EXHIBIT 99.4

“Management of Metastatic Breast Cancer to Bone”
Adam Brufsky, MD, PhD
Co-Director, Assistant Professor of Medicine
Magee-Womens Cancer Program at
UPMC Cancer Centers
Pittsburgh, PA
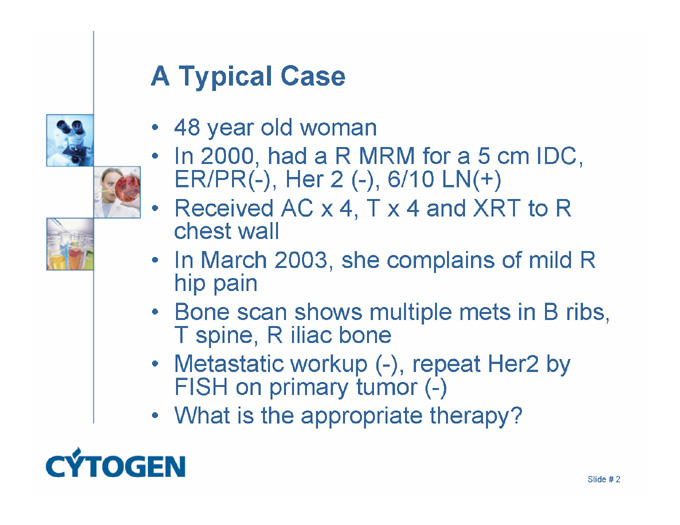
A Typical Case
48 year old woman
In 2000, had a R MRM for a 5 cm IDC, ER/PR(-), Her 2 (-), 6/10 LN(+)
Received AC x 4, T x 4 and XRT to R chest wall
In March 2003, she complains of mild R hip pain
Bone scan shows multiple mets in B ribs, T spine, R iliac bone
Metastatic workup (-), repeat Her2 by FISH on primary tumor (-)
What is the appropriate therapy?
Slide # 2
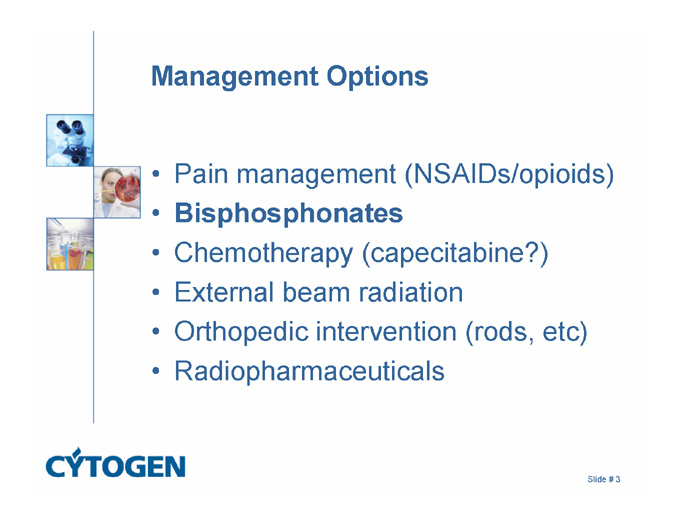
Management Options
Pain management (NSAIDs/opioids)
Bisphosphonates
Chemotherapy (capecitabine?)
External beam radiation
Orthopedic intervention (rods, etc)
Radiopharmaceuticals
Slide # 3
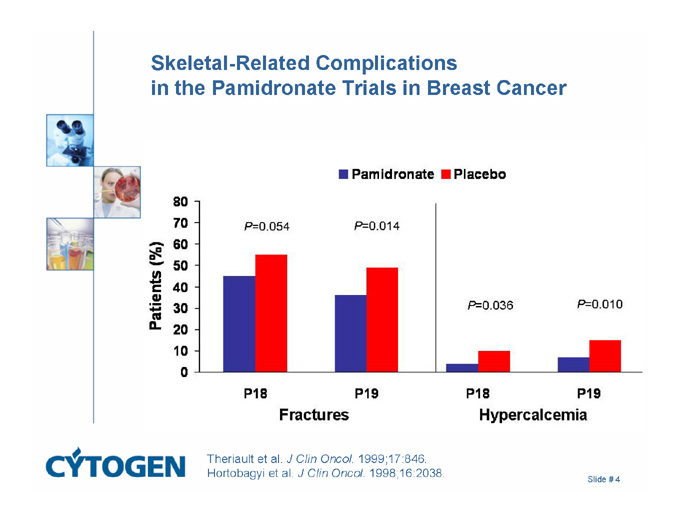
Skeletal-Related Complications in the Pamidronate Trials in Breast Cancer
Pamidronate
Placebo
Patients (%)
Fractures
Hypercalcemia
P=0.054
P=0.014
P=0.036
P=0.010
P18
P19
P18
P19
80
70
60
50
40
30
20
10
0
Theriault et al. J Clin Oncol. 1999;17:846.
Hortobagyi et al. J Clin Oncol. 1998;16:2038.
Slide # 4
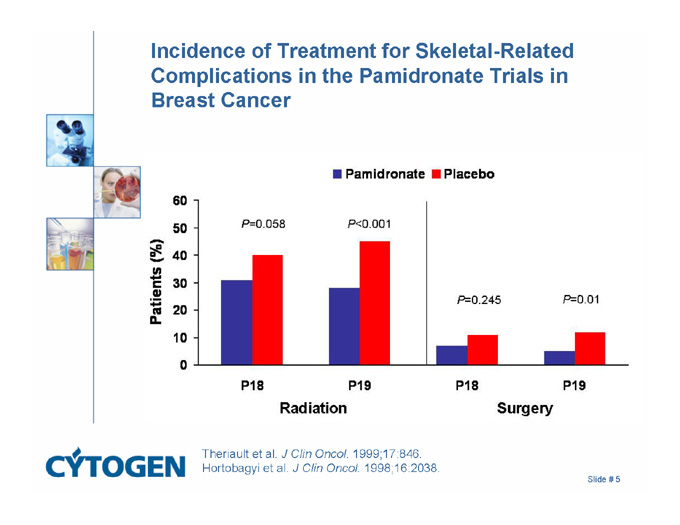
Incidence of Treatment for Skeletal-Related Complications in the Pamidronate Trials in Breast Cancer
Pamidronate
Placebo
Patients (%)
Radiation
Surgery
P=0.058
P<0.001
P=0.245
P=0.01
P18
P19
P18
P19
60
50
40
30
20
10
0
Theriault et al. J Clin Oncol. 1999;17:846.
