UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
| | | |
| þ | | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
For the quarterly period ended December 31, 2006
OR
| | | |
| o | | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934. |
For the transition period from to .
Commission File No. 1-15803
AVANIR PHARMACEUTICALS
(Exact name of registrant as specified in its charter)
| | | | | |
California
(State or other jurisdiction of
incorporation or organization) | | | | 33-0314804
(I.R.S. Employer Identification No.) |
| | | |
101 Enterprise Suite 300, Aliso Viejo, California
(Address of principal executive offices) | | 92656
(Zip Code) |
(949) 389-6700
(Registrant’s telephone number, including area code)
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities and Exchange Act of 1934 during the preceding 12 months (or for such shorter periods that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. YESþ NOo
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer or a non-accelerated filer. (See definition of “accelerated filer” and “large accelerated filer” in Rule 12b-2 of the Exchange Act). Check one:
Large Accelerated Filero Accelerated Filerþ Non-accelerated Filero
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). YESo NOþ
As of February 12, 2007, the registrant had 39,598,709 shares of common stock issued and outstanding.
PART I. FINANCIAL INFORMATION
Item 1. FINANCIAL STATEMENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
AvanirPharmaceuticals
We have reviewed the accompanying condensed consolidated balance sheet ofAvanir Pharmaceuticals and subsidiaries (the “Company”) as of December 31, 2006 and the related condensed consolidated statements of operations and of cash flows for the three-month periods ended December 31, 2006 and 2005. These interim financial statements are the responsibility of the Company’s management.
We conducted our reviews in accordance with the standards of the Public Company Accounting Oversight Board (United States). A review of interim financial information consists principally of applying analytical procedures and making inquiries of persons responsible for financial and accounting matters. It is substantially less in scope than an audit conducted in accordance with the standards of the Public Company Accounting Oversight Board (United States), the objective of which is the expression of an opinion regarding the financial statements taken as a whole. Accordingly, we do not express such an opinion.
Based on our reviews, we are not aware of any material modifications that should be made to such condensed consolidated interim financial statements for them to be in conformity with accounting principles generally accepted in the United States of America.
We have previously audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheet ofAvanir Pharmaceuticals and subsidiaries as of September 30, 2006, and the related consolidated statements of operations, shareholders’ deficit, and cash flows for the year then ended (not presented herein); and in our report dated December 15, 2006, we expressed an unqualified opinion on those consolidated financial statements. In our opinion, the information set forth in the accompanying condensed consolidated balance sheet as of September 30, 2006 is fairly stated, in all material respects, in relation to the consolidated balance sheet from which it has been derived.
DELOITTE & TOUCHE LLP
Costa Mesa, California
February 14, 2007
3
AVANIR Pharmaceuticals
CONDENSED CONSOLIDATED BALANCE SHEETS (UNAUDITED)
| | | | | | | | | |
| | | December 31, | | | September 30, | |
| | | 2006 | | | 2006 | |
ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 14,457,274 | | | $ | 4,898,214 | |
| Short-term investments in securities | | | 1,968,663 | | | | 16,778,267 | |
| Receivables, net | | | 2,316,187 | | | | 3,042,468 | |
| Inventories | | | 3,531,111 | | | | 2,835,203 | |
| Prepaid expenses | | | 1,683,447 | | | | 1,778,918 | |
| | | | | | | |
| Total current assets | | | 23,956,682 | | | | 29,333,070 | |
| | | | | | | | | |
| Investments in securities | | | 245,040 | | | | 2,216,995 | |
| Restricted investments in securities | | | 856,597 | | | | 856,597 | |
| Property and equipment, net | | | 5,221,484 | | | | 6,047,729 | |
| Intangible assets, net | | | 9,678,740 | | | | 10,113,329 | |
| Goodwill | | | 19,042,439 | | | | 22,110,328 | |
| Other assets | | | 438,614 | | | | 784,289 | |
| | | | | | | |
| TOTAL ASSETS | | $ | 59,439,596 | | | $ | 71,462,337 | |
| | | | | | | |
| | | | | | | | | |
LIABILITIES AND SHAREHOLDERS’ DEFICIT | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 4,813,554 | | | $ | 10,845,057 | |
| Accrued expenses | | | 15,047,773 | | | | 12,983,501 | |
| Assumed liabilities for returns and other discounts | | | — | | | | 3,980,229 | |
| Deferred revenues, net | | | 4,705,633 | | | | 7,592,563 | |
| Notes payable | | | 592,101 | | | | 670,737 | |
| Capital lease obligations | | | 224,000 | | | | 230,760 | |
| | | | | | | |
| Total current liabilities | | | 25,383,061 | | | | 36,302,847 | |
| |
| Other liabilities | | | 219,563 | | | | 230,450 | |
| Deferred revenues, net of current portion | | | 15,047,365 | | | | 15,716,762 | |
| Notes payable, net of current portion | | | 21,820,992 | | | | 24,715,905 | |
| Capital lease obligations, net of current portion | | | 116,815 | | | | 170,908 | |
| | | | | | | |
| Total liabilities | | | 62,587,796 | | | | 77,136,872 | |
| | | | | | | |
| | | | | | | | | |
COMMITMENTS AND CONTINGENCIES(Note 10) | | | | | | | | |
| | | | | | | | | |
SHAREHOLDERS’ DEFICIT | | | | | | | | |
| Preferred stock — no par value, 10,000,000 shares authorized, no shares issued and outstanding as of December 31, 2006 and September 30, 2006 | | | — | | | | — | |
| Common stock — no par value, Class A, 200,000,000 shares authorized, 37,158,572 and 31,708,461 shares issued and outstanding as of December 31, 2006 and September 30, 2006, respectively | | | 228,069,110 | | | | 211,993,249 | |
| Accumulated deficit | | | (231,182,807 | ) | | | (217,565,280 | ) |
| Accumulated other comprehensive loss | | | (34,503 | ) | | | (102,504 | ) |
| | | | | | | |
| Total shareholders’ deficit | | | (3,148,200 | ) | | | (5,674,535 | ) |
| | | | | | | |
| TOTAL LIABILITIES AND SHAREHOLDERS’ DEFICIT | | $ | 59,439,596 | | | $ | 71,462,337 | |
| | | | | | | |
See notes to condensed consolidated financial statements.
4
AVANIR Pharmaceuticals
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS (UNAUDITED)
| | | | | | | | | |
| | | Three months ended | |
| | | December 31, | |
| | | 2006 | | | 2005 | |
PRODUCT SALES | | | | | | | | |
| Net revenues | | $ | 6,270,704 | | | $ | — | |
| Cost of revenues | | | 1,347,184 | | | | — | |
| | | | | | | |
| Product gross margin | | | 4,923,520 | | | | — | |
| | | | | | | |
| | | | | | | | | |
LICENSES, RESEARCH SERVICES AND GRANTS | | | | | | | | |
| Revenues from research services | | | 1,270,244 | | | | 2,478,018 | |
| Cost of research services | | | (1,164,075 | ) | | | (2,018,799 | ) |
| Revenues from government research grant services | | | 86,348 | | | | 84,825 | |
| Cost of government research grant services | | | (95,727 | ) | | | (71,123 | ) |
| Revenues from license agreements | | | 57,265 | | | | 5,000,000 | |
| Revenue from royalties and royalty rights | | | 723,040 | | | | 582,045 | |
| | | | | | | |
| License, research services, and grants gross margin | | | 877,095 | | | | 6,054,966 | |
| | | | | | | |
| | | | | | | | | |
| Total gross margin | | | 5,800,615 | | | | 6,054,966 | |
| | | | | | | | | |
OPERATING EXPENSES | | | | | | | | |
| Research and development | | | 5,906,001 | | | | 7,198,958 | |
| Selling, general and administrative | | | 13,245,934 | | | | 4,768,743 | |
| | | | | | | |
| | | | | | | | | |
| Loss from operations | | | (13,351,320 | ) | | | (5,912,735 | ) |
| | | | | | | | | |
OTHER INCOME (EXPENSE) | | | | | | | | |
| Interest expense | | | (603,028 | ) | | | (23,438 | ) |
| Interest income | | | 191,476 | | | | 328,166 | |
| Other | | | 145,345 | | | | 10,512 | |
| | | | | | | |
| |
| Loss before income taxes | | | (13,617,527 | ) | | | (5,597,495 | ) |
| | | | | | | | | |
| Provision for income taxes | | | — | | | | (2,417 | ) |
| | | | | | | |
| | | | | | | | | |
NET LOSS BEFORE CUMULATIVE EFFECT OF CHANGE IN ACCOUNTING PRINCIPLE | | | (13,617,527 | ) | | | (5,599,912 | ) |
| | | | | | | | | |
| Cumulative effect of change in accounting principle | | | — | | | | (3,616,058 | ) |
| | | | | | | |
| | | | | | | | | |
NET LOSS | | $ | (13,617,527 | ) | | $ | (9,215,970 | ) |
| | | | | | | |
| | | | | | | | | |
Basic and diluted net loss per share | | | | | | | | |
| Loss before cumulative effect of change in accounting principle | | $ | (0.39 | ) | | $ | (0.20 | ) |
| Cumulative effect of change in accounting principle | | | — | | | | (0.12 | ) |
| | | | | | | |
Net loss per share | | $ | (0.39 | ) | | $ | (0.32 | ) |
| | | | | | | |
| Basic and diluted weighted average number of common shares outstanding | | | 34,626,117 | | | | 28,579,357 | |
| | | | | | | |
See notes to condensed consolidated financial statements.
5
AVANIR Pharmaceuticals
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS (UNAUDITED)
| | | | | | | | | |
| | | Three Months Ended | |
| | | December 31, | |
| | | 2006 | | | 2005 | |
OPERATING ACTIVITIES: | | | | | | | | |
| Net loss | | $ | (13,617,527 | ) | | $ | (9,215,970 | ) |
| Adjustments to reconcile net loss to net cash used for operating activities: | | | | | | | | |
| Cumulative effect of change in accounting principle | | | — | | | | 3,616,058 | |
| Depreciation and amortization | | | 1,415,040 | | | | 415,019 | |
| Share-based compensation expense | | | 844,517 | | | | 462,013 | |
| Loss on disposal of assets | | | — | | | | 1,225 | |
| Changes in operating assets and liabilities: | | | | | | | | |
| Receivables | | | 964,646 | | | | (5,428,921 | ) |
| Inventories | | | (348,484 | ) | | | (364,567 | ) |
| Prepaid expenses and other assets | | | 86,709 | | | | (679,390 | ) |
| Accounts payable | | | (6,031,503 | ) | | | (3,581,490 | ) |
| Accrued expenses | | | 2,053,385 | | | | 2,803,655 | |
| Assumed liabilities for returns and other discounts, net of non-cash adjustment of purchase price | | | (912,340 | ) | | | — | |
| Deferred revenues | | | (3,556,327 | ) | | | (553,597 | ) |
| | | | | | | |
| Net cash used for operating activities | | | (19,101,884 | ) | | | (12,525,965 | ) |
| | | | | | | |
| |
INVESTING ACTIVITIES: | | | | | | | | |
| Investments in securities | | | (50,440 | ) | | | (13,532,124 | ) |
| Proceeds from sales and maturities of investments in securities | | | 16,900,000 | | | | 11,850,000 | |
| Purchases of property and equipment | | | (86,193 | ) | | | (543,300 | ) |
| | | | | | | |
| Net cash (used for) provided by investing activities | | | 16,763,367 | | | | (2,225,424 | ) |
| | | | | | | |
| | | | | | | | | |
FINANCING ACTIVITIES: | | | | | | | | |
| Proceeds from issuance of common stock, net | | | 14,992,979 | | | | 37,016,041 | |
| Payments on notes and capital lease obligations | | | (3,095,402 | ) | | | (82,901 | ) |
| | | | | | | |
| Net cash provided by financing activities | | | 11,897,577 | | | | 36,933,140 | |
| | | | | | | |
| |
| Net increase in cash and cash equivalents | | | 9,559,060 | | | | 22,181,751 | |
| Cash and cash equivalents at beginning of period | | | 4,898,214 | | | | 8,620,143 | |
| | | | | | | |
| Cash and cash equivalents at end of period | | $ | 14,457,274 | | | $ | 30,801,894 | |
| | | | | | | |
| | | | | | | | | |
SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | | | | | | | | |
| Interest paid | | $ | 542,028 | | | $ | 23,438 | |
| Income taxes paid | | $ | — | | | $ | 2,417 | |
| | | | | | | | | |
NONCASH INVESTING AND FINANCING ACTIVITIES: | | | | | | | | |
| Stock subscription receivable | | $ | 238,365 | | | | — | |
| Purchase price adjustment of assumed liabilities | | | 3,067,889 | | | | — | |
| Purchases of property and equipment which are included in accounts payable and accrued expenses | | $ | — | | | $ | 253,538 | |
| Elimination of unearned compensation against common stock | | $ | — | | | $ | 3,477,144 | |
See notes to condensed consolidated financial statements.
6
AVANIR Pharmaceuticals
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS (UNAUDITED)
1. NATURE OF BUSINESS AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Nature of Business
AvanirPharmaceuticals (“Avanir,” “we,” or the “Company”) prepared the accompanying unaudited condensed consolidated financial statements in accordance with the rules and regulations of the Securities and Exchange Commission (“SEC”) for interim reporting. These condensed statements do not include all disclosures required by accounting principles generally accepted in the United States of America (“U.S. GAAP”) for annual audited financial statements and should be read in conjunction with the Company’s audited consolidated financial statements and related notes for the year ended September 30, 2006. We believe these condensed consolidated financial statements reflect all adjustments (consisting only of normal, recurring adjustments) that are necessary for a fair presentation of our financial position and results of operations for the periods presented. Results of operations for the interim periods presented are not necessarily indicative of results to be expected for the year.
The preparation of financial statements in conformity with U.S GAAP requires management to make estimates and assumptions that affect the reported amounts and the disclosure of contingent amounts in the financial statements and accompanying notes. Actual results could differ from those estimates.
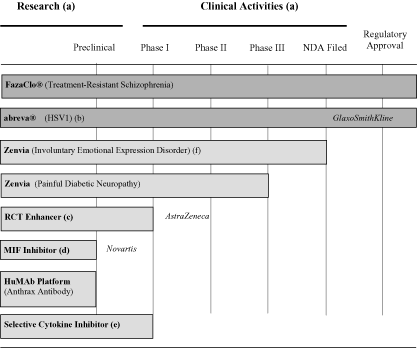
Since our inception, the Company has reported accumulated net losses of approximately $231 million and recurring negative cash flows from operations. In order to maintain sufficient cash and investments to fund future operations, we will seek to raise additional capital in 2007 through various financing alternatives. The balance of securities available for sale under our existing shelf registration was approximately $48.0 million as of December 31, 2006. We believe that these anticipated offering proceeds, plus our cash, cash equivalents and unrestricted investments in securities of approximately $16.7 million at December 31, 2006, as well as anticipated future cash flows generated from licensed technologies and sales from the shipments of FazaClo, will be sufficient to sustain our planned level of operations for at least the next 12 months. However, the Company cannot provide assurances that our plans will not change, or that changed circumstances will not result in the depletion of capital resources more rapidly than anticipated. If we are unable to generate sufficient cash flows from licensed technologies or sales from the shipments of FazaClo and are unable to raise sufficient capital, management believes that planned expenditures could be curtailed in order to continue operations for the next 12 months.
Revenue recognition
The Company generates revenues from product sales, collaborative research and development arrangements, and other activities such as, royalties, the sale of royalty rights and sales of technology rights. Payments received under such arrangements may include non-refundable fees at the inception of the arrangements, fully burdened funding for services performed under collaborative arrangements, milestone payments for specific achievements designated in the collaborative arrangements, royalties on sales of products resulting from collaborative arrangements, and payments for the sale of rights to future royalties.
