Below is a reproduction of the contents of the poster entitled “Low-Dose Controlled-Release Phentermine/Topiramate for Weight Loss and Management of Type 2 Diabetes Mellitus”:
Authors: W. Timothy Garvey, MD(a); Craig A. Peterson, MS(b); Barbara Troupin, MD, MBA(b); Charles H. Bowden, MD(b)
(a)University of Alabama at Birmingham, Birmingham, Alabama, USA; (b)Vivus, Inc., Mountain View, California, USA
· Introduction
· Obesity is a global epidemic associated with comorbidities such as cardiovascular disease and type 2 diabetes mellitus (T2DM), as well as increased mortality.(1)-(4)
· Weight loss is gaining recognition as an important factor in management of T2DM(5)-(6) and has been shown to improve multiple cardiometabolic risk factors.(6)-(8)
· Moreover, weight loss of 5% to 10% and lifestyle intervention have been shown to prevent progression of T2DM in at-risk patients with T2DM.(6),(9),(10) The National Heart, Lung, and Blood Institute recommends 10% weight loss as an initial target.(11)
· Phentermine (PHEN) and topiramate (TPM) are 2 pharmacologic agents with demonstrated weight-loss properties. PHEN is approved for short-term weight loss (recommended dose: 37.5 mg/day) as an adjunct to lifestyle modifications.(12) TPM is indicated for treatment of seizures (recommended dose: 400 mg/day) and prevention of migraine headaches (recommended dose: 100 mg/day).(13)
· A novel investigational combination of low-dose, controlled-release (CR) PHEN/TPM for once-daily oral dosing was developed to maximize weight loss and cardiometabolic benefits, while minimizing dose-related adverse events (AEs).
· Objectives
· To evaluate the capacity of PHEN/TPM CR to promote weight loss in overweight/obese subjects with and without T2DM.
· To evaluate changes in insulin sensitivity, glucose tolerance, and HbA1c over a 56-week period in the subset of this population with T2DM at baseline.
· Methods
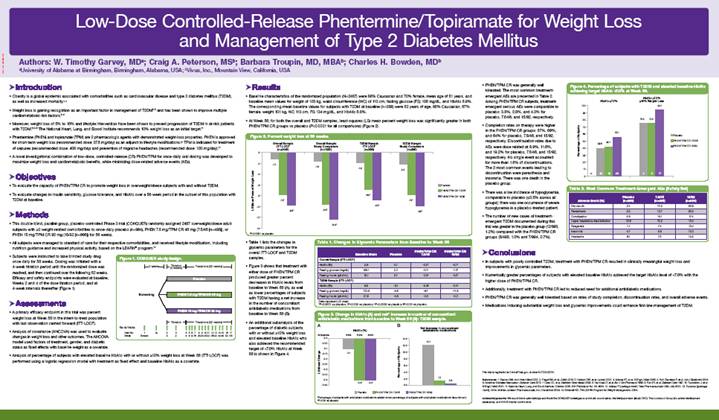
· This double-blind, parallel-group, placebo-controlled Phase 3 trial (CONQUER) randomly assigned 2487 overweight/obese adult subjects with >2 weight-related comorbidities to once-daily placebo (n=994), PHEN 7.5 mg/TPM CR 46 mg (7.5/46 [n=498]), or PHEN 15 mg/TPM CR 92 mg (15/92 [n=995]) for 56 weeks.
· All subjects were managed to standard of care for their respective comorbidities, and received lifestyle modification, including nutrition guidance and increased physical activity, based on the LEARN® program.(14)
· Subjects were instructed to take blinded study drug once daily for 56 weeks. Dosing was initiated with a 4-week titration period until the randomized dose was reached, and then continued over the following 52 weeks. Efficacy and safety endpoints were evaluated at baseline, Weeks 2 and 4 of the dose titration period, and at 4-week intervals thereafter (Figure 1).
· Assessments
· A primary efficacy endpoint in this trial was percent weight loss at Week 56 in the intent-to-treat population with last observation carried forward (ITT-LOCF).
· Analysis of covariance (ANCOVA) was used to evaluate changes in weight loss and other outcomes. The ANCOVA model used factors of treatment, gender, and diabetic status as fixed effects with baseline weight as a covariate.