Hortobagyi et al. J Clin Oncol. 1998;16:2038.
Slide # 5
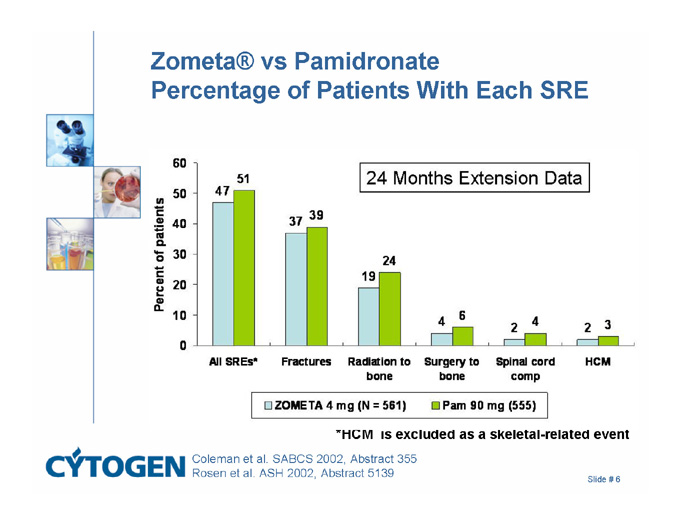
Zometa® vs Pamidronate Percentage of Patients With Each SRE
*HCM is excluded as a skeletal-related event
24 Months Extension Data
Percent of patients
All SREs*
Fractures
Radiation to bone
Surgery to Bone
Spinal cord comp
HCM
47
51
37
39
19
24
4
6
2
4
2
3
60
50
40
30
20
10
0
ZOMETA 4 mg (N = 581)
Pam 90 mg (555)
Coleman et al. SABCS 2002, Abstract 355
Rosen et al. ASH 2002, Abstract 5139
Slide # 6
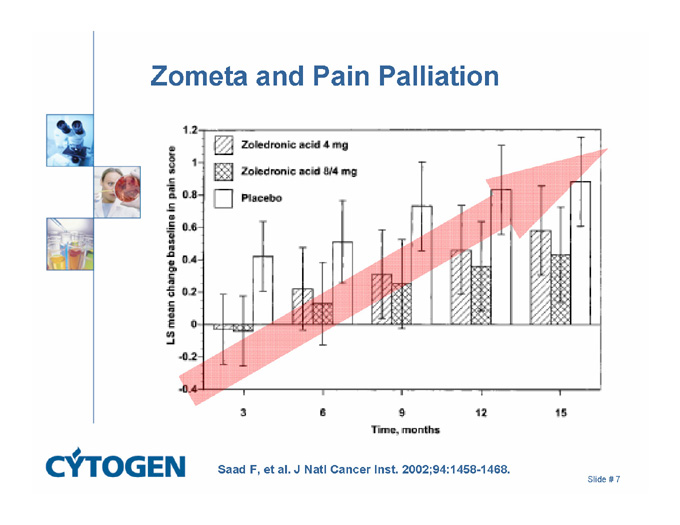
Zometa and Pain Palliation
Zoledronic acid 4 mg
Zoledronic acid 8/4 mg
Placebo
LS mean change baseline in pain score
Time, months
1.2
1
0.8
0.6
0.4
0.2
0
-0.2
-0.4
3
6
9
12
16
Saad F, et al. J Natl Cancer Inst. 2002;94:1458-1468.
Slide # 7
Management Options
Pain management (NSAIDs/opioids)
Bisphosphonates
Chemotherapy (capecitabine?)
External beam radiation
Orthopedic intervention (rods, etc)
Radiopharmaceuticals
Slide # 8
Chemotherapy
Taxanes, vinca alkaloids, capecitabine, anthracyclines
All have potential toxicity
Difficult to measure benefit in bone-only MBC
Risk-benefit ratio in asymptomatic patient
Slide # 9
Management Options
Pain management (NSAIDs/opioids)
Bisphosphonates
Chemotherapy (capecitabine?)
External beam radiation
Orthopedic intervention (rods, etc)
Radiopharmaceuticals
Slide # 10
External Beam Radiation: Local Field
Pain relief efficacy
Periods of complete pain relief in 50% of patients
Partial pain relief in 40% of patients
Onset of pain relief: few days to 4 weeks
>50% of patients with some pain relief have reemergence of pretreatment pain levels
Duration of pain relief: 3 to 6 months
Adverse effect: myelosuppression depending on dose and volume irradiated
Only targets a single area, repeat treatments difficult
Slide # 11
Management Options
Pain management (NSAIDs/opioids)
Bisphosphonates
Chemotherapy (capecitabine?)
External beam radiation
Orthopedic intervention (rods, etc)
Radiopharmaceuticals
Slide # 12
Orthopedic intervention
Elective internal fixation for weight-bearing long bone metastases
Lesions involving 50% or more of the diaphysis
Lesions destroying 50% or more of the cortex
Lesions >2.5 cm in the femoral neck, subtrochanteric, or intertrochanteric region or in supracondylar regions of the femur
Lytic lesions located in high-stress areas
Only targets a specific area, surgical morbidity
Slide # 13

Management Options
Pain management (NSAIDs/opioids)
Bisphosphonates
Chemotherapy (capecitabine?)
