Below is a reproduction of the contents of the poster entitled “Low-Dose, Controlled-Release Phentermine/Topiramate Reduces Hunger and Increases Satiety, Leading to Weight Loss in Overweight/Obese Adults”:
Authors: Wesley W. Day, PhD; Thomas Najarian, MD; Barbara Troupin, MD, MBA; Peter Tam, MBA; Craig A. Peterson, MS
VIVUS, Inc., Mountain View, California, USA
· Introduction
· Obesity is an epidemic in the United States associated with significant comorbidities such as cardiovascular disease and type 2 diabetes mellitus (T2DM), as well as increased mortality.(1)-(4)
· Weight loss reduces the incidence of cardiometabolic comorbidities and improves multiple cardiovascular risk factors such as lipids, blood pressure, sleep apnea, and glycemia.(5),(6)
· Patient perceptions of hunger and satiety play an integral role in modulating food intake(7), and a successful weight-loss strategy addresses hunger and satiety.
· Phentermine (PHEN) and topiramate (TPM) are 2 pharmacologic agents with demonstrated weight-loss properties and mechanisms of action that may impact appetite and satiety. PHEN is currently approved in the United States for short-term weight loss (recommended dose: 37.5 mg/day) as an adjunct to lifestyle modifications. TPM is indicated for treatment of seizures (recommended dose: 400 mg/day) and prevention of migraines (recommended dose: 100 mg/day).(8),(9)
· A novel, investigational combination of controlled-release (CR) phentermine/topiramate (PHEN/TPM CR) comprised of lower than currently approved doses was developed for once-daily oral dosing to maximize weight loss and cardiometabolic benefits, while minimizing adverse events (AEs).
· Objective
· To examine the effect of PHEN/TPM CR on weight loss and subjects’ perceptions of hunger and satiety.
· Methods
· 2 clinical studies are included in this evaluation:
· CONQUER: double-blind, placebo-controlled Phase 3 trial of 2487 overweight/obese adult subjects with >2 weight-related comorbidities randomly assigned to placebo, PHEN 7.5 mg/TPM CR 46 mg (7.5/46), or PHEN 15 mg/TPM CR 92 mg (15/92) for 56 weeks. All subjects received lifestyle guidance using the LEARN® Program for Weight Management.(10)
· EQUIP: double-blind, placebo-controlled Phase 3 trial of 1267 overweight/obese subjects, excluding subjects with T2DM, randomly assigned to placebo, PHEN 3.75 mg/TPM CR 23 mg (3.75/23), or 15/92 for 56 weeks.
· A pooled analysis of CONQUER and EQUIP was conducted in order to determine the effects of PHEN/TPM CR over a broad range of subjects.
· Visual analogue scale (VAS) questionnaires were administered throughout the trials and included questions about subjects’ perceived levels of hunger and satiety; lower scores indicated reduction in hunger and increase in satiety.
· Assessments
· Subjects’ perceptions of hunger and satiety were measured based on VAS at baseline, 28 weeks, and 56 weeks.
· Percent weight loss at Week 56 with last observation carried forward (LOCF) for the intent-to-treat (ITT) sample was the primary efficacy endpoint for both Phase 3 studies. This endpoint was also assessed among study completers.
· For VAS quartile comparison, the estimates (least-squares [LS] mean) are from an analysis of covariance (ANCOVA) with treatment and gender as fixed effects and with baseline weight as a covariate.
· Results
· Baseline characteristics of the pooled ITT 1-year cohort are presented in Table 1. Subjects were mostly female (74.3%), with a mean age of 48.3 years and mean body mass index (BMI) of 38.4 kg/m2.
Table 1. Baseline Characteristics of Pooled Analysis of EQUIP and CONQUER: ITT Set - 1 Year Cohort
| | Placebo
(n=1477) | | PHEN/TPM CR
3.75/23
(n=234) | | PHEN/TPM CR
7.5/46
(n=488) | | PHEN/TPM CR
15/92
(n=1479) | |
Age (years) | | | | | | | | | |
Mean (SD) | | 48.5 (11.4) | | 43.0 (11.1) | | 51.1 (10.4) | | 48.0 (12.0) | |
Age, n (%) | | | | | | | | | |
<65 years | | 1382 (93.6) | | 230 (98.3) | | 442 (90.6) | | 1376 (93.0) | |
Gender, n (%) | | | | | | | | | |
Female | | 1102 (74.6) | | 194 (82.9) | | 341 (69.9) | | 1094 (74.0) | |
Weight (kg) | | | | | | | | | |
Mean (SD) | | 107.5 (20.2) | | 118.6 (21.9) | | 102.8 (18.2) | | 107.1 (19.6) | |
Waist circumference (cm) | | | | | | | | | |
Mean (SD) | | 115.8 (13.3) | | 121.5 (15.2) | | 112.7 (12.4) | | 115.5 (13.5) | |
Body mass index (kg/m2) | | | | | | | | | |
Mean (SD) | | 38.5 (5.7) | | 42.5 (6.5) | | 36.3 (4.4) | | 38.4 (5.7) | |
Body mass index category n (%) | | | | | | | | | |
>30 and <40 kg/m2 | | 904 (61.2) | | 91 (38.9) | | 344 (70.5) | | 887 (60.0) | |
>40 kg/m2 | | 502 (34.0) | | 143 (61.1) | | 111 (22.7) | | 521 (35.2) | |
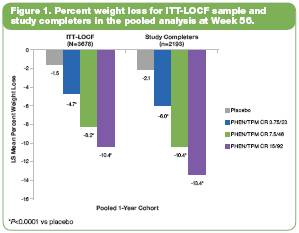
· LS mean percent weight loss at 56 weeks in the ITT-LOCF sample and the study completer sample was statistically significant (P<0.0001 vs placebo; Figure 1).
