QuickLinks -- Click here to rapidly navigate through this documentSCHEDULE 14A
(Rule 14a-101)
INFORMATION REQUIRED IN PROXY STATEMENT
SCHEDULE 14A INFORMATION
Proxy Statement Pursuant to Section 14(a) of
the Securities Exchange Act of 1934 (Amendment No. )
| Filed by the Registrantý |
Filed by a Party other than the Registranto |
Check the appropriate box: |
o |
|
Preliminary Proxy Statement |
| o | | Confidential, for Use of the Commission only (as permitted by Rule 14a-6(e)(2)) |
ý |
|
Definitive Proxy Statement |
o |
|
Definitive Additional Materials |
o |
|
Soliciting Material Pursuant to Rule 14a-11(c) or Rule 14a-12 |
Dyax Corp. |
(Name of Registrant as Specified in Its Charter) |
|
(Name of Person(s) Filing Proxy Statement, if other than the Registrant) |
| | | | | |
| Payment of Filing Fee (Check the appropriate box): |
ý |
|
No fee required. |
o |
|
Fee computed on table below per Exchange Act Rules 14a-6(i)(4) and 0-11. |
| | | (1) | | Title of each class of securities to which transaction applies:
|
| | | (2) | | Aggregate number of securities to which transaction applies:
|
| | | (3) | | Per unit price or other underlying value of transaction computed pursuant to Exchange Act Rule 0-11 (Set forth the amount on which the filing fee is calculated and state how it was determined):
|
| | | (4) | | Proposed maximum aggregate value of transaction:
|
| | | (5) | | Total fee paid:
|
o |
|
Fee paid previously with preliminary materials. |
o |
|
Check box if any part of the fee is offset as provided by Exchange Act Rule 0-11(a)(2) and identify the filing for which the offsetting fee was paid previously. Identify the previous filing by registration statement number, or the form or schedule and the date of its filing. |
|
|
(1) |
|
Amount Previously Paid:
|
| | | (2) | | Form, Schedule or Registration Statement No.:
|
| | | (3) | | Filing Party:
|
| | | (4) | | Date Filed:
|
DYAX CORP.
300 TECHNOLOGY SQUARE
CAMBRIDGE, MA 02139
(617) 250-5500
NOTICE OF ANNUAL MEETING OF STOCKHOLDERS
To Be Held May 20, 2004
The 2004 Annual Meeting of Stockholders of Dyax Corp., a Delaware corporation ("Dyax"), will be held at the offices of Dyax Corp., 300 Technology Square, Cambridge, Massachusetts, at 2:00 p.m. on Thursday, May 20, 2004, for the following purposes:
- 1.
- To elect two Class I directors to serve until the 2007 Annual Meeting of Stockholders.
- 2.
- To vote on an amendment to Dyax's Restated Certificate of Incorporation to increase the number of authorized shares of our common stock by 75,000,000 shares from 50,000,000 shares to 125,000,000 shares.
- 3.
- To transact any other business that may properly come before the meeting or any adjournment of the meeting.
Only stockholders of record at the close of business on March 26, 2004 will be entitled to vote at the meeting or any adjournment of the meeting.
It is important that your shares be represented at the meeting.Therefore, whether or not you plan to attend the meeting, please complete your proxy and return it in the enclosed envelope, which requires no postage if mailed in the United States. If you attend the meeting and wish to vote in person, your proxy will not be used.
| | | By order of the Board of Directors, |
|
|
Nathaniel S. Gardiner
Secretary |
April 8, 2004
DYAX CORP.
PROXY STATEMENT
FOR ANNUAL MEETING OF STOCKHOLDERS
TO BE HELD
MAY 20, 2004
Our Board of Directors is soliciting your proxy with the enclosed proxy card for use at the 2004 Annual Meeting of Stockholders of Dyax Corp. to be held at our offices at 300 Technology Square, Cambridge, Massachusetts at 2:00 p.m. on Thursday, May 20, 2004, and at any adjournments of the meeting. The approximate date on which this proxy statement and accompanying proxy are first being sent or given to stockholders is April 8, 2004.
General Information About Voting
Who can vote. You will be entitled to vote your shares of Dyax Common Stock at the annual meeting if you were a stockholder of record at the close of business on March 26, 2004. As of that date, 31,130,614 shares of Common Stock were outstanding. You are entitled to one vote for each share of Common Stock that you held at that date.
How to vote your shares. You can vote your shares either by attending the annual meeting and voting in person or by voting by proxy. If you choose to vote by proxy, please complete, sign, date and return the enclosed proxy card. The proxies named in the enclosed proxy card will vote your shares as you have instructed. If you sign and return the proxy card without indicating how you wish your shares to be voted, the proxies will vote your shares in favor of the proposals contained in this proxy statement, as recommended by our Board of Directors. Even if you plan to attend the meeting, please complete and mail your proxy card to ensure that your shares are represented at the meeting. If you attend the meeting, you can still revoke your proxy by voting in person.
How you may revoke your proxy. You may revoke the authority granted by your executed proxy at any time before its exercise by filing with Dyax, Attention: Nathaniel S. Gardiner, Secretary, a written revocation or a duly executed proxy bearing a later date, or by voting in person at the meeting.
Quorum. A quorum of stockholders is required in order to transact business at the annual meeting. A majority of the outstanding shares of Common Stock entitled to vote must be present at the meeting, represented either in person or by proxy, to constitute a quorum for the transaction of business. If your shares are held in a brokerage account, you must make arrangements with your broker or bank to vote your shares in person or to revoke your proxy.
Abstentions and broker non-votes. "Broker non-votes" are proxies submitted by brokers that do not indicate a vote for one or more proposals because the brokers do not have discretionary voting authority and have not received instructions from the beneficial owners on how to vote on these proposals. Abstentions and broker non-votes will be considered present for purposes of determining a quorum for a matter.
Householding of Annual Meeting Materials. Some banks, brokers and other nominee record holders may be "householding" our proxy statements and annual reports. This means that only one copy of our proxy statement and annual report to stockholders may have been sent to multiple stockholders in your household. We will promptly deliver a separate copy of either document to you if you call or write us at our principal executive offices, 300 Technology Square, Cambridge, Massachusetts 02139, Attn: Investor Relations, telephone: (617) 225-2500. If you want to receive
1
separate copies of the proxy statement or annual report to stockholders in the future, or if you are receiving multiple copies and would like to receive only one copy per household, you should contact your bank, broker, or other nominee record holder, or you may contact us at the above address and telephone number.
Share Ownership
The following table and footnotes set forth certain information regarding the beneficial ownership of our Common Stock as of March 15, 2004 by (i) persons known by us to be beneficial owners of more than 5% of our Common Stock, (ii) our current executive officers and our named executive officers, (iii) our directors and (iv) all our current executive officers and directors as a group.
| | Number of Shares
Beneficially Owned
| |
|---|
Beneficial Owner
| |
|---|
| | Shares(1)
| | Percent
| |
|---|
Federated Investors, Inc. and certain related entities(2)
Federated Investors Tower
Pittsburgh, PA 15222-3779 | | 3,891,625 | | 12.50 | % |
HealthCare Ventures V, L.P. and certain related entities(3)
44 Nassau Street
Princeton, NJ 08542 | | 1,651,376 | | 5.31 | % |
Francis H. and Margrit A. Kelly(4)
1 Voltastrasse
8044 Zurich, Switzerland | | 1,623,750 | | 5.22 | % |
Thomas L. Kempner(5)
c/o Loeb Partners Corporation
61 Broadway
New York, NY 10006 | | 1,546,415 | | 4.96 | % |
| Susan B. Bayh(6) | | 6,750 | | * | |
| Henry E. Blair(7) | | 930,926 | | 2.97 | % |
| Constantine E. Anagnostopoulos(8) | | 62,895 | | * | |
| James W. Fordyce(9) | | 59,090 | | * | |
| Mary Ann Gray(10) | | 3,600 | | * | |
| Henry R. Lewis(11) | | 92,657 | | * | |
| John W. Littlechild(12) | | 1,674,251 | | 5.37 | % |
| David J. McLachlan(13) | | 44,950 | | * | |
| Gregory D. Phelps(14) | | 275,792 | | * | |
| Lynn G. Baird, Ph.D.(15) | | 62,968 | | * | |
| Stephen S. Galliker(16) | | 260,826 | | * | |
| Ivana Magovcevic, Ph.D., J.D.(17) | | 55,003 | | * | |
| Jack H. Morgan(18) | | 90,588 | | * | |
| David B. Patteson(19) | | 112,000 | | * | |
| Anthony H. Williams, M.D.(20) | | 79,114 | | * | |
| Clive R. Wood, Ph.D.(21) | | 12,500 | | * | |
| All Current Directors and Executive Officers as a Group (15 Persons)(22) | | 5,167,737 | | 16.02 | % |
- *
- Less than 1%
- (1)
- The persons and entities named in the table have sole voting and investment power with respect to all shares beneficially owned by them, except as noted below.
2
- (2)
- Based on the Schedule 13G filed by Federated Investors, Inc. with the SEC on February 13, 2004, Federated Investors, Inc. is the parent holding company of Federated Investment Management Company, Federated Investment Counseling, and Federated Global Investment Management Corp. (the "Investment Advisers"), which act as investment advisers to registered investment companies and separate accounts that own shares of common stock in us (the "Reported Securities"). The Investment Advisers are wholly owned subsidiaries of FII Holdings, Inc., which is a wholly owned subsidiary of Federated Investors, Inc. All of Federated Investors' outstanding voting stock is held in the Voting Shares Irrevocable Trust for which John F. Donahue, Rhodora J. Donahue and J. Christopher Donahue act as trustees.
- (3)
- Based on the Schedule 13G/A filed by HealthCare Ventures V, L.P. with the SEC on February 17, 2004, HealthCare Partners V, L.P. is the general partner of HealthCare Ventures V, L.P. The natural persons who control the 1,651,376 shares owned by HealthCare Ventures V., L.P. are John W. Littlechild, William Crouse, Harold R. Werner, Christopher Mirabelli, Ph.D., Augustine Lawlor, and James H. Cavanaugh, Ph.D.
- (4)
- Based on the Schedule 13D/A filed by Francis H. and Margrit A. Kelly with the SEC on April 9, 2003, Mr. and Mrs. Kelly share beneficial ownership of 1,623,750 shares, as joint tenants. Mr. and Mrs. Kelly also share voting and dispositive control over 985,450 shares. Mr. Kelly has sole voting and dispositive control over 1,000 shares and Mrs. Kelly has sole voting and dispositive control over 637,300 shares.
