2006 (remaining nine months) | | $ | 192,000 | |
| 2007 | | | 257,000 | |
| 2008 | | | 257,000 | |
| 2009 | | | 248,000 | |
| 2010 | | | 226,000 | |
| Thereafter | | | 2,386,000 | |
| | | | |
| Total | | $ | 3,566,000 | |
| | | | |
17
9. DEFERRED REVENUE
The following table sets forth as of December 31, 2005 the deferred revenue balances for our sale of future abreva royalty rights to Drug Royalty USA, which was completed in December 2002, and other agreements. The portion of deferred revenue classified as a current liability represents the amount we expect to realize as revenue within the next 12 months.
| | | | | | | | | | | | | |
| | | Drug Royalty | | | Other | | | | |
| | | USA Agreement | | | Agreements | | | Total | |
| Deferred revenue as of September 30, 2005 | | $ | 19,049,877 | | | $ | 108,333 | | | $ | 19,158,210 | |
| Changes during the period: | | | | | | | | | | | | |
| Additions during period | | | — | | | | 28,300 | | | | 28,300 | |
| Recognized as revenue during period | | | (581,897 | ) | | | — | | | | (581,897 | ) |
| | | | | | | | | | |
| Deferred revenue as of December 31, 2005 | | $ | 18,467,980 | | | $ | 136,633 | | | $ | 18,604,613 | |
| | | | | | | | | | |
| Classified and reported as: | | | | | | | | | | | | |
| Current portion of deferred revenue | | $ | 1,941,289 | | | $ | 61,633 | | | $ | 2,002,922 | |
| Deferred revenue, net of current portion | | | 16,526,691 | | | | 75,000 | | | | 16,601,691 | |
| | | | | | | | | | |
| Total deferred revenue | | $ | 18,467,980 | | | $ | 136,633 | | | $ | 18,604,613 | |
| | | | | | | | | | |
10. COMPUTATION OF NET LOSS PER COMMON SHARE
Basic net (loss) earnings per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period, excluding restricted stock that has been issued but is not yet vested. Diluted net (loss) earnings per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period plus additional weighted average common equivalent shares outstanding during the period. Common equivalent shares result from the assumed exercise of outstanding stock options and warrants (the proceeds of which are then presumed to have been used to repurchase outstanding stock using the treasury stock method) and the vesting of unvested restricted shares of common stock. In the loss periods, the common equivalent shares have been excluded from the computation of diluted net loss per share, because their effect would have been anti-dilutive. For the quarters ended December 31, 2005 and 2004, options to purchase 1,752,845 and 1,477,260 shares of common stock, respectively, were excluded from the computation of diluted net loss per share. For the quarters ended December 31, 2005 and 2004, warrants to purchase 968,414 and 1,326,827 shares of common stock, respectively, were excluded from the computation of diluted net loss per share. The share amounts have been adjusted for a one-for-four reverse stock split (see Note 2, “Reverse Stock Split”).
11. COMPREHENSIVE LOSS
Comprehensive loss consists of the following:
| | | | | | | | | |
| | | Three Months Ended | |
| | | December 31, | |
| | | 2005 | | | 2004 | |
| Net loss | | $ | (5,674,434 | ) | | $ | (7,088,589 | ) |
| Other comprehensive loss, net of tax: | | | | | | | | |
| Unrealized (loss) gain on available-for-sale securities | | | (59,337 | ) | | | 40,935 | |
| | | | | | | |
| Total comprehensive loss | | $ | (5,733,771 | ) | | $ | (7,047,654 | ) |
| | | | | | | |
18
12. SHAREHOLDERS’ EQUITY
The share amounts and share prices have been adjusted for a one-for-four reverse stock split. See Note 2, “Reverse Stock Split”.
Unearned compensation. Pursuant to SFAS 123(R), unearned compensation with the balance of $3.5 million at September 30, 2005 was eliminated against common stock upon the adoption of SFAS 123(R) on October 1, 2005. The unearned compensation was related to a restricted stock award granted to our Chief Executive Officer prior to the adoption of SFAS 123(R). See Note 3, “Significant Accounting Policies — Change in Accounting Method for Share-based Compensation.”
Common stock. In October 2005, we issued and sold to certain institutional investors 1,523,585 shares of our common stock at a price of $10.60 per share, for aggregate net offering proceeds of approximately $16.15 million. In December 2005, we issued and sold to certain institutional investors 1,492,538 shares of our common stock at $13.40 per share, for aggregate offering proceeds of approximately $20.0 million and net offering proceeds of approximately $19.4 million, after deducting commissions and offering fees and expenses. These offerings were made pursuant to our shelf registration statement on Form S-3, filed with the SEC in June 2005.
During the quarter ended December 31, 2005, we also issued an aggregate of 211,639 shares of common stock in connection with the exercises of Class A stock purchase warrants (153,639 shares at a weighted average exercise price of $7.73) and employee stock options (58,000 shares at a weighted average exercise price of $3.29) for cash in the aggregate amount of approximately $1,378,000.
As of December 31, 2005 and 2004, warrants to purchase 968,414 and 1,326,827 shares of common stock, respectively, at a weighted-average price per share of $7.54 and $7.28, respectively, remained outstanding, of which all are exercisable. As of December 31, 2005 and 2004, options to purchase 1,752,845 and 1,477,260 shares of common stock, respectively, at a weighted-average price per share of $9.27 and $8.51, respectively, remained outstanding; of these, 1,245,735 and 1,165,319 options were exercisable at December 31, 2005 and 2004, respectively. See Note 16, “Subsequent Events.”
13. COMMITMENTS AND CONTINGENCIES
In the ordinary course of business, we may face various claims brought by third parties, including claims relating to employment and the safety or efficacy of our products. Any of these claims could subject us to costly litigation and, while we generally believe that we have adequate insurance to cover many different types of liabilities, our insurance carriers may deny coverage or our policy limits may be inadequate to fully satisfy any damage awards or settlements. If this were to happen, the payment of any such awards could have a material adverse effect on our operations, cash flows and financial position. Additionally, any such claims, whether or not successful, could damage our reputation and business. Management believes the outcome of currently identified claims and lawsuits will not have a material adverse effect on our operations or financial position.
14. ASTRAZENECA LICENSE AGREEMENT
In July 2005, we entered into an exclusive licensing and research collaboration agreement with AstraZeneca to discover, develop and commercialize reverse cholesterol transport enhancing compounds for the treatment of cardiovascular disease. Under the terms of the agreement, we will be eligible to receive royalty payments, assuming the product is successfully developed and approved for marketing by the FDA. We are also eligible to receive up to $330 million in milestone payments contingent upon achievement of certain development and regulatory milestones, which could take several years of further development, including achievement of certain sales targets, if a licensed compound is approved for marketing by the FDA. Under this agreement, we received an up-front payment of $10 million in July 2005 and will also receive research funding of between $2.5 million to $4.0 million per
19
year for providing research and development services to AstraZeneca for up to three years. AstraZeneca will assume responsibility for overall development and for the development costs, with both parties contributing scientific expertise in the research collaboration.
Payments related to substantive, performance-based milestones are recognized as revenue upon the achievement of the milestones as specified in the agreement. Revenue from research and development services is recognized during the period in which the services are performed and is based upon the number of FTE personnel working on the project at the agreed-upon rates.
During the quarter ended December 31, 2005, we recognized $5.0 million revenue upon achievement of the first milestone and $2.0 million revenues from research services and reimbursement of direct costs in connection with the license agreement with AstraZeneca.
14. SEGMENT INFORMATION
We operate our business on the basis of a single reportable segment, which is the business of discovery, development and commercialization of novel therapeutics for chronic diseases. Our chief operating decision-maker reviews our operating results on an aggregate basis and manages our operations as a single operating segment. We have developed one commercial product, docosanol 10% cream, known as abreva in North America, and we have several other product candidates in various stages of development. We have licensed docosanol 10% cream to other companies in the world that market the product and provide us royalties on product sales. We also have exclusively licensed our reverse cholesterol transport and macrophage migration inhibitory factor programs to AstraZeneca and Novartis International Pharmaceutical Ltd., respectively, which provide us with license fee and research funding revenues. These collaborative agreements will also provide: (1) milestone payments if certain development and regulatory milestones are achieved, which could take several years of further development, (2) milestone payments upon achievement of certain sales targets, if the products are approved for marketing by the FDA, and (3) royalties on sales, if the products are approved for marketing by the FDA.
We categorize revenues by geographic area based on selling location. All our operations are currently located in the United States; therefore, total revenues for the quarters ended December 31, 2005 and 2004 are attributed to the United States. All long-lived assets at December 31, 2005 and September 30, 2005 are located in the United States.
For the quarter ended December 31, 2005, 24% and 7% of our total revenues were derived from our license agreement with AstraZeneca and the sale of rights to royalties under the GlaxoSmithKline license agreement, respectively. For the quarter ended December 31, 2004, 57% of our total revenues were derived from the sale of rights to royalties under the GlaxoSmithKline license agreement. As of December 31, 2005 and September 30, 2005, receivables from AstraZeneca accounted for approximately 97% and 0%, respectively, of our total net receivables.
15. RECENT ACCOUNTING PRONOUNCEMENTS
Financial Accounting Standards No. 151 (“SFAS 151”). In November 2004, the FASB issued SFAS 151, “Inventory Costs”, which requires abnormal amounts of idle facility expense, freight, handling costs and wasted material (spoilage), as well as unallocated overhead, to be recognized as current period charges. SFAS 151 will be effective for inventory costs incurred during the fiscal years beginning after June 15, 2005. Adoption of SFAS 151 did not significantly affect our financial condition or results of operations.
Financial Accounting Standards No. 154 (“SFAS 154”). In May 2005, the FASB issued SFAS 154, “Accounting Changes and Error Corrections.” SFAS 154 establishes retrospective application as the required method for reporting a change in accounting principle in the absence of explicit transition requirements specific to the newly adopted accounting principle. SFAS 154 also provides guidance for determining whether retrospective application of a change in accounting principle is impracticable and for reporting a change when retrospective application is impracticable. SFAS 154 is effective for accounting changes and corrections of errors made in fiscal years beginning after December 15, 2005. We do not expect the adoption of SFAS 154 to significantly affect our financial condition or results of operations.
