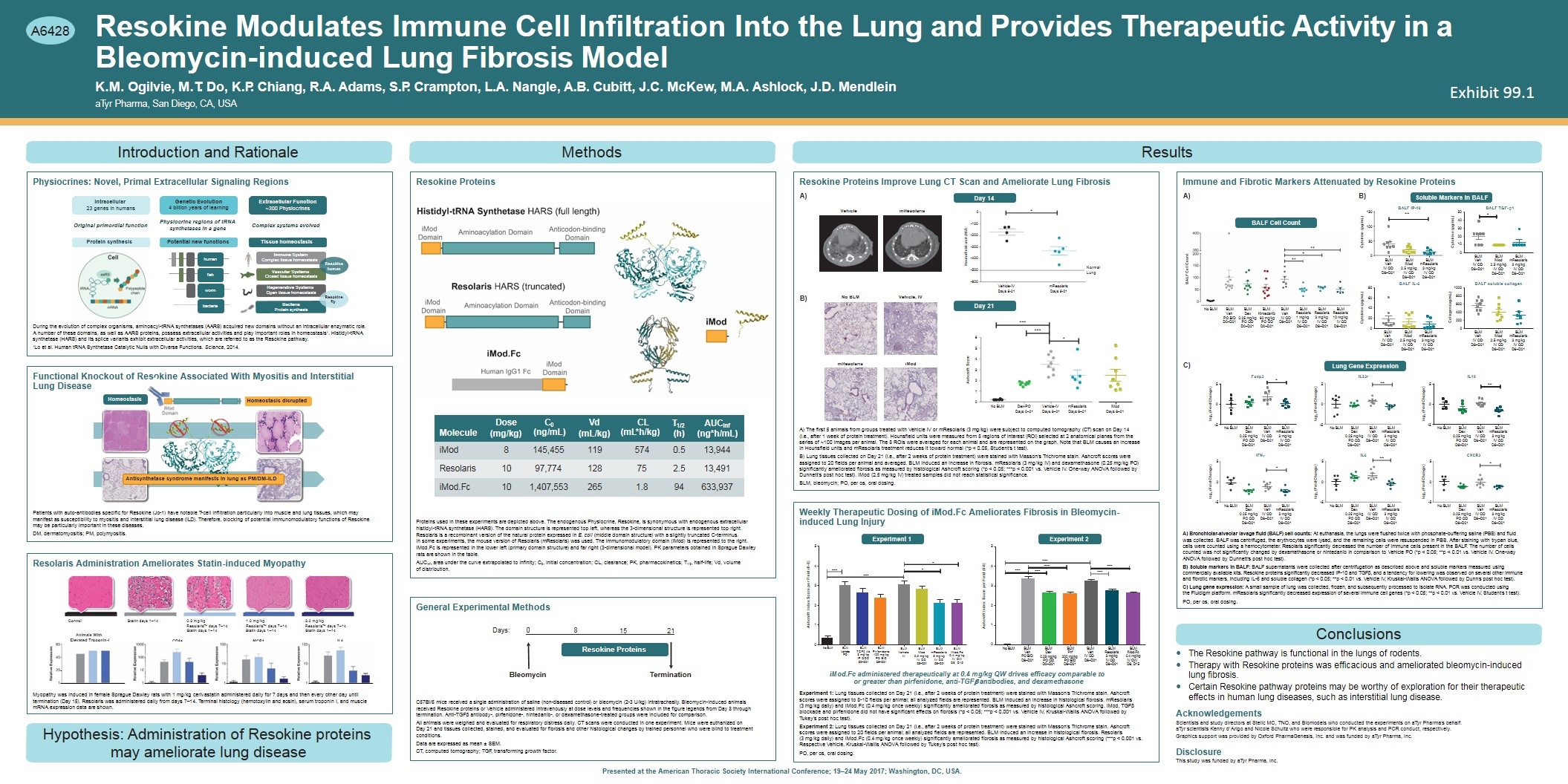
** Immune System Complex tissue homeostasis human Vascular Systems Closed tissue homeostasis Resokine Modulates Immune Cell Infiltration Into the Lung and Provides Therapeutic Activity in a Bleomycin-induced Lung Fibrosis Model K.M. Ogilvie, M.T. Do, K.P. Chiang, R.A. Adams, S.P. Crampton, L.A. Nangle, A.B. Cubitt, J.C. McKew, M.A. Ashlock, J.D. Mendlein aTyr Pharma, San Diego, CA, USA Introduction and Rationale Protein synthesis Original primordial function Intracellular 23 genes in humans Physiocrine regions of tRNA synthetases in a gene Genetic Evolution 4 billion years of learning bacteria fish worm human Regenerative Systems Open tissue homeostasis Bacteria Protein synthesis Complex systems evolved Extracellular Function ~300 Physiocrines Tissue homeostasis Potential new functions Physiocrines: Novel, Primal Extracellular Signaling Regions During the evolution of complex organisms, aminoacyl-tRNA synthetases (AARS) acquired new domains without an intracellular enzymatic role. A number of these domains, as well as AARS proteins, possess extracellular activities and play important roles in homeostasis1. Histidyl-tRNA synthetase (HARS) and its splice variants exhibit extracellular activities, which are referred to as the Resokine pathway. 1Lo et al. Human tRNA Synthetase Catalytic Nulls with Diverse Functions. Science, 2014. Homeostasis Homeostasis disrupted od Domain iM Antisynthetase syndrome manifests in lung as PM/DM-ILD Functional Knockout of Resokine Associated With Myositis and Interstitial Lung Disease Patients with auto-antibodies specific for Resokine (Jo-1) have notable T-cell infiltration particularly into muscle and lung tissues, which may manifest as susceptibility to myositis and interstitial lung disease (ILD). Therefore, blocking of potential immunomodulatory functions of Resokine may be particularly important in these diseases. DM, dermatomyositis; PM, polymyositis. Control Statin days 1–14 Animals With Elevated Troponin-I CD8a MCP1 IL6 0.3 mg/kg Resolaris™ days 7–14 Statin days 1–14 1.0 mg/kg Resolaris™ days 7–14 Statin days 1–14 3.0 mg/kg Resolaris™ days 7–14 Statin days 1–14 Myopathy was induced in female Sprague Dawley rats with 1 mg/kg cerivastatin administered daily for 7 days and then every other day until termination (Day 15). Resolaris was administered daily from days 7–14. Terminal histology (hemotoxylin and eosin), serum troponin I, and muscle mRNA expression data are shown. Resolaris Administration Ameliorates Statin-induced Myopathy Hypothesis: Administration of Resokine proteins may ameliorate lung disease Day 14 Day 21 -600 -500 -400 -300 -200 -100 0 Hounsfield unit (HU) * Vehicle-IV Days 8-21 mResolaris Days 8-21 Normal Lung No BLM Dex-PO Days 0–21 Vehicle-IV Days 8–21 mResolaris Days 8–21 0 1 2 3 4 5 6 Ashcroft Score *** *** * iMod Days 8–21 No BLM Vehicle, IV mResolaris iMod Vehicle mResolaris Resokine Proteins Improve Lung CT Scan and Ameliorate Lung Fibrosis The first 5 animals