- INVA Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Innoviva (INVA) 8-KOther Events
Filed: 4 Sep 12, 12:00am
Exhibit 99.14
POSTER P2161
Efficacy of fluticasone furoate (FF) and vilanterol (VI), separately and in combination (FF/VI), in an allergen challenge model
Oliver A(1), Bjermer L(2), Quinn D(3), Saggu P(1), Thomas P(4), Lötvall J(5)
(1)GlaxoSmithKline, Uxbridge, UK; (2)Skane University Hospital, Lund, Sweden; (3)P3 Research, Wellington, NZ; (4)The University of New South Wales, Sydney, Australia; (5)*University of Gothenburg, Gothenburg, Sweden
INTRODUCTION
· In sensitised asthma patients the response to allergen exposure is often evident as a bi-phasic decline in lung function.
· The early asthmatic response (EAR) starts shortly after a single inhaled allergen challenge, and the late asthmatic response (LAR) commences 2–4h later;(1),(2) the LAR is associated with development of non-specific airway hyper-responsiveness (AHR).(3)
· FF(4) and VI(5) are promising agents for a combined, long-acting, once-daily ICS/LABA treatment of asthma.
OBJECTIVES
· Primary: to compare the effect of FF/VI combination on EAR (vs. FF or VI monotherapy) and LAR (vs. placebo).
· Secondary: to compare the effects of treatments on AHR (more detailed results presented separately).(6)
METHODS
· Randomised, double-blind, 4-way complete crossover study.
· Following a 2-week run-in, patients received 21-days treatment administered in the morning via a novel dry powder inhaler (Fig. 1).
· Forced expiratory volume in 1 sec (FEV1) was measured at 5, 10, 15, 20, 30, 45, 60 minutes and every 30 minutes until 10h post-final dose of allergen
· EAR: minimum (min) FEV1 (0–2h post-allergen challenge); LAR: wmFEV1 (4–10h)
· treatment differences were assessed by a mixed effects ANCOVA model.
Figure 1. Study design

F/V = FF/VI 100/25mcg; F = FF 100mcg; V = VI 25mcg; P = Placebo; R = Randomisation; F-U = Follow-Up
* Allergen challenge on Day 21, 1h post-final dose
† Assessment of AHR on Day 22, 24-h post-allergen challenge (25h post-dose) using doubling concentrations of methacholine (MCh) to induce a 20% fall in FEV1 (PC20)
RESULTS
Study population and demographics
· Patient demographics, baseline lung function and allergen details are summarised in Table 1.
· Of the 27 randomised patients, 26 completed the study; one withdrew consent and four protocol deviations during treatment Period 1 (received incorrect allergen bolus dose) led to those data being excluded from the analysis.
Table 1. Patient demographics, baseline lung function and allergen details
Patient demographics
Age (years), mean (range) |
| 30.8 (18–49) |
|
Female, n (%) |
| 8 (30) |
|
White race, n (%) |
| 25 (93) |
|
Lung function
Pre-bronchodilator FEV1 (L), mean (range) |
| 3.7 |
|
|
| (2.7–5.0) |
|
Pre-bronchodilator FEV1 (% pred), mean (range) |
| 92.3 |
|
|
| (71.3–119.8) |
|
Methacholine PC20 , mg/mL |
| <8 |
|
Allergen, n (%)
House dust mite |
| 15 (56) |
|
Cat hair/dander |
| 10 (37) |
|
Birch tree |
| 1 (4) |
|
Grass pollen |
| 1 (4) |
|
Pre-challenge lung function
· FEV1 improved from Day 1 to 21 prior to allergen challenge with FF/VI (230mL [145, 315]), FF (116mL [30, 202]) and VI (183mL [95, 272]) but declined by 61mL [–147, 24] with placebo (Fig. 2).
Allergen challenge
· At all time points assessed, FF/VI generally exhibited the greatest mean attenuation of the allergen-induced response (Fig. 2).
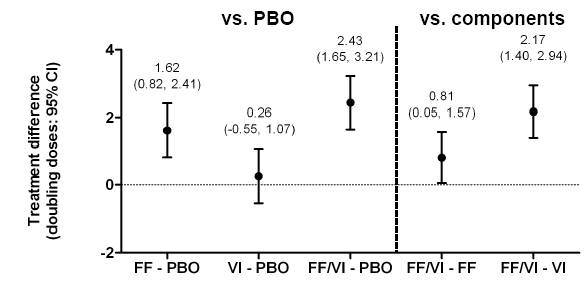
· FF/VI and FF were superior to placebo on the EAR and LAR; VI was superior to placebo on the LAR only. FF/VI was superior to FF and VI on the EAR and to VI on the LAR (Fig. 3).
· Alleviation of the AHR relative to placebo was seen with FF/VI and FF, but not with VI. FF/VI was superior to FF and VI (Fig. 4).
Figure 2. Absolute FEV1 from Day 1 to 21, over the allergen challenge time course, up to pre-methacholine challenge on Day 22

Figure 3. Treatment differences for allergen challenge on (a) the EAR assessed by minimum FEV1 (minFEV1) and (b) the LAR assessed by wmFEV1

PBO=placebo
Figure 4. Treatment differences for doubling doses of methacholine required to achieve PC20 24h post-allergen challenge
Adverse events and withdrawals
· No serious adverse events (AE) or withdrawals were reported.
· The number of treatment-related AEs were similar between FF/VI, FF, VI and placebo.(6)
CONCLUSIONS
· FF was highly effective in reducing both the EAR and LAR, and the addition of VI to FF further reduced the allergen-induced EAR.
· Adding VI to FF also provided further reduction of AHR as measured by methacholine responsiveness 24h after the allergen provocation.
REFERENCES
(1) O’Byrne PM. Allergy Asthma Immunol Rev 2009;1:3–9.
(2) Cockroft DW, et al. Can Respir J 2007;14:414–418.
(3) Gauvreau GM, Evans MY. Contrib Microbiol 2007;14:21–32.
(4) Woodcock A, et al. Respir Res 2011;12:160.
(5) Lötvall J, et al. Eur Respir J 2012 [Epub ahead of print].
(6) Oliver A, et al. European Academy of Allergy and Clinical Immunology (EAACI) June 2012.
ACKNOWLEDGEMENTS
· The presenting author, J Lötvall, declares the following real or perceived statements of interest during the last 3 years in relation to this presentation: consultancy and lecture fees from AstraZeneca, GlaxoSmithKline, Merck Sharpe and Dohme, Novartis and UCB Pharma; partly covered by some of these companies to attend scientific meetings including the ERS and the AAAAI; and has participated in clinical trials sponsored by AstraZeneca, GlaxoSmithKline, Merck Sharpe and Dohme, and Novartis.
· This study was funded by GlaxoSmithKline; GSK Study Code HZA113126, Clinicaltrials.gov NCT01128595.
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Lisa Moore at Gardiner-Caldwell Communications and was funded by GlaxoSmithKline.
![]()
Presented at the European Respiratory Society Annual Congress 2012 Vienna, Austria, 1–5 September, 2012