7
We recognize revenue in accordance with the SEC’s Staff Accounting Bulletin Topic 13 (“Topic 13”),“Revenue Recognition.”Revenue is recognized when all of the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the seller’s price to the buyer is fixed and determinable; and (4) collectibility is reasonably assured. Certain product sales are subject to rights of return. In accordance with Statement of Financial Accounting Standards No. 48,“Revenue Recognition When Right of Return Exists”(“FAS 48”), we recognize such product revenues at the time of sale only if we have met all the criteria of FAS 48, including the ability to reasonably estimate future returns. FAS 48 states that revenue from sales transactions where the buyer has the right to return the product shall be recognized at the time of sale only if (1) the seller’s price to the buyer is substantially fixed or determinable at the date of sale, (2) the buyer has paid the seller, or the buyer is obligated to pay the seller and the obligation is not contingent on resale of the product, (3) the buyer’s obligation to the seller would not be changed in the event of theft or physical destruction or damage of the product, (4) the buyer acquiring the product for resale has economic substance apart from that provided by the seller, (5) the seller does not have significant obligations for future performance to directly bring about resale of the product by the buyer, and (6) the amount of future returns can be reasonably estimated. We recognize such product revenues when either we have met all the criteria of FAS 48, including that ability to reasonably estimate future returns, when we can reasonably estimate that the return privilege has substantially expired, or when the return privilege has substantially expired, whichever occurs first.
We allocate amounts to separate elements in multiple element arrangements in accordance with Emerging Issues Task Force No. 00-21 (“EITF 00-21”),“Accounting for Revenue Arrangements with Multiple Deliverables.”Revenues are allocated to a delivered product or service when all of the following criteria are met: (1) the delivered item has value to the customer on a standalone basis; (2) there is objective and reliable evidence of the fair value of the undelivered item; and (3) if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered item is considered probable and substantially in our control. We use the relative fair values of the separate deliverables to allocate revenue. For arrangements with multiple elements that are separated, we recognize revenues in accordance with the SEC’s Staff Accounting Bulletin Topic 13 (“Topic 13”),“Revenue Recognition.”Revenue is recognized when all of the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the seller’s price to the buyer is fixed and determinable; and (4) collectibility is reasonably assured. Certain sales transactions include multiple deliverables.
Product Sales
| | | Active Pharmaceutical Ingredient Docosanol (“API Docosanol”).Revenues from the sale of our API Docosanol are recognized when title and risk of loss have passed to the buyer and provided the criteria in SAB Topic 13 are met. We sell the API Docosanol to various licensees upon receipt of a written order for the materials. Shipments generally occur fewer than five times a year. Our contracts for sales of the API Docosanol include buyer acceptance provisions that give our buyers the right of replacement if the delivered product does not meet specified criteria. That right requires that they give us notice within 30 days after receipt of the product. We have the option to refund or replace any such defective materials; however, we have historically demonstrated that the materials shipped from the same pre-inspected lot have consistently met the specified criteria and no buyer has rejected any of our shipments from the same pre-inspected lot to date. Therefore, we recognize revenue at the time of delivery without providing any returns reserve. |
| |
| | | FazaClo.We acquired Alamo Pharmaceuticals LLC (“Alamo”) on May 24, 2006, with one marketed product, FazaClo (clozapine, USP), that began shipping to wholesale customers in July 2004 in 48-pill units. At that time, FazaClo had a two-year shelf life. In June 2005, Alamo received FDA approval to extend the product expiration date to three years. In October 2005, Alamo began shipping 96-pill units and accepted returns of unsold or undispensed 48-pill units. |
| |
| | | We sell FazaClo to pharmaceutical wholesalers, the three largest of which account for approximately 84% of our net wholesale shipments for the quarter ended December 31, 2006. They resell our product to outlets such as pharmacies, hospitals and other dispensing organizations. We have agreements with our wholesale customers, various states, hospitals, certain other medical institutions and third-party payers throughout the U.S. These agreements frequently contain commercial incentives, which may include pricing allowances and discounts payable at the time the product is sold to the dispensing outlet or upon dispensing the product to patients. Consistent with pharmaceutical industry practice, wholesale customers can return purchased product during an 18-month period that begins six months prior to the product’s expiration date and ends 12 months after the expiration date. Additionally, several of our dispensing outlets have the right to return expired product at any time. Once products have been dispensed to patients the right of return expires. |
8
| | | Prior to the quarter ended December 31, 2006, we did not have sufficient historical information and analytical support to reasonably estimate future product returns, nor to reasonably estimate the extent to which the right of return had expired on product we shipped. Accordingly, prior to the first quarter of fiscal 2007, we were unable to recognize revenues for product sales subject to return under FAS 48 and deferred the recognition of revenues on all shipments of FazaClo. We would have recognized revenues when we obtained reliable evidence that helped us reasonably determine that the right of return no longer exists, such as when the products have been dispensed to patients or the return period had otherwise expired. We previously disclosed in the notes to our fiscal 2006 financial statements included in our Annual Report on Form 10-K, that for purposes of applying this sell-through method, we would determine when products that we shipped are dispensed to patients based only on those instances when rebate requests are submitted to us by various state agencies and others. Based on the information we had available to us at the time, we had no other means to determine that product had been dispensed until we received the rebate requests, however, rebate requests are only expected to be received for a portion of the products dispensed to patients since not all products dispensed are subject to rebates. Rebates are received in approximately 90-120 days from when the products are dispensed. As of September 30, 2006, we had not received any rebate requests that we believed related to product that we shipped after our acquisition of Alamo. Further, as previously disclosed in the notes to our fiscal 2006 financial statements included in our Annual Report on Form 10-K, in conjunction with our evaluation of our assumed liability for returns, we had been working with our key wholesalers to obtain sufficient information about the amount and type of FazaClo product in the distribution channel. |
| |
| | | During the second quarter of fiscal 2007, we obtained and substantially completed our analysis of third-party information regarding certain wholesaler inventory levels, a sample of outlet inventory levels and third-party market research data. The third-party data includes, (i) IMS Health Audit — National Sales Perspective reports (“NSP”), which is a projection of near-census data of wholesaler shipments of product to all outlet types, including retail and non-retail and; (ii) IMS Health National Prescription Audit (“NPA”) Syndicated data which captures end user consumption from retail dispensed prescriptions based upon projected data from pharmacies estimated to represent approximately 60% to 70% of the U.S. prescription universe . Finally, we completed our analysis of historical rebates and chargebacks earned by State Medicaid, Medicare Part D and managed care customers for a trailing twelve months. Based upon this additional information and analysis obtained in this quarter, we now believe we can reasonably estimate the amount of product that we have shipped that is no longer in the wholesale or outlet channels as of December 31, 2006, and hence no longer subject to a right of return. Accordingly, since January 2007, we have received sufficient additional information that allows us to reasonably estimate the amount of product no longer subject to the right of return as of December 31, 2006. Therefore, we began recognizing revenues, net of returns, chargebacks, rebates, and discounts, in the first quarter of fiscal 2007, for product that we estimate have been sold to patients and that is no longer subject to a right of return. |
| |
| | | As a result, we have recognized revenues on FazaClo product sales of $6.3 million in the quarter ended December 31, 2006, which includes the recognition of deferred revenues of FazaClo that were deferred as of September 30, 2006. Had we continued to recognize revenue only upon receipt of rebate requests as we did in the prior quarter, management estimates that minimal revenue would have been recorded in the quarter ended December 31, 2006. We received approximately $1.2 million of rebate requests in the first quarter of 2007 that pertain to product that was shipped prior to our acquisition of Alamo. |
| |
| | | Additionally, we continue to accumulate historical product return data. To date we have accumulated return data for four lots which are within the 18-month return window, which have varying historical return rates. We are continuing to accumulate historical product return information, but at this time believe that we have insufficient information to reasonably estimate future product returns for revenue recognition purposes. Accordingly, we continue to defer recognizing revenues on all estimated in-channel inventories that are subject to the right of return until such time as we can reasonably estimate product returns. |
| |
| | | Revenues are recorded net of provisions for estimated product pricing allowances including: State Medicaid base and supplemental rebates, Medicare Part D discounts, managed care contract discounts and prompt payment discounts at an aggregate rate of approximately 29.2% of gross revenues as of December 31, 2006. Provisions for these allowances are estimated based upon contractual terms and require management to make subjective judgments on customer mix to reach this judgment. We considered our current contractual rates with States related to Medicaid base and supplemental rebates, with private organizations for Medicare Part D discounts and contracts with managed care organizations over a trailing twelve months. We review these rates at least quarterly and make adjustments, if necessary. |
9
Multiple Element Arrangements.
We have arrangements whereby we deliver to the customer multiple elements including technology and/or services. Such arrangements have generally included some combination of the following: antibody generation services; licensed rights to technology, patented products, compounds, data and other intellectual property; and research and development services. In accordance with EITF 00-21, we analyze our multiple element arrangements to determine whether the elements can be separated. We perform our analysis at the inception of the arrangement and as each product or service is delivered. If a product or service is not separable, the combined deliverables will be accounted for as a single unit of accounting.
When a delivered element meets the criteria for separation in accordance with EITF 00-21, we allocate amounts based upon the relative fair values of each element. We determine the fair value of a separate deliverable using the price we charge other customers when we sell that product or service separately; however if we do not sell the product or service separately, we use third-party evidence of fair value. We consider licensed rights or technology to have standalone value to our customers if we or others have sold such rights or technology separately or our customers can sell such rights or technology separately without the need for our continuing involvement.
| | | License Arrangements.License arrangements may consist of non-refundable upfront license fees, data transfer fees, research reimbursement payments, exclusive licensed rights to patented or patent pending compounds, technology access fees, various performance or sales milestones and future product royalty payments. These arrangements are often multiple element arrangements. |
| |
| | | Non-refundable, up-front fees that are not contingent on any future performance by us, and require no consequential continuing involvement on our part, are recognized as revenue when the license term commences and the licensed data, technology and/or compound is delivered. Such deliverables may include physical quantities of compounds, design of the compounds and structure-activity relationships, the conceptual framework and mechanism of action, and rights to the patents or patents pending for such compounds. We defer recognition of non-refundable upfront fees if we have continuing performance obligations without which the technology, right, product or service conveyed in conjunction with the non-refundable fee has no utility to the licensee that is separate and independent of our performance under the other elements of the arrangement. In addition, if we have required continuing involvement through research and development services that are related to our proprietary know-how and expertise of the delivered technology, or can only be performed by us, then such up-front fees are deferred and recognized over the period of continuing involvement. |
| |
| | | Payments related to substantive, performance-based milestones in a research and development arrangement are recognized as revenues upon the achievement of the milestones as specified in the underlying agreements when they represent the culmination of the earnings process. |
| |
| | | Research Services Arrangements.Revenues from research services are recognized during the period in which the services are performed and are based upon the number of full-time-equivalent personnel working on the specific project at the agreed-upon rate. Reimbursements from collaborative partners for agreed upon direct costs including direct materials and outsourced services, or subcontracted, pre-clinical studies are classified as revenues in accordance with EITF Issue No. 99-19,“Reporting Revenue Gross as a Principal versus Net as an Agent,”and recognized in the period the reimbursable expenses are incurred. Payments received in advance are deferred until the research services are performed or costs are incurred. These arrangements are often multiple element arrangements. |
10
| | | Royalty Arrangements.We recognize royalty revenues from licensed products when earned in accordance with the terms of the license agreements. Net sales amounts generally required to be used for calculating royalties include deductions for returned product, pricing allowances, cash discounts, freight and warehousing. These arrangements are often multiple element arrangements. |
| |
| | | When we sell our rights to future royalties under license agreements and also maintain continuing involvement in earning such royalties, we defer recognition of any upfront payments and recognize them as revenues over the life of the license agreement. We recognize revenues for the sale of an undivided interest of our Abreva® license agreement to Drug Royalty USA under the “units-of-revenue method.” Under this method, the amount of deferred revenues to be recognized in each period is calculated by multiplying the ratio of the royalty payments due to Drug Royalty USA by GlaxoSmithKline for the period to the total remaining royalties that is expect GlaxoSmithKline will pay Drug Royalty USA over the remaining term of the agreement by the unamortized deferred revenues. |
Government Research Grant Revenues.We recognize revenues from federal research grants during the period in which the related expenditures are incurred.
Cost of Revenues
Cost of product revenues includes direct and indirect costs to manufacture, manufacturer royalties, write-off of obsolete inventories, and the amortization of the acquired FazaClo product rights. The cost of research and grant services includes the direct and indirect cost to provide such services
Capitalization and Valuation of Long-Lived and Intangible Assets
In accordance with Statement of Financial Accounting Standards No. 141,“Business Combinations” (“FAS 141”) and Statement of Financial Accounting Standards No. 142,“Goodwill and Other Intangible Assets”(“FAS 142”), goodwill and intangible assets acquired in a purchase business combination and determined to have an indefinite useful life are not amortized, but instead are tested for impairment on an annual basis or more frequently if certain indicators arise. Goodwill represents the excess of purchase price of an acquired business over the fair value of the underlying net tangible and intangible assets. The Company operates in one segment and goodwill is evaluated at the company level as there is only one reporting unit. Goodwill is evaluated in the fourth quarter of each fiscal year. There was no impairment of goodwill as of December 31, 2006.
Intangible assets with finite useful lives are amortized over their respective useful lives and reviewed for impairment in accordance with Statement of Financial Accounting Standards No. 144,“Accounting for the Impairment or Disposal of Long-Lived Assets”(“FAS 144”.) The method of amortization reflects the pattern in which the economic benefits of the intangible asset are consumed or otherwise used up. If that pattern cannot be reliably determined, a straight-line amortization method will be used. Intangible assets with finite useful lives include product rights, customer relationships, trade name, non-compete agreement and license agreement, which are being amortized over their estimated useful lives ranging from one to 15.5 years.
In accordance with FAS 144, intangible assets with finite useful lives are evaluated for impairment whenever events or changes in circumstances indicate that their carrying value may not be recoverable. If the review indicates that intangible assets or long-lived assets are not recoverable (i.e. the carrying
11
amount is less than the future projected undiscounted cash flows), their carrying amount would be reduced to fair value. Factors we consider important that could trigger an impairment review include a significant underperformance relative to expected historical or projected future operating results; a significant change in the manner of our use of the acquired asset or the strategy for our overall business; and/or a significant negative industry or economic trend.
Prior to October 1, 2005, intangible assets with finite useful lives also include capitalized legal costs incurred in connection with approved patents and patent applications pending. We amortized the costs of patents and patent applications that are pending over their estimated useful lives. For patents pending, we amortized the costs over the shorter of a period of twenty years from the date of filing the application or, if licensed, the term of the license agreement. For patent and patent applications pending and trademarks that we abandon, we charge the remaining unamortized accumulated costs to expense.
Share-Based Compensation
We adopted the provisions of revised Statement of Financial Accounting Standards No. 123 (“FAS 123R”), “Share-Based Payment,” including the provisions of Staff Accounting Bulletin No. 107 (“SAB 107”) on October 1, 2005, the first day of our fiscal 2006, using the modified prospective transition method to account for our employee share-based awards. The valuation provisions of FAS 123R apply to new awards and to awards that are outstanding at the effective date and subsequently modified or cancelled. Estimated compensation expense for awards outstanding at the effective date are being recognized over the remaining service period using the compensation cost calculated for pro forma disclosure purposes under Statement of Financial Accounting Standards No. 123, "Accounting for Stock-Based Compensation” (“FAS 123”).