External beam radiation
Orthopedic intervention (rods, etc)
Radiopharmaceuticals
Slide # 14

Radiopharmaceuticals in Management of Bone Metastases
Home to areas of increased osteoblastic activity
Primary side effect: transient myelosuppression
Three FDA approved agents
Samarium-153 lexidronam injection (IV)
Strontium-89 chloride injection (IV)
Phosphorus-32 (IV or PO)
Slide # 15
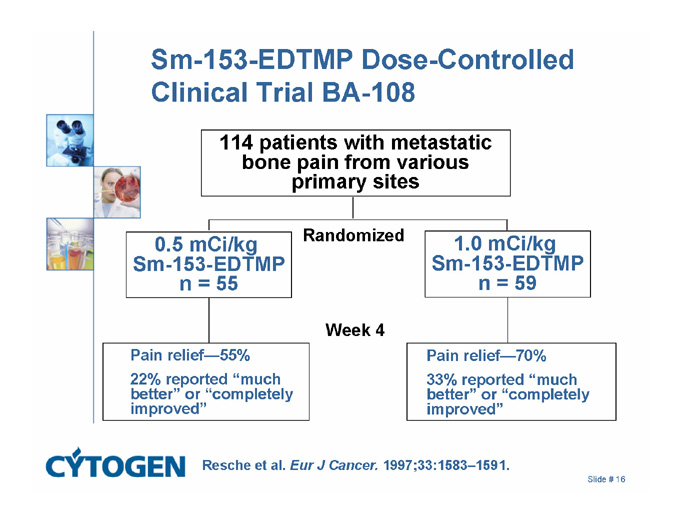
Sm-153-EDTMP Dose-Controlled Clinical Trial BA-108
114 patients with metastatic bone pain from various primary sites
Randomized
0.5 mCi/kg
Sm-153-EDTMP
n = 55
1.0 mCi/kg
Sm-153-EDTMP
n = 59
Week 4
Pain relief - 55%
22% reported “much better” or “completely improved”
Pain relief - 70%
33% reported “much better” or “completely improved”
Resche et al. Eur J Cancer. 1997;33:1583–1591.
Slide # 16
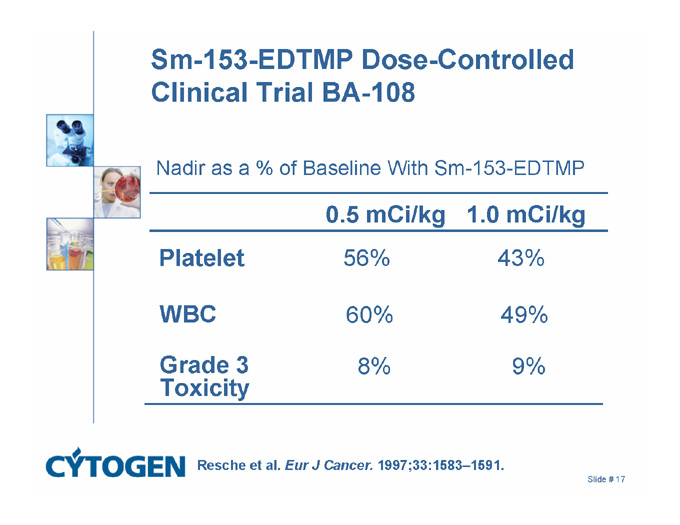
Sm-153-EDTMP Dose-Controlled Clinical Trial BA-108
Nadir as a % of Baseline With Sm-153-EDTMP
0.5 mCi/kg 1.0 mCi/kg
56% 43%
Platelet
60% 49%
WBC
8% 9%
Grade 3
Toxicity
Resche et al. Eur J Cancer. 1997;33:1583–1591.
Slide # 17
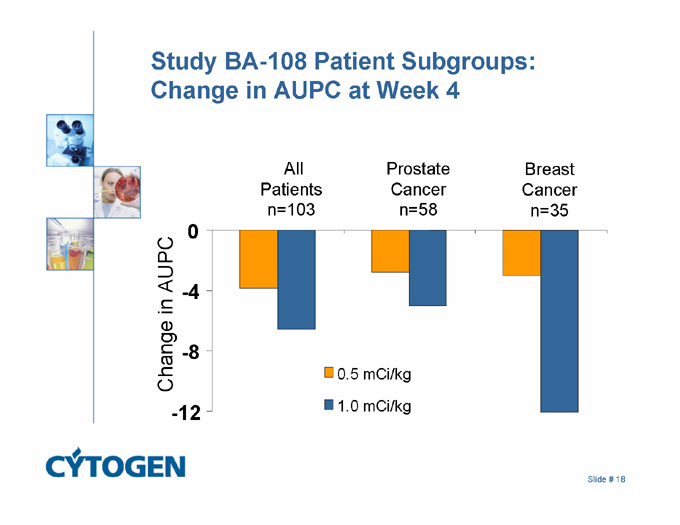
Study BA-108 Patient Subgroups: Change in AUPC at Week 4
All
Patients
n=103
Breast
Cancer
n=35
Prostate
Cancer
n=58
0
-4
Change in AUPC
-8
0.5 mCi/kg
-12
1.0 mCi/kg
Slide # 18
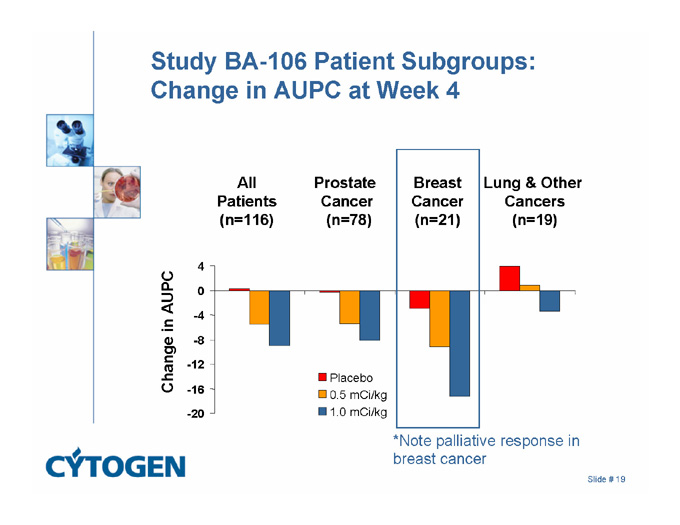
Study BA-106 Patient Subgroups: Change in AUPC at Week 4
Breast
Cancer
(n=21)
Prostate
Cancer
(n=78)
All
Patients
(n=116)
Lung & Other
Cancers
(n=19)
4
0
Change in AUPC
-4
-8
-12
Placebo
0.5 mCi/kg
-16
-20
1.0 mCi/kg
*Note palliative response in breast cancer
Slide # 19
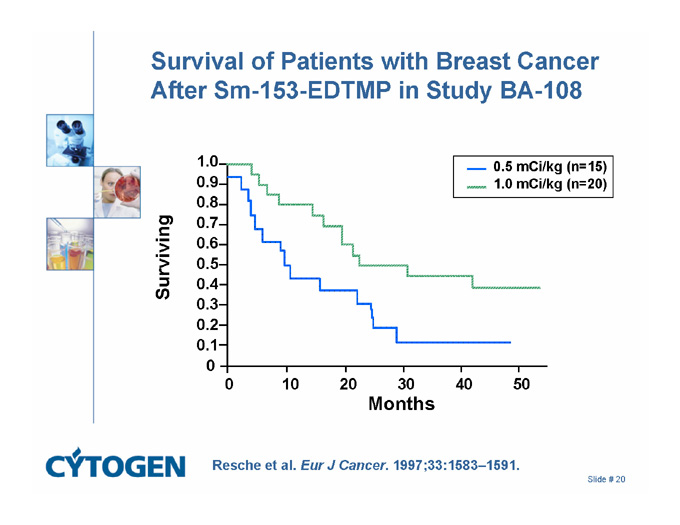
Survival of Patients with Breast Cancer After Sm-153-EDTMP in Study BA-108
0.5 mCi/kg (n=15)
