Below is a reproduction of the contents of the poster entitled “Low-Dose, Controlled-Release Phentermine/Topiramate Reduces Adiposity, Improves Cardiometabolic Risk in Overweight/Obese Patients”:
Authors: Dympna Gallagher, EdD(a); Mark Punyanitya, MA(a); Charles H. Bowden, MD(b); Craig A. Peterson, MS(b)
(a)St. Luke’s-Roosevelt Hospital Center at Columbia University, New York, New York, USA; (b)Vivus, Inc., Mountain View, California, USA
· Introduction
· Obesity is a significant health problem which is associated with comorbidities such as cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM), as well as increased risk of mortality.(1)-(4)
· Increased adiposity, especially central adiposity, is associated with increased cardiometabolic risk.(5),(6) Weight loss has been shown to improve cardiometabolic risk parameters, thereby delaying or preventing CVD and T2DM.(7)-(9)
· Phentermine (PHEN) and topiramate (TPM) are 2 pharmacologic agents with demonstrated weight-loss properties. PHEN is currently approved in the United States as a short-term treatment for weight loss (recommended dose: 37.5 mg/day) as an adjunct to lifestyle modifications, while TPM is indicated for treatment of seizures (recommended dose: 400 mg/day) and prevention of migraine headaches (recommended dose: 100 mg/day).(10),(11)
· A previous formulation of TPM at doses titrated up to 384 mg/day demonstrated weight loss in clinical trials.(12) Studies have also demonstrated significant improvements in blood pressure and glycemic and lipid parameters;(13)-(17) however, dose-related side effects were significant, and prevented further development of TPM as monotherapy for weight loss.(12)-(17)
· An investigational, controlled-release (CR) formulation of low-dose PHEN/TPM provides a combination therapy which potentially achieves greater improvements in weight and CVD risk factors at lower doses than monotherapeutic weight-loss agents.
· Objectives
· To determine percent weight loss at 56 weeks.
· To determine the impact of degree of adiposity lost in treatment with PHEN/TPM CR and to determine effect on cardiometabolic risk over a 56-week period.
· Methods
· Pooled 1-year data were analyzed from two double-blind, placebo-controlled Phase 3 clinical trials:
· EQUIP: 1267 obese subjects without T2DM (BMI ³35 kg/m2), randomly assigned to placebo, PHEN 3.75 mg/TPM CR 23 mg (3.75/23), or PHEN 15 mg/TPM CR 92 mg (15/92) for 56 weeks.
· CONQUER: 2487 overweight/obese adult subjects (BMI ³27 kg/m2 and £45 kg/m2) with ³2 weight-related comorbidities randomly assigned to placebo, PHEN 7.5 mg/TPM CR 46 mg (7.5/46), or 15/92 for 56 weeks.
· All subjects received lifestyle and exercise guidance using the LEARN® Program for Weight Management.(18)
· Dual energy X-ray absorptiometry (DEXA) scans were conducted in 288 subjects at 16 sites, to derive body composition data. All scans were read at a central site; readings at baseline and Week 56 were available for 202 subjects.
· Efficacy and safety endpoints were evaluated at baseline and Weeks 2 and 4 of a 4-week titration period, and then at 4-week intervals throughout the trials.
· Assessments
· A primary efficacy endpoint for both Phase 3 studies was percent weight loss at Week 56 in the intent-to-treat (ITT) population with last observation carried forward (LOCF).
· Additional endpoints included changes in adiposity, lean body mass, waist circumference (WC), where WC is an accepted surrogate marker of central adiposity(6), and lipid and glycemic parameters.
· Analysis of percent weight loss at Week 56 was performed using an analysis of covariance (ANCOVA) model with treatment, study, and gender as fixed effects and baseline weight as a covariate.
· Results
· Baseline characteristics of the pooled ITT 1-year cohort are presented in Table 1. Subjects were mostly female (74.3%), age <65 years (93.3%), and overweight or obese (60.5% BMI ³30 kg/m2 and <40 kg/m2; 34.7% BMI ³40 kg/m2).