- (5)
- Includes (i) 1,441,048 shares of Common Stock held in trusts for the benefit of Mr. Kempner's brother, Mr. Kempner's brother's children, Mr. Kempner's children, and Mr. Kempner, of which Mr. Kempner is a trustee (ii) 11,792 shares held by Pinpoint Partners Corporation, of which Mr. Kempner is President, and (iii) 53,764 shares owned by Loeb Investors Co. IX, of which Mr. Kempner is the Managing Partner. Also includes 32,563 shares of Common Stock issuable to Mr. Kempner upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (6)
- Consists entirely of shares of Common Stock issuable to Ms. Bayh upon the exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (7)
- Includes (i) 114,100 shares which are held in trust for the benefit of Mr. Blair's spouse and child, as to which Mr. Blair disclaims beneficial ownership, and (ii) 259,905 shares of Common Stock issuable to Mr. Blair upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (8)
- Includes 49,310 shares of Common Stock issuable to Dr. Anagnostopoulos upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (9)
- Includes 40,549 shares of Common Stock issuable to Mr. Fordyce upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (10)
- Consists entirely of shares of Common Stock issuable to Dr. Gray upon the exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (11)
- Includes 49,310 shares of Common Stock issuable to Dr. Lewis upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (12)
- Includes 1,651,376 shares held by HealthCare Ventures V, L.P. Mr. Littlechild is the general partner of HealthCare Partners, LP, which is the general partner of HealthCare Ventures V, L.P. Mr. Littlechild disclaims beneficial ownership of these shares except to the extent of his pecuniary interest in the limited partnerships. Also includes 22,875 shares of Common Stock issuable to Mr. Littlechild upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (13)
- Includes 39,750 shares of Common Stock issuable to Mr. McLachlan upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
3
- (14)
- Includes 206,479 shares of Common Stock issuable to Mr. Phelps upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (15)
- Consists entirely of shares of Common Stock issuable to Dr. Baird upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (16)
- Includes 202,684 shares of Common Stock issuable to Mr. Galliker upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (17)
- Consists entirely of shares of Common Stock issuable to Dr. Magovcevic upon the exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (18)
- Includes 89,999 shares of Common Stock issuable to Mr. Morgan upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (19)
- Consists entirely of shares of Common Stock issuable to Mr. Patteson upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (20)
- Consists entirely of shares of Common Stock issuable to Dr. Williams upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
- (21)
- Consists entirely of shares of Common Stock issuable to Dr. Wood upon the exercise of outstanding options exercisable with the 60-day period following March 15, 2004.
- (22)
- See Notes 5 through 17, 20 and 21. Includes 1,123,360 shares of Common Stock issuable upon exercise of outstanding options exercisable within the 60-day period following March 15, 2004.
Section 16(a) Beneficial Ownership Reporting Compliance
Our executive officers and directors and persons who own beneficially more than ten percent of our equity securities are required under Section 16(a) of the Securities Exchange Act of 1934 to file reports of ownership and changes in their ownership of our securities with the Securities and Exchange Commission. They must also furnish copies of these reports to us. Based solely on a review of the copies of reports furnished to us and written representations that no other reports were required, we believe that for 2003 our executive officers, directors and 10% beneficial owners complied with all applicable Section 16(a) filing requirements, except that a Form 5 report was not filed in 2002 on behalf of Mr. Lewis covering three gift transactions that were later reported on a Form 4 report, two Form 4 reports were filed late on behalf of Mr. Fordyce covering two sale transactions, and a Form 4 report was filed late on behalf of Dr. Wood covering a grant of stock options.
4
PROPOSAL 1
ELECTION OF DIRECTORS
Our Board of Directors has fixed the number of directors at eight (8) for the coming year. Under our charter, our Board is divided into three classes, with each class having as nearly equal number of directors as possible. The term of one class expires, with their successors being subsequently elected to a three-year term, at each annual meeting of stockholders. At the 2004 Annual Meeting two Class I Directors will be elected to hold office for three years until their successors are elected and qualified. Our Board of Directors has nominated Henry E. Blair and Susan B. Bayh for re-election as Class I Directors at the upcoming annual meeting. Each has consented to serve, if elected. If any nominee is unable to serve, proxies will be voted for any replacement candidate nominated by our Board of Directors.
Votes Required
Directors will be elected by a plurality of the votes cast by the stockholders entitled to vote on this proposal at the meeting. Abstentions, broker non-votes and votes withheld will not be treated as votes cast for this purpose and, therefore, will not affect the outcome of the election.
Nominees for Director
The following table contains certain information as of March 15, 2004 about the nominees for Class I Director and current directors whose term of office will continue after the annual meeting.
Name and Age
| | Business Experience During Past Five Years
and Other Directorships
| | Director Since
|
|---|
| | | Class I Directors
(present term expires in 2004) | | |
Henry E. Blair
Age: 60 |
|
Henry E. Blairhas served as Chairman of the Board and President of Dyax Corp. since its merger with Protein Engineering Corporation in August 1995, and as acting Chief Executive Officer from August 1995 until his appointment as Chief Executive Officer in April 1997. He has been a director and officer of the Company since co-founding it in 1989. Mr. Blair is a director of Genzyme Corporation, a biotechnology company he co-founded in 1981. He is also a director of Esperion Therapeutics, Inc., a biotechnology company, a trustee of the CBR Institute for Biomedical Research, Inc., and a member of the Board of Overseers at Tufts University School of Medicine. |
|
1989 |
| | | | | |
5
Susan B. Bayh
Age: 44 |
|
Susan B. Bayh has been a director of Dyax since July 2003. Ms. Bayh currently serves as a director of Cubist Pharmaceuticals, Inc., Curis Inc., Dendreon International, Inc. and Esperion Therapeutics, Inc., all publicly-held biotechnology companies, as well as Anthem Inc., a publicly-held health benefits company, and Emmis Communications, a publicly-held diversified media company. Ms. Bayh is also a director of E-Bank and of Golden State Foods, both privately-held companies. From 1994 to January 2001, Ms. Bayh served as the Commissioner of the International Commission between the United States and Canada, overseeing compliance with environmental and water level treaties for the United States-Canadian border. Since 1994, Ms. Bayh has served as a Distinguished Visiting Professor at the College of Business Administration at Butler University, Indianapolis, Indiana. From 1989 to 1994, Ms. Bayh was an attorney in the Pharmaceutical Division of Eli Lilly and Company, where she handled federal regulatory issues for marketing and medical affairs. Prior to 1989, Ms. Bayh worked at the law firms of Barnes & Thornburg and Gibson, Dunn & Crutcher LLP, where she specialized in litigation, antitrust and corporate law. She received a J.D. from the University of Southern California Law Center in California. |
|
2003 |
|
|
Class II Directors
(present term expires 2005) |
|
|
James W. Fordyce
Age: 61 |
|
James W. Fordyce has been a director of Dyax since August 1995. From 1981 to 2003, he served as a general partner of Prince Ventures Limited Partnership, a venture capital management firm that specialized in early stage investments in companies involved in medical and life science businesses. Mr. Fordyce has been the Managing Member of Fordyce & Gabrielson LLC, a private investment management firm, since 1998. In addition, he is Chairman of the Board of Directors of the Albert and Mary Lasker Foundation. |
|
1995 |
| | | | | |
6
Thomas L. Kempner
Age: 76 |
|
Thomas L. Kempner has been a director of Dyax since August 1995, and previously was a director of Protein Engineering Corporation before its merger with Dyax. Mr. Kempner is the Chairman and Chief Executive Officer of Loeb Partners Corporation, an investment banking, registered broker/dealer and registered investment advisory firm. He is also President of Pinpoint Partners Corporation, the general partner of the Loeb Investment Partnerships. Mr. Kempner is a director of Alcide Corporation, a publicly-held biotechnology company, CCC Information Services Group, Inc., a publicly-held automobile insurance-related software company, FuelCell Energy, IGENE BioTechnology, Inc., a publicly-held biotechnology and nutrition company, Insight Communications Company, Inc., a publicly-held cable television company, and Intermagnetics General Corporation, a publicly-held producer of magnetic resonance imaging technology. Mr. Kempner also serves as a director emeritus of Northwest Airlines, Inc. |
|
1995 |
Mary Ann Gray
Age: 51 |
|
Mary Ann Gray, Ph.D. has been a director of Dyax since February 2004. Dr. Gray has served as a director of Telik, Inc., a public biotech company, since September 2003. Dr. Gray has been the President of Gray Strategic Advisors, LLC, a company that provides strategic advice to both public and private biotechnology companies, since August 2003. Previously, she was Senior Analyst and Portfolio Manager of the Federated Kaufmann Fund, focusing on both public and private healthcare investments, from 1999 to July 2003. Prior to joining the Kaufmann Fund, Dr. Gray was a sell-side biotechnology analyst with Kidder Peabody from 1992 to 1995, and held similar positions with Warburg Dillon Read from 1996 to 1998 and with Raymond James & Associates from 1998 to 1999. Additionally, Dr. Gray has over twelve years of experience as a scientist in academia and industry. She held scientific positions at Schering Plough Corporation and NeoRx Corporation, and early in her career, Dr. Gray managed pre-clinical toxicology studies for the National Cancer Institute through Battelle Memorial Institute. Dr. Gray has a Ph.D. in Pharmacology from the University of Vermont where she focused on novel chemotherapeutic agents for the treatment of cancer. She did post-doctoral work at Northwestern University Medical School as well as Yale University School of Medicine. |
|
2004 |
| | | | | |
7
|
|
Class III Directors
(present term expires in 2006) |
|
|
Constantine E. Anagnostopoulos
Age: 81 |
|
Constantine E. Anagnostopoulos, Ph.D. has been a director of Dyax since 1991. He has been a Managing General Partner of Gateway Associates L.P., a venture capital management firm, since 1987. Dr. Anagnostopoulos is a retired corporate officer of Monsanto Company. He is also a director of a number of other biotechnology companies, including Genzyme Corporation. |
|
1991 |
Henry R. Lewis
Age: 78 |
|
Henry R. Lewis, Ph.D. has been a director of Dyax since August 1995, and previously was a director of Protein Engineering Corporation before its merger with Dyax. Dr. Lewis is a consultant to several companies. From 1986 to February 1991, Dr. Lewis was the Vice Chairman of the board of directors of Dennison Manufacturing Company, a manufacturer and distributor of products for the stationery, technical paper and industrial and retail systems markets. From 1982 to 1986, he was a Senior Vice President of Dennison Manufacturing Company. Dr. Lewis was also a director of Genzyme Corporation, a biotechnology company, from 1986 until 2000. |
|
1995 |
David J. McLachlan
Age: 65 |
|
David J. McLachlan has been a director of Dyax since May 1999. Since June 1999, Mr. McLachlan has been a Senior Advisor to Genzyme Corporation, where he held the position of Executive Vice President and Chief Financial Officer from 1989 through 1999. Mr. McLachlan currently serves on the board of directors of: HearUSA Inc., a hearing care company; Skyworks Solutions, Inc., a wireless semiconductor company; and Peptimmune, Inc., a private biotechnology company. He is also a director of the Massachusetts Biotechnology Council. |
|
1999 |
Board and Committee Matters
Independence. Our Board of Directors has determined that each of the current directors, as well as those standing for re-election, are independent directors as defined by applicable Nasdaq National Market standards governing the independence of directors, except for Henry E. Blair, our Chairman, President and Chief Executive Officer, and Gregory D. Phelps, who was formerly a senior executive of Dyax until December 2002.
