EXHIBIT 99.6

“Radiopharmaceutical Approaches To Skeletal Metastases from Prostate Cancer”
Howard I. Scher, MD
Chief, Genitourinary Oncology Service
Memorial Sloan-Kettering Cancer Center
New York, NY
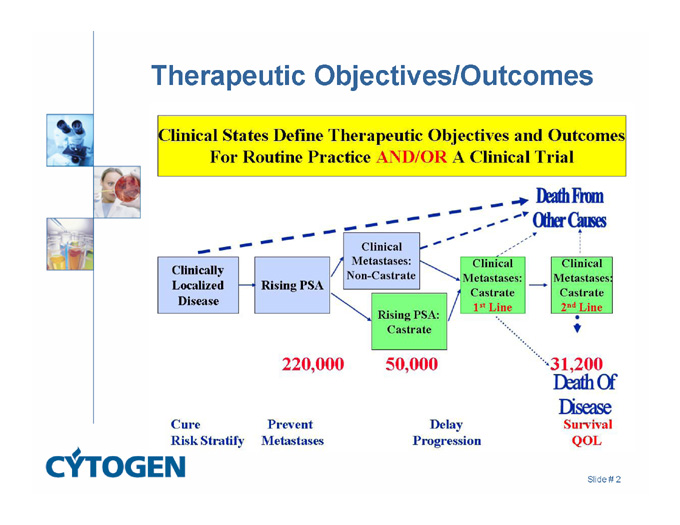
Therapeutic Objectives/Outcomes
Death From Other Causes
220,000
50,000
31,200
Death Of Disease Survival QOL
Cure Risk Stratify
Prevent Metastases
Delay Progression
Slide # 2
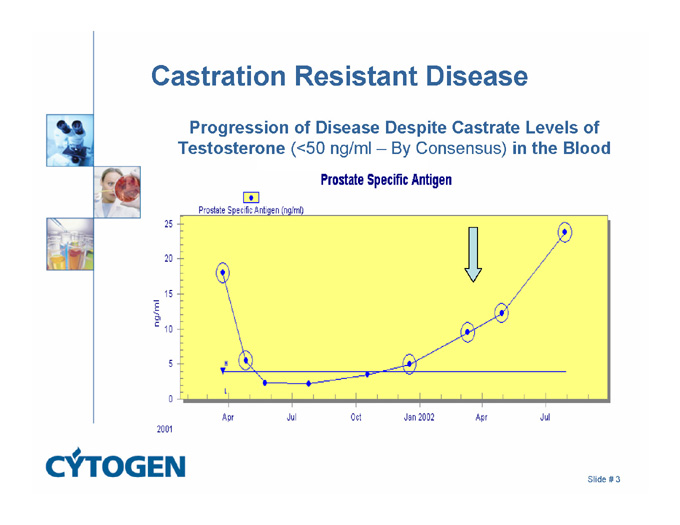
Castration Resistant Disease
Progression of Disease Despite Castrate Levels of Testosterone (<50 ng/ml – By Consensus) in the Blood
Prostate Specific Antigen
Prostate Specific Antigen (ng/ml)