16. SUBSEQUENT EVENTS
On January 6, 2006, we entered into a manufacturing services agreement with Patheon Inc. (“Patheon”), a provider of outsourced drug manufacturing services, under which Patheon will initially serve as our sole provider of drug manufacturing services for Neurodex™ (the “Manufacturing Agreement”). Under the terms of the Manufacturing Agreement, Patheon will be responsible for manufacturing, quality control, quality assurance and stability testing of Neurodex. The Manufacturing Agreement is effective until December 31, 2011.
On January 10, 2006, we signed an exclusive license agreement with Kobayashi Pharmaceutical Co., Ltd. (“Kobayashi”), a Japanese corporation, (the “License Agreement”) to allow Kobayashi to market in Japan finished or semi-finished medical products that are curative of episodic outbreaks of herpes simplex or
20
herpes labialis and that contain a therapeutic concentration of our docosanol 10% cream either as the sole active ingredient or in combination with any other ingredient, substance or compound (the “Products”). Under the terms of the License Agreement, Kobayashi will be responsible for all sales and marketing activities and the manufacturing and distribution of the Products. The License Agreement automatically expires upon the latest to occur of (1) the tenth anniversary of the first commercial sale in Japan, (2) the last expiration date of any patent licensed under the License Agreement, or (3) the last date of expiration of the post marketing surveillance period in Japan. Pursuant to the terms of the License Agreement, we expect to receive a non-refundable know-how and data transfer fee of 100 million Japanese Yen (or approximately U.S. $874,000) during the quarter ending March 31, 2006. In addition, we will be eligible to receive milestone payments of up to 450 million Japanese Yen (or up to U.S. $3.9 million), subject to achievement of certain milestones relating to the regulatory approval and commercialization of docosanol in Japan and patent and know-how royalties for sales of Products in Japan, if commercial sales commence.
In December 2003, we issued warrants to purchase a total of up to 807,347 shares (adjusted for a one-for-four reverse stock split, see Note 2, “Reverse Stock Split”) of our Class A common stock at an exercise price of $7.00 per share (the “Warrants”). The Warrants had a five-year term, but included a provision that we could redeem the Warrants for $1.00 each if our stock price traded above twice the Warrant exercise price for a certain period of time (the “Redemption Right”). On January 24, 2006, we sent the Warrant holders notice that the Redemption Right had been triggered and that the Warrants would expire, to the extent unexercised, on February 7, 2006. Between January 26, 2006 and February 7, 2006, we received Warrant subscriptions for 671,923 shares, representing $4.7 million in proceeds to us. At the close of business on February 7, 2006, a single warrant to purchase 25,167 shares of Class A common stock expired unexercised.
21
Item 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This Quarterly Report on Form 10-Q contains forward-looking statements concerning future events and performance of the Company. When used in this report, the words “intend,” “estimate,” “anticipate,” “believe,” “plan” or “expect” and similar expressions are included to identify forward-looking statements. These forward-looking statements are based on our current expectations and assumptions and many factors could cause our actual results to differ materially from those indicated in these forward-looking statements. You should review carefully the factors identified in this report under the caption, “Risk Factors” and in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission (“SEC”). We disclaim any obligation to update or announce revisions to any forward-looking statements to reflect actual events or developments. Except as otherwise indicated herein, all dates referred to in this report represent periods or dates fixed with reference to the calendar year, rather than our fiscal year ending September 30. The three-month period ended December 31, 2005, may also be referred to as the first quarter of fiscal 2006.
EXECUTIVE OVERVIEW
Avanir Pharmaceuticals is a pharmaceutical company focused on developing and commercializing novel therapeutic products for the treatment of chronic diseases. Our product candidates address therapeutic markets that include the central nervous system, atherosclerosis, inflammatory diseases and infectious diseases. Our research and drug discovery programs are focused primarily on small molecules that can be taken orally as therapeutic treatments.
On January 5, 2006, our Board of Directors approved a one-for-four reverse stock split of our common stock. The reverse stock split was previously approved by our shareholders at our annual shareholder meeting held on March 17, 2005 and was implemented on January 17, 2006. Our common stock began trading on a split-adjusted basis on January 18, 2006, under the new ticker symbol of “AVN.R”. All share and per share information herein (including shares outstanding, earnings per share and warrant and stock option exercise prices) reflect this reverse stock split.
We are currently developing Neurodex™ for the treatment of Involuntary Emotional Expression Disorder (“IEED”) also known as pseudobulbar affect (“PBA”) or emotional lability, and for the treatment of chronic diabetic neuropathic pain as well as evaluating other applications for this drug. IEED/PBA is a complex neurological syndrome that is characterized by a lack of control of emotional expression, typically episodes of involuntary or exaggerated motor expression of emotion such as laughing and/or crying or weeping at the wrong time or in an exaggerated amount. IEED/PBA afflicts patients with neurological disorders such as amyotrophic lateral sclerosis (“ALS”), Alzheimer’s disease, multiple sclerosis (“MS”), stroke, traumatic brain injury and Parkinson’s disease. While the exact number is unknown, the medical literature indicates that there are approximately 800,000 to 1,000,000 patients who have IEED/PBA in North America. We completed the submission to the U.S. Federal Drug and Administration (“FDA”) of our new drug application (“NDA”) for Neurodex for the treatment of IEED/PBA in January 2006. If the FDA approves Neurodex, we expect it would be the first drug approved for the treatment of IEED/PBA. We are also currently engaged in a Phase III clinical trial with Neurodex in patients with diabetic neuropathic pain.
We have two programs in Phase I clinical development. One development program is for the treatment of atherosclerosis through the use of reverse cholesterol transport and is partnered with AstraZeneca. In the second half of December 2005, we successfully submitted an investigational new drug application (“IND”) to the FDA for AZD2479 (also known as AVP-26452), a compound under development as a reverse cholesterol transport enhancer which resulted in our achievement of a $5.0 million milestone under the terms of the agreement with AstraZeneca. Our other development program is for AVP13358 and various backup compounds that are currently being evaluated in the treatment of systemic lupus erythematosus (“SLE”).
22
Our pre-clinical research program targeting macrophage migration inhibitory factor (“MIF”) in the treatment of inflammatory diseases is partnered with Novartis International Pharmaceutical Ltd. (“Novartis”). Using our proprietary Xenerex™ technology, we continue to conduct research to develop injectable human monoclonal antibody products for anthrax and other infectious diseases.
Our first commercialized product, docosanol 10% cream, (sold as abreva® by our marketing partner GlaxoSmithKline Consumer Healthcare in North America) is the only over-the-counter treatment for cold sores that has been approved by the FDA. In January 2006, we signed an exclusive license agreement with Kobayashi Pharmaceutical Co., Ltd. (“Kobayashi”) giving them the rights to manufacture and sell docosanol 10% cream as a treatment for cold sores in Japan.
During the quarter ended December 31, 2005, we issued and sold to certain institutional investors a total of 3,016,122 (adjusted for a one-for-four reverse stock split) shares of our common stock at a weighted average price of $11.99 per share (adjusted for a one-for-four reverse stock split), for aggregate net offering proceeds of approximately $35.5 million. The offering was made pursuant to our shelf registration statement on Form S-3, filed in June 2005.
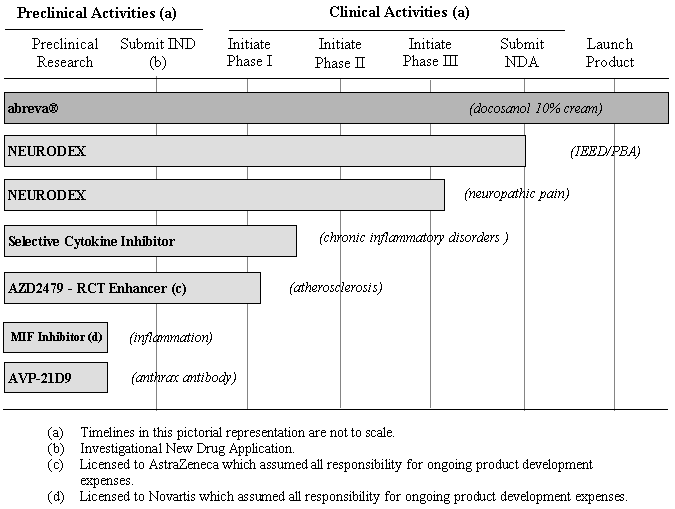
The following chart illustrates the status of research activities for our products, product candidates and licensed technologies that are under development as of January 31, 2006.
| (a) Timelines in this pictorial representation are not to scale. (b) Investigational New Drug Application. (c) Licensed to AstraZeneca which assumed all responsibility for ongoing product development expenses. (d) Licensed to Novartis which assumed all responsibility for ongoing product development expenses. |
We strive to maintain flexibility in our cost structure by actively managing outsourced functions, such as clinical trials, legal counsel, documentation and testing of internal controls, pre-clinical development work, manufacturing, warehousing and distribution, rather than maintaining all of these
23
functions in house. We believe the benefits of outsourcing, being flexible and being able to rapidly respond to program delays or successes far outweigh the higher costs often associated with outsourcing at this stage of our development, although some of these functions could be brought in-house as we grow.
If the Neurodex NDA is approved by the FDA, we intend to begin marketing and selling the product within several months of receiving product approval. We are in the process of transforming from a research and development organization into a commercially viable pharmaceutical company. In order to facilitate that transformation, we are starting to build the necessary infrastructure to support the planned commercial launch of Neurodex if it is approved by the FDA. In preparation for the commercial launch of Neurodex, we are in the process of developing our sales and marketing strategy and are recruiting sales and marketing personnel for key positions within the organization. In January 2006, we entered into a manufacturing services agreement with Patheon, Inc. (“Patheon”) under which Patheon will initially serve as our sole provider of drug manufacturing services for Neurodex (the “Manufacturing Agreement”). Under the terms of the Manufacturing Agreement, Patheon will be responsible for manufacturing, quality control, quality assurance and stability testing of Neurodex. The Manufacturing Agreement is effective until December 31, 2011.
We intend to continue to seek partnerships with pharmaceutical companies to help fund other research and development programs in exchange for sharing in the rights to commercialize new drugs. We may also seek to develop other drug candidates through research collaborations with larger pharmaceutical companies, allowing us to share the risks and the opportunities that come from such development efforts.
