
Interstitial Lung Disease and the Immune System Introduction to the iMod.Fc Program aTyr Pharma Investor and Analyst ILD and iMod.Fc Educational Webinar American Thoracic Society International Conference May 23, 2017 Exhibit 99.3

Forward-Looking Statements The following slides and any accompanying oral presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” “opportunity,” or “continue,” and other similar expressions are intended to identify forward-looking statements. For example, all statements we make regarding the potential therapeutic benefits of Physiocrines and our product candidates, including Resolaris™ and our iMod.Fc program, the ability to successfully advance our pipeline or product candidates, the timing within which we expect to initiate, receive and report data from, and complete our planned clinical trials, and our ability to receive regulatory approvals for, and commercialize, our product candidates, our ability to identify and discover additional product candidates, our projected cash expenditures, and the ability of our intellectual property portfolio to provide protection are forward-looking statements. All forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These risks, uncertainties and other factors are more fully described in our filings with the U.S. Securities and Exchange Commission, including our most recent Quarterly Report on Form 10-Q, our Annual Report on Form 10-K and in our other filings. The forward-looking statements in this presentation speak only as of the date of this presentation and neither we nor any other person assume responsibility for the accuracy and completeness of any forward-looking statement. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. We own various U.S. federal trademark applications and unregistered trademarks, including our company name and Resolaris™. All other trademarks or trade names referred to in this presentation are the property of their respective owners. Solely for convenience, the trademarks and trade names in this presentation are referred to without the symbols ® and ™, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.

Agenda Introduction Mark Johnson, Senior Director Investor Relations, aTyr Pharma Resokine Pathway Sanuj Ravindran, MD, Chief Business Officer, aTyr Pharma ILD Overview Steven Nathan, MD, Director of the Advanced Lung Disease Program and Medical Director of the Lung Transplant Program at Inova Fairfax Hospital, Falls Church, Virginia iMod.Fc Program Sanjay Shukla, MD, MS, Chief Medical Officer, aTyr Pharma Question & Answer Session