On November 10, 2005, the FASB issued FASB Staff Position No. FAS 123R-3, “Transition Election Related to Accounting for Tax Effects of Share-Based Payment Awards” (“FAS 123R-3”). We have elected to adopt the alternative transition method provided in FAS 123R-3. The alternative transition method includes a simplified method to establish the beginning balance of the additional paid-in capital pool (“APIC pool”) related to the tax effects of employee share-based compensation, which is available to absorb tax deficiencies recognized subsequent to the adoption of FAS 123R.
Share-based compensation expense recognized during a period is based on the value of the portion of share-based payment awards that is ultimately expected to vest during the period. Share-based compensation expense recognized in our condensed consolidated statements of operations for the fiscal quarters ended December 31, 2006 and 2005 include compensation expense for share-based payment awards granted prior to, but not yet vested as of September 30, 2005 based on the grant date fair value estimated in accordance with the pro forma provisions of FAS 123, adjusted for estimated forfeitures, and share-based payment awards granted subsequent to September 30, 2005 based on the grant date fair value estimated in accordance with FAS 123R. For share awards granted prior to October 1, 2006, expenses are amortized under the straight-line single option method prescribed by FAS 123. As share-based compensation expense recognized in the condensed consolidated statements of operations for the fiscal quarters ended December 31, 2006 and 2005 is based on awards ultimately expected to vest, it has been reduced for estimated forfeitures. FAS 123R requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Pre-vesting forfeitures in the first quarters of fiscal 2007 and 2006 were estimated to be approximately 4% and 8%, respectively, for both officers and directors and 4% and 13%, respectively, for other employees based on our historical experience.
12
The adoption of FAS 123R resulted in incremental share-based compensation expense of $844,517 and $462,013 in the first quarters of fiscal 2007 and 2006, respectively. The incremental share-based compensation caused our net loss from operations and net loss to increase by the same amounts and the basic and diluted loss per share to decrease $0.02 per share in the first quarters of fiscal 2007 and 2006. Total compensation expense related to all of our share-based awards, recognized under FAS 123R, for the first quarters of fiscal 2007 and 2006 was comprised of the following:
| | | | | | | | | |
| | | For the 3 months ended December 31, | |
| | | 2006 | | | 2005 | |
| Share-based compensation expense from: | | | | | | | | |
| Stock options | | $ | 414,847 | | | $ | 246,698 | |
| Restricted stock awards | | | 416,405 | | | | 215,315 | |
| Restricted stock units | | | 13,265 | | | | — | |
| | | | | | | |
| Total | | $ | 844,517 | | | $ | 462,013 | |
| | | | | | | |
Since we have a net operating loss carry-forward as of December 31, 2006, no excess tax benefits for the tax deductions related to share-based awards were recognized in the consolidated statement of operations. Additionally, no incremental tax benefits were recognized from stock options exercised in the first quarters of fiscal 2007 and 2006 that would have resulted in a reclassification to reduce net cash provided by operating activities with an offsetting increase in net cash provided by financing activities.
Change in Accounting for Patent-Related Costs
In the fourth quarter of fiscal 2006, we changed our method of accounting, effective October 1, 2005 for legal costs which included only external legal costs and were associated with the application for patents. Prior to the change, we expensed as incurred all internal costs associated with the application for patents and capitalized external legal costs associated with the application for patents. Costs of approved patents were amortized over their estimated useful lives or if licensed, the terms of the license agreement, whichever was shorter, while costs for patents pending were amortized over the shorter period of twenty years from the date of the filing application or if licensed, the term of the license agreement. Amortization expense for these capitalized costs was classified as research and development expenses in our consolidated statements of operations because the patents related to underlying technologies that constituted research and development activities. Under the new method, external legal costs are expensed as incurred and classified as research and development expenses in our consolidated statements of operations. We believe that this change is preferable because it will result in a consistent treatment for all costs, that is, under our new method both internal and external costs associated with the application for patents are expensed as incurred. In addition, the change will provide a better comparison with our industry peers. The $3.6 million cumulative effect of the change on prior years as of October 1, 2005 is included as a charge to net loss in fiscal 2006, retrospectively applied to the first quarter. The effect of the change for fiscal 2006 was to increase the net loss by approximately the cumulative effect of the change on prior years, or $0.12 per basic and diluted share.
2. ALAMO ACQUISITION
On May 24, 2006, we acquired all of the outstanding equity interests in Alamo from the former members of Alamo (the “Selling Holders”) for approximately $30.0 million in consideration, consisting of approximately $4.0 million in cash, $25.1 million in promissory notes and the payment of $912,000 in acquisition-related transaction costs. The purchase price exceeded the net assets acquired, resulting in the
13
recognition of $19.0 million of goodwill. The results of operations of Alamo have been included in our consolidated financial statements since the date of acquisition.
We also agreed to pay the Selling Holders up to an additional $39,450,000 in revenue-based earn-out payments, based on future sales of FazaClo (clozapine USP), Alamo’s orally disintegrating drug for the treatment of refractory schizophrenia. These earn-out payments are based on FazaClo sales in the U.S. from the closing date of the acquisition through December 31, 2018 (the “Contingent Payment Period”) and at present are payable as follows:
| | • | | A promissory note, in the principal amount of $2,000,000, generally payable on the third anniversary date if monthly FazaClo net product sales, as reported by us, exceed $1,000,000 for each of the three months in a given fiscal quarter during the Contingent Payment Period, and an additional promissory note in the principal amount of $2,000,000, generally payable on the third anniversary date if monthly FazaClo net product sales, as reported by us, exceed $1,500,000 for each of the three months in a given fiscal quarter during the Contingent Payment Period. As of December 31, 2007, we did not meet these conditions. |
| |
| | • | | A one-time cash payment of $10,450,000 if FazaClo net product sales, as reported by us, exceed $40.0 million over four consecutive fiscal quarters during the Contingent Payment Period. |
| |
| | • | | An additional one-time cash payment of $25,000,000 if FazaClo net product sales, as reported by us, exceed $50.0 million over four consecutive fiscal quarters during the Contingent Payment Period. |
Any of these additional revenue-based earn-out payments that are ultimately paid upon satisfying the contingent conditions above will be treated as additional consideration and recorded as goodwill.
We have also agreed to pay the Selling Holders one-half of all net licensing revenues that we may receive during the Contingent Payment Period from licenses of FazaClo outside of the U.S., if any (“Non-US Licensing Revenues”). Any amounts paid to the Selling Holders on Non-US Licensing Revenues will be recognized in the consolidated statement of operations in the period such amounts are paid. We also agreed to apply 20% of any future net offering proceeds to repay the promissory notes.
Purchase Price Allocation
In accordance with FAS 141, we allocated the total purchase price to the tangible and identifiable intangible assets acquired and liabilities assumed based on their preliminary estimated fair values as of the date of acquisition, using the purchase method of accounting. The fair values of the assets acquired and liabilities assumed were determined at the date of the acquisition (May 2006) on a preliminary basis. We adjusted our preliminary purchase price in the fourth quarter of fiscal 2006 and again in the first quarter of fiscal 2007 as described below.
14
====================================================================================================================================
The components of the final purchase price allocation are as follows:
| | | | | |
| Purchase price: | | | | |
| Cash paid at closing | | $ | 4,040,000 | |
| Estimated fair value of notes payable issued, net of imputed discount | | | 24,343,000 | |
| Transaction costs | | | 911,536 | |
| | | | |
| | | $ | 29,294,536 | |
| | | | |
| Allocation: | | | | |
| Net tangible assets acquired: | | | | |
| Cash | | $ | 157,507 | |
| Accounts receivable | | | 1,026,740 | |
| Inventories | | | 2,297,117 | |
| Property and equipment | | | 1,845,077 | |
| Other assets | | | 423,457 | |
| Identifiable intangible assets acquired: | | | | |
| Product rights | | | 7,200,000 | |
| In-process research and development | | | 1,300,000 | |
| Customer relationships | | | 2,900,000 | |
| Trade name | | | 400,000 | |
| Non-compete agreement | | | 160,000 | |
| Goodwill | | | 19,042,439 | |
| | | | |
| Total assets acquired | | | 36,752,337 | |
| | | | |
| Liabilities assumed: | | | | |
| Accounts payable and accrued expenses | | | (3,611,653 | ) |
| Assumed liabilities for returns and other discounts | | | (3,333,432 | ) |
| Capital lease obligations | | | (512,716 | ) |
| | | | |
| Total liabilities assumed | | | (7,457,801 | ) |
| | | | |
| | | $ | 29,294,536 | |
| | | | |
Pursuant to EITF 01-3,“Accounting in a Business Combination for Deferred Revenue of an Acquiree,” we did not assume Alamo’s deferred revenue balance as of the acquisition date, and accordingly will not record revenue associated with product that was shipped prior to the acquisition date. However, in connection with the acquisition, we assumed an obligation for future product returns, pricing allowances and royalties associated with pre-acquisition shipments of FazaClo. As such, we recorded preliminary estimated liabilities for such returns and other discounts of $6.4 million based on our estimate of the fair values of the liabilities at the acquisition date, which is classified as assumed liabilities for returns and other discounts in the accompanying consolidated balance sheets. Since the acquisition in May 2006 the Company has been receiving and analyzing historical data regarding FazaClo product returns and product pricing allowances (See Note 1 Revenue Recognition). Based on this analysis, the Company recorded in the first quarter of fiscal year 2007, an adjustment to reduce the preliminary estimate of these assumed liabilities by $3.1 million and recorded a corresponding reduction in goodwill.
The preliminary purchase price allocation was first adjusted in the fourth quarter of fiscal 2006 to increase goodwill by $22.1 million and record a corresponding reduction in value of identifiable intangible assets acquired. The lower allocation to intangible assets reflects a higher risk-adjusted discount rate for the identifiable intangible assets than other assets purchased in the transaction, which lowered the fair values of the intangible assets acquired and increased the residual being applied to goodwill. We acquired Alamo to support our strategic plan to become a fully integrated pharmaceutical company. Specially, we sought to acquire a trained and skilled workforce, with an established commercial organization giving us the opportunity to cross-sell future Avanir products, such as Zenvia, if approved. We believe this transaction places the Company in a position of market recognition as a provider of treatment for resistant schizophrenia. This could afford us the opportunity to acquire new customers in the future that we would not otherwise acquire. Further, this market position achieved through the transaction could also provide us with the ability to sell new products into the market as older
15
products are phased out and/or replaced. All of these factors have contributed to the recognition of goodwill.
Identifiable Intangible Assets
We determined fair values of identifiable intangible assets acquired based on estimates and assumptions by management on projected sales and product returns, pricing allowances and discounts. Identifiable intangible assets acquired represent expected benefits of the FazaClo product rights, customer relationships, trade name and non-compete agreement. The fair values of the customer relationships, technology, trade name and covenants not to compete were determined using an income approach and discounted cash flow (“DCF”) techniques. The fair value of the software registry and assembled workforce were determined using a cost approach. The remaining goodwill value of the Company was determined using a residual approach, by comparing the total fair market value of the assumed liabilities and equity consideration paid less the fair value of the tangible and identified intangible assets.
The identifiable intangible assets are being amortized, with the annual amortization amount based on the rate of consumption of the expected benefits of the intangible, if identifiable, or the straight-line method over the remaining estimated economic life ranging from one to 12 years if the rate of consumption of the expected benefits cannot be reasonably determined otherwise.
The following are the estimated amortization percentages by year for amortizable intangible assets:
| | | | | | | | | | | | | | | | | |
| | | Product | | Customer | | Trade | | Non-Compete |
| Year | | Rights | | Relationships | | Name | | Agreements |
| 1 | | | 14 | % | | | 15 | % | | | 8 | % | | | 100 | % |
| 2 | | | 15 | % | | | 22 | % | | | 9 | % | | | — | |
| 3 | | | 15 | % | | | 18 | % | | | 8 | % | | | — | |
| 4 | | | 14 | % | | | 13 | % | | | 9 | % | | | — | |
| 5 | | | 14 | % | | | 9 | % | | | 8 | % | | | — | |
| 6 | | | 14 | % | | | 8 | % | | | 9 | % | | | — | |
| 7 | | | 14 | % | | | 8 | % | | | 8 | % | | | — | |
| 8 | | | — | | | | 7 | % | | | 9 | % | | | — | |
| 9 | | | — | | | | — | | | | 8 | % | | | — | |
| 10 | | | — | | | | — | | | | 9 | % | | | — | |
| 11 | | | — | | | | — | | | | 8 | % | | | — | |
| 12 | | | — | | | | — | | | | 8 | % | | | — | |
In-Process Research and Development
We evaluated research and development projects including new manufacturing technology for FazaClo under development by CIMA Labs. As the basis for identifying whether or not the development projects represented in-process research and development (“IPR&D”), we conducted an evaluation in the context of FASB Interpretation 4 (“FIN 4: Applicability of FASB Statement No. 2 to Business Combinations Accounted for by the Purchase Method”). In accordance with these provisions, we examined the research and development projects to determine whether any alternative future uses existed. Such evaluation consisted of a specific review of the efforts, including the overall objectives of the project, progress
16
toward the objectives, and the uniqueness of the developments of these objectives as well as our intended use of the developments. Further, we reviewed each development project to determine whether technological feasibility had been achieved. Based on our analysis, we determined that the DuraSolv technology, a certain technology being developed in collaboration with CIMA Labs for manufacturing FazaClo, was IPR&D. We expect this technology will be commercialized in fiscal year 2007.
In order to estimate the fair value of the DuraSolv technology, we used the relief from royalty valuation approach on incremental product revenues that could result from manufacturing with such technology. The fair value of the IPR&D is determined by measuring the present value of the after-tax cash flows from revenues from such technology based on an appropriate technology royalty rate applied over 12 years commencing after FDA approval (if approved), discounted at a risk-adjusted rate of 29%. DuraSolv technology allows for the product to be packaged in a bottle, which is more convenient to open than the current blister packaging for FazaClo. We expect to use the DuraSolv manufacturing technology to replace the current OraSolv technology for manufacturing FazaClo, assuming the manufacturing process is approved by the FDA. We determined the future economic benefits from the purchased IPR&D to be uncertain because such technology has not been approved by the FDA. No material change in pricing or manufacturing cost is anticipated. As DuraSolv was determined to be IPR&D, the estimated fair value of DuraSolv of $1.3 million was expensed in fiscal 2006, under guidelines in FAS 141.
Pro Forma Results of Operations
The following unaudited financial information presents the pro forma results of operations and gives effect to the Alamo acquisition as if the acquisition was consummated at the beginning of fiscal 2005.
| | | | | |
| | | For the 3 months |
| | | ended December 31, |
| | | 2005 |
| Pro forma net revenues(1) | | $ | 9,369,049 | |
| Pro forma net loss(2) | | $ | (11,163,405 | ) |
| Pro forma loss per basic and diluted share | | $ | (0.39 | ) |
| Shares used for basic and diluted computation | | | 28,579,357 | |
| | |
| (1) | | In accordance with the provisions of EITF 01-3, we will not recognize deferred revenues recorded as of the acquisition date, resulting in lower net revenues in the periods following the merger than Alamo would likely have achieved as a separate company. |
| |
| (2) | | Pro forma net loss for the periods presented includes the amortization of identifiable intangible assets, interest expense associated with the notes payable issued as part of the purchase price, elimination of interest expense associated with Alamo’s historical debt that was not assumed by us in the acquisition, reduction of interest income by an amount determined by applying the average rate of return for the respective periods to the decrease in our cash balance of $4.0 million used to fund the acquisition, amortization of discount associated with the notes payable. The charge of $1.3 million for purchased IPR&D is not included in the pro forma results of operations because it reflects a one-time charge directly related to the acquisition and does not have a continuing impact on our future operations. |
17
3. RELOCATION OF COMMERCIAL AND GENERAL AND ADMINISTRATIVE OPERATIONS
In fiscal 2006, we relocated all operations other than research and development from San Diego, California to Aliso Viejo, California (the “Relocation”). In the first quarter of fiscal 2007, we recorded restructuring and other related expenses of $772,000, for employee severance and relocation benefits.