We expect that our selling, marketing, development and other operational costs will continue to exceed revenues from existing sources through at least fiscal 2007. Trends in revenues and various types of expenses are discussed further in the “Results of Operations.” We expect that we will have to raise additional capital to prepare for and execute a product launch of Neurodex for IEED/PBA, if approved by the FDA for marketing, and to fund our ongoing clinical trials for Neurodex for neuropathic pain, as well as selected research and other operating activities. Our future capital needs will depend substantially on our ability to reach predetermined milestones under our existing collaboration agreements, as well as the economic terms and the timing of any new partnerships or collaborative arrangements with pharmaceutical companies under which they will share the costs of such activities. If we are unable to raise capital as needed to fund our operations, or if we are unable to reach these milestones or enter into any such collaborative arrangements, then we may need to slow the rate of development of some of our programs or sell the rights to one or more of our drug candidates, and our commercialization plans for Neurodex may be adversely affected. For additional information about the risks and uncertainties that may affect our business and prospects, please see “Risk Factors.”
Our offices and research facilities are located at 11388 Sorrento Valley Road, San Diego, California 92121. Our telephone number is (858) 622-5200 and our e-mail address isinfo@avanir.com. Additional information aboutAvanir can be found on our website, atwww.avanir.com, and in our periodic and current reports filed with the SEC. Copies of our current and periodic reports filed with the SEC are available at the SEC Public Reference Room at 450 Fifth Street, N.W., Washington, D.C. 20549, and online atwww.sec.govand our website.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Our discussion and analysis of our financial condition and results of operations is based upon our condensed consolidated financial statements prepared in accordance with generally accepted accounting principles in the United States. The preparation of these financial statements requires us to make a number of assumptions and estimates that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reported periods. These items are monitored and analyzed by management for changes in facts and circumstances, and material changes in these estimates could occur in the future. Changes in estimates are recorded in the period in which they become known.
24
We base our estimates on historical experience and various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ from our estimates if past experience or other assumptions do not turn out to be substantially accurate.
We believe that the application of our accounting policies for share-based compensation expense, revenue recognition, expenses in outsourced contracts, research and development expenses and valuation of long-lived and intangible assets, all of which are important to our financial position and results of operations, require significant judgments and estimates on the part of management.
Share-based compensation expense
We grant options to purchase our common stock to our employees, directors and consultants under our stock option plans. The benefits provided under these plans are share-based payments subject to the provisions of revised Statement of Financial Accounting Standards No. 123, “Share-Based Payment” (“SFAS 123 (R)”). Effective October 1, 2005, we adopted SFAS 123(R) and use the fair value method to account for share-based payments with a modified prospective application which provides for certain changes to the method for valuing share-based compensation. The valuation provisions of SFAS 123(R) apply to new awards and to awards that are outstanding on the effective date and subsequently modified or cancelled. Under the modified prospective application, prior periods are not revised for comparative purposes. Total compensation cost for our share-based payments recognized in the first quarter of fiscal 2006 was $462,000. Selling, general and administrative expense and research and development expense in the first quarter of fiscal 2006 included share-based compensation of $386,000 and $76,000, respectively. As of December 31, 2005, $6.0 million of total unrecognized compensation costs related to nonvested awards is expected to be recognized over a weighted average period of 2.7 years.
The fair value of each option award is estimated on the date of grant using a Black-Scholes-Merton option pricing model (“Black-Scholes model”) that uses assumptions regarding a number of complex and subjective variables. These variables include, but are not limited to, our expected stock price volatility, actual and projected employee stock option exercise behaviors, risk-free interest rate and expected dividends. Expected volatilities are based on historical volatility of our common stock and other factors. The expected term of options granted is based on analyses of historical employee termination rates and option exercises. The risk-free interest rates are based on the U.S. Treasury yield in effect at the time of the grant. Since we do not expect to pay dividends on our common stock in the foreseeable future, we estimated the dividend yield to be 0%. SFAS 123(R) requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. We estimate pre-vesting forfeitures based on our historical experience.
If factors change and we employ different assumptions in the application of SFAS 123(R) in future periods, the compensation expense that we record under SFAS 123(R) may differ significantly from what we have recorded in the current period. There is a high degree of subjectivity involved when using option pricing models to estimate share-based compensation under SFAS 123(R). Because changes in the subjective input assumptions can materially affect our estimates of fair values of our share-based compensation, in our opinion, existing valuation models, including the Black-Scholes and lattice binomial models, may not provide reliable measures of the fair values of our share-based compensation. Consequently, there is a risk that our estimates of the fair values of our share-based compensation awards on the grant dates may bear little resemblance to the actual values realized upon the exercise, early termination or forfeiture of those share-based payments in the future. Certain share-based payments, such as employee stock options, may expire worthless or otherwise result in zero intrinsic value as compared to the fair values originally estimated on the grant date and reported in our financial statements. Alternatively, values may be realized from these instruments that are significantly in excess of the fair values originally estimated on the grant date and reported in our financial statements. There is currently no market-based mechanism or other practical application to verify the reliability and accuracy of the estimates stemming from these valuation models, nor is there a means to compare and adjust the estimates to actual values. Although the fair value of employee share-based awards is determined in accordance
25
with SFAS 123(R) and the SEC’s Staff Accounting Bulletin No. 107 (“SAB 107”) using an option- pricing model, that value may not be indicative of the fair value observed in a willing buyer/willing seller market transaction.
The guidance in SFAS 123 (R) and SAB 107 is relatively new, and best practices are not well established. The application of these principles may be subject to further interpretation and refinement over time. There are significant differences among valuation models, and there is a possibility that we will adopt different valuation models in the future. This may result in a lack of consistency in future periods and materially affect the fair value estimate of share-based payments. It may also result in a lack of comparability with other companies that use different models, methods and assumptions.
Theoretical valuation models and market-based methods are evolving and may result in lower or higher fair value estimates for share-based compensation. The timing, readiness, adoption, general acceptance, reliability and testing of these methods is uncertain. Sophisticated mathematical models may require voluminous historical information, modeling expertise, financial analyses, correlation analyses, integrated software and databases, consulting fees, customization and testing for adequacy of internal controls. Market-based methods are emerging that, if employed by us, may dilute our earnings per share and involve significant transaction fees and ongoing administrative expenses. The uncertainties and costs of these extensive valuation efforts may outweigh the benefits to investors.
For purpose of estimating the fair value of stock options granted during the quarter ended December 31, 2005 using the Black-Scholes model, we have made an estimate regarding our stock price volatility (weighted average of 79.1%). If our stock price volatility assumption were increased to 89.1%, the weighted average estimated fair value per share of stock options granted during the quarter ended December 31, 2005 would increase by $0.15, or 8%. The volatility percentage assumed in the quarter ended December 31, 2005 was based on the historical prices of our common stock.
The expected term of options granted is based on our analyses of historical employee terminations and option exercises (weighted average of 4.5 years for the quarter ended December 31, 2005) which, if increased to 5.5 years, would increase the weighted average estimated fair value per share of stock options granted during the quarter ended December 31, 2005 by $0.14 or 8%.
The risk-free interest rate is based on the U.S. Treasury yield for a period consistent with the expected term of the option in effect at the time of grant (weighted average of 4.2% for the quarter ended December 31, 2005) which, if increased to 5.2%, would increase the weighted average estimated fair value per share of stock options granted during the quarter ended December 31, 2005 by $0.02, or 1%.
The pre-vesting forfeiture rate is estimated using historical option cancellation information (weighted average of 8.0% for both officers and directors and 13% for employees for the quarter ended December 31, 2005) which, if decreased to 3% for officers and directors and 8% for employees, would increase the share-based compensation expense for the quarter ended December 31, 2005 by $96,000, or 21%. See Note 3, “Significant Accounting Policies — Change in Accounting Method for Share-Based Compensation,” in the Notes to Condensed Consolidated Financial Statements (Unaudited) for a detailed discussion.
Revenue Recognition
We recognize revenue in accordance with SEC Staff Accounting Bulletin Topic 13, “Revenue Recognition” and Emerging Issues Task Force No. 00-21 (“EITF 00-21”), “Accounting for Revenue Arrangements with Multiple Deliverables.” Revenue is recognized when four basic criteria of revenue recognition are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services rendered; (3) the fee is fixed or determinable; and (4) collectibility is reasonably assured.
Contract Revenue from Collaborations. Collaborative arrangements typically consist of non-refundable and/or exclusive technology access or data transfer fees, reimbursements for costs of specific research and development, and various milestone and future product royalty payments. If the delivered technology does not have standalone value or if we do not have objective or reliable evidence of the fair value of the
26
undelivered component, the amount of revenue allocable to the delivered technology is deferred. Nonrefundable, up-front license or technology access fees with standalone value that are not dependent on any future performance by us under the arrangements are recognized as revenue upon the earlier of when payments are received or collection is assured, but are deferred if we have continuing performance obligations. If we have continuing involvement through research and development collaborations or other contractual obligations, such up-front fees are deferred and recognized over the collaboration period or the period for which we continue to have a performance obligation, unless all of the following criteria exist: (1) the collaboration or contractual obligations have standalone value to the customer, (2) there is objective and reliable evidence of the fair value of the undelivered item(s), and (3) there is no general right to return the delivered item(s).
Payments related to substantive, performance-based milestones are recognized as revenue upon the achievement of the milestones as specified in the underlying agreements, which represent the culmination of the earnings process. Revenue from research services is recognized during the period in which the services are performed and is based upon the number of FTE personnel working on specific project at the agreed-upon rate. Reimbursements from collaborative partners for agreed upon direct costs including research stock and outsourced pre-clinical studies are recognized as revenues in the period the reimbursable expenses are incurred. Payments received in advance are recorded as deferred revenue until the research is performed, costs are incurred, or milestone is reached.
Royalty Revenues.We recognize royalty revenues from licensed products when earned in accordance with the terms of the license agreements. Net sales figures used for calculating royalties include deductions for costs of unsaleable returns, managed care chargebacks, cash discounts, freight and warehousing, and miscellaneous write-offs, which may vary over the course of the license agreement.
