Exhibit 99.7

“Radiolabeled 7E11 antibody targeting prostate-specific membrane antigen (PSMA)”
Howard I. Scher, MD
Chief, Genitourinary Oncology Service
Memorial Sloan-Kettering Cancer Center
New York, NY
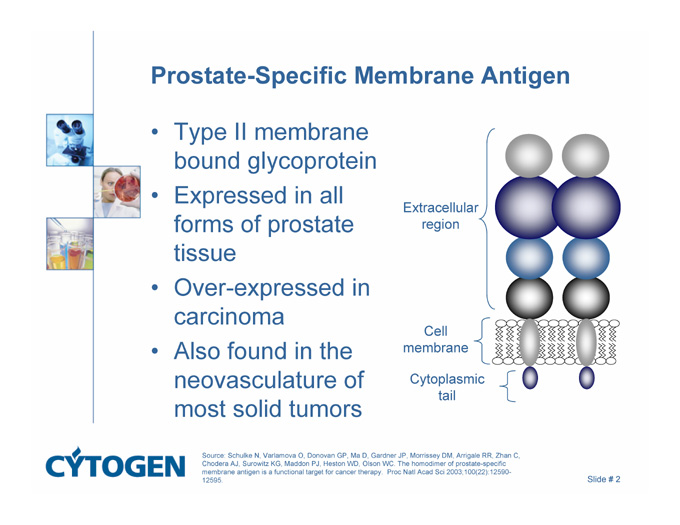
Prostate-Specific Membrane Antigen
Type II membrane bound glycoprotein
Expressed in all forms of prostate tissue
Over-expressed in carcinoma
Also found in the neovasculature of most solid tumors
Extracellular
region
Cell
membrane
Cytoplasmic
tail
Source: Schulke N, Varlamova O, Donovan GP, Ma D, Gardner JP, Morrissey DM, Arrigale RR, Zhan C, Chodera AJ, Surowitz KG, Maddon PJ, Heston WD, Olson WC. The homodimer of prostate-specific membrane antigen is a
functional target for cancer therapy. Proc Natl Acad Sci 2003;100(22):12590-12595.
Slide # 2
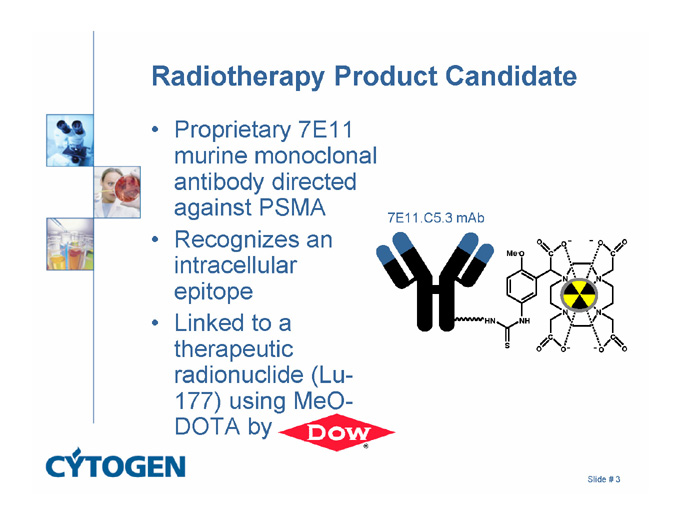
Radiotherapy Product Candidate
Proprietary 7E11 murine monoclonal antibody directed against PSMA
Recognizes an intracellular epitope
Linked to a therapeutic radionuclide (Lu-177) using MeO-DOTA by
7E11.C5.3 mAb
O
O
O
O
e
M
C
C
O
N
N
3
+
7
7
1
u
L
N
N
N
H
H
N
C
C
S
O
O
O
O
Slide # 3

7E11 Monoclonal Antibody
ProstaScint (7E11.C5.3) monoclonal antibody has been safely administered to more than 60,000 patients
7E11 monoclonal antibody has established intellectual property
When linked to a therapeutic radionuclide can deliver lethal dose of radiation to target cells
IND filing expected in Q1 2006
Slide # 4
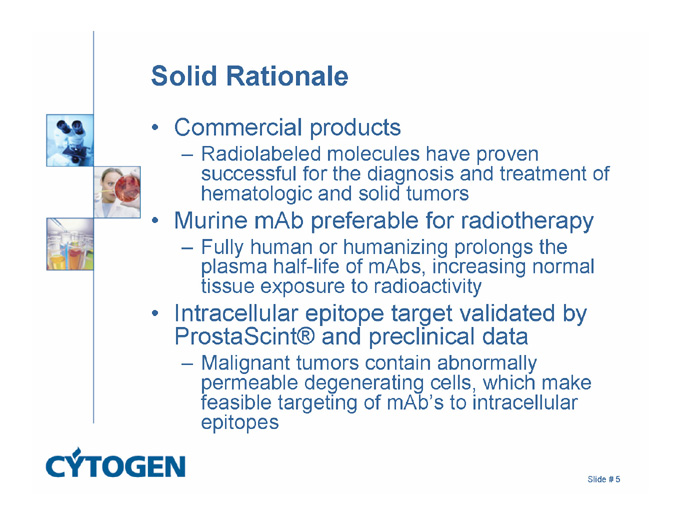
Solid Rationale
Commercial products