2001
25
20
15
10
5
0
Apr
Jul
Oct
Jan 2002
Apr
Jul
ng/ml
Slide # 3
Clinical Management of Castration Resistant Prostate Cancer
1. Therapeutic objectives:
Why offer therapy – eliminate/relieve and/or prevent/delay
2. Chemotherapy for hormone-refractory prostate cancer
3. Radiopharmaceutical approaches to skeletal metastases from prostate cancer
Slide # 4
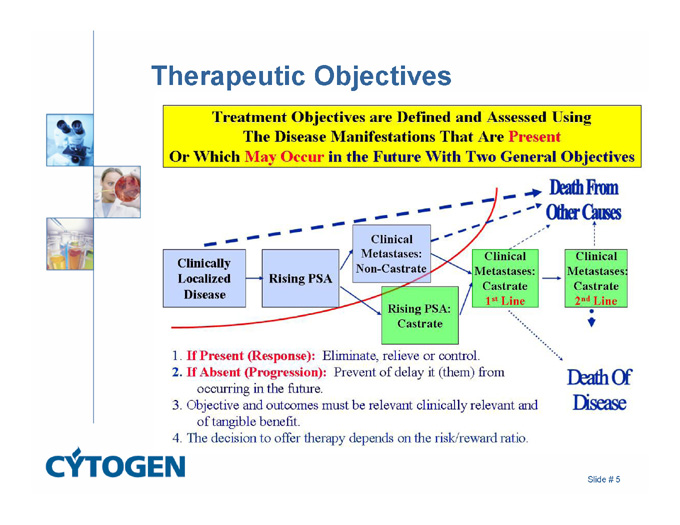
Therapeutic Objectives
Death From Other Causes
1. If Present (Response): Eliminate, relieve or control.
2. If Absent (Progression): Prevent of delay it (them) from occurring in the future.
3. Objective and outcomes must be relevant clinically relevant and of tangible benefit.
4. The decision to offer therapy depends on the risk/reward ratio.
Death Of Disease
Slide # 5
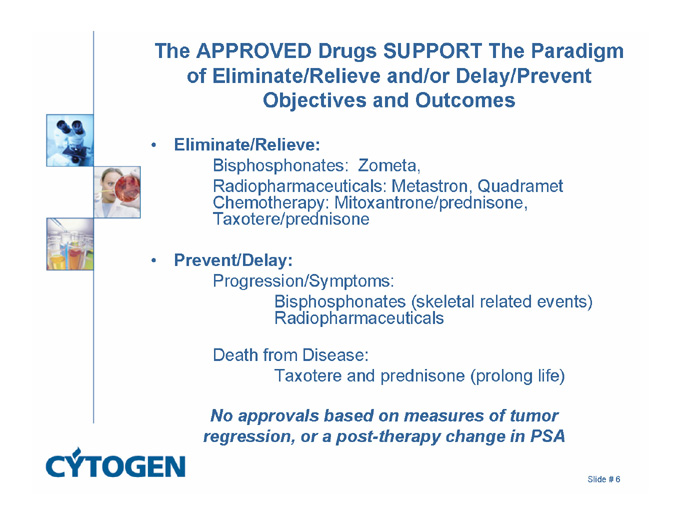
The APPROVED Drugs SUPPORT The Paradigm of Eliminate/Relieve and/or Delay/Prevent Objectives and Outcomes
Eliminate/Relieve:
Bisphosphonates: Zometa,
Radiopharmaceuticals: Metastron, Quadramet Chemotherapy: Mitoxantrone/prednisone, Taxotere/prednisone
Prevent/Delay:
Progression/Symptoms:
Bisphosphonates (skeletal related events) Radiopharmaceuticals
Death from Disease:
Taxotere and prednisone (prolong life)
No approvals based on measures of tumor regression, or a post-therapy change in PSA
Slide # 6
Clinical Management of Castration Resistant Prostate Cancer
1. Therapeutic objectives:
Why offer therapy – eliminate/relieve and/or prevent/delay
2. Chemotherapy for hormone-refractory prostate cancer
3. Radiopharmaceutical approaches to skeletal metastases from prostate cancer
Slide # 7
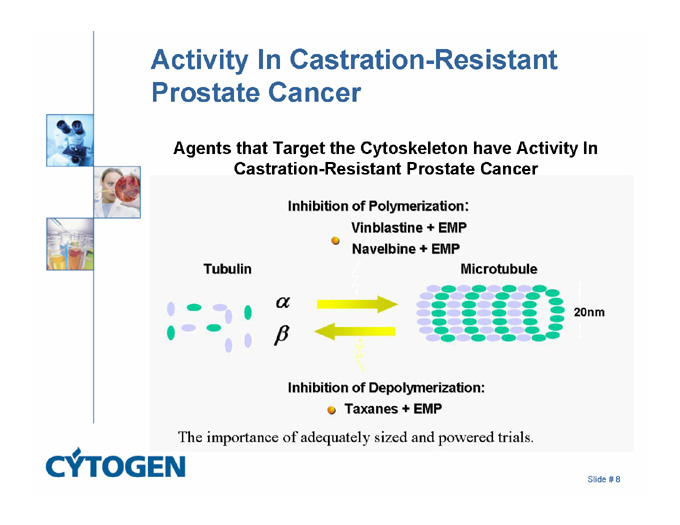
Activity In Castration-Resistant Prostate Cancer
Agents that Target the Cytoskeleton have Activity In Castration-Resistant Prostate Cancer
Inhibition of Polymerization:
Vinblastine + EMP
Navelbine + EMP
Tubulin
Microtubule
20nm
Inhibition of Depolymerization:
Taxanes + EMP
The importance of adequately sized and powered trials.