8
Board Meetings and Committees. Our Board of Directors held twelve (12) meetings during 2003. Each of the directors then in office attended at least 75% of the aggregate of all meetings of the Board of Directors and all meetings of the committees of the Board of Directors on which such director then served, except that Dr. Marduel attended 50% of the meetings of the Board of Directors and 50% of the meetings of the committees on which she served. In 2003, eight directors attended the annual meeting of shareholders. Nominees for election as directors in a given year are required to attend the annual meeting of shareholders barring significant commitments or special circumstances.
Shareholder Communications. Any shareholder wishing to communicate with the Company's Board of Directors, a particular director or any committee of the Board of Directors may do so by sending written correspondence to the Company's principal executive offices, c/o Senior Vice President, Legal Affairs. All such communications will be delivered to the Board of Directors or the applicable director or committee chair.
Our Board of Directors has three standing committees: the Audit Committee, Compensation Committee and Nominating and Governance Committee.
Audit Committee. The Audit Committee has authority to select and engage our independent auditors and is responsible for reviewing our audited financial statements, accounting processes and reporting systems and discusses the adequacy of our internal financial controls with our management and our auditors. The Audit Committee also is responsible for overseeing the independence of, and approving all services provided by our independent auditors.
The members of the Audit Committee are David McLachlan (Chair), Henry Lewis, Thomas Kempner and Mary Ann Gray. Our Board of Directors has considered and concluded that each of the members of the Audit Committee satisfies the independence, financial literacy and expertise requirements as defined by applicable Nasdaq National Market standards governing the qualifications of Audit Committee members. Additionally, our Board of Directors has determined that Mr. McLachlan qualifies as an audit committee financial expert under the rules of the SEC.
The Audit Committee held seven (7) meetings during fiscal 2003. The Audit Committee operates under a written charter adopted by the Board and attached to this proxy statement asAppendix A. For more information about the Audit Committee, including its audit services pre-approval procedures, see "Audit Committee Report" and "Information Concerning Our Auditors" in this proxy statement.
Compensation Committee. Our Compensation Committee is responsible for establishing cash compensation policies with respect to our executive officers, employees, directors and consultants, determining the compensation to be paid to our executive officers and administering our equity incentive and stock purchase plans. The members of the Compensation Committee are James Fordyce (Chair), Constantine Anagnostopoulos, Henry Lewis and Susan Bayh. The Compensation Committee held seven (7) meetings during fiscal 2003.
Nominating and Governance Committee. Our Nominating and Governance Committee identifies individuals qualified to become Board members and recommends to the Board the director nominees for the next annual meeting of shareholders and candidates to fill vacancies on the Board. Additionally, the committee recommends to the Board the directors to be appointed to Board committees. The committee also develops and recommends to the Board a set of corporate governance guidelines applicable to the Board and to the Company and oversees the effectiveness of our corporate governance in accordance with those guidelines. The Nominating and Governance Committee currently consists of all the independent directors, namely Henry Lewis (Chair), Constantine Anagnostopoulos, Thomas Kempner, James Fordyce, Susan Bayh, John Littlechild, David McLachlan and Mary Ann Gray, each of whom the Board has determined meets the independence requirements as defined by applicable Nasdaq National Market standards governing the independence of directors. The committee held two (2) meetings during fiscal 2003 in addition to four (4) executive sessions conducted in
9
conjunction with meetings of the Board. The Nominating and Governance Committee operates pursuant to a written charter, which is attached to this proxy statement asAppendix B.
The Nominating and Governance Committee considers candidates for Board membership suggested by its members and other Board members. Additionally, in selecting nominees for directors, the Nominating and Governance Committee will review candidates recommended by stockholders in the same manner and using the same general criteria as candidates recruited by the Committee and/or recommended by the Board. Any stockholder wishing to recommend a candidate for consideration by the committee as a nominee for director should follow the procedures set forth in "Shareholder Recommendations for Director Nominations" below. The Nominating and Governance Committee will also consider whether to nominate any person proposed by a shareholder in accordance with the provisions of the Company's bylaws relating to shareholder nominations as described in "Deadline for Stockholder Proposals and Director Nominations," below.
Once the Nominating and Governance Committee has identified a prospective nominee, the Committee makes an initial determination as to whether to conduct a full evaluation of the candidate. This initial determination is based on the information provided to the Committee with the recommendation of the prospective candidate, as well as the Committee's own knowledge of the prospective candidate, which may be supplemented by inquiries of the person making the recommendation or others. The preliminary determination is based primarily on the need for additional Board members to fill vacancies or expand the size of the Board and the likelihood that the prospective nominee can satisfy the evaluation factors described below. The Committee then evaluates the prospective nominee against the standards and qualifications set out in the Company's Corporate Governance Guidelines, which include among others:
- •
- whether the prospective nominee meets the independence requirements defined under applicable Nasdaq National Market listing standards and audit committee financial expert requirements defined under applicable SEC rules and regulations;
- •
- the extent to which the prospective nominee's skills, experience and perspective adds to the range of talent appropriate for the Board and whether such attributes are relevant to our industry;
- •
- the prospective nominee's ability to dedicate the time and resources sufficient for the diligent performance of Board duties; and
- •
- the extent to which the prospective nominee holds any position that would conflict with responsibilities to the Company.
If the committee's internal evaluation is positive, a sub-group of the committee will interview the candidate. Upon completion of this evaluation and interview process, the committee makes a recommendation to the full Board as to whether the candidate should be nominated by the Board and the Board determines the whether to approve the nominee after considering the recommendation and report of the committee.
Director Compensation
Director Fees. Our directors who are not employees of Dyax receive compensation for their services as directors in the form of a retainer of $15,000, payable in quarterly installments, a fee of $2,000 for each Board meeting attended ($1,000 for attendance by conference call), and a fee of $1,000 for each committee meeting attended ($500 for attendance by conference call), other than meetings of the Nominating and Governance Committee held in conjunction with a Board meeting, plus reimbursement for travel expenses. We pay non-employee directors who serve as the chairman of a committee of the Board of Directors an additional $3,000 per year. All other non-employee directors who serve on a committee of the Board of Directors receive $1,000 per year. Directors who are also our employees receive no additional compensation for serving as directors.
10
Stock Options. In addition, our non-employee directors elected at the 2003 Annual Meeting automatically received stock options under our Amended and Restated 1995 Equity Incentive Plan to purchase 9,000 shares of our Common Stock for each year of their three-year term, as will non-employee directors elected at the 2004 Annual Meeting. Non-employee directors elected between annual meetings automatically receive options to purchase 9,000 shares of our Common Stock for each year or portion of a year remaining in the three-year term of the class of directors to which they have been elected.
Certain Relationships and Related Transactions
In October 1998, we loaned $1,300,000 to Henry Blair, our Chairman and Chief Executive Officer, in connection with a purchase of real property. This loan was secured by Mr. Blair's interest in the real property and by his shares of our capital stock. Interest accrued on the unpaid principal balance at the rate of one and a half percent less than the base rate of Fleet National Bank, provided that the interest rate would not be less than the minimum rate required to avoid imputed interest for federal income tax purposes. The outstanding principal and accrued interest totaling $1,196,707.19 was repaid on June 30, 2003.
Mr. Blair serves as an outside director of Genzyme Corporation. Dr. Anagnostopoulos is also a director of Genzyme and Mr. McLachlan is a Senior Advisor to Genzyme.
We have a collaboration agreement with Genzyme Corporation for the development and commercialization of DX-88. Under this agreement, which was amended on May 31, 2002, and again effective as of September 30, 2003, we were responsible for all expenses incurred in connection with the development of DX-88 for the treatment of HAE through the completion of the first Phase II clinical trial for HAE, which occurred during the second quarter of 2003. In June 2003, Genzyme exercised its option to create Kallikrein LLC, a jointly owned limited liability company, to manage the development and commercialization of DX-88. Through the creation of Kallikrein LLC, Genzyme acquired a 49.99% financial interest in the DX-88 program and is now responsible for 49.99% of all costs incurred in connection with the development of DX-88 subsequent to completion of the first Phase II clinical trial. Upon dosing the first patient in a pivotal clinical trial of DX-88 for HAE, Genzyme will also be obligated to pay us a milestone payment anticipated to be approximately $3.0 million. In addition, we will be entitled to receive potential milestone payments of $10.0 million for the first FDA approved product derived from DX-88, and up to $15.0 million for additional therapeutic indications developed under the collaboration, as well as approximately 50% of the profits from sales of such products. The term of this collaboration is perpetual unless terminated by either party with prior written notice, upon a material breach by the other party or immediately upon a change of control or bankruptcy of the other party. We currently anticipate that this collaboration will not terminate until the parties determine that no commercial products will result from the collaboration or, if commercial products are eventually sold, until the sale of those products is no longer profitable. Because the drug discovery and approval process is lengthy and uncertain, we do not expect to be able to determine whether any commercial products will result under this collaboration until the completion of clinical trials.
