Exhibit 99.10
POSTER P2123
Efficacy and safety of once-daily fluticasone furoate (FF) in patients with persistent asthma: a 24-week randomised trial
Lötvall J(1), Bleecker ER(2), Busse WW(3), O’Byrne PM(4), Woodcock A(5), Bateman ED(6), Kerwin EM(7), Stone S(8), Forth R(9), Jacques L(8)
(1)Krefting Research Centre, University of Gothenburg, Sweden; (2)Center for Genomics and Personalized Medicine, Wake Forest University Health Sciences Winston-Salem, NC, USA; (3)Department of Medicine, University of Wisconsin, Madison, WI, USA; (4)Michael G DeGroote School of Medicine, Hamilton, Ontario, Canada; (5)School of Translational Medicine, University of Manchester, Manchester, UK; (6)Department of Medicine, University of Cape Town, Cape Town, South Africa; (7)Clinical Research Institute of Southern Oregon, Medford, OR, USA; (8)Respiratory Medicine Development Centre, GlaxoSmithKline, London, UK; (9)Quantitative Sciences Division, GlaxoSmithKline, Research Triangle Park, NC, USA
INTRODUCTION
· Inhaled corticosteroids (ICS) are considered the most effective therapy for all severities of persistent asthma.(1)
· FF is a new ICS under development as once-daily monotherapy for asthma and in combination with the long-acting beta2 agonist vilanterol as a once-daily treatment for asthma and COPD.
OBJECTIVES
· To evaluate the efficacy and safety of once-daily FF 100mcg vs. placebo, with twice-daily fluticasone propionate (FP) 250mcg as an active control, in patients with persistent asthma uncontrolled on a stable low-to-mid dose of ICS (<500mcg FP equivalent total daily dose).
METHODS
· Multi-centre, randomised, placebo-controlled, double-blind, double-dummy, parallel-group study.
· Patients: age >12 years; asthma for >12 weeks; pre-bronchodilator FEV1 40–90% predicted; >12% and >200mL evening reversibility of FEV1 following salbutamol.
· Following a 4-week run-in, patients were randomised (1:1:1) to receive one of the following for 24 weeks
· FF 100mcg once daily (evening dosing) via novel dry powder inhaler
· FP 250mcg twice daily via DISKUS™/ACCUHALER™
· placebo.
RESULTS
Study population and demographics (Table 1)
· Of 1036 patients screened, 349 were randomised (ITT population=343) and 255 completed the study.
Efficacy
· Mean SE change from baseline in trough evening (pre-bronchodilator and pre-dose) FEV1 at 24 weeks (primary endpoint; ANCOVA analysis): FF 161mL (39.8), FP 159mL (40.6), placebo 15mL (39.4)
· treatment differences (95% CI) vs. placebo were statistically significant: FF 146mL (36, 257; p=0.009), FP 145mL (33, 257; p=0.011)
· Fig. 1 shows the 24-week time course for trough FEV1 (repeated measures analysis).
Table 1. Baseline characteristics (ITT population)
|
| Placebo |
| FF 100 OD |
| FP 250 BD |
|
|
| N=115 |
| N=114 |
| N=114 |
|
Age (yr) |
| 40.3 (17.68) |
| 40.1 (16.17) |
| 41.4 (15.64) |
|
Female, % |
| 59 |
| 55 |
| 63 |
|
% Predicted baseline FEV1 |
| 72.32 (10.871) |
| 72.18 (10.387) |
| 73.04 (11.936) |
|
% Reversibility |
| 25.43 (12.959) |
| 27.32 (15.252) |
| 25.07 (14.537) |
|
% Symptom-free 24h, mean |
| 3.9 |
| 7.9 |
| 7.0 |
|
% Rescue-free 24h, mean |
| 18.5 |
| 13.3 |
| 17.1 |
|
Values are mean (SD) unless otherwise stated
· Significant increase from baseline in % rescue-free 24-h periods (powered secondary endpoint) with FF (14.8%) and FP (17.9%) vs. placebo (both p<0.001)
· the 24-week time course is illustrated in Fig. 2.
· Numerical treatment differences in favour of FF and FP vs. placebo for secondary endpoints
· evening PEF, L/min (95% CI)*†: FF=5.8 (–1.9, 13.6); FP=8.3 (0.6, 16.1)
· morning PEF, L/min (95% CI)*†: FF=12.1 (4.0, 20.2); FP=7.6 (–0.5, 15.7)
· symptom-free 24-h periods, % (95% CI)*: FF=8.9 (1.1, 16.7); FP=8.8 (1.1, 16.6)
· change from baseline in Total AQLQ12+ at Week 24, score (95% CI)*: FF=0.33 (0.09, 0.57); FP=0.16 (–0.08, 0.41).