Evolved from Cellular Homeostasis Genes over 400 Million Years New Immunological Pathway: Resokine
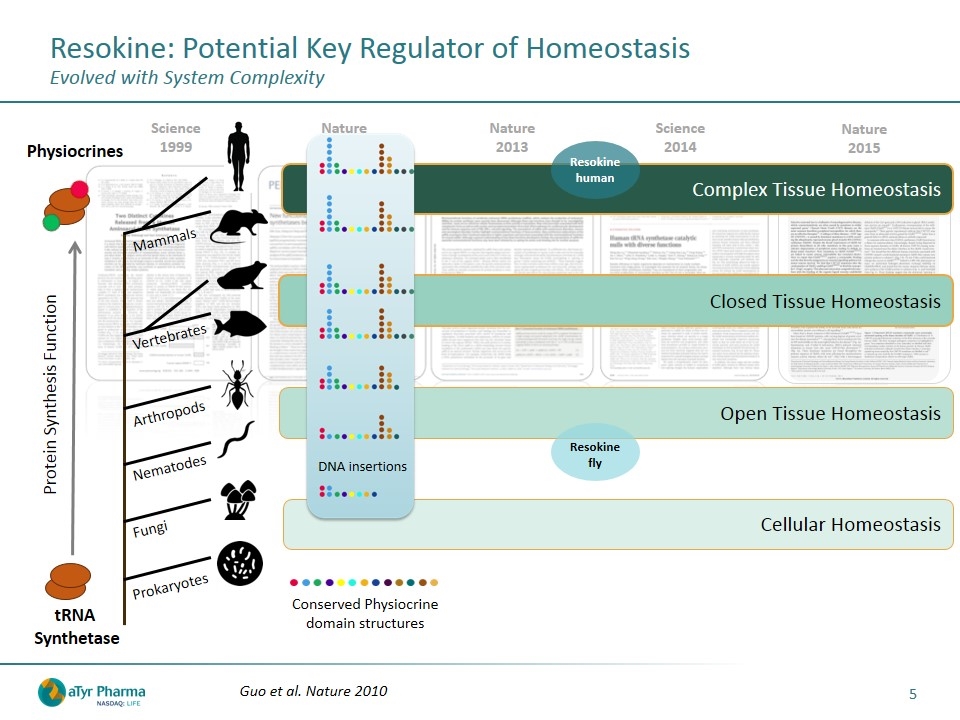
Resokine: Potential Key Regulator of Homeostasis Evolved with System Complexity Nature 2015 Science 2014 Nature 2013 Nature 2010 Science 1999 Closed Tissue Homeostasis Complex Tissue Homeostasis Open Tissue Homeostasis Conserved Physiocrine domain structures Cellular Homeostasis Protein Synthesis Function tRNA Synthetase Fungi Nematodes Arthropods Vertebrates Mammals Prokaryotes Physiocrines DNA insertions Guo et al. Nature 2010 Resokine fly Resokine human
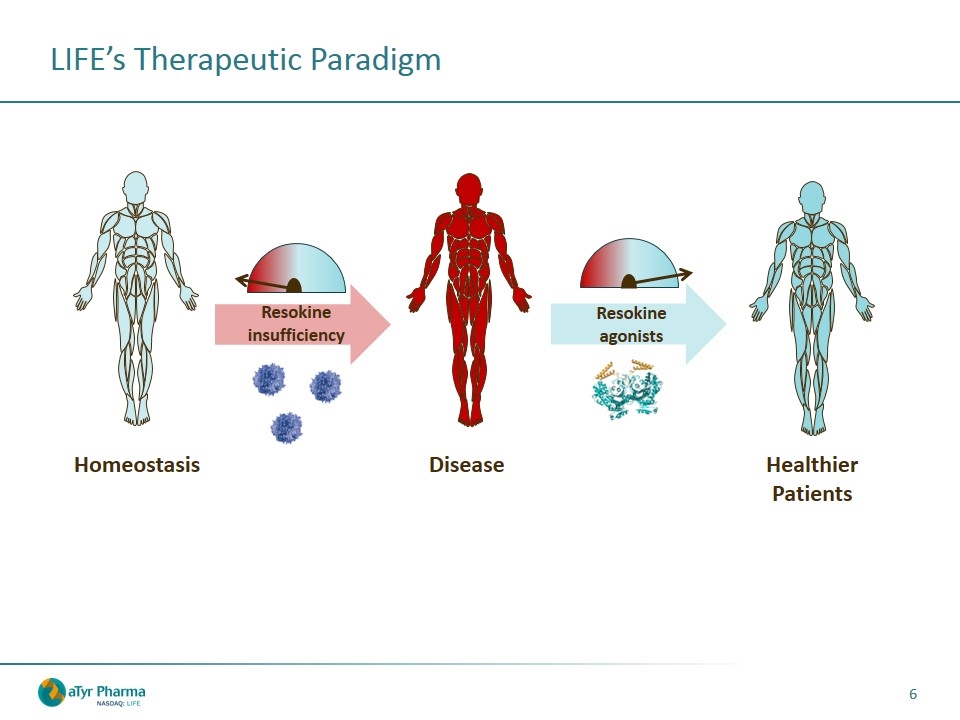
LIFE’s Therapeutic Paradigm Homeostasis Disease Healthier Patients Resokine insufficiency Resokine agonists
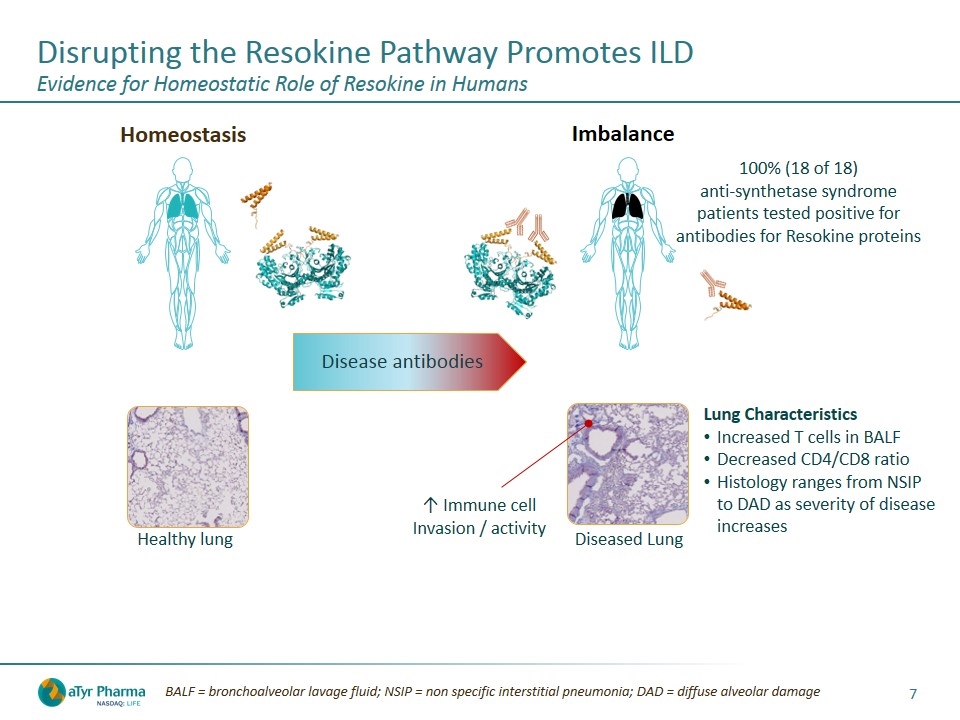
Disease antibodies Homeostasis Imbalance 100% (18 of 18) anti-synthetase syndrome patients tested positive for antibodies for Resokine proteins Diseased Lung Healthy lung ↑ Immune cell Invasion / activity Lung Characteristics Increased T cells in BALF Decreased CD4/CD8 ratio Histology ranges from NSIP to DAD as severity of disease increases BALF = bronchoalveolar lavage fluid; NSIP = non specific interstitial pneumonia; DAD = diffuse alveolar damage Disrupting the Resokine Pathway Promotes ILD Evidence for Homeostatic Role of Resokine in Humans
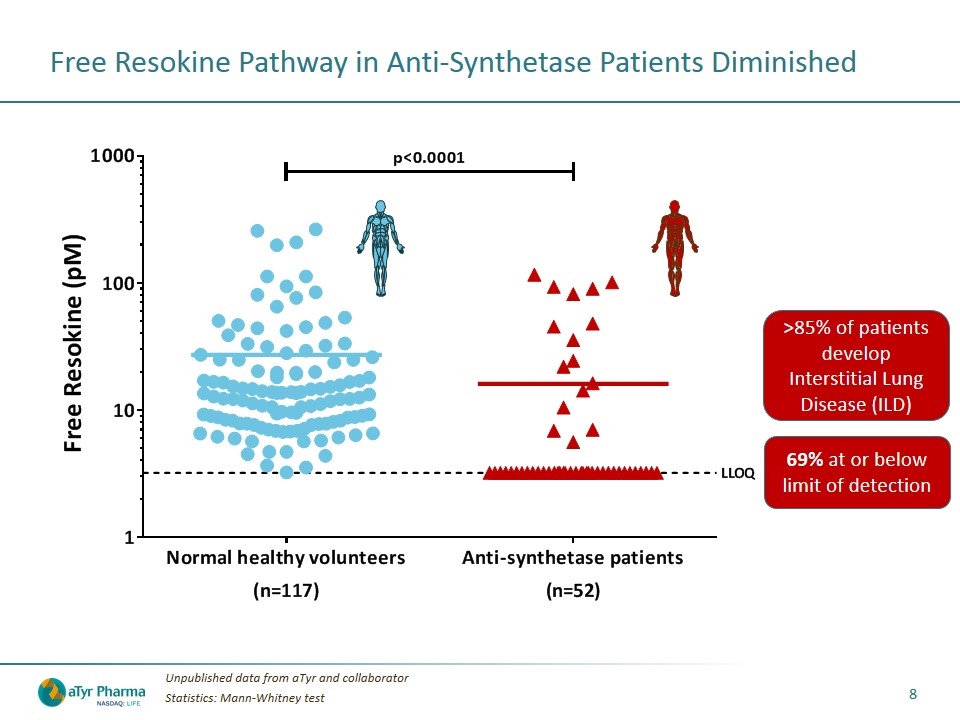
Free Resokine Pathway in Anti-Synthetase Patients Diminished Unpublished data from aTyr and collaborator Statistics: Mann-Whitney test 69% at or below limit of detection >85% of patients develop Interstitial Lung Disease (ILD)
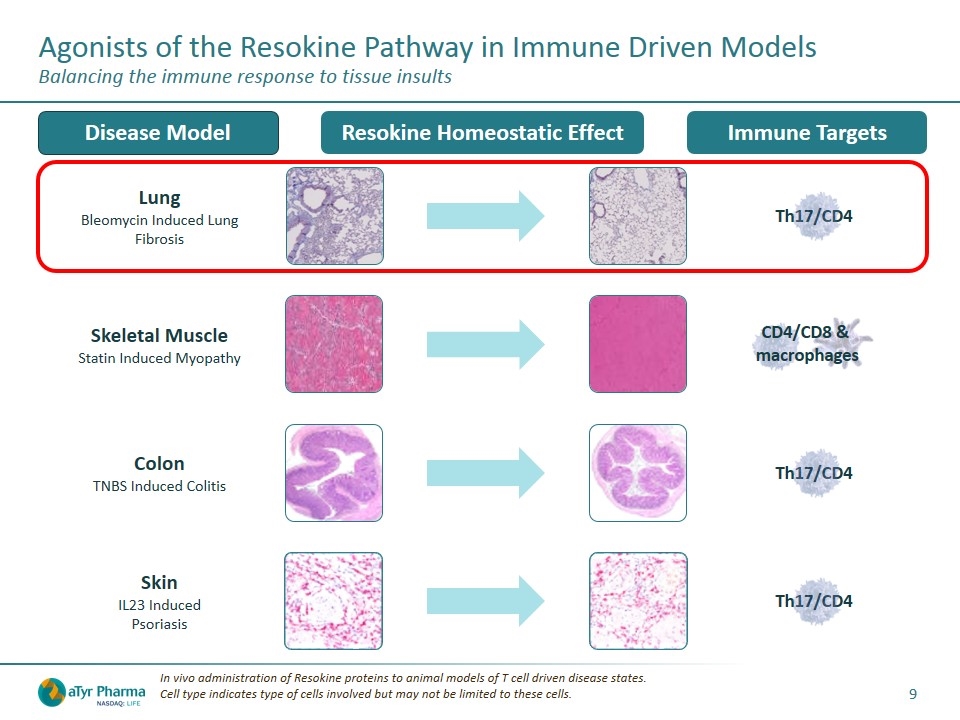
Lung Bleomycin Induced Lung Fibrosis Th17/CD4 Agonists of the Resokine Pathway in Immune Driven Models Balancing the immune response to tissue insults In vivo administration of Resokine proteins to animal models of T cell driven disease states. Cell type indicates type of cells involved but may not be limited to these cells. Immune Targets Disease Model Resokine Homeostatic Effect Skeletal Muscle Statin Induced Myopathy CD4/CD8 & macrophages Colon TNBS Induced Colitis Th17/CD4 Skin IL23 Induced Psoriasis Th17/CD4
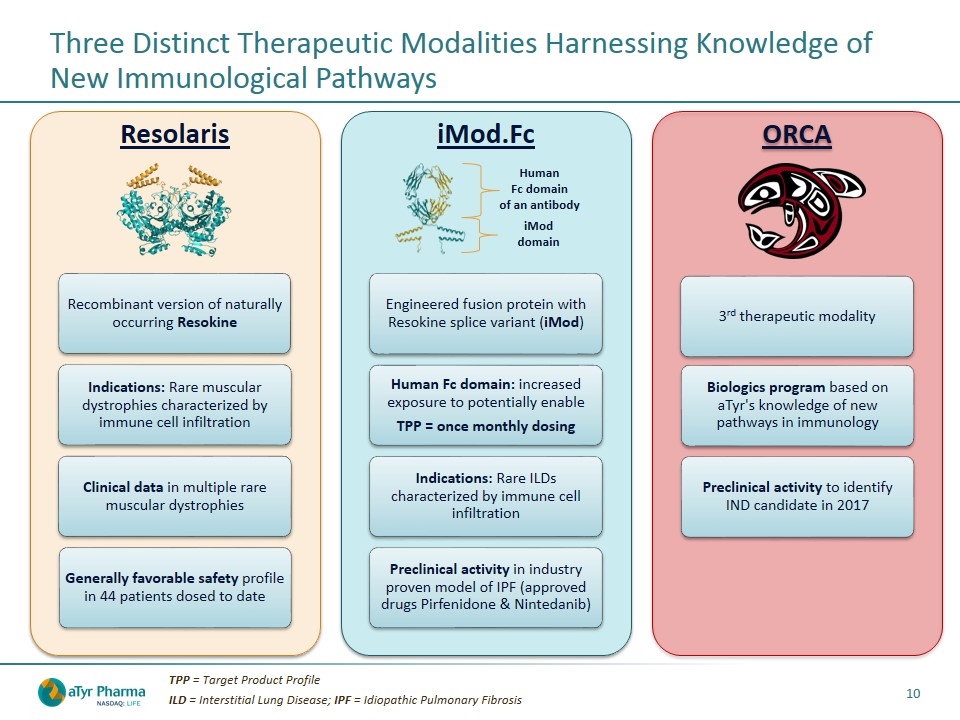
