Exhibit 99.1
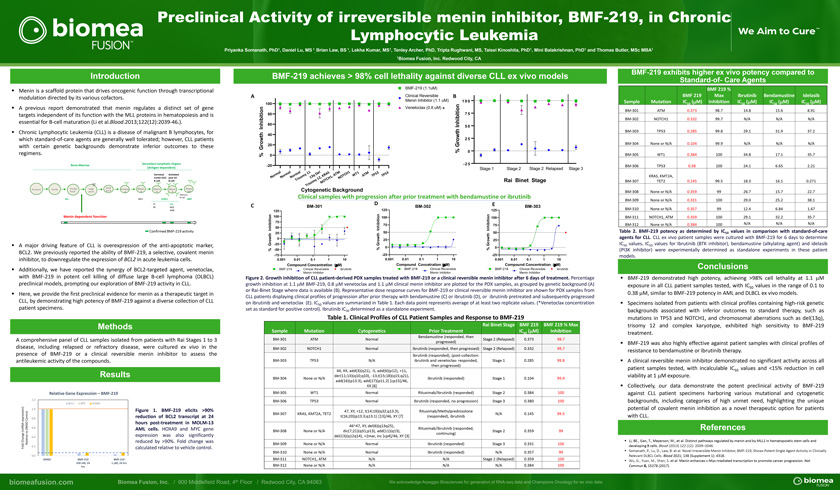
Preclinical Activity of irreversible menin inhibitor, BMF-219, in Chronic Lymphocytic LeukemiaPriyanka Somanath, PhD1, Daniel Lu, MS 1 Brian Law, BS 1, Lekha Kumar, MS1, Tenley Archer, PhD, Tripta Rughwani, MS, Taisei Kinoshita, PhD1, Mini Balakrishnan, PhD1 and Thomas Butler, MSc MBA1,1Biomea Fusion, Inc. Redwood City, CAIntroductionMenin is a scaffold protein that drives oncogenic function through transcriptional modulation directed by its various cofactors.A previous report demonstrated that menin regulates a distinct set of gene targets independent of its function with the MLL proteins in hematopoiesis and is essential for B-cell maturation (Li et al.Blood.2013;122(12):2039-46.).Chronic Lymphocytic Leukemia (CLL) is a disease of malignant B lymphocytes, for which standard-of-care agents are generally well tolerated; however, CLL patients with certain genetic backgrounds demonstrate inferior outcomes to these regimens.Secondary Lymphatic Organs Bone Marrow (Antigen-dependent)Germinal Activated Center (GC) post-GC y B-cell B-cellSmall Plasma Pre-pro B Pro-B1 Pro-B2/ Large pre-B Immature Mature Mature Mature Pre-B pre-B B B B B Cell Memory B-cellALL MCL DLBCL MMFL CLLBL MZL WMMenin dependent functionConfirmed BMF-219 activityA major driving feature of CLL is overexpression of the anti-apoptotic marker, BCL2. We previously reported the ability of BMF-219, a selective, covalent menin inhibitor, to downregulate the expression of BCL2 in acute leukemia cells.Additionally, we have reported the synergy of BCL2-targeted agent, venetoclax, with BMF-219 in potent cell killing of diffuse large B-cell lymphoma (DLBCL) preclinical models, prompting our exploration of BMF-219 activity in CLL.Here, we provide the first preclinical evidence for menin as a therapeutic target in CLL, by demonstrating high potency of BMF-219 against a diverse collection of CLL patient specimens.MethodsA comprehensive panel of CLL samples isolated from patients with Rai Stages 1 to 3 disease, including relapsed or refractory disease, were cultured ex vivo in the presence of BMF-219 or a clinical reversible menin inhibitor to assess the antileukemic activity of the compounds.ResultsRelative Gene Expression – BMF-2191.2BCL2 MYC HOXA91.0 Figure 1. BMF-219 elicits >90% reduction of BCL2 transcript at 24Control 0.8 expression)hours post-treatment in MOLM-13DMSO 0.6 AML cells. HOXA9 and MYC gene(mRNA tonge expression was also significantly0.4- Cha Relative reduced by >90%. Fold change was Fold 0.2 calculated relative to vehicle control.0.0DMSO BMF-219 BMF-219500 nM, 24 1 M, 24 hrsBMF-219 achieves > 98% cell lethality against diverse CLL ex vivo modelsBMF-219 (1.1uM)A Clinical Reversible B100 Menin Inhibitor (1.1 uM) 1 0 0 on Venetoclax (0.8 uM) *ti 80 7 5 hib i60 Inhibitionn 5 0I hth40t ow 2 520 G rGrow% 0% 0-20 -2 5Stage 1 Stage 2 Stage 2 Relapsed Stage 3al