· Change from baseline in Asthma Control Test™ (‘Other’ endpoint) at Week 24, score (95% CI)*: FF=1.4 (0.4, 2.5); FP=1.1 (0.1, 2.1).
Safety
· On-treatment and treatment-related adverse events (AEs) were higher with FF vs. FP and placebo (Table 2).
· Most frequently reported treatment-related AEs were oral candidiasis and oropharyngeal candidiasis (for both: FF 3%, FP <1%, placebo 0%) (oropharyngeal examinations were performed at each clinic visit).
*A lack of significance in the original analysis (including outlier†) in evening PEF between FF 100mcg and placebo meant that statistical inference could not be drawn on the subsequent efficacy endpoints in the hierarchy (daily AM PEF, symptom-free 24-h periods and AQLQ12+ score). Confidence intervals for comparisons are shown to describe the data more fully
†Data are from a sensitivity analysis with an outlier removed (due to a malfunctioning mouthpiece on the peak flow meter)
· Incidence of on-treatment severe asthma exacerbations: FF 3%, FP 2%, placebo 7%; none resulted in hospitalisation.
· Statistically significant urinary cortisol suppression was seen with FF (ratio=0.76; p=0.030) and FP (0.77; p=0.036) relative to placebo.
Figure 1. Repeated measures analysis of change from baseline in trough FEV1 over 24 weeks (ITT Population)

Figure 2. Change from baseline in % rescue-free 24h periods over time (ITT population)
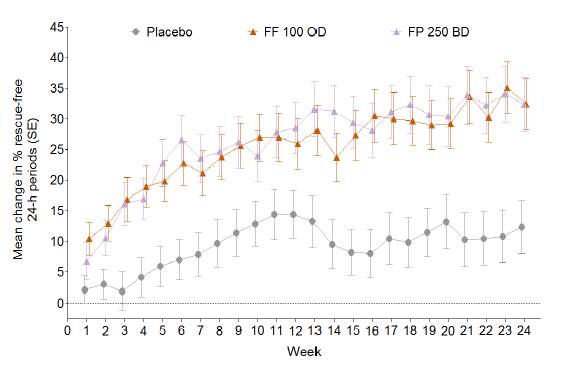
Table 2. Summary of AEs and frequent on-treatment AEs (ITT population)
|
| Placebo |
| FF 100 OD |
| FP 250 BD |
|
n (%) |
| (N=115) |
| (N=114) |
| (N=114) |
|
On-treatment AEs |
| 46 (40%) |
| 60 (53%) |
| 48 (42%) |
|
Bronchitis |
| 7 (6%) |
| 8 (7%) |
| 4 (4%) |
|
Headache |
| 5 (4%) |
| 7 (6%) |
| 7 (6%) |
|
Nasopharyngitis |
| 6 (5%) |
| 9 (8%) |
| 4 (4%) |
|
Upper respiratory tract infection |
| 6 (5%) |
| 7 (6%) |
| 6 (5%) |
|
Drug-related AEs |
| 7 (6%) |
| 11 (10%) |
| 7 (6%) |
|
AEs leading to permanent discontinuation of study drug or withdrawal from study |
| 2 (2%) |
| 3 (3%) |
| 3 (3%) |
|
On-treatment SAEs* |
| 2 (2%) |
| 4 (4%) |
| 1 (<1%) |
|
AEs occurring in >5% of patients in any treatment group are presented
*There were 9 on-treatment SAEs in 7 patients (none were fatal or considered treatment-related): FF: abscess, Crohn’s disease, epididymal cyst, Escherichia bacteraemia, prostate cancer and pyelonephritis; FP: supraventricular tachycardia; placebo: meningitis and pyelonephritis
CONCLUSIONS
· FF 100mcg administered once a day significantly improved trough FEV1 to a similar extent to FP 250mcg twice a day, and reduced rescue medication use relative to placebo.
· FF was well tolerated and had a similar AE profile to FP.
REFERENCE
(1) Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention 2011. http://www.ginasthma.org/Guidelines/guidelines-resources.html (accessed 14 August 2012).
ACKNOWLEDGEMENTS
· The presenting author, J Lötvall, declares the following real or perceived conflicts of interest during the last 3 years in relation to this presentation: has served as a consultant to and received lecture fees from AstraZeneca, GlaxoSmithKline, Merck Sharpe and Dohme, Novartis and UCB Pharma; has been partly covered by some of these companies to attend previous scientific meetings including the ERS and the AAAAI; and has participated in clinical research studies sponsored by AstraZeneca, GlaxoSmithKline, Merck Sharpe and Dohme, and Novartis.
· This study was funded by GlaxoSmithKline; GSK Study Code FFA112059, Clinicaltrials.gov NCT01159912.
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Tom Gallagher at Gardiner-Caldwell Communications and was funded by GlaxoSmithKline.

Presented at the European Respiratory Society Annual Congress 2012 Vienna, Austria, 1–5 September, 2012