Table 1. Baseline Characteristics of Pooled 1-Year Analysis of EQUIP and CONQUER (ITT)
| | Placebo
(n=1477) | | PHEN/TPM CR
3.75/23
(n=234) | | PHEN/TPM CR
7.5/46
(n=488) | | PHEN/TPM CR
15/92
(n=1479) |
Age (years) | | | | | | | | |
Mean (SD) | | 48.5 (11.4) | | 43.0 (11.1) | | 51.1 (10.4) | | 48.0 (12.0) |
Race, n (%) | | | | | | | | |
Caucasian | | 1251 (84.7) | | 189 (80.8) | | 424 (86.9) | | 1235 (83.5) |
African American | | 199 (13.5) | | 35 (15.0) | | 51 (10.5) | | 210 (14.2) |
Weight (kg) | | | | | | | | |
Mean (SD) | | 107.5 (20.2) | | 118.6 (21.9) | | 102.8 (18.2) | | 107.1 (19.6) |
Waist circumference (cm) | | | | | | | | |
Mean (SD) | | 115.8 (13.3) | | 121.5 (15.2) | | 112.7 (12.4) | | 115.5 (13.5) |
· At Week 56, least-squares (LS) mean percent weight loss was significantly greater in all PHEN/TPM CR groups vs placebo (P<0.0001) in both the ITT-LOCF sample and in subjects completing the trials on study drug (completers) (Figure 1).

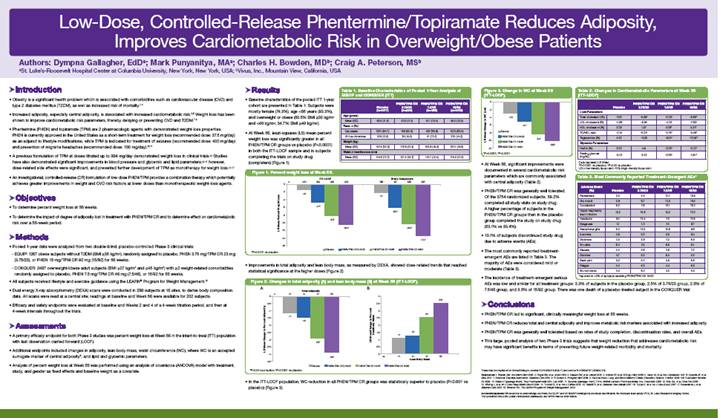
· Improvements in total adiposity and lean body mass, as measured by DEXA, showed dose-related trends that reached statistical significance at the higher doses (Figure 2).
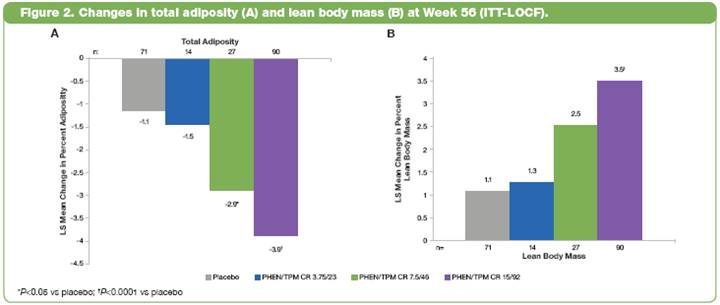
· In the ITT-LOCF population, WC reduction in all PHEN/TPM CR groups was statistically superior to placebo (P<0.001 vs placebo) (Figure 3).
· At Week 56, significant improvements were documented in several cardiometabolic risk parameters which are commonly associated with central adiposity (Table 2).
Table 2. Changes in Cardiometabolic Parameters at Week 56 (ITT-LOCF)
| | Placebo | | PHEN/TPM CR
3.75/23 | | PHEN/TPM CR
7.5/46 | | PHEN/TPM CR
15/92 | |
Lipid Parameters | | | | | | | | | |
Total cholesterol (%) | | -3.81 | | -5.85† | | -5.35† | | -6.68* | |
LDL cholesterol (%) | | -4.88 | | -6.94 | | -4.36 | | -7.69† | |
HDL cholesterol (%) | | 0.39 | | 1.57 | | 4.06* | | 5.31* | |
TC/HDL ratio | | -0.15 | | -0.31† | | -0.38* | | -0.46* | |
Triglycerides (%) | | 4.22 | | -0.00 | | -9.04* | | -10.62* | |
Glycemic Parameters | | | | | | | | | |
HbA1c (%) | | 0.03 | | n/a | | -0.08* | | -0.10* | |
Fasting glucose (mg/dL) | | -0.43 | | -2.02 | | -2.88† | | -3.61* | |
Data represent LS Mean
*P<0.0001 vs placebo; †P<0.05 vs placebo
LDL=low-density lipoprotein; HDL=high-density lipoprotein
· PHEN/TPM CR was generally well tolerated. Of the 3754 randomized subjects, 59.2% completed all study visits on study drug. A higher percentage of subjects in the PHEN/TPM CR groups than in the placebo group completed the study on study drug (63.1% vs 53.4%).