Slide # 8
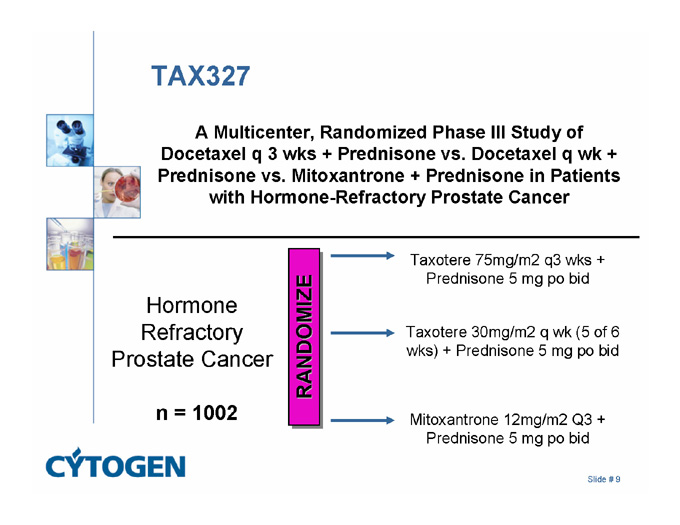
TAX327
A Multicenter, Randomized Phase III Study of Docetaxel q 3 wks + Prednisone vs. Docetaxel q wk + Prednisone vs. Mitoxantrone + Prednisone in Patients with Hormone-Refractory Prostate Cancer
Taxotere 75mg/m2 q3 wks +
Prednisone 5 mg po bid
Hormone Refractory Prostate Cancer
RANDOMIZE
Taxotere 30mg/m2 q wk (5 of 6 wks) + Prednisone 5 mg po bid
n = 1002
Mitoxantrone 12mg/m2 Q3 + Prednisone 5 mg po bid
Slide # 9
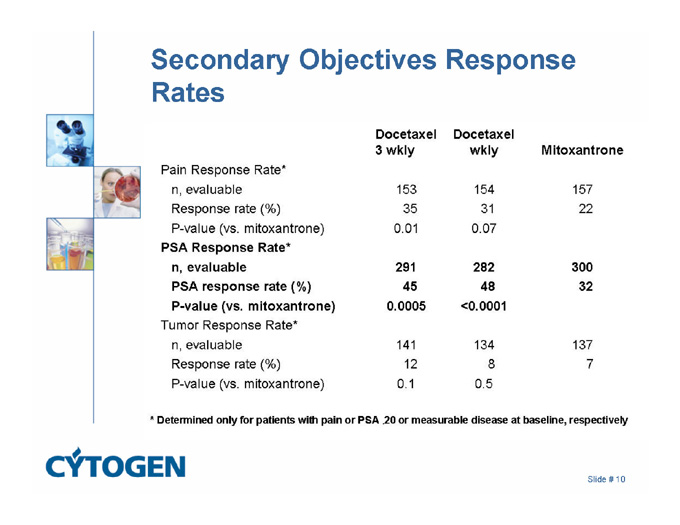
Secondary Objectives Response Rates
Docetaxel Docetaxel
3 wkly wkly Mltoxantrone
Pain Response Rate*
n, evaluable 153 154 157
Response rate (%) 35 31 22
P-value (vs. mitoxantrone) 0.01 0.07
PSA Response Rate*
n, evaluable 291 282 300
PSA response rate (%) 45 48 32
P-value (vs. mitoxantrone) 0.0005 <0.0001
Tumor Response Rate*
n, evaluable 141 134 137
Response rate (%) 12 8 7
P-value (vs. mitoxantrone) 0.1 0.5
* Determined only for patients with pain or PSA .20 or measurable disease at baseline, respectively
Slide # 10
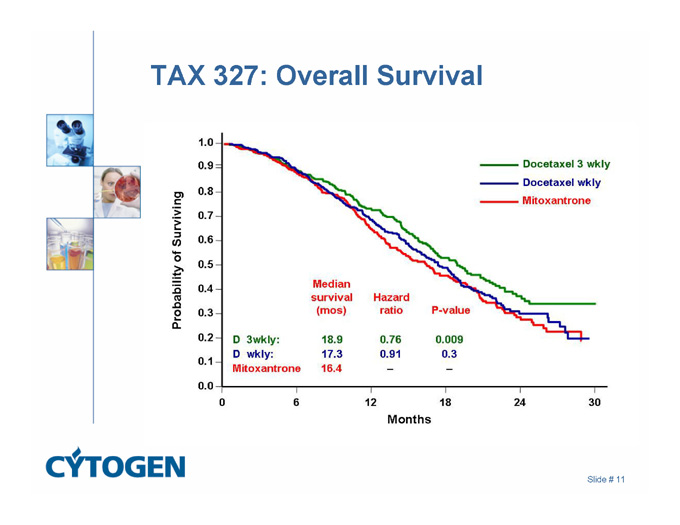
TAX 327: Overall Survival
Docetaxel 3 wkly
Docetaxel wkly
Mitoxantrone
Probability of Surviving
1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0.0
Median
survival Hazard
(mos) ratio P-value