The following table presents the restructuring activities in the first quarter of fiscal 2007:
| | | | | | | | | | | | | | | | | |
| | | September 30, | | | | | | | | | | | December 31, | |
| | | 2006 | | | Additions | | | Payments | | | 2006 | |
Accrued Restructuring: | | | | | | | | | | | | | | | | |
| Employee severance and relocation benefits | | $ | 237,050 | | | $ | 771,852 | | | $ | (334,251 | ) | | $ | 674,651 | |
| Lease restructuring liability | | | 273,998 | | | | — | | | | (10,887 | ) | | | 263,111 | |
| | | | | | | | | | | | | |
| Total | | $ | 511,048 | | | $ | 771,852 | | | $ | (345,138 | ) | | $ | 937,762 | |
| | | | | | | | | | | | | |
4. INVENTORIES
Components of inventories were:
| | | | | | | | | |
| | | December 31, | | | September 30, | |
| | | 2006 | | | 2006 | |
| Finished goods | | $ | 1,381,355 | | | $ | 1,642,208 | |
| Raw materials | | | 1,994,180 | | | | 923,822 | |
| Work in progress | | | — | | | | 80,580 | |
| Inventories subject to return | | | 155,576 | | | | 536,017 | |
| | | | | | | |
| Total inventories | | $ | 3,531,111 | | | $ | 3,182,627 | |
| | | | | | | |
Inventories include costs associated with marketed products and certain products prior to regulatory approval based upon estimated probable future use. We could be required to expense these costs related to pre-approval or pre-launch inventory upon a change in such judgment, due to a denial or delay of approval by regulators, a delay in commercialization, or other potential factors. At December 31, 2006, we had $1.1 million of inventory costs related to the pre-approved drug Zenvia. As of September 30, 2006 we had $347,424 of Abreva classified long-term in other assets.
Inventories subject to return are the costs associated with FazaClo shipments not yet recognized as revenues.
5. NET DEFERRED REVENUES
The following table sets forth as of December 31, 2006 the net deferred revenue balances for our sale of future Abreva® royalty rights to Drug Royalty USA, FazaClo product shipments and other agreements.
18
| | | | | | | | | | | | | | | | | |
| | | Drug Royalty | | | FazaClo | | | | | | | |
| | | USA | | | Net | | | Other | | | | |
| | | Agreement | | | Shipments | | | Agreements | | | Total | |
| Net deferred revenues as of October 1, 2006 | | $ | 17,111,913 | | | $ | 3,955,150 | | | $ | 2,242,262 | | | $ | 23,309,325 | |
| Changes during the period: | | | | | | | | | | | | | | | | |
| Shipments, net | | | — | | | | 3,536,205 | | | | — | | | | 3,536,205 | |
| Recognized as revenues during period | | | (723,040 | ) | | | (6,270,704 | ) | | | (98,788 | ) | | | (7,092,532 | ) |
| | | | | | | | | | | | | |
| Net deferred revenues as of December 31, 2006 | | $ | 16,388,873 | | | $ | 1,220,651 | | | $ | 2,143,474 | | | $ | 19,752,998 | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Classified and reported as: | | | | | | | | | | | | | | | | |
| Current portion of deferred revenues | | $ | 1,870,891 | | | $ | 1,220,651 | | | $ | 1,614,091 | | | $ | 4,705,633 | |
| Deferred revenues, net of current portion | | | 14,517,982 | | | | — | | | | 529,383 | | | | 15,047,365 | |
| | | | | | | | | | | | | |
| Total deferred revenues | | $ | 16,388,873 | | | $ | 1,220,651 | | | $ | 2,143,474 | | | $ | 19,752,998 | |
| | | | | | | | | | | | | |
6. COMPUTATION OF NET LOSS PER COMMON SHARE
Basic net loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period, excluding restricted stock that has been issued but is not yet vested. Diluted net loss per common share is computed by dividing net loss by the weighted-average number of common shares outstanding during the period plus additional weighted average common equivalent shares outstanding during the period. Common equivalent shares result from the assumed exercise of outstanding stock options and warrants (the proceeds of which are then presumed to have been used to repurchase outstanding stock using the treasury stock method) and the vesting of restricted shares of common stock. In the loss periods, the common equivalent shares have been excluded from the computation of diluted net loss per share, because their effect would have been anti-dilutive. For the first quarter of fiscal 2007 a total of 1,155,537 stock options, 1,322,305 stock warrants, 211,082 restricted stock awards and 647,510 restricted stock units were excluded from the computation of diluted net loss per share. For the first quarter of fiscal 2006 a total of 1,752,845 stock options, 968,414 stock warrants, 223,915 restricted stock awards and 51,480 restricted stock units were excluded from the computation of diluted net loss per share.
7. COMPREHENSIVE LOSS
Comprehensive loss consists of the following:
| | | | | | | | | |
| | | For the 3 months
ended December 31, | |
| | | 2006 | | | 2005 | |
| Net loss | | $ | (13,617,527 | ) | | $ | (9,215,970 | ) |
| Other comprehensive loss, net of tax: | | | | | | | | |
| Unrealized (loss) gain on available-for-sale securities | | | 68,001 | | | | (59,337 | ) |
| | | | | | | |
| Total comprehensive loss | | $ | (13,549,526 | ) | | $ | (9,275,307 | ) |
| | | | | | | |
8. SHAREHOLDERS’ EQUITY
In November 2006, we sold and issued 5,263,158 shares of our Class A common stock for aggregate gross offering proceeds of $15.0 million ($14.4 million after expenses). In connection with this offering, we issued warrants to purchase a total of 1,053,000 shares of our Class A common stock at an exercise price of $3.30 per share. The warrants become exercisable beginning in May 2007 and all unexercised warrants expire in November 2007. In December 2006, we sold 243,060 shares (settled and issued 133,900 shares at December 31, 2006) of our Class A common stock under our financing facility with
19
Brinson Patrick Securities Corporation, raising aggregate gross offering proceeds of $589,037 ($562,512 after expenses). These offerings were made pursuant to our shelf registration statement on Form S-3 filed on July 22, 2005. Approximately $3.0 million of the net proceeds from these offerings were used to partially repay the outstanding principal balance of a note payable issued in the Alamo acquisition, with such repayment being made in accordance with the terms of the note.
In the three months ended December 31, 2006, we also issued to employees 15,000 shares of restricted stock at a weighted average exercise price of $7.35. We also awarded an additional 613,930 shares that were not issued at a weighted average exercise price of $2.79.
As of December 31, 2006 and 2005, warrants to purchase 1,322,305 and 968,414 shares of common stock, respectively, at a weighted-average price per share of $4.44 and $7.54, respectively, remained outstanding, of which all are exercisable.
9. EMPLOYEE EQUITY INCENTIVE PLANS
We currently have five equity incentive plans (the “Plans”): the 2005 Equity Incentive Plan (the “2005 Plan”), the 2003 Equity Incentive Plan (the “2003 Plan”), the 2000 Stock Option Plan (the “2000 Plan”), the 1998 Stock Option Plan (the “1998 Plan”) and the 1994 Stock Option Plan (the “1994 Plan”), which are described below. All of the Plans were approved by the shareholders, except for the 2003 Equity Incentive Plan, which was approved solely by the Board of Directors. Stock-based awards are subject to terms and conditions established by the Compensation Committee of our Board of Directors. Our policy is to issue new common shares upon the exercise of stock options, conversion of share units or purchase of restricted stock.
During the first quarters of fiscal 2007 and 2006, we granted share-based awards under both the 2003 Plan and the 2005 Plan. Under the 2003 Plan and 2005 Plan, options to purchase shares, restricted stock units, restricted stock and other share-based awards may be granted to our employees and consultants. Under the Plans, as of December 31, 2006, we had an aggregate of 3,265,008 shares of our common stock reserved for issuance. Of those shares, 1,155,537 were subject to outstanding options and other awards and 2,109,471 shares were available for future grants of share-based awards. We also issued share-based awards outside of the Plans. As of December 31, 2006, options to purchase 140,000 shares of our common stock that were issued outside of the Plans (inducement option grants) are outstanding. None of the share-based awards is classified as a liability as of December 31, 2006.
Stock Options. Stock options are granted with an exercise price equal to the current market price of our common stock at the grant date and have 10-year contractual terms. For option grants to employees, 25% of the option shares vest and become exercisable on the first anniversary of the grant date and the remaining 75% of the option shares vest and become exercisable quarterly in equal installments thereafter over three years; for option grants to non-employee directors, one-third of the option shares vest and become exercisable on the first anniversary of the grant date and the remaining two-thirds of the option shares vest and become exercisable daily or quarterly in equal installments thereafter over two years; and for certain option grants to non-employee directors, options fully vest and become exercisable at the date of grant. Certain option awards provide for accelerated vesting if there is a change in control (as defined in the Plans).
20
Summaries of stock options outstanding and changes during first quarter of fiscal 2007 are presented below.
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | Weighted | | |
| | | | | | | Weighted | | average | | |
| | | | | | | average | | remaining | | |
| | | | | | | exercise | | contractual | | Aggregate |
| | | | | | | price per | | term | | intrinsic |
| | | Number of shares | | share | | (in years) | | value |
| Outstanding, October 1, 2006 | | | 1,587,070 | | | $ | 10.07 | | | | 7.2 | | | $ | 817,000 | |
| | | | | | | | | | | | | | | | | |
| Granted | | | 198,420 | | | | 8.36 | | | | | | | | | |
| Exercised | | | (65,571 | ) | | | 4.38 | | | | | | | | | |
| Forfeited | | | (420,757 | ) | | | 9.98 | | | | | | | | | |
| Expired | | | (3,625 | ) | | | 6.38 | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Outstanding, December 31, 2006 | | | 1,295,537 | | | | 9.35 | | | | 7.7 | | | | 537,105 | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Vested and expected to vest in the future, December 31, 2006 | | | 1,234,324 | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Exercisable, December 31, 2006 | | | 537,105 | | | | | | | | | | | | | |
The weighted average grant-date fair values of options granted during the first quarters of fiscal 2007 and 2006 were $3.00 and $7.44 per share, respectively. The total intrinsic value of options exercised during the first quarters of fiscal 2007 and 2006 was $269,803 and $604,000, respectively, based on the differences in market prices on the dates of exercise and the option exercise prices.
The fair value of each option award is estimated on the date of grant using the Black-Scholes-Merton option pricing model (“Black-Scholes model”), which uses the assumptions noted in the following table. Expected volatilities are based on historical volatility of our common stock and other factors. The expected term of options granted is based on analyses of historical employee termination rates and option exercises. The risk-free interest rates are based on the U.S. Treasury yield for a period consistent with the expected term of the option in effect at the time of the grant.
Assumptions used in the Black-Scholes model for options granted during the first quarters of fiscal 2007 and 2006 were as follows:
| | | | | | | | | |
| | | 2007 | | 2006 |
| Expected volatility | | | 75 | % | | | 77.4% - 80.4 | % |
| Weighted-average volatility | | | 75 | % | | | 78.4 | % |
| Average expected term in years | | | 6.0 | | | | 4.5 | |
| Risk-fee interest rate (zero coupon U.S. Treasury Note) | | | 4.6 | % | | | 4.5 | % |
| Expected dividend yield | | | 0 | % | | | 0 | % |
The following table summarizes information concerning outstanding and exercisable Class A stock options as of December 31, 2006:
| | | | | | | | | | | | | | | | | | | | | |
| | | Options Outstanding | | Options Exercisable |
| | | | | | | Weighted | | | | | | | | |
| | | | | | | Average | | Weighted | | | | | | Weighted |
| | | | | | | Remaining | | Average | | | | | | Average |
| Range of | | Number | | Contractual | | Exercise | | Number | | Exercise |
| Exercise Prices | | Outstanding | | Life in Years | | Price | | Exercisable | | Price |
| $1.20 - $4.64 | | | 159,396 | | | | 4.8 | | | $ | 3.92 | | | | 136,896 | | | $ | 3.93 | |
| $5.12 - $6.92 | | | 236,661 | | | | 7.8 | | | $ | 6.26 | | | | 58,911 | | | $ | 6.02 | |
| $7.12 - $9.92 | | | 172,525 | | | | 7.7 | | | $ | 8.67 | | | | 81,704 | | | $ | 8.80 | |
| $10.10 - $10.70 | | | 146,250 | | | | 9.3 | | | $ | 10.68 | | | | 3,646 | | | $ | 10.24 | |
| $11.08 - $11.76 | | | 245,605 | | | | 8.4 | | | $ | 11.70 | | | | 95,519 | | | $ | 11.69 | |
| $12.12 - $13.84 | | | 183,650 | | | | 7.3 | | | $ | 13.25 | | | | 127,104 | | | $ | 13.26 | |
| $14.28 - $19.38 | | | 151,450 | | | | 8.2 | | | $ | 15.86 | | | | 33,325 | | | $ | 17.06 | |
| | | | | | | | | | | | | | | | | | | | | |
| | | | 1,295,537 | | | | 7.7 | | | $ | 9.94 | | | | 537,105 | | | $ | 9.35 | |
| | | | | | | | | | | | | | | | | | | | | |
21
Restricted stock units.RSUs generally vest based on three years of continuous service and may not be sold or transferred until the awardee’s termination of service. The following table summarizes the RSU activities for the first quarter of fiscal 2007:
| | | | | | | | | |
| | | | | | | Weighted |
| | | Number of | | average grant |
| | | shares | | date fair value |
| Unvested, October 1, 2006 | | | 51,480 | | | $ | 15.54 | |
| Granted | | | 613,930 | | | $ | 2.79 | |
| Vested | | | — | | | $ | — | |
| Forfeited | | | (17,900 | ) | | $ | — | |
| | | | | | | | | |
| Unvested, December 31, 2006 | | | 647,510 | | | $ | 3.78 | |
| | | | | | | | | |
The grant-date fair value of RSUs granted during the first quarter of fiscal 2007 was $1,713,689. No RSUs were granted during the first quarter of fiscal 2006. As of December 31, 2006, the total unrecognized compensation cost related to unvested shares was $2,513,689, which is expected to be recognized over a weighted-average period of 2.9 years, based on the vesting schedules.
Restricted stock awards. Restricted stock awards are grants that entitle the holder to acquire shares of restricted common stock at a fixed price, which is typically nominal. The shares of restricted stock cannot be sold, pledged or otherwise disposed of until the award vests, and any unvested shares may be reacquired by us for the original purchase price following the awardee’s termination of service. The restricted stock awards typically vest on the second or third anniversary of the grant date or on a graded vesting schedule over three years of employment. A summary of our unvested restricted stock awards as of December 31, 2006 and changes during the quarter then ended are presented below.
| | | | | | | | | |
| | | | | | | Weighted |
| | | Number of | | average grant |
| | | shares | | date fair value |
| Unvested, October 1, 2006 | | | 223,915 | | | $ | 13.48 | |
| Granted | | | 15,000 | | | $ | 7.35 | |
| Vested | | | (20,833 | ) | | $ | 2.90 | |
| Forfeited | | | ( 7,000 | ) | | $ | — | |
| | | | | | | | | |
| Unvested, December 31, 2006 | | | 211,082 | | | $ | 14.54 | |
| | | | | | | | | |
The grant-date fair value of restricted stock awards granted in the first quarters of fiscal 2007 and 2006 was $110,025 and $164,000, respectively. As of December 31, 2006, the total unrecognized compensation cost related to unvested shares was $2,513,689, which is expected to be recognized over a weighted-average period of 2.9 years.