Radiolabeled molecules have proven successful for the diagnosis and treatment of hematologic and solid tumors
Murine mAb preferable for radiotherapy
Fully human or humanizing prolongs the plasma half-life of mAbs, increasing normal tissue exposure to radioactivity
Intracellular epitope target validated by ProstaScint® and preclinical data
Malignant tumors contain abnormally permeable degenerating cells, which make feasible targeting of mAb’s to intracellular epitopes
Slide # 5
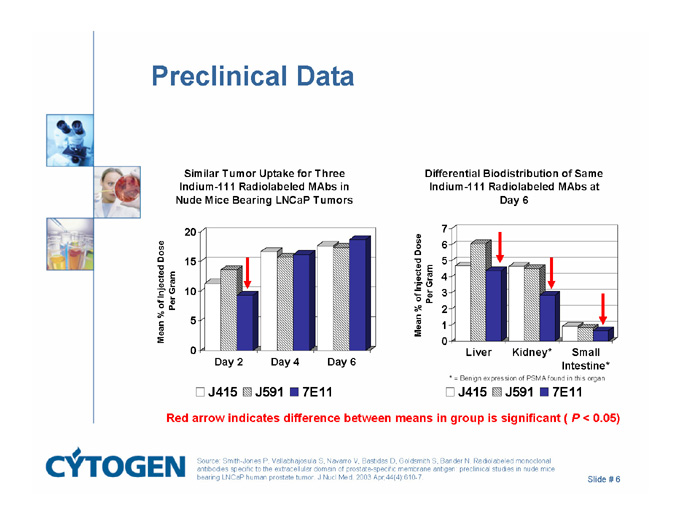
Preclinical Data
* = Benign expression of PSMA found in this organ
Red arrow indicates difference between means in group is significant ( P < 0.05)
Source: Smith-Jones P, Vallabhajosula S, Navarro V, Bastidas D, Goldsmith S, Bander N. Radiolabeled monoclonal antibodies specific to the extracellular domain of prostate-specific membrane antigen: preclinical studies in nude mice bearing LNCaP human prostate tumor. J Nucl Med. 2003 Apr;44(4):610-7.
Slide # 6
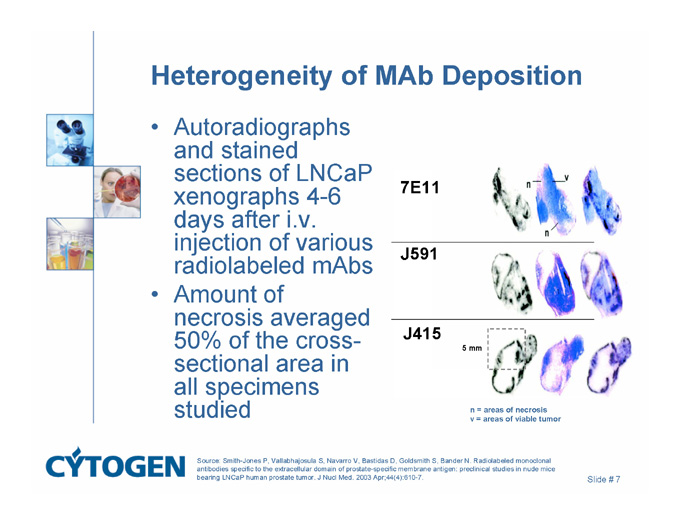
Heterogeneity of MAb Deposition
Autoradiographs and stained sections of LNCaP xenographs 4-6 days after i.v. injection of various radiolabeled mAbs
Amount of necrosis averaged 50% of the cross-sectional area in all specimens studied
7E11
J591
J415
5 mm
n = areas of necrosis
v = areas of viable tumor
Source: Smith-Jones P, Vallabhajosula S, Navarro V, Bastidas D, Goldsmith S, Bander N. Radiolabeled monoclonal antibodies specific to the extracellular domain of prostate-specific membrane antigen: preclinical studies in nude mice bearing LNCaP human prostate tumor. J Nucl Med. 2003 Apr;44(4):610-7.
