Below is a reproduction of the contents of the poster entitled “Weight Loss With Once-Daily, Low-Dose, Controlled-Release Phentermine/Topiramate Improves Plasma Alanine Transaminase Concentrations”:
Authors: Samuel Klein, MD(a); Thomas Najarian, MD(b); Wesley W. Day, PhD(b)
(a)Washington University School of Medicine, Saint Louis, Missouri, USA; (b)Vivus, Inc., Mountain View, California, USA
· Introduction
· Nonalcoholic fatty liver disease (NAFLD) represents a constellation of liver abnormalities manifested by increased intrahepatic triglyceride(1) with or without inflammation and fibrosis.
· Prevalence of NAFLD increases with increasing BMI values, and is associated with insulin resistance, diabetes, hypertension, and dyslipidemia.(2)
· Increases in concentrations of plasma alanine transaminase (ALT) are associated with NAFLD(3)-(5) such that ALT is a commonly used surrogate marker of NAFLD.(6)
· Weight reduction is a common approach for the management of NAFLD.(7)
· Phentermine (PHEN) and topiramate (TPM) are 2 pharmacologic agents with demonstrated weight-loss properties. PHEN is currently approved in the United States as a short-term treatment for weight loss (recommended dose: 37.5 mg/day) as an adjunct to lifestyle modifications, while TPM is indicated for treatment of seizures (recommended dose: 400 mg/day) and prevention of migraine headaches (recommended dose: 100 mg/day).(8),(9)
· A novel investigational combination of low-dose, controlled-release (CR) formulation of PHEN/TPM for once-daily oral dosing was developed to maximize weight loss while minimizing adverse events (AEs).
· Objective
· To determine the effect of increasing degrees of weight loss achieved during PHEN/TPM CR clinical trials on plasma ALT concentrations, a surrogate marker of NAFLD, in overweight and obese subjects.
· Methods
· Data from 2 clinical studies of PHEN/TPM CR were pooled for this evaluation:
· EQUIP: double-blind, placebo-controlled Phase 3 trial of 1267 obese subjects (BMI >35 kg/m2), excluding subjects with type 2 diabetes mellitus (T2DM), randomly assigned to placebo, PHEN 3.75 mg/TPM CR 23 mg (3.75/23), or PHEN 15 mg/TPM CR 92 mg (15/92) for 56 weeks.
· CONQUER: double-blind, placebo-controlled Phase 3 trial of 2487 overweight/obese adult subjects (BMI >27 kg/m2 and <45 kg/m2) with >2 weight-related comorbidities randomly assigned to placebo, PHEN 7.5 mg/TPM CR 46 mg (7.5/46), or 15/92 for 56 weeks.
· All subjects received lifestyle counseling based on the LEARN® program for weight management.(10)
· Assessments
· The primary endpoint for this secondary analysis was the effect of weight loss on NAFLD using serum ALT values as the surrogate marker. Subjects with elevated ALT concentrations (>30 IU/mL for males, >19 IU/mL for females)(11) at baseline were included in this analysis.
· A primary endpoint for both studies was percent weight loss at 56 weeks in the intent-to-treat (ITT) set with last observation carried forward (LOCF).
· Analysis of the percent weight loss at Week 56 was performed using an analysis of covariance (ANCOVA) model with treatment, study, and gender as fixed effects and baseline weight as a covariate. Least-squares (LS) means for ALT were derived from an ANCOVA model with weight loss category as the fixed effect and baseline ALT as a covariate.
· Results
· Baseline demographics for the 2 studies are presented in Table 1.
Table 1. Baseline Demographics for EQUIP and CONQUER
| | EQUIP (N=1267) | | CONQUER (N=2487) | |
Mean age, years (SD) | | 43 (11.8) | | 51 (10.4) | |
Female, % | | 83 | | 70 | |
Mean weight, kg (SD) | | 116.1 (21.2) | | 103.1 (17.9) | |
Mean BMI, kg/m2 (SD) | | 42.1 (6.2) | | 36.6 (4.5) | |
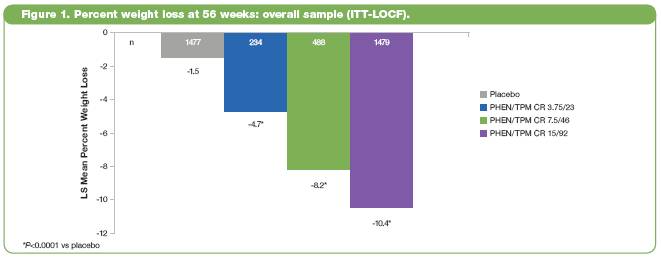
· LS mean percent weight loss in the ITT-LOCF population was significantly greater for all PHEN/TPM CR doses than placebo (P<0.0001 vs placebo for all comparisons) (Figure 1).
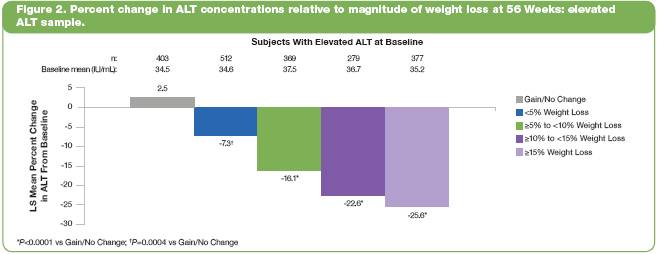
· Of the 3754 subjects in the two trials, 2234 (60%) had elevated plasma ALT concentrations at baseline. To determine how weight loss affected ALT concentrations in these subjects, changes in ALT concentration were evaluated relative to the magnitude of weight loss, ranging from gain/no change to >15%, at Week 56.