D 3 wkly: 18.9 0.76 0.009
D wkly: 17.3 0.91 0.3
Mitoxantrone 16.4 - -
0 6 12 18 24 30
Months
Slide # 11
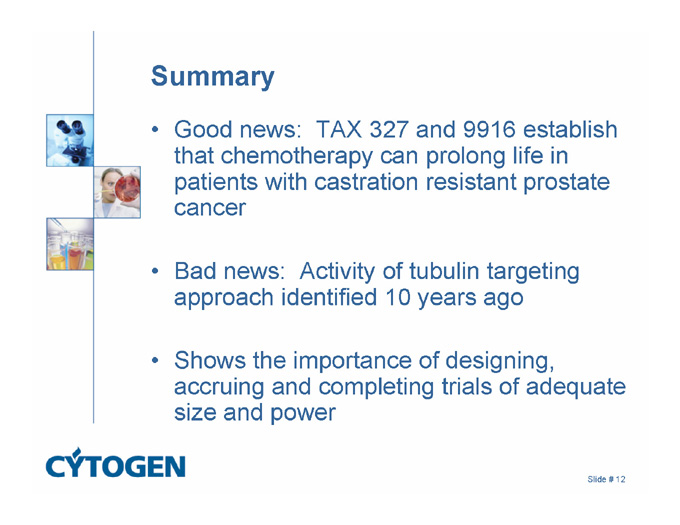
Summary
Good news: TAX 327 and 9916 establish that chemotherapy can prolong life in patients with castration resistant prostate cancer
Bad news: Activity of tubulin targeting approach identified 10 years ago
Shows the importance of designing, accruing and completing trials of adequate size and power
Slide # 12
Clinical Management of Castration Resistant Prostate Cancer
1. Therapeutic objectives:
Why offer therapy – eliminate/relieve and/or prevent/delay
2. Chemotherapy for hormone-refractory prostate cancer
3. Radiopharmaceutical approaches to skeletal metastases from prostate cancer
Slide # 13
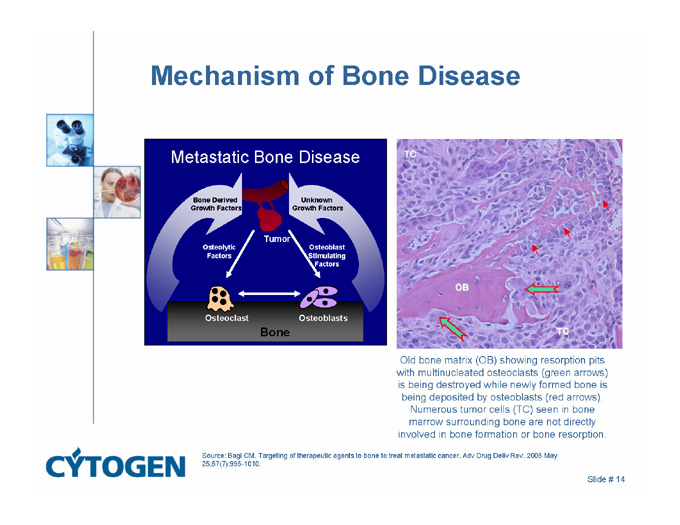
Mechanism of Bone Disease
Metastatic Bone Disease
Bone Derived
Growth Factors
Unknown
Growth Factors
Tumor
Osteoblast
Stimulating
Factors
Osteolytic
Factors
Osteoclast
Osteoblasts
Bone
Old bone matrix (OB) showing resorption pits with multinucleated osteoclasts (green arrows) is being destroyed while newly formed bone is being deposited by osteoblasts (red arrows). Numerous tumor cells (TC) seen in bone marrow surrounding bone are not directly involved in bone formation or bone resorption.