from groups treated with Vehicle IV or mResolaris (3 mg/kg) were subject to computed tomography (CT) scan on Day 14 (i.e., after 1 week of protein treatment). Hounsfield units were measured from 8 regions of interest (ROI) selected at 2 anatomical planes from the series of ~100 images per animal. The 8 ROIs were averaged for each animal and are represented on the graph. Note that BLM causes an increase in Hounsfield units and mResolaris treatment reduces it toward normal (*p < 0.05, Student’s t test). Lung tissues collected on Day 21 (i.e., after 2 weeks of protein treatment) were stained with Masson’s Trichrome stain. Ashcroft scores were assigned to 20 fields per animal and averaged. BLM induced an increase in fibrosis. mResolaris (3 mg/kg IV) and dexamethasone (0.25 mg/kg PO) significantly ameliorated fibrosis as measured by histological Ashcroft scoring (*p < 0.05; ***p < 0.001 vs. Vehicle IV. One-way ANOVA followed by Dunnett’s post hoc test). iMod (2.5 mg/kg IV) treated samples did not reach statistical significance. BLM, bleomycin; PO, per os, oral dosing. Ashcroft Index Score per Field (0-8) 0 1 2 3 4 5 *** *** *** No BLM BLM Veh PO BID D8–D21 BLM Pirf 0.25 mg/kg 200 mg/kg PO QDPO BID D0–D21 D8–D21 BLM Veh IV QD D8–D21 BLM Resolaris 3 mg/kg IV QD D8–D21 BLM iMod.Fc 0.4 mg/kg IV QW D8, D15 BLM Dex *** *** *** Experiment 1Experiment 2 iMod.Fc administered therapeutically at 0.4 mg/kg QW drives efficacy comparable to or greater than pirfenidone, anti-TGFb antibodies, and dexamethasone Experiment 1: Lung tissues collected on Day 21 (i.e., after 2 weeks of protein treatment) were stained with Masson’s Trichrome stain. Ashcroft scores were assigned to 8-10 fields per animal; all analyzed fields are represented. BLM induced an increase in histological fibrosis. mResolaris (3 mg/kg daily) and iMod.Fc (0.4 mg/kg once weekly) significantly ameliorated fibrosis as measured by histological Ashcroft scoring. iMod, TGFb blockade and pirfenidone did not have significant effects on fibrosis (*p < 0.05; ***p < 0.001 vs. Vehicle IV, Kruskal-Wallis ANOVA followed by Tukey’s post hoc test). Experiment 2: Lung tissues collected on Day 21 (i.e., after 2 weeks of protein treatment) were stained with Masson’s Trichrome stain. Ashcroft scores were assigned to 20 fields per animal; all analyzed fields are represented. BLM induced an increase in histological fibrosis. Resolaris (3 mg/kg daily) and iMod.Fc (0.4 mg/kg once weekly) significantly ameliorated fibrosis as measured by histological Ashcroft scoring (***p < 0.001 vs. Respective Vehicle, Kruskal-Wallis ANOVA followed by Tukey’s post hoc test). PO, per os, oral dosing. 