Under the collaboration agreement, as amended, we had the option to purchase Genzyme's interest in the application of DX-88 for the prevention of blood loss and other systemic inflammatory responses in on-pump open-heart surgery and other surgical indications for $1.0 million. We exercised this option in the first quarter of 2003.
When we first amended the collaboration agreement in May 2002, we also executed a senior secured promissory note and security agreement under which Genzyme agreed to loan us up to $7.0 million and we agreed to grant Genzyme a continuing security interest in certain tangible and intangible personal property arising out of the DX-88 program. In addition, under the terms of the security agreement, once we exercised our option to purchase Genzyme's interest in the application of
11
DX-88 in on-pump open-heart surgery and other surgical indications, we were required to pledge to Genzyme a percentage interest in our wholly owned subsidiary, Biotage. Under an amendment to the security agreement executed on October 15, 2003, Genzyme agreed to release the interest in Biotage pledged to it in exchange for a continuing security interest in Dyax's rights to revenues from licenses of its fundamental phage display patent portfolio known as the Ladner patents. The security agreement, as amended, contains certain financial covenants under which the Company must maintain (i) at least $20.0 million in cash or cash equivalents based on the Company's quarterly consolidated financial statements and (ii) at least one continued listing standard for the Nasdaq National Market. As of December 31, 2003, we had borrowed the full $7.0 million available under the note.
Compensation Committee Interlocks And Insider Participation
Our Compensation Committee determines salaries, incentives and other compensation for our directors and officers. The Compensation Committee also administers our equity incentive and stock purchase plans. The Compensation Committee currently consists of Drs. Anagnostopoulos and Lewis, and Mr. Fordyce. For more information regarding the relationship of Dr. Anagnostopoulos with Genzyme Corporation and its relationships with Dyax, see the sections of this proxy statement entitled "Share Ownership," "Election of Directors" and "Certain Relationships and Related Transactions."
PROPOSAL 2
APPROVAL OF AN AMENDMENT TO OUR RESTATED CERTIFICATE OF INCORPORATION
General
Our Restated Certificate of Incorporation currently authorizes the issuance of 50,000,000 shares of common stock and 1,000,000 shares of preferred stock. On March 19, 2004, our Board of Directors approved an amendment to our Restated Certificate of Incorporation to increase the number of authorized shares of our common stock from 50,000,000 shares to 125,000,000 shares, subject to stockholder approval.
Current Use of Shares
As of March 15, 2004, there were 31,128,558 shares of common stock outstanding or reserved for issuance (including shares subject to outstanding options), with no shares held by us in treasury. This total number of shares includes 8,950,568 shares reserved for issuance or issued under our Amended and Restated 1995 Equity Incentive Plan, and 400,000 shares reserved for issuance or issued under our 1998 Employee Stock Purchase Plan. As of the date of this proxy statement, there were no shares of preferred stock issued or outstanding.
Purpose of the Proposed Amendment
Our Board of Directors believes that increasing the number of authorized shares of our common stock is essential to ensure that we continue to have an adequate number of shares of common stock available for future use. Our Board of Directors believes that the proposed increase will make available a sufficient number of authorized shares of common stock for future issuances including, financings, corporate mergers and acquisitions, use in employee benefit plans, stock splits, stock dividends or other corporate purposes. The availability of additional shares of common stock will provide us with greater flexibility in taking any of these actions and would allow us to issue shares of our common stock without the delay or expense of obtaining stockholder approval, except to the extent required by state law or Nasdaq requirements for particular transactions. As of the date of this proxy statement, we had no agreements, commitments or plans with respect to the sale or issuance of additional shares of
12
common stock, other than with respect to those shares of common stock reserved for issuance as noted above or subject to our existing registration statement.
Effects of the Proposed Amendment
The proposed amendment would increase the number of shares of our common stock available for issuance, but would have no effect upon the terms of our common stock or the rights of holders of our common stock. Common stockholders are not now, and will not be, entitled to preemptive or other rights to subscribe for additional shares of our common stock. If this proposal is adopted, additional shares of authorized common stock (as well as all currently authorized but unissued shares of common stock) would be available for issuance without further action by the stockholders, subject to Nasdaq stockholder approval requirements for certain issuances of additional shares of common stock. While our Board of Directors will authorize the issuance of additional shares of common stock based on its judgment as to our best interests and that of our stockholders, future issuances of common stock could have a dilutive effect on existing stockholders and on earnings per share. In addition, the issuance of additional shares of common stock, as well as the availability of preferred stock that the Board may issue on such terms as it selects, could have the effect of making it more difficult for a third party to acquire a majority of our outstanding voting stock.
OUR BOARD OF DIRECTORS BELIEVES THAT THE PROPOSED AMENDMENT IS IN OUR AND OUR STOCKHOLDERS BEST INTERESTS AND RECOMMENDS A VOTEFOR THIS PROPOSAL.
EXECUTIVE OFFICERS AND KEY EMPLOYEES
The following contains certain information as of March 15, 2004 about the current executive officers and key employees of Dyax:
Name
| | Age
| | Position
|
|---|
| Henry E. Blair* | | 60 | | Chairman of the Board, President and Chief Executive Officer |
| Stephen S. Galliker* | | 57 | | Executive Vice President, Finance and Administration, and Chief Financial Officer |
| Lynn G. Baird, Ph.D.* | | 56 | | Senior Vice President, Development |
| Anthony H. Williams, M.D.* | | 48 | | Senior Vice President, Medical Affairs and Clinical Operations |
| Clive R. Wood, Ph.D.* | | 43 | | Senior Vice President Discovery Research & Chief Science Officer |
| Ivana Magovcevic, Ph.D., J.D.* | | 36 | | Senior Vice President Legal Affairs and Chief Patent Counsel |
| Robert Charles Ladner, Ph.D. | | 59 | | Senior Vice President |
- *
- Executive officer
Henry E. Blair has served as Chairman of the Board and President of Dyax Corp. since its merger with Protein Engineering Corporation in August 1995, and as acting Chief Executive Officer from August 1995 until his appointment as Chief Executive Officer in April 1997. He has been a director and officer of the Company since co-founding it in 1989. Mr. Blair is a director of Genzyme Corporation, a biotechnology company he co-founded in 1981, as well as being a co-founder of Biocode, Inc. and GelTex Pharmaceuticals, Inc, both also biotechnology companies. He is also a director of Esperion Therapeutics, Inc., a member of the Board of Trustees of the CBR Institute for Biomedical Research, Inc., and a member of the Board of Overseers at the Tufts University School of Medicine and at the Lahey Hitchcock Clinic.
13
Stephen S. Galliker has served Dyax as Executive Vice President, Finance and Administration, and Chief Financial Officer since September 1999. He was Chief Financial Officer of Excel Switching Corporation, a developer and manufacturer of open switching platforms for telecommunications networks, from July 1996 to September 1999, and was Excel's Vice President, Finance and Administration from September 1997. Mr. Galliker was employed by Ultracision, Inc., a developer and manufacturer of ultrasonically powered surgical instruments from September 1992 to June 1996. At Ultracision, Inc., Mr. Galliker was Chief Financial Officer and Vice President of Finance until November 1995 and Chief Operating Officer from December 1995 to June 1996.
Lynn G. Baird, Ph.D. has served as Senior Vice President of Development for Dyax since March 2002. She joined the company in October 2001 as Senior Vice President of Preclinical and Regulatory Affairs. From 1998 to 2001, she held the title of Vice President at Reprogenesis, Inc. and its successor Curis, Inc., a biotechnology company. Her responsibilities at various times during her tenure included Regulatory Affairs, Quality, Clinical Development and Preclinical Development. Prior to Reprogenesis, Dr. Baird was at CytoTherapeutics, Inc., a biotechnology company, from 1995 to 1998 and was Vice President of Regulatory, Quality and Clinical Development at her departure. Prior to that she held positions at Johnson and Johnson, a pharmaceutical company, from 1990 to 1995 and at Creative BioMolecules, a biotechnology company, from 1985 to 1990.
Anthony H. Williams, M.D. has served as Dyax's Senior Vice President, Medical Affairs and Clinical Operations since September 2001. Previously, Dr. Williams was a Corporate Officer & Vice President for Aronex Pharmaceuticals, Inc. from 1999 to September 2001, where he was responsible for the implementation of pre-existing clinical development programs and product safety, and Director of Experimental Medicine at Medeva plc from 1993 to 1999, where he was responsible for early clinical development up to NDA studies. Since 1985, he has held a number of positions in the pharmaceutical industry with Merrell Dow Pharmaceuticals, Inc. and GlaxoSmithKline. Dr. Williams graduated from the University of London, England in 1980, with a degree in medicine. He had previously obtained a Master of Arts degree from the University of Cambridge in England. Dr. Williams specialized in infectious diseases and oncology during his hospital training in London.
Clive R. Wood, Ph.D. has served as Dyax's Senior Vice President, Research Discovery and Chief Scientific Officer since August 2003. Prior to this, Dr. Wood spent 17 years at Genetics Institute (GI) and Wyeth Research, where he held a number of drug discovery research positions of increasing scope and responsibility. Most recently, Dr. Wood held the position of Senior Director and Acting Head of Inflammation Discovery Research at Wyeth Research in Cambridge, MA. At Wyeth (formerly GI), Mr. Wood focused on respiratory diseases, transplantation, immunology, hematopoiesis and antibody technologies. Prior to joining GI in 1986, Dr. Wood worked for four years at Celltech Ltd. and contributed to the first work on the production of recombinant antibodies. He is an Adjunct Professor in the Department of Pharmacology and Experimental Therapeutics of Boston University School of Medicine, and received his Biochemistry B.Sc. as well as his Ph.D. from the University of London in 1982 and 1986, respectively.
Ivana Magovcevic, Ph.D., J.D., has served as Senior Vice President of Legal Affairs and Chief Patent Counsel for Dyax Corp. since February 2004. She heads the Legal and Investor Relations & Corporate Communications departments at Dyax. She joined the company in April of 2001 as Vice President of Intellectual Property. Prior to joining Dyax, Dr. Magovcevic was Director of Intellectual Property and Patent Counsel for Transkaryotic Therapies, Inc. She previously served as a patent agent at Fish and Richardson and Lahive & Cockfield, two Boston patent law firms. Dr. Magovcevic holds a doctorate degree in genetics from Harvard University and a law degree from Suffolk University.
