- INVA Dashboard
- Financials
- Filings
-
Holdings
- Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
8-K Filing
Innoviva (INVA) 8-KOther Events
Filed: 4 Sep 12, 12:00am
Exhibit 99.13
POSTER P2090
The efficacy of inhaled fluticasone furoate (FF) and vilanterol (VI) administered in combination in asthma is comparable when administered in the morning or evening
Rodger Kempsford(1), Amanda Oliver(2), Lee Tombs(3), Joanne Bal(4)
GlaxoSmithKline R&D Respiratory Medicines Development Centre (1)Stevenage, UK and (2)Uxbridge, UK; (3)Synergy, Slough, UK; (4)GlaxoSmithKline R&D Clinical Pharmacology Science & Study Operations, Uxbridge, UK
INTRODUCTION
· FF/VI, a novel inhaled corticosteroid/long-acting beta2-agonist combination (ICS/LABA), is being developed as a once-daily inhaled treatment for asthma and COPD.
· Phase IIb studies in subjects with asthma demonstrated that both FF and VI were efficacious when dosed once daily(1),(2). Phase III clinical studies in asthma with FF/VI were conducted with evening dosing.
· This study was conducted to compare the efficacy of FF/VI when administered in the morning (AM) or evening (PM) in subjects with asthma. The results provide supporting information to understand the implications of time of dosing on the therapeutic response to FF/VI.
OBJECTIVES
· To investigate the effect of time of day of dosing (AM or PM) on the efficacy of FF/VI (100/25mcg).
METHODS
· This was a randomised, double-blind, placebo-controlled, repeat-dose, three-way crossover study.
· Subjects had asthma (>60% predicted FEV1; receiving inhaled corticosteroids [ICS] ± short-acting beta2-agonist [SABA] for 12 weeks prior to screening [total ICS dose 200-500 mcg/day fluticasone propionate or equivalent]).
· ICS were stopped and subjects were switched to SABA only for 2 weeks prior to dosing and throughout the study.
· Subjects received the following treatments for 14 (±2) days
· FF/VI 100/25 mcg once daily (AM dosing) with placebo (PM)
· FF/VI 100/25 mcg once daily (PM dosing) with placebo (AM)
· Placebo (AM and PM).
· FF/VI doses were administered at approximately 09:00 h (AM) and 21:00 h (PM). Dosing started with the PM dose on Day 1. The final FF/VI doses were administered PM on Day 14 and AM on Day 15. The washout period was 14–21 days.
· FEV1 was measured on the last study day (i.e. on the nominal ‘Day 14’ which was Day 14 (±2) days).
· FEV1 was determined every 3 h for 0–24 h on Day 14 (i.e. from approximately 21:00 h on Day 14 until 21:00 h on Day 15).
· The primary endpoint was Day 14 weighted mean FEV1 (0–24 h).
· Secondary endpoints included Day 14 trough FEV1 (AM and PM) and mean pre-treatment peak expiratory flow (PEF).
RESULTS
Table 1. Demographics (N=26)
Characteristic |
| Value |
|
Mean age, years (range) |
| 38.1 (24–64) |
|
Gender, F/M: n (%) |
| 8/18 (31/69) |
|
Height, cm: mean (range) |
| 171.5 (153–186) |
|
Race: n (%) |
|
|
|
White – White/Caucasian/European Heritage |
| 25 (96) |
|
Native Hawaiian or Other Pacific Islander |
| 1 (4) |
|
Primary endpoints
· Weighted mean FEV1 (Day 14; 0–24h) was clinically significantly higher after both AM and PM dosing with FF/VI 100/25 mcg compared with placebo (Table 2). The values were similar for AM or PM dosing and the AM-PM difference was not considered to be clinically significant.
· FF/VI dosed AM or PM resulted in clinically significant increases in FEV1 compared with placebo at all time points 0–24 h on Day 14 (Figure 1). There was no discernible difference between AM and PM dosing with FF/VI despite the final AM dose being administered in the middle of the 24 h assessment period.
· There was little evidence of diurnal variation in FEV1 (Day 14: 0–24 h) with either AM or PM dosing with FF/VI. There was no evidence of the early morning nadir in FEV1 seen with placebo between approximately 6–12 h post-PM dose (03:00 h–09:00 h; Figure 1).
Figure 1. Mean (95% CI) FEV1 (L) on Day 14 following administration of FF/VI (100/25 mcg) AM or PM and placebo in subjects with asthma (n=26)

Table 2. Summary of statistical analysis of FEV1 (L) weighted mean (Day 14; 0–24 h)
|
| Adjusted |
| Difference from |
| Difference from FF/VI PM |
|
Treatment |
| Mean (L) |
| Placebo (90% CI) |
| (90% CI) |
|
FF/VI AM |
| 3.188 |
| 0.377 (0.293, 0.462) |
| -0.044 (-0.125, 0.036) |
|
FF/VI PM |
| 3.233 |
| 0.422 (0.337, 0.507) |
| N/A |
|
Placebo |
| 2.811 |
| N/A |
| N/A |
|
N/A – not applicable
Secondary endpoints
· Mean (90% CI) AM trough FEV1 on Day 14 improved by 0.403 L (0.272, 0.533) with AM dosing compared with placebo.
· Mean (90% CI) PM trough FEV1 on Day 14 improved by 0.309 L (0.205, 0.413) with PM dosing compared with placebo.
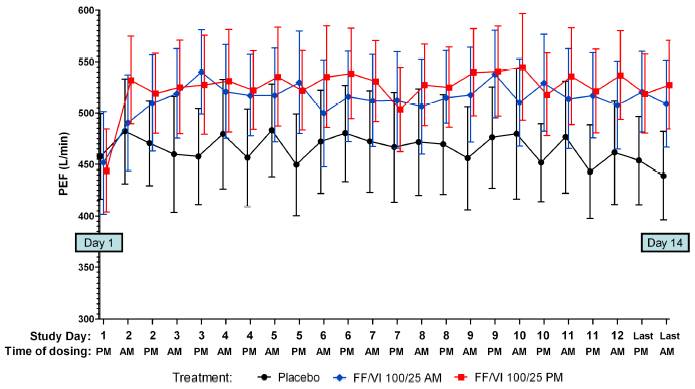
· FF/VI AM or PM produced clinically significant increases in pre-treatment PEF after the first dose, which were maintained throughout the 14 day treatment period (Figure 2).
· AM and PM dosing with FF/VI 100/25 mcg for 14 days were well tolerated in subjects with asthma. No serious adverse events were reported. There were no withdrawals due to worsening asthma or asthma adverse events.
Figure 2. Mean (95% CI) PEF (L/min) following AM or PM FF/VI (100/25 mcg) administration and placebo in subjects with asthma (Day 1-14; n=26)
CONCLUSIONS
· The efficacy of FF/VI (100/25mcg) was comparable when dosed in the morning or evening in subjects with persistent asthma.
REFERENCES
(1) Lötvall J, et al. Eur Respir J 2012 Feb 23 [Epub ahead of print].
(2) Busse WW, et al. Thorax 2012;67:35–41.
ACKNOWLEDGEMENTS
· R. Kempsford is an employee of GlaxoSmithKline.
· This study was funded by GlaxoSmithKline; GSK Study Code HZA114624, Clinicaltrials.gov NCT01287065.
· Editorial support (in the form of copyediting) was provided by Tom Gallagher at Gardiner-Caldwell Communications and was funded by GlaxoSmithKline.

Presented at the European Respiratory Society Annual Congress 2012 Vienna, Austria, 1–5 September, 2012