Slide # 7
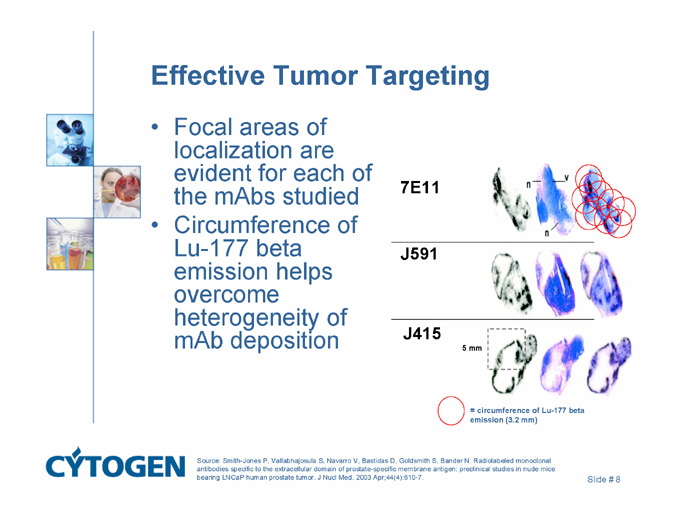
Effective Tumor Targeting
Focal areas of localization are evident for each of the mAbs studied
Circumference of Lu-177 beta emission helps overcome heterogeneity of mAb deposition
7E11
J591
J415
5 mm
= circumference of Lu-177 beta emission (3.2 mm)
Source: Smith-Jones P, Vallabhajosula S, Navarro V, Bastidas D, Goldsmith S, Bander N. Radiolabeled monoclonal antibodies specific to the extracellular domain of prostate-specific membrane antigen: preclinical studies in nude mice bearing LNCaP human prostate tumor. J Nucl Med. 2003 Apr;44(4):610-7.
Slide # 8
Clinical Experience with J591 MAb
Lu-177 labeled J591 mAb
Humanized J591 mAb labeled with Lu-177 using a DOTA based linker/chelator
Escalating doses (10-75 mCi/m2) administered to 35 patients
Dose limiting toxicity was myelosuppression
Maximum tolerated single dose: 70 mCi/m2
Repeated doses tolerated at 30 mCi/m2
PSA responses:
14 progressive disease (PSA increase =25%)
20 stable disease (PSA increase <25%)
4 partial response (PSA decrease =50%)
Median duration of PSA stabilization: 60 days (range 28-601+ days)
Source: Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005 Jul 20;23(21):4591-601.
Slide # 9
Clinical Experience with 7E11 MAb
Initial clinical experience with prior therapeutic 7E11 mAb construct
7E11 mAb labeled with Y-90 using the same GYK-DTPA linker/chelator used in ProstaScint
9 mCi/m2 dose administered to 8 patients
Significant hematologic toxicity associated with loss of radiolabel from antibody
Administration of a chelating agent to scavenge “free” radioisotope (2 patients) significantly reduced hematologic toxicity
Reduction of PSA slope observed in 7/8 patients, however, no PSA declines >50%
Source: Kahn D, Austin JC, Maguire RT, Miller SJ, Gerstbrein J, Williams RD. A phase II study of [90Y] yttrium-capromab pendetide in the treatment of men with prostate cancer recurrence following radical prostatectomy. Cancer Biother Radiopharm. 1999 Apr;14(2):99-111.