0 1 2 3 4 5 *** Ashcroft Index Score per Field (0-8) * * *** BLM Vehicle IV Weekly Therapeutic Dosing of iMod.Fc Ameliorates Fibrosis in Bleomycin- induced Lung Injury Lung Gene Expression IL23r ** BALF IP-10 Cytokine (pg/mL) Cytokine (pg/mL) BALF Cell Count Cytokine (pg/mL) Collagen (ug/mL) 0 50 100 BALF IL-6 0 20 40 60 80 BALF soluble collagen 0 200 400 600 800 1000 BLM Veh IV QD D8–D21 Bronchiolar-alveolar lavage fluid (BALF) cell counts: At euthanasia, the lungs were flushed twice with phosphate-buffering saline (PBS) and fluid was collected. BALF was centrifuged, the erythrocytes were lysed, and the remaining cells were resuspended in PBS. After staining with trypan blue, cells were counted using a hemocytometer. Resolaris significantly decreased the number of immune cells present in the BALF. The number of cells counted was not significantly changed by dexamethasone or nintedanib in comparison to Vehicle PO (*p < 0.05; **p < 0.01 vs. Vehicle IV. One-way ANOVA followed by Dunnett’s post hoc test). Soluble markers in BALF: BALF supernatants were collected after centrifugation as described above and soluble markers measured using commercially available kits. Resokine proteins significantly decreased IP-10 and TGFb, and a tendency for lowering was observed on several other immune and fibrotic markers, including IL-6 and soluble collagen (*p < 0.05; **p < 0.01 vs. Vehicle IV, Kruskal-Wallis ANOVA followed by Dunn’s post hoc test). Lung gene expression: A small sample of lung was collected, frozen, and subsequently processed to isolate RNA. PCR was conducted using the Fluidigm platform. mResolaris significantly decreased expression of several immune cell genes (*p < 0.05; **p < 0.01 vs. Vehicle IV, Student’s t test). PO, per os, oral dosing. Conclusions The Resokine pathway is functional in the lungs of rodents. Therapy with Resokine proteins was efficacious and ameliorated bleomycin-induced lung fibrosis. Certain Resokine pathway proteins may be worthy of exploration for their therapeutic effects in human lung diseases, such as interstitial lung disease. Acknowledgements Scientists and study directors at Stelic MC, TNO, and Biomodels who conducted the experiments on aTyr Pharma’s behalf. aTyr scientists Kenny d’Arigo and Nicole Schultz who were responsible for PK analysis and PCR conduct, respectively. Graphics support was provided by Oxford PharmaGenesis, Inc. and was funded by aTyr Pharma, Inc. Disclosure This study was funded by aTyr Pharma, Inc. Anticodon-binding Domain iMod Domain Molecule Dose (mg/kg) C0 (ng/mL) Vd (mL/kg) CL (mL*h/kg) T1/2AUCinf (h)(ng*h/mL) iMod 8 145,455 119 574 0.5 13,944 Resolaris 10 97,774 128 75 2.5 13,491 iMod.Fc 10 1,407,553 265 1.8 94 633,937 Proteins used in these experiments are depicted above. The endogenous Physiocrine, Resokine, is synonymous with endogenous extracellular histidyl-tRNA synthetase (HARS). The domain structure is represented top left, whereas the 3-dimensional structure is represented top right. Resolaris is a recombinant version of the natural protein expressed in E. coli (middle domain structure) with a slightly truncated C-terminus. In some experiments, the mouse version of Resolaris (mResolaris) was used. The immunomodulatory domain (iMod) is represented to the right. iMod.Fc is represented in the lower left (primary domain structure) and far right (3-dimensional model). PK parameters obtained in Sprague Dawley rats are shown in the table. AUCinf, area under the curve extrapolated to infinity; C0, initial concentration; CL, clearance; PK, pharmacokinetics; T1/2, half-life; Vd, volume of distribution. 