Revenues from Sale of Royalty Rights.In agreements where we have sold our rights to future royalties under license agreements and we maintain continuing involvement in earning such royalties, we defer revenues and recognize them over the life of the license agreement. For example, in the sale of an undivided interest of our abreva license agreement to Drug Royalty USA, revenue recognition is being determined under the units-of-revenue method. Under this method, the amount of deferred revenue to be recognized as revenue in each period is calculated by multiplying the following: (1) the ratio of the royalty payments due to Drug Royalty USA for the period to the total remaining royalties that we expect GlaxoSmithKline will pay Drug Royalty USA over the term of the agreement by (2) the unamortized deferred revenue amount.
Revenue Arrangements with Multiple Deliverables.We have revenue arrangements whereby we are obligated to deliver to the customer multiple products and/or services (multiple deliverables). Such arrangements may include antibody generation services agreements and other forms of research collaborations. In accordance with EITF 00-21, we analyze our multiple element arrangements to determine whether the elements can be separated and accounted for individually as separate units of accounting. The evaluation is performed at the inception of the arrangement. The delivered item(s) is (are) considered a separate unit of accounting if all of the following criteria are met: (1) the delivered item(s) has (have) value to the customer on a standalone basis; (2) there is objective and reliable evidence of the fair value of the undelivered item(s); and (3) if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered item(s) is (are) considered probable and substantially in our control.
Government Research Grant Revenue.We recognize revenues from federal research grants during the period in which the related expenditures are incurred.
Product Sales — Active Pharmaceutical Ingredient Docosanol.Revenue from product sales, which consist of sales of our active pharmaceutical ingredient docosanol, is recorded when the above criteria are met, generally when title and risk of loss have passed to the buyer upon delivery. We sell the active pharmaceutical ingredient docosanol to various licensees and ship only on written order for the materials.
27
Total shipments generally occur fewer than five times a year. Our contracts for sales of the active pharmaceutical ingredient docosanol include buyer acceptance provisions that give our buyers the right of replacement if the delivered materials do not meet specified criteria by giving notice within 30 days after receipt of such defective materials. We have the option to refund or replace any such defective materials; however, we have historically demonstrated that the materials shipped from the same pre-inspected lot have consistently met the specified criteria and no buyer has rejected any of our shipments from the same pre-inspected lot to date. Therefore, we recognize revenue from sales of the active pharmaceutical ingredient docosanol without providing for such contingency upon shipment.
Recognition of Expenses in Outsourced Contracts
Pursuant to management’s assessment of the services that have been performed on clinical trials and other contracts, we recognize expenses as the services are provided. Such management assessments include, but are not limited to: (1) an evaluation by the project manager of the work that has been completed during the period, (2) measurement of progress prepared internally and/or provided by the third-party service provider, (3) analyses of data that justify the progress, and (4) management’s judgment. Several of our contracts extend across multiple reporting periods, including our largest contract, representing an $8.5 million Phase III clinical trial contract as of December 31, 2005. A 3% variance in our estimate of the work completed in our largest contract could increase or decrease our quarterly operating expenses by approximately $255,000.
Research and Development Expenses
Research and development expenses consist of expenses incurred in performing research and development activities including salaries and benefits, facilities and other overhead expenses, clinical trials, contract services and other outside expenses. Research and development expenses are charged to operations as they are incurred. Up-front payments to collaborators made in exchange for the avoidance of potential future milestone and royalty payments on licensed technology are also charged to research and development expense when the drug is still in the development stage, has not been approved by the FDA for commercialization and concurrently has no alternative uses.
We assess our obligations to make milestone payments that may become due under licensed or acquired technology to determine whether the payments should be expensed or capitalized. We charge milestone payments to research and development expense when:
| | • | | The technology is in the early stage of development and has no alternative uses; |
| |
| | • | | There is substantial uncertainty of the technology or product being successful; |
| |
| | • | | There will be difficulty in completing the remaining development; and |
| |
| | • | | There is substantial cost to complete the work. |
Acquired contractual rights. Payments to acquire contractual rights to a licensed technology or drug candidate are expensed as incurred when there is uncertainty in receiving future economic benefits from the acquired contractual rights. We consider the future economic benefits from the acquired contractual rights to a drug candidate to be uncertain until such drug candidate is approved by the FDA or when other significant risk factors are abated.
Capitalization and Valuation of Long-Lived and Intangible Assets
Intangible assets with finite useful lives consist of capitalized legal costs incurred in connection with patents, patent applications pending and license agreements. We amortize costs of approved patents, patent applications pending and license agreements over their estimated useful lives, or terms of the agreements, whichever are shorter. For patents pending, we amortize the costs over the shorter of a period of twenty years from the date of filing the application or, if licensed, the term of the license
28
agreement. We re-assess the useful lives of patents when they are issued, or whenever events or changes in circumstances indicate the useful lives may have changed. For patent and patent applications pending and trademarks that we abandon, we charge the remaining unamortized accumulated costs to expense.
Intangible assets and long-lived assets are evaluated for impairment whenever events or changes in circumstances indicate that their carrying value may not be recoverable. If the review indicates that intangible assets or long-lived assets are not recoverable (i.e. the carrying amount is less than the future projected undiscounted cash flows), their carrying amount would be reduced to fair value. Factors we consider important that could trigger an impairment review include the following:
| | • | | A significant underperformance relative to expected historical or projected future operating results; |
| |
| | • | | A significant change in the manner of our use of the acquired asset or the strategy for our overall business; and/or |
| |
| | • | | A significant negative industry or economic trend. |
When we determine that the carrying value of intangible assets or long-lived assets are not recoverable based upon the existence of one or more of the above indicators of impairment, we may be required to record impairment charges for these assets.
RESULTS OF OPERATIONS
COMPARISON OF THREE MONTHS ENDED DECEMBER 31, 2005 AND 2004
Revenues
Total revenues increased to $8.1 million for the first quarter of fiscal 2006, compared to $888,000 for the first quarter of fiscal 2005. Revenues in the first quarter of fiscal 2006 included $5.0 million relating to the achievement of a milestone under the AztraZeneca license agreement, $2.5 million in research and development service revenues generated from our collaborative agreements with AstraZeneca and Novartis executed in July 2005 and April 2005, respectively, and $582,000 from the recognition of revenue related to the sale of an undivided interest in our abreva license agreement to Drug Royalty USA. Revenue in the first quarter of fiscal 2005 included $508,000 from the recognition of deferred revenue and $200,000 relating to the achievement of milestones under license agreements.
Revenue-generating contracts that remained active as of February 8, 2006 include license agreements with AstraZeneca and Novartis, eight docosanol 10% cream license agreements and one Neurodex sublicense. Partnering, licensing and research collaborations have been, and will continue to be, an important part of our business development strategy. We intend to partner with pharmaceutical companies that can help fund our research in exchange for sharing in the rights to commercialize new drugs resulting from this research. We have licensed and continue to seek licensees in other countries for docosanol 10% cream and other potential products in our development pipeline. Research collaborations also represent an important way to achieve our development goals, while sharing in the risks and the opportunities that come from such development efforts.
Operating Expenses
Total operating expenses increased by $6.1 million, or 76%, to $14.1 million for the first quarter of fiscal 2006, compared to $8.0 million for the same period in fiscal 2005. The increase in operating expenses was caused by a $4.3 million, or 85%, increase in research and development expenses and a $1.8 million, or 62%, increase in selling, general and administrative expenses. These and other costs and trends are more fully described below.
29
| | | | | | | | | |
| | | Three Months Ended | |
| | | December 31, | |
| | | 2005 | | | 2004 | |
| Operating expenses: | | | | | | | | |
| Research and development | | | 66 | % | | | 63 | % |
| Selling, general and administrative | | | 34 | % | | | 37 | % |
| Costs of sales | | | — | % | | | — | % |
| | | | | | | |
| Total operating expenses | | | 100 | % | | | 100 | % |
| | | | | | | |
Research and development (“R&D”) expenses.R&D expenses for the first quarter of fiscal 2006 and 2005 were $9.4 million and $5.1 million, respectively. R&D expenses in the first quarter of fiscal 2006 were related to continuation of the open label safety study of Neurodex in the treatment for IEED/PBA, a Phase III clinical trial of Neurodex for the treatment of neuropathic pain, and a Phase I clinical trial of our leading compound for the selective cytokine inhibitor program. R&D expenses also included pre-clinical research related to the MIF inhibitor, reverse cholesterol transport and antibody development programs. The increase in R&D expenses is due to a $2.0 million increase in spending for the open label safety study of Neurodex for the treatment of IEED/PBA, a $1.6 million increase in spending for a Phase III clinical trial of Neurodex for the treatment of neuropathic pain and a $363,000 increase in pre-clinical development of our reverse cholesterol transport program.
The following table sets forth the status of, and costs attributable to, our proprietary research and clinical development programs.
Research and Development Projects and Expenses
| | | | | | | | | | | | | | | |
| | | Three Months Ended | | | Inception Through | | | Estimated Cost to |
| | | December 31, | | | December 31, | | | License or Complete |
| | | 2005 (1) | | | 2004 (1)(4) | | | 2005 (1)(2)(4) | | | Project (1)(3) |
Company-funded Projects: | | | | | | | | | | | | | | |
| Develop programs for Neurodex, selective cytokine inhibitor and other programs projected through fiscal 2007 (3) | | $ | 7,273,480 | | | $ | 3,384,312 | | | $ | 86,021,878 | | | $35M to $45M |
| | | | | | | | | | | | | | | |
Partner-funded Projects: | | | | | | | | | | | | | | |
| Reverse cholesterol transport and MIF inhibitor research programs | | | 2,018,799 | | | | 1,508,751 | | | | 21,464,714 | | | Funded by Partners |
| | | | | | | | | | | | | | | |
Government-funded Projects: | | | | | | | | | | | | | | |
| Preclinical research on various projects. Estimated timing to complete the current anthrax research project is less than 12 months | | | 71,123 | | | | 161,178 | | | | 2,594,575 | | | $0.1 M |
| | | | | | | | | | | | |
| Total | | $ | 9,363,402 | | | $ | 5,054,241 | | | $ | 110,081,167 | | | |
| | | | | | | | | | | | |
| | |
| (1) | | Each project includes an allocation of laboratory occupancy costs. “M” refers to millions. Estimated costs and timing to complete the projects are subject to the availability of funds. For each of the projects set forth in the table, other than Neurodex for IEED/PBA (which we intend to market ourselves) and the reverse cholesterol transport and the MIF inhibitor programs (which we have partnered), we may seek development partners or licensees to defray part or all of the ongoing development costs. |
| |
| (2) | | Inception dates are on or after October 1, 1998, at which time we began identifying and tracking program costs. |
| |
| (3) | | Assumes completion of development of Neurodex in the treatment of IEED/PBA and for one Phase III clinical trial in the treatment of neuropathic pain and continuation of Phase I studies of our selective cytokine inhibitor program. Projected spending thereafter will be subject to progress made in research that is currently underway. |
| |
| (4) | | Includes expenses funded by us prior to partnering these projects in the amounts of $1.5 million and $17.1 million for the three-month period ended December 31, 2004 and since inception through December 31, 2005, respectively. |
30
We expect that increases in R&D spending for neuropathic pain and potentially other indications will partially offset the expected decline in spending related to IEED/PBA in the coming years as we continue Phase III clinical trials of Neurodex in the treatment of neuropathic pain. All future R&D spending on MIF and reverse cholesterol transport programs is expected to be fully reimbursed by our collaborative partners. We expect that spending on our selective cytokine inhibitor program and on development of monoclonal antibodies will depend in part on the progress that we make in these programs and on our strategy for partnering these programs or in obtaining additional government grants, so that we are able to defray part or all of these ongoing development costs.