Robert Charles Ladner, Ph.D. became Senior Vice President of Dyax in August 1995 and served as our Chief Scientific Officer from 1995 to 2003. He was a co-founder of Protein Engineering Corporation where he was an inventor of our fundamental phage display technology and served as
14
Senior Vice President and Scientific Director from 1987 until its merger with Dyax in August 1995. Previously, Dr. Ladner served as Senior Scientist of Genex Corp., where he was an inventor of single chain antibodies.
EXECUTIVE COMPENSATION
Compensation Committee Report on Executive Compensation
The Compensation Committee of the Dyax Board of Directors determines the compensation to be paid to Dyax's executive officers, including the Chief Executive Officer. The Committee also administers Dyax's equity incentive plan and its employee stock purchase plan, including the grant of stock options and other awards under those plans. The Committee is currently composed of Mr. Fordyce (Chairman) and Drs. Anagnostopoulos and Lewis, as well as Ms. Bayh, who has just been elected to the Committee after the Committee's most recent meeting in February 2004. This report is submitted by the Committee and addresses the compensation policies for fiscal year 2003 as they affected Mr. Blair, as Chairman, President and Chief Executive Officer, and Dyax's other executive officers, including the five most highly compensated executive officers other than Mr. Blair who are named in this year's Summary Compensation Table.
Compensation Philosophy
Dyax's executive compensation policy is designed to attract, retain and reward executive officers who contribute to Dyax's long-term success and to maintain a competitive salary structure as compared with other biotechnology companies. The compensation program seeks to align compensation with the achievement of business objectives and individual and corporate performance. Bonuses are included to encourage effective individual performance relative to Dyax's current plans and objectives. Stock option grants are key components of the executive compensation program and are intended to provide executives with an equity interest in Dyax in order to link a meaningful portion of the executive's compensation with the performance of Dyax's Common Stock.
In executing its compensation policy, the Committee seeks to reward each executive's achievement of designated objectives relating to Dyax's annual and long-term performance and individual fulfillment of responsibilities. While compensation survey data are useful guides for comparative purposes, the Committee believes that a successful compensation program also requires the application of judgment and subjective determinations of individual performance, and to that extent the Committee applies its judgment in reconciling the program's objectives with the realities of retaining valued employees.
Executive Compensation Program
Dyax's executive compensation package for the Chief Executive Officer and the other named executive officers is composed of three elements:
- •
- base salary;
- •
- annual incentive bonuses based on corporate and individual performance; and
- •
- initial, annual and other periodic grants of stock options under the Amended and Restated 1995 Equity Incentive Plan.
Named Executive Officers. The Compensation Committee reviews and determines annually the base salaries provided for each of the named executive officers based upon the executive's salary history and internal and external equity considerations. The annual base salary for 2003 for each named executive officer was adjusted in light of the executive's prior performance, tenure and responsibilities, as well as independent compensation data.
15
For fiscal 2003, the Committee established a target bonus opportunity for each of the senior executives, other than Mr. Blair, expressed as a percentage of base salary. The target bonus opportunity, which ranged from 25% to 30% for these senior executives, could be exceeded by up to 20% of the target for exceptional performance. For example, an executive with a target bonus of 25% who had outstanding performance could receive a bonus of as much as 30% of base salary. One half of the 2003 bonuses was tied to Dyax's corporate performance and one half was tied to the Committee's judgment regarding individual performance against objectives determined by Mr. Blair. In February 2004, the Compensation Committee reviewed with Mr. Blair the performance of each named executive officer and determined the bonus to be paid to each of them based on company performance against a number of corporate objectives and the achievement of individual's performance goals for 2003. The portion of bonuses based on corporate objectives included subjective assessment of a number of objectives in the areas of Dyax's clinical development, cash position, collaborations and technology development, among other factors agreed upon among the Committee and Mr. Blair. In connection with the sale of Dyax's Biotage operations, Mr. Patteson also received special incentive compensation that was payable only upon the successful completion of the sale in October 2003, as noted in the Summary Compensation Table.
Executive officer compensation also includes long-term incentives afforded by options to purchase Common Stock. The purposes of the stock option grant program are to reinforce the mutuality of long-term interests between Dyax's employees and stockholders, and to assist in the attraction and retention of important key executives, managers and individual contributors who are essential to Dyax's growth and development.
In June 2003, the Committee approved option grants of 27,500 shares to Mr. Galliker, 32,500 shares to Dr. Williams, 32,500 shares to Dr. Baird, 22,500 shares to Mr. Patteson, and 25,000 shares to Mr. Morgan, as shown in the Summary Compensation Table.
Chief Executive Officer. The Compensation Committee established a 2003 compensation package for Mr. Blair based on an analysis of compensation data for chief executive officers in comparable companies gathered from surveys prepared by independent compensation consultants.
The Committee established a 2003 base salary of $450,000 for Mr. Blair, which at his request did not increase from his 2002 base compensation. Mr. Blair's target bonus percentage was fixed at 33% of his base salary and was based entirely on corporate performance. Mr. Blair's target bonus opportunity was based on the Committee's assessment and evaluation of the same corporate objectives used for the other named executive officers. In February 2004, the Committee awarded Mr. Blair a bonus of $165,000, representing 111% of his target bonus opportunity for 2003. In addition, in June 2003, the Committee awarded Mr. Blair options to purchase 55,000 shares of Common Stock.
Compensation Deductibility
Section 162(m) of the Internal Revenue Code denies a tax deduction to a public corporation for annual compensation in excess of one million dollars paid to its Chief Executive Officer and its four other highest compensated officers. This provision excludes certain types of "performance based compensation" from the compensation subject to the limit. Although Dyax currently does not expect to have compensation exceeding this one million dollar limit, the Amended and Restated 1995 Equity Incentive Plan contains an individual annual limit on the number of stock options and stock appreciation rights that may be granted under the plan so that the awards will qualify for the exclusion from the limitation on deductibility for performance-based compensation. The Committee will continue to assess the impact of Section 162(m) on its compensation practices and determine what further action, if any, is appropriate.
By the Compensation Committee,
James W. Fordyce (Chair)
Constantine E. Anagnostopoulos
Susan B. Bayh
Henry R. Lewis
16
Stock Performance Graph
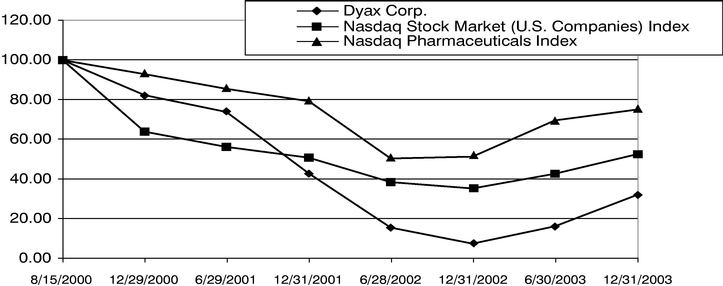
The following graph shows a comparison of the cumulative total stockholder returns on our Common Stock over the period from August 15, 2000 (the first trading day of our Common Stock) to December 31, 2003 as compared with that of the Nasdaq US Index and Nasdaq Pharmaceuticals Index, based on an initial investment of $100 in each on August 15, 2000. Total stockholder return is measured by dividing share price change plus dividends, if any, for each period by the share price at the beginning of the respective period, assuming reinvestment of any dividends.
Comparison of Cumulative Total Return of Dyax Corp.,
Nasdaq Stock Market (U.S. Companies) Index and Nasdaq Pharmaceuticals Index
| | 8/15/00
| | 12/29/00
| | 6/29/01
| | 12/31/01
| | 6/28/02
| | 12/31/02
| | 6/30/03
| | 12/31/03
|
|---|
| Dyax Corp. | | 100 | | 82.27 | | 73.72 | | 42.56 | | 15.13 | | 6.98 | | 15.83 | | 31.62 |
| Nasdaq Stock Market (U.S. Companies) Index | | 100 | | 63.71 | | 56.05 | | 50.56 | | 38.18 | | 34.94 | | 42.41 | | 52.27 |
| Nasdaq Pharmaceuticals Index | | 100 | | 92.90 | | 85.47 | | 79.18 | | 50.22 | | 51.15 | | 69.47 | | 74.98 |
17
Summary Compensation Table
The following table sets forth certain compensation information for our Chief Executive Officer, each of the other three most highly compensated executive officers whose salary and bonus for the year ended December 31, 2003 exceeded $100,000, and two additional individuals who would have been one of the most highly compensated executive officers but for the fact that he was not an executive officer at December 31, 2003. We refer to these persons as the named executive officers.
Summary Compensation Table
| |
| |
| |
| | Long-Term
Compensation Awards
| |
| |
|---|
| | Annual Compensation
| |
| |
|---|
Name and Principal Position
| | Shares Of Common Stock
Underlying Options(#)
| | All Other
Compensation($)(a)
| |
|---|
| | Year
| | Salary($)
| | Bonus($)
| |
|---|
Henry E. Blair
President and Chief Executive Officer | | 2003
2002
2001 | | 467,308
448,846
418,269 | | 165,000
89,100
124,740 | | 55,000
30,000
100,000 | | 9,701
7,786
6,003 | (b)
(b)
(c) |
Stephen S. Galliker
Executive Vice President, Finance and Administration, and Chief Financial Officer |
|
2003
2002
2001 |
|
257,077
253,995
230,193 |
|
85,956
55,020
62,370 |
|
27,500
35,000
40,000 |
|
23,412
14,803
21,026 |
(b)(d)
(b)(e)
(f)(d) |
Anthony H. Williams, M.D.(g)
Senior Vice President, Medical Affairs and Clinical Operations |
|
2003
2002
2001 |
|
251,308
241,923
58,154 |
|
66,369
48,400
15,238 |
|
32,500
60,000
75,000 |
|
5,861
4,836
7,633 |
(h)
(i)
(j)(k) |
Lynn G. Baird, Ph.D.(l)
Senior Vice President, Development |
|
2003
2002
2001 |
|
236,769
222,952
43,365 |
|
63,014
45,600
6,900 |
|
32,500
60,000
50,00 |
|
8,163
7,703
1,412 |
(m)
(n)
(o) |
David B. Patteson(p)
Executive Vice President, Separations Division, President of Biotage, Inc. |
|
2003
2002
2001 |
|
230,000
234,423
219,231 |
|
330,547
94,000
66,000 |
(q)
|
22,500
45,000
35,000 |
|
3,459
4,047
3,901 |
(r)
(s)
(t) |
Jack H. Morgan(u)
Senior Vice President, Corporate Development and Business Operations |
|
2003
2002
2001 |
|
256,692
229,616
126,923 |
|
29,167
64,400
57,529 |
|
25,000
60,000
85,000 |
|
6,286
5,932
3,029 |
(v)
(v)
(w) |
- (a)
- Unless otherwise noted, this amount represents premiums paid by Dyax for group term life insurance.