Source: Bagi CM. Targeting of therapeutic agents to bone to treat metastatic cancer. Adv Drug Deliv Rev. 2005 May 25;57(7):995-1010.
Slide # 14

Questions in Development of Radiopharmaceuticals
Do they provide clinical benefit(s) and for how long?
Can they be used more than once?
Can effects be enhanced if used BEFORE a patient demonstrates progressive/resistant disease?
Can effects be enhanced if combined with an effective chemotherapy that also functions as a radiosensitizer?
Slide # 15
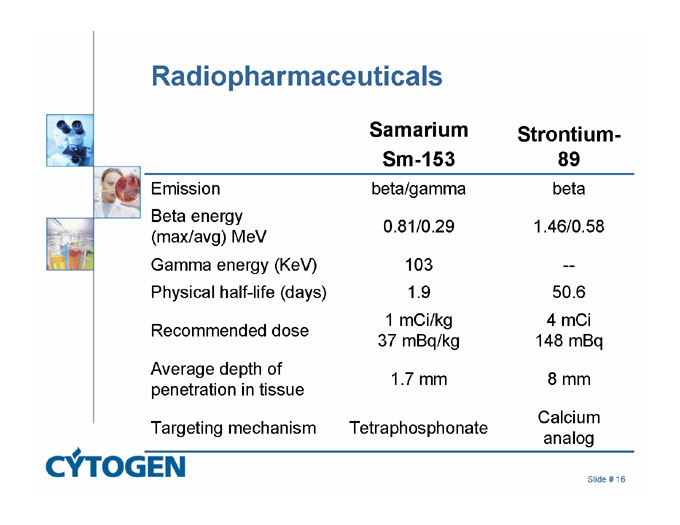
Radiopharmaceuticals
Samarium
Sm-153
Strontium-89
Emission
beta/gamma
beta
Beta energy
(max/avg) MeV
0.81/0.29
1.46/0.58
Gamma energy (KeV)
103
—
Physical half-life (days)
1.9
50.6
Recommended dose
1 mCi/kg
37 mBq/kg
4 mCi
148 mBq
Average depth of penetration in tissue
1.7 mm
8 mm
Targeting mechanism
Tetraphosphonate
Calcium analog
Slide # 16
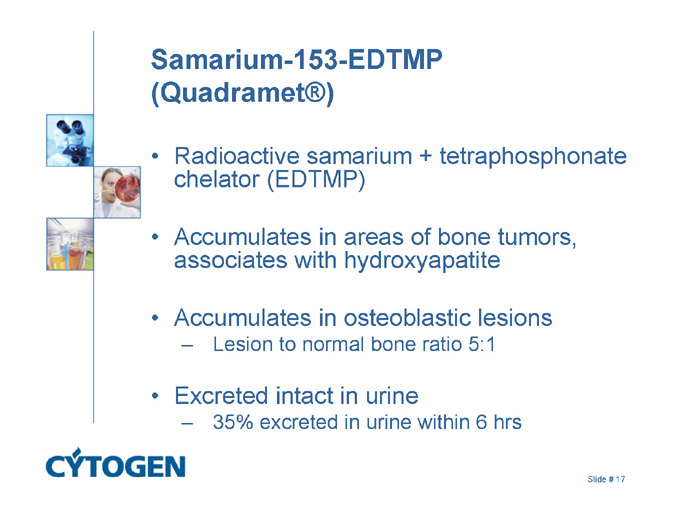
Samarium-153-EDTMP (Quadramet®)
Radioactive samarium + tetraphosphonate chelator (EDTMP)
Accumulates in areas of bone tumors, associates with hydroxyapatite
Accumulates in osteoblastic lesions
Lesion to normal bone ratio 5:1
Excreted intact in urine