Status of R&D Programs and Plans — Company-funded Projects
Neurodex for the treatment of Involuntary Emotional Expression Disorder/Pseudobulbar Affect.We completed the submission of the Neurodex NDA to the FDA in January 2006. Assuming the NDA is accepted by the FDA for review, we expect the FDA will take six months or ten months from the submission date to complete its review of the NDA, depending on whether it is a priority review or standard review, respectively. We will learn of the review timing (i.e., priority or standard review) when and if the FDA notifies us of acceptance of the submission. We have been engaged in an open-label safety study for the treatment of IEED/PBA in a broad pool of patients who have IEED/PBA associated with their underlying neurodegenerative disease or condition. We expect to continue this open-label study until we have a decision from the FDA on approval of the drug for marketing, plus any additional time until launch, if approved. In June 2004, we successfully completed the treatment phase of a Phase III clinical trial of Neurodex for the treatment of IEED/PBA in 150 patients with MS. Prior to engaging in these recent and current ongoing studies, we successfully completed the initial Phase III clinical trial of IEED/PBA in patients with ALS in May 2002.
Neurodex for the treatment of neuropathic pain.In June 2005, we initiated our first Phase III clinical trial of Neurodex in patients who have diabetic neuropathic pain and we are currently evaluating the balance of our development plan. Simultaneously, we are evaluating commercial development alternatives for this indication, including continuing development on our own (including conducting a second Phase III clinical trial) or co-promotion/licensing opportunities in which a partner would fund the second trial. Estimated timing to complete the first Phase III clinical trial and potentially partner this indication is up to two years. There can be no assurances that we will choose or be able to negotiate a licensing and/or co-promotion arrangement for Neurodex in the treatment of diabetic neuropathic pain on attractive terms, if at all.
Development program for selective cytokine inhibitor (IgE regulator).In November 2005, we completed a multi-rising dose Phase Ib safety trial of AVP-13358 and are currently in the process of evaluating the results of the study. Assuming the results of the multi-rising dose study are favorable, we intend to proceed with proof of concept studies for the clinical indications to be explored. In 2004 we completed the first Phase Ia clinical trial of AVP-13358 in 54 healthy volunteers. The placebo-controlled study was intended to assess safety, tolerability and pharmacokinetics following single rising oral doses. Results of the Phase Ia study suggest AVP-13358 was well tolerated at all single rising doses up through 16 milligrams. The study also demonstrated AVP-13358 was detectable in the bloodstream at all doses administered and remains in circulation long enough to allow potentially once or twice daily dosing.
Status of R&D Programs and Plans — Partner-funded Projects
Development program for reverse cholesterol transport.In July 2005 we entered into an exclusive licensing and research collaboration agreement with AstraZeneca to discover, develop and commercialize reverse cholesterol transport enhancing compounds for the treatment of cardiovascular disease. Under the terms of the agreement, we are eligible to receive royalty payments, assuming the product is successfully developed and approved for marketing by the FDA. We are also eligible to receive up to $330 million in milestone payments contingent upon achievement of certain development and regulatory milestones,
31
which could take several years of further development, including achievement of certain sales targets, if a licensed compound is approved for marketing by the FDA. Under the terms of the agreement, we received an up-front payment of $10.0 million in July 2005 and will also receive research funding of between $2.5 million and $4.0 million per year for providing research and development services to AstraZeneca for up to three years. AstraZeneca has the responsibility for all development costs. In December 2005, we submitted an IND to the FDA for the compound AZD 2479 (also known as AVP-26452) and began enrolling patients in a Phase I study to evaluate the safety of this compound, which triggered a $5.0 million milestone payment from AstraZeneca under the terms of the collaboration agreement. We expect spending on this program will remain about the same in the remaining quarters of fiscal 2006; however, AstraZeneca will be paying us for such services.
Anti-inflammatory research program (MIF inhibitor).In April 2005, we entered into a research, development and commercialization agreement with Novartis International Pharmaceutical Ltd. for orally active small molecule therapeutics utilizing our novel MIF technology as a potential treatment for inflammatory diseases. Under the terms of the agreement, we will be eligible to receive royalty payments, assuming the product is successfully developed and approved for marketing by the FDA. We are also eligible to receive up to $169 million in milestone payments contingent upon achievement of certain development and regulatory milestones, which could take several years of further development, including achievement of certain sales targets, if a MIF compound is approved for marketing by the FDA. Additionally, we received an up-front payment of $2.5 million in May 2005 and will also receive research funding of between $1.5 million and $2.5 million per year for providing research and development services to Novartis for two years from the date of the agreement, or longer upon mutual agreement of the parties. Novartis assumes responsibility for all development expenses.
Status of R&D programs and plans — government-funded projects
Government research grants have helped us fund research programs, including the development of antibodies to anthrax toxins and docosanol-based formulations for the treatment of genital herpes. Subject to certain conditions, we, as the awardee organization, retain the principal worldwide patent rights to any invention developed with the United States government support.
Two of our most potent anthrax antibodies, AVP-21D9 and AVP-22G12, appear unique both in mechanism of action and in terms of the binding site on an anthrax toxin. AVP-21D9 is currently in preclinical development for use as a prophylactic and therapeutic drug to treat anthrax infections and is being funded by a two-year $750,000 federal (SBIR) research grant. As of December 31, 2005, approximately $76,000 remains to be spent in the remainder of 2006 under this research grant. Much of the work related to anthrax has been funded by government research grants, and our progress in this area will substantially depend on future grants. Because all of our monoclonal antibody research is at a very early preclinical stage of development and is unpredictable in terms of the outcome, we are unable to predict the cost and timing for development of any antibody or drug.
Our genital herpes project came to a formal end during the third quarter of fiscal 2005 and we do not anticipate that we will perform any further work on that project. We have no grant requests pending nor do we anticipate submitting in the future any grant requests for further research related to genital herpes.
Selling, general and administrative expenses.Our selling, general and administrative expenses increased by $1.8 million, or 62%, to $4.8 million for the first quarter of fiscal 2006, compared to $3.0 million for the first quarter of fiscal 2005. These increased expenses primarily relate to:
| | • | | $848,000 increase in expenses related to the continued expansion of our medical education and awareness programs for IEED/PBA, market research, and pre-launch planning activities for Neurodex and hiring of additional sales and marketing personnel; |
32
| | • | | $411,000 increase in expenses related to increases in headcount and compensation levels in general and administrative areas; |
| |
| | • | | $386,000 increase in share-based compensation expense; and |
| |
| | • | | $207,000 increase in professional services mainly associated with corporate governance and SEC reporting, internal controls documentation and testing under the Sarbanes-Oxley Act of 2002. |
Based on our current commercial development plans for Neurodex for the treatment of IEED/PBA, we expect sales and marketing expenses in fiscal 2006 will continue to increase, although the timing and pace of these increases is difficult to predict and will be subject to fluctuation depending on the status of the FDA’s review of our NDA and any approval decisions by the FDA. We expect that costs related to professional services mainly associated with compliance with the Sarbanes-Oxley Act of 2002 will continue to be near the levels experienced in fiscal 2005.
Share-Based Compensation
Through fiscal year 2005, we accounted for our stock plans using the intrinsic value method. Effective at the beginning of fiscal year 2006, we adopted Statement of Financial Accounting Standards No. 123(R) (“SFAS 123(R)”), “Share-Based Payment,” and elected to adopt the modified prospective application method. SFAS No. 123(R) requires us to use a fair-valued based method to account for share-based compensation. Accordingly, share-based compensation cost is measured at the grant date, based on the fair value of the award, and is recognized as expense over the employees’ requisite service period. Total compensation cost for our share-based payments in the first quarter of fiscal 2006 was $462,000. Selling, general and administrative expense and research and development expense in the first quarter of fiscal 2006 include share-based compensation of $386,000 and $76,000, respectively. As of December 31, 2005, $6.0 million of total unrecognized compensation costs related to nonvested awards is expected to be recognized over a weighted average period of 2.7 years. See Note 3, “Critical Accounting Policies — Change in Accounting Method for Share-Based Compensation” in the Notes to Condensed Consolidated Financial Statements (unaudited) for further discussion.
Interest Income
For the first quarter of fiscal 2006, interest income increased to $328,000, compared to $122,000 for the same period in the prior year. The increase is primarily due to a 50% increase in average balance of cash, cash equivalents and investments in securities for the quarter ended December 31, 2005, compared to the same period in the prior year.
Net Loss
Net loss was $5.7 million, or $0.20 per share, in the first quarter of fiscal 2006, compared to a net loss of $7.1 million, or $0.30 per share (adjusted for a one-for-four reverse stock split), in the first quarter of fiscal 2005. A lower net loss and a higher weighted average number of shares outstanding in the first quarter of fiscal 2006 accounted for the lower loss per share.
We expect to continue to pursue our drug development strategy focused on the development of Neurodex, followed by other programs in earlier stages of development that are in large therapeutic areas and that have significant partnering and licensing potential. Effective April 2005, Novartis assumed all responsibility for ongoing product development expenses for our MIF technology. Effective July 2005, AstraZeneca assumed all responsibility for ongoing product development expenses for our reverse cholesterol transport program. To help fund and develop our other product candidates, we may elect to license those technologies and drug candidates to help offset the costs of development. These potential license arrangements could materially change our outlook for future revenues and costs. However, the timing of such potential arrangements is unpredictable.