1.0
1.0 mCi/kg (n=20)
0.9
0.8
0.7
0.6
Surviving
0.5
0.4
0.3
0.2
0.1
0
40
30
20
10
0
50
Months
Resche et al. Eur J Cancer. 1997;33:1583–1591.
Slide # 20

What about an antineoplastic effect – aren’t we radiating the tumor?
Should we use radiopharmaceuticals earlier in the course of MBC?
Slide # 21

Barriers to Early Radiopharmaceutical Use
1. Are there negative interactions with bisphosphonates?
2. Are there negative interactions with EBXRT?
3. Can we dose multiple times?
4. Are there negative interactions with chemotherapy?
5. Are there negative interactions with concurrent chemotherapy?
Slide # 22
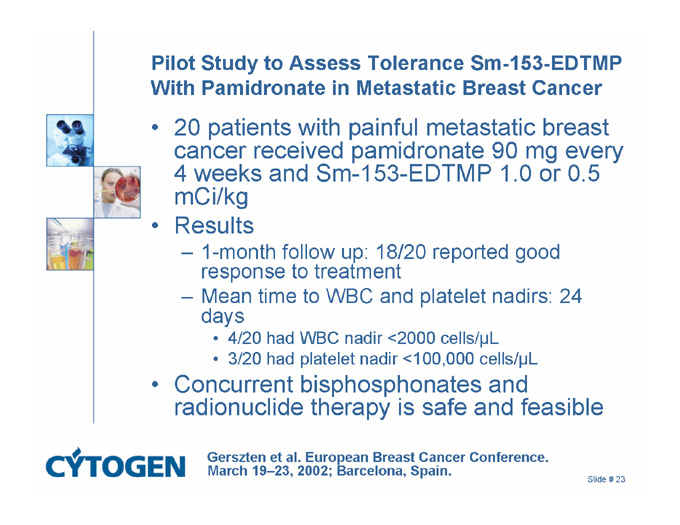
Pilot Study to Assess Tolerance Sm-153-EDTMP With Pamidronate in Metastatic Breast Cancer
20 patients with painful metastatic breast cancer received pamidronate 90 mg every 4 weeks and Sm-153-EDTMP 1.0 or 0.5 mCi/kg
Results
1-month follow up: 18/20 reported good response to treatment
Mean time to WBC and platelet nadirs: 24 days
4/20 had WBC nadir <2000 cells/µL
3/20 had platelet nadir <100,000 cells/µL
Concurrent bisphosphonates and radionuclide therapy is safe and feasible
Gerszten et al. European Breast Cancer Conference. March 19���23, 2002; Barcelona, Spain.
Slide # 23
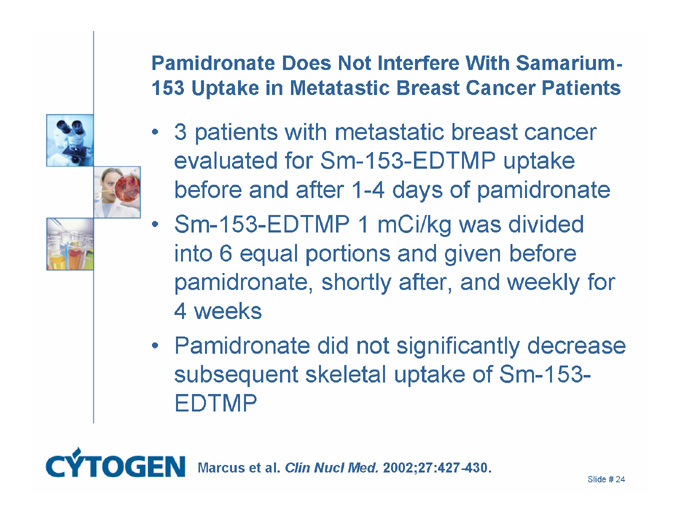
Pamidronate Does Not Interfere With Samarium-153 Uptake in Metatastic Breast Cancer Patients
3 patients with metastatic breast cancer evaluated for Sm-153-EDTMP uptake before and after 1-4 days of pamidronate
Sm-153-EDTMP 1 mCi/kg was divided into 6 equal portions and given before pamidronate, shortly after, and weekly for 4 weeks
Pamidronate did not significantly decrease subsequent skeletal uptake of Sm-153-EDTMP
Marcus et al. Clin Nucl Med. 2002;27:427-430.