Three Distinct Therapeutic Modalities Harnessing Knowledge of New Immunological Pathways TPP = Target Product Profile ILD = Interstitial Lung Disease; IPF = Idiopathic Pulmonary Fibrosis iMod domain Human Fc domain of an antibody Resolaris Recombinant version of naturally occurring Resokine iMod.Fc ORCA Preclinical activity to id entify IND candidate in 2017 Clinical data in multiple rare muscular dystrophies Generally favorable safety profile in 44 patients dosed to date Human Fc domain: increased exposure to potentially enable TPP = once monthly dosing Indications: Rare ILDs characterized by immune cell infiltration Indications: Rare muscular dystrophies characterized by immune cell infiltration Preclinical activity in industry proven model of IPF (approved drugs Pirfenidone & Nintedanib) Biologics program based on aTyr's knowledge of new pathways in immunology Engineered fusion protein with Resokine splice variant ( iMod ) 3 rd therapeutic modality
Overview of Interstitial Lung Disease Steven Nathan, MD Medical Director, Advanced Lung Disease & Transplant Program Inova Fairfax Hospital Falls Church, Virginia USA Inova Advanced Lung Disease & Transplant Program

Disclosures: Steven Nathan, MD Personal financial relationships with commercial interests relevant to this presentation during the past 12 months: Consultant: aTyr Pharma, Bayer Pharmaceuticals, Boerhinger-Ingelheim, Genentech-Roche, Gilead, Third Pole, United Therapeutics. Speaker’s Bureau: Bayer, Boerhinger-Ingelheim, Genentech, Gilead, Grifols, United Therapeutics. Research Funding: Actelion, Bayer, Boerhinger-Ingelheim, Gilead, Genentech-Roche, United Therapeutics, Veracyte.
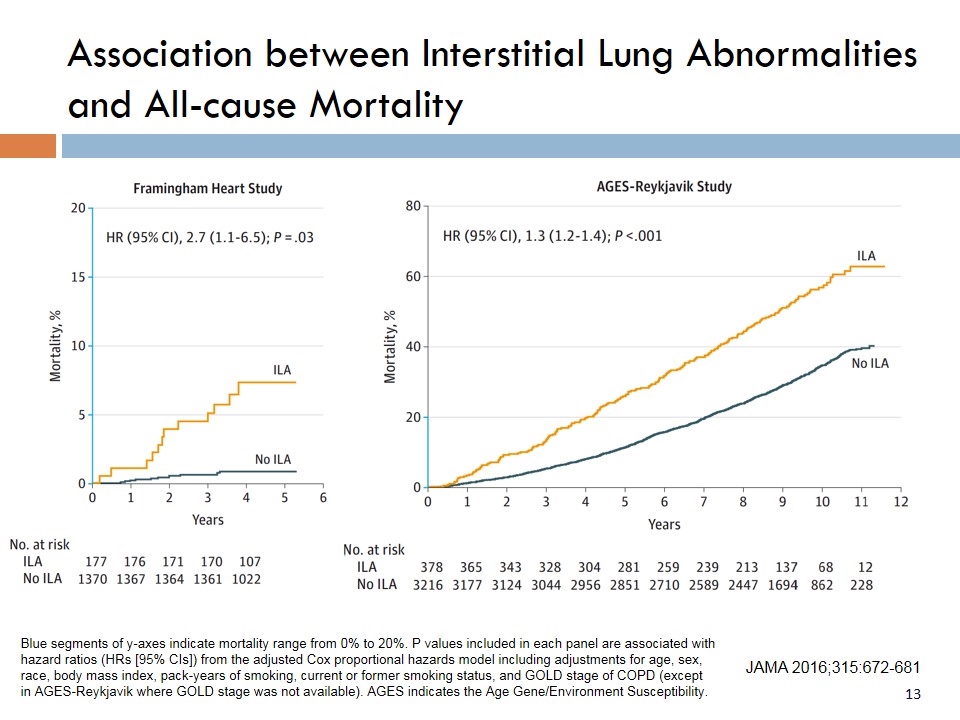
Association between Interstitial Lung Abnormalities and All-cause Mortality JAMA 2016;315:672-681 Blue segments of y-axes indicate mortality range from 0% to 20%. P values included in each panel are associated with hazard ratios (HRs [95% CIs]) from the adjusted Cox proportional hazards model including adjustments for age, sex, race, body mass index, pack-years of smoking, current or former smoking status, and GOLD stage of COPD (except in AGES-Reykjavik where GOLD stage was not available). AGES indicates the Age Gene/Environment Susceptibility.
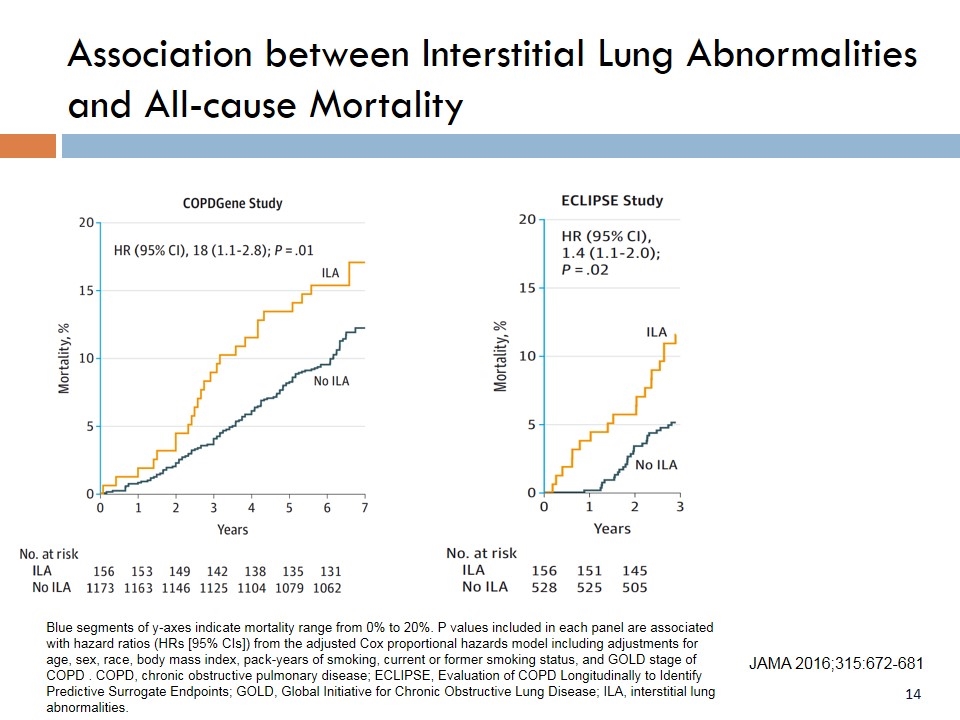
JAMA 2016;315:672-681 Blue segments of y-axes indicate mortality range from 0% to 20%. P values included in each panel are associated with hazard ratios (HRs [95% CIs]) from the adjusted Cox proportional hazards model including adjustments for age, sex, race, body mass index, pack-years of smoking, current or former smoking status, and GOLD stage of COPD . COPD, chronic obstructive pulmonary disease; ECLIPSE, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ILA, interstitial lung abnormalities. Association between Interstitial Lung Abnormalities and All-cause Mortality
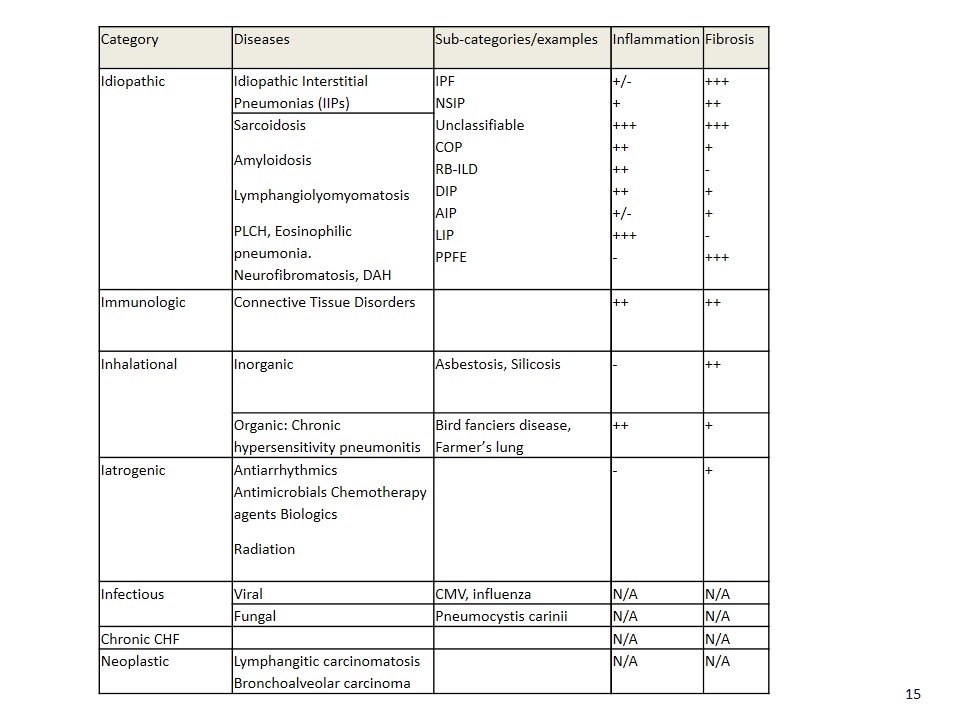