33
LIQUIDITY AND CAPITAL RESOURCES
As of December 31, 2005, we had cash, cash equivalents and investments in securities totaling $51.3 million, including cash and cash equivalents of $30.8 million, short- and long-term investments of $19.7 million and restricted investments in securities of approximately $857,000. Our net working capital balance as of December 31, 2005 was $44.4 million. As of September 30, 2005, we had cash, cash equivalents and investments in securities totaling $27.5 million, including cash and cash equivalents of $8.6 million, short- and long-term investments of $18.1 million, and restricted investments of approximately $857,000. Our net working capital balance as of September 30, 2005 was $12.0 million. Explanations of net cash provided by or used for operating, investing and financing activities are provided in the table below.
| | | | | | | | | | | | | |
| | | December 31, | | | Increase (Decrease) | | | September 30, | |
| | | 2005 | | | During Period | | | 2005 | |
| Cash, cash equivalents and investment in securities | | $ | 51,342,124 | | | $ | 23,804,538 | | | $ | 27,537,586 | |
| Cash and cash equivalents | | $ | 30,801,894 | | | $ | 22,181,751 | | | $ | 8,620,143 | |
| Net working capital | | $ | 44,356,945 | | | $ | 32,387,495 | | | $ | 11,969,450 | |
| | | | | | | | | | | | | |
| | | Three Months | | | | | | | |
| | | Ended | | | Change | | | Three Months Ended | |
| | | December 31, | | | Between | | | December 31, | |
| | | 2005 | | | Periods | | | 2004 | |
| Net cash used for operating activities | | $ | (12,500,651 | ) | | $ | (6,141,267 | ) | | $ | (6,359,384 | ) |
| Net cash (used for) provided by investing activities | | | (2,250,738 | ) | | | (2,629,484 | ) | | | 378,746 | |
| Net cash provided by financing activities | | | 36,933,140 | | | | 29,831,191 | | | | 7,101,949 | |
| | | | | | | | | | |
| Net increase in cash and cash equivalents | | $ | 22,181,751 | | | $ | 21,060,440 | | | $ | 1,121,311 | |
| | | | | | | | | | |
Operating activities.Net cash used for operating activities amounted to $12.5 million in the first quarter of fiscal 2006, $6.1 million higher than the same period a year ago. The net cash used for operating activities in the first quarter of fiscal 2005 amounted to $6.4 million. The increase in cash used is due to spending on an open label study and consulting fees relating to Neurodex in the treatment of IEED/PBA and a Phase III clinical study for Neurodex in the treatment of diabetic neuropathic pain. The increase in cash used is also due to the continued expansion of our medical education and awareness programs for IEED/PBA, market research, and pre-launch activities for Neurodex, assuming the drug is approved by the FDA for marketing. Based on our current commercial development plans for Neurodex for the treatment of IEED/PBA, we expect sales and marketing expenses will continue to increase, although the timing and rate of increase will depend on whether the FDA accepts our submission of the NDA for Neurodex and whether the agency grants us a standard review or priority review. We expect R&D spending will continue at about the same rate for the remainder of fiscal 2006, including clinical development of Neurodex in the treatment of diabetic neuropathic pain and the Phase III clinical trial. Additionally, we expect to continue spending at about the same rate on preclinical and clinical research services related to the development of compounds for the MIF and RCT programs in fiscal 2006. AstraZeneca and Novartis are funding the RCT and MIF programs, respectively, and pay us for these research services.
Our net receivables increased by $5.4 million, which is primarily due to a billing for a $5.0 million milestone and a $434,000 increase in unbilled receivables for research services earned in the quarter ended December 31, 2005 in connection with the AstraZeneca collaborative agreement.
Accounts payable decreased by $3.7 million and is primarily due to payments of invoices in connection with our medical education and awareness programs and market research regarding IEED/PBA. A $3.0 million increase in accrued expenses resulted from accruals for goods and services received in the quarter ended December 31, 2005 but were not yet invoiced by the vendors.
Investing activities.Net cash used for investing activities was $2.3 million in the first quarter of fiscal 2006, compared to $378,000 provided by investing activities in the first quarter of fiscal 2005. Our investments in securities increased by $1.7 million in the first quarter of fiscal 2006 and by $640,000 in first quarter of fiscal 2005, net of proceeds from sales and maturities of investments in securities. We invested $543,000 in property and
34
equipment in the first quarter of fiscal 2006, compared to $58,000 in the first quarter of fiscal 2005. The increased spending was related primarily to product tooling, additional computer equipment, and in making improvements in office space utilization to accommodate additional personnel within existing leased space. We expect that capital expenditures for property and equipment will likely increase as we make further modifications to improve office space utilization and to make accommodations in our existing facilities for additional sales and marketing personnel that will be necessary to support commercialization of Neurodex, assuming the drug is approved by the FDA. (See Management’s Discussion and Analysis of Financial Condition and Results of Operations, “Selling, General and Administrative Expenses.”)
Financing activities.Net cash provided by financing activities was $36.9 million in the first quarter of fiscal 2006, consisting of $35.6 million in net proceeds from sales of our common stock through private placements and $1.4 million from exercises of warrants and stock options to purchase our common stock. Net cash provided by financing activities amounted to $7.1 million in the first quarter of fiscal 2005, consisting of $7.0 million received from the sale of our common stock and $100,000 from exercises of stock options.
In June 2005, we filed shelf registration statement on Form S-3 with the SEC to sell an aggregate of up to $100 million in Class A common stock and preferred stock, depositary shares, debt securities and warrants. This shelf registration statement was declared effective on August 3, 2005. In October 2005, we sold 1,523,585 shares (adjusted for a one-for-four reverse stock split) of Class A common stock to certain institutional investors at $10.60 per share for aggregate net offering proceeds of $16.15 million. In December 2005, we sold to certain institutional investors 1,492,538 shares (adjusted for a one-for-four reverse stock split) of our common stock at $13.40 per share, for aggregate offering proceeds of approximately $20.0 million and net offering proceeds of approximately $19.4 million, after deducting commissions and offering fees and expenses.
In December 2003, we issued warrants to purchase a total of up to 807,347 shares (adjusted for a one-for-four reverse stock split, see Note 2, “Reverse Stock Split”) of our Class A common stock at an exercise price of $7.00 per share (the “Warrants”). The Warrants had a five-year term, but included a provision that we could redeem the Warrants for $1.00 each if our stock price traded above twice the Warrant exercise price for a certain period of time (the “Redemption Right”). On January 24, 2006, we sent the warrant holders notice that the Redemption Right had been triggered and that the Warrants would expire, to the extent unexercised, on February 7, 2006. Between January 26, 2006 and February 7, 2006, we received Warrant subscriptions for 671,923 shares, representing $4.7 million in proceeds to us. At the close of business on February 7, 2006, a single warrant to purchase 25,167 shares of Class A common stock expired unexercised.
In September 2004, we entered into an equipment line of credit with GE Healthcare Financial Services for financing of up to $1.4 million. As of December 31, 2005, the outstanding balance due under the line of credit was approximately $878,000.
Under the terms of a license agreement with the Center for Neurologic Study (“CNS”), we will be obligated to pay CNS milestone payments upon achievement of certain future events relating to the FDA’s regulatory approval process for Neurodex and a royalty on commercial sales of Neurodex, if and when the drug is approved by the FDA for commercialization. Under certain circumstances, we may have the obligation to pay CNS a share of net revenues received if we sublicense Neurodex to a third party for certain licensed indications. We expect to pay CNS a $75,000 milestone in 2006 if the FDA approves our NDA.
35
MANAGEMENT OUTLOOK
We believe that cash, cash equivalents, and investments in securities of approximately $59.3 million at February 8, 2006, plus anticipated future cash from licensed technologies, should be sufficient to sustain our planned level of operations for at least the next 12 months. During fiscal 2006, we expect to earn $4.0 million to $6.5 million for the year in revenues from R&D services that we are providing under collaborative agreements. These payments will fully offset the expenses that we incur in connection with providing those services. Potential milestone payments to be received under existing license agreements are outside of our control and far less predictable. In fiscal 2006, we do not expect to earn any additional milestones under the AstraZeneca license agreement. Fiscal 2006 revenues from new sources, such as license fees and milestone payments, will depend substantially on whether or not we enter into additional license arrangements and whether or not we achieve milestones under those arrangements. Such arrangements could be in the form of licensing or partnering agreements for docosanol 10% cream, Neurodex, or for our other product development programs including development of a selective cytokine inhibitor. Many of our product development programs could take years of additional development before they reach the stage of being licensable to other pharmaceutical companies.
In anticipation of a product launch of Neurodex within several months of approval of the drug, if approved by FDA for marketing, we expect to raise additional capital to support the potential launch and marketing of the drug. Potential alternatives that we could pursue for raising capital include, but are not limited to, partnering arrangements where partners share development costs, issuance of debt or equity securities, and licensing or sales of one or more of our platform technologies or new drug candidates. The balance of securities available for sale under the existing shelf registration is approximately $64.0 million as of December 31, 2005. If we are unable to raise capital as needed to fund our operations, or if we are unable to reach milestones in our license agreements or enter into any new collaborative arrangements, then we may need to slow the rate of development of some of our programs or sell the rights to one or more of our drug candidates. For information regarding the risks associated with our need to raise capital to fund our ongoing and planned operations, please see “Risk Factors.”
RECENT ACCOUNTING PRONOUNCEMENTS
See Note 3, “Significant Accounting Policies — Change in Accounting Method for Share-based Compensation” and Note 15, “Recent Accounting Pronouncements” in the Notes to Condensed Consolidated Financial Statements (Unaudited) for a discussion of recent accounting pronouncements and their effect, if any, on our financial condition or results of operations.
RISK FACTORS
Risks Relating to Our Business
We have a history of losses and we may never achieve or maintain profitability.
To date, we have experienced significant operating losses in funding the research, development and clinical testing of our drug candidates and we expect to continue to incur substantial operating losses through at least fiscal 2007. As of December 31, 2005, our accumulated deficit was approximately $160.7 million. To achieve profitability, we would need to generate significant additional revenue with positive gross margins to offset our other operating expenses, which we expect will increase as we increase our pre-launch activities and sales and marketing efforts over the next several quarters while awaiting the FDA’s decision regarding the approval of Neurodex for the treatment of IEED/PBA.