Slide # 24
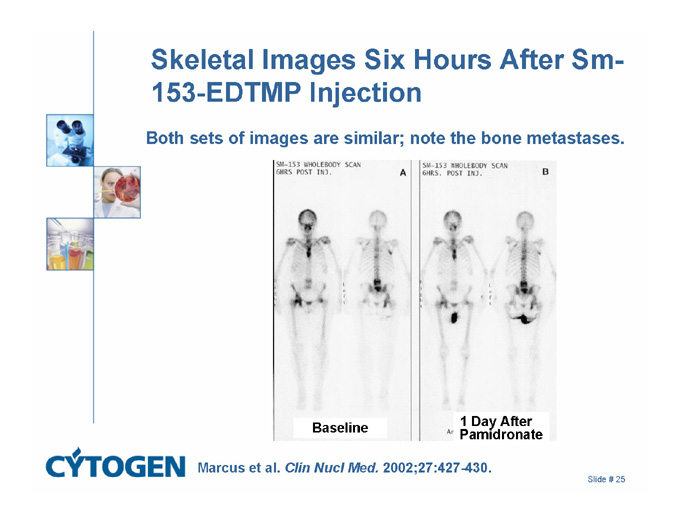
Skeletal Images Six Hours After Sm-153-EDTMP Injection
Both sets of images are similar; note the bone metastases.
Baseline
1 Day After
Pamidronate
Marcus et al. Clin Nucl Med. 2002;27:427-430.
Slide # 25

Barriers to Early Radiopharmaceutical Use
1. Are there negative interactions with bisphosphonates? (no)
2. Are there negative interactions with EBXRT?
3. Can we dose multiple times?
4. Are there negative interactions with chemotherapy?
5. Are there negative interactions with concurrent chemotherapy?
Slide # 26
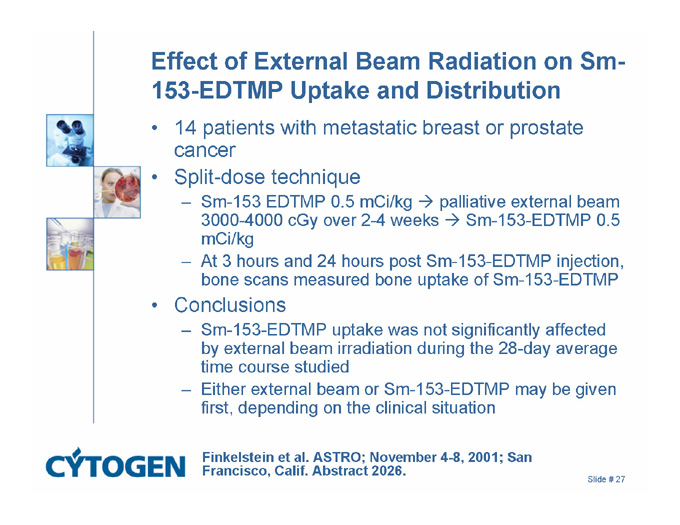
Effect of External Beam Radiation on Sm-153-EDTMP Uptake and Distribution
14 patients with metastatic breast or prostate cancer
Split-dose technique
Sm-153 EDTMP 0.5 mCi/kgð palliative external beam 3000-4000 cGy over 2-4 weeksð Sm-153-EDTMP 0.5 mCi/kg
At 3 hours and 24 hours post Sm-153-EDTMP injection, bone scans measured bone uptake of Sm-153-EDTMP
Conclusions
Sm-153-EDTMP uptake was not significantly affected by external beam irradiation during the 28-day average time course studied
Either external beam or Sm-153-EDTMP may be given first, depending on the clinical situation
Finkelstein et al. ASTRO; November 4-8, 2001; San Francisco, Calif. Abstract 2026.