al al 1 el S M 1 1 3 3 m m m1D A T C H T TM 5 5 r r r y q RA T W A T P T P o o o om 3 , K 1 , ON N N i s 1 2 H N T r1 C y T Rai Binet Stage m O i s o NT rCytogenetic BackgroundClinical samples with progression after prior treatment with bendamustine or ibrutinibD EC BM-301 BM-302 BM-303125 125 125 n 100 o n n o 100 i o 100 t i ib i 7575 hibit 75 nh 50 nhibiti II In25 50 50 w th th0 25 ow 25 ro G -25 Growth Gr% 0 0% -50 % -75 -25 -250.001 0.01 0.1 1 10 0.001 0.01 0.1 1 10 0.001 0.01 0.1 1 10Compound Concentration (mM) Compound Concetration (mM) Compound Concentration (mM)BMF-219 Clinical Reversible ibrutinib BMF-219 Clinical Reversible BMF-219 Clinical Reversible ibrutinib Menin Inhibitor Menin Inhibitor Menin InhibitorFigure 2. Growth inhibition of CLL patient-derived PDX samples treated with BMF-219 or a clinical reversible menin inhibitor after 6 days of treatment. Percentage growth inhibition at 1.1 mM BMF-219, 0.8 mM venetoclax and 1.1 mM clinical menin inhibitor are plotted for the PDX samples, as grouped by genetic background (A) or Rai-Binet Stage where data is available (B). Representative dose response curves for BMF-219 or clinical reversible menin inhibitor are shown for PDX samples from CLL patients displaying clinical profiles of progression after prior therapy with bendamustine (C) or ibrutinib (D), or ibrutinib pretreated and subsequently progressed on ibrutinib and venetoclax (E). IC50 values are summarized in Table 1. Each data point represents average of at least two replicate values. (*Venetoclax concentration set as standard for positive control). Ibrutinib IC50 determined as a standalone experiment.Table 1. Clinical Profiles of CLL Patient Samples and Response to BMF-219Rai Binet Stage BMF 219 BMF 219 % Max Sample Mutation Cytogenetics Prior Treatment IC (mM) Inhibition50Bendamustine (responded, thenBM-301 ATM Normal Stage 2 (Relapsed) 0.373 98.7 progressed) BM-302 NOTCH1 Normal Ibrutinib (responded, then progressed) Stage 2 (Relapsed) 0.332 99.7 Ibrutinib (responded), (post-collection: BM-303 TP53 N/A ibrutinib and venetoclax- responded, Stage 1 0.285 99.8 then progressed) 44, XX, add(3)(q21), -5, add(6)(p12), +11, der(11;13)(q10;q10), -13,t(15;18)(q15;q21), BM-304 None or N/A Ibrutinib (responded) Stage 1 0.104 99.9 add(16)(p13.3), add(17)(p11.2) [cp15]/46, XX [6] BM-305 WT1 Normal Rituximab/Ibrutinib (responded) Stage 2 0.384 100BM-306 TP53 Normal Ibrutinib (responded, no progression) Stage 3 0.380 10047, XY, +12, t(14;19)(q32;q13.3), Rituximab/MethylprednisoloneBM-307 KRAS, KMT2A, TET2 N/A 0.145 99.5 t(16;20)(p13.3;q13.1) [13]/46, XY [7] (responded), ibrutinib46~47, XY, del(6)(q13q25),Rituximab/Ibrutinib (responded,BM-308 None or N/A dic(7;21)(q31;p13), add(11)(q13), Stage 2 0.359 99 continuing) del(13)(q12q14), +2mar, inc [cp4]/46, XY [3]BM-309 None or N/A Normal Ibrutinib (responded) Stage 3 0.331 100BM-310 None or N/A Normal Ibrutinib (responded) N/A 