We may also increase spending on our clinical and pre-clinical programs to the extent our progress in development is favorable. Although we have recently entered into research collaborations with major pharmaceutical companies for two of our research programs, we continue to seek and to negotiate revenue-generating licenses for docosanol 10% cream, and for our other research programs for a selective
36
cytokine inhibitor and our antibody technology. However, we may not find additional attractive arrangements, if at all, and any such arrangements may not provide adequate revenues to cover future operating expenses. Increases in expenditures may not be offset by new or adequate sources of revenues, and as a result, we may not achieve profitability.
We have experienced delays in obtaining the FDA’s acceptance for filing of our new drug application for Neurodex due to requests by the Agency for additional discussions about the data. Any further delays or any adverse decisions in the regulatory review or approval process may harm our prospects and could harm our stock price.
In late August 2005, the FDA informed us that the Agency had restarted the review date for the Neurodex NDA to August 10, 2005 (from June 29, 2005) because of a submission that we had made at the request of the Agency containing supplemental data with certain pre-clinical information. The Agency has the discretion to determine whether responses to the Agency are sufficient to reset the start date for the review. At the time that we submitted the additional data, we did not know our submission would reset the review timeline for the Neurodex NDA. On September 21, 2005, the FDA informed us that there were formatting and summarization issues with our NDA, which we clarified in detail at a meeting with the Agency in October 2005.
Based on commentary that we received from the FDA, we have strengthened and enhanced our NDA to include data from additional patients who had completed treatment for more than six months in our open-label study, bringing the total number above the relevant requirements for chronic exposure. We re-submitted the Neurodex NDA with expanded data, refined narratives and re-formatted materials in January 2006. Thereafter, the Agency will have 60 days to determine whether the file is complete and assign a review timeline, namely whether it will be a standard or priority review. These filing and acceptance delays, and any subsequent delays, might extend our operating losses, which would likely increase our cash requirements over the long run.
We also cannot be certain that Neurodex will ultimately be approved by the FDA for marketing or that we will be able to obtain the labeling claims desirable for the promotion of the product or that the FDA may adjust our claims adversely. Additional, recent public announcements regarding safety problems with certain approved drugs may also affect the FDA’s policies regarding safety data for all new drug applications and may result in the FDA requiring additional safety data before approving Neurodex, and FDA may require closer surveillance after commercialization if the drug is approved.
We have yet to market or sell Neurodex or any of our other potential products.
We have never before directly marketed or sold any pharmaceutical products. In order to market Neurodex, assuming it is approved by the FDA, or market any other drug candidates, we will need to hire additional personnel with relevant pharmaceutical experience to staff our sales management and marketing group to help ensure the potential success in marketing our products. If we cannot develop the required marketing and sales expertise internally, our ability to generate revenue from product sales will likely suffer.
In international markets, we may rely on collaborative partners to obtain regulatory approvals, and to market and sell our products in those markets. We have not yet entered into any collaborative arrangement with respect to marketing or selling Neurodex, with the exception of one such agreement relating to Israel. We cannot guarantee that we will be able to enter into any other arrangements on terms favorable to us, or at all. If we are able to enter into sales and marketing arrangements with collaborative partners, we cannot assure you that such collaborators will apply adequate resources and skills to their responsibilities, or that their marketing efforts will be successful. If we are unable to enter into favorable collaborative arrangements with respect to marketing or selling Neurodex or docosanol 10% cream or our collaborators’ efforts are not successful, our ability to generate revenue from product sales will suffer.
37
We expect that over the next 12 to 24 months we will need to raise additional capital to fund ongoing operations and support our planned product launch. If we are unable to raise additional capital, we may be forced to curtail operations. If we succeed in raising additional capital through a licensing or financing transaction, it may affect our stock price and future revenues.
In order to maintain sufficient cash and investments to fund future operations and to prepare for the commercialization of Neurodex, we will need to raise additional capital over the next 12 to 24 months through various alternatives, including licensing or sales of our technologies and drug candidates, selling shares of our Class A common stock or preferred stock or through the issuance of one or more forms of senior or subordinated debt. The balance of securities available for sale under an existing shelf registration is approximately $64.0 million. See “Management Outlook” in Management’s Discussion and Analysis of Financial Condition and Results of Operations.
If we raise capital by issuing additional shares of Class A common stock at a price per share less than the then-current market price per share, the per-share value of our Class A common stock may be reduced. Further, even if we sell shares of common stock at prices equal to or higher than the then-current market price, the issuance of additional shares may depress the market price of our Class A common stock and will dilute voting rights of our other shareholders. If we raise capital through licensing or sales of one or more of our technologies or drug candidates, as we have with our RCT and MIF technologies, then we will likely need to share a significant portion of future revenues from these drug candidates with our licensees. Additionally, the development of drug candidates licensed or sold to third parties will no longer be in our control. Because license arrangements typically provide us with revenue only as the drug candidate is successfully developed and marketed, and because the development of any out-licensed drug candidates will typically be outside of our control, we may not realize a significant portion of the potential value of any such license arrangements.
If we are unable to raise additional capital to fund future operations, then we may not be able to execute our commercialization plans for Neurodex and may be required to reduce operations or defer or abandon one or more of our clinical or pre-clinical research programs.
Changes in board and management composition that are intended to strengthen the board and management team could adversely disrupt our operations.
We have recently made significant changes to our senior management team and board of directors to add to our pharmaceutical experience, significantly enhance our scientific and clinical expertise, and provide depth in managing profitable pharmaceutical businesses. Our President and Chief Executive Officer joined the Company in September 2005, our Senior Vice President of Sales and Marketing joined the Company in July 2005 and our Vice President of Medical Affairs joined in January 2006. Since September 2004, seven new directors have joined our board and we continue to recruit senior-level sales and marketing and regulatory personnel to add to our management team. Accordingly, we anticipate that we could experience other changes in management and infrastructure as we expand our organization, prepare for the commercialization of Neurodex and effect our transition from a research and development company to a pharmaceutical company. These changes could be disruptive, and we may experience difficulties in retaining new directors, attracting and integrating new members of the management team and in transitioning our operating activities.
Our inability to attract and retain key management and scientific personnel could negatively affect our business.
The industry in which we compete has a high level of employee mobility and aggressive recruiting of skilled personnel. This type of environment creates intense competition for qualified personnel, particularly in product research and development, sales and marketing and accounting and finance. The loss of certain executive officers and other key employees could adversely affect our operations.
38
Additionally, in order to expand the Company as planned and to effect our transition to a pharmaceutical company, we will need to hire, train, retain and motivate high quality personnel, including sales and marketing personnel for the commercialization of Neurodex. Any inability to hire qualified sales and marketing personnel would harm our commercialization plans. If we were to lose one or more of our key scientists, then we would likely lose some portion of our institutional knowledge and technical know-how, which could potentially cause a substantial delay in one or more of our development programs until adequate replacement personnel could be hired and trained.
Our patents may be challenged and our pending patents may be denied. Either result would seriously jeopardize our ability to compete in the intended markets for our proposed products.
We have invested in an extensive patent portfolio and we rely substantially on the protection of our intellectual property through our ownership or control of issued patents and patent applications. Such patents and patents pending cover Neurodex, docosanol 10% cream and other potential drug candidates that could come from our technologies such as reverse cholesterol transport, selective cytokine inhibitors, anti-inflammatory compounds and antibodies. Because of the competitive nature of the biopharmaceutical industry, we cannot assure you that:
| | • | | The claims in any pending patent applications will be allowed or that patents will be granted; |
| |
| | • | | Competitors will not develop similar or superior technologies independently, duplicate our technologies, or design around the patented aspects of our technologies; |
| |
| | • | | Our proposed technologies will not infringe other patents or rights owned by others, including licenses that may be not be available to us; |
| |
| | • | | Any of our issued patents will provide us with significant competitive advantages; or |
| |
| | • | | Challenges will not be instituted against the validity or enforceability of any patent that we own or, if instituted, that these challenges will not be successful. |
Even if we successfully preserve our intellectual property rights, other biotechnology or pharmaceutical companies may allege that our technology infringes on their rights. Intellectual property litigation is costly, and even if we were to prevail in such a dispute, the cost of litigation could adversely affect our business, financial condition, and results of operations. Litigation is also time-consuming and would divert management’s attention and resources away from our operations and other activities. If we were to lose any litigation, in addition to any damages we would have to pay, we could be required to stop the infringing activity or obtain a license. Any required license might not be available to us on acceptable terms, or at all. Some licenses might be non-exclusive, and our competitors could have access to the same technology licensed to us. If we were to fail to obtain a required license or were unable to design around a competitor’s patent, we would be unable to sell or continue to develop some of our products, which would have a material adverse effect on our business, financial condition and results of operations.
We depend on third parties to manufacture compounds for our drugs and drug candidates. The failure of these third parties to perform successfully could harm our business.
We have utilized, and intend to continue utilizing, third parties to manufacture docosanol 10% cream, Neurodex and active pharmaceutical ingredients and supplies for our other drug candidates. We have no experience in manufacturing and do not have any manufacturing facilities. Currently, we have sole suppliers for the active pharmaceutical ingredients (“API”) for docosanol and Neurodex, and a sole manufacturer of Neurodex. Further, we do not have any long-term agreements in place with our current docosanol supplier or Neurodex API suppliers. Any delays or difficulties in obtaining API or in manufacturing Neurodex could delay our clinical trials for neuropathic pain and delay the commercialization of Neurodex for IEED/PBA. Additionally, although we and GlaxoSmithKline maintain a strategic reserve of docosanol to mitigate against a short-term supply disruption, any sustained disruption of our docosanol supply could harm our operations.
39
Because we depend on clinical research centers and other contractors for clinical testing and for certain research and development activities, the results of our clinical trials and such research activities are, to a certain extent, beyond our control.
The nature of clinical trials and our business strategy of outsourcing a substantial portion of our research require that we rely on clinical research centers and other contractors to assist us with research and development, clinical testing activities, patient enrollment and regulatory submissions to the FDA. As a result, our success depends partially on the success of these third parties in performing their responsibilities. Although we pre-qualify our contractors and we believe that they are fully capable of performing their contractual obligations, we cannot directly control the adequacy and timeliness of the resources and expertise that they apply to these activities. If our contractors do not perform their activities in an adequate or timely manner, the pace of clinical development, regulatory approval and commercialization of our drug candidates could have to be abandoned or delayed.