Category Diseases Sub-categories/examples Idiopathic Idiopathic Interstitial Pneumonias (IIPs) IPF NSIP Unclassifiable COP RB-ILD DIP AIP LIP PPFE Sarcoidosis Amyloidosis Lymphangiolyomyomatosis PLCH, Eosinophilic pneumonia. Neurofibromatosis, DAH Immunologic Connective Tissue Disorders Inhalational Inorganic Asbestosis, Silicosis Organic: Chronic hypersensitivity pneumonitis Bird fanciers disease, Farmer’s lung Iatrogenic Antiarrhythmics Antimicrobials Chemotherapy agents Biologics Radiation Infectious Viral CMV, influenza Fungal Pneumocystis carinii Chronic CHF Neoplastic Lymphangitic carcinomatosis Bronchoalveolar carcinoma Inflammation Fibrosis +/- + +++ ++ ++ ++ +/- +++ - +++ ++ +++ + - + + - +++ ++ ++ - ++ ++ + - + N/A N/A N/A N/A N/A N/A N/A N/A
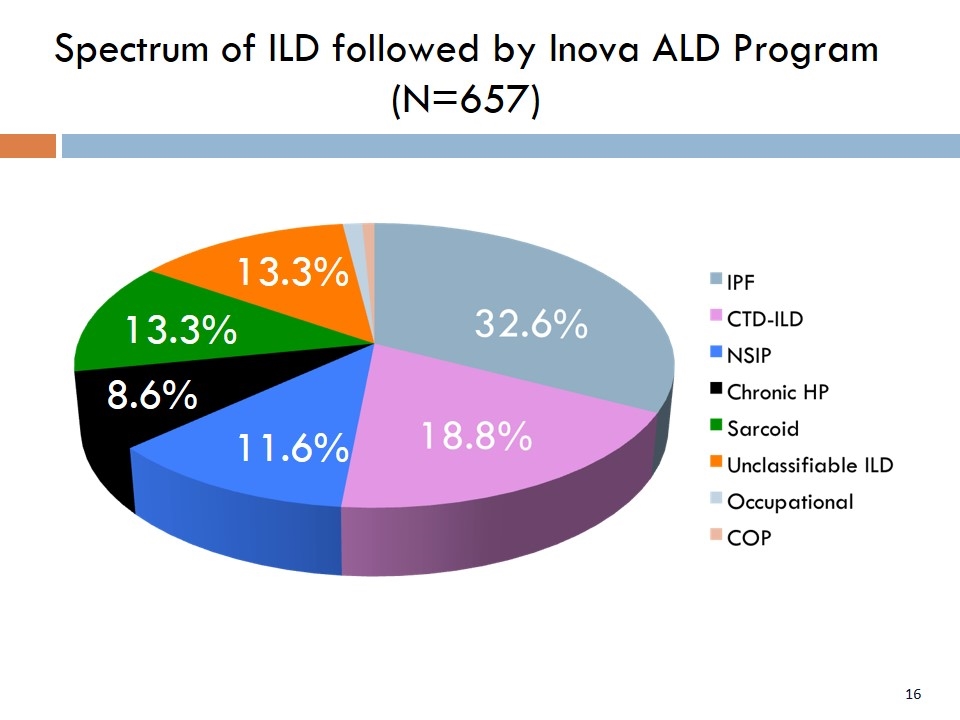
Spectrum of ILD followed by Inova ALD Program (N=657) 11.6% 8.6% 13.3% 13.3%
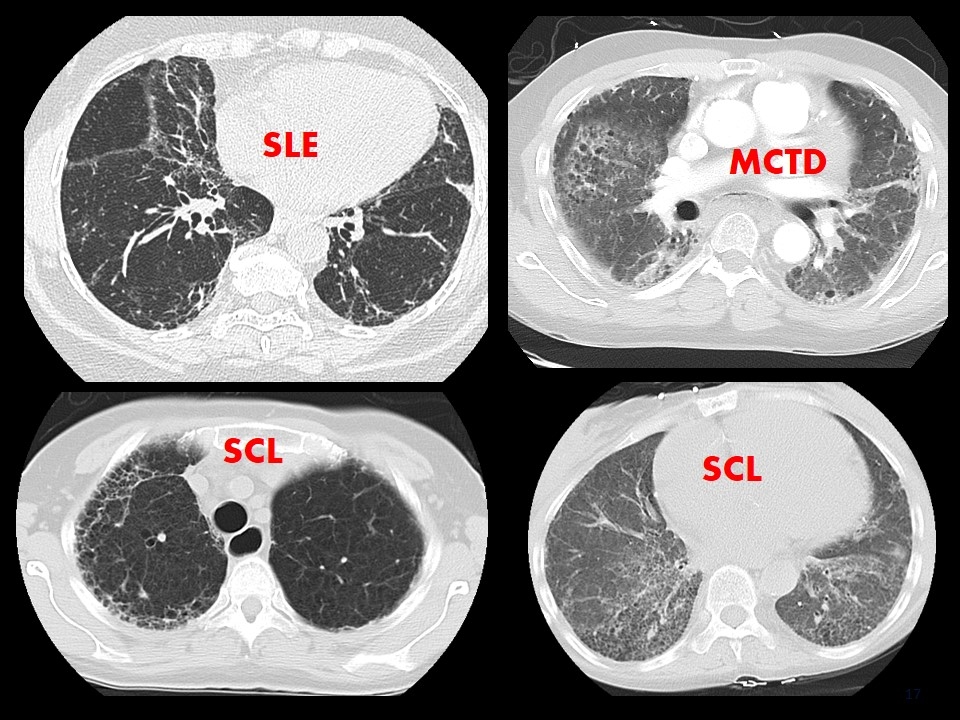
SLE MCTD SCL SCL
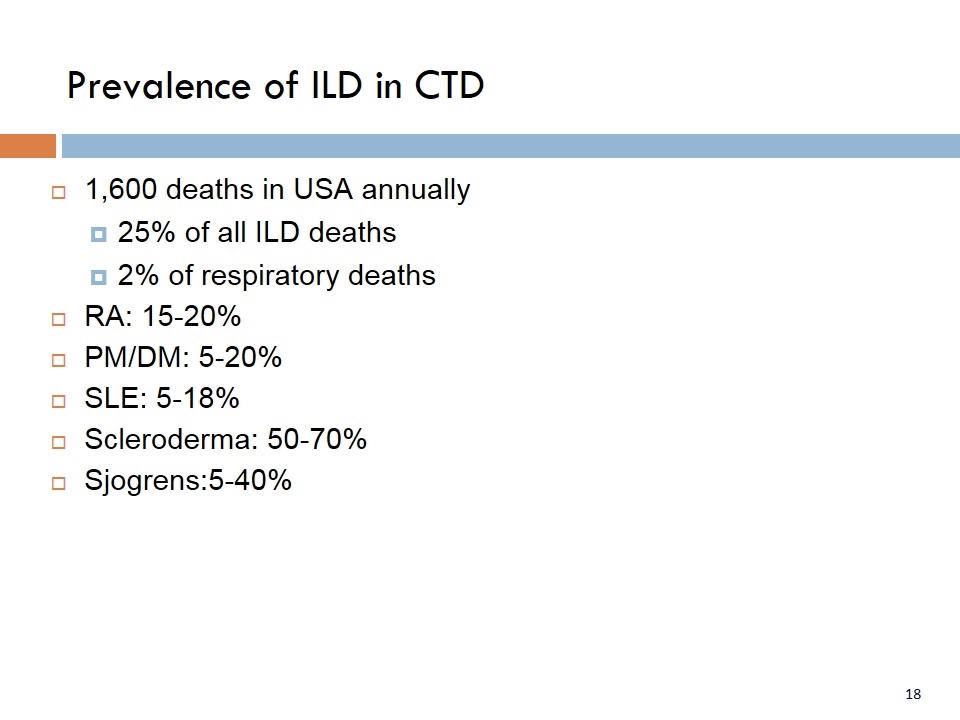
Prevalence of ILD in CTD 1,600 deaths in USA annually 25% of all ILD deaths 2% of respiratory deaths RA: 15-20% PM/DM: 5-20% SLE: 5-18% Scleroderma: 50-70% Sjogrens:5-40%

Adapted from Chartrand, Fischer. Sarc Vasc;2015:32:2-21 APPROACH TO THE TREATMENT OF CTD-ILD Disease impairment Subjective: Objective: -functional capacity - 6MWT -dypsnea - HRCT/PFT Mild and non-progressive Clinically non-significant ILD Moderate-severe and/or progressive Clinically significant ILD Clinical surveillance q3-6 months Treat Non-pharmacologic Smoking cessation Pulm Rehab Oxygen Immunizations PJP prophylaxis GERD PH CTEPH Pharmacologic Induction High dose oral or pulsed IV steroids Maintenance Steroid-sparing agent: MMF, CSA, AZA, FK506, CSA Cyclophosphamide in severe cases Evaluate treatment response (q3-6 months) Stable or improved -continue Rx -continue clinical surveillance Progressive disease, consider: -maintain/titrate up steroids -Change agents (consider cyclophos) -Severe refractory-consider rituximab - ?lung transplant
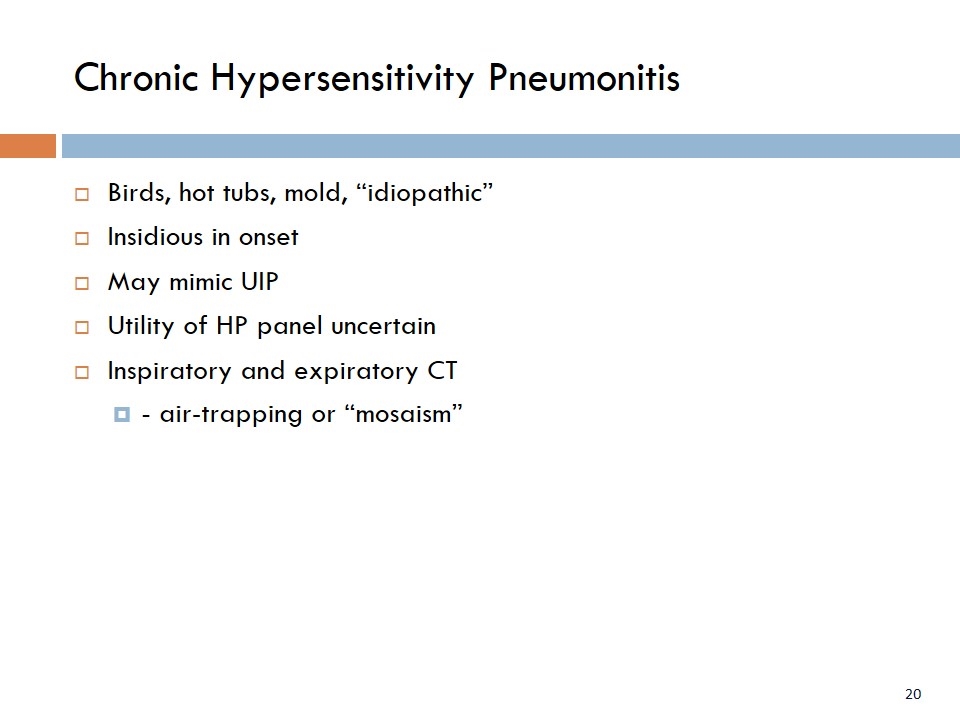
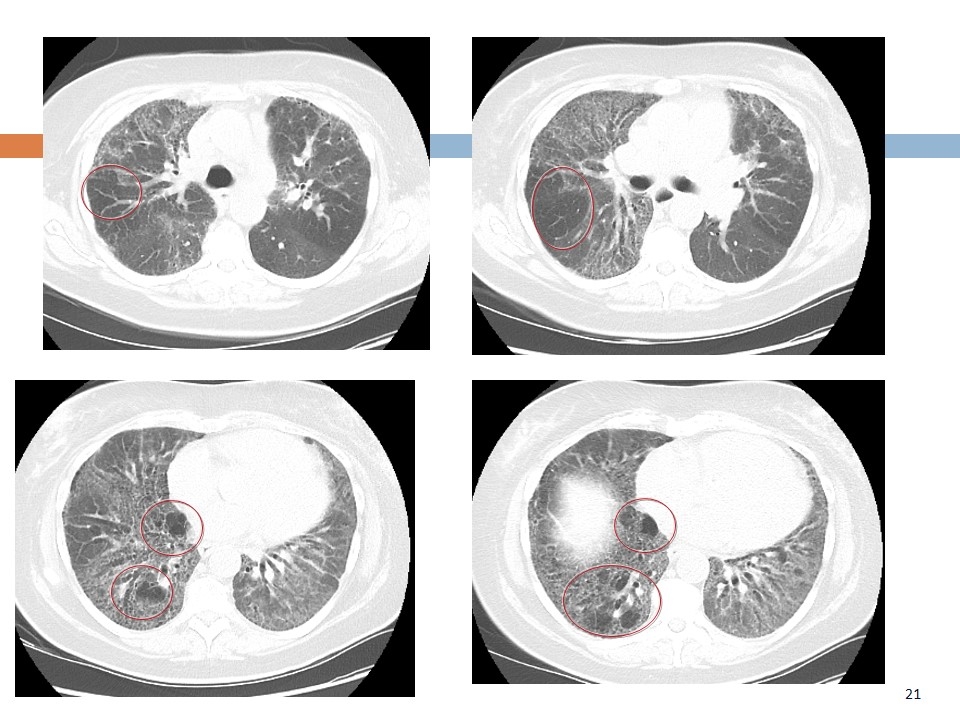
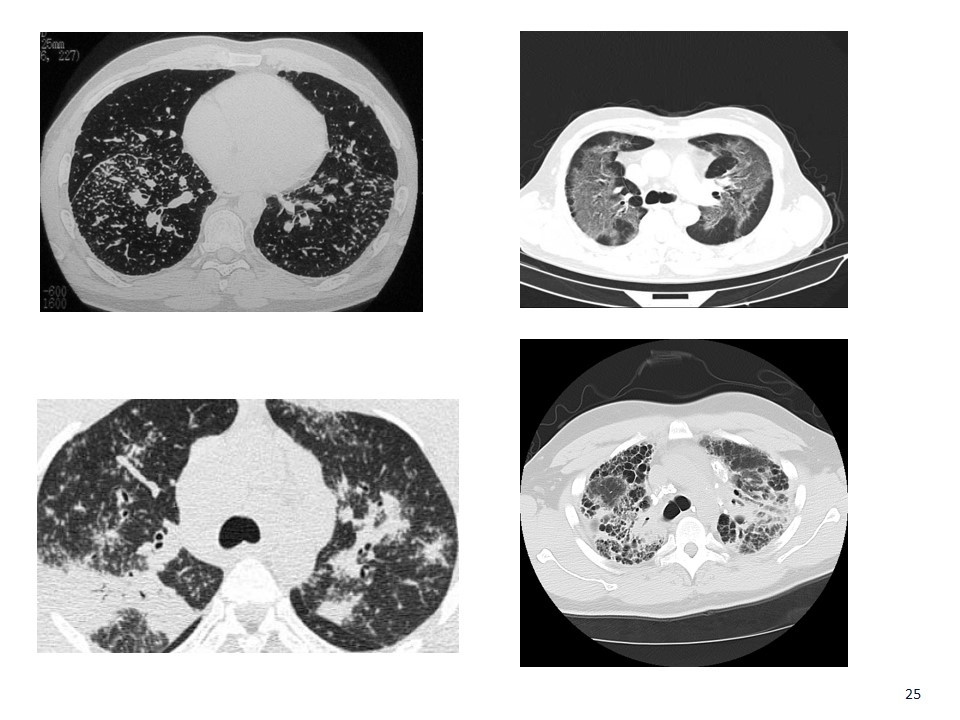
Chronic Hypersensitivity Pneumonitis Birds, hot tubs, mold, “idiopathic” Insidious in onset May mimic UIP Utility of HP panel uncertain Inspiratory and expiratory CT - air-trapping or “mosaism”
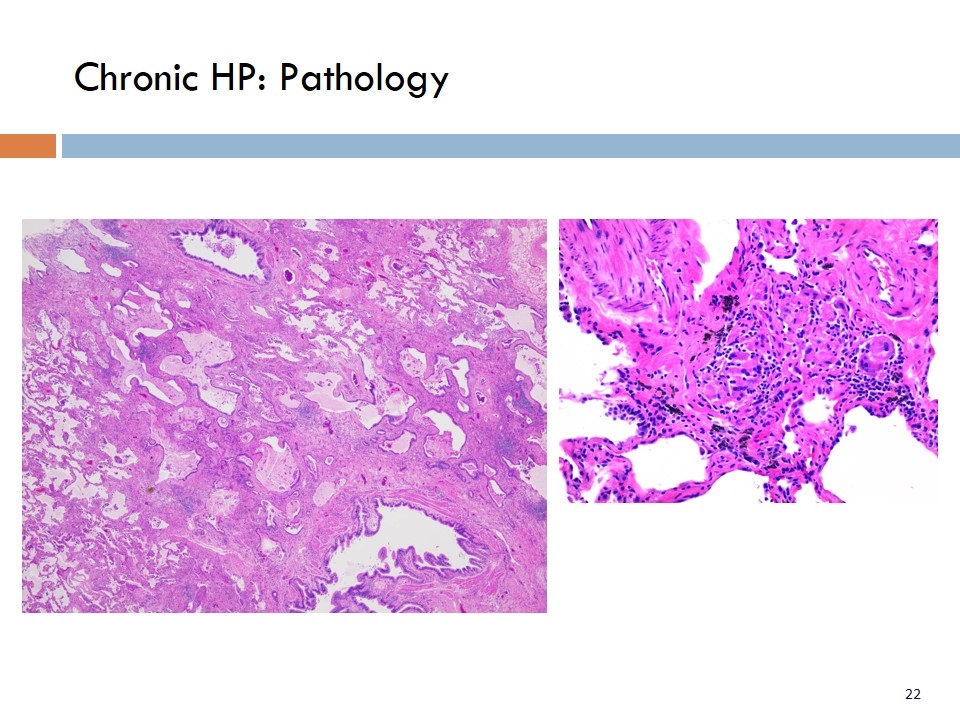
Chronic HP: Pathology
Sarcoidosis: Systemic Disease A multisystem disease Unknown etiology Granulomatous disorder Affects individuals world wide Most often affects young adults Prevalence of 10-20 per 100,000 population Incidence is unknown Varies among geographical groups Lifetime incidence in blacks is 2.4%, in whites 0.85%