0 8 15 21 Bleomycin Termination Days: A)B) BALF TGF- b1 0 20 10 30 40 50 * Soluble Markers in BALF BALF Cell Count Immune and Fibrotic Markers Attenuated by Resokine Proteins Methods Resokine Proteins Resokine Proteins General Experimental Methods C57Bl/6 mice received a single administration of saline (non-diseased control) or bleomycin (2-3 U/kg) intratracheally. Bleomycin-induced animals received Resokine proteins or Vehicle administered intravenously at dose levels and frequencies shown in the figure legends from Day 8 through termination. Anti-TGFb antibody-, pirfenidone-, nintedanib-, or dexamethasone-treated groups were included for comparison. All animals were weighed and evaluated for respiratory distress daily. CT scans were conducted in one experiment. Mice were euthanized on Day 21 and tissues collected, stained, and evaluated for fibrosis and other histological changes by trained personnel who were blind to treatment conditions. Data are expressed as mean ± SEM. CT, computed tomography; TGF, transforming growth factor. Presented at the American Thoracic Society International Conference; 19–24 May 2017; Washington, DC, USA. Results A) B) C) A6428 0.25 mg/kg PO QD D0–D21 60 mg/kg PO QD D0–D21 BLM iMod 2.5 mg/kg IV QD D8–D21 BLM mResolaris 3 mg/kg IV QD D8–D21 BLM Veh IV QD D8–D21 BLM iMod 2.5 mg/kg IV QD D8–D21 BLM mResolaris 3 mg/kg IV QD D8–D21 150 BLM Veh IV QD D8–D21 BLM iMod 2.5 mg/kg IV QD D8–D21 BLM mResolaris 3 mg/kg IV QD D8–D21 Lung Gene Expression BLM Veh IV QD D8–D21 BLM iMod 2.5 mg/kg IV QD D8–D21 BLM mResolaris 3 mg/kg IV QD D8–D21 No BLM BLM Vehicle PO BLM TGFb Ab 3 mg/kg IP QOD D0–D21 BLM Pirfenidone 100 mg/kg PO BID D8–D21 BLM iMod 2.5 mg/kg IV QD D8–D21 BLM mResolaris 3 mg/kg IV QD D8–D21 BLM iMod.Fc 0.4 mg/kg IV QW D8, D15 Resokine human Resokine fly No BLM BLMBLMBLMBLM VehDex Nintedanib Veh BLMBLMBLM Resolaris Resolaris Resolaris 1 mg/kg 3 mg/kg 10 mg/kg IV QD IV QD IV QD D8–D21 D8–D21 D8–D21 PO BID D0–D21 IV QD D8–D21 -5 0 5 * ** -5 0 5 -5 0 5 -5 0 5 -5 0 5 -5 0 5 * ** * log2 (Fold Change) Foxp3 IL6 IL10 log2 (Fold Change) log2 (Fold Change) log2 (Fold Change) log2 (Fold Change) log2 (Fold Change) No BLM BLM Dex 0.25 mg/kg PO QD D8–D21 BLM Veh IV QD D8–D21 BLM mResolaris 3 mg/kg IV QD D8–D21 No BLM BLM Dex 0.25 mg/kg PO QD D8–D21 BLM Veh IV QD D8–D21 BLM mResolaris 3 mg/kg IV QD D8–D21 IFNg No BLM BLM Dex 0.25 mg/kg PO QD D8–D21 BLM Veh IV QD D8–D21 BLM mResolaris 3 mg/kg IV QD D8–D21 No BLM BLM Dex 0.25 mg/kg PO QD D8–D21 BLM Veh IV QD D8–D21 BLM mResolaris 3 mg/kg IV QD D8–D21 No BLM BLM Dex 0.25 mg/kg PO QD D8–D21 BLM Veh IV QD D8–D21 BLM mResolaris 3 mg/kg IV QD D8–D21 No BLM BLM Dex 0.25 mg/kg PO QD D8–D21 BLM Veh IV QD D8–D21 BLM mResolaris 3 mg/kg IV QD D8–D21 CXCR3 Exhibit 99.1