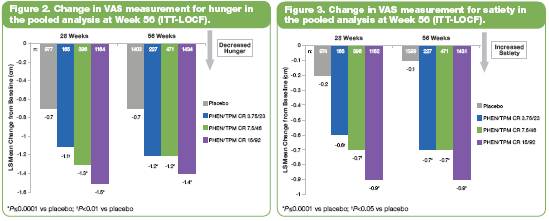
· Change in subjects’ perception of hunger and satiety, measured as LS mean change in VAS scores, was significantly improved for 15/92 vs placebo (P<0.0001) after 28 and 56 weeks of treatment (Figures 2 and 3).
· A comparison of the upper and lower quartiles of VAS response demonstrated that subjects in the lower VAS quartile, those with the greatest improvements in hunger and satiety, lost significantly more weight than those subjects in the upper quartile (least improvement in hunger and satiety) (P<0.0001 vs upper quartile; Figure 4).

· PHEN/TPM CR was generally well tolerated. Of the 3754 randomized subjects, 59.2% completed all study visits on study drug. A higher percentage of subjects in the PHEN/TPM CR groups than in the placebo group completed the study on study drug (63.1% vs. 53.4%).
· 13.1% of subjects discontinued study drug due to AEs, and rates were dose dependent.
· The most commonly reported treatment-emergent AEs are listed in Table 2. The majority of AEs were considered mild or moderate.
Table 2. Most Commonly Reported Treatment-Emergent AEs*
Adverse Event
(%) | | Placebo | | PHEN/TPM CR
3.75/23 | | PHEN/TPM CR
7.5/46 | | PHEN/TPM CR
15/92 | |
Paresthesia | | 2.0 | | 4.2 | | 13.7 | | 19.9 | |
Dry mouth | | 2.9 | | 6.7 | | 13.5 | | 19.5 | |
Constipation | | 6.2 | | 7.9 | | 15.1 | | 16.3 | |
Upper respiratory tract infection | | 12.2 | | 15.8 | | 12.2 | | 13.0 | |
Headache | | 9.4 | | 10.4 | | 7.0 | | 10.8 | |
Dysgeusia | | 1.1 | | 1.3 | | 7.4 | | 9.7 | |
Nasopharyngitis | | 8.2 | | 12.5 | | 10.6 | | 9.6 | |
Insomnia | | 4.8 | | 5.0 | | 5.8 | | 9.4 | |
Dizziness | | 3.5 | | 2.9 | | 7.2 | | 8.5 | |
Sinusitis | | 6.3 | | 7.5 | | 6.8 | | 8.1 | |
Nausea | | 4.4 | | 5.8 | | 3.6 | | 7.0 | |
Diarrhea | | 4.7 | | 5.0 | | 6.4 | | 5.4 | |
Back pain | | 5.0 | | 5.4 | | 5.6 | | 6.6 | |
Fatigue | | 4.4 | | 5.0 | | 4.4 | | 6.0 | |
Blurred vision | | 3.5 | | 6.3 | | 4.0 | | 5.5 | |
*reported in >5% of subjects receiving PHEN/TPM CR 15/92
· The incidence of treatment-emergent serious AEs was low and similar for all treatment groups: 3.3% of subjects in the placebo group, 2.5% of 3.75/23 group, 2.8% of 7.5/46 group, and 3.5% of 15/92 group.
· Conclusions
· PHEN/TPM CR treatment is associated with significant and clinically meaningful weight loss in overweight/obese subjects at all 3 doses tested.
· PHEN/TPM CR was generally well tolerated based on rates of study completion, discontinuation rates, and overall adverse events.
· PHEN/TPM CR treatment led to clinically meaningful reduction in hunger and increased satiety as measured by VAS; with greater VAS effects corresponding to greater weight loss.
These trials are registered at ClinicalTrials.gov, number NCT00554216 (EQUIP) and number NCT00553787 (CONQUER).
References: (1) Reaven GM. Ann Intern Med 2003. (2) Flegal KM, et al. JAMA 2010. (3) Haslam DW, et al. Lancet 2005. (4) Adams KF, et al. N Engl J Med 2006. (5) Van Gaal LF, et al. Int J Obes Relat Metab Disord 1997. (6) Kopp HP, et al. Arterioscler Thromb Vasc Biol 2003. (7) National Heart, Lung, and Blood Institute Obesity Education Initiative. October 2000. NIH Publication Number 00-4084. (8) Adipex-P [package insert]. Teva Pharmaceuticals USA; July 2005. (9) Topamax [package insert]. Ortho-McNeil-Janssen Pharmaceuticals, Inc.; December 2009. (10) Brownell KD. The LEARN Program for Weight Management. 2004.
Acknowledgements: We would like to acknowledge and thank the EQUIP and CONQUER investigators and study coordinators, the Medpace team (study CRO), The Lockwood Group (for poster development assistance), and VIVUS internal contributors.