Developing new pharmaceutical products for human use involves product liability risks, for which we currently have limited insurance coverage.
The testing, marketing and sale of pharmaceutical products involves the risk of product liability claims by consumers and other third parties. We maintain product liability insurance coverage for our clinical trials in the amount of $5 million per incident and $5 million in the aggregate. We expect to increase this coverage if we receive a favorable marketing approval of Neurodex by the FDA. However, product liability claims can be high in the pharmaceutical industry and our insurance may not sufficiently cover our actual liabilities. Additionally, our insurance carriers may deny, or attempt to deny, coverage in certain instances. If a suit against our business or proposed products is successful, then the lack of or insufficiency of insurance coverage could affect materially and adversely our business and financial condition. Furthermore, various distributors of pharmaceutical products require minimum product liability insurance coverage before their purchase or acceptance of products for distribution. Failure to satisfy these insurance requirements could impede our ability to achieve broad distribution of our proposed products.
Risks Relating to Our Stock
Our stock price has historically been volatile and we expect that this volatility may continue for the foreseeable future.
The market price of our Class A common stock has been, and is likely to continue to be, highly volatile. This volatility can be attributed to many factors, including the following:
| | • | | Unfavorable announcements by us regarding our Neurodex NDA submission, clinical trial results or results of operations; |
| |
| | • | | Announcements of developments regarding our agreements with Novartis and AstraZeneca, including delays in meeting goals or performance milestones by us or our partners; |
| |
| | • | | Comments made by securities analysts, including changes in their recommendations; |
| |
| | • | | Announcements by us of financing transactions and/or future sales of equity or debt securities; |
| |
| | • | | Announcements by us of significant acquisitions, strategic partnerships, joint ventures, or capital commitments; |
| |
| | • | | Sales of our Class A common stock by our directors, officers, or significant shareholders; |
| |
| | • | | Announcements by our competitors of clinical trial results or product approvals; |
| |
| | • | | Changes in operations and increase in operating expenses as we prepare for the commercial launch of Neurodex, assuming it is approved by the FDA; and |
| |
| | • | | Market and economic conditions. |
Additionally, our stock price has been volatile as a result of periodic variations in our operating results. We expect our operating results to continue to vary from quarter-to-quarter due to the foregoing factors, as well as other factors, and these variations may be significant. Variations may result from the following factors:
40
| | • | | Performance under partnering arrangements— The recognition of the revenue under our partnering arrangements, including our license agreements for our MIF and RCT programs, will partially depend on the efforts and performance of our licensees in reaching milestones that are outside of our control, such as regulatory approval, product launch, or reaching a sales threshold. |
| |
| | • | | Timing of FDA regulatory decisions— We are in the process of building a sales force for the planned commercialization of Neurodex. The timing and extent of these development expenditures will vary depending on the status and timing of the FDA’s review and decision on approval of our NDA for Neurodex. As a result, our expenses could vary significantly from quarter-to-quarter while we await regulatory decisions and complete this building process. |
| |
| | • | | Acquisitions/alliances— Our acquisition of certain rights relating to Neurodex in fiscal 2005 resulted in charges of approximately $7.2 million. In the future, if we acquire complementary technologies, products, or businesses, we will incur potentially significant charges in connection with such acquisitions and may have ongoing charges after the closing of any such transaction. Any such acquisitions could adversely affect our results of operations. |
As a result of these factors, our stock price may continue to be volatile and investors may be unable to sell their shares at a price equal to, or above, the price paid.
Risks Relating to Our Industry
The pharmaceutical industry is highly competitive and most of our competitors are larger and have greater resources. As a result, we face significant competitive hurdles.
The pharmaceutical and biotechnology industries are highly competitive and subject to significant and rapid technological change. We compete with hundreds of companies that develop and market products and technologies in similar areas as our research. For example, we expect that Neurodex, if approved by the FDA for marketing as a treatment of IEED/PBA, will compete against antidepressants, atypical anti-psychotic agents and other agents for the treatment of this condition.
Our competitors may have specific expertise and technologies that are better than ours and many of these companies, either alone or together with their research partners, have substantially greater financial resources, larger research and development staffs and substantially greater experience than we do. Accordingly, our competitors may successfully develop competing products. If we commence commercial sales for Neurodex for IEED/PBA, we may potentially be competing with other companies and their products with respect to manufacturing efficiencies and marketing capabilities, areas where we have limited or no direct experience.
Our industry is highly regulated and our failure or inability to comply with government regulations regarding the development, production, testing, manufacturing and marketing of our products may adversely affect our operations.
Government authorities in the U.S., including the FDA, and other countries highly regulate the development, production, testing, manufacturing and marketing of pharmaceutical products. The clinical testing and regulatory approval process can take many years and requires the expenditure of substantial resources. Failure to obtain, or delays in obtaining, these approvals will adversely affect our business operations, including our ability to commence marketing of any of the proposed products. We may find it necessary to use a significant portion of our financial resources for research and development and the clinical trials necessary to obtain these approvals for our proposed products. We will continue to incur costs of development without any assurance that we will ever obtain regulatory approvals for any of our products under development. Additionally, we cannot predict the extent to which adverse government
41
regulations might arise from future U.S. or foreign legislative or administrative actions. Moreover, we cannot predict with accuracy the effects of any future changes in the regulatory approval process and in the domestic health care system for which we develop our products, or the costs of on-going compliance regulations after marketing approval has been obtained. Future changes could affect adversely the time frame required for regulatory review, our financial resources, and the sales prices of our proposed products, if approved for sale.
Item 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As described below, we are exposed to market risks related to changes in interest rates. Because substantially all of our revenue, expenses, and capital purchasing activities are transacted in U.S. dollars, our exposure to foreign currency exchange rates is immaterial. However, in the future we could face increasing exposure to foreign currency exchange rates as we expand international distribution of docosanol 10% cream and purchase additional services from outside the U.S. Until such time as we are faced with material amounts of foreign currency exchange rate risks, we do not plan to use derivative financial instruments, which can be used to hedge such risks. We will evaluate the use of derivative financial instruments to hedge our exposure as the needs and risks should arise.
Interest rate sensitivity
Our investment portfolio consists primarily of fixed income instruments with an average duration of 0.9 year as of December 31, 2005 (1.2 years as of September 30, 2005). The primary objective of our investments in debt securities is to preserve principal while achieving attractive yields, without significantly increasing risk. We classify our investments in securities as of December 31, 2005 as available-for-sale and our restricted investments in securities as held-to-maturity. These available-for-sale securities are subject to interest rate risk. In general, we would expect that the volatility of this portfolio would decrease as its duration decreases. Based on the average duration of our investments as of December 31, 2005 and 2004, an increase of one percentage point in the interest rates would have resulted in increases in comprehensive losses of approximately $183,000 and $137,000, respectively.
Item 4. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our chief executive officer and chief financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in the Securities Exchange Act of 1934, Rules 13a-15(e) and 15d-15(e), as of the end of the period covered by this report, have concluded that, based on such evaluation, as of December 31, 2005 our disclosure controls and procedures were effective and designed to provide reasonable assurance that the information required to be disclosed is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission rules and forms. In designing and evaluating the disclosure controls and procedures, our management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Changes in Internal Controls over Financial Reporting
There has been no change in the Company’s internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) during the Company’s fiscal quarter ended December 31, 2005, that has materially affected, or is reasonably likely to materially affect the Company’s internal control over financial reporting.
42
PART II OTHER INFORMATION
Item 1. LEGAL PROCEEDINGS
In the ordinary course of business, we may face various claims brought by third parties. Any of these claims could subject us to costly litigation. Management believes the outcome of currently identified claims and lawsuits will not have a material adverse effect on our financial condition or results of operations.
Item 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
Not applicable
Item 3. DEFAULTS UPON SENIOR SECURITIES
Not applicable.
Item 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
Not applicable.
Item 5. OTHER INFORMATION
On December 23, 2005, we entered into entered into an employment agreement with Randall E. Kaye, M.D. pursuant to which Dr. Kaye will serve as our Vice President of Medical Affairs in an at-will capacity. Dr. Kaye’s employment commenced on January 16, 2006. A copy of Dr. Kaye’s employment agreement is attached hereto as Exhibit 10.1.
Item 6. EXHIBITS
| | | |
| Exhibits | | |
| 10.1 | | Employment Agreement with Randall Kaye, dated December 23, 2005. |
| | | |
| 15.1 | | Letter on unaudited interim financial information. |
| | | |
| 31.1 | | Certification of Principal Executive Officer Required Under Rule 13a-14(a) of the Securities Exchange Act of 1934, as amended. |
| | | |
| 31.2 | | Certification of Principal Financial Officer Required Under Rule 13a-14(a) of the Securities Exchange Act of 1934, as amended. |
| | | |
| 32.1 | | Certification of Principal Executive Officer Required Under Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. 1350. |
| | | |
| 32.2 | | Certification of Principal Financial Officer Required Under Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. 1350. |
43
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | | |
| Signature | | Title | | Date |
| | | | | |
| /s/Eric K. Brandt Eric K. Brandt | | President and Chief Executive Officer (Principal Executive Officer) | | February 9, 2006 |
| |
| | | | | |
| /s/Gregory P. Hanson, CMA Gregory P. Hanson, CMA | | Vice President, Finance and Chief Financial Officer (Principal Financial and Accounting Officer) | | February 9, 2006 |
44
EXHIBIT INDEX
| | | |
| Exhibits | | |
| 10.1 | | Employment Agreement with Randall Kaye, dated December 23, 2005. |
| | | |
| 15.1 | | Letter on unaudited interim financial information. |
| | | |
| 31.1 | | Certification of Principal Executive Officer Required Under Rule 13a-14(a) of the Securities Exchange Act of 1934, as amended. |
| | | |
| 31.2 | | Certification of Principal Financial Officer Required Under Rule 13a-14(a) of the Securities Exchange Act of 1934, as amended. |
| | | |
| 32.1 | | Certification of Principal Executive Officer Required Under Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. 1350. |
| | | |
| 32.2 | | Certification of Principal Financial Officer Required Under Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. 1350. |