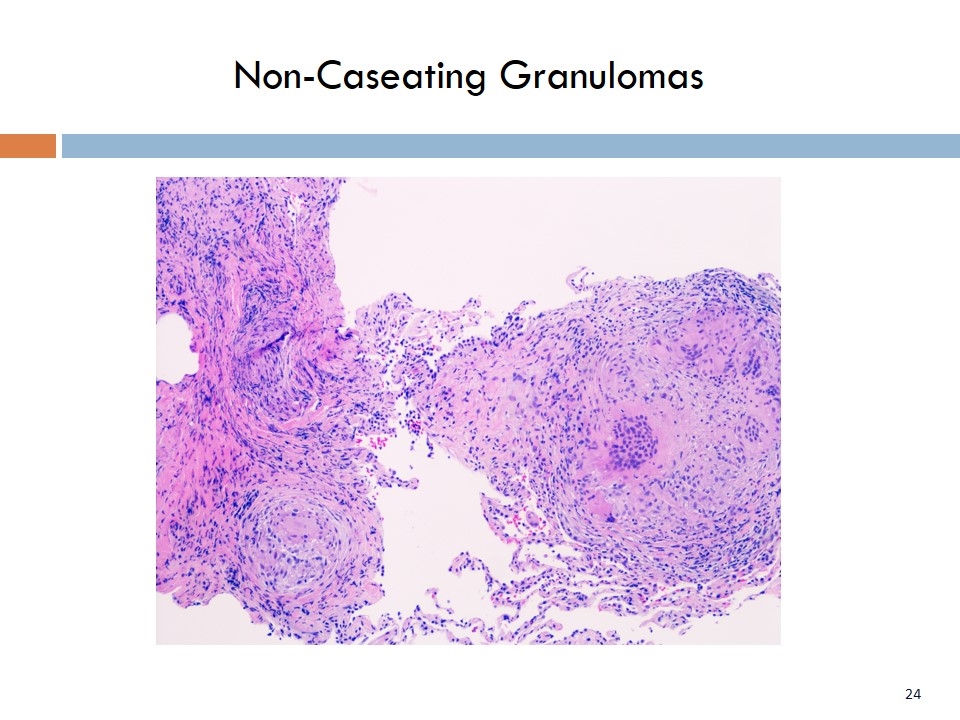
Non-Caseating Granulomas
Treatment of Sarcoidosis Not all patients require therapy for sarcoidosis About half never get treated Pulmonary, ocular, neuro, cardiac, hypercalcemia Treatment strategies are different based on phase of disease Acute Chronic Refractory Steroids, methotrexate, azathioprine, mycophenolate, leflunomide, infliximab, acthar gel
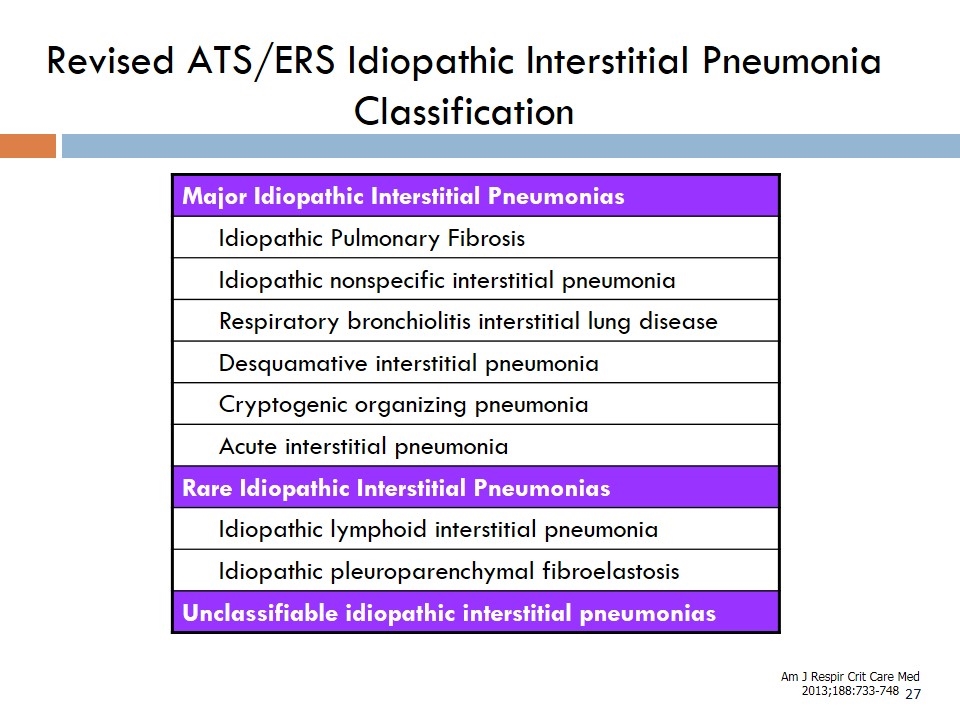
Revised ATS/ERS Idiopathic Interstitial Pneumonia Classification Major Idiopathic Interstitial Pneumonias Idiopathic Pulmonary Fibrosis Idiopathic nonspecific interstitial pneumonia Respiratory bronchiolitis interstitial lung disease Desquamative interstitial pneumonia Cryptogenic organizing pneumonia Acute interstitial pneumonia Rare Idiopathic Interstitial Pneumonias Idiopathic lymphoid interstitial pneumonia Idiopathic pleuroparenchymal fibroelastosis Unclassifiable idiopathic interstitial pneumonias Am J Respir Crit Care Med 2013;188:733-748
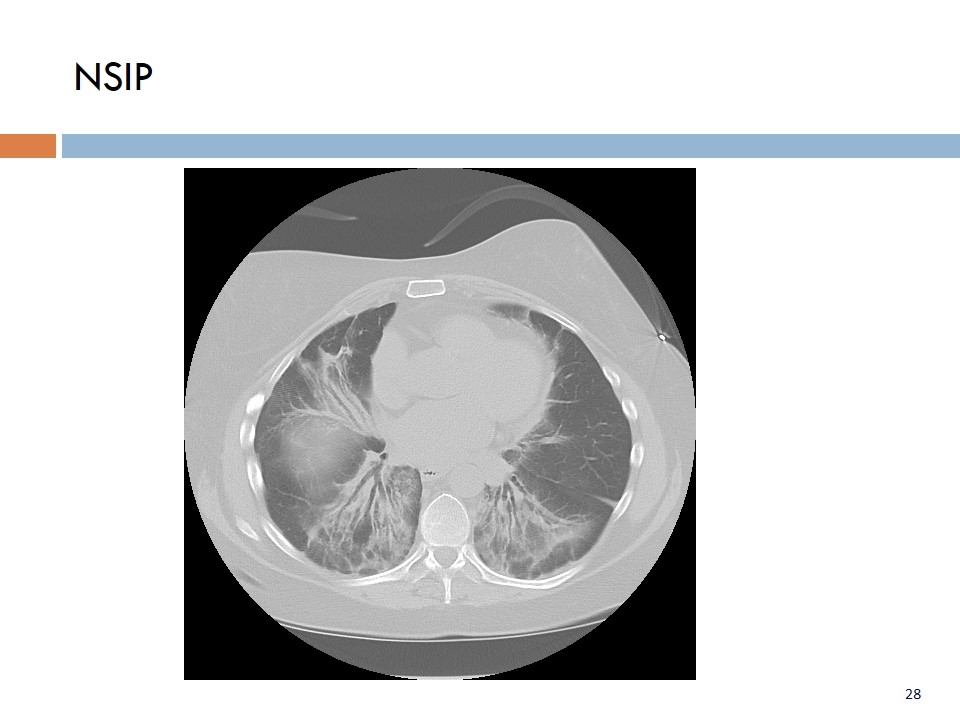
NSIP
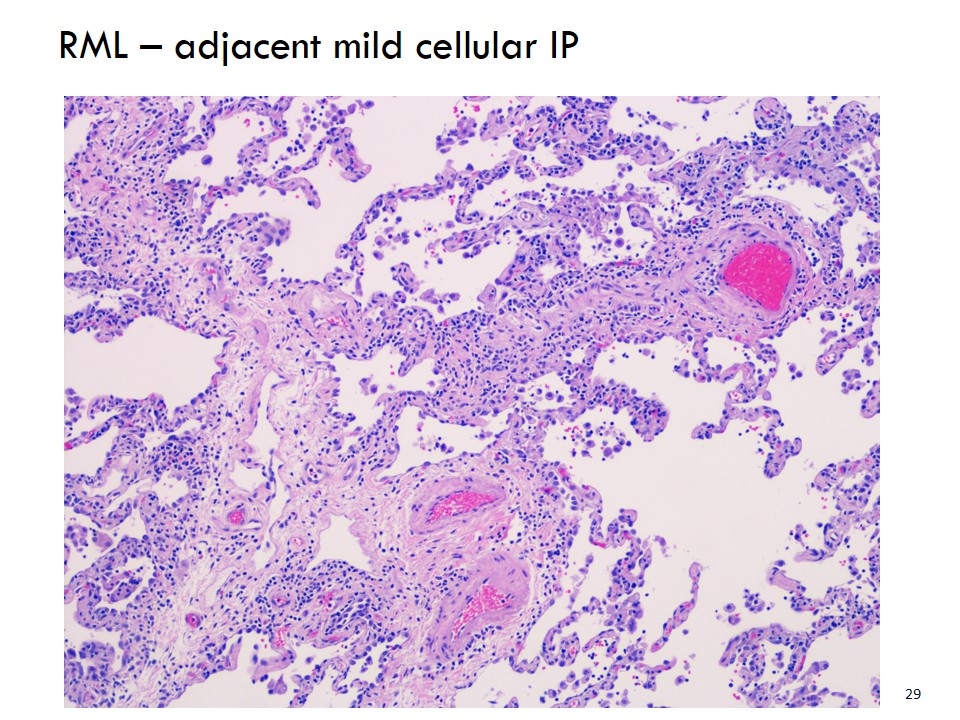
RML – adjacent mild cellular IP
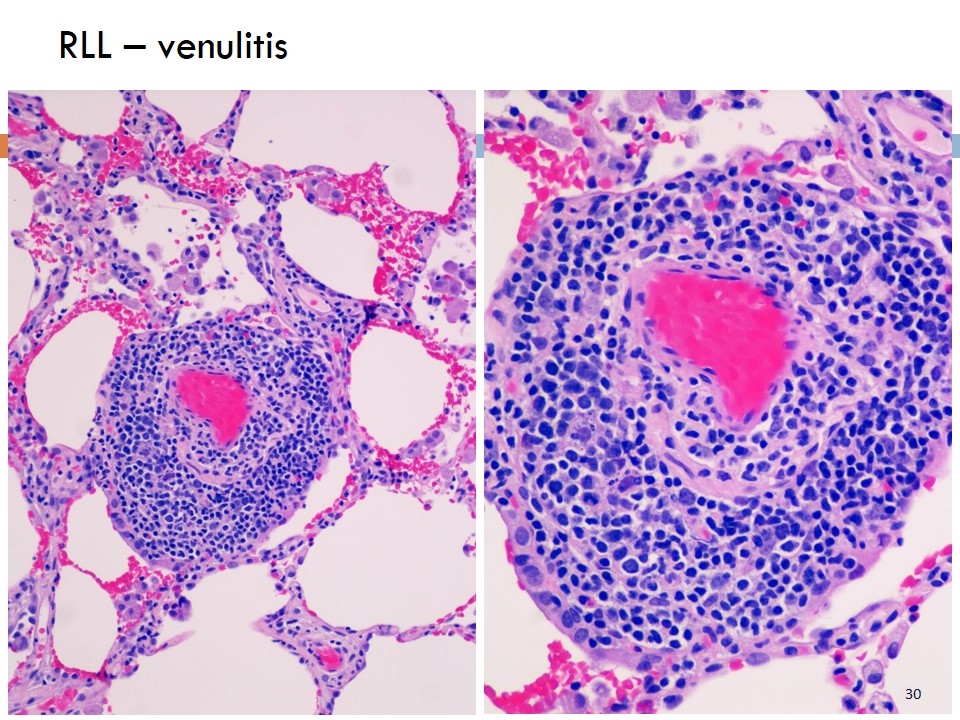
RLL – venulitis
Current Definition of IPF Specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause Occurring primarily in older adults Limited to the lungs ATS/ERS/JRS/ALAT consensus statement Am J Respir Crit Care Med. 2011;183:788-824
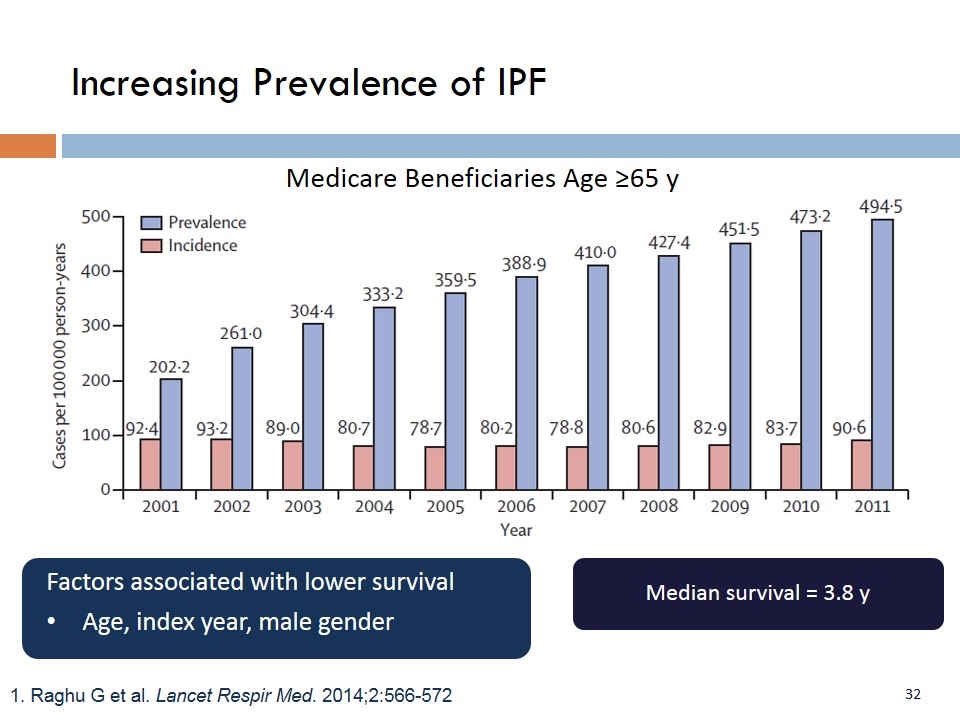
1. Raghu G et al. Lancet Respir Med. 2014;2:566-572 Medicare Beneficiaries Age ≥65 y Median survival = 3.8 y Factors associated with lower survival Age, index year, male gender Increasing Prevalence of IPF
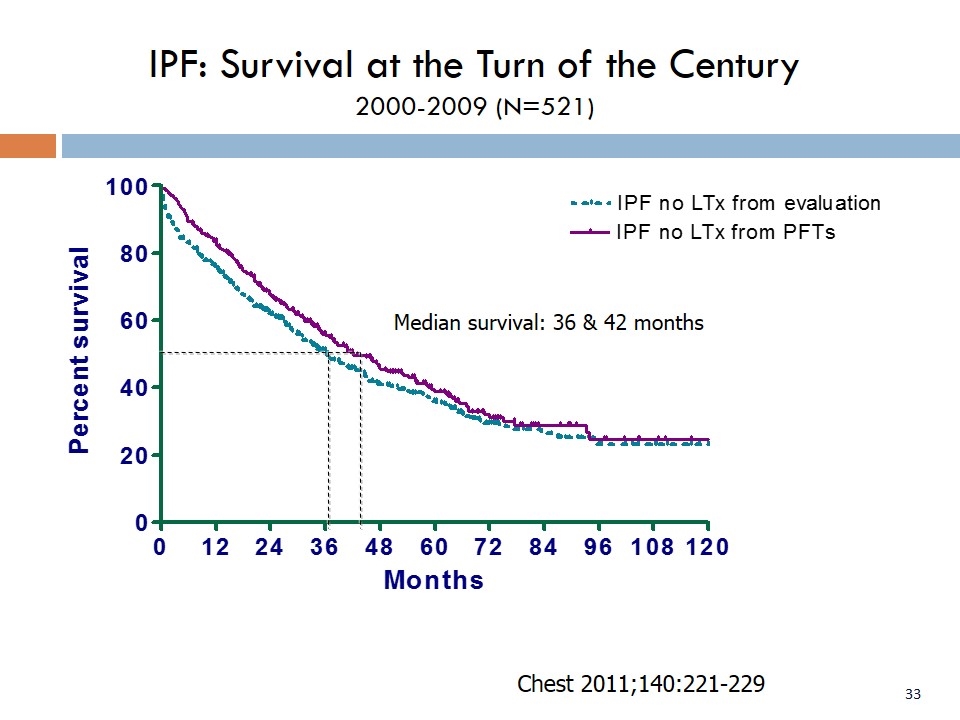
IPF: Survival at the Turn of the Century 2000-2009 (N=521) Median survival: 36 & 42 months Chest 2011;140:221-229
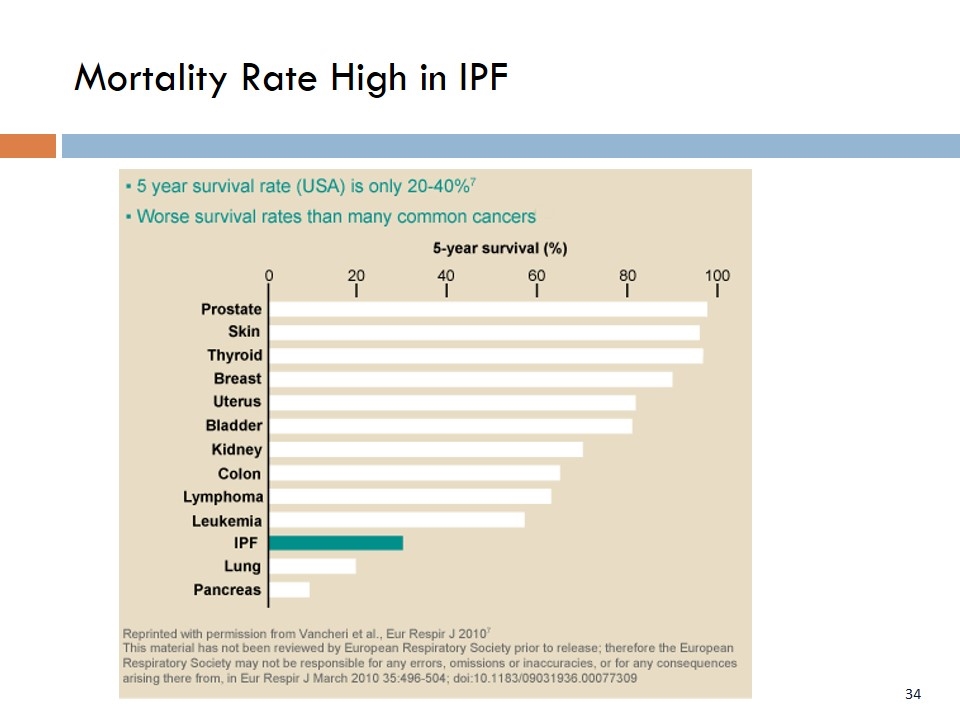
Mortality Rate High in IPF
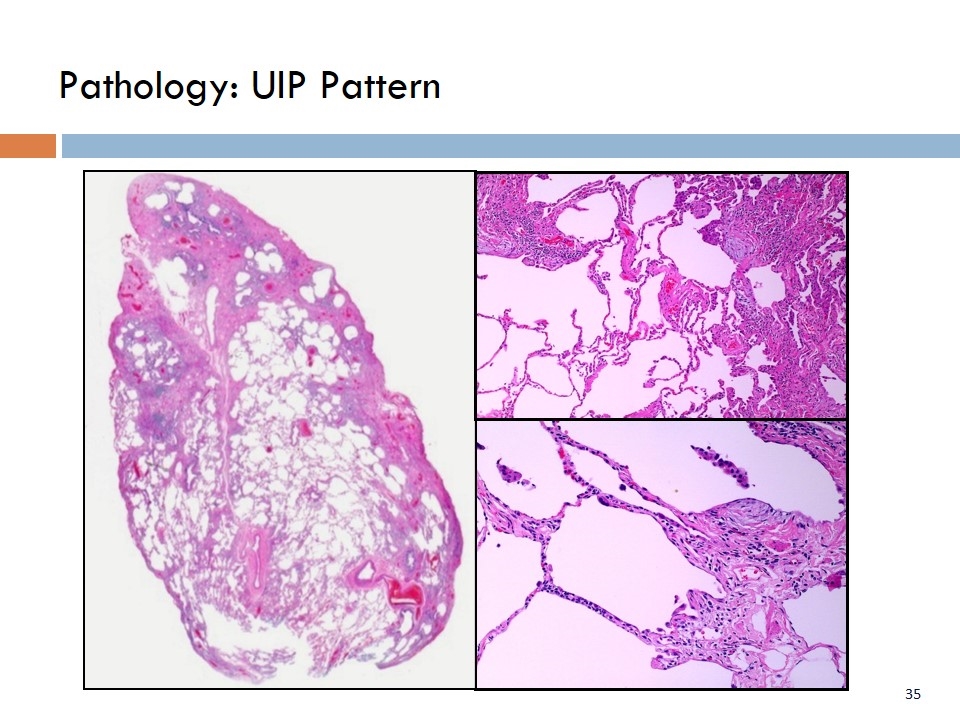
Pathology: UIP Pattern
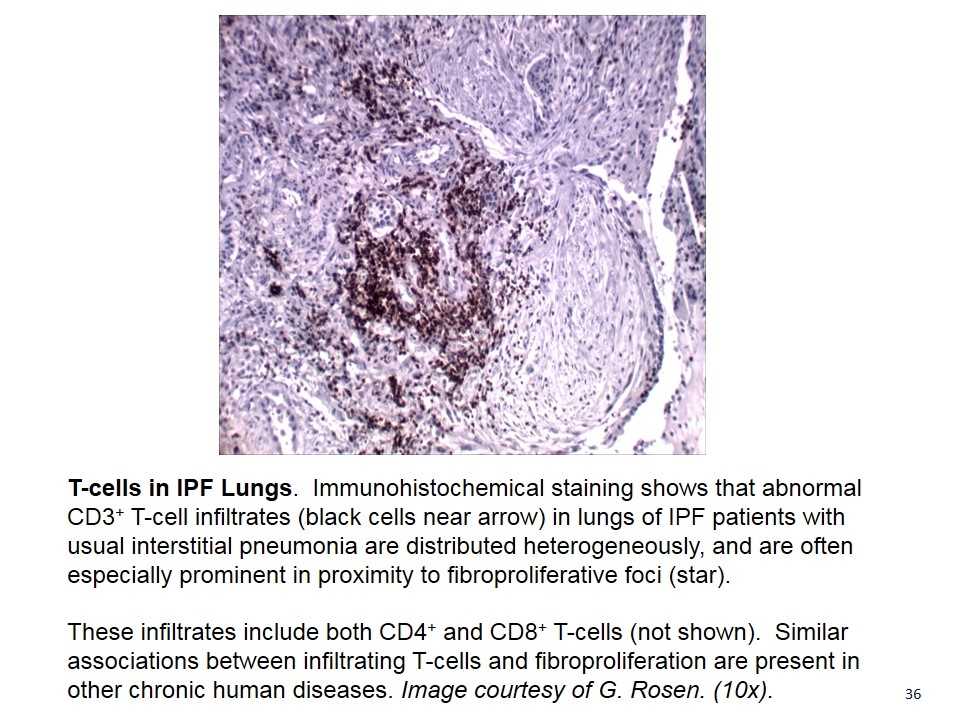
T-cells in IPF Lungs. Immunohistochemical staining shows that abnormal CD3+ T-cell infiltrates (black cells near arrow) in lungs of IPF patients with usual interstitial pneumonia are distributed heterogeneously, and are often especially prominent in proximity to fibroproliferative foci (star). These infiltrates include both CD4+ and CD8+ T-cells (not shown). Similar associations between infiltrating T-cells and fibroproliferation are present in other chronic human diseases. Image courtesy of G. Rosen. (10x).