· Baseline ALT concentrations were similar across weight-loss groups (Figure 2). At 56 weeks, LS mean percent reduction in ALT was significantly greater in all weight-loss groups vs the gain/no change group (P<0.0004 for all comparisons).
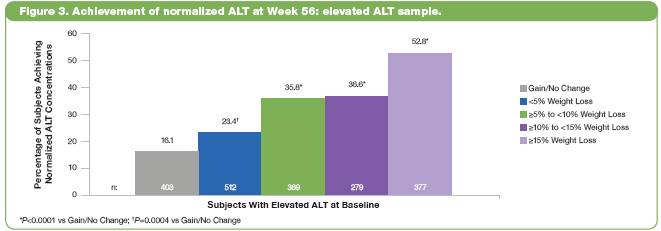
· The percentage of subjects with elevated ALT at baseline who achieved normalized ALT at Week 56 was greater in subjects with greater weight loss (Figure 3). Percentages ranged from 16.1 in the gain/no change group to 52.8 in the >15% group (P<0.0005 vs gain/no change).
· PHEN/TPM CR was generally well tolerated. Of the 3754 randomized subjects, 59.2% completed all study visits on study drug. A higher percentage of subjects in the PHEN/TPM CR groups than in the placebo group completed the study on study drug (63.1% vs. 53.4%).
· 13.1% of subjects discontinued study drug due to AEs.
· The most commonly reported treatment-emergent AEs are listed in Table 2. The majority of AEs were considered mild or moderate (Table 2).
Table 2. Most Commonly Reported Treatment-Emergent AEs*
Adverse Events (%) | | Placebo | | PHEN/TPM CR
3.75/23 | | PHEN/TPM CR
7.5/46 | | PHEN/TPM CR
15/92 | |
Paresthesia | | 2.0 | | 4.2 | | 13.7 | | 19.9 | |
Dry mouth | | 2.9 | | 6.7 | | 13.5 | | 19.5 | |
Constipation | | 6.2 | | 7.9 | | 15.1 | | 16.3 | |
Upper respiratory tract infection | | 12.2 | | 15.8 | | 12.2 | | 13.0 | |
Headache | | 9.4 | | 10.4 | | 7.0 | | 10.8 | |
Dysgeusia | | 1.1 | | 1.3 | | 7.4 | | 9.7 | |
Nasopharyngitis | | 8.2 | | 12.5 | | 10.6 | | 9.6 | |
Insomnia | | 4.8 | | 5.0 | | 5.8 | | 9.4 | |
Dizziness | | 3.5 | | 2.9 | | 7.2 | | 8.5 | |
Sinusitis | | 6.3 | | 7.5 | | 6.8 | | 8.1 | |
Nausea | | 4.4 | | 5.8 | | 3.6 | | 7.0 | |
Diarrhea | | 4.7 | | 5.0 | | 6.4 | | 5.4 | |
Back pain | | 5.0 | | 5.4 | | 5.6 | | 6.6 | |
Fatigue | | 4.4 | | 5.0 | | 4.4 | | 6.0 | |
Blurred vision | | 3.5 | | 6.3 | | 4.0 | | 5.5 | |
*reported in >5% of subjects receiving PHEN/TPM CR 15/92
· The incidence of treatment-emergent serious AEs was low and similar for all treatment groups: 3.3% of subjects in the placebo group, 2.5% of 3.75/23 group, 2.8% of 7.5/46 group, and 3.5% of 15/92 group. There was one death of a placebo-treated subject in the CONQUER trial.
· Conclusions
· Increasing weight loss in overweight and obese subjects is associated with a progressive decline in plasma ALT concentrations, presumably by reducing liver fat content and improving NAFLD.
· Greater weight loss achieved with PHEN/TPM CR resulted in greater reductions in plasma ALT concentrations than seen in placebo-treated subjects.
· PHEN/TPM CR was generally well tolerated based on rates of AEs, discontinuation, and overall study completion.
These trials are registered at ClinicalTrials.gov, number NCT00554216 (EQUIP), and number NCT00553787 (CONQUER).
References: (1) Li ZZ, et al. J Biol Chem 2009. (2) Patel AA, et al. J Clin Gastroenterol 2009. (3) Ghouri N, et al. Hepatology 2010. (4) Radetti G, et al. Acta Paediatr 2006. (5) Clark JM, et al. Am J Gastroenterol. 2003. (6) Schwimmer JB, et al. Pediatrics 2005. (7) Diehl AM. Semin Liver Dis 1999. (8) Adipex-P [package insert]. Teva Pharmaceuticals USA; July 2005. (9) Topamax [package insert]. Ortho-McNeil-Janssen Pharmaceuticals, Inc.; December 2009. (10) Brownell KD. The LEARN Program for Weight Management. 2004. (11) Prati D, et al. Ann Intern Med 2002.
Acknowledgements: We would like to acknowledge and thank the CONQUER, EQUIP, and DM-230 investigators and study coordinators, the Medpace team (study CRO), The Lockwood Group (for poster development assistance), and VIVUS internal contributors.