During the first quarters of fiscal 2007 and 2006, we received a total of $289,023 and $191,000, respectively, in cash from exercised options and restricted stock awards under all share-based payment
22
arrangements. No tax benefit was realized for the tax deductions from option exercise of the share-base payment arrangements in the first quarters of fiscal 2007 and 2006.
10. COMMITMENTS AND CONTINGENCIES
In the ordinary course of business, we may face various claims brought by third parties and we may, from time to time, make claims or take legal actions to assert or protect our rights. Any of these claims could subject us to costly litigation and, while we generally believe that we have adequate insurance to cover many different types of liabilities, our insurance carriers may deny coverage or our policy limits may be inadequate to fully satisfy any damage awards or settlements. If this were to happen, the payment of any such awards could have a material adverse effect on our operations, cash flows and financial position. Additionally, any such claims, whether or not successful, could damage our reputation and business. Management believes the outcome of currently pending claims and lawsuits will not likely have a material effect on our operations or financial position.
11. SEGMENT INFORMATION
We operate our business on the basis of a single reportable segment, which is the business of discovery, development and commercialization of novel therapeutics for chronic diseases. Our chief operating decision-maker is the Chief Executive Officer, who evaluates our Company as a single operating segment.
We categorize revenues by geographic area based on selling location. All our operations are currently located in the U.S.; therefore, total revenues for the first fiscal quarter of 2007, fiscal 2006, 2005 and 2004 are attributed to the U.S. All long-lived assets at the first fiscal quarter of 2007, September 30, 2006 and 2005 are located in the U.S. For purposes of the evaluation for impairment of goodwill, the Company’s operating segment represents its single reporting unit.
For the first quarter of fiscal 2007, 9% and 9% of our total net revenues were derived from our license agreement with AstraZeneca and the sale of rights to royalties under the GlaxoSmithKline license agreement, respectively. For the first quarter of fiscal 2006, 24% and 7% of our total net revenues were derived from our license agreement with AstraZeneca and the sale of rights to royalties under the GlaxoSmithKline license agreement, respectively. Net receivables from AstraZeneca and Novartis accounted for approximately 11% and 3%, respectively, of our net receivables at December 31, 2006 and 26% and 3%, respectively, of our net receivables at September 30, 2006.
The wholesale value of FazaClo shipments, net of returns, to McKesson Corporation, AmeriSourceBergen Corporation and Cardinal Health were 41%, 21% and 22%, respectively, of our total net revenues of $6.3 million in the first quarter of fiscal 2007. Net receivables from McKesson Corporation, AmeriSourceBergen Corporation and Cardinal Health accounted for 19%, 13% and 25%, respectively, of our total net receivables at December 31, 2006 and 27%, 11% and 14%, respectively, of our total net receivables at September 30, 2006.
12. SUBSEQUENT EVENTS
Subsequent to December 31, 2006, the Company sold and issued a total of 2,329,790 shares of our Class A common stock for aggregate gross offering proceeds of $5.6 million ($5.4 million after offering expenses, including underwriting discounts and commissions). Approximately $1.1 million of these net offering proceeds were used to partially repay the outstanding principle balance of a note issued in connection with the Alamo transaction. The repayment of such amount is required under the terms of the note.
23
Item 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This Quarterly Report on Form 10-Q contains forward-looking statements concerning future events and performance of the Company. When used in this report, the words “intend,” “estimate,” “anticipate,” “believe,” “plan” or “expect” and similar expressions are included to identify forward-looking statements. These forward-looking statements are based on our current expectations and assumptions and many factors could cause our actual results to differ materially from those indicated in these forward-looking statements. You should review carefully the factors identified in this report under the caption, “Risk Factors” and in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission (“SEC”). We disclaim any intent to update or announce revisions to any forward-looking statements to reflect actual events or developments. Except as otherwise indicated herein, all dates referred to in this report represent periods or dates fixed with reference to the calendar year, rather than our fiscal year ending September 30. The three-month period ended December 31, 2006, may also be referred to as the first quarter of fiscal 2007.
EXECUTIVE OVERVIEW
Avanir Pharmaceuticals is a pharmaceutical company focused on developing, acquiring and commercializing novel therapeutic products for the treatment of chronic diseases. Our product candidates address therapeutic markets that include the central nervous system, cardiovascular disorders, inflammatory and infectious diseases.
We currently market FazaClo®, acquired with the purchase of Alamo Pharmaceuticals, LLC (“Alamo”) in May 2006, the only orally disintegrating formulation of clozapine for the management of treatment resistant schizophrenia and reduction in the risk of recurrent suicidal behavior in schizophrenia or schizoaffective disorders. On January 25, 2007, we received an “approval letter” from the FDA for our new formulation and dosing strength of FazaClo (DuraSolv). An approvable letter is an official notification from the FDA that certain additional conditions must be satisfied prior to obtaining U.S. marketing approval for a new drug. The Company has responded to the two CMC issues that were discussed in the approval letter and anticipate approval in the coming months.
Our lead product candidate, Zenvia™ (formerly referred to as Neurodex™) for the treatment of involuntary emotional expression disorder (“IEED”), also known as pseudobulbar affect (“PBA”) or emotional liability, has completed two Phase III clinical trials. On October 30, 2006, we received an approvable letter from the FDA for our NDA submission for Zenvia for the treatment of IEED/PBA. The approvable letter that we received from the FDA outlined concerns that the agency has regarding the efficacy and safety data contained in our NDA submission, which may require additional clinical trials and data in order to obtain marketing approval. Details of the contents of the approvable letter are contained in our Annual Report on Form 10-K for the year ended September 30, 2006.
Because the approvable letter for Zenvia did not specify the exact data and what additional clinical trials, if any, may be required for approval, we have scheduled a meeting with the FDA in late February of 2007 to clarify what would be needed for marketing approval. Until we meet with the FDA, we will not know how extensive any required additional data and/or trials are likely to be. However, we believe that it is likely that the FDA’s requirements for additional data may be substantial and that we may be required to undertake additional trials that would be costly and time consuming. Accordingly, we cannot be certain that, once we have met with the FDA, we will continue the development of Zenvia as previously planned.
We are also currently developing Zenvia for the treatment of chronic diabetic neuropathic pain and we are evaluating Zenvia for use for other clinical indications. We are currently engaged in a Phase III clinical
24
trial of Zenvia in patients with painful diabetic neuropathy under a special protocol assessment (“SPA”). An SPA is an agreement between the FDA and the sponsor of a clinical trial documenting that if the study endpoints are met, the results should be acceptable to support a New Drug Application (NDA). Our future development plans for Zenvia for this indication may be affected by our upcoming meeting with the FDA.
Our research and drug discovery programs have historically been focused primarily on small molecules that can be taken orally as therapeutic treatments. We have one Phase I development program, which is for the treatment of atherosclerosis and is partnered with AstraZeneca UK Limited (“AstraZeneca”). In November 2006, we announced that we have placed on hold the development of our other Phase I program, AVP-13358, which was being developed as a potential treatment for lupus. We are currently evaluating strategic options as it relates to AVP-13358. Our pre-clinical research program targeting macrophage migration inhibitory factor (“MIF”) in the treatment of inflammatory diseases is partnered with Novartis International Pharmaceutical Ltd. (“Novartis”). We also have developed an anthrax antibody using our proprietary Xenerex™ technology, which is currently being funded by a $2.0 million grant from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (“NIH”). In the future we may choose to pursue further grants or partner this technology.
Our first commercialized product, docosanol 10% cream, (sold as Abreva® by our marketing partner GlaxoSmithKline Consumer Healthcare in North America) is the only over-the-counter treatment for cold sores that has been approved by the FDA.
Avanir Marketed Products and Product Pipeline
The following chart illustrates the status of research and development activities for our products, product candidates and licensed technologies.
| | |
| (a) | | Timelines in this pictorial representation are not to scale. |
| |
| (b) | | Granted to GlaxoSmithKline exclusive rights to market docosanol 10% cream in North America. |
| |
| (c) | | Licensed to AstraZeneca, which assumed all ongoing product development expenses. |
| |
| (d) | | Licensed to Novartis, which assumed all ongoing product development expenses. |
| |
| (e) | | Effective November 9, 2006, we place on hold activities associated with the selective cytokine. |
| |
| (f) | | Pending outcome of planned meeting with the FDA in February 2007. |
25
We have historically sought to maintain flexibility in our cost structure by actively managing several outsourced functions, such as clinical trials, legal counsel, documentation and testing of internal controls, pre-clinical development work, and manufacturing, warehousing and distribution services, rather than maintaining all of these functions in house. We believe the benefits of outsourcing, including being flexible and being able to rapidly respond to program delays or successes far outweigh the higher costs often associated with outsourcing at this stage of our development. Assuming successful growth of FazaClo sales and if Zenvia is successfully developed or other products are acquired, we expect more of these functions may be brought in-house.
We may continue to seek partnerships with pharmaceutical companies to help fund research and development programs in exchange for sharing in the rights to commercialize new drugs. Additionally, we may acquire other drugs to leverage the current infrastructure and sales organization being used for the marketing and sales of FazaClo. We are unable to determine if and when we might be able to reach profitability until we know the outcome of future discussions with the FDA regarding Zenvia. Trends in revenues and various types of expenses are discussed further in the “Results of Operations.”
We will need to raise additional capital to fund our operations, and we will need to raise a significant amount of additional capital if we continue to develop Zenvia for IEED/PBA and for painful diabetic neuropathy. We expect to attempt to raise additional capital through the continued sales of common stock under our financing facility with Brinson Patrick Securities Corporation (“Brinson Patrick”) and may seek to raise additional capital from time to time through various financing alternatives, including licensing or sales of our technologies and drug candidates, selling shares of common or preferred stock in other transactions, or through the issuance of one or more forms of senior or subordinated debt. Our future capital needs will also depend substantially on our ability to reach predetermined milestones under our existing collaboration agreements, as well as the economic terms and the timing of any new partnerships or collaborative arrangements with pharmaceutical companies under which we would expect our partners to fund the costs of such activities. If we are unable to raise capital as needed to fund our operations, or if we are unable to reach these milestones or enter into any such collaborative arrangements, then we may need to slow the rate of development of some of our programs or sell the rights to one or more of our drug candidates. For additional information about the risks and uncertainties that may affect our business and prospects, please see “Risk Factors.”
Our principal executive offices are located at 101 Enterprise, Suite 300, Aliso Viejo, California 92656. Our telephone number is (949) 389-6700 and our e-mail address isinfo@avanir.com. Our Internet website address iswww.avanir.com.We make our periodic and current reports available on our Internet website, free of charge, as soon as reasonably practicable after such material is electronically filed with, or furnished to, the Securities and Exchange Commission (“SEC”). No portion of our website is incorporated by reference into this Report on Form 10-Q.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Management’s Discussion and Analysis of our financial condition and results of operations is based upon our consolidated financial statements prepared in accordance with generally accepted accounting principles in the United States. The preparation of these financial statements requires us to make a number of assumptions and estimates that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amounts of revenues and expenses during the reported periods. These items are monitored and analyzed by management for changes in facts and circumstances, and material changes in these estimates could occur in the future. Changes in estimates are recorded in the period in which they become known. We base our estimates on historical experience and various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ from our estimates if past experience or other assumptions do not turn out to be substantially accurate.
26
A summary of significant accounting policies and a description of accounting policies that are considered critical may be found in Part II, Item 7 of our Annual Report on Form 10-K for the year ended September 30, 2006 in the “Critical Accounting Policies and Estimates” section and in Note 1 of the Notes to our condensed consolidated financial statements included herein.
We recognize revenue in accordance with the SEC’s Staff Accounting Bulletin Topic 13 (“Topic 13”),“Revenue Recognition.”Revenue is recognized when all of the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the seller’s price to the buyer is fixed and determinable; and (4) collectibility is reasonably assured. Certain product sales are subject to rights of return. In accordance with Statement of Financial Accounting Standards No. 48,“Revenue Recognition When Right of Return Exists”(“FAS 48”), we recognize such product revenues at the time of sale only if we have met all the criteria of FAS 48, including the ability to reasonably estimate future returns. FAS 48 states that revenue from sales transactions where the buyer has the right to return the product shall be recognized at the time of sale only if (1) the seller’s price to the buyer is substantially fixed or determinable at the date of sale, (2) the buyer has paid the seller, or the buyer is obligated to pay the seller and the obligation is not contingent on resale of the product, (3) the buyer’s obligation to the seller would not be changed in the event of theft or physical destruction or damage of the product, (4) the buyer acquiring the product for resale has economic substance apart from that provided by the seller, (5) the seller does not have significant obligations for future performance to directly bring about resale of the product by the buyer, and (6) the amount of future returns can be reasonably estimated. We recognize such product revenues when either we have met all the criteria of FAS 48, including that ability to reasonably estimate future returns, when we can reasonably estimate that the return privilege has substantially expired, or when the return privilege has substantially expired, whichever occurs first.
We allocate amounts to separate elements in multiple element arrangements in accordance with Emerging Issues Task Force No. 00-21 (“EITF 00-21”),“Accounting for Revenue Arrangements with Multiple Deliverables.”Revenues are allocated to a delivered product or service when all of the following criteria are met: (1) the delivered item has value to the customer on a standalone basis; (2) there is objective and reliable evidence of the fair value of the undelivered item; and (3) if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered item is considered probable and substantially in our control. We use the relative fair values of the separate deliverables to allocate revenue. For arrangements with multiple elements that are separated, we recognize revenues in accordance with the SEC’s Staff Accounting Bulletin Topic 13 (“Topic 13”),“Revenue Recognition.”Revenue is recognized when all of the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the seller’s price to the buyer is fixed and determinable; and (4) collectibility is reasonably assured. Certain sales transactions include multiple deliverables.