Slide # 27
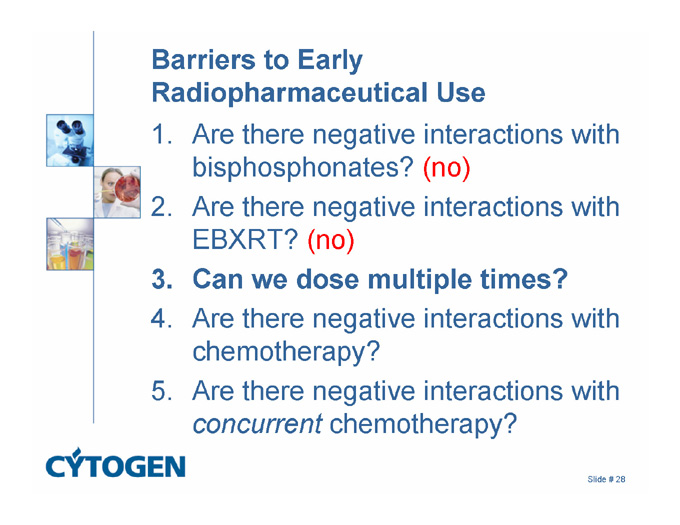
Barriers to Early Radiopharmaceutical Use
1. Are there negative interactions with bisphosphonates? (no)
2. Are there negative interactions with EBXRT? (no)
3. Can we dose multiple times?
4. Are there negative interactions with chemotherapy?
5. Are there negative interactions with concurrent chemotherapy?
Slide # 28
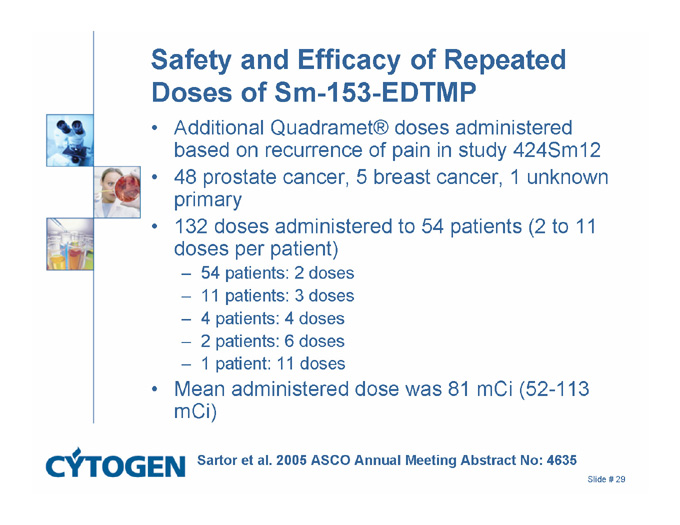
Safety and Efficacy of Repeated Doses of Sm-153-EDTMP
Additional Quadramet® doses administered based on recurrence of pain in study 424Sm12
48 prostate cancer, 5 breast cancer, 1 unknown primary
132 doses administered to 54 patients (2 to 11 doses per patient)
54 patients: 2 doses
11 patients: 3 doses
4 patients: 4 doses
2 patients: 6 doses
1 patient: 11 doses
Mean administered dose was 81 mCi (52-113 mCi)
Sartor et al. 2005 ASCO Annual Meeting Abstract No: 4635
Slide # 29
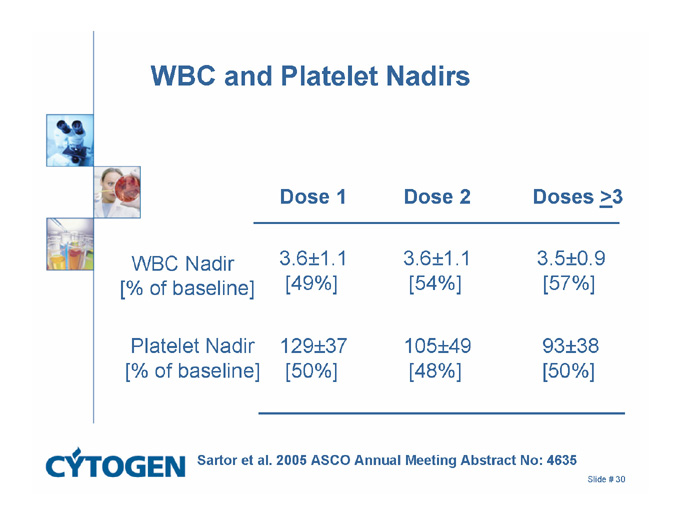
WBC and Platelet Nadirs
Dose 1 Dose 2 Doses >3
3.6±1.1
[54%]
3.6±1.1
[49%]
WBC Nadir
[% of baseline]
3.5±0.9
[57%]
105±49
[48%]
129±37
[50%]
Platelet Nadir
[% of baseline]
93±38
[50%]
