Exhibit 99.1

“Quadramet® Overview”
William F Goeckeler, PhD
Senior Vice President, Operations
CYTOGEN Corp

Introduction
Skeleton is the most common organ to be affected by metastatic cancer
Tumors arising from the breast, prostate, myeloma, and lung possess a special propensity to spread to bone
Bone metastases serve as a secondary site of tumor spread, represent the highest tumor burden in the body, and appear to be more resistant to treatment than visceral metastases
Source: Bagi CM. Targeting of therapeutic agents to bone to treat metastatic cancer. Adv Drug Deliv Rev. 2005 May 25;57(7):995-1010.
Slide # 2
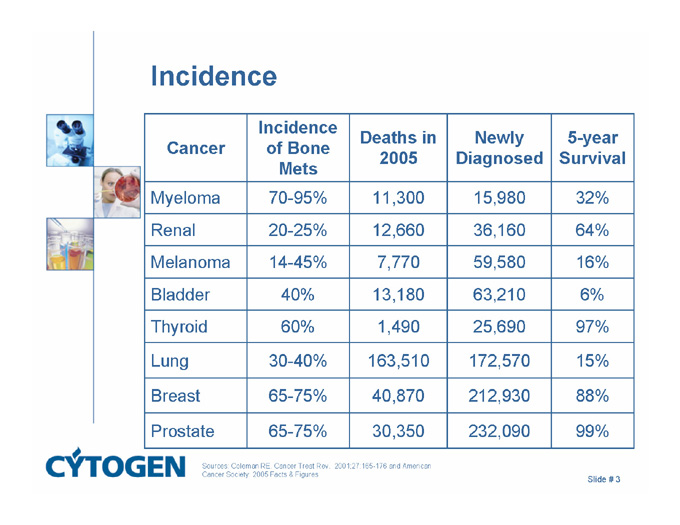
Incidence
Cancer
Incidence of Bone Mets
Deaths in 2005
5-year Survival
Newly Diagnosed
Myeloma
70-95%
11,300
32%
15,980
Renal
20-25%
12,660
64%
36,160
Melanoma
14-45%
7,770
16%
59,580
Bladder
40%
13,180
6%
63,210
Thyroid
60%
1,490
97%
25,690
Lung
30-40%
163,510
15%
172,570
Breast
65-75%
40,870
88%
212,930
Prostate
65-75%
30,350
99%
232,090
Sources: Coleman RE. Cancer Treat Rev. 2001;27:165-176 and American Cancer Society: 2005 Facts & Figures
Slide # 3
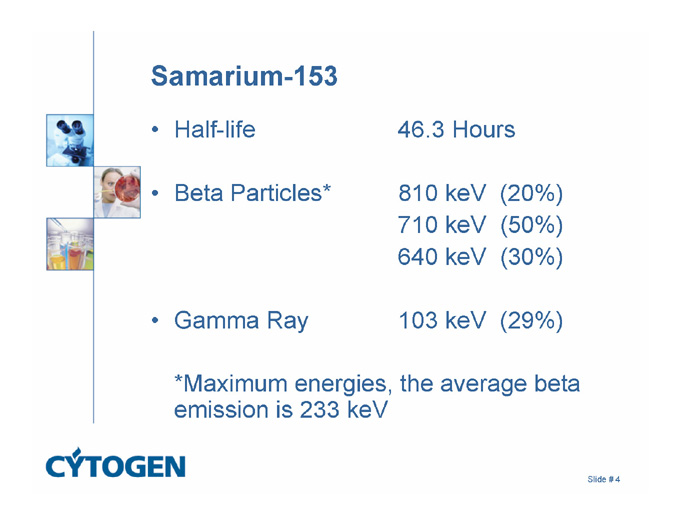
Samarium-153
Half-life 46.3 Hours
Beta Particles* 810 keV (20%)
710 keV (50%)
640 keV (30%)
Gamma Ray 103 keV (29%)
*Maximum energies, the average beta emission is 233 keV
Slide # 4
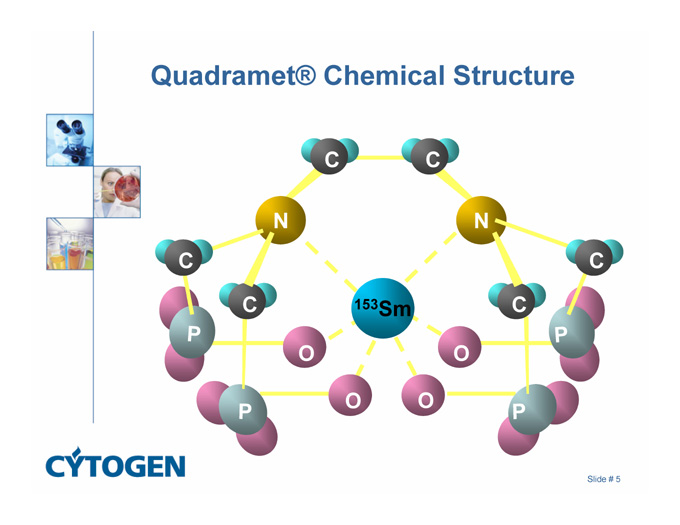
Quadramet® Chemical Structure
C
C
N
N
C
C
153Sm
C
C
P
P
O
O
O
O
P
P
Slide # 5
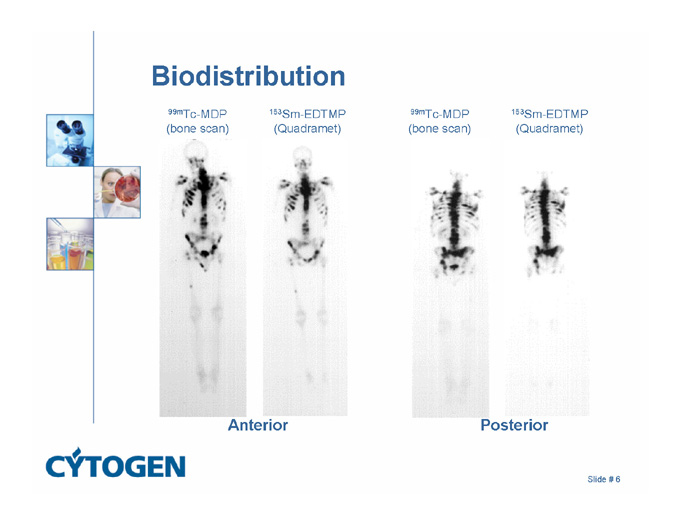
Biodistribution
99mTc-MDP
(bone scan)
153Sm-EDTMP
(Quadramet)
99mTc-MDP
(bone scan)
153Sm-EDTMP
(Quadramet)
Anterior
Posterior
Slide # 6
Controlled Clinical Studies
Study BA-108
114 patients, variety of primary malignancies
Publication: Resche, et. al., Eur. J. Cancer, 33, 10, 1583-1591 (1997)
Study BA-106/110
118 patients, variety of primary malignancies
Publication: Serafini, et. al., J. Clin. Oncol., 16, 4, 1574-1581 (1998)
Study 424Sm10/11
152 patients, hormone refractory prostate cancer
Publication: Sartor, et. al., Urology, 63, 5, 940-945 (2004)
Slide # 7
Indication Statement
Quadramet® is indicated for relief of pain in patients with confirmed osteoblastic metastatic bone lesions that enhance on radionuclide bone scan
Slide # 8
Features and Benefits
Onset
Pain relief usually begins within one week of administration
Duration
Single dose relieves pain for a median of 16 weeks in patients who respond
Opioid-sparing
Reduces concomitant opioid analgesic use
Mild side effects
Transient myelosuppression tends to resolve within eight weeks
Slide # 9