Product Sales
| | | Active Pharmaceutical Ingredient Docosanol (“API Docosanol”).Revenues from the sale of our API Docosanol are recognized when title and risk of loss have passed to the buyer and provided the criteria in SAB Topic 13 are met. We sell the API Docosanol to various licensees upon receipt of a written order for the materials. Shipments generally occur fewer than five times a year. Our contracts for sales of the API Docosanol include buyer acceptance provisions that give our buyers the right of replacement if the delivered product does not meet specified criteria. That right requires that they give us notice within 30 days after receipt of the product. We have the option to refund or replace any such defective materials; however, we have historically demonstrated that the materials shipped from the same pre-inspected lot have consistently met the specified criteria and no buyer has rejected any of our shipments from the same pre-inspected lot to date. Therefore, we recognize revenue at the time of delivery without providing any returns reserve. |
| |
| | | FazaClo.We acquired Alamo Pharmaceuticals LLC (“Alamo”) on May 24, 2006, with one marketed product, FazaClo (clozapine, USP), that began shipping to wholesale customers in July 2004 in 48-pill units. At that time, FazaClo had a two-year shelf life. In June 2005, Alamo received FDA approval to extend the product expiration date to three years. In October 2005, Alamo began shipping 96-pill units and accepted returns of unsold or undispensed 48-pill units. |
| |
| | | We sell FazaClo to pharmaceutical wholesalers, the three largest of which account for approximately 84% of our net wholesale shipments for the quarter ended December 31, 2006. They resell our product to outlets such as pharmacies, hospitals and other dispensing organizations. We have agreements with our wholesale customers, various states, hospitals, certain other medical institutions and third-party payers throughout the U.S. These agreements frequently contain commercial incentives, which may include pricing allowances and discounts payable at the time the product is sold to the dispensing outlet or upon dispensing the product to patients. Consistent with pharmaceutical industry practice, wholesale customers can return purchased product during an 18-month period that begins six months prior to the product’s expiration date and ends 12 months after the expiration date. Additionally, several of our dispensing outlets have the right to return expired product at any time. Once products have been dispensed to patients the right of return expires. |
27
| | | Prior to the quarter ended December 31, 2006, we did not have sufficient historical information and analytical support to reasonably estimate future product returns, nor to reasonably estimate the extent to which the right of return had expired on product we shipped. Accordingly, prior to the first quarter of fiscal 2007, we were unable to recognize revenues for product sales subject to return under FAS 48 and deferred the recognition of revenues on all shipments of FazaClo. We would have recognized revenues when we obtained reliable evidence that helped us reasonably determine that the right of return no longer exists, such as when the products have been dispensed to patients or the return period had otherwise expired. We previously disclosed in the notes to our fiscal 2006 financial statements included in our Annual Report on Form 10-K, that for purposes of applying this sell-through method, we would determine when products that we shipped are dispensed to patients based only on those instances when rebate requests are submitted to us by various state agencies and others. Based on the information we had available to us at the time, we had no other means to determine that product had been dispensed until we received the rebate requests, however, rebate requests are only expected to be received for a portion of the products dispensed to patients since not all products dispensed are subject to rebates. Rebates are received in approximately 90-120 days from when the products are dispensed. As of September 30, 2006, we had not received any rebate requests that we believed related to product that we shipped after our acquisition of Alamo. Further, as previously disclosed in the notes to our fiscal 2006 financial statements included in our Annual Report on Form 10-K, in conjunction with our evaluation of our assumed liability for returns, we had been working with our key wholesalers to obtain sufficient information about the amount and type of FazaClo product in the distribution channel. |
| |
| | | During the second quarter of fiscal 2007, we obtained and substantially completed our analysis of third-party information regarding certain wholesaler inventory levels, a sample of outlet inventory levels and third-party market research data. The third-party data includes, (i) IMS Health Audit — National Sales Perspective reports (“NSP”), which is a projection of near-census data of wholesaler shipments of product to all outlet types, including retail and non-retail and; (ii) IMS Health National Prescription Audit (“NPA”) Syndicated data which captures end user consumption from retail dispensed prescriptions based upon projected data from pharmacies estimated to represent approximately 60% to 70% of the U.S. prescription universe . Finally, we completed our analysis of historical rebates and chargebacks earned by State Medicaid, Medicare Part D and managed care customers for a trailing twelve months. Based upon this additional information and analysis obtained in this quarter, we now believe we can reasonably estimate the amount of product that we have shipped that is no longer in the wholesale or outlet channels as of December 31, 2006, and hence no longer subject to a right of return. Accordingly, since January 2007, we have received sufficient additional information that allows us to reasonably estimate the amount of product no longer subject to the right of return as of December 31, 2006. Therefore, we began recognizing revenues, net of returns, chargebacks, rebates, and discounts, in the first quarter of fiscal 2007, for product that we estimate have been sold to patients and that is no longer subject to a right of return. |
| |
| | | As a result, we have recognized revenues on FazaClo product sales of $6.3 million in the quarter ended December 31, 2006, which includes the recognition of deferred revenues of FazaClo that were deferred as of September 30, 2006. Had we continued to recognize revenue only upon receipt of rebate requests as we did in the prior quarter, management estimates that minimal revenue would have been recorded in the quarter ended December 31, 2006. We received approximately $1.2 million of rebate requests in the first quarter of 2007 that pertain to product that was shipped prior to our acquisition of Alamo. |
| |
| | | Additionally, we continue to accumulate historical product return data. To date we have accumulated return data for four lots which are within the 18-month return window, which have varying historical return rates. We are continuing to accumulate historical product return information, but at this time believe that we have insufficient information to reasonably estimate future product returns for revenue recognition purposes. Accordingly, we continue to defer recognizing revenues on all estimated in-channel inventories that are subject to the right of return until such time as we can reasonably estimate product returns. |
| |
| | | Revenues are recorded net of provisions for estimated product pricing allowances including: State Medicaid base and supplemental rebates, Medicare Part D discounts, managed care contract discounts and prompt payment discounts at an aggregate rate of approximately 29.2% of gross revenues as of December 31, 2006. Provisions for these allowances are estimated based upon contractual terms and require management to make subjective judgments on customer mix to reach this judgment. We considered our current contractual rates with States related to Medicaid base and supplemental rebates, with private organizations for Medicare Part D discounts and contracts with managed care organizations over a trailing twelve months. We review these rates at least quarterly and make adjustments, if necessary. |
28
RESULTS OF OPERATIONS
COMPARISON OF THREE MONTHS ENDED DECEMBER 31, 2006 AND 2005
| | | | | | | | | | | | | | | | | |
| | | Three Months ended | | | | | | | |
| | | December 31 | | | | | | | |
| | | 2006 | | | 2005 | | | $ Change | | | % Change | |
PRODUCT SALES | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Net revenues | | $ | 6,270,704 | | | $ | — | | | $ | 6,270,704 | | | | 100 | % |
| Cost of revenues | | | (1,347,184 | ) | | | — | | | | (1,347,184 | ) | | | 100 | % |
| | | | | | | | | | | | | | |
Gross margin | | $ | 4,923,520 | | | $ | — | | | $ | 4,923,520 | | | | 100 | % |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
LICENSES, RESEARCH SERVICES AND GRANTS | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| Revenues: | | | | | | | | | | | | | | | | |
| License agreements | | $ | 57,265 | | | $ | 5,000,000 | | | $ | (4,942,735 | ) | | | -98.9 | % |
| Research services | | | 1,270,244 | | | | 2,478,018 | | | | (1,207,774 | ) | | | -48.7 | % |
| Government research grant | | | 86,348 | | | | 84,825 | | | | 1,523 | | | | 1.8 | % |
| Royalty and sale of royalty rights | | | 723,040 | | | | 582,045 | | | | 140,995 | | | | 24.2 | % |
| | | | | | | | | | | | | | |
| Total Revenues | | | 2,136,897 | | | | 8,144,888 | | | | (6,007,991 | ) | | | -73.8 | % |
| | | | | | | | | | | | | | | | | |
| Costs: | | | | | | | | | | | | | | | | |
| Research services | | | 1,164,075 | | | | 2,018,799 | | | | (854,724 | ) | | | -42.3 | % |
| Government research grant | | | 95,727 | | | | 71,123 | | | | 24,604 | | | | 34.6 | % |
| | | | | | | | | | | | | | |
| Total Costs | | | 1,259,802 | | | | 2,089,922 | | | | (830,120 | ) | | | -39.7 | % |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Gross margin | | $ | 877,095 | | | $ | 6,054,966 | | | $ | (5,177,871 | ) | | | -85.5 | % |
| | | | | | | | | | | | | | |
Revenues
Product revenues for the first quarter of fiscal year 2007, include sales of FazaClo (acquired with the acquisition of Alamo in May 2006) of $6.3 million, including revenue that was deferred as of September 30, 2006. Product revenues are recorded net of product pricing allowances including: State Medicaid base and supplemental rebates, Medicare Part D discounts, managed care contract discounts and prompt payment discounts aggregating approximately 29.2% of gross revenues.
Revenues from licenses, research services and grants declined to $2.1 million compared to $8.1 million for the first quarter of fiscal 2006. This decline was principally due to a $5.0 million milestone earned under the AztraZeneca license agreement in the first quarter of fiscal year 2006 which was not repeated in the first quarter of fiscal year 2007 and a decrease in revenues of $1.2 million from research services earned from our collaborative agreements with AstraZeneca and Novartis as AstraZeneca has elected to rely less upon our scientists and to run more of the projects themselves.
Potential revenue-generating contracts that remained active as of December 31, 2006 include license agreements with AstraZeneca and Novartis, several docosanol 10% cream license agreements and one Zenvia sublicense. AstraZeneca and Novartis are currently engaged in preclinical and/or clinical development efforts of our licensed RCT and MIF programs. To the extent that these development efforts
29
produce negative or inconclusive results, our partners may terminate the development services we are providing, which would negatively affect revenues in future periods and would limit the potential financial returns from these licensing arrangements. Partnering, licensing and research collaborations have been, and will continue to be, an important part of our business development strategy. We may continue to seek partnerships with pharmaceutical companies that can help fund our remaining research programs in exchange for sharing in the rights to commercialize new drugs resulting from this research.
Cost of revenues
Cost of product revenues was $1.3 million or 21.5% of net product revenues for the three months ended December 31, 2006. Costs include the direct and indirect costs to manufacture of $776,000 or 12.4% of net product revenues, royalties to CIMA of $314,000 or 5% of net product revenues and amortization of acquired FazaClo product rights of $257,000 or 4.1% of net product revenue.
Cost of licenses, research services and grants declined to $1.3 million or 59.0% of revenues compared with $2.1 million or 25.7% of revenues for the first quarter of fiscal year 2006. The cost of licenses, research services and grants includes primarily direct and indirect payroll costs and the costs of outside vendors.
| | | | | | | | | | | | | | | | | |
| | | Three Months ended | | | | | | | |
| | | December 31 | | | | | | | |
| | | 2006 | | | 2005 | | | $ Change | | | % Change | |
OPERATING EXPENSES | | | | | | | | | | | | | | | | |
| Research and development | | $ | 5,906,001 | | | $ | 7,198,958 | | | $ | 1,292,957 | | | | 18.0 | % |
| Selling, general and administrative | | | 13,245,934 | | | | 4,768,743 | | | | (8,477,191 | ) | | | -177.8 | % |
| | | | | | | | | | | | | | |
Total Operating Expenses | | $ | 19,151,935 | | | $ | 11,967,701 | | | $ | (7,184,234 | ) | | | -60.0 | % |
| | | | | | | | | | | | | | |
Selling, general and administrative expenses
Selling, general and administrative expenses increased $8.5 million, or 178% for the first quarter of fiscal 2007, compared to the first quarter of fiscal year 2006. This increase is due primarily to a $6.4 million increase in expenses related to the expansion of our pre-launch activities and market research for Zenvia for the treatment of IEED/PBA, as well as the hiring of additional sales and marketing personnel; $722,000 in share-based compensation expense; a $1.0 million increase in expenses related to increases in headcount and compensation levels in general and administrative areas partially as a result of the Alamo acquisition in May 2006, and partially due to a modified Board of Directors compensation plan that became effective February 2006; and a $335,000 increase in legal fees.
Research and Development Expenses
R&D expenses decreased $1.3 million or 18% for the first quarter of fiscal 2007 compared to the first quarter of fiscal year 2006. The decrease in R&D expenses is due to reduced spending compared to the prior year period for the Zenvia open label safety study for the treatment of IEED/PBA, and a decrease in spending compared to the prior period for Phase III clinical trial for the treatment of neuropathic pain.
30
Until we have our planned meeting with the FDA in late February, we do not know what additional clinical spending, if any, will be needed for the approval of Zenvia for the treatment of IEED/PBA or in connection with the completion of the Zenvia Phase III clinical trials for the treatment of neuropathic pain. Depending on the outcome of this meeting, we may also need to undertake significant additional clinical trial work, and incur related expenses, for these programs. All future R&D spending on MIF and reverse cholesterol transport programs is expected to be fully reimbursed by our collaborative partners. We expect that spending on our monoclonal antibodies will depend in part on the progress that we make in these programs and on our strategy for partnering these programs or in obtaining additional government grants, so that we are able to defray part or all of these ongoing development costs.
Company-funded R&D Programs
Zenvia — Involuntary Emotional Expression Disorder (IEED)/Pseudobulbar Affect (PBA)
IEED/PBA is a complex neurological syndrome that is characterized by a lack of control of emotional expression, typically episodes of involuntary or exaggerated motor expression of emotion such as laughing and/or crying or weeping when the patient does not feel those emotions or in an exaggerated amount. IEED/PBA afflicts patients with neurological disorders such as amyotrophic lateral sclerosis (“ALS”), Alzheimer’s disease (“AD”), multiple sclerosis (“MS”), stroke, traumatic brain injury and Parkinson’s disease. While the exact number is unknown, based on our review of medical literature, independent surveys and our latest market research, we believe that there are likely over one million patients in the U.S. suffering from the symptoms of IEED/PBA. In addition, we believe that the availability of an FDA-approved treatment option for these patients may lead to the diagnosis of additional patients. If the FDA approves Zenvia, it would be the first drug approved for the treatment of IEED/PBA. Zenvia is a patented, orally administered combination of two well-characterized compounds, the active ingredient dextromethorphan and the enzyme inhibitor quinidine, which serves to increase the bioavailability of dextromethorphan in the human body.
As discussed above, we received an “approvable letter” from the FDA in October 2006 for our NDA submission for Zenvia for the treatment of IEED/PBA. Because the approvable letter did not specify the exact data and what additional clinical trials may be required, if any, for approval of Zenvia, we will not know how extensive any required additional data and/or trials are likely to be until our planned meeting with the FDA. However, we believe that it is likely that the FDA’s requirements for additional data may be substantial and that we may be required to undertake additional trials that would be costly and time consuming. Accordingly, we cannot be certain that, once we have met with the FDA, we will continue the development of Zenvia as previously planned.
We have been engaged in an open-label safety study for the treatment of IEED/PBA in a broad pool of patients who experience the symptoms of IEED/PBA associated with their underlying neurodegenerative disease or condition. We have ceased enrolling new patients in this study and, depending on the outcome of our meeting with the FDA, we may or may not continue with the open-label safety study.
Zenvia — Painful Diabetic Neuropathy Indication
Painful diabetic neuropathy, which arises from nerve injury, can result in a chronic and debilitating form of pain that has historically been poorly diagnosed and treated. Conditions that can cause neuropathic pain include trauma (e.g. car accidents), cancer, viral infection (e.g. herpes zoster) and metabolic disease (e.g. diabetic neuropathy). According to the American Diabetes Association at least half of the 15.7 million Americans who have diabetes are estimated to suffer from nerve damage caused by the disease. The damaged nerves can alter the sensitivity of pain centers in the spinal cord and consequently intensify pain transmission within the central nervous system. Painful diabetic neuropathy currently is most commonly
31
treated with tricyclic antidepressants, anticonvulsants, opioid analgesics and local anesthetics. Most of these treatments have limited effectiveness or undesirable side effects. It is estimated that the potential annual market size for drugs that treat painful diabetic neuropathy is at least $1 billion.
As of November 2006, we have enrolled the necessary number of patients needed to assess the efficacy endpoint in our ongoing Zenvia Phase III painful diabetic neuropathy trial. The protocol for the Phase III study was reviewed by the FDA through the SPA process. Assuming positive outcomes, we currently expect to use the data from this study as one of the pivotal Phase III clinical trials required before we would be able to submit an NDA for this indication. The data from the trial is currently anticipated in mid-2007.
AVP-13358 — Selective Cytokine Inhibitor
In November 2006, in an effort to reduce operating expenses we decided to place on hold activities associated with the selective cytokine inhibitor clinical development program. AVP-13358 is a novel, orally active drug molecule discovered by our scientists that appears to have anti-inflammatory and other pharmacological properties that could be useful against certain disease targets. Experiments in animals have shown that it inhibits or prevents the production of one such target, immunoglobulin epsilon (“IgE”), a pro-inflammatory mediator, and certain cytokines associated with chronic inflammatory diseases. For example, the compound suppresses markers of disease in mouse models of asthma and Systemic Lupus Erythematosus (SLE), which could indicate that the compound has the potential to be effective in those diseases.
We completed a multi-rising dose Phase Ib safety trial of AVP-13358 in November 2005, which was being developed as a potential treatment for Asthma/Lupus, and we announced that we had ended the development of our other Phase I program. We have evaluated several therapeutic indications for this program and may elect to continue with its development in the future. We are currently evaluating strategic options for this program.