35% excreted in urine within 6 hrs
Slide # 17
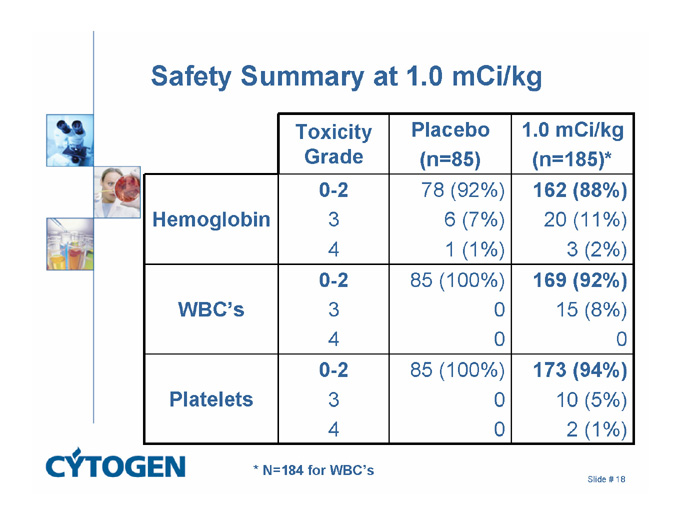
Safety Summary at 1.0 mCi/kg
Toxicity Grade
Placebo
(n=85)
1.0 mCi/kg
(n=185)*
Hemoglobin
0-2
3
4
78 (92%)
6 (7%)
1 (1%)
162 (88%)
20 (11%)
3 (2%)
WBC’s
0-2
3
4
85 (100%)
0
0
169 (92%)
15 (8%)
0
Platelets
0-2
3
4
85 (100%)
0
0
173 (94%)
10 (5%)
2 (1%)
* N=184 for WBC’s
Slide # 18
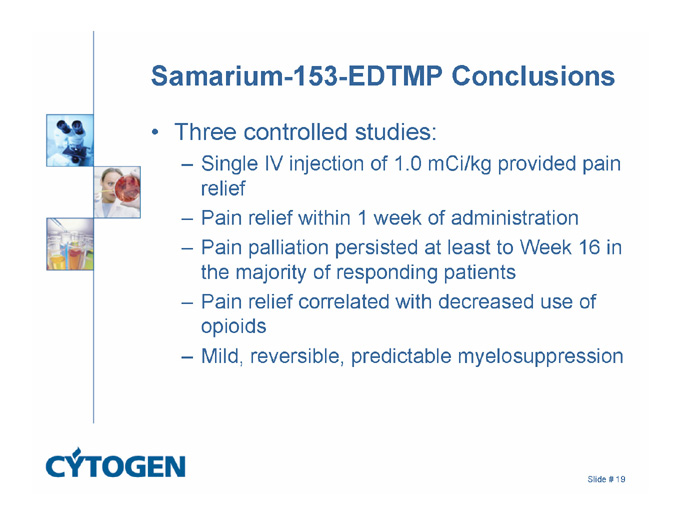
Samarium-153-EDTMP Conclusions
Three controlled studies:
Single IV injection of 1.0 mCi/kg provided pain relief
Pain relief within 1 week of administration
Pain palliation persisted at least to Week 16 in the majority of responding patients
Pain relief correlated with decreased use of opioids
Mild, reversible, predictable myelosuppression
Slide # 19

Radiopharmaceuticals Summary
Bone-targeted radioisotopes provide long-lasting pain relief
PSA declines are observed in 10% of patients
Cell kill appears not to be a prerequisite for palliation of pain
No trial of any one bone-targeted radioisotope alone has been shown to prolong life
Slide # 20

Questions in Development of Radiopharmaceuticals
Do they provide clinical benefit(s) and for how long?