· Analysis of percentage of subjects with elevated baseline HbA1c with or without >10% weight loss at Week 56 (ITT-LOCF) was performed using a logistic regression model with treatment as fixed effect and baseline HbA1c as a covariate.
· Results
· Baseline characteristics of the randomized population (N=2487) were 86% Caucasian and 70% female, mean age of 51 years, and baseline mean values for weight of 103 kg, waist circumference (WC) of 113 cm, fasting glucose (FG) 106 mg/dL, and HbA1c 5.9%. The corresponding mean baseline values for subjects with T2DM at baseline (n=388) were 52 years of age, 85% Caucasian, 67% female, weight 101 kg, WC 113 cm, FG 134 mg/dL, and HbA1c 6.8%.
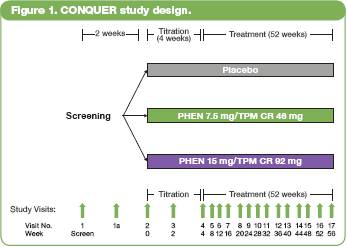
· At Week 56, for both the overall and T2DM samples, least-squares (LS) mean percent weight loss was significantly greater in both PHEN/TPM CR groups vs placebo (P<0.0001 for all comparisons) (Figure 2).
· Table 1 lists the changes in glycemic parameters for the overall ITT-LOCF and T2DM samples.
Table 1. Changes in Glycemic Parameters from Baseline to Week 56
| | | | | | PHEN/TPM CR | | PHEN/TPM CR | |
| | Baseline Mean | | Placebo | | 7.5/46 | | 15/92 | |
Overall Sample (ITT-LOCF) | | | | | | | | | |
HbA1c (%) | | 5.9 | | 0.1 | | -0.0* | | -0.1* | |
Fasting glucose (mg/dL) | | 106.1 | | 2.3 | | -0.1† | | -1.3* | |
Fasting insulin (mIU/mL) | | 18.1 | | 0.7 | | -3.5‡ | | -4.0* | |
T2DM Sample (ITT-LOCF) | | | | | | | | | |
HbA1c (%) | | 6.8 | | -0.1 | | -0.4§ | | -0.4† | |
Fasting glucose (mg/dL) | | 133.9 | | -5.6 | | -9.7 | | -11.9 | |
Fasting insulin (mIU/mL) | | 21.6 | | -4.6 | | -4.0 | | -5.3 | |
Data represent LS mean
*P<0.0001 vs placebo; †P<0.005 vs placebo; ‡P<0.0005 vs placebo; §P<0.05 vs placebo
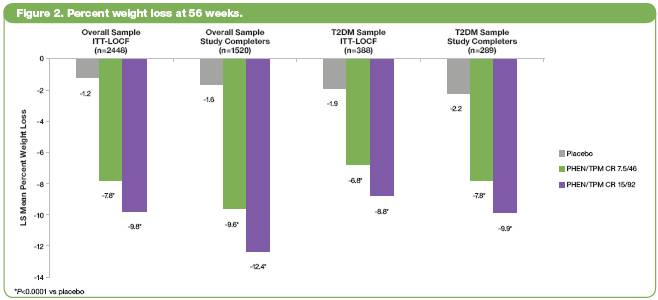
· Figure 3 shows that treatment with either dose of PHEN/TPM CR produced greater percent decreases in HbA1c levels from baseline to Week 56 (A), as well as lower percentages of subjects with T2DM having a net increase in the number of concomitant antidiabetic medications from baseline to Week 56 (B).
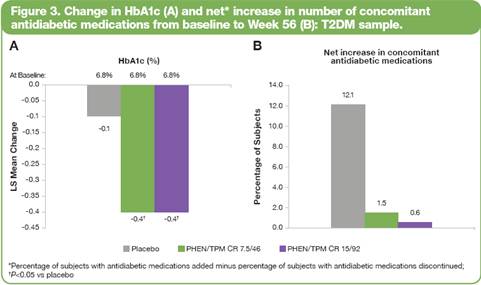
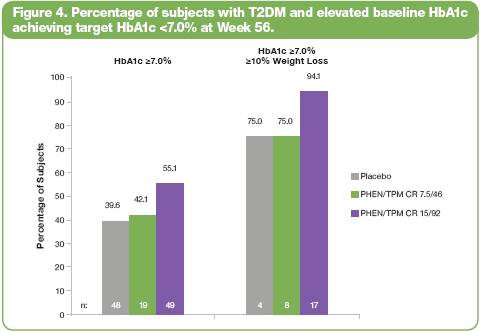
· An additional subanalysis of the percentage of diabetic subjects with or without >10% weight loss and elevated baseline HbA1c who also achieved the recommended target of <7.0% HbA1c at Week 56 is shown in Figure 4.