Partner-funded R&D Programs
Reverse Cholesterol Transport Technology — Atherosclerosis
In July 2005, we entered into an exclusive license and research collaboration agreement with AstraZeneca regarding the license of certain compounds we discovered for the potential treatment of cardiovascular disease. Under the terms of the agreement, AstraZeneca is responsible for the development of the licensed compounds and, if a licensed compound is successfully developed by AstraZeneca and approved for marketing by the FDA, we will then be eligible to receive royalty payments. We are also eligible to receive up to $330 million in milestone payments contingent upon AstraZeneca’s performance and achievement of certain development and regulatory milestones, which could take several years of further development, including achievement of certain sales targets, if a licensed compound is approved for marketing by the FDA. Pursuant to the agreement with AstraZeneca, we also currently perform certain research support activities directed and funded by AstraZeneca.
Macrophage Migration Inhibitory Factor (“MIF”) — Inflammation
In April 2005, we entered into an exclusive license and research collaboration agreement with Novartis regarding the license of certain compounds that regulate macrophage migration inhibitory factor (“MIF”) in the treatment of various inflammatory diseases. Under the terms of the agreement, Novartis is responsible for the development of the licensed compounds and, if a licensed compound is successfully developed by Novartis and approved for marketing by the FDA, we will then be eligible to receive royalty payments. We are also eligible to receive up to $198 million in milestone payments contingent upon Novartis’ performance and achievement of certain development and regulatory milestones, including
32
approval for certain additional indications, and regulatory milestones, which could take several years of further development by Novartis, including achievement of certain sales targets, if a licensed compound is approved for marketing by the FDA. Pursuant to the agreement with Novartis, we also currently perform certain support research activities directed and funded by Novartis.
Government-funded R&D programs
Government research grants have, in prior periods, helped us fund research programs, including the development of antibodies to anthrax toxins and docosanol-based formulations for the treatment of genital herpes.Subject to certain conditions, we, as the awardee organization, retain the principal worldwide patent rights to any invention developed with U.S. government support.
Our patented Xenerex antibody technology can be used to develop human monoclonal antibodies for use as prophylactic and therapeutic drugs to prevent and treat anthrax and other infectious diseases. The proprietary technology provides a platform for accessing human monoclonal antibodies against disease antigens. The Xenerex technology is capable of generating fully human antibodies to target antigens and draws on the natural diversity of the human donor population. Using Xenerex technology, we have discovered a human monoclonal antibody, AVP-21D9, that provides immediate post-exposure neutralization and immediate immunity to animals exposed to a lethal dose of recombinant anthrax toxins.
Our anthrax antibody is in preclinical development and is currently being funded by a grant from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (“NIH”). In June 2006, we were notified that we had been awarded a $2.0 million research grant from the NIH for ongoing research and development related to our anthrax antibody. Under the terms of the grant, the NIH will reimburse us for up to $2.0 million in certain expenses related to the establishment of a cGMP manufacturing process and the testing of efficacy of the anthrax antibody. Subject to certain conditions, we, as the awardee organization, retain the principal worldwide patent rights to any invention developed with U.S. government support. Our progress on this program will substantially depend on future grants, as well as our business priorities. Currently, we expect that we will continue with the development of this program only to the extent that its development is funded by research grants. Currently we are actively seeking additional grants and partnering opportunities. Because all of our monoclonal antibody research is at a very early preclinical stage of development and is unpredictable in terms of the outcome, we are unable to predict the cost and timing for development of this antibody.
Share-Based Compensation
Effective at the beginning of fiscal year 2006, we adopted Statement of Financial Accounting Standards No. 123(R) (“SFAS 123(R)”), “Share-Based Payment,” and elected to adopt the modified prospective application method. SFAS No. 123(R) requires us to use a fair-valued based method to account for share-based compensation. Accordingly, share-based compensation cost is measured at the grant date, based on the fair value of the award, and is recognized as expense over the employees’ requisite service period. Total compensation cost for our share-based payments in the first quarter of fiscal 2007 and 2006 was $845,000 and $462,000, respectively. Selling, general and administrative expense in the first quarter of fiscal 2007 and 2006 include share-based compensation of $722,000 and $386,000, respectively. Research and development expense in the first quarter of fiscal 2007 and 2006 include share-based compensation of $123,000 and $76,000, respectively. As of December 31, 2006, $8.3 million of total unrecognized compensation costs related to nonvested awards is expected to be recognized over a weighted average period of 2.9 years. See Note 1, “Nature of Business and Significant Accounting Policies –Share-Based Compensation” in the Notes to Condensed Consolidated Financial Statements (Unaudited) for further discussion.
33
Interest Expense and Interest Income
For the first quarter of fiscal 2007, interest expense increased to $603,000, compared to $23,000 for the same period in the prior year. The increase is primarily due to the Seller Notes with an original balance of $251 million issued in May 2006 in connection with the purchase of Alamo.
For the first quarter of fiscal 2007, interest income decreased to $191,000, compared to $328,000 for the same period in the prior year. The decrease is due to approximately a 66% decrease in the average balance of cash, cash equivalents and investments in securities for the quarter ended December 31, 2006, compared to the same period in the prior year.
Net Loss
Net loss was $13.6 million, or $0.39 per share, in the first quarter of fiscal 2007, compared to a net loss of $9.2 million, or $0.32 per share for the first quarter of fiscal 2006 including a loss of $0.12 related to the cumulative effect of the change in accounting principle. A significantly higher net loss only partially mitigated by the effect of a higher weighted average number of shares outstanding in the first quarter of fiscal 2007 accounted for the higher loss per share.
LIQUIDITY AND CAPITAL RESOURCES
We assess our liquidity by our ability to generate cash to fund future operations. Key factors in the management of our liquidity are: cash required to fund operating activities including expected operating losses and the levels of accounts receivable, inventories, accounts payable and capital expenditures; the timing and extent of cash received from milestone payments under license agreements; funds required for acquisitions; funds required to repay notes payable and capital lease obligations as they become due; adequate credit facilities; and financial flexibility to attract long-term equity capital on satisfactory terms. Historically, cash required to fund on-going business operations has been provided by financing activities and used to fund operations and working capital requirements and investing activities.
Cash, cash equivalents and investments, as well as, net cash provided by or used for operating, investing and financing activities are summarized in the table below.
| | | | | | | | | | | | | |
| | | | | | | Increase | | |
| | | December 31, | | (Decrease) | | September 30, |
| | | 2006 | | During Period | | 2006 |
| Cash, cash equivalents and investment in securities | | $ | 17,527,574 | | | $ | (7,222,499 | ) | | $ | 24,750,073 | |
| Cash and cash equivalents | | $ | 14,457,274 | | | $ | 9,559,060 | | | $ | 4,898,214 | |
| Net working capital | | $ | (1,426,379 | ) | | $ | (5,543,398 | ) | | $ | (6,969,777 | ) |
| | | | | | | | | | | | | |
| | | Three Months | | | | | | | Three Months | |
| | | Ended | | | Change | | | Ended | |
| | | December 31, | | | Between | | | December 31, | |
| | | 2006 | | | Periods | | | 2005 | |
| Net cash used for operating activities | | $ | (19,101,884 | ) | | $ | (6,575,919 | ) | | $ | (12,525,965 | ) |
| Net cash (used for) provided by investing activities | | | 16,763,367 | | | | 18,988,791 | | | | (2,225,424 | ) |
| Net cash provided by financing activities | | | 11,897,577 | | | | (25,035,563 | ) | | | 36,933,140 | |
| | | | | | | | | | |
| Net increase in cash and cash equivalents | | $ | 9,559,060 | | | $ | (12,622,691 | ) | | $ | 22,181,751 | |
| | | | | | | | | | |
Operating activities.Net cash used for operating activities amounted to $19.1 million in the first quarter of fiscal 2007 compared to $12.5 million in the first quarter of fiscal 2006. The increase in cash used is related to continuation of the open label safety study of Zenvia in the treatment for IEED/PBA and a Phase III clinical trial of Zenvia for the treatment of neuropathic pain, increased expenses primarily relate to the expansion of our pre-launch activities and market research for Zenvia for the treatment of IEED/PBA, as well as the hiring of additional sales and marketing personnel, expenses from
34
operations of Alamo acquired in May 2006, increase in expenses related to increases in headcount and compensation levels in general and administrative areas and increased legal fees.
Our net trade receivables decreased slightly by $726,000, which is primarily due a decrease in unbilled receivables for research services earned in the quarter and a reduction of FazaClo wholesale receivables. Accounts payable decreased by $6.0 million and is primarily due to payments of invoices in connection with our pre-launch activities and market research for Zenvia which is now on hold and payments of other clinical activities currently on hold. A $2.1 million increase in accrued expenses resulted from accruals for goods and services received in the quarter ended December 31, 2006 but were not yet invoiced by the vendors.
Investing activities.Net cash provided by investing activities was $16.8 million in the first quarter of fiscal 2007, compared to $2.2 million used in the first quarter of fiscal 2006. Our investments in securities, net of maturities, decreased by $16.9 million in the first quarter of fiscal 2007 compared with only $11.9 million in first quarter of fiscal 2006. We invested $86,000 in property and equipment in the first quarter of fiscal 2007, compared to $543,000 in the first quarter of fiscal 2006.
Financing activities.Net cash provided by financing activities was $11.9 million in the first quarter of fiscal 2007, consisting of $15.0 million in net proceeds from sales of our common stock offset by $3.1 million payment of long-term debt. Net cash provided by financing activities amounted to $36.9 million in the first quarter of fiscal 2006, consisting of $37.0 million received from the sale of our common stock reduced by $83,000 for payments on capital lease obligations.
In June 2005, we filed shelf registration statement on Form S-3 with the SEC to sell an aggregate of up to $100 million in Class A common stock and preferred stock, depositary shares, debt securities and warrants. This shelf registration statement was declared effective on August 3, 2005 and through December 31, 2006, we have sold a total of 8,524,181 shares of Class A common stock under this registration statement, raising gross offering proceeds of approximately $51.7 million and net offering proceeds of approximately $50.6 million. We have also issued under this registration statement common stock warrants to purchase a total of 1,053,000 shares of our Class A common stock at an exercise price of $3.30 per share. The warrants become exercisable beginning in May 2007 and all unexercised warrants expire in November 2007.
Subsequent to December 31, 2006, the Company sold and issued 2,329,790 shares of our Class A common stock under this registration statement for aggregate gross offering proceeds of $5.6 million ($5.4 million after expenses). Approximately $1.1 million of the net proceeds from this offering were used to reduce the principal balance of a note payable as required under the terms of the note. These offerings and sales were made under our financing facility with Brinson Patrick Securities Corporation, which we entered into in December 2006. Under this facility, we may offer and sell up to 5,684,000 shares of Class A common stock. As of the date of this filing 3,111,150 shares remained available for sale by us from time to time.
In September 2004, we entered into an equipment line of credit with GE Healthcare Financial Services for financing of up to $1.4 million. As of December 31, 2006, the available borrowing under the line of credit was approximately $227,000. This equipment line expires February 28, 2007. In December 2006 we entered into a fleet operating lease agreement with GE Commercial Finance Fleet Services for up to $1.4 million of financing for our sales fleet. No obligations are currently outstanding under this facility.
35
As of December 31, 2006, we have contractual obligations for long-term debt, capital (finance) lease obligations and operating lease obligations, as summarized in the table that follows.
| | | | | | | | | | | | | | | | | | | | | |
| | | Payments Due by Period |
| | | | | | | Less than | | | | | | | | | | More than |
| | | Total | | 1 Year | | 1-3 Years | | 3-5 Years | | 5 Years |
| Long-term debt (principal and interest) | | $ | 26,757,840 | | | $ | 2,260,026 | | | $ | 24,497,814 | | | $ | — | | | $ | — | |
| Capital (finance) lease obligations | | | 372,443 | | | | 255,186 | | | | 117,257 | | | | — | | | | — | |
| Operating lease obligations | | | 11,020,911 | | | | 2,452,443 | | | | 4,066,814 | | | | 3,233,084 | | | | 1,268,570 | |
| Purchase obligations (1) | | | 15,179,376 | | | | 13,661,438 | | | | 1,517,938 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | | |
| Total | | $ | 53,330,570 | | | $ | 18,629,093 | | | $ | 30,199,823 | | | $ | 3,233,084 | | | $ | 1,268,570 | |
| | |
| (1) | | Purchase obligations consist of the total of accounts payable and accrued expenses at December 31, 2006 which approximates our contractual commitments for goods and services in the normal course of our business. |
As part of the purchase consideration of the Alamo acquisition, we issued three promissory notes in the initial respective principal amounts of $14,400,000, $6,675,000 and $4,000,000 (the “First Note,” “Second Note” and “Third Note” respectively) (collectively, the “Notes”). The Notes bear interest at an average rate equal to the London Inter-Bank Offered Rate, or “LIBOR,” plus 1.33%. Interest accruing on the Notes is payable monthly and the principal amount of the Notes matures on May 24, 2009, provided that (i) the Selling Holders may demand early repayment of the First Note if the closing price of our common stock, as reported on the NASDAQ Global Market, equals or exceeds $15.00 per share for a total of 20 trading days in any 30 consecutive trading-day period (the “Stock Contingency”), and (ii) we must apply 20% of any future net offering proceeds from equity offerings and other financing transactions to repay the Notes (starting with the First Note), and must repay the Notes in full if we have raised in an offering more than $100,000,000 in future aggregate net proceeds. In connection with the equity offering we completed subsequent to fiscal 2006 and in accordance with the terms of the Notes, we used $2.9 million or 20% of the net proceeds to pay down the First Note. The principal balance of the First Note is $10.5 million as of February 7, 2007.
If the Selling Holders demand repayment of the First Note following satisfaction of the Stock Contingency, we must repay the First Note within 180 days from the demand in our choice of cash or shares of common stock. If we elect to repay the First Note in shares of common stock, the shares will be valued at 95% of the average closing price of the common stock, as reported on the NASDAQ Global Market, for the five trading days prior to repayment, subject to a price floor.
We have the right to prepay, in cash or in common stock, the amounts due under the Notes at any time, provided that we may only pay the Notes in common stock if the Stock Contingency has occurred prior to the maturity date and if we have registered the shares on an effective registration statement filed with the SEC. If we elect to prepay the Notes with common stock, the shares will be valued at 95% of the average
36
closing price of the common stock, as reported on the NASDAQ Global Market, for the five trading days prior to repayment, subject to a price floor.
Alamo Earn-Out Payments.In connection with the Alamo acquisition, we agreed to pay up to an additional $39,450,000 in revenue-based earn-out payments, based on future sales of FazaClo. These earn-out payments are based on FazaClo sales in the U.S. from the closing date of the acquisition through December 31, 2018 (the “Contingent Payment Period”) and are currently payable to the Selling Holders as follows:
| | • | | A promissory note, in the principal amount of $2,000,000, generally payable on the third anniversary date if monthly FazaClo net product sales, as reported by us, exceed $1,000,000 for each of the three months in a given fiscal quarter during the Contingent Payment Period, and an additional promissory note in the principal amount of $2,000,000, generally payable on the third anniversary date if monthly FazaClo net product sales, as reported by us, exceed $1,500,000 for each of the three months in a given fiscal quarter during the Contingent Payment Period. None of these conditions were satisfied as of December 31, 2006. |
| |
| | • | | A one-time cash payment of $10,450,000 if FazaClo net product sales, as reported by us, exceed $40.0 million over four consecutive fiscal quarters during the Contingent Payment Period. |
| |
| | • | | An additional one-time cash payment of $25,000,000 if FazaClo net product sales, as reported by us, exceed $50.0 million over four consecutive fiscal quarters during the Contingent Payment Period. |
We have also agreed to pay the Selling Holders one-half of all net licensing revenues that we may receive, if any, during the Contingent Payment Period from licenses of FazaClo outside of the U.S. (“Non-US Licensing Revenues”). Any amounts paid to the Selling Holders on Non-US Licensing Revenues will be recognized in the consolidated statement of operations in the period such amounts are paid.