Lessons Learned
Radiopharmaceuticals are relegated to end-stage use largely due to the safety profile of first-generation agents
Cytogen’s actions:
Educate prospective users about the unique physical properties of Quadramet, including short half-life of radioactivity and targeting ligand
Facilitate Quadramet trial, usage, and new approaches to pain management through registry program
Slide # 10

Lessons Learned
Prior to Cytogen’s reacquisition of marketing rights in August 2003, marketing activities excluded medical oncologists – a key prescribing audience
Cytogen’s actions:
Introduce medical oncologists to Quadramet through medical meetings and journals
Expand sales and marketing infrastructure to focus on this additional call point
Facilitate a multidisciplinary approach to treating metastatic bone disease through registry program
New clinical development initiatives tailored to medical oncologists
Slide # 11
Lessons Learned
While Quadramet has a broad cancer indication, existing data is primarily in prostate cancer – with the majority of product use in this setting
Cytogen’s actions:
Communicate the role of Quadramet in various cancers using existing clinical data in breast cancer
New clinical development initiatives designed to generate data in cancers other than prostate
Slide # 12

Lessons Learned
There is interest among physicians to understand the role of Quadramet in a contemporary oncology setting
Cytogen’s actions:
Publication and presentation of existing clinical data regarding repeat administration of Quadramet and the use of this product in various contemporary clinical settings
New clinical development initiatives designed to generate data in various contemporary clinical settings
Slide # 13

Lessons Learned
Beyond its demonstrated supportive care role, there is interest among physicians to understand the potential tumoricidal attributes of Quadramet
Cytogen’s actions:
Leverage the ability to deliver a highly specific therapeutic in clinical settings where treatment of the bone component of disease represents a significant unmet medical need
New clinical development initiatives designed to explore the tumoricidal attributes of Quadramet
Slide # 14

Applying the Lessons
More than a dozen clinical development programs are underway in response to physician interest in Quadramet®
During today’s presentations, you will hear from some of these physicians (highlighted in the following slides)
Cytogen’s extensive ongoing clinical development for Quaramet also reflects Cytogen’s commitment to building the brand
Slide # 15