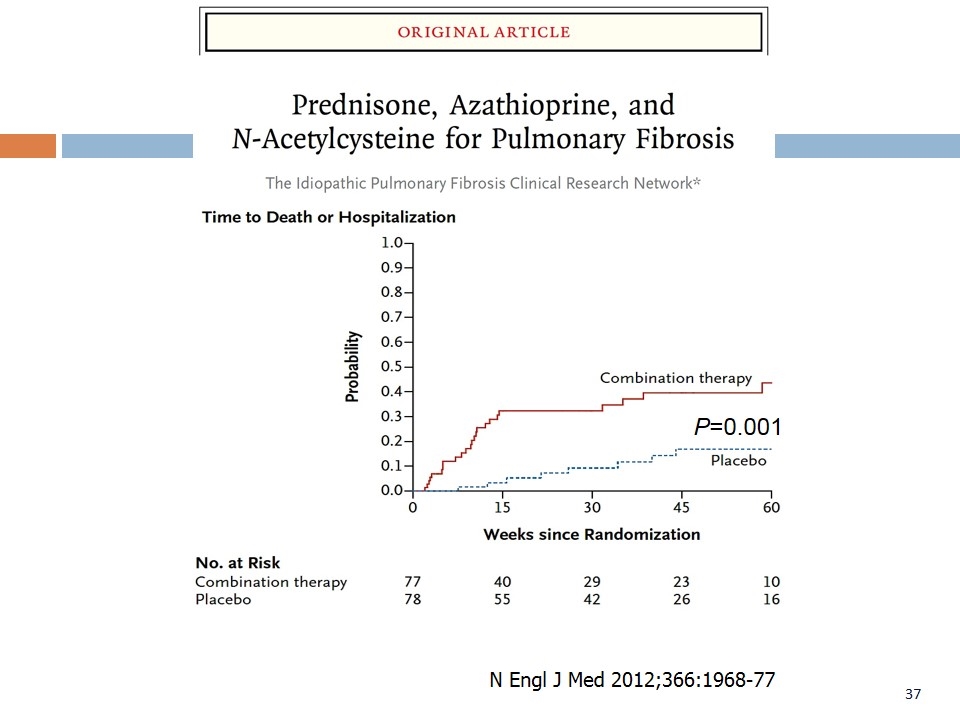
P=0.001 Collaborators. N Engl J Med. 2012;366:1968-1977. N Engl J Med 2012;366:1968-77
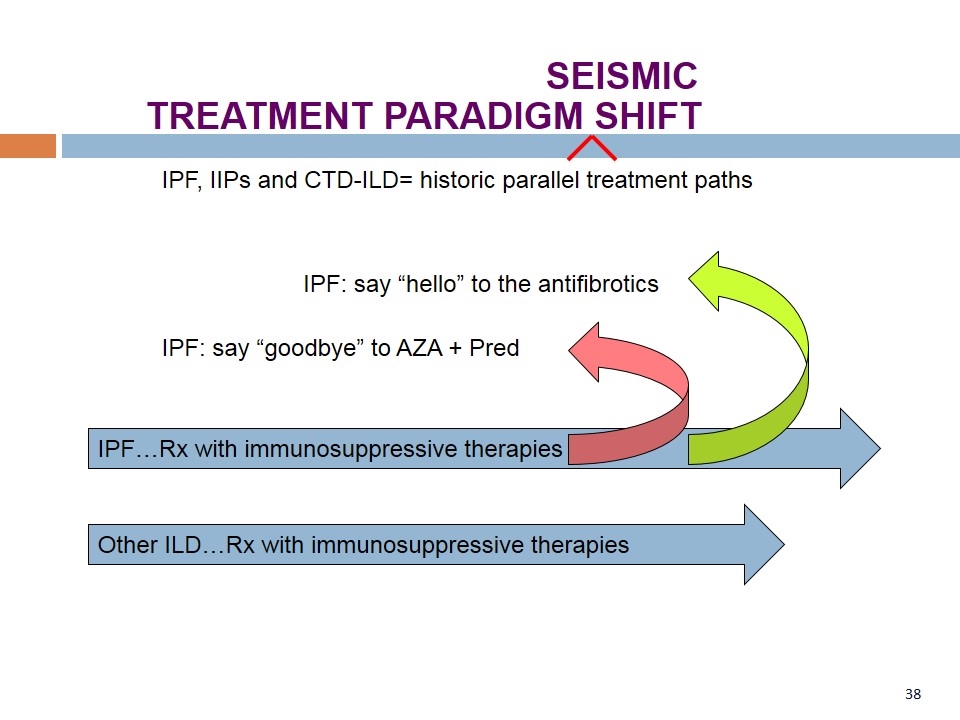
IPF…Rx with immunosuppressive therapies Other ILD…Rx with immunosuppressive therapies IPF, IIPs and CTD-ILD= historic parallel treatment paths IPF: say “goodbye” to AZA + Pred IPF: say “hello” to the antifibrotics TREATMENT PARADIGM SHIFT SEISMIC
King TE Jr., et al. N Engl J Med. 2014;370:2083-2092. Richeldi L, et al. N Engl J Med. 2014;370:2071-2082.
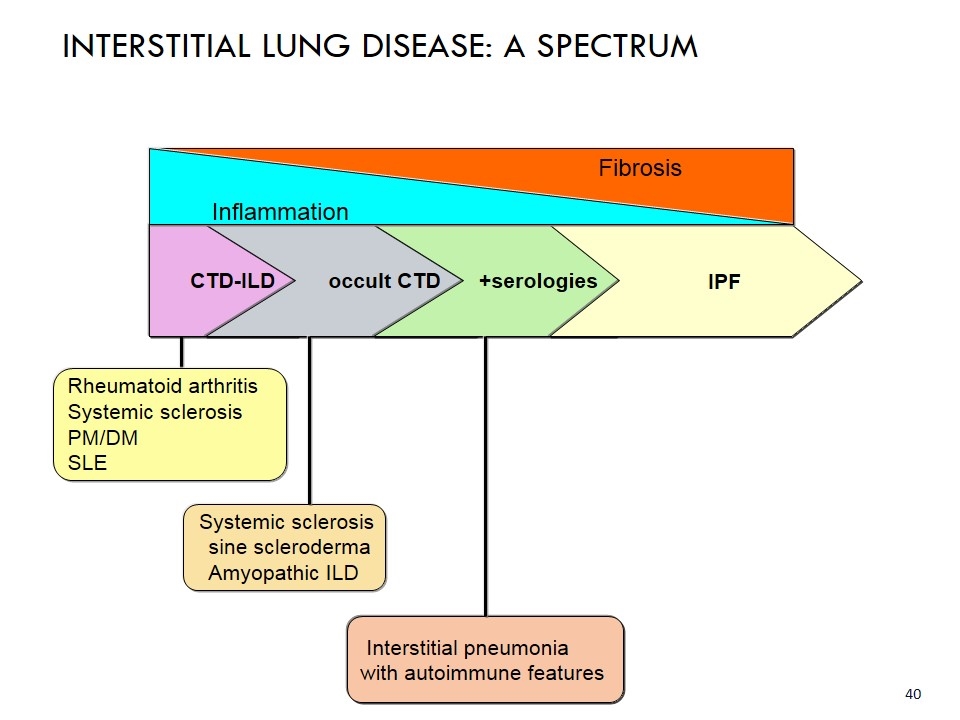
Fibrosis CTD-ILD occult CTD +serologies IPF Interstitial pneumonia with autoimmune features Systemic sclerosis sine scleroderma Amyopathic ILD Rheumatoid arthritis Systemic sclerosis PM/DM SLE INTERSTITIAL LUNG DISEASE: A SPECTRUM Inflammation
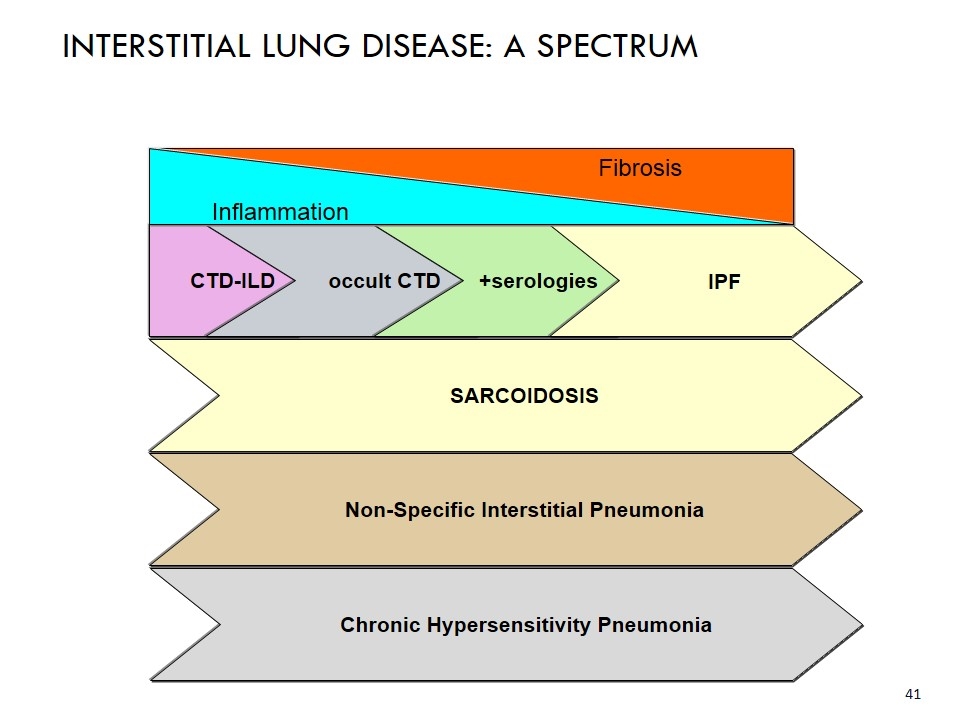
Fibrosis CTD-ILD occult CTD +serologies IPF INTERSTITIAL LUNG DISEASE: A SPECTRUM Inflammation SARCOIDOSIS Non-Specific Interstitial Pneumonia Chronic Hypersensitivity Pneumonia
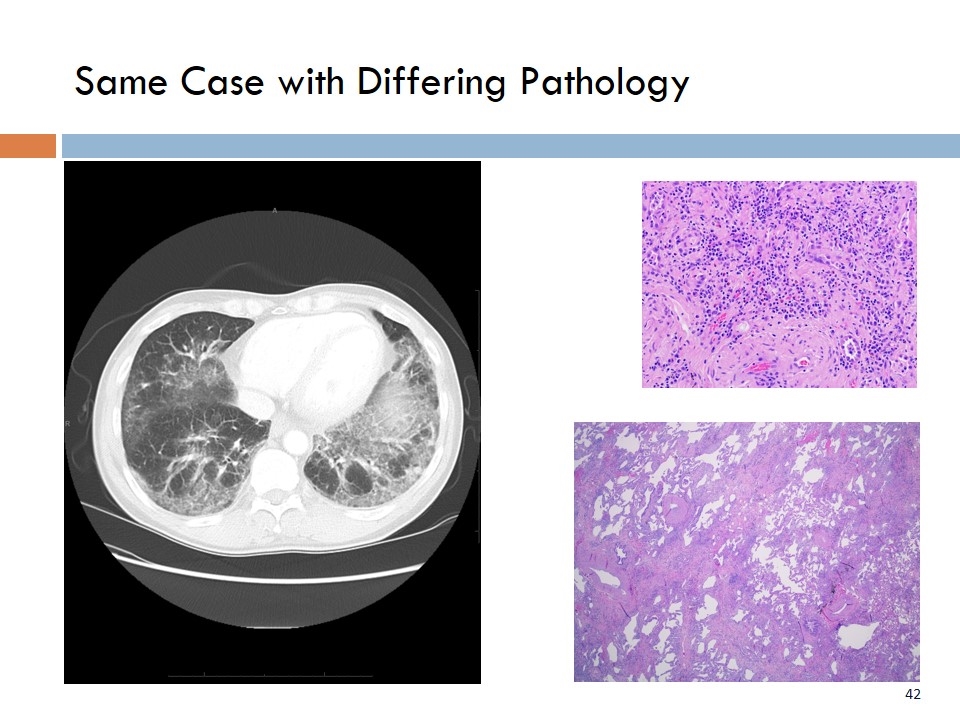
Same Case with Differing Pathology
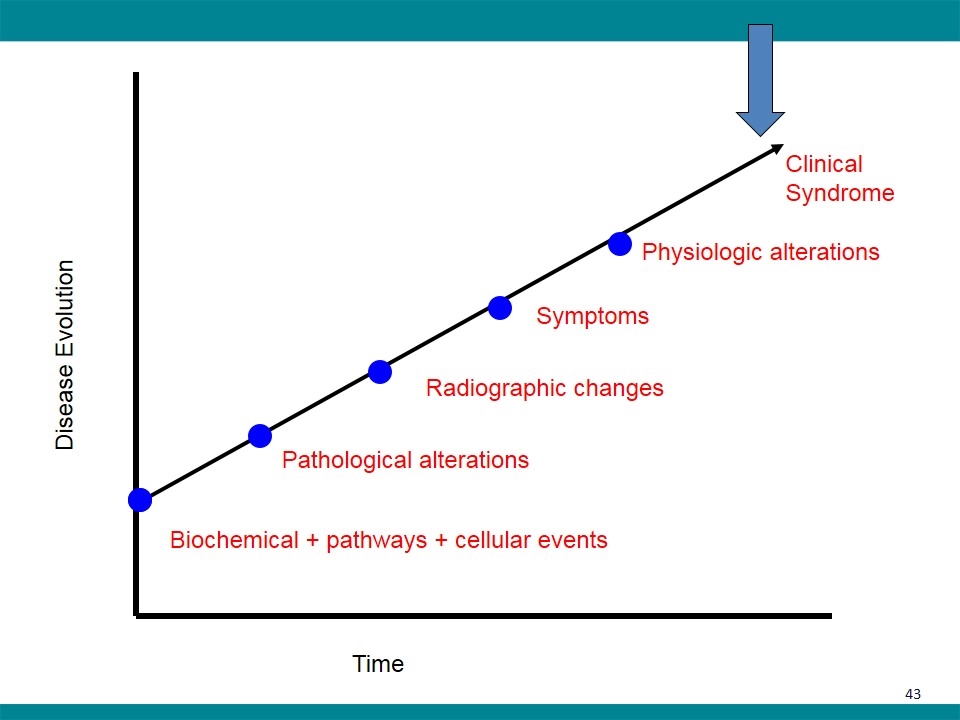
Disease Evolution Time Biochemical + pathways + cellular events Pathological alterations Radiographic changes Symptoms Physiologic alterations Clinical Syndrome
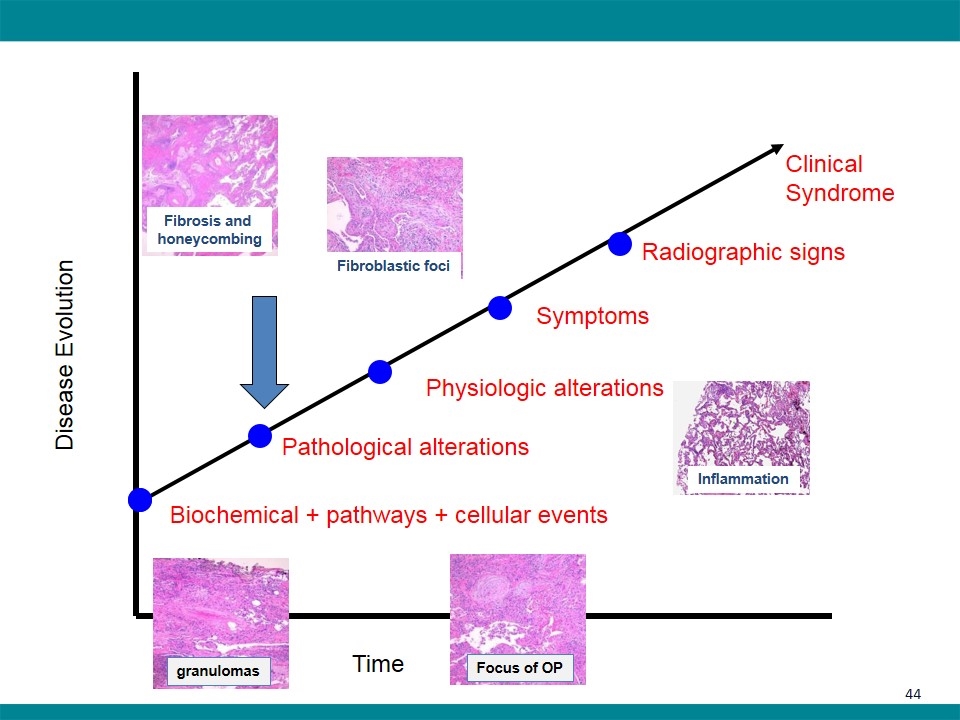
Disease Evolution Time Biochemical + pathways + cellular events Pathological alterations Physiologic alterations Symptoms Radiographic signs Clinical Syndrome Fibrosis and honeycombing Fibroblastic foci granulomas Focus of OP Inflammation
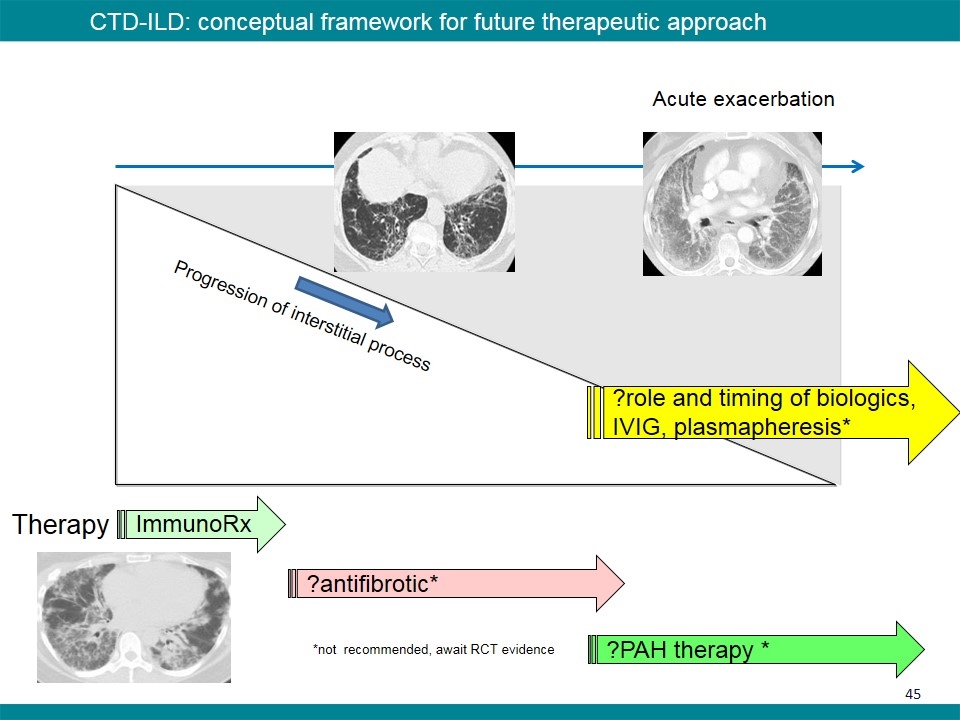