- (b)
- Includes $6,000 in 401(k) matching paid by Dyax.
- (c)
- Includes $5,100 in 401(k) matching paid by Dyax.
- (d)
- Includes $15,000 paid to Mr. Galliker as a housing allowance.
- (e)
- Includes $6,923 paid to Mr. Galliker as a housing allowance.
- (f)
- Includes $5,088 in 401(k) matching paid by Dyax.
- (g)
- Dr. Williams joined us in September 2001.
- (h)
- Includes $5,050 in 401(k) matching paid by Dyax.
18
- (i)
- Includes $4,199 in 401(k) matching paid by Dyax.
- (j)
- Includes $1,833 in 401(k) matching paid by Dyax.
- (k)
- Includes $5,703 paid to Dr. Williams as a relocation expense.
- (l)
- Dr. Baird joined us in October 2001.
- (m)
- Includes $5,998 in 401(k) matching paid by Dyax.
- (n)
- Includes $6,000 in 401(k) matching paid by Dyax.
- (o)
- Includes $1,300 in 401(k) matching paid by Dyax.
- (p)
- Mr. Patteson ceased to be one of our executive officers and employees upon the sale of Biotage in October 2003.
- (q)
- Includes $278,547 paid to Mr. Patteson pursuant to an incentive agreement executed in connection with and in contemplation of the successful sale of Biotage in October 2003.
- (r)
- Includes $3,180 in 401(k) matching paid by Dyax.
- (s)
- Includes $3,732 in 401(k) matching paid by Dyax.
- (t)
- Includes $3,586 in 401(k) matching paid by Dyax.
- (u)
- Mr. Morgan joined us in May 2001 and ceased to be an executive officer and employee in December 2003.
- (v)
- Includes $5,000 in 401(k) matching paid by Dyax.
- (w)
- Includes $2,750 in 401(k) matching paid by Dyax.
Option Grants and Potential Realizable Values Table
The following table sets forth certain information concerning option grants made to the named executive officers through December 31, 2003.
Option Grants In Last Fiscal Year
| |
| |
| |
| |
| | Potential Realizable Value at Assumed Annual Rates of Stock Price Appreciation For Option Term(b)
|
|---|
| | Individual Grants
|
|---|
| | Number Of
Securities
Underlying Options
Granted (#)(a)
| | Percent Of Total
Options Granted
To Employees In
Fiscal Year
| |
| |
|
|---|
Name(a)
| | Exercise or
Base Price
($/Sh)
| | Expiration
Date
|
|---|
| | 5%($)
| | 10%($)
|
|---|
| Henry E. Blair | | 55,000 | | 5.41 | | 3.36 | | 7/22/2013 | | 116,220 | | 294,524 |
| Stephen S. Galliker | | 27,500 | | 2.70 | | 3.36 | | 7/22/2013 | | 58,110 | | 147,262 |
| Anthony H. Williams, M.D. | | 32,500 | (c) | 3.20 | | 3.36 | | 7/22/2013 | | 68,675 | | 174,037 |
| Lynn G. Baird, Ph.D. | | 32,500 | (c) | 3.20 | | 3.36 | | 7/22/2013 | | 68,675 | | 174,037 |
| David B. Patteson | | 22,500 | (d) | 2.21 | | 3.36 | | 7/22/2013 | | 47,544 | | 120,487 |
| Jack H. Morgan | | 25,000 | (e) | 2.46 | | 3.36 | | 7/22/2013 | | 52,827 | | 133,874 |
- (a)
- All options reported are Incentive Stock Options, except as noted. These options vest as to 1/48th of the total shares per month beginning on the date of grant.
- (b)
- The values in this column are given for illustrative purposes; they do not reflect our estimate or projection of future stock prices. The values are based on an assumption that our Common Stock's market price will appreciate at the stated rate, compounded annually, from the date of the option
19
grant until the end of the option's 10-year term. Actual gains, if any, on stock option exercises will depend upon the future performance of our Common Stock's price, which will benefit all stockholders proportionately.
- (c)
- Includes 19,636 nonstatutory stock options.
- (d)
- Includes 21,094 nonstatutory stock options.
- (e)
- Includes 8,854 nonstatutory stock options.
Option Exercises and Year-End Values Table
The following table sets forth certain information concerning exercisable and unexercisable stock options held by the named executive officers as of December 31, 2003.
Aggregated Option Exercises In Last Fiscal Year And
Fiscal Year-End Option Value
Name
| | Shares
Acquired on
Exercise(#)
| | Value
Realized($)
| | Number of Securities Underlying
Unexercised Options at Fiscal Year-End(#)
Exercisable/Unexercisable
| | Value of Unexercised
in-the-Money Options at
Fiscal Year-End($)
Exercisable/Unexercisable(a)
|
|---|
| Henry E. Blair | | — | | — | | 234,280 / 143,020 | | 738,230 / 380,296 |
| Stephen S. Galliker | | — | | — | | 189,315 / 79,221 | | 863,212 / 286,339 |
| Anthony H. Williams, M.D. | | — | | — | | 64,115 / 103,385 | | 22,208 / 198,370 |
| Lynn G. Baird, Ph.D. | | — | | — | | 49,011 / 93,489 | | 132,455 / 402,220 |
| David B. Patteson | | 62,000 | | 223,226 | | 155,793 / - | | 658,200 / - |
| Jack H. Morgan | | — | | — | | 74,583 / 95,417 | | 128,709 / 374,041 |
- (a)
- Based on the difference between the exercise price of the option and the $8.15 closing price of the underlying Common Stock on December 31, 2003.
Executive Employment Agreements
Mr. Galliker and Dr. Baird each has an agreement with us under which they are entitled to certain benefits under particular conditions if they are terminated in connection with, or within twelve months after, a change in control of Dyax. Under the agreements, each officer is entitled to receive, as severance, his or her base salary for a period of six months if they are terminated without cause, or if they resign for good reason due to a the material diminution of their duties, a reduction in their base salary, or a relocation of their place of business that is more than 50 miles from their prior place of business. Additionally, following the termination of any of the officers' employment, that person's outstanding unvested options will be fully accelerated and he or she will also be entitled to receive full benefits during that time, as well as outplacement services. In addition, each of the officers is entitled to these benefits if we terminate their employment within 90 days prior to a change in control, where their termination was a condition to the change in control transaction.
Under his 1999 employment agreement, Mr. Galliker is entitled to receive a minimum base salary of $185,000. We also pay Mr. Galliker a housing allowance of no more than $15,000 a year. If we terminate Mr. Galliker without cause, we must continue to pay him at his current salary for six months, reduced by any compensation that Mr. Galliker earns for other work performed during this six-month period.
20
Under his 2001 employment agreement, Dr. Williams is entitled to receive a base salary of $240,000, which is subject to annual review. If we terminate Dr. Williams without cause or, following a change in control of the Company, he is terminated or quits because the terms of his employment have been adversely changed, we must continue to pay him at his current salary for six months.
Under her 2001 employment agreement, Dr. Baird is entitled to receive a base salary of $205,000, which is subject to annual review. If we terminate Dr. Baird without cause or, following a change of control of the Company, she is terminated or quits because the terms of her employment have been adversely changed, we must continue to pay her at her current salary for six months.
In preparation of the sale of Biotage, we entered into an agreement with Mr. Patteson that provided him with a retention bonus based upon the purchase price received by Dyax in any sale of Biotage and his continual employment with the Company through the date of sale. Under this agreement, Mr. Patteson received $278,547 and will be entitled to receive up to approximately $60,000 from any refund distributed to Dyax from the sale escrow. Prior to our sale of Biotage, Inc. to Pyrosequencing AB, Mr. Patteson had an agreement with us under which he was entitled to certain benefits under particular conditions if he was terminated in connection with a change in control of Biotage. Upon the sale of Biotage, Pyrosequencing assumed the obligations to Mr. Patteson under this employment agreement.
In connection with Mr. Morgan's departure, we entered into an agreement with Mr. Morgan under which we agreed to pay him his monthly base salary for six months and to continue to make such payments for an additional six months if he has not by that time found comparable employment with compensation that was not materially less than his base salary and bonus with Dyax. We also agreed to provide Mr. Morgan with health and dental benefits through this period of time. Additionally, all of Mr. Morgan's stock options will continue to vest for a period of six months following his departure and he is permitted to exercise any vested options until 90 days after the end of that six month period. In consideration for these terms, Mr. Morgan agreed to execute a general release and to not compete with us or solicit our employees for a period of one year from the date of his termination.
Report of the Audit Committee
The members of the Audit Committee are David McLachlan (Chair), Henry Lewis, Thomas Kempner and Mary Ann Gray, who joined the committee in March 2004. In the course of its oversight of Dyax's financial reporting process, the Audit Committee of the Board of Directors has:
- •
- reviewed and discussed with management and PricewaterhouseCoopers LLP, Dyax's auditor, Dyax's audited financial statements for the fiscal year ended December 31, 2003;
- •
- discussed with the auditor the matters required to be discussed by Statement on Auditing Standards No. 61,Communication with Audit Committees;
- •
- received the written disclosures and the letter from the auditor required by Independence Standards Board Standard No. 1,Independence Discussions with Audit Committees;
- •
- reviewed with management and the auditor Dyax's critical accounting policies;
- •
- discussed with management the quality and adequacy of Dyax's internal controls;
- •
- discussed with the auditor any relationships that may impact their objectivity and independence; and
- •
- considered whether the provision of non-audit services by the auditor is compatible with maintaining the auditor's independence.
21
Based on the foregoing review and discussions, the Committee recommended to the Board of Directors that the audited financial statements be included in Dyax's Annual Report on Form 10-K for the year ended December 31, 2003 for filing with the Securities and Exchange Commission.
The Committee has also reviewed, and upon its recommendation, the Nominating and Governance has approved a revision of the Audit Committee Charter. The current form of the Audit Committee Charter is attached to this proxy statement asAppendix A.