Slide # 10
Clinical Experience with 7E11 MAb
Human anti-murine antibodies (HAMA)
The incidence of HAMA following administration of ProstaScint® (7E11 protein dose of 0.5 mg) is < 10%
In the study of Y-90 labeled 7E11 (protein dose of 10 mg) one of the eight patients developed a positive* HAMA titer
In prior ProstaScint studies involving higher doses of 7E11 mAb, the rate of HAMA positivity* was:
0/10 at a protein dose of 5 mg
1/18 at a protein dose of 10 mg
The projected protein dose for the Phase I study of Lu-177 labeled 7E11 is between 5 to 12.5 mg
* Based on a HAMA serum titer >125 ng/mL
Source: Kahn D, Austin JC, Maguire RT, Miller SJ, Gerstbrein J, Williams RD. A phase II study of [90Y] yttrium-capromab pendetide in the treatment of men with prostate cancer recurrence following radical prostatectomy. Cancer Biother Radiopharm. 1999 Apr;14(2):99-111.
Slide # 11
Clinical Experience with 7E11 MAb
Conclusions:
Future radioimmunotherapy studies in this patient population might investigate alternative methods including:
Using a lower dose of 7E11 mAb
Using radionuclides with particulate emissions that would be more likely to deposit their energy within tumor volume, such as I-131 or Lu-177
Cytogen’s current 7E11 mAb development initiative addresses both of these topics
Slide # 12
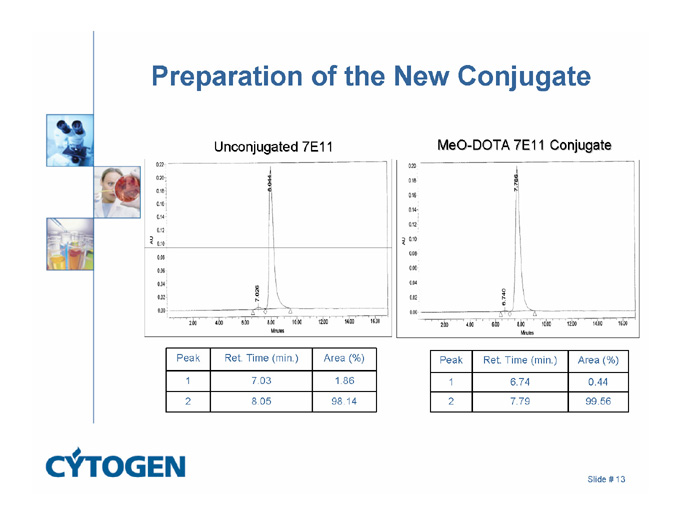
Preparation of the New Conjugate
Unconjugated 7E11
MeO-DOTA 7E11 Conjugate
Peak
Ret. Time (min.)
Area (%)
Peak
Ret. Time (min.)
Area (%)
1
7.03
1.86
1
6.74
0.44
2
8.05
98.14
2
7.79
99.56
Slide # 13
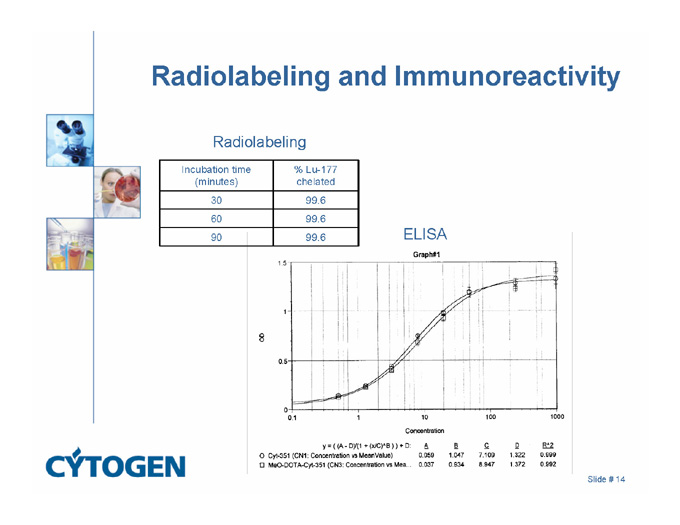
Radiolabeling and Immunoreactivity
Radiolabeling
Incubation time (minutes)
% Lu-177 chelated
30
99.6
60
99.6
90
99.6
ELISA
Slide # 14
Select Future PSMA Therapies
Toxin conjugated fully human mAbs:
Synthetic, cell-killing auristatin ADC payloads licensed from Seattle Genetics
Production of clinical-grade antibodies underway
Viral Vector Vaccine:
Novel alphavirus vaccine
Induces both antibodies and killer T cells
Completing preclinical development activities
Slide # 15