Can they be used more than once?
Can effects be enhanced if used BEFORE a patient demonstrates progressive/resistant disease?
Can effects be enhanced if combined with an effective chemotherapy that also functions as a radiosensitizer?
Slide # 21
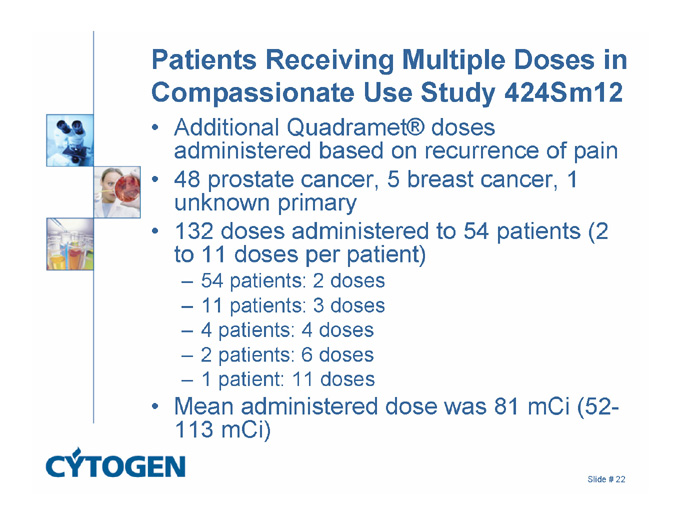
Patients Receiving Multiple Doses in Compassionate Use Study 424Sm12
Additional Quadramet® doses administered based on recurrence of pain
48 prostate cancer, 5 breast cancer, 1 unknown primary
132 doses administered to 54 patients (2 to 11 doses per patient)
54 patients: 2 doses
11 patients: 3 doses
4 patients: 4 doses
2 patients: 6 doses
1 patient: 11 doses
Mean administered dose was 81 mCi (52-113 mCi)
Slide # 22
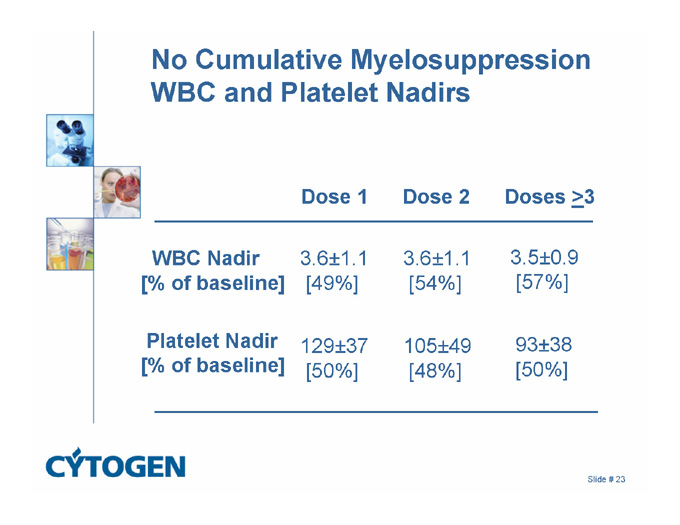
No Cumulative Myelosuppression
WBC and Platelet Nadirs
Dose 1 Dose 2 Doses >3
3.6±1.1
[54%]
3.6±1.1
[49%]
WBC Nadir
[% of baseline]
3.5±0.9
[57%]
93±38
[50%]
105±49
[48%]
129±37
[50%]
Platelet Nadir
[% of baseline]
Slide # 23

Questions in Development of Radiopharmaceuticals
Do they provide clinical benefit(s) and for how long?
Can they be used more than once?
Can effects be enhanced if used BEFORE a patient demonstrates progressive/resistant disease?
Can effects be enhanced if combined with an effective chemotherapy that also functions as a radiosensitizer?