· 13.1% of subjects discontinued study drug due to adverse events (AEs).
· The most commonly reported treatment-emergent AEs are listed in Table 3. The majority of AEs were considered mild or moderate (Table 3).
Table 3. Most Commonly Reported Treatment-Emergent AEs*
Adverse Event
(%) | | Placebo | | PHEN/TPM CR
3.75/23 | | PHEN/TPM CR
7.5/46 | | PHEN/TPM CR
15/92 | |
Paresthesia | | 2.0 | | 4.2 | | 13.7 | | 19.9 | |
Dry mouth | | 2.9 | | 6.7 | | 13.5 | | 19.5 | |
Constipation | | 6.2 | | 7.9 | | 15.1 | | 16.3 | |
Upper respiratory tract infection | | 12.2 | | 15.8 | | 12.2 | | 13.0 | |
Headache | | 9.4 | | 10.4 | | 7.0 | | 10.8 | |
Dysgeusia | | 1.1 | | 1.3 | | 7.4 | | 9.7 | |
Nasopharyngitis | | 8.2 | | 12.5 | | 10.6 | | 9.6 | |
Insomnia | | 4.8 | | 5.0 | | 5.8 | | 9.4 | |
Dizziness | | 3.5 | | 2.9 | | 7.2 | | 8.5 | |
Sinusitis | | 6.3 | | 7.5 | | 6.8 | | 8.1 | |
Nausea | | 4.4 | | 5.8 | | 3.6 | | 7.0 | |
Diarrhea | | 4.7 | | 5.0 | | 6.4 | | 5.4 | |
Back pain | | 5.0 | | 5.4 | | 5.6 | | 6.6 | |
Fatigue | | 4.4 | | 5.0 | | 4.4 | | 6.0 | |
Blurred vision | | 3.5 | | 6.3 | | 4.0 | | 5.5 | |
*reported in >5% of subjects receiving PHEN/TPM CR 15/92
· The incidence of treatment-emergent serious AEs was low and similar for all treatment groups: 3.3% of subjects in the placebo group, 2.5% of 3.75/23 group, 2.8% of 7.5/46 group, and 3.5% of 15/92 group. There was one death of a placebo-treated subject in the CONQUER trial.
· Conclusions
· PHEN/TPM CR led to significant, clinically meaningful weight loss at 56 weeks.
· PHEN/TPM CR reduces total and central adiposity and improves metabolic risk markers associated with increased adiposity.
· PHEN/TPM CR was generally well tolerated based on rates of study completion, discontinuation rates, and overall AEs.
· This large, pooled analysis of two Phase 3 trials suggests that weight reduction that addresses cardiometabolic risk may have significant benefits in terms of preventing future weight-related morbidity and mortality.
These trials are registered at ClinicalTrials.gov, number NCT00554216 (EQUIP) and number NCT00553787 (CONQUER).
References: (1) Reaven GM. Ann Intern Med 2003. (2) Flegal KM, et al. JAMA 2010. (3) Haslam DW, et al. Lancet 2005. (4) Adams KF, et al. N Engl J Med 2006. (5) Carey VJ, et al. Am J Epidemiol 1997. (6) Després JP, et al. BMJ 2001. (7) American Diabetes Association. Diabetes Care 2010. (8) Pi-Sunyer X. Postgrad Med 2009. (9) National Heart, Lung, and Blood Institute Obesity Education Initiative. October 2000. NIH Publication Number 00-4084. (10) Adipex-P [package insert]. Teva Pharmaceuticals USA; July 2005. (11) Topamax [package insert]. Ortho-McNeil-Janssen Pharmaceuticals, Inc.; December 2009. (12) Bray GA, et al. Obes Res 2003. (13) Wilding J, et al. Int J Obes Relat Metab Disord 2004. (14) Tonstad S, et al. Am J Cardiol 2005. (15) Stenlöf K, et al. Diabetes Obes Metab 2007. (16) Toplak H, et al. Int J Obes (Lond) 2007. (17) Rosenstock J, et al. Diabetes Care 2007. (18) Brownell KD. The LEARN Program for Weight Management. 2004.
Acknowledgements: We would like to acknowledge and thank the EQUIP and CONQUER investigators and study coordinators, the Medpace team (study CRO), St. Luke-Roosevelt’s Imaging Center, The Lockwood Group (for poster development assistance), and VIVUS internal contributors.