Sartor et al. 2005 ASCO Annual Meeting Abstract No: 4635
Slide # 30
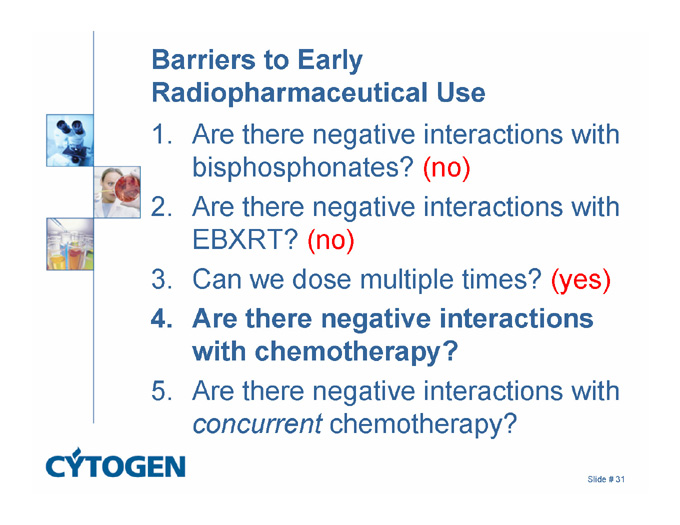
Barriers to Early Radiopharmaceutical Use
1. Are there negative interactions with bisphosphonates? (no)
2. Are there negative interactions with EBXRT? (no)
3. Can we dose multiple times? (yes)
4. Are there negative interactions with chemotherapy?
5. Are there negative interactions with concurrent chemotherapy?
Slide # 31
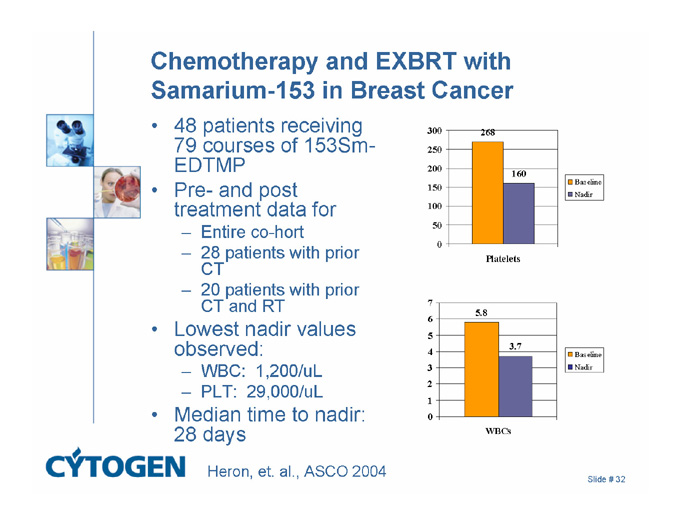
Chemotherapy and EXBRT with Samarium-153 in Breast Cancer
48 patients receiving 79 courses of 153Sm-EDTMP
Pre- and post treatment data for
Entire co-hort
28 patients with prior CT
20 patients with prior CT and RT
Lowest nadir values observed:
WBC: 1,200/uL
PLT: 29,000/uL
Median time to nadir: 28 days
Heron, et. al., ASCO 2004
300
250
200
150
100
50
0
268
160
Platelets
Baseline
Nadir
7
6
5
4
3
2
1
0
5.8
3.7
WBCs
Baseline
Nadir
Slide # 32
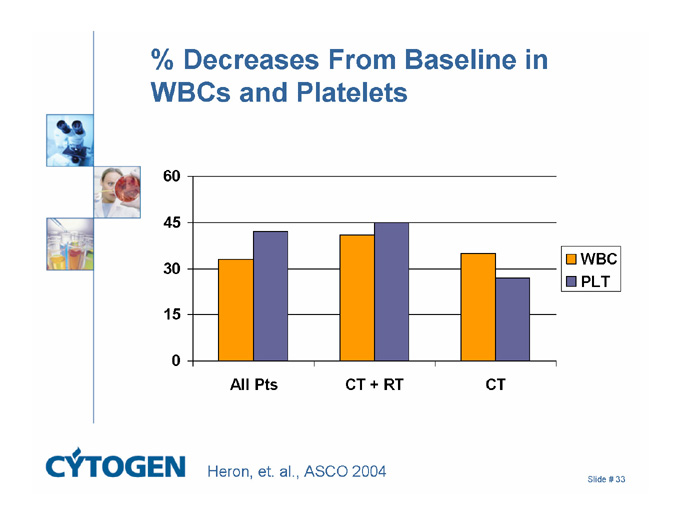
% Decreases From Baseline in WBCs and Platelets
60
45
30
15
0
All Pts
CT + RT
CT
WBC
PLT
Heron, et. al., ASCO 2004
Slide # 33

Barriers to Early Radiopharmaceutical Use
1. Are there negative interactions with bisphosphonates? (no)
2. Are there negative interactions with EBXRT? (no)
3. Can we dose multiple times? (yes)
4. Are there negative interactions with chemotherapy? (no)
5. Are there negative interactions with concurrent chemotherapy? (we’re not sure)
Slide # 34

Bevacizumab is part of the new standard of care for MBC
Bevacizumab is a humanized monoclonal antibody directed against VEGF
Recognizes all VEGF-A isoforms
Active in metastatic colon cancer and NSCLC
Toxicity manageable
A randomized phase III trial of paclitaxel +/- bevaciumab as first line MBC therapy was presented at ASCO 2005
Slide # 35
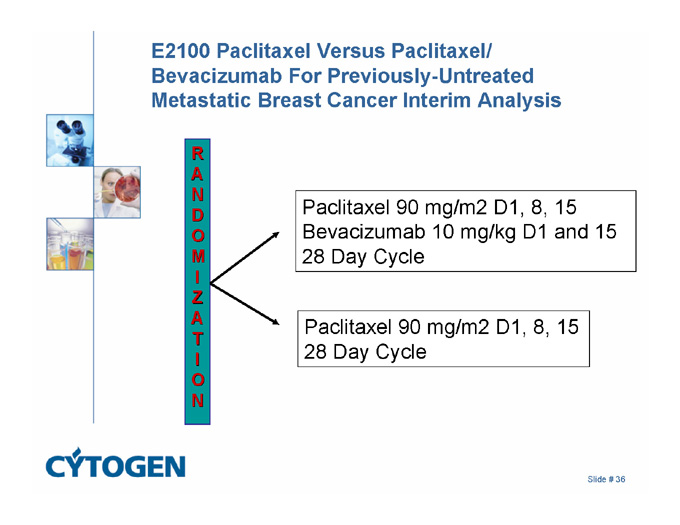
E2100 Paclitaxel Versus Paclitaxel/ Bevacizumab For Previously-Untreated Metastatic Breast Cancer Interim Analysis
RANDOMIZATION