Quadramet Clinical Development
TAXane-based chemotherapy and SAMarium Sm-153 lexidronam injection
Trial
Description
Status
TAXSAM1
(prostate)
Docetaxel and Quadramet
(European study)
Phase II
TAXSAM2
(prostate)
Paclitaxel and dose escalation of Quadramet
(Northwestern University in Illinois)
Phase I/II
TAXSAM3
(prostate)
Weekly docetaxel with multiple doses of standard Quadramet
(M D Anderson Cancer Center)
Phase I/II
TAXSAM4
(prostate)
Loading dose of docetaxel with Quadramet
(Johns Hopkins Kimmel Cancer Center)
Phase I/II
TAXSAM5
(prostate)
Standard docetaxel with escalating, multiple doses of Quadramet
(Memorial Sloan-Kettering Cancer Center)
Phase I/II
TAXSAM6
(breast)
Paclitaxel, bevacizumab, and Quadramet
(University of Pittsburgh Medical Center)
IRB review
Slide # 16
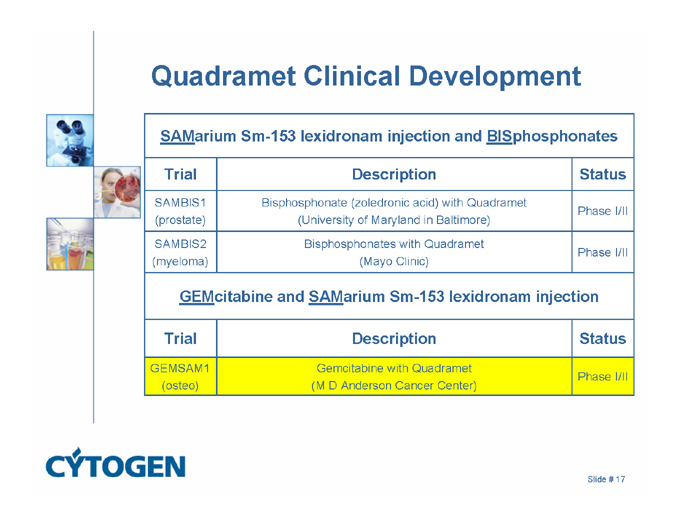
Quadramet Clinical Development
SAMarium Sm-153 lexidronam injection and BISphosphonates
Trial
Description
Status
SAMBIS1
(prostate)
Bisphosphonate (zoledronic acid) with Quadramet
(University of Maryland in Baltimore)
Phase I/II
SAMBIS2
(myeloma)
Bisphosphonates with Quadramet
(Mayo Clinic)
Phase I/II
GEMcitabine and SAMarium Sm-153 lexidronam injection
Trial
Description
Status
GEMSAM1
(osteo)
Gemcitabine with Quadramet
(M D Anderson Cancer Center)
Phase I/II
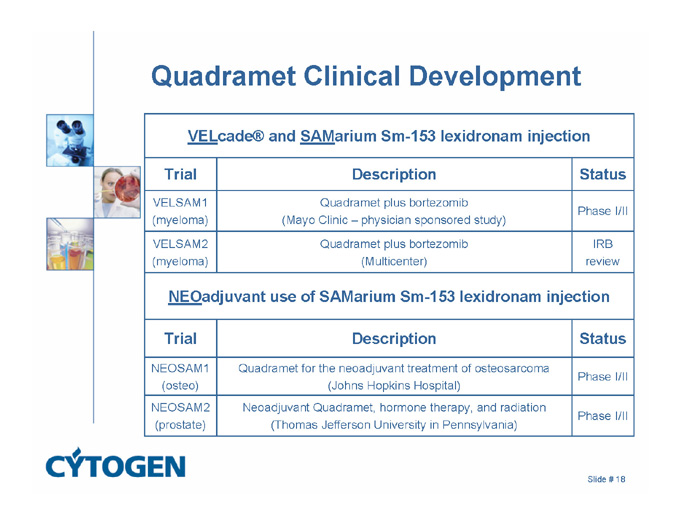
Quadramet Clinical Development
VELcade® and SAMarium Sm-153 lexidronam injection
Trial
Description
Status
VELSAM1
(myeloma)
Slide # 17
Quadramet plus bortezomib
(Mayo Clinic – physician sponsored study)
Phase I/II
VELSAM2
(myeloma)
Quadramet plus bortezomib
(Multicenter)
IRB
review
NEOadjuvant use of SAMarium Sm-153 lexidronam injection
Trial
Description
Status
NEOSAM1
(osteo)
Quadramet for the neoadjuvant treatment of osteosarcoma
(Johns Hopkins Hospital)
Phase I/II
NEOSAM2
(prostate)
Neoadjuvant Quadramet, hormone therapy, and radiation
(Thomas Jefferson University in Pennsylvania)
Phase I/II
Slide # 18

