Slide # 24
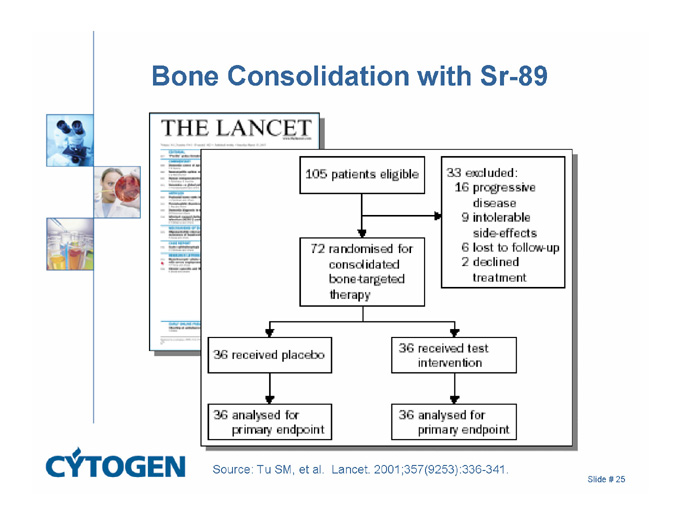
Bone Consolidation with Sr-89
Source: Tu SM, et al. Lancet. 2001;357(9253):336-341.
Slide # 25
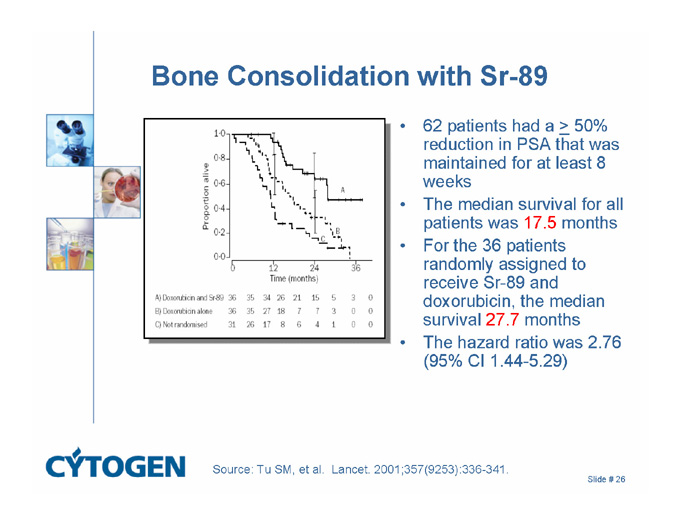
Bone Consolidation with Sr-89
62 patients had a > 50% reduction in PSA that was maintained for at least 8 weeks
The median survival for all patients was 17.5 months
For the 36 patients randomly assigned to receive Sr-89 and doxorubicin, the median survival 27.7 months
The hazard ratio was 2.76 (95% CI 1.44-5.29)
Source: Tu SM, et al. Lancet. 2001;357(9253):336-341.
Slide # 26
Questions in Development of Radiopharmaceuticals
Do they provide clinical benefit(s) and for how long?
Can they be used more than once?
Can effects be enhanced if used BEFORE a patient demonstrates progressive/resistant disease?
Can effects be enhanced if combined with an effective chemotherapy that also functions as a radiosensitizer?
Slide # 27
Rationale
Both chemotherapy and bone-targeted radioisotopes do not eliminate disease in bone
The combination of the two modalities has shown promising results in a randomized Phase II trial
The approved chemotherapy for prostate cancer is a radiosensitizer
The combination of chemotherapy and bone-targeted radioisotopes should be pursued
Slide # 28
MSKCC Combination Study
Approach
Use the approved dosing schedule for docetaxel (q3wk)
Administer Quadramet® with the first cycle and again at 12 weeks
Study Design
Dose escalate docetaxel from 65®75 mg/m2 (2 steps) at a Quadramet dose of 0.5 mCi/kg, then
Dose escalate Quadramet from 0.5®1.0 mCi/kg (2 steps)
First dose cohort treated with no dose limiting toxicity
Slide # 29




