Paclitaxel 90 mg/m2 D1, 8, 15
Bevacizumab 10 mg/kg D1 and 15
28 Day Cycle
Paclitaxel 90 mg/m2 D1, 8, 15
28 Day Cycle
Slide # 36
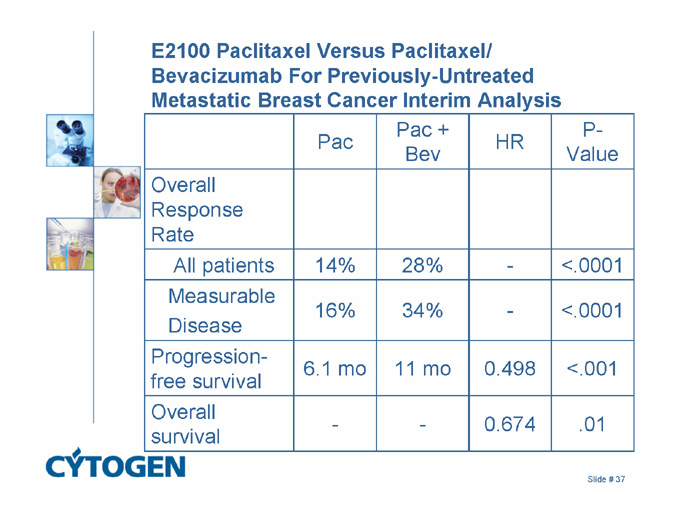
E2100 Paclitaxel Versus Paclitaxel/ Bevacizumab For Previously-Untreated Metastatic Breast Cancer Interim Analysis
Pac
Pac + Bev
HR
P-Value
Overall Response Rate
All patients
14%
28%
-
<.0001
Measurable
Disease
16%
34%
-
<.0001
Progression-free survival
6.1 mo
11 mo
0.498
<.001
Overall survival
-
-
0.674
.01
Slide # 37
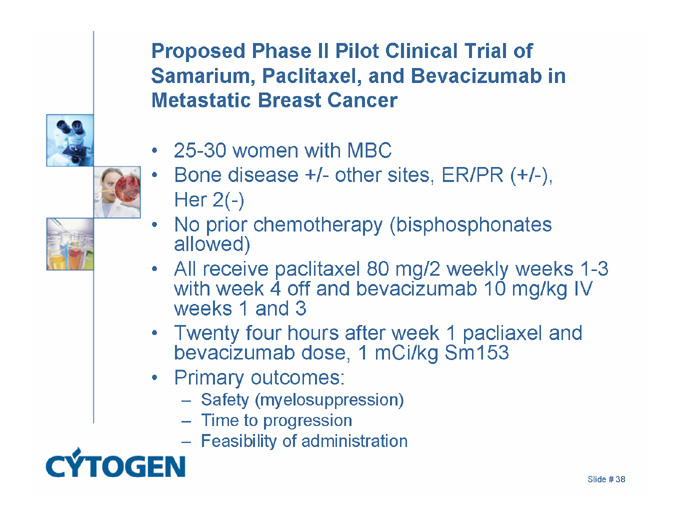
Proposed Phase II Pilot Clinical Trial of Samarium, Paclitaxel, and Bevacizumab in Metastatic Breast Cancer
25-30 women with MBC
Bone disease +/- other sites, ER/PR (+/-),
Her 2(-)
No prior chemotherapy (bisphosphonates allowed)
All receive paclitaxel 80 mg/2 weekly weeks 1-3 with week 4 off and bevacizumab 10 mg/kg IV weeks 1 and 3
Twenty four hours after week 1 pacliaxel and bevacizumab dose, 1 mCi/kg Sm153
Primary outcomes:
Safety (myelosuppression)
Time to progression
Feasibility of administration
Slide # 38
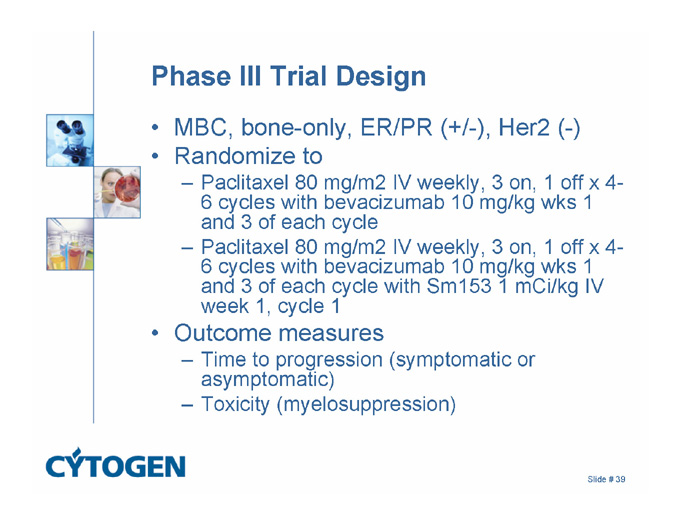
Phase III Trial Design
MBC, bone-only, ER/PR (+/-), Her2 (-)
Randomize to
Paclitaxel 80 mg/m2 IV weekly, 3 on, 1 off x 4-6 cycles with bevacizumab 10 mg/kg wks 1 and 3 of each cycle
Paclitaxel 80 mg/m2 IV weekly, 3 on, 1 off x 4-6 cycles with bevacizumab 10 mg/kg wks 1 and 3 of each cycle with Sm153 1 mCi/kg IV week 1, cycle 1
Outcome measures
Time to progression (symptomatic or asymptomatic)
Toxicity (myelosuppression)
Slide # 39
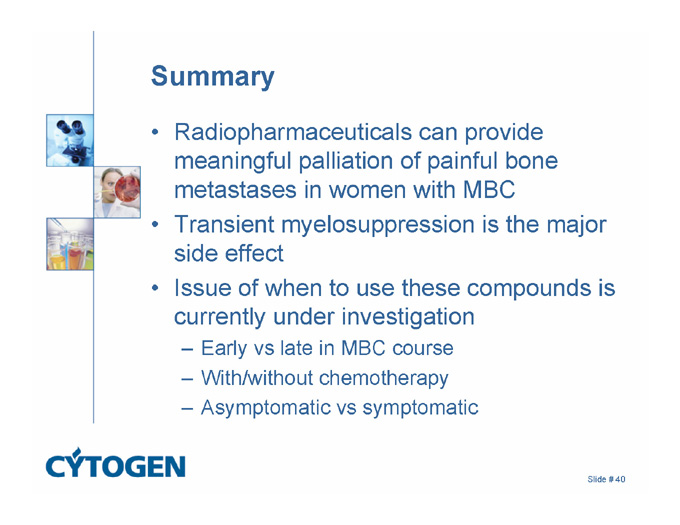
Summary
Radiopharmaceuticals can provide meaningful palliation of painful bone metastases in women with MBC
Transient myelosuppression is the major side effect
Issue of when to use these compounds is currently under investigation
Early vs late in MBC course
With/without chemotherapy
Asymptomatic vs symptomatic
Slide # 40







