Progression of interstitial process Therapy ImmunoRx ?antifibrotic* ?PAH therapy * CTD-ILD: conceptual framework for future therapeutic approach *not recommended, await RCT evidence Acute exacerbation ?role and timing of biologics, IVIG, plasmapheresis*
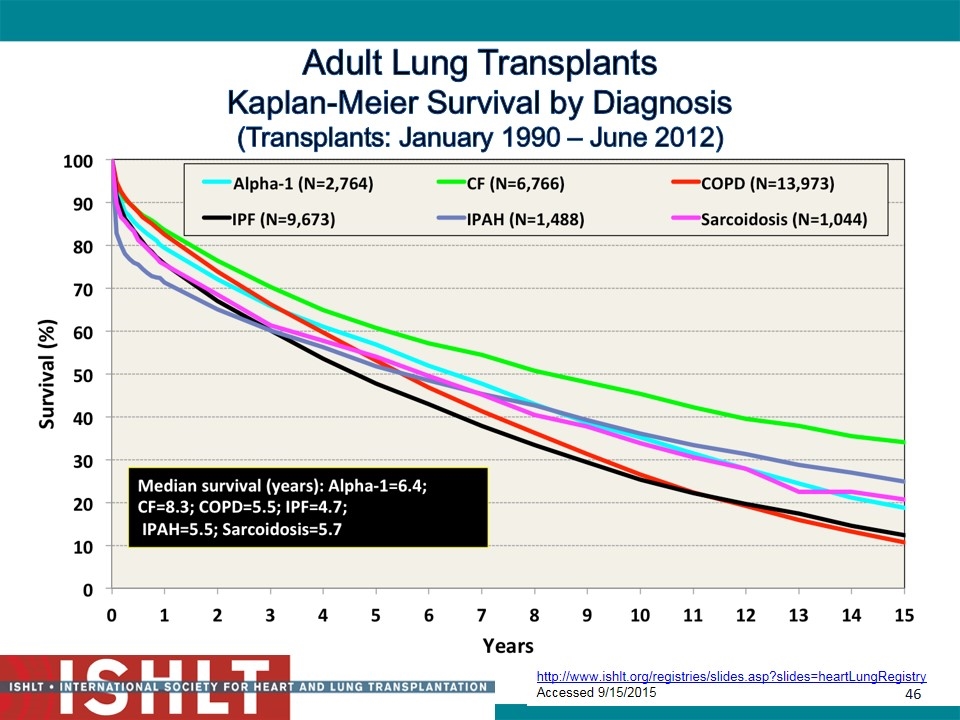
Adult Lung Transplants Kaplan-Meier Survival by Diagnosis (Transplants: January 1990 – June 2012) 2014 JHLT. 2014 Oct; 33(10): 1009-1024 http://www.ishlt.org/registries/slides.asp?slides=heartLungRegistry Accessed 9/15/2015

Lung Physiocrine Engineered to Treat Multiple Pulmonary Diseases iMod.Fc Program
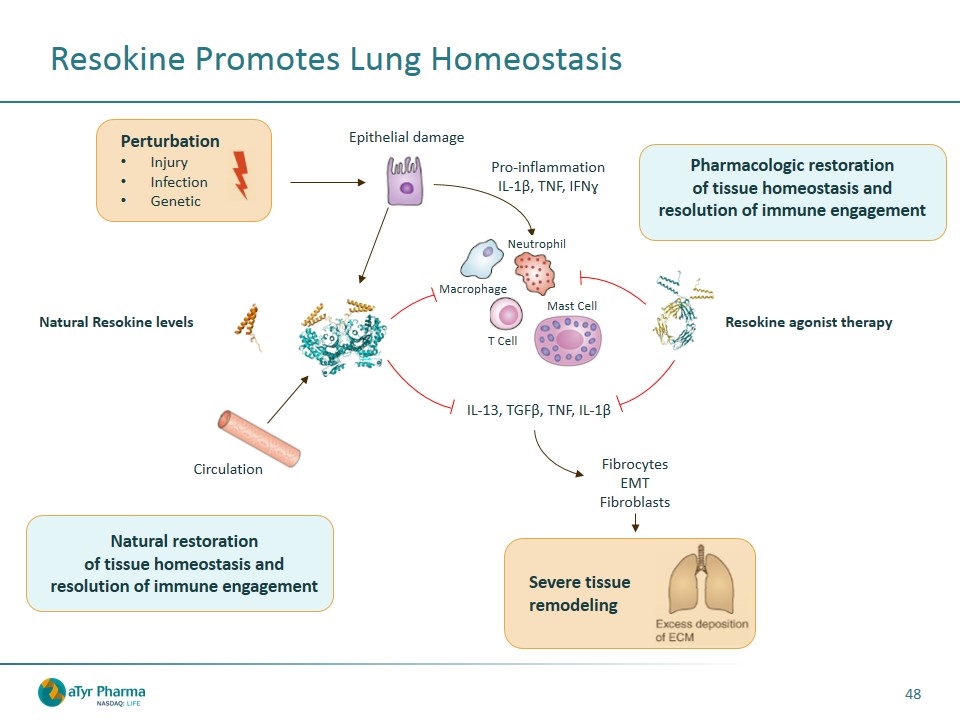
Resokine Promotes Lung Homeostasis Epithelial damage IL-13, TGFβ, TNF, IL-1β Resokine agonist therapy Pharmacologic restoration of tissue homeostasis and resolution of immune engagement Natural restoration of tissue homeostasis and resolution of immune engagement Perturbation Injury Infection Genetic Fibrocytes EMT Fibroblasts Severe tissue remodeling Circulation Natural Resokine levels Pro-inflammation IL-1β, TNF, IFNɣ T Cell Neutrophil Macrophage Mast Cell
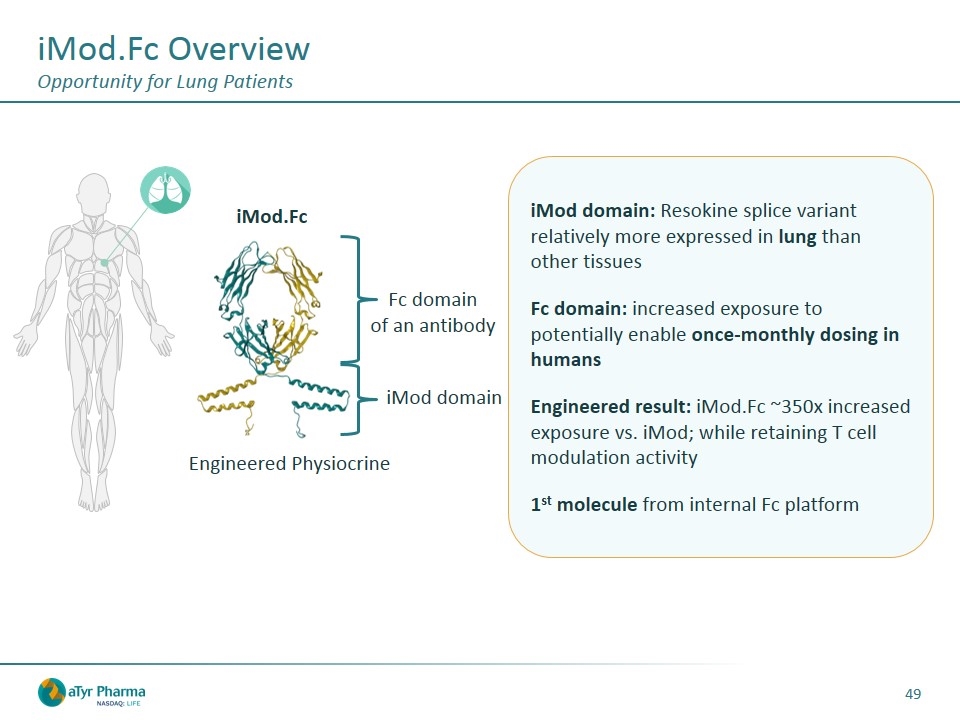
iMod.Fc Overview Opportunity for Lung Patients iMod domain Fc domain of an antibody Engineered Physiocrine iMod.Fc iMod domain: Resokine splice variant relatively more expressed in lung than other tissues Fc domain: increased exposure to potentially enable once-monthly dosing in humans Engineered result: iMod.Fc ~350x increased exposure vs. iMod; while retaining T cell modulation activity 1st molecule from internal Fc platform
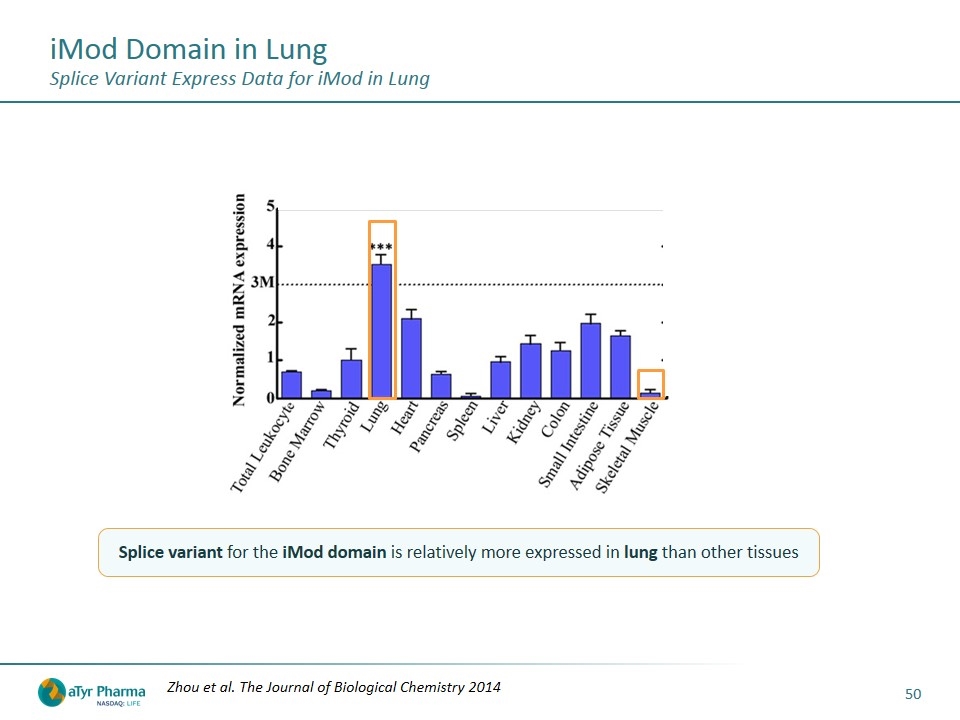
iMod Domain in Lung Splice Variant Express Data for iMod in Lung Splice variant for the iMod domain is relatively more expressed in lung than other tissues Zhou et al. The Journal of Biological Chemistry 2014