0.357 99 BM-311 NOTCH1, ATM N/A N/A Stage 2 (Relapsed) 0.359 100 BM-312 None or N/A N/A N/A N/A 0.384 100BMF-219 exhibits higher ex vivo potency compared toStandard-of- Care AgentsBMF 219 %BMF 219 Max Ibrutinib Bendamustine Idelasib Sample Mutation IC (mM) Inhibition IC (mM) IC (mM) IC (mM)50 50 50 50BM-301 ATM 0.373 98.7 14.8 15.6 8.91BM-302 NOTCH1 0.332 99.7 N/A N/A N/ABM-303 TP53 0.285 99.8 29.1 31.9 37.2 BM-304 None or N/A 0.104 99.9 N/A N/A N/A BM-305 WT1 0.384 100 34.8 17.1 35.7 BM-306 TP53 0.38 100 24.1 6.65 2.21KRAS, KMT2A,BM-307 TET2 0.145 99.5 18.3 16.1 0.271BM-308 None or N/A 0.359 99 26.7 15.7 22.7 BM-309 None or N/A 0.331 100 29.0 25.2 38.1 BM-310 None or N/A 0.357 99 12.4 6.84 1.67BM-311 NOTCH1, ATM 0.359 100 29.1 32.2 35.7 BM-312 None or N/A 0.384 100 N/A N/A N/ATable 2. BMF-219 potency as determined by IC50 values in comparison with standard-of-care agents for CLL. CLL ex vivo patient samples were cultured with BMF-219 for 6 days to determine IC50 values. IC50 values for Ibrutinib (BTK inhibitor), bendamustine (alkylating agent) and idelasib (PI3K inhibitor) were experimentally determined as standalone experiments in these patient models.Conclusionsâ–ª BMF-219 demonstrated high potency, achieving >98% cell lethality at 1.1 mM exposure in all CLL patient samples tested, with IC50 values in the range of 0.1 to 0.38 mM, similar to BMF-219 potency in AML and DLBCL ex vivo models.â–ª Specimens isolated from patients with clinical profiles containing high-risk genetic backgrounds associated with inferior outcomes to standard therapy, such as mutations in TP53 and NOTCH1, and chromosomal aberrations such as del(13q), trisomy 12 and complex karyotype, exhibited high sensitivity to BMF-219 treatment.â–ª BMF-219 was also highly effective against patient samples with clinical profiles of resistance to bendamustine or ibrutinib therapy.â–ª A clinical reversible menin inhibitor demonstrated no significant activity across all patient samples tested, with incalculable IC50 values and <15% reduction in cell viability at 1 mM exposure.â–ª Collectively, our data demonstrate the potent preclinical activity of BMF-219 against CLL patient specimens harboring various mutational and cytogenetic backgrounds, including categories of high unmet need, highlighting the unique potential of covalent menin inhibition as a novel therapeutic option for patients with CLL.Referencesâ–ª Li, BE., Gan, T., Meyerson, M., et al. Distinct pathways regulated by menin and by MLL1 in hematopoietic stem cells and developing B cells. Blood (2013) 122 (12): 2039–2046.â–ª Somanath, P., Lu, D., Law, B. et al. Novel Irreversible Menin Inhibitor, BMF-219, Shows Potent Single Agent Activity in ClinicallyRelevant DLBCL Cells. Blood 2021; 138 (Supplement 1): 4318.â–ª Wu, G., Yuan, M., Shen, S. et al. Menin enhances c-Myc-mediated transcription to promote cancer progression. Nat Commun 8, 15278 (2017).