CIMA Royalty payments.In connection with the Alamo acquisition, we acquired a development, license and supply agreement with CIMA Labs Inc. (“CIMA”), which holds intellectual property rights related to certain aspects of the development and production of FazaClo (the “FazaClo Supply Agreement”). The FazaClo Supply Agreement grants, through our Alamo subsidiary, an exclusive license to us to market, distribute and sell FazaClo. The FazaClo Supply Agreement provides royalty rates of 5% to 6%, based on annual net sales and minimum annual royalty targets set forth in the agreement. The FazaClo Supply Agreement extends through the life of the longest patent with is currently the OraSolv patent expiring in 2012. The agreement terminates for insolvency by either party, with 60-day notice if terms of agreement are not met, with 60-days notice if we fail to pay royalties, and CIMA can terminate region-by-region if Alamo/Avanir does not commercialize in other regions. Minimum future annual royalty payments under the agreement are as follows:
| | | | | |
| Twelve-month period ending December 31: | | | | |
| 2006 | | $ | 250,000 | |
| 2007 | | $ | 300,000 | |
| 2008 and each year thereafter | | $ | 400,000 | |
Eurand Milestone and Royalty Payments.In August 2006, we entered into a development and license agreement (“Eurand Agreement”) with Eurand, Inc. (“Eurand”), under which Eurand will provide R&D services using Eurand’s certain proprietary technology to develop a once-a-day controlled release capsule, a new formulation, of Zenvia for the treatment of IEED/PBA (“Controlled-Release Zenvia”). Under the terms of the Eurand Agreement, we will pay Eurand for development services on time and
37
material basis. We will be required to make payments up to $7.6 million contingent upon achievement of certain development milestones and up to $14.0 million contingent upon achievement of certain sales targets. In addition, we will be required to make royalty payments based on sales of Controlled-Release Zenvia, if it is approved for commercialization. Development milestone events include program initiation, delivery of prototypes, delivery of clinical trial material for phase 1, achieving target PK Profile in the pilot clinical study, delivery of clinical trial material for phase 3, filing of the first NDA for the Product with the FDA, completion of manufacturing validation and approval of the NDA with the FDA. Sales target milestones are $2.0 million upon achieving $100 million of U.S. net revenues, $4.0 million upon achieving $200 million of U.S. net revenues and $8.0 million upon achieving $400 million of U.S. net revenues. The agreement remains in effect on a country by country basis for the longer of 10 years after first commercial sale or the life of any Eurand patent, unless earlier terminated in accordance with the agreement. The Company may terminate the agreement upon 30 days notice in the event the company receives a response from the FDA that is something other than an unconditional approval of the original formulation of Zenvia. Upon expiration of the agreement the Company shall own a fully-paid irrevocable license. Effective December 2006, we suspended further work under this agreement until resolution of further development plans for Zenvia resulting from our meeting with the FDA in late February 2007. All material remaining obligations would only be due in the event we initiate the agreement in future.
Zenvia License Milestone Payments.We hold the exclusive worldwide marketing rights to Zenvia for certain indications pursuant to an exclusive license agreement with the Center for Neurologic Study (“CNS”). We will be obligated to pay CNS up to $400,000 in the aggregate in milestones to continue to develop both indications, assuming they are both approved for marketing by the FDA. We are not currently developing, nor do we have an obligation to develop, any other indications under the CNS license agreement. In fiscal 2005, we paid $75,000 to CNS under the CNS license agreement, and will need to pay a $75,000 milestone if the FDA approves our NDA for Zenvia for the treatment of IEED/PBA. In addition, we are obligated to pay CNS a royalty on commercial sales of Zenvia with respect to each indication, if and when the drug is approved by the FDA for commercialization. Under certain circumstances, we may have the obligation to pay CNS a portion of net revenues received if we sublicense Zenvia to a third party.
Management Outlook
In order to maintain sufficient cash and investments to fund future operations, including sales of FazaClo, and to prepare for the additional clinical work that may be required for the commercialization of Zenvia, we will need to raise additional capital in the near term. We may seek to raise this additional capital at any time and may do so through various financing alternatives, including licensing or sales of our technologies and drug candidates, selling shares of common or preferred stock, or through the issuance of one or more forms of senior or subordinated debt. The balance of securities available for sale under our existing shelf registration was approximately $48 million as of December 31, 2006. We believe that these anticipated offering proceeds plus our cash, cash equivalents and unrestricted investments in securities of approximately $16.7 million at December 31, 2007 as well as anticipated future cash flows generated from licensed technologies and sales from the shipments of FazaClo, will be sufficient to sustain our planned level of operations for at least the next 12 months. However, the Company cannot provide assurances that our plans will not change, or that changed circumstances will not result in the depletion of capital resources more rapidly than anticipated. If we are unable to generate sufficient cash flows from licensed technologies or sales from the shipments of FazaClo and are unable to raise sufficient capital, management believes that planned expenditures could be curtailed in order to continue operations for the next 12 months.
During fiscal 2007, we expect to earn sufficient revenues from R&D services, under license agreements with AstraZeneca and Novartis, to fully offset the expenses that we incur in connection with providing those services. If either AstraZeneca or Novartis were to reduce or terminate development efforts and
38
funding, then we would expect to reduce or terminate our own research and development spending associated with these programs, although we may incur unreimbursed charges associated with the reduction or termination of these programs. In general, potential milestone payments to be received under existing license agreements are outside of our control and the timing of potential payment cannot be predicted. Revenues from new sources in fiscal 2007, such as license fees and milestone payments, will depend substantially on whether or not we enter into additional license arrangements and whether or not we achieve milestones under existing arrangements. Such arrangements may be in the form of licensing or partnering agreements for Zenvia or for our other product development programs including development of a selective cytokine inhibitor. Many of our product development programs could take years of additional development before they reach the stage of being licensable to other pharmaceutical companies.
For information regarding the risks associated with our need to raise capital to fund our ongoing and planned operations, please see “Risk Factors.”
Item 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As described below, we are exposed to market risks related to changes in interest rates. Because substantially all of our revenue, expenses, and capital purchasing activities are transacted in U.S. dollars, our exposure to foreign currency exchange rates is immaterial. However, in the future we could face increasing exposure to foreign currency exchange rates as we expand international distribution of docosanol 10% cream and purchase additional services from outside the U.S. Until such time as we are faced with material amounts of foreign currency exchange rate risks, we do not plan to use derivative financial instruments, which can be used to hedge such risks. We will evaluate the use of derivative financial instruments to hedge our exposure as the needs and risks should arise.
Interest rate sensitivity
Our investment portfolio consists primarily of fixed income instruments with an average duration of approximately 9-12 months. The primary objective of our investments in debt securities is to preserve principal while achieving attractive yields, without significantly increasing risk. We classify our investments in securities as of December 31, 2006 as available-for-sale and our restricted investments in securities as held-to-maturity. These available-for-sale securities are subject to interest rate risk. In general, we would expect that the volatility of this portfolio would decrease as its duration decreases. Based on the average duration of our investments as of December 31, 2006 and 2005, an increase of one percentage point in the interest rates would have resulted in increases in comprehensive losses of approximately $27,000 and $183,000, respectively.
Item 4. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our chief executive officer and chief financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in the Securities Exchange Act of 1934, Rules 13a-15(e) and 15d-15(e), as of the end of the period covered by this report, have concluded that, based on such evaluation, as of December 31, 2006 our disclosure controls and procedures were effective and designed to provide reasonable assurance that the information required to be disclosed is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission rules and forms. In designing and evaluating the disclosure controls and procedures, our management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable
39
assurance of achieving the desired control objectives, and our management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
Changes in Internal Controls over Financial Reporting
There has been no change in the Company’s internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) during the Company’s fiscal quarter ended December 31, 2006, that has materially affected, or is reasonably likely to materially affect the Company’s internal control over financial reporting.
PART II OTHER INFORMATION
Item 1. LEGAL PROCEEDINGS
In the ordinary course of business, we may face various claims brought by third parties. Any of these claims could subject us to costly litigation. Management believes the outcome of currently identified claims and lawsuits will not have a material adverse effect on our financial condition or results of operations.
Item 1A. RISK FACTORS
Below are the risk factors that have been revised since the filing of our annual report on Form 10-K for the year ended September 30, 2006 (the “2006 Form 10-K”). We face significant additional risks, which are set forth in the other risk factors contained in our 2006 Form 10-K under the caption “Risk Factors.” You are urged to read these risk factors in the 2006 Form 10-K, in addition to the following revised risk factors set forth below, before making an investment decision with regard to our securities.
We have limited capital resources and will need to raise additional funds to support our operations.
We have experienced significant operating losses in funding the research, development and clinical testing of our drug candidates, accumulating operating losses totaling $231 million as of December 31, 2006, and we expect to continue to incur substantial operating losses for the foreseeable future. As of December 31, 2006 and February 9, 2007, we had approximately $16.7 million and $14.7 million, respectively, in cash and cash equivalents and unrestricted investments in securities and we do not expect to generate positive net cash flows from FazaClo sales unless we can significantly reduce operating expenses and/or increase sales.
We will need to raise significant amounts of additional capital to finance our ongoing operations. Based on our current loss rate and existing capital resources as of the date of this filing, we estimate that we have sufficient funds to sustain our operations at their current levels for approximately 3-4 months. Although we expect to be able to raise additional capital and/or curtail current levels of operation to be able to be able to continue to fund our operations beyond that time, there can be no assurance that we will be able to do so. Additionally, because we do not yet know the extent of the additional clinical development efforts that may be required by the FDA to allow us to resubmit our NDA for Zenvia, it is difficult to estimate our projected capital needs beyond our current spending levels. If the FDA requires substantial additional clinical data, our capital requirements would be significant and we may have difficulty financing the continued development of Zenvia and/or our other product candidates.
We will seek to raise additional capital and may do so at any time and may do so through various financing alternatives, including licensing or sales of our technologies and drug candidates, selling shares of common or preferred stock, or through the issuance of one or more forms of senior or subordinated debt. Each of these financing alternatives carries certain risks. Raising capital
40
through the issuance of common stock may depress the market price of our stock and any such financing will dilute our existing shareholders. If we instead seek to raise capital through licensing transactions or sales of one or more of our technologies or drug candidates, as we have with our RCT and MIF technologies, then we will likely need to share a significant portion of future revenues from these drug candidates with our licensees. Additionally, the development of any drug candidates licensed or sold to third parties will no longer be in our control and thus we may not realize the full value of any such relationships.
If we are unable to raise additional capital to fund future operations, then we may be unable to execute our commercialization plans for FazaClo or our development plans for Zenvia and may be required to reduce operations or defer or abandon one or more of our clinical or pre-clinical research programs.
We may face challenges attracting and retaining members of management and other key personnel, particularly following our receipt of the Zenvia approvable letter.
The industry in which we compete has a high level of employee mobility and aggressive recruiting of skilled personnel. This type of environment creates intense competition for qualified personnel, particularly in product research and development, sales and marketing and accounting and finance. Additionally, we have a relatively small organization and the loss of certain executive officers and other key employees could adversely affect our operations.
For example, if we were to lose one or more of the senior members of our sales and marketing team, we could experience potentially significant disruptions in our FazaClo commercialization activities. Additionally, many members of the management team have recently joined after the initial filing of the NDA for Zenvia with the objective of launching Zenvia for IEED/PBA. Given the uncertainty around the commercialization opportunities for Zenvia following receipt of the FDA approvable letter, some of these employees may choose to pursue other opportunities.
Since receiving the approvable letter for Zenvia, we have lost several employees and we may lose key employees in the future for the reasons discussed above. The loss of any of our key employees could adversely affect our business and cause significant disruption in our operations.
We generally do not control the development of compounds licensed to third parties and, as a result, we may not realize a significant portion of the potential value of any such license arrangements.
Under our license arrangements for our RCT and MIF compounds, we have no direct control over the development of these drug candidates and have only limited, if any, input on the direction of development efforts. These development efforts are ongoing by our licensing partners and if the results of their development efforts are negative or inconclusive, it is possible that our licensing partners could elect to defer or abandon further development of these programs. In the case of our RCT program licensed to AstraZeneca, we learned in the second quarter of fiscal 2007 of certain inconclusive preclinical data obtained by AstraZeneca and we do not know whether they will continue with the development of the licensed compound.
Because much of the potential value of these license arrangements is contingent upon the successful development and commercialization of the licensed technology, the ultimate value of these licenses will depend on the efforts of our licensing partners. If our licensing partners do not succeed in developing the licensed technology for whatever reason, or elect to discontinue the development of these programs, we may be unable to realize the potential value of these arrangements.
Item 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
Not applicable.
41
Item 3. DEFAULTS UPON SENIOR SECURITIES
Not applicable.
Item 4. SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS
Not applicable.
Item 5. OTHER INFORMATION
On February 12, 2007, Martin J. Sturgeon became the Vice President and Chief Accounting Officer of the Company. Mr. Sturgeon, 48,previously spent three years with the financial consultancy firm DLC, Inc. In this capacity, he acted as interim Chief Accounting Officer and interim Corporate Controller responsible for managing the finance and accounting departments of several companies. Prior to joining DLC, he served as Vice President, Corporate Controller for Corinthian Colleges, Inc. and as Vice President, Group Controller at Toshiba America Information Systems, Inc. He previously held various positions with increasing responsibility in accounting and finance departments at several large corporations. He holds a bachelor’s of business administration degree in accounting from the University of San Diego and a MBA in finance from IESE, a European MBA program sponsored by Harvard University. Additionally, Mr. Sturgeon is a licensed certified public accountant in California.
Mr. Sturgeon will be serving in an at-will capacity and will be paid an annual base salary of $200,000 and will be eligible for an annual bonus with a target value equal to 25% of his then current base salary. Mr. Sturgeon will be awarded a stock option to purchase 20,000 shares of Class A common stock at a price equal to the fair market value of the underlying shares on the date of grant, with this option to vest and become exercisable with respect to one-quarter of the underlying shares on the first anniversary of the date of grant and then with respect to the remaining shares quarterly thereafter for the next three years. He will also be awarded 10,000 shares of restricted stock, with such shares to vest on the second anniversary of his employment. Finally, Mr. Sturgeon will be eligible to enter into the Company’s standard form of change of control agreement, with potential severance benefits equal to 12 months of base salary, plus bonus.
Item 6. EXHIBITS
Exhibits
| | | |
| 15.1 | | Letter on unaudited interim financial information. |
| | | |
| 31.1 | | Certification of Principal Executive Officer Required Under Rule 13a-14(a) of the Securities Exchange Act of 1934, as amended. |
| | | |
| 31.2 | | Certification of Principal Financial Officer Required Under Rule 13a-14(a) of the Securities Exchange Act of 1934, as amended. |
| | | |
| 32.1 | | Certification of Principal Executive Officer Required Under Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. 1350. |
| | | |
| 32.2 | | Certification of Principal Financial Officer Required Under Rule 13a-14(b) of the Securities Exchange Act of 1934, as amended, and 18 U.S.C. 1350. |
42
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | | | | |
| Signature | | Title | | Date |
| | | | | |
/s/ Eric K. Brandt Eric K. Brandt | | President and Chief Executive Officer (Principal Executive Officer) | | February 14, 2007 |
| | | | | |
/s/ Michael J. Puntoriero Michael J. Puntoriero | | Vice President, Finance and Chief Financial Officer (Principal Financial and Accounting Officer) | | February 14, 2007 |
43