Information Concerning Our Auditors
The firm of PricewaterhouseCoopers LLP, independent accountants, examined our financial statements for the year ended December 31, 2003. The Board of Directors has appointed PricewaterhouseCoopers LLP to serve as our independent auditors for its fiscal year ending December 31, 2004. Representatives of PricewaterhouseCoopers LLP are expected to attend the annual meeting to respond to appropriate questions, and will have the opportunity to make a statement if they desire.
The following table shows the fees paid or accrued by us for the audit and other services provided by PricewaterhouseCoopers LLP for fiscal years 2003 and 2002:
| | 2003
| | 2002
|
|---|
| Audit Fees(1) | | $ | 390,585 | | $ | 317,638 |
| Audit-Related Fees(2) | | $ | 40,000 | | $ | 17,000 |
| Tax Fees(3) | | $ | 53,666 | | $ | 31,947 |
| All Other Fees(4) | | | 1,400 | | | — |
| | |
| |
|
| Total | | $ | 485,651 | | $ | 366,585 |
- (1)
- Audit fees represent fees for professional services provided in connection with the audit of our financial statements and review of our quarterly financial statements and audit services provided in connection with other statutory or regulatory filings.
- (2)
- Audit-related fees consisted primarily of accounting consultations, services related to a business divestiture and other attestation services.
- (3)
- For fiscal 2003 and 2002, tax fees principally included tax advice and tax planning fees. Tax fees for 2003 included $33,059 in connection with the sale of our subsidiary, Biotage.
- (4)
- All other fees include technical research materials.
Our Audit Committee has adopted procedures requiring its pre-approval of all non-audit (including tax) services performed by the independent auditor in order to assure that these services do not impair the auditor's independence. These procedures generally approve the performance of specific services subject to a cost limit for all such services. This general approval is to be reviewed, and if necessary modified, at least annually. Management must obtain the specific prior approval of the Audit Committee for each engagement of the independent auditor to perform other audit-related or other non-audit services. The Audit Committee does not delegate its responsibility to approve services performed by the independent auditor to any member of management.
22
The standard applied by the Audit Committee in determining whether to grant approval of any type of non-audit service, or of any specific engagement to perform a non-audit service, is whether the services to be performed, the compensation to be paid therefor and other related factors are consistent with the independent auditor's independence under guidelines of the Securities and Exchange Commission and applicable professional standards. Relevant considerations include whether the work product is likely to be subject to, or implicated in, audit procedures during the audit of our financial statements, whether the independent auditor would be functioning in the role of management or in an advocacy role, whether the independent auditor's performance of the service would enhance our ability to manage or control risk or improve audit quality, whether such performance would increase efficiency because of the independent auditor's familiarity with our business, personnel, culture, systems, risk profile and other factors, and whether the amount of fees involved, or the non-audit services portion of the total fees payable to the independent auditor in the period would tend to reduce the independent auditor's ability to exercise independent judgment in performing the audit.
All of the non-audit services rendered by PricewaterhouseCoopers LLP with respect to the 2003 fiscal year were pre-approved by the Audit Committee in accordance with this policy.
Other Matters
The Board of Directors does not know of any business to come before the meeting other than the matters described in the notice. If other business is properly presented for consideration at the meeting, the enclosed proxy authorizes the persons named therein to vote the shares in their discretion.
Shareholder Recommendations for Director Nominations
Any shareholder wishing to recommend a director candidate for consideration by the Nominating and Governance Committee should provide the following information to the Chair of the Nominating and Governance Committee, Dyax Corp., 300 Technology Square, Cambridge, Massachusetts 02139: (a) a brief statement outlining the reasons the nominee would be an effective director for Dyax; (b) (i) the name, age, and business and residence addresses of the candidate, (ii) the principal occupation or employment of the candidate for the past five years, as well as information about any other board of directors and board committee on which the candidate has served during that period, (iii) the number of shares of Dyax stock, if any, beneficially owned by the candidate and (iv) details of any business or other significant relationship the candidate has ever had with Dyax; and (c) (i) the shareholder's name and record address and the name and address of the beneficial owner of Dyax shares, if any, on whose behalf the proposal is made and (ii) the number of shares of Dyax stock that the shareholder and any such other beneficial owner beneficially own. The Committee may seek further information from or about the shareholder making the recommendation, the candidate, or any such other beneficial owner, including information about all business and other relationships between the candidate and the shareholder and between the candidate and any such other beneficial owner.
Deadline for Stockholder Proposals and Director Nominations
In order for a stockholder proposal to be considered for inclusion in Dyax's proxy materials for the 2005 Annual Meeting of Stockholders, it must be received by Dyax at 300 Technology Square, Cambridge, Massachusetts 02139 (or such other address as is listed as Dyax's primary executive offices in its periodic reports under the Securities Exchange Act of 1934) no later than December 6, 2004.
In addition, Dyax's Bylaws require a stockholder who wishes to bring business before or propose director nominations at an annual meeting to give advance written notice to Dyax's Secretary between March 21, 2005 and April 5, 2005 (assuming the 2005 annual meeting of stockholders is held on May 20, 2005).
23
Expenses Of Solicitation
We will bear the cost of the solicitation of proxies, including the charges and expenses of brokerage firms and others of forwarding solicitation material to beneficial owners of Common Stock. We have retained Georgeson Shareholder to assist with the solicitation of proxies for a fee of approximately $7,500, plus reimbursement of out-of-pocket expenses. In addition to the use of mails, proxies may be solicited by officers and any of our regular employees in person or by telephone, facsimile and e-mail.
24
Appendix A
DYAX CORP.
Audit Committee Charter
Purpose
The purpose of the Audit Committee (the "Committee") of the Board of Directors (the "Board") of Dyax Corp. is to:
- •
- Assist the Board in fulfilling its responsibility to oversee the Company's accounting and financial reporting processes and audits of the Company's financial statements;
- •
- Review the financial reports and other financial information provided by the Company, the Company's disclosure controls and procedures, and its internal accounting and financial controls;
- •
- Assume direct responsibility for the appointment, compensation, retention (and where appropriate, replacement), and oversight of the work of the outside auditor in preparing or issuing an audit report or related work;
- •
- Oversee the independence of the outside auditor and approve all auditing services and permitted non-audit services provided by the outside auditor;
- •
- Receive direct reports from the outside auditor and resolve any disagreements between management and the outside auditor regarding financial reporting; and
- •
- Carry out the specific responsibilities set forth below in furtherance of this stated purpose.
Committee Membership and Procedures
Committee members shall be appointed by the Board. The Board shall designate one member of the Committee as its Chair.
The Committee shall have at least three directors, all of whom satisfy the independence requirements of The NASDAQ Stock Marketand:
- •
- Have no relationship to the Company that may interfere with the exercise of their independent judgment;
- •
- Do not receive, directly or indirectly, any consulting, advisory or other compensatory fee from the Company, other than in the member's capacity as a member of the Board or any of its committees;
- •
- Are not "affiliated persons" (as defined by applicable law or regulation) of the Company or any subsidiary, other than as members of the Board or any of its committees; and
- •
- Are able to read and understand fundamental financial statements in accordance with the requirements of The NASDAQ Stock Market.
In addition, at least one member of the Committee will have sufficient accounting or related financial management expertise and experience to be designated an "audit committee financial expert" (as that term is defined by the Securities and Exchange Commission (the "SEC")) and to satisfy any other requirements of The NASDAQ Stock Market regarding financial sophistication.
The Committee shall conduct its meetings in accordance with this Charter, the procedures of the Board set forth in the by-laws for the Board's meetings, and such other procedures as the Committee may adopt.
A-1
Resources and Authority
In discharging its oversight role, the Committee is granted the authority to investigate any matter brought to its attention with full access to all books, records, facilities and personnel of the Company and the authority to engage independent legal, accounting or other advisors to obtain such advice and assistance as the Committee determines necessary to carry out its duties. The Committee may request any officer or employee of the Company or the Company's outside counsel to attend a meeting of the Committee or to meet with any member of, or consultants to, the Committee.
The Company shall provide the Committee all appropriate funding, as determined by the Committee, for payment of compensation to any such advisors and any outside auditor, as well as for any ordinary administrative expenses of the Committee that it determines are necessary or appropriate in carrying out its responsibilities.
Key Responsibilities
The Committee's role is one of oversight, and it is recognized that the Company's management is responsible for preparing the Company's financial statements and that the outside auditor is ultimately accountable to the Board and the Committee, as representatives of the stockholders, and is responsible for auditing those financial statements.
The following functions shall be the common recurring activities of the Committee in carrying out its oversight role. The functions are set forth as a guide and may be varied and supplemented from time to time as appropriate under the circumstances.
Appointment of Outside Auditor. The Committee shall have direct responsibility for the appointment, compensation, retention (and where appropriate, replacement), and oversight of the work of any registered public accounting firm selected to be the Company's outside auditor for the purpose of preparing or issuing an audit report or performing other audit, review or attest services for the Company.
Disclosure Controls and Procedures. The Committee shall review periodically with management the Company's disclosure controls and procedures.
Internal Controls. The Committee shall discuss periodically with management and the outside auditor the quality and adequacy of the Company's internal controls and internal auditing procedures, if any, including any significant deficiencies in the design or operation of those controls which could adversely affect the Company's ability to record, process, summarize and report financial data and any fraud, whether or not material, that involves management or other employees who have a significant role in the Company's internal controls, and discuss with the outside auditor how the Company's financial systems and controls compare with industry practices.
Accounting Policies. The Committee shall review periodically with management and the outside auditor the quality, as well as acceptability, of the Company's accounting policies, and discuss with the outside auditor how the Company's accounting policies compare with those in the industry and all alternative treatments of financial information within generally accepted accounting principles that have been discussed with management, the ramifications of use of such alternative disclosures and treatments and the treatment preferred by the outside auditor.
Pre-approval of All Audit Services and Permitted Non-Audit Services. The Committee shall approve, in advance, all audit services and all permitted non-audit services to be provided to the Company by the outside auditor.
A-2
Annual Audit. In connection with the annual audit of the Company's financial statements, the Committee shall:
- •
- request from the outside auditor a formal written statement delineating all relationships between the auditor and the Company consistent with Independence Standards Board Standard No. 1 and such other requirements as may be established by the Public Company Accounting Oversight Board, discuss with the outside auditor any such disclosed relationships and their impact on the outside auditor's objectivity and independence, and take appropriate action to oversee the independence of the outside auditor.
- •
- approve the selection and the terms of the engagement of the outside auditor.