· PHEN/TPM CR was generally well tolerated. The most common treatment-emergent AEs are presented in Table 2. Among PHEN/TPM CR subjects, treatment-emergent serious AEs were comparable to placebo: 3.8%, 2.8%, and 4.3% for placebo, 7.5/46, and 15/92, respectively.
Table 2. Most Common Treatment-Emergent AEs (Safety Set)
Adverse Event (%) | | Placebo
(n=993) | | 7.5/46
(n=498) | | 15/92
(n=994) | |
Dry mouth | | 2.4 | | 13.5 | | 20.8 | |
Paresthesia | | 2.0 | | 13.7 | | 20.5 | |
Constipation | | 5.9 | | 15.1 | | 17.4 | |
Upper respiratory tract infection | | 12.9 | | 12.2 | | 13.4 | |
Dysgeusia | | 1.1 | | 7.4 | | 10.4 | |
Insomnia | | 4.7 | | 5.8 | | 10.3 | |
Headache | | 9.1 | | 7.0 | | 10.2 | |
· Completion rates on therapy were higher in the PHEN/TPM CR groups: 57%, 69%, and 64% for placebo, 7.5/46, and 15/92, respectively. Discontinuation rates due to AEs were dose related at 8.9%, 11.6%, and 19.2% for placebo, 7.5/46, and 15/92, respectively. No single event accounted for more than 1.6% of discontinuations. The 2 most common events leading to discontinuation were paresthesia and insomnia. There was one death in the placebo group.
· There was a low incidence of hypoglycemia, comparable to placebo (<0.5% across all groups); there was one occurrence of severe hypoglycemia in a placebo-treated patient.
· The number of new cases of treatment-emergent T2DM documented during this trial was greater in the placebo group (12/993, 1.2%) compared with the PHEN/TPM CR groups (5/498, 1.0% and 7/994, 0.7%).
· Conclusions
· In subjects with poorly controlled T2DM, treatment with PHEN/TPM CR resulted in clinically meaningful weight loss and improvements in glycemic parameters.
· Numerically greater percentages of subjects with elevated baseline HbA1c achieved the target HbA1c level of <7.0% with the higher dose of PHEN/TPM CR.
· Additionally, treatment with PHEN/TPM CR led to reduced need for additional antidiabetic medications.
· PHEN/TPM CR was generally well tolerated based on rates of study completion, discontinuation rates, and overall adverse events.
· Medications inducing substantial weight loss and glycemic improvements could enhance first-line management of T2DM.
This trial is registered at ClinicalTrials.gov, number NCT00553787.
References: (1) Reaven GM. Ann Intern Med 2003. (2) Flegal KM, et al. JAMA 2010. (3) Haslam DW, et al. Lancet 2005. (4) Adams KF, et al. N Engl J Med 2006. (5) Koh-Banerjee P, et al. Am J Epidemiol 2004. (6) American Diabetes Association. Diabetes Care 2010. (7) Case CC, et al. Diabetes Obes Metab 2002. (8) Van Gaal LF, et al. Eur J Clin Pharmacol 1998. (9) Pan XR, et al. Diabetes Care 1997. (10) Tuomilehto J, et al. N Engl J Med 2001. (11) National Heart, Lung, and Blood Institute. October 2000. NIH Publication No. 00-4084. (12) Adipex-P [package insert]. Teva Pharmaceuticals USA; July 2005. (13) Topamax [package insert]. Ortho-McNeil-Janssen Pharmaceuticals, Inc.; December 2009. (14) Brownell KD. The LEARN Program for Weight Management. 2004.
Acknowledgements: We would like to acknowledge and thank the CONQUER investigators and study coordinators, the Medpace team (study CRO), The Lockwood Group (for poster development assistance), and VIVUS internal contributors.