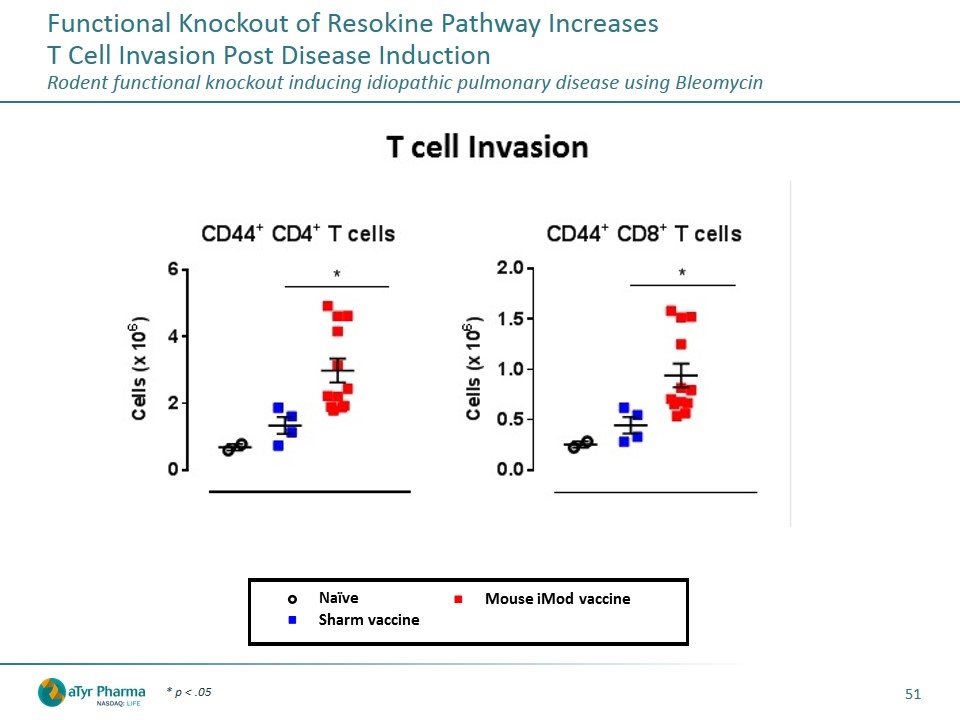
Functional Knockout of Resokine Pathway Increases T Cell Invasion Post Disease Induction Rodent functional knockout inducing idiopathic pulmonary disease using Bleomycin * p < .05
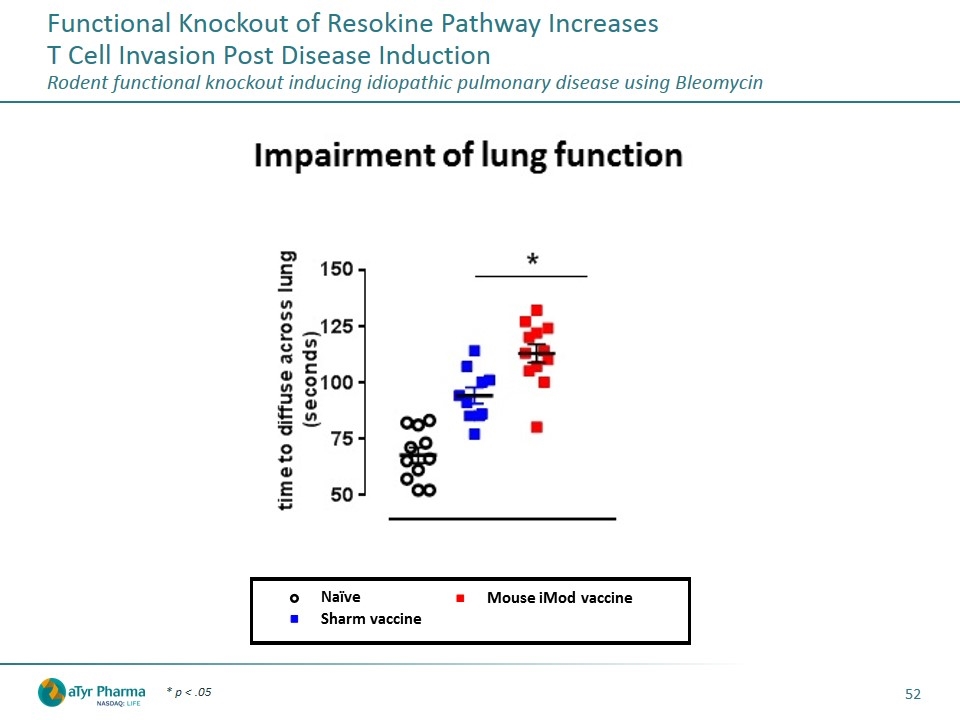
Functional Knockout of Resokine Pathway Increases T Cell Invasion Post Disease Induction Rodent functional knockout inducing idiopathic pulmonary disease using Bleomycin * p < .05
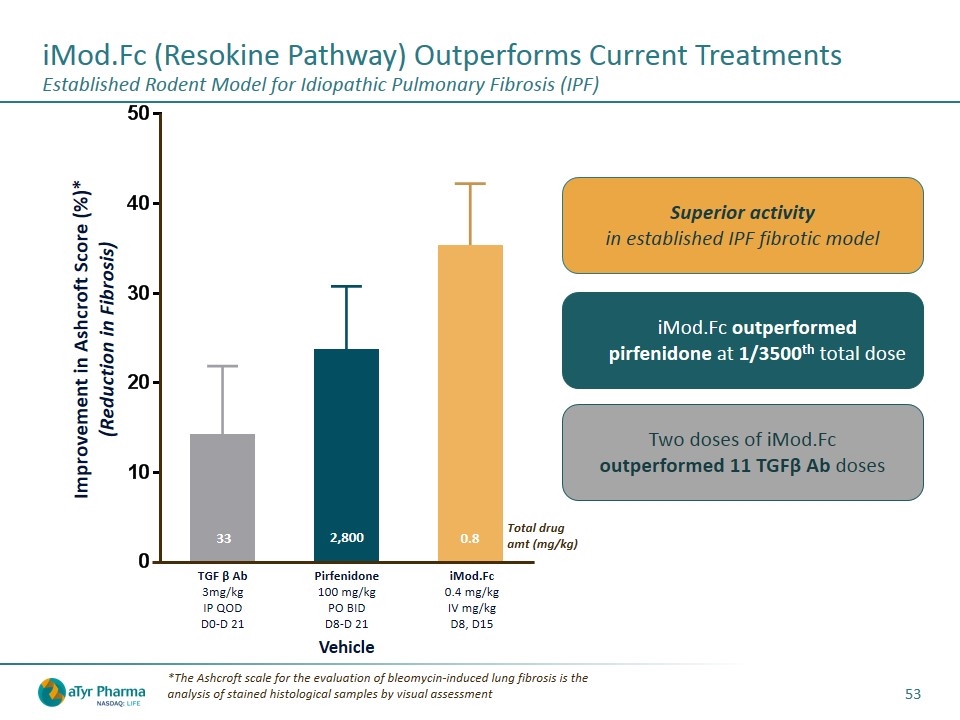
iMod.Fc (Resokine Pathway) Outperforms Current Treatments Established Rodent Model for Idiopathic Pulmonary Fibrosis (IPF) *The Ashcroft scale for the evaluation of bleomycin-induced lung fibrosis is the analysis of stained histological samples by visual assessment Improvement in Ashcroft Score (%)* (Reduction in Fibrosis) TGF β Ab 3mg/kg IP QOD D0-D 21 Pirfenidone 100 mg/kg PO BID D8-D 21 iMod.Fc 0.4 mg/kg IV mg/kg D8, D15 33 Total drug amt (mg/kg) 2,800 0.8 iMod.Fc outperformed pirfenidone at 1/3500th total dose Two doses of iMod.Fc outperformed 11 TGFβ Ab doses Superior activity in established IPF fibrotic model Vehicle

Biomarker/MOA: Introduce mechanistic/PD assay GMP Manufacturing: Complete initial clinical trial supply IND Enabling: Initiate preclinical safety studies Activity in industry proven model of IPF (approved drugs Pirfenidone & Nintedanib) GMP manufacturing kicked off Rat/non-human primate non-GLP safety & PK data support advancement to IND Milestones: 2017 Development Goals: iMod.Fc: Status and 2017 Development Goals Clinical Trial: Initiate first in human clinical trial

Questions?






















