- •
- review with management and the outside auditor the audited financial statements to be included in the Company's Annual Report on Form 10-K and the Annual Report to Stockholders, and review and consider with the outside auditor the matters required to be discussed by Statement on Auditing Standards No. 61.
- •
- perform the procedures set forth below in"Financial Reporting Procedures" with respect to the annual financial statements to be reported.
- •
- review with management and the outside auditor the Company's critical accounting policies and practices.
- •
- recommend to the Board whether, based on the reviews and discussions referred to above, the annual financial statements should be included in the Company's Annual Report on Form 10-K.
Quarterly Reports. In connection with the Company's preparation of its interim financial information to be included in the Company's Quarterly Reports on Form 10-Q, the Committee shall
- •
- review with the outside auditor the Company's interim financial information and the matters required to be discussed by SAS No. 61.
- •
- perform the procedures set forth below in "Financial Reporting Procedures" with respect to the interim financial information to be reported.
- •
- by action of a majority of the Committee or through the Committee Chair, review with the outside auditor, prior to filing with the SEC, the Company's interim financial information to be included in the Company's Quarterly Reports on Form 10-Q.
Financial Reporting Procedures. In connection with the Committee's review of each reporting of the Company's annual or interim financial information, the Committee shall:
- •
- discuss with the outside auditor whether all material correcting adjustments identified by the outside auditor in accordance with generally accepted accounting principles and the rules of the SEC are reflected in the Company's financial statements.
- •
- review with the outside auditor all material communications between the outside auditor and management, such as any management letter or schedule of unadjusted differences.
- •
- review with management and the outside auditor any material financial or other arrangements of the Company which do not appear on the Company's financial statements and any transactions or courses of dealing with third parties that are significant in size or involve terms or other aspects that differ from those that would likely be negotiated with independent parties, and which arrangements or transactions are relevant to an understanding of the Company's financial statements.
- •
- resolve any disagreements between management and the outside auditor regarding financial reporting.
A-3
Charter. The Committee shall review and reassess at least annually the adequacy of this Charter and recommend any proposed changes to the Board for approval.
Complaint Procedures
Any issue of significant financial misconduct shall be brought to the attention of the Committee for its consideration. In this connection, the Committee shall establish procedures for (i) the receipt, retention and treatment of complaints received by the Company regarding accounting, internal accounting controls or auditing matters and (ii) the confidential, anonymous submission by employees of the Company of concerns regarding questionable accounting or auditing matters.
A-4
Appendix B
DYAX CORP.
Nominating and Governance Committee Charter
Purpose.
The purpose of the Nominating and Governance Committee (the "Committee") of the Board of Directors is to:
- •
- Identify individuals qualified to become Board members and recommend to the Board the director nominees for the next annual meeting of shareholders and candidates to fill vacancies on the Board;
- •
- Recommend to the Board the directors to be appointed to Board committees;
- •
- Develop and recommend to the Board a set of corporate governance guidelines applicable to the Board and the Company;
- •
- Oversee the effectiveness of the Company's corporate governance in accordance with the guidelines; and
- •
- Carry out the specific responsibilities set forth below in furtherance of this stated purpose.
Committee Membership and Procedures.
The Committee shall have at least three directors who satisfy the independence requirements of the The NASDAQ Stock Market. Committee members shall be appointed by the Board. The Board shall designate one member of the Committee as its Chairman.
The Committee shall meet at least once each year and hold such other meetings from time to time as may be called by its Chairman or any two members of the Committee.
The Committee shall conduct its meetings in accordance with this Charter, the procedures of the Board set forth in the by-laws for the Board's meetings, and such other procedures as the Committee may adopt.
Committee Authority and Responsibilities.
Nominees for Election as Directors. The Committee shall recommend to the Board the director nominees for the next annual meeting of shareholders and persons to fill vacancies on the Board that occur between meetings of shareholders. In carrying out this responsibility, the Committee shall:
- •
- Develop criteria for selecting directors and, when appropriate, conduct searches for prospective Board members whose skills and attributes meet those criteria;
- •
- Consider nominees submitted to the Board by shareholders; and
- •
- Prior to recommending a nominee for election, determine that the election of the nominee as a director would effectively further the policies set forth in the corporate governance guidelines.
Appointments to Board Committees. At least annually, the Committee shall review and recommend to the Board the directors to be appointed to the various committees of the Board and the Chair of each committee. The Committee shall consider the desired qualifications for membership on each committee, the availability of each director to meet the time commitment required for membership on the particular committee, and the extent to which there should be rotation of committee members.
B-1
Evaluations. The Committee shall oversee the process of evaluations of the Board, its committees and executive management of the Company, and make recommendations to the Board as appropriate.
Corporate Governance Guidelines. The Committee shall recommend to the Board a set of corporate governance guidelines that are appropriate for the Company and in compliance with applicable laws, regulations and listing requirements of The Nasdaq Stock Market, Inc.. The Committee shall periodically review the Company's corporate governance guidelines for the purposes of:
- •
- Determining whether the guidelines are being effectively adhered to and implemented;
- •
- Ensuring that they continue to be appropriate for the Company and comply with applicable laws, regulations and listing requirements; and
- •
- Recommending any desirable changes in the guidelines to the Board.
In addition, the Committee shall consider any other corporate governance issues that may arise from time to time, and develop appropriate recommendations to the Board.
Code of Business Conduct and Ethics. The Committee shall develop and recommend to the Board a code (or separate codes) of business conduct and ethics, and shall consider any requests for waivers thereunder or recommend to the Board delegation of authority to another committee of the Board to consider such requests. The Company shall make disclosure of such waivers as required by applicable law, regulations or listing requirements.
Board Compensation. The Committee shall advise the Board with respect to compensation of non-employee directors.
Orientation and Education. The Committee shall oversee the orientation and education of directors with respect to the Company's business and financial matters, corporate governance and other appropriate subjects.
Engagement of Advisors. The Committee shall have the sole authority to retain and terminate any search firm used to identify director candidates and shall have sole authority to approve such search firm's fees and other retention terms. The Committee shall also have authority to obtain advice and assistance from internal or external legal, accounting or other advisors. The Committee may request any officer or employee of the Company or the Company's outside counsel to attend a meeting of the Committee or to meet with any member of, or consultants to, the Committee.
Charter. The Committee shall review and reassess at least annually the adequacy of this Charter and recommend any proposed changes to the Board for approval.
B-2
(Front of Proxy Card)
DYAX CORP.
2004 ANNUAL MEETING OF STOCKHOLDERS
THIS PROXY IS BEING SOLICITED ON BEHALF OF THE BOARD OF DIRECTORS
The undersigned stockholder of Dyax Corp. hereby appoints Henry E. Blair, Stephen S. Galliker and Nathaniel S. Gardiner, or any of them, with full power of substitution in each, as proxies to cast all votes which the undersigned stockholder is entitled to cast at the annual meeting of stockholders (the "2004 Annual Meeting") to be held at 2:00 p.m., local time, on Thursday, May 20, 2004, at the offices of Dyax Corp., 300 Technology Square, Cambridge, Massachusetts 02139, and at any adjournments of the meeting, upon the following matters. The undersigned stockholder hereby revokes any proxy or proxies heretofore given.
This proxy will be voted as directed by the undersigned stockholder. Unless contrary direction is given, this proxy will be voted FOR the election of the nominees listed in Proposal 1 and FOR the amendment as described in Proposal 2, and in accordance with the determination of a majority of the Board of Directors as to any other matters. The undersigned stockholder may revoke this proxy at any time before it is voted by delivering either a written notice of revocation of the proxy or a duly executed proxy bearing a later date to the Secretary, or by attending the 2004 Annual Meeting and voting in person. The undersigned stockholder hereby acknowledges receipt of the Notice of Annual Meeting and Proxy Statement for the 2004 Annual Meeting.
If you receive more than one proxy card, please sign and return all cards in the accompanying envelope.
(CONTINUED AND TO BE DATED AND SIGNED ON REVERSE SIDE.)
(Reverse of Proxy Card)
PLEASE DATE, SIGN AND MAIL YOUR PROXY CARD BACK AS SOON AS POSSIBLE
2004 ANNUAL MEETING OF STOCKHOLDERS
DYAX CORP.
MAY 20, 2004
- ý
- PLEASE MARK YOUR VOTES AS IN THIS EXAMPLE.
- 1.
- To elect two directors to serve for three-year terms (Class I).
| | | WITHHOLD | | NOMINEES |
| FOR | | AUTHORITY | | Henry E. Blair |
| all nominees listed | | to vote for all nominees listed | | Susan B. Bayh |
| o | | o | | |
- o
- FOR, EXCEPT WITHHOLD AUTHORITY to vote for the following nominees only:
(write the name of the nominee(s) in the space below),
- 2.
- To amend Dyax's Restated Certificate of Incorporation to increase the number of authorized shares of our common stock by 75,000,000 shares from 50,000,000 shares to 125,000,000 shares.
| o FOR | | o AGAINST | | o ABSTAIN |
SIGNATURE(S) OF STOCKHOLDER(S) OR AUTHORIZED REPRESENTATIVE(S)
| NOTE: | | Please date and sign exactly as your name(s) appear(s) hereon. Each executor, administrator, trustee, guardian, attorney-in-fact and other fiduciary should sign and indicate his or her full title. When stock has been issued in the name of two or more persons, all should sign. |
|
|
|
|
|
|
|
| | | Date: | | | | , 2004 |
| | | |
| | |
| Signature(s) of Stockholder(s) or Authorized Representative(s) | | | | | | |
QuickLinks
DYAX CORP. 300 TECHNOLOGY SQUARE CAMBRIDGE, MA 02139 (617) 250-5500NOTICE OF ANNUAL MEETING OF STOCKHOLDERS To Be Held May 20, 2004PROPOSAL 1 ELECTION OF DIRECTORSPROPOSAL 2 APPROVAL OF AN AMENDMENT TO OUR RESTATED CERTIFICATE OF INCORPORATIONEXECUTIVE OFFICERS AND KEY EMPLOYEESEXECUTIVE COMPENSATION Compensation Committee Report on Executive CompensationComparison of Cumulative Total Return of Dyax Corp., Nasdaq Stock Market (U.S. Companies) Index and Nasdaq Pharmaceuticals IndexReport of the Audit CommitteeDYAX CORP. Audit Committee CharterDYAX CORP. Nominating